Abstract
Summary background
The coronavirus 2019 pandemic was caused by a new single-strand RNA virus that originated from Wuhan, China, and infected more than 190 countries. The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) coinfection with tuberculosis posed a serious public health concern and complicated the prognosis and treatment of patients. Since both are respiratory diseases, the sign and symptoms may overlap and could have synergistic effects on the host that can increase mortality during coinfection. The present investigation reported the clinical characteristics of patients having coinfection of COVID-19 and tuberculosis (COVID-TB).
Methods
We performed a retrospective investigation on COVID-19 infection in tuberculosis patients between the years 2020 and 2021. The SARS-CoV-2 was confirmed by PCR and the COVID-TB epidemiological and clinical findings were recorded on the day of admission and followed up for 25 days.
Results
The mean age of the COVID-19 patients was 50 ± 15 years, 76.36% were male and 23.64% were female. Weight loss, sore throat, whooping cough, chest pain, and vomiting were common symptoms, and asthma, diabetes, arthritis, and hypertension were found as co-morbidities in COVID-TB. The D-dimer, lactate dehydrogenase, C-reactive protein, erythrocyte sedimentation rate, and creatine kinase levels increased 14-fold, 12.5-fold, 11-fold, 10-fold, and 7-fold respectively during COVID-TB. The patients suffered from hyperferritinemia and lymphocytopenia which increased the likelihood of death. The levels of D-dimer, lactate dehydrogenase, C-reactive protein, erythrocyte sedimentation rate, and creatinine kinase were positively correlated with patient age. The chest radiograph showed the infectious agents have consolidated opacity and peripheral dissemination in the lungs.
Conclusion
Tuberculosis coinfection augmented the severity of COVID-19 and the likelihood of death, and high vigilance is recommended for respiratory pathogens in COVID-19.
Keywords: SARS-CoV-2, Tuberculosis, D-dimer, Lactate dehydrogenase, Creatine kinase, Erythrocyte sedimentation rate
1. Introduction
Coronaviruses are emerging pathogens that have stopped the world in a unidirectional way and caused enormous human losses and socioeconomic damage to the world economy. Coronaviruses can cause disease in humans, bats, pigs, cats, dogs, horses [1], [2], and target the respiratory tract, gastrointestinal tract, lungs, and can cause neurological disorders in their hosts [3]. To date, bats are considered natural reservoirs while snakes and pangolins are proposed to be the intermediate hosts [4]. The study of coronaviruses begins in the 1960 s when two Human coronaviruses HCoV-229E and HCoV-OC43 were identified as the causative agents of respiratory illnesses [5], [6]. In 2003, the severe acute respiratory syndrome coronavirus (SARS-CoV) epidemic occurred in China which affected around 30 countries and caused approximately 800 deaths [6]. In 2019, the outbreak of an unknown agent that caused severe pneumonia in patients in Wuhan, China, leads to a global pandemic. The unknown agent has been named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses [7]. The SARS-CoV-2 showed an 89% sequence identity with Bat SARS-CoV and 82% resemblance with human SARS-CoV. The reports from China revealed that the virus spread from the Wuhan seafood market from animal sources to humans and due to the annual spring festival when a large number of people travel to their homes in China, it disseminated in different cities and took the shape of the epidemic [8], [9]. Overall, SARS-CoV-2 is the seventh coronavirus with the ability to replicate in the upper and lower respiratory tract [10]. The clinical data from various reports suggested that COVID-19 patients with preexisting comorbidities are at higher risk such as diabetes patients [11], arthritis patients [12], hypertension, cardiovascular, and lung diseases posed serious health complications in COVID-19 patients [13]. Tuberculosis (TB) is an infectious disease widely present in developing countries and had caused greater health risks during the COVID-19 pandemic [14]. Although COVID-19 and TB are caused by two different etiological agents, they shared some common attributes during infections such as the route of transmission via respiratory droplets, symptoms such as coughing, infection site lungs, and diagnosis methods via PCR are technically similar [15], [16]. TB was the most common cause of death for a single infectious pathogen in the world before COVID-19 but the pandemic has disrupted TB control programs and global spending on essential TB services [15]. Thus, SARS-CoV-2 emerged as the most contagious pathogen at present and the clinical characteristics of patients infected with COVID-TB are of high importance for medical research. Bacterial coinfection is defined as the concurrent infection with the initial infection and several pathogens have caused coinfection in COVID-19 patients including Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Pseudomonas aeruginosa, Haemophilus influenzae, and Mycobacterium tuberculosis [17], [18], [19]. So far, few countries reported COVID-19 coinfection with TB and their clinical characteristics, for instance, researchers from Italy reported 49 cases of COVID-TB of which 26 patients had TB before and 14 had TB after SARS-CoV-2 infection [16]. A meta-analysis of 36 studies reported 89 COVID-TB cases of which 88.76% were active TB, 8.99% were previous TB, and 2.25% cases were latent TB [20]. From India, 32 cases of COVID-19 with previously diagnosed TB were reported of which 15 had pulmonary TB whereas 12 had extrapulmonary TB [21]. A cross-sectional study from China reported 45 cases of COVID-TB, with 100% active TB, and severe comorbidities [22]. Pakistan is the sixth-largest country by population with more than 220 million people and has faced the most difficult epidemic that had caused lockdowns across the country and a shortage of medical facilities in combating the SARS-CoV-2 [23], [24]. Pakistan ranked 53 among coronavirus-affected countries as of February 15, 2023, and accounted for 5.8% of global TB cases [25]. Although tuberculosis cases are regularly reported in Pakistan, the demographic and clinical characteristics of TB patients with COVID-19 illness have not been reported. In a previous study, we investigated the COVID-19 epidemic in Pakistan and revealed that the warm and humid climate of Pakistan paused the community transmission of SARS-CoV-2 [24]. We also revealed the emergence of antibiotic-resistant strains in COVID-19 patients and documented S. aureus superinfections as a greater risk for patient health [18]. In the present study, we evaluated the clinical characteristics of COVID-TB infection such as signs and symptoms, demographic and clinical characteristics, laboratory indices, and comorbidities in COVID-TB patients. Since Pakistan is a developing country with a high number of TB cases emerging each year (510000 cases/year) according to World Health Organization [26], therefore, it is highly noteworthy to explore the clinical features of COVID-TB in Pakistan to increase the understanding of the coinfection.
2. Methods
2.1. Data collection and laboratory tests of COVID-19 patients
A total of 165 patient data were collected from four hospitals in Khyber-Pakhtunkhwa and three hospitals in Sindh. Among the 165 COVID-TB cases, 76.36% (126/165) were male and 23.64% (39/165) were female. The data on TB was obtained from the patient's medical history, and COVID-19 was confirmed as we previously described [18]. COVID-TB patient’s data was monitored from the day of admission to 25 days due to the limited stay period of patients at hospitals. In exclusion criteria, those COVID-TB cases that showed major clinical symptoms of the SARS-CoV-2 but were not confirmed by COVID-19 polymerase chain reaction (PCR), were not included in this study. Those COVID-19 cases that were confirmed by PCR testing but remain asymptomatic for TB or TB was not confirmed by GENE-XPERT in medical records, were also excluded. The inclusion criteria include TB confirmation through GENE-XPERT in medical records and further SARS-CoV-2 testing and validation by PCR. The general information, laboratory tests, chest radiology, and treatment were performed by relevant hospitals and diagnostic labs. All the blood biochemical tests such as; D-dimer, ferritin, lactate dehydrogenase (LDH), creatine kinase (CK), erythrocyte sedimentation rate (ESR), alanine aminotransferase (ALT), blood urea, serum creatinine, alkaline phosphatase (ALP), troponin-I, C-reactive protein (CRP), lymphocytes, neutrophils, monocytes, eosinophils, RBCs, WBCs, and platelets counts were performed on the day of admission and followed up for 25 days.
2.2. RNA extraction and real-time PCR
The COVID-TB patient samples were collected with nasopharyngeal and oropharyngeal swabs. The RNA was extracted using the TANBead nucleic acid extraction kit. The genesig real-time PCR coronavirus kit (Primerdesign Ltd, UK) was used for the detection of COVID-19 viral RNA. The PCR program (55 °C for 10-min, 95 °C for 2-min, 45-cycles of 95 °C for 10-sec, and 60 °C for 60-sec) was run by the Agilent AriaMx RT-PCR G-8830 system according to the kit manufacturer instructions.
2.3. Statistical analysis
The data was statistically analyzed and represented as means ± standard deviations or means and range values. The correlation between patient age and laboratory parameters was determined by Pearson correlation and aligned by linear regression by Graph pad prism 8. P < 0.05 was considered significant.
3. Results
3.1. Demographic assessment of COVID-TB patients
Pakistan detected the first SARS-CoV-2 case in late February 2020, and the SARS-CoV-2 recovered from Pakistan was sequenced that revealed the virus showed more than 99% sequence similarity with Wuhan, China SARS-CoV-2 (accession number MT500122). Among the 165 COVID-TB cases, the mean age was 50 ± 15; 76.36% were males and 23.64% were females. TB was confirmed from the medical records as well as from patient caregivers and their relatives who accompany them. The COVID-19 clinical symptoms were evident before hospital admission and patients were using TB control medicines and had other coexisting illnesses as well. All the COVID-TB cases were active TB, among which 88% were of pulmonary TB while 12% reported extrapulmonary TB. Among 165 COVID-TB patients, 56.36% showed comorbidities such as 18.1% with diabetes, 15.1% with asthma, 13.9% had hypertension, 4.2% with liver diseases (hepatitis B & C), 3% had pulmonary, and 2% with kidney disease. All the COVID-TB patients showed typical symptoms of COVID-19 infection on the day of admission such as high fever (38 °C–39.5 °C), chest pain, whole body aches, loss of appetite, dyspnea, etc. whereas whooping cough was detected in 92.1% patients, weight loss in 73.9%, sore throat in 81.8%, and vomiting in 78.78% COVID-TB patients. Also, 61.2% of COVID-TB patients got infection by SARS-CoV-2 asymptomatic carrier and 26.5% by someone who was presymptomatic. Most of the cases were from urban areas (74.55%) compared to rural areas (25.45%) (Table 1).
Table 1.
The table provides demographic and clinical data of COVID-TB patients.
| Characteristics of patients | Number of patients (%) | |
|---|---|---|
| Mean age in years | 50 ± 15 | |
| Male | 76.36 | |
| Female | 23.64 | |
| Asthma | 15.1 | |
| Diabetes | 18.1 | |
| Hypertension | 13.9 | |
| Liver diseases (hepatitis B & C) | 4.2 | |
| Whooping cough | 92.1 | |
| Dyspnea | 80 | |
| Flu | 6.6 | |
| Sore throat | 81.8 | |
| Vomiting | 78.78 | |
| Weight loss | 73.9 | |
| Chest pain | 91.5 | |
| Body aches | 72.7 | |
| Loss of taste | 10.3 | |
| Loss of appetite | 86 | |
| Difficulty in walking | 83.6 | |
| Fever (38 °C–39.5 °C) | 80 | |
| Heart rate >95 beats per mint | 52.1 | |
| Lymphocytopenia | 90.9 | |
| Neutrophilia | 89.6 | |
| Infected by asymptomatic carrier | 61.2 | |
| Infected by presymptomatic carrier | 26.5 | |
| Aged (≥50-years) | 60.6 | |
| Young (<50-years) | 39.4 | |
| Mortality in patients aged ≥ 50-years | 20 | |
| Mortality in patients aged < 50-years | 8.4 | |
| Urban area COVID-19 cases | 74.5 | |
| Rural area COVID-19 cases | 25.45 | |
| Died | 18.78 | |
| Recovered | 81.2 | |
| Laboratory indices | Mean (Interquartile range) | Reference values |
| WBC | 14e103 (8–34 e103/µl) | 4–11 e103/µl |
| RBC | 4e106 (3–7.5 e106/µl) | 4–6 e106/µl |
| Platelets | 240e103(210–390 e103/µl) | 150–450 e103/µl |
| Neutrophils | 82.51 (70–98%) | 40–75% |
| Lymphocytes | 10.36 (6–14%) | 20–45% |
| Monocytes | 1.52 (0.8–10%) | 2–10% |
| Eosinophils | 0.29 (0.2–0.5%) | 0–0.6% |
| D-dimer | 3306 (430–8500 ng/ml) | 200 ng/ml |
| LDH | 1463 (350–2680 U/L) | 91–180 U/L |
| Ferritin | 1671 (510–2200 ng/ml) | 30–400 ng/ml |
| CK | 1348 (90–3033 U/L) | 40–175 U/L |
| ESR | 98.94 (70–120 mm/h) | 1–20 mm/h |
| ALT | 124 (17–310 U/L) | 10–50 U/L |
| ALP | 126.5 (45–290 U/L) | 40–130 U/L |
| CRP | 33 (8–45 mg/L) | <6.0 mg/L |
| Troponin-I | 0.87 (0.7–1.5 ng/ml) | <0.6 ng/ml |
| Blood urea | 23.5 (18–55 mg/dl) | 18–45 mg/dl |
| Creatinine | 0.98 (0.7–1.5 mg/dl) | 0.5–1.08 mg/dl |
3.2. An elevated level of blood biomarkers was detected in COVID-TB patients
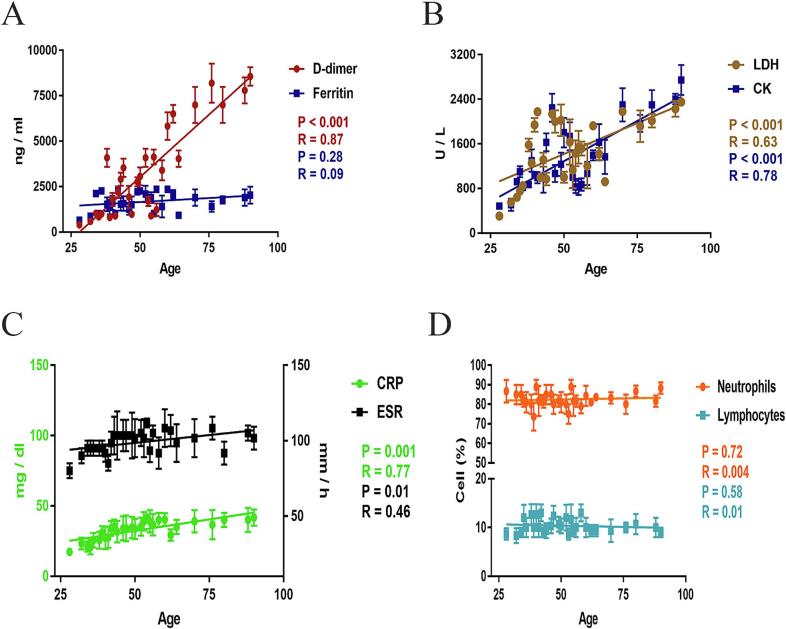
Among 165 subjects, the average increase in D-dimer, LDH, CRP, ESR, CK, and ferritin levels was 14-fold, 12.5-fold, 11-fold, 10-fold, 7-fold, and 6.5-fold respectively compared to the reference values. The blood indicators mean values were; D-dimer was 3306 ng/ml, LDH was 1463 U/L, CK was 1348 U/L, CRP was 33 mg/L, ESR was 98.94 mm/h, and ferritin was 1671 ng/ml in COVID-TB patients (Table 1). The mean values of ALT and ALP were 124 U/L and 126.5 U/L respectively while troponin-1, creatinine, and blood urea values did not show any significant changes (Table 1). The immune system of patients was suppressed by the SARS-CoV-2 coinfection with Mycobacterium and the patients suffered lymphocytopenia and neutrophilia. The lymphocyte count in COVID-TB patients was 6–14% compared to the reference values of 20–45% while the neutrophils percentage was 70–98% compared to the reference value of 40–75%. The lymphocyte and the neutrophil mean values were 10.36% and 82.51% respectively. The WBC cell count was 8–34 e103/µl compared to the reference value of 4–11 e103/µl while the eosinophil, monocytes, platelets, and RBCs cell counts were normal (Table 1). Moreover, we found that the D-dimer, LDH, ESR, CRP, and CK were positively correlated with patient age and significantly increased (P < 0.001) in aged patients (≥50 years) compared to the young ones (<50 years) (Fig. 1A, B, & C) while ferritin level, ALT, ALP, neutrophils, and lymphocyte were not changed significantly (Fig. 1A & D). The D-dimer, LDH, ESR, CRP, and CK values give raised a linear slope with significant changes with the increase of age while the ferritin, ALT, ALP, neutrophils, and lymphocytes values were not elevated significantly. Further, we observed that the chest radiographs of COVID-TB patients showed bilateral pulmonary opacities with the peripheral spread of the SARS-CoV-2. The most common manifestations in the patient's radiographs were multiple infiltrative shadows (93.33%), consolidation (95.75%), ground-glass opacity (61.21%), and the majority of the patient required intubation (63.03%).
Fig. 1.
Laboratory blood indicators correlated with the severity of COVID-. Association between patient age and D-dimer, ferritin, LDH, ESR, CRP, CK, neutrophils, and lymphocytes was determined. The correlation was determined by Pearson correlation and aligned by linear regression. The D-dimer, LDH, CK, ESR, and CRP were positively correlated with patient age and significantly changed (A, B, & C) while no significant changes were detected between age and ferritin, neutrophils, and lymphocytes (A & D).
4. Discussion
As of February 15, 2023, Pakistan ranked 53 among coronavirus-affected countries and reported more than 1,576,600 positive cases and 30,610 deaths but the epidemiological and clinical characteristics of patients with COVID-TB have not been reported yet. Our study delineated the COVID-TB cases of Pakistan in 165 hospitalized patients with a particular focus on clinical characteristics and laboratory indices. Previously, we summarized how the environmental variables affected COVID-19 transmission in Pakistan and also reported superinfection in hospitalized patients [18], [24]. Here, we found fever, dyspnea, sore throat, vomiting, loss of taste, loss of appetite, weight loss, and whooping cough as common symptoms among COVID-TB patients. Our findings were in accordance with the report of a large cohort study of 767 patients from 34 countries where the patient's median age was 44 years; the majority of patients were male; common symptoms were fever, dry cough, dyspnea, taste disorders, and ground-glass opacities in lung radiograph [27]. Furthermore, their study had TB in 74% of cases before COVID-19 while our all cases had TB before COVID-19. Notably, their study under-represented Asian countries and had a lack of laboratory data [27], whereas the present study provides a thorough perspective of COVID-TB in Pakistan with laboratory and clinical characteristics of patients. From the chest radiographs analysis, we found consolidated and hazy opacities on the lungs that indicated the spread of the virus to the body organs, which is akin to the previous report where the lung is the most affected body part in SARS-CoV-2 infection [28]. Besides, we found a 14-fold increase in D-dimer in COVID-TB while Huang et al reported a 5-fold increase in COVID-19 [9], and Tang et al reported a 3.5-fold increase in patients with severe COVID-19 compared to those without illness [29]. The increase in D-dimer level is an indication of blood clotting as well as the presence of severe underlying disease and was used in the prognosis of COVID-19 infection [30]. According to Han et al, LDH has been considered one of the predictive factors for lung injury in coronavirus-infected patients [28] and our results concluded a 12.5-fold increase in LDH in COVID-TB patients. For the first time, we detected an elevated level of CRP (11-fold), ESR (10-fold), and CK (7-fold) in COVID-TB patients and discovered that D-dimer, LDH, CRP, ESR, and CK concentrations were positively correlated with patient age which might be a reason that aged patients have a higher death risk. Moreover, the ferritin level was increased 6.5-fold and its level was similar in aged and young patients. The level of ferritin is vital for the use of iron in the body because a low amount of ferritin creates iron deficiency while hyperferritinemia indicated viral and bacterial infections [31]. Clinically, a higher level of serum LDH is related to viral infection and is a good prognostic indicator for lung and liver diseases [32]. We collectively discerned that D-dimer, LDH, CRP, ESR, CK, and ferritin levels increased during COVID-TB illness and were found to be negatively associated with lymphocyte cells. From our findings, we witnessed a rapid decrease in lymphocyte cells, an increase in neutrophil count, and recovery after three weeks of infection, and urban populations were more vulnerable to COVID-TB than rural regions. The timing of the SARS-CoV-2 pandemic does not allow us to analyze MDR-TB, therapy for TB, co-infection with HIV, and TB testing in COVID-19 patients. These factors are the main limitations in our study that need further investigation, in particular, disclosing the MDR emergence and the antibiotics therapy for COVID-TB. To our knowledge, this is the first study that reported the clinical characteristics of COVID-TB in Pakistan that will help in understanding Mycobacterium co-infection in COVID-19 illness.
5. Conclusion
The source of COVID-TB infection in Pakistan was the community spread of SARS-CoV-2 and the male patients were more infected than females. The laboratory indices such as D-dimer, LDH, CRP, ESR, CK, and ferritin levels significantly increased in COVID-TB patients while the level of lymphocytes decreased considerably. We presumed that the variations in blood biomarkers are associated with coinfection that could be helpful in the prognosis of SARS-CoV-2 infection in TB patients in a country with a high number of TB cases and limited medical resources.
Ethical approval
This study was approved by the Abbottabad University of Science and Technology (September 10, 2020). The Pakistan COVID-19 data can be accessed at the National Institute of Health website (https://www.nih.org.pk/). The data records were deidentified and informed consent was waived.
CRediT authorship contribution statement
Gul Habib: Conceptualization, Investigation, Writing – original draft. Khalid Mahmood: Investigation. Latif Ahmad: Investigation. Haji Gul: Investigation. Azam Hayat: Writing – review & editing. Mujaddad Ur Rehman: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Prof. Dr. Tahir Irfan Khan (Vice-Chancellor Abbottabad University of Science and Technology) for technical assistance. The study received partial funding from the Office of Research Innovation and Commercialization, Abbottabad University of Science and Technology (Grant no: AUST/ORIC/2021/375).
References
- 1.Hu B., Ge X., Wang L.-F., Shi Z. Bat origin of human coronaviruses. Virol J. 2015;12:1–10. doi: 10.1186/s12985-015-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. 2020;368:1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganesh B., Rajakumar T., Malathi M., Manikandan N., Nagaraj J., Santhakumar A., et al. Epidemiology and pathobiology of SARS-CoV-2 (COVID-19) in comparison with SARS, MERS: An updated overview of current knowledge and future perspectives. Clin Epidemiol Global Health. 2021 doi: 10.1016/j.cegh.2020.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao J., Cui W., Tian B.-P. The potential intermediate hosts for SARS-CoV-2. Front Microbiol. 2020:11. doi: 10.3389/fmicb.2020.580137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yesudhas D., Srivastava A., Gromiha M.M. COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics. Infection. 2020:1–15. doi: 10.1007/s15010-020-01516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu R.-H., He J.-F., Evans M.R., Peng G.-W., Field H.E., Yu D.-W., et al. Epidemiologic clues to SARS origin in China. Emerg Infect Dis. 2004;10:1030. doi: 10.3201/eid1006.030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viruses C.S.G.o.t.I.C.o.T.o. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corona G., Pizzocaro A., Vena W., Rastrelli G., Semeraro F., Isidori A.M., et al. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: Systematic review and meta-analysis. Rev. Endocrine Metabolic Dis. 2021;22:275–296. doi: 10.1007/s11154-021-09630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson K., Shah A., Grunbaum A., Oyesanmi O. Investigation into the effect of COVID-19 infection on length of hospital stay and mortality in patients with rheumatoid arthritis. Cureus. 2022:14. doi: 10.7759/cureus.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awan H.A., Sahito A.M., Sukaina M., Khatri G., Waheed S., Sohail F., et al. Tuberculosis amidst COVID-19 in Pakistan: a massive threat of overlapping crises for the fragile healthcare systems. J Epidemiology. 2022:150. doi: 10.1017/S0950268822000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakaya J., Khan M., Ntoumi F., Aklillu E., Fatima R., Mwaba P., et al. Global Tuberculosis report 2020–reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021;113:S7–S12. doi: 10.1016/j.ijid.2021.02.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tadolini M., Codecasa L.R., García-García J.-M., Blanc F.-X., Borisov S., Alffenaar J.-W., et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. 2020;56 doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu X., Gong W., Peng Z., Zeng F., Liu F. High resolution CT imaging dynamic follow-up study of novel coronavirus pneumonia. Front Med. 2020;7:168. doi: 10.3389/fmed.2020.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habib G., Mahmood K., Gul H., Tariq M., Ain Q.U., Hayat A., et al. Pathophysiology of methicillin-resistant Staphylococcus aureus Superinfection in COVID-19 Patients. Pathophysiology. 2022;29:405–413. doi: 10.3390/pathophysiology29030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westblade L.F., Simon M.S., Satlin M.J. Bacterial coinfections in coronavirus disease 2019. Trends Microbiol. 2021;29:930–941. doi: 10.1016/j.tim.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daneshvar P., Hajikhani B., Sameni F., Noorisepehr N., Zare F., Bostanshirin N., et al. COVID-19 and tuberculosis coinfection: an overview of case reports/case series and meta-analysis of prevalence studies. Heliyon. 2023 doi: 10.1016/j.heliyon.2023.e13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel N., Kaur M., Aggarwal R., Ganesh V., Ayub A., Kumar R., et al. Clinical characteristics, course and outcome of critically ill COVID-19 patients with previous or current TB admitted in ICU of a tertiary care COVID centre of Indian subcontinent. Lung India. 2023;40:91–93. doi: 10.4103/lungindia.lungindia_249_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gou J., Zhang G. Characteristics of COVID-19 and Tuber-culosis Co-Infection: a Cross-Sectional Study in Henan Province. J Clin Med Img. 2022;6:1–8. doi: 10.1007/s44231-022-00018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abid K., Bari Y.A., Younas M., Tahir Javaid S., Imran A. Progress of COVID-19 epidemic in Pakistan. Asia Pac J Public Health. 2020;32:154–156. doi: 10.1177/1010539520927259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habib, G.; Khan, M.S.Z.; Gul, H.; Hayat, A.; Rehman, M.U. A persistent high ambient temperature waned the community spread of Severe Acute Respiratory Syndrome Coronavirus-2 in Pakistan. 2022, 100961. [DOI] [PMC free article] [PubMed]
- 25.Organization, W.H. Global tuberculosis report 2021: supplementary material. 2022.
- 26.from, W.H.O.R. https://www.emro.who.int/pak/programmes/stop-tuberculosis.html. 2023, February 14.
- 27.Group T.c.-g.s. Tuberculosis and COVID-19 co-infection: description of the global cohort. Eur Respir J. 2022;59 doi: 10.1183/13993003.02538-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han Y., Zhang H., Mu S., Wei W., Jin C., Tong C., et al. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging (Albany NY) 2020;12:11245–11258. doi: 10.18632/aging.103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schafer K., Goldschmidt E., Oostra D., Fish J., Russell T., Lurie F. The clinical significance of ultra-high D-dimer levels. J Vascular Surgery: Venous Lymphatic Dis. 2022;10:8–13. doi: 10.1016/j.jvsv.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kernan K.F., Carcillo J.A. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ede L.C., O’Brien J., Chonmaitree T., Han Y., Patel J.A. Lactate dehydrogenase as a marker of nasopharyngeal inflammatory injury during viral upper respiratory infection: implications for acute otitis media. Pediatr Res. 2013;73:349–354. doi: 10.1038/pr.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]