Although sympathetic activation and altered cardiac β-receptor number and function accompany heart failure, the requirement of cardiac beta2-adrenoceptor (β2AR) expression and its lineage-specific (patho)physiological roles remain unclear. The literature suggests cardio-protective roles of β2AR in attenuating myocyte apoptosis induced by chronic adrenergic stress, hypoxia, and ischemia-reperfusion. 1 β2AR expression is elevated relative to β1AR in heart failure, this is thought to provide a tempting opportunity to treat heart failure with reduced ejection fractions via use of β2AR agonists. 2 However, studies indicate that β2AR contributes to cardiac fibrosis and dysfunction in cardiomyopathy induced by a high fat diet (HFD). 3 This discrepancy highlights a potential dichotomy of β2AR in cardiac remodeling in a cell- and disease-specific manner. To explore these possibilities, we developed lineage-restricted deletion of β2AR in cardiomyocytes (β2AR-cKO) or myofibroblasts (β2AR-fKO) with β2AR-flox mice 4. β2AR-cKO and β2AR-fKO displayed normal cardiac function relative to β2AR-flox controls. When subjected to HFD feeding, all strains displayed comparable cardiac hypertrophy accompanied by similar body weight gain and glucose intolerance (Figure 1A). However, β2AR-cKO hearts were the most fibrotic while β2AR-fKO hearts were least affected (Figure 1B). Consequently, β2AR-cKO mice had impaired cardiac function relative to β2AR-flox controls while β2AR-fKO mice displayed a reverse cardiac phenotype (Figure 1A). RNAseq analysis revealed that HFD-fed β2AR-cKO and β2AR-flox hearts display alterations in gene expression of pathways associated with cardiac remodeling, including pathways for extracellular matrix (ECM) disorganization. In contrast, HFD-fed β2AR-fKO hearts had minimal changes in gene expression relative to normal chow (NC) fed controls (Figure 1C). These data highlight the divergent and lineage-restricted roles of myofibroblast versus cardiomyocyte expression of β2AR in HFD-induced cardiac remodeling. To examine whether these observations are HFD-specific, we subjected mice to myocardial infarction. β2AR-cKO hearts display larger infarct size with concomitant increased fibrosis and reduced ejection fraction relative to β2AR-flox controls, whereas β2AR-fKO display the opposite phenotype with smaller infarct size, less fibrosis, and a higher ejection fraction (Figure 1D). Thus, myofibroblast versus cardiomyocyte β2AR expression display disparate effects on cardiac remodeling in diverse pathological models. We then explored whether mouse embryonic fibroblasts (MEFs) from WT and β2AR-KO mice may differentially affect profibrotic transforming growth factor β (TGF-β) signaling. Deleting β2AR did not affect TGF-β-induced canonical Smad pathways but significantly attenuated non-canonical MAPK/ERK profibrotic pathway 5 (Figure 1E). β2AR deletion also resulted in diminished pro-fibrotic connective tissue growth factor (CTGF) and α smooth muscle actin (α-SMA) deposition. In agreement, HFD promoted MAPK/ERK signaling in β2AR-flox hearts relative to NC, which was attenuated in β2AR-fKO mice (Figure 1E). Together, our data highlight a detrimental role of β2AR in promoting profibrotic TGF-β-MAPK pathway in myofibroblasts, opposing the cardio-protective role of β2AR in cardiomyocytes. Our results highlight the cell-type specific β2AR signaling in regulating cardiac remodeling and identify important potential pitfalls in treating comorbidities of lung and heart diseases via use of β2AR agonists.
Figure.
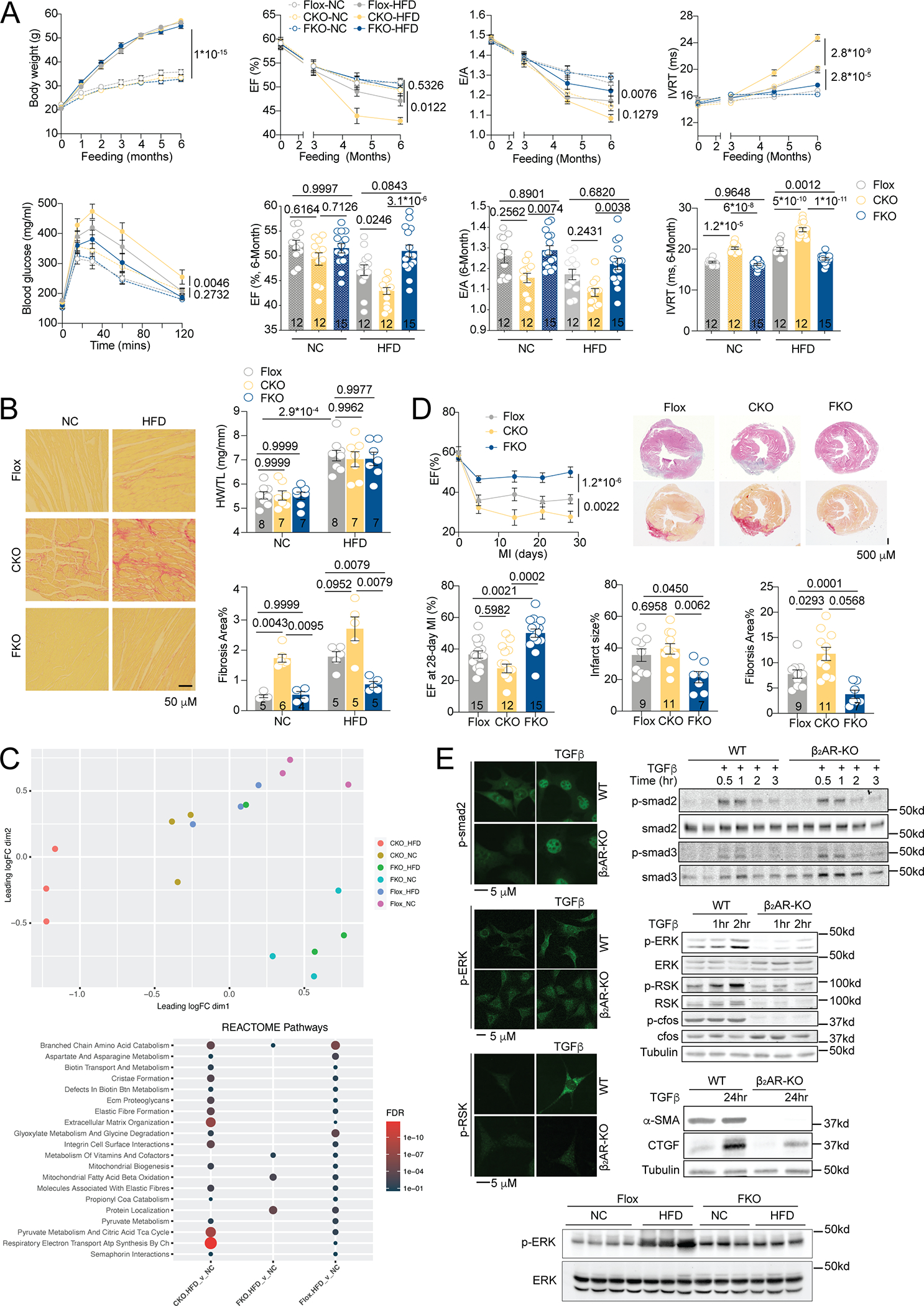
A) Quantification of body weights during six months HFD in β12AR-flox and β2AR-cKO and fKO transgenic mice. Plasma glucose levels were measured after a 6-hour fast followed by intraperitoneal injection of glucose (1g/kg), and cardiac function was measured by echocardiography, including EF, E/A ratio, and IVRT.
B) Picrosirius-red staining for collagen in heart tissue was imaged and quantified; heart weights were normalized to tibia lengths.
C) RNAseq analysis of β2ARflox, cKO and fKO mouse hearts after NC and HFD feeding. Principal Component Analysis (PCA) and affected reactome pathways are summarized (NCBI #PRJNA891331). The circle size and color correspond to the FDR in reactome pathway analysis.
D) After MI surgery, hematoxylin and eosin and picrosirius-red staining of heart collagen was imaged and quantified; cardiac EF was measured by echocardiography in β2ARflox, cKO and fKO mice.
E) Immunofluorescent and western analysis of phospho- and total smad2, smad3, ERK, RSK, and cfos, and CTGF and α-SMA in overnight serum starved WT and β2AR-KO MEFs following stimulation with TGFβ (10 ng/ml) for indicated times and in NC- and HFD-fed β2AR-flox and fKO mouse hearts in vivo.
Images are representative of mean quantified data. Dot-plots show mean ± SEM; and the numbers of independent samples are indicated. For fibrosis data in Figure 1B, p values were obtained by Mann Whitney test. All other datasets passed the Shapiro-Wilk normality test. p values were obtained after repeated measures AVNOA (time courses), two-way (Figure 1A, dot plots), or one-way (Figure 1D, dot plots) ANOVA followed by Tukey’s test.
Sources of funding
This work was supported by NIH-HL127764, HL112413, HL147263, HL148165, and VA-BX005100 and AHA postdoctoral fellowships (YW, CZ).
Footnotes
Disclosure
None.
Data Availability
The methods, data, and materials are available upon request.
β2AR-flox, β2AR-cKO (MHC-cre, JAX, 009074), β2AR-fKO (Postn-cre, Conway SJ at Indiana University) male mice (6–8-week-old) were randomly assigned to 6-month HFD or NC (D12492 and D12450B, Research Diets) feeding as females are resistant to HFD-induced cardiomyopathy. Male mice (10–12-week-old) were randomly assigned for myocardial infarction via ligation of left descending coronary artery for 4-week. Heart and body weights, glucose tolerance test, hematoxylin and eosin and picrosirius-red staining, and echocardiogram were performed. Data were blinded for statistical analysis. Animal procedures were performed according to the Guide for the Care and Use of Laboratory Animals (NIH) and approved by the University of California Davis IACUC.
References:
- 1.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK and Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98:1607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao RP, Zhang SJ, Chakir K, Avdonin P, Zhu W, Bond RA, Balke CW, Lakatta EG and Cheng H. Enhanced G(i) signaling selectively negates beta2-adrenergic receptor (AR)--but not beta1-AR-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation. 2003;108:1633–9. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Liu Y, Fu Q, Xu B, Zhang Y, Kim S, Tan R, Barbagallo F, West T, Anderson E, et al. Inhibiting Insulin-Mediated beta2-Adrenergic Receptor Activation Prevents Diabetes-Associated Cardiac Dysfunction. Circulation. 2017;135:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG Jr., Chua SC Jr., Kim JK, Kaestner KH and Karsenty G. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schafer S, Viswanathan S, Widjaja AA, Lim WW, Moreno-Moral A, DeLaughter DM, Ng B, Patone G, Chow K, Khin E, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The methods, data, and materials are available upon request.
β2AR-flox, β2AR-cKO (MHC-cre, JAX, 009074), β2AR-fKO (Postn-cre, Conway SJ at Indiana University) male mice (6–8-week-old) were randomly assigned to 6-month HFD or NC (D12492 and D12450B, Research Diets) feeding as females are resistant to HFD-induced cardiomyopathy. Male mice (10–12-week-old) were randomly assigned for myocardial infarction via ligation of left descending coronary artery for 4-week. Heart and body weights, glucose tolerance test, hematoxylin and eosin and picrosirius-red staining, and echocardiogram were performed. Data were blinded for statistical analysis. Animal procedures were performed according to the Guide for the Care and Use of Laboratory Animals (NIH) and approved by the University of California Davis IACUC.


