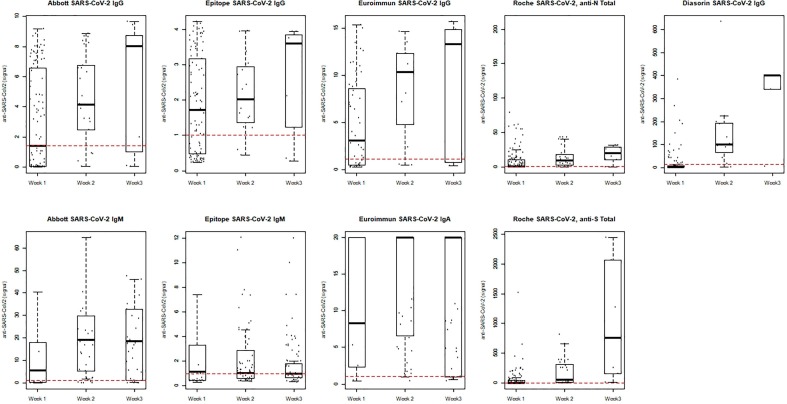
Abstract
Background
Serologic assays for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been proposed to assist with the acute diagnosis of infection, support epidemiological studies, identify convalescent plasma donors, and evaluate vaccine response.
Methods
We report an evaluation of nine serologic assays: Abbott (AB) and Epitope (EP) IgG and IgM, EUROIMMUN (EU) IgG and IgA, Roche anti-N (RN TOT) and anti-S (RS TOT) total antibody, and DiaSorin (DS) IgG. We evaluated 291 negative controls (NEG CTRL), 91 PCR positive (PCR POS) patients (179 samples), 126 convalescent plasma donors (CPD), 27 healthy vaccinated donors (VD), and 20 allogeneic hematopoietic stem cell transplant (HSCT) recipients (45 samples).
Results
We observed good agreement with the method performance claims for specificity (93–100%) in NEG CTRL but only 85% for EU IgA. The sensitivity claims in the first 2 weeks of symptom onset was lower (26–61%) than performance claims based on > 2 weeks since PCR positivity. We observed high sensitivities (94–100%) in CPD except for AB IgM (77%), EP IgM (0%). Significantly higher RS TOT was observed for Moderna vaccine recipients then Pfizer (p-values < 0.0001). A sustained RS TOT response was observed for the five months following vaccination. HSCT recipients demonstrated significantly lower RS TOT than healthy VD (p < 0.0001) at dose 2 and 4 weeks after.
Conclusions
Our data suggests against the use of anti-SARS-CoV-2 assays to aid in acute diagnosis. RN TOT and RS TOT can readily identify past-resolved infection and vaccine response in the absence of native infection. We provide an estimate of expected antibody response in healthy VD over the time course of vaccination for which to compare antibody responses in immunosuppressed patients.
Keywords: Coronavirus disease 2019 (COVID-19), Convalescent plasma donors, Epidemiologic studies, COVID-19 vaccination, Estimate of expected antibody response, Immunosuppressed patients
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11] which is an enveloped, single-stranded RNA virus of the family Coronaviridae that contains four structural glycoproteins: envelope (E), membrane (M), nucleocapsid (N), and spike (S) [4], [10]. As significant immunogenic components of SARS-CoV-2, the N and S proteins serve as major antigen targets of virus-specific antibodies [10].
In the US, the Moderna (ModernaTX, mRNA-1273) and Pfizer (Pfizer-BioNTech, BNT162b2) SARS-CoV-2 messenger RNA (mRNA) vaccines were authorized by the Food and Drug Administration (FDA) for emergency use in January 2022 and August 2021, respectively [12], [13]. These mRNA vaccines are administered in a two-injection series (second injection at 21 and 28 days after the first for Pfizer and Moderna, respectively). They elicit antibodies against the viral S protein where the serologic response measured as SARS-CoV-2 S protein neutralizing antibody levels has been correlated with protective immunity [14], [15], [16] and has been studied in neutralization escape with viral evolution [17]. Further, the correlation of neutralizing antibody levels to commercial anti-S levels had been demonstrated in previously infected individuals [18], [19], [20] and vaccinated immunocompetent individuals [21].
Now widely available, serologic assays for SARS-CoV-2 have been designed, validated, and manufactured for clinical, epidemiologic, and research purposes [22], [23], [24], [25], [26], [27], [28]. Over 90 SARS-CoV-2 assays have received emergency use authorization (EUA) from the FDA since the beginning of the COVID-19 pandemic [29]. SARS-CoV-2 serology tests differ by the antigen target (N or S protein) and the antibody detected (IgM, IgG, IgA, or total combined), which contribute to the assay performance characteristics (e.g. specificity, sensitivity). In general, the diagnostic performance of serologic assays improves when combining SARS-CoV-2 antibody panels [21], [30] and when the time from symptom onset or PCR confirmation is > 10 days [31], [32], [33].
Based on the recommendations of professional societies, serologic assays for SARS-CoV-2 have been proposed to (1) assist with the diagnosis of COVID-19 in patients with high clinical suspicion but who are repeatedly negative for SARS-CoV-2 viral RNA, (2) determine the prevalence of COVID-19 in the general population, particularly in asymptomatic individuals, (3) aid in the identification of convalescent plasma donors, and (4) evaluate vaccine response in healthy individuals as well as those who are immunosuppressed [34], [35], [36], [37]. Studies evaluating immune response are of great interest to the global community as they will help us to better understand the presence, durability, and duration of protective immunity following infection and/or vaccination to inform public health measures as the pandemic evolves to a pervasive endemic.
The evaluation of many assays have been published previously, but most studies lack comprehensive validation of multiple manufacturers using sample cohorts necessary to evaluate the performance across the wide-breath of applications considered [30], [32], [33], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]. In general, prior studies are limited to distinct evaluations of 1–4 different manufacturer’s methods, lacking robust clinical cohorts representing the intended populations, and do not provide sufficient detail to compare the performance of most major manufacturer’s methods available on the market today across various timepoints post infection or vaccination. We report a detailed assessment of our comprehensive evaluation of nine commercially available SARS-CoV-2 serologic assays produced by manufacturers of high-volume automated instrumentation commonly found in clinical diagnostic laboratories in comprehensive cohorts to evaluate the clinical and epidemiologic utility proposed by professional societies. Here, we evaluated methods detecting various immunoglobulin classes and antigen targets in pre-pandemic negative controls (NEG CTRL), COVID-19 patients confirmed by RT-PCR for SARS-CoV-2 (PCR POS), and eligible convalescent plasma donors (CPD). Further, following the adoption of the Roche Elecsys Anti-N and Anti-S SARS-CoV-2 total immunoglobulin methods in clinical practice, we demonstrate their performance in the evaluation of vaccine response in apparently healthy vaccinated donors (VD) more than five months past completion of the initial vaccination series, and a cohort of allogeneic hematopoietic stem cell transplant (HSCT) recipients.
2. Methods
Brigham and Women’s Hospital (BWH) is a 783-bed tertiary care academic hospital in Boston, MA and one of the founding members of Mass General Brigham (MGB) health system (previously Partners Healthcare) which is affiliated with the Dana-Farber Cancer Institute. All studies were conducted under the approval of the MGB Institutional Review Board (IRB 2020P001074). In the clinical chemistry laboratory, the Roche cobas 8000 chemistry analyzer series and an Abbott i1000 were used for evaluating Elecsys Anti-N and Anti-S SARS-CoV-2 total immunoglobulin and Abbott SARS-CoV-2 IgG and IgM methods. The immunology laboratory evaluated both the Epitope IgG and IgM as well as the EUROIMMUN IgG and IgA manual plate-based methods. The microbiology laboratory is equipped with a DiaSorin LIASION on which the IgG method evaluation was performed.
2.1. Study design
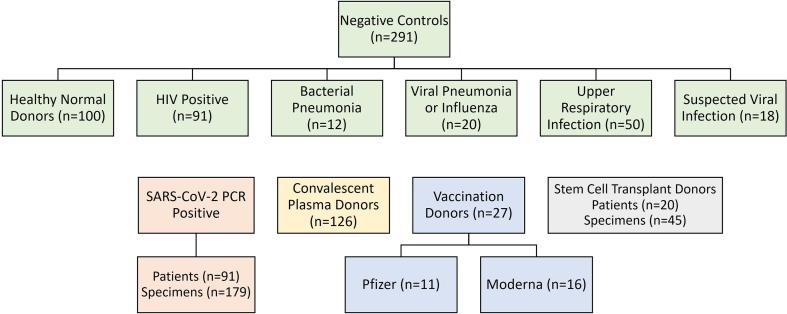
We evaluated the performance of nine commercially available anti-SARS-CoV-2 assays in five different population cohorts, as shown in Fig. 1 : NEG CTRL samples, PCR POS hospitalized patients, and eligible CPD with a history of PCR-confirmed COVID-19 infection within the previous 6 months. Subsequently, we evaluated the Roche anti-N and anti-S SARS-CoV-2 total immunoglobulin methods for clinical use, to include an extended set of eligible CPD, apparently healthy VD, and fully-vaccinated HSCT recipients. Due to limited sample volume and not wanting to introduce any sample instability variables (prolonged time refrigerated or freeze–thaw cycles that would otherwise be required for coordinating testing across three separate specialty laboratories) not all samples were run on each assay (Table S1 provides detailed information). Note that borderline results have been excluded from the sensitivity calculations for EP and EU methods.
Fig. 1.
Evaluation of nine commercially available anti-SARS-CoV-2 assays in five different population cohorts. Samples included pre-pandemic negative controls (n = 291) comprised of apparently healthy normal donors (n = 100), HIV positive patients (n = 91), and individuals with non-SARS-CoV-2 upper respiratory infections (n = 100); SARS-CoV-2 PCR positive hospitalized patients (n = 91); CPD (n = 126); vaccination donors (n = 27), and fully-vaccinated HSCT recipients (n = 20). Note: not all samples were run on each assay, additional information is presented in Table S1.
2.2. Study cohorts
2.2.1. Negative controls (NEG CTRL)
The NEG CTRL cohort was used to evaluate the analytical specificity of methods. The performance of methods in this cohort can be helpful to project the rates of false-positive results reported in the general population and impact on epidemiological studies. Pre-pandemic negative control specimens included 91 residual serum samples from HIV positive individuals collected and stored frozen at −20C our institution before the emergence of SARS-CoV-2 in 2019. Another 200 pre-pandemic serum samples obtained from the MGB Biobank, stored frozen at −80C, were included and consisted of consented donors: 100 healthy individuals and 100 individuals with non-SARS-CoV-2 upper respiratory infections (12 with bacterial pneumonia within the past 14 days, 20 with viral pneumonia and/or influenza within the past 60 days or with unspecified pneumonia within the past 31 days, 50 with any upper respiratory infection within the past 60 days, and 18 where a viral panel was ordered within the past 31 days).
2.2.2. SARS-CoV-2 Positive Patients (PCR POS)
The PCR POS cohort was used to evaluate the analytical sensitivity of methods and determine the biases introduced when manufacturer’s base performance claims on data using time since symptom onset versus first PCR positivity. The performance of methods in this cohort directly demonstrates their varying ability to assist with the diagnosis of acute COVID-19 infection. Over the period from April 3 to April 17, 2020, residual serums samples were collected from 91 hospitalized patients diagnosed with COVID-19 by RT-PCR for SARS-CoV-2 using nasopharyngeal swab samples. Aliquots were made, depending on the amount of residual sample volume, and were frozen and maintained at −20C until the day of testing. A total of 164 serum samples were collected, including serial serum samples from 28 patients over this 14-day period and an additional 15 paired plasma samples for the purposes of validating plasma sample type for clinical testing. Electronic medical records (EMR) were reviewed for all patients to include first-time PCR positive results and date of symptom onset [55].
2.2.3. Convalescent Plasma Donors (CPD)
The CPD cohort was designed to evaluate the ability to quickly screen individuals for potential convalescent plasma donation prior to enduring the cost of directly evaluating patients for neutralizing antibody titers. Initial evaluation across all nine antibody assays was performed using 60 samples collected from participants at least 1 week after confirming SARS-CoV-2 infection by PCR, who were eligible for convalescent plasma donation. An additional 66 CPD were included in the subsequent Roche anti-N and anti-S validation studies. As previously described [21], this CPD cohort was comprised of patients who were eligible for convalescent plasma donation with a history of PCR-confirmed COVID-19 infection within the previous 6 months. All patients with neutralizing antibody titers of ID50 > 100 were eligible for enrollment, following the minimum neutralizing titer recommended by the FDA [56], [57]. These samples were collected from consenting patients and samples were stored frozen at −80F until the day of testing.
2.2.4. Healthy vaccinated donors (VD)
The VD cohort was designed to evaluate the ability to monitor vaccine response in healthy individuals. Consenting VD included adults ≥ 18 years old at BWH (n = 27) who received a 2-dose series of either Pfizer (n = 11) or Moderna (n = 16) mRNA vaccines [21]. As previously reported, exclusion criteria included: immunosuppressive conditions, asplenia, HIV with CD4 count < 200 and/or detectable HIV viral load in the last year, receipt of systemic immunosuppressive or immune-modifying therapies for ≥ 14 days within 6 months of enrollment, or pregnancy [21]. Samples were collected at baseline at the time of initial vaccination dose (D1), approximately 2 weeks (D1 + 2) after the first dose, at the time of receiving the second dose (D2) (approximately 21 days and 28 days for Pfizer and Moderna vaccinations, respectively), and again approximately 4 weeks (D2 + 4), 12 weeks (D2 + 12), and 22 weeks (D2 + 22) after receiving the second dose. Samples were stored frozen at −80F until the day of testing.
2.2.5. Hematopoietic stem cell transplant (HSCT) recipients
As opposed to the vaccine response from apparently healthy VDs, the HSCT cohort was tested to evaluate the variable response in immunosuppressed individuals. Consenting adult allogeneic HSCT who were ≥ 18 years old and ≥ 100 days after transplantation were included in the study and received the 2-dose series of either the Pfizer (n = 15) or Moderna (n = 5) mRNA vaccines [58]. None had a reported history of COVID-19 before enrollment or during the study or had received intravenous immunoglobulin within 3 months of vaccination. Samples were collected at D1 and approximately D2 + 4 for a total of 45 specimens. Samples were stored frozen at −80F until the day of testing.
2.3. Anti-SARS-CoV-2 methods
The nine-method comparison included the anti-SARS-CoV-2 IgG and IgM assays from Abbott Laboratories (Abbott Park, IL) and Epitope Diagnostics, Inc. (San Diego, CA), IgG and IgA assays from EUROIMMUN (Lubeck, Germany), anti-N and anti-S total antibody assays from Roche Diagnostics (Indianapolis, IN), and the IgG assay from DiaSorin (Saluggia, Italy).
2.3.1. Abbott SARS-CoV-2 IgG and IgM
The automated, two-step Abbott (AB) IgG and IgM methods are qualitative chemiluminescent microparticle immunoassays that detect IgG or IgM binding to the SARS-CoV-2 N protein in serum and plasma. The AB IgG assay can be qualitatively interpreted as positive with a signal to cutoff ratio (S/CO) ≥ 1.4. The diagnostic specificity reported in the instructions for use (IFU) is 99.4% in 176 pre-pandemic specimens including donors with potentially interfering medical conditions and respiratory illnesses. According to the IFU, while the sensitivity was reported to be 100% in 88 subjects>14 days after PCR confirmed SARS-CoV-2 infection, the sensitivity was 61.8% in 34 subjects collected within the first two weeks since PCR positivity. The AB IgM assay qualitatively reported as positive with a S/CO ≥ 1.0 and the specificity is demonstrated to be 99% in a total of 207 negative control subjects. In the same cohort, the AB IgM assay sensitivity is reported as 95% in samples collected from subjects 14 – 30 days after PCR positivity, but 63.8% in 130 subjects within 14 days of PCR positivity.
2.3.2. DiaSorin LIAISON SARS-CoV-2 S1/S2 IgG
The DiaSorin (DS) method is an indirect qualitative chemiluminescent immunoassay designed to qualitatively detect IgG antibodies against recombinant S1 and S2 antigens in serum and plasma. The DiaSorin assay can be interpreted as positive (≥15.0 AU/mL) or negative (<15.0 AU/mL). The diagnostic specificity is reported in the IFU to be 98.2% in 168 pre-pandemic negative controls. While the sensitivity was reported in the IFU to be 97.6% in subjects>14 days after PCR confirmed SARS-CoV-2 infection, the sensitivity was 59.1% in 93 samples collected from subjects within 14 days of PCR positivity.
2.3.3. Epitope Diagnostics SARS-CoV-2 IgG and IgM
The Epitope (EP) methods utilize a microtiter plate-based enzyme immunoassay developed for the qualitative measurement of the human anti-COVID-19 IgG and IgM antibody directed towards a recombinant full-length form of the viral N protein. Results are interpreted in context of the average absorbance of the negative control (xNC). EP IgG positive samples are those greater than the positive cutoff of 1.1*(xNC + 0.18); negative samples are less than or equal to the negative cutoff of 0.9*(xNC + 0.18); and borderline results are those between the negative and positive cutoffs. The diagnostic specificity was determined to be 99.8% in 624 pre-pandemic negative samples. The sensitivity was reported in the IFU to be 98.4% in 187 PCR confirmed COVID-19 positive patients, but without any indication of the time since symptom onset or PCR test positivity. The EP IgM assay is reported as positive when the results are above the positive cutoff of 1.1*(xNC + 0.10); negative when less than or equal to 0.9(xNC + 0.10); and borderline results are those between the negative and positive cutoffs. In the same 624 pre-pandemic negative samples, the EP IgM is reported to have a specificity of 99.8% according to the IFU. Again, sensitivity was 73.2% in 41 PCR confirmed COVID-19 patients without information on time since PCR positivity according to the IFU.
2.3.4. EUROIMMUN SARS-CoV-2 IgG and IgA
The EUROIMMUN (EU) method is a microtiter plate-based enzyme-linked immunosorbent assay that provides a qualitative determination of SARS-CoV-2 IgG and IgA against recombinant structural protein (S1 domain) of SARS-CoV-2 S protein in serum or plasma. Results can be derived from the ratio of the absorbance of the sample over that of the calibrator. The SARS-CoV-2 IgG or IgA can be interpreted as positive (ratio ≥ 1.1), borderline (ratio < 1.1 to ≥ 0.8), or negative (ratio < 0.8). The EU IgG method has a reported diagnostic specificity of 98.6% in 1445 pre-pandemic negative controls according to the IFU. Despite reporting a 100% sensitivity in 6 subjects at least 21 days after symptom onset, the EU IgG sensitivity was 29.1% in 55 patients with symptom onset of PCR confirmed SARS-CoV-2 infection within the prior two weeks, according to the IFU. The EU IgA method was reported in the IFU to have a diagnostic specificity of 92.5% in 200 samples and a very limited dataset (n = 9) of PCR-confirmed positive patients was used to report a diagnostic sensitivity of 100% (>10 days after onset of symptoms) in the IFU. However, in the 6 samples that were collected within 14 days of symptom onset, a 66.7% sensitivity can be derived from the IFU.
2.3.5. Roche Elecsys Anti-N and Anti-S SARS-CoV-2 total immunoglobulin
The automated Roche Elecsys methods for anti-N (RN TOT) and anti-S (RS TOT) employ a high throughout double-antigen sandwich assay electrochemiluminescence assay format that uses a recombinant protein representing the SARS-CoV-2 N or receptor binding domain (RBD) of the S antigen to detect antibodies (including IgG, IgA, and IgM) to SARS-CoV-2 in serum and plasma. The threshold for positivity is a cutoff index (COI) ≥ 1.0 and is ≥ 0.8 U/mL for RN TOT and RN TOT, respectively. For the RN TOT assay, the reported specificity is 99.8% in 5272 pre-pandemic negative controls including potentially cross-reactive infectious respiratory diseases, according to the IFU. While the sensitivity was reported in the IFU to be 100% in 29 samples collected at or after 14 days after PCR confirmed SARS-CoV-2 infection, the method was 73.1% sensitive in 175 samples collected within the first two weeks of PCR positivity, according to the IFU. For the RS TOT assay, the analytic specificity was reported in the IFU as 100% in 1100 pre-pandemic negative controls. The sensitivity was 96.6% in 233 samples collected at least 15 days after PCR positivity but 88.1% in 109 samples collected within two weeks of a SARS-CoV-2 PCR positive test result, according to the IFU.
2.4. Data analysis
Data analysis and visualization was performed using RStudio (RStudio 2022.07.1, PBC, Boston, MA, USA) and the Rlab v4.0 package. Statistical analysis was performed using GraphPad (GraphPad Software, San Diego, CA, USA). A p-value of ≤ 0.05 is defined as significant for the two-tailed unpaired t-test comparison of populations.
3. Results
3.1. Evaluation of nine commercial anti-SARS-CoV-2 assays
A summary of the number of samples run by each method, the number of false-positive (FP) and -negative (FN) results, and the calculated specificity and sensitivity in each patient cohort: NEG CTRL, PCR POS, CPD, VD, and HSCT recipients are shown in Table S1.
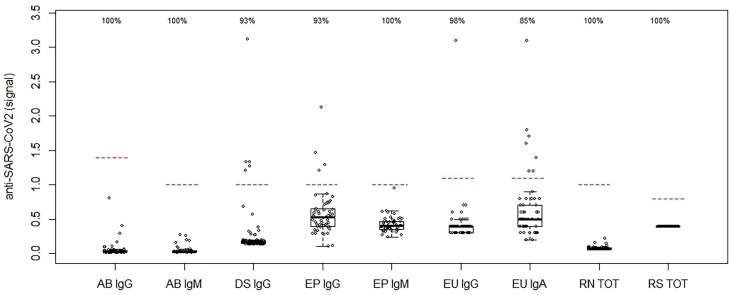
The distribution of NEG CTRL results of the nine methods is shown in Fig. 2 as compared to the threshold for positivity (dashed line) for each method, along with the calculated specificity: 100% for AB IgG, AB IgM, EP IgM, RN TOT and RS TOT; 98% for EU IgG; 93% for DS IgG, EP IgG; but only 85% for EU IgA with the highest rate of false-positive results in this cohort (8 of 52; 15%). As compared to the manufacturer’s package inserts (Table S1), we observed a slightly lower specificity for DS IgG (93% vs. claim of 98.2%), EP IgG (93% vs. claim of 99.8%), and EU IgA (85% vs. claim of 92.5%).
Fig. 2.
Distribution of results for nine anti-SARS-CoV-2 assays in pre-pandemic negative controls. A total of 291 negative controls were evaluated and assay specificity is shown at the top, where the dashed horizontal lines represent the signal cutoff or concentration threshold for positivity: Abbott (AB) IgG (S/CO = 1.4), and IgM (S/CO = 1.0), DiaSorin (DS) IgG (S/CO = 1.1), Epitope (EP) IgG (S/CO = 0.999) and IgM (S/CO = 0.999), EUROIMMUN (EU) IgG and IgA (S/CO = 1.1), and Roche total anti-N (RN TOT) (COI = 1.0) and anti-S (RS TOT) (0.8 U/mL). Note: DS IgG data and cut-off is normalized for visualization purposes; Borderline results have been excluded for sensitivity and specificity calculations.
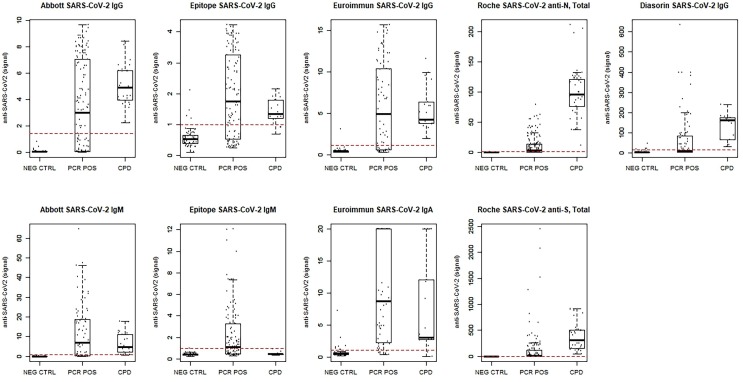
The distribution of results for each the NEG CTRL, PCR POS, and CPD cohorts is shown in Fig. 3 , in relation to the method threshold for positivity (dashed line). A wide distribution is observed for PCR POS as this, not unexpectedly, varies as a function of time since symptom onset (Fig. 4 ) which is also seen for the time since PCR positivity (Fig. S1). Here, we observe a number of samples throughout three weeks since symptom onset where various methods remain negative despite PCR positivity (below the cut-off shown as a horizontal dashed line). Considering only samples collected within the first 2 weeks since symptom onset, EU IgA has the highest sensitivity at 93%, followed by EP IgG and EU IgG at 61%, AB IgM at 54%, RS TOT at 50%, EP IgM at 49%, AB IgG at 43%, RN TOT at 38% and DS IgG with the lowest sensitivity at 26% for this cohort (Table S1). As compared to sensitivity derived from the IFU, results obtained for patients in the two weeks following the first PCR positive result documented within the EMR are in good agreement (Table S1), with the exception for RN TOT (53% vs. claim of 73.1%), RS TOT (60% vs. claim of 88.1%), DS IgG (42% vs. claim of 59.1%), and a much higher sensitivity for EU IgG than reported (69% vs. claim of 29.1%). We generally observed acceptable assay performance in the ability to detect antibodies in CPD with neutralizing antibody titers of ID50 > 100 following native SARS-CoV-2 infection. In this cohort, we found sensitivities of 100% for AB IgG, DS IgG, EU IgG, and RS TOT; 99% for RN TOT; 94% for EP IgG and EU IgA, and 77% for AB IgM, but EP IgM demonstrated 0% sensitivity (Table S1).
Fig. 3.
Distribution of results for each of the nine methods evaluated in pre-pandemic negative controls (NEG CTRL), SARS-CoV-2 PCR positive patients (PCR POS), and a subset of convalescent plasma donors (CPD). The assay cutoff for positivity is shown with dashed line for each method.
Fig. 4.
Distribution of results for each of the nine methods evaluated in SARS-CoV-2 PCR positive patients. Results are shown as a function of time (in weeks) since symptom onset, as reported in the medical record.
3.2. Validation of Roche methods for clinical use
Subsequently, the RN TOT and RS TOT were implemented clinically (cobas e601), given their ability for high-throughput automation, high specificity in negative controls, and excellent sensitivity in CPD. Additionally, linearity studies using commercial materials (CalCheck Anti-SARS-CoV-2 S, Roche Diagnostics) and standard manual dilution studies of patient samples were conducted to allow for robust determination of COI or concentration for both assays, as well as, extend the upper limit of the reportable range from 2500 U/mL to 25,000 U/mL for the anti-S assay (data not shown).
3.3. Examination of Roche anti-SARS-CoV-2 methods
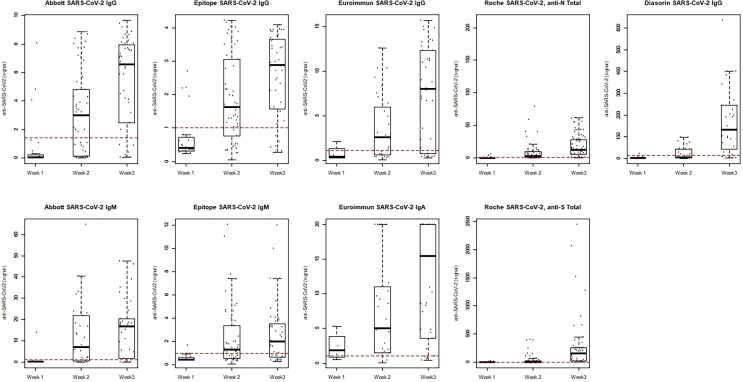
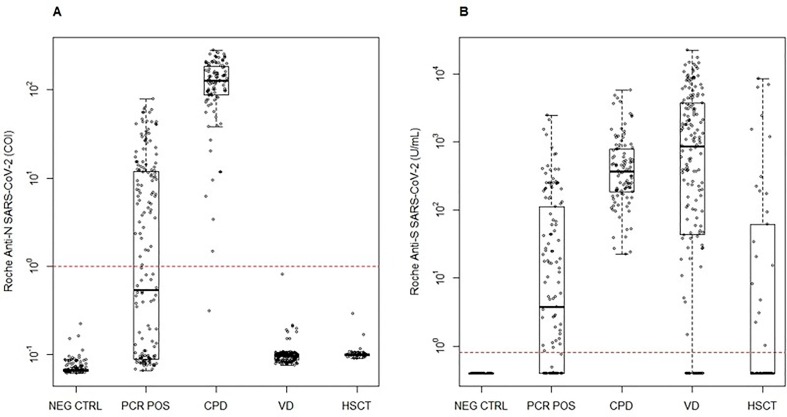
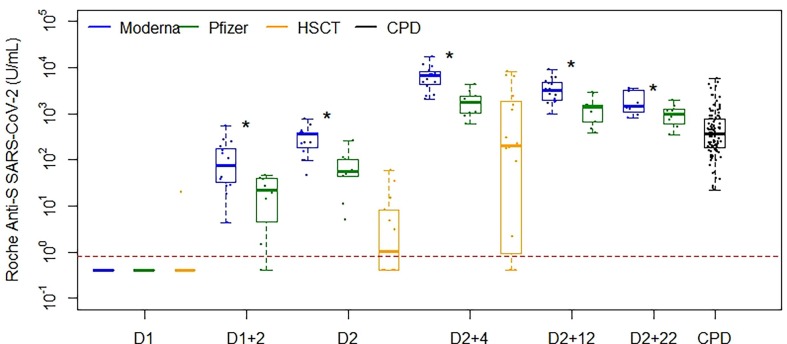
The performance of RN TOT (Fig. 5 A) and RS TOT (Fig. 5B) assay is shown in NEG CTRL, POS PCR, CPD (including an additional 66 patients), VD (across nine timepoints throughout the vaccination series) and HSCT cohorts (at three timepoints throughout the vaccination series). VD and HSCT recipients were all negative for RN TOT, consistent with the reported history of no native COVID-19 infection before or during the study period. Statistically significant differences in antibody concentrations were observed between donors receiving Moderna and Pfizer vaccinations (Fig. 6 , Table S2) for all timepoints beyond the initial dose (p < 0.0001), where the median antibody concentrations and interquartile range (IQR) for each are detailed in Table S2. We observed sustained antibody responses for both vaccination types more than five months following vaccination series completion. Further, HSCT demonstrated significantly lower antibody responses as compared to healthy VD (Moderna and Pfizer combined) at D2 and D2 + 4 (p < 0.0001) (Fig. 6, Table S2). This was a result of 4 of 16 HSCT recipients not mounting an antibody response by series completion whereas the 16 who did, had a median (IRQ) concentration of 744 (185–3375) U/mL.
Fig. 5.
(A) Roche anti-N and (B) anti-S SARS-CoV-2 total immunoglobulin performance. Distribution of results are shown in NEG CTRL, PCR POS, CPD, VD (across nine timepoints throughout the vaccination series) and HSCT recipients (at three timepoints in the vaccination series).
Fig. 6.
Roche anti-S SARS-CoV-2 total immunoglobulin results in VD administered Moderna (blue) and Pfizer (green) vaccinations, as compared to HSCT (orange) recipients and CPD (black). Distribution of VD samples are shown as a function of time after D1 or D2 in weeks. Statistically significant differences in antibody concentrations were observed between donors receiving Moderna and Pfizer vaccinations for all timepoints beyond the initial dose (p < 0.0001) and strong responses were seen for both vaccination type cohorts more than five months post series completion. HSCT demonstrated significantly lower antibody responses from healthy VD (Moderna and Pfizer combined) at both D2 and D2 + 4 (p < 0.0001). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We have demonstrated a comprehensive evaluation of nine commercially available anti-SARS-CoV-2 assays across five study cohorts to help support or oppose their effectiveness for use in (1) aiding in the diagnosis of acute SARS-CoV-2 infection (2) epidemiologic studies estimating the prevalence of infection (3) identifying eligible CPD and (4) evaluating vaccine response in healthy and immunosuppressed individuals.
First, while anti-SARS-CoV-2 assay sensitivity is commonly reported in terms of days after PCR positivity, we find that this is generally a less meaningful timeline given that symptom onset can be days, if not weeks, before seeking medical care and obtaining a PCR test confirming infection. The reported sensitivities in the IFU range from 96.6 to 100% in the nine methods evaluated. However, not only is this derived from testing hospitalized individuals more than two weeks since their initial PCR positive result, but it may also be weeks since symptom on set and even longer since initial infection. As shown in Fig. 4 and Table S1, we observed sensitivities ranging from 26 to 61% when using days since reported symptom onset; which we believe more accurately reflects the expected performance of these assays in their use aiding in the diagnosis of acute infection despite repeatedly negative PCR results. We did observe a 93% sensitivity of the EU IgA method, however, given a specificity of 85% and a high rate of false-positives, these results are less reliable. It is also important to consider that patients with intrinsic or acquired immune suppression may have a limited antibody response and may return negative results despite recent native infection.
Second, the prevalence of SARS-CoV-2 infection in epidemiologic studies is highly dependent upon the performance of the assay used. Confirming initial findings [59], [60], our data suggests against the use of IgM or IgA assays, demonstrating sensitivities of 0% for EP IgM, 77% for AB IgM and 94% for EU IgA (with known higher rates of false-positivity, 8/52 NEG CTRL) in CPD with neutralizing antibody titers ID50 > 100 following native infection. Considering the time to mount a serologic response to infection, even with highly sensitive IgG or total antibody assays, testing>1–2 weeks post exposure or possible infection may be necessary in surveillance studies to reduce false-negatives. This can be particularly challenging for population-wide screening, including asymptomatic individuals, where repeated testing may be required for accurate estimates.
Third, the rapid identification of individuals who are eligible for convalescent plasma donation would benefit from a high-throughput, automated method with high sensitivity, with testing occurring at least two weeks since symptom onset following native infection; where more time consuming and costly neutralizing antibody titers would then serve as confirmation. Our findings suggest that the EP IgM, AB IgM, EP IgG, and EU IgA assays would not be suited as screening methods with sensitivities ranging from 0 to 94% in the CPD cohort. More recently, NIH has indicated that there is insufficient evidence for administering convalescent plasma in hospitalized COVID-19 patients with impaired humoral immunity [61]. However, we demonstrate that given the correlation between anti-SARS-CoV-2 assays and neutralizing antibody assays, some methods (not all) could have been used to identify potential donors more rapidly and efficiently that direct neutralization testing, to preferentially select those expected to have the highest antibody titers [14], [15], [16], [39]. The CPD cohort is helpful to demonstrate the performance of these assays in PCR-confirmed COVID-19 patients with neutralizing antibody titers ID50 > 100 through 6 months following infection.
Finally, our data confirms that the RN TOT and RS TOT are highly effective assays for differentiating recent past-resolved infection (positive RN TOT and RS TOT) and vaccine response in the absence of native infection (negative RN TOT and positive RS TOT). However, with continued resurgence of SARS-CoV-2, it’s important to consider the pattern of serologic results expected with recent infection in the vaccinated population. In the case of RS TOT positive results, unless the degree of response to vaccination has been documented just prior to infection, this would be indistinguishable from a recent native infection without vaccination response as antibody titers of vaccinated individuals and unvaccinated individuals with SARS-CoV-2 infection has many contributing factors, including the type of vaccination received, number of vaccination doses, and time since last vaccination or infection. RN TOT may be an effective marker of native infection in vaccinated individuals, however, the durability of this serologic response is not well known, particularly in recurrent infections. Clearly, additional work is needed to monitor the effects of increasing mutations in contemporary strains on the performance of these assays. While a large systematic review including most of the major manufacturers with samples from more recently infected PCR POS patients is desired, like our study, it is challenging to coordinate the analysis on every platform for true sample-to-sample comparison of assay performance.
Further, we provide an estimate of the expected antibody response for RS TOT in healthy donors along the time course of vaccination (Fig. 6, Table S2). Our results support previous findings of immunological responses to vaccination [21], [62], [63] but also expand upon this work to demonstrate the immunological responses through more than five months following the completion of the two-injection series of both Moderna and Pfizer vaccinations in apparently health VDs as compared to immunosuppressed HSCT patients and CPD with neutralizing antibody titers ID50 > 100. While the CDC does not recommend population-wide testing for vaccination response [37], there is an interest to routinely evaluate the efficacy of vaccination in immunosuppressed patients, in order to actively manage their risk of exposure. We observed a reproducible response distribution for RS TOT along the various points measured throughout the vaccination series in apparently healthy vaccination donors, establishing a curve to compare antibody responses in immunosuppressed individuals against. Further studies are necessary to establish the correlation of RS TOT concentrations in immunosuppressed individuals and neutralization assays, as correlates of infection protection. This is a heterogenous cohort with complex immune characteristics and many variables that impact response to vaccination. Further, non-antibody mediated immune protection must be considered carefully when interpreting anti-SARS-CoV-2 results. However, achieving an expected antibody response along the course of vaccination would be reassuring to these individuals, particularly when immunosuppressive drugs are withheld prior to and during the vaccination series, when considering the reduction in risk of severe COVID-19.
5. Conclusions
Most methods, aside from those targeting only IgA and IgM and those with poor specificity/high false-positive rates, are capable of detecting serologic response to native infection for the purposes of epidemiologic studies, and identifying potential CPD. Further, we have demonstrated the ability to monitor vaccine response using RS TOT in immunosuppressed individuals as compared to apparently healthy vaccinated donors. However, we find that most methods are not suitable for aiding in the acute diagnosis of SARS-CoV-2 infection due to poor clinical sensitivity early in the course of disease. We recommend evaluating the performance of serologic assays in the first 1–2 weeks post symptom onset as it is a more accurate reflection of how these assays will perform in clinical use, where the anti-SARS-CoV-2 assay is thought to help elucidate an underlying COVID-19 infection in patients who have cleared the virus and are repeatedly PCR negative. Our data suggests that if the serologic test is positive, it may be helpful, so long as the specificity of the assay is acceptable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to sincerely thank Tolu Terebo for her support in manuscript preparation and Kristi Rollins, Gail Kinchla, Lisa Bernhard, Jacquelyn Thomas, and Rebecca Zaffini for their time and dedication to acquiring the data for this report. We would like to acknowledge the support of Dr. David Grenache for testing additional samples at TriCore Reference Laboratories and the BWH Crimson Core and MGB Biobank for access to high-quality residual waste samples for this investigation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinbiochem.2023.03.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Figure S1. Distribution of results for each of the nine methods evaluated in SARS-CoV-2 PCR positive patients. Results are shown as a function of time (in weeks) since SARS-CoV-2 PCR positive result, as reported in the MGB medical record system.
References
- 1.Cao Y., Liu X., Xiong L., Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J. Med. Virol. 2020;92(9):1449–1459. doi: 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet (London, England) 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J.A., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.V. Coronaviridae Study Group of the International Committee on Taxonomy of, The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China, The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. J. Am. Med. Assoc. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA, Spikevax and Moderna COVID-19 Vaccine, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/spikevax-and-moderna-covid-19-vaccine. (Accessed 2021/03/31).
- 13.FDA, Commissioner of the FDA Authorizes the Pfizer-BioNTech COVID-19 Vaccine 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine. (Accessed 2021/03/31).
- 14.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 15.McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., Liu J., Peter L., Atyeo C., Zhu A., Bondzie E.A., Dagotto G., Gebre M.S., Jacob-Dolan C., Li Z., Nampanya F., Patel S., Pessaint L., Van Ry A., Blade K., Yalley-Ogunro J., Cabus M., Brown R., Cook A., Teow E., Andersen H., Lewis M.G., Lauffenburger D.A., Alter G., Barouch D.H. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.T., Limbo O., Smith C., Song G., Woehl J., Yang L., Abbott R.K., Callaghan S., Garcia E., Hurtado J., Parren M., Peng L., Ramirez S., Ricketts J., Ricciardi M.J., Rawlings S.A., Wu N.C., Yuan M., Smith D.M., Nemazee D., Teijaro J.R., Voss J.E., Wilson I.A., Andrabi R., Briney B., Landais E., Sok D., Jardine J.G., Burton D.R. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369(6506):956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabel K.G., Clark S.A., Shankar S., Pan J., Clark L.E., Yang P., Coscia A., McKay L.G.A., Varnum H.H., Brusic V., Tolan N.V., Zhou G., Desjardins M., Turbett S.E., Kanjilal S., Sherman A.C., Dighe A., LaRocque R.C., Ryan E.T., Tylek C., Cohen-Solal J.F., Darcy A.T., Tavella D., Clabbers A., Fan Y., Griffiths A., Correia I.R., Seagal J., Baden L.R., Charles R.C., Abraham J. Structural basis for continued antibody evasion by the SARS-CoV-2 receptor binding domain. Science. 2022;375(6578):eabl6251. doi: 10.1126/science.abl6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., Mills R., Teng E., Kamruzzaman M., Garcia-Beltran W.F., Astudillo M., Yang D., Miller T.E., Oliver E., Fischinger S., Atyeo C., Iafrate A.J., Calderwood S.B., Lauer S.A., Yu J., Li Z., Feldman J., Hauser B.M., Caradonna T.M., Branda J.A., Turbett S.E., LaRocque R.C., Mellon G., Barouch D.H., Schmidt A.G., Azman A.S., Alter G., Ryan E.T., Harris J.B., Charles R.C. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020;5(52) doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterhoff D., Glück V., Vogel M., Schuster P., Schütz A., Neubert P., Albert V., Frisch S., Kiessling M., Pervan P., Werner M., Ritter N., Babl L., Deichner M., Hanses F., Lubnow M., Müller T., Lunz D., Hitzenbichler F., Audebert F., Hähnel V., Offner R., Müller M., Schmid S., Burkhardt R., Glück T., Koller M., Niller H.H., Graf B., Salzberger B., Wenzel J.J., Jantsch J., Gessner A., Schmidt B., Wagner R. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection. 2021;49(1):75–82. doi: 10.1007/s15010-020-01503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., McMahon M., Meade P., Mendu D.R., Muellers K., Stadlbauer D., Stone K., Strohmeier S., Simon V., Aberg J., Reich D.L., Krammer F., Cordon-Cardo C. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolan N.V., Sherman A.C., Zhou G., Nabel K.G., Desjardins M., Melanson S., Kanjilal S., Moheed S., Kupelian J., Kaufman R.M., Ryan E.T., LaRocque R.C., Branda J.A., Dighe A.S., Abraham J., Baden L.R., Charles R.C., Turbett S.E. The Effect of Vaccine Type and SARS-CoV-2 Lineage on Commercial SARS-CoV-2 Serologic and Pseudotype Neutralization Assays in mRNA Vaccine Recipients. Microbiology spectrum. 2022;10(2):e0021122. doi: 10.1128/spectrum.00211-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., Wang D.Q., Hu Y., Ren J.H., Tang N., Xu Y.Y., Yu L.H., Mo Z., Gong F., Zhang X.L., Tian W.G., Hu L., Zhang X.X., Xiang J.L., Du H.X., Liu H.W., Lang C.H., Luo X.H., Wu S.B., Cui X.P., Zhou Z., Zhu M.M., Wang J., Xue C.J., Li X.F., Wang L., Li Z.J., Wang K., Niu C.C., Yang Q.J., Tang X.J., Zhang Y., Liu X.M., Li J.J., Zhang D.C., Zhang F., Liu P., Yuan J., Li Q., Hu J.L., Chen J., Huang A.L. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W., Wang Q., Tan L., Wu W., Tang S., Xiong Z., Zheng S. Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;58(6) doi: 10.1128/JCM.00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rongqing Z., Li M., Song H., Chen J., Ren W., Feng Y., Gao G.F., Song J., Peng Y., Su B., Guo X., Wang Y., Chen J., Li J., Sun H., Bai Z., Cao W., Zhu J., Zhang Q., Sun Y., Sun S., Mao X., Su J., Chen X., He A., Gao W., Jin R., Jiang Y., Sun L. Early Detection of Severe Acute Respiratory Syndrome Coronavirus 2 Antibodies as a Serologic Marker of Infection in Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2020;71(16):2066–2072. doi: 10.1093/cid/ciaa523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J., Zhou Q., Ye H., Ma Y., Li H., Wei X., Cai P., Ma W.-L. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin. Infect. Dis. 2020;71(8):1930–1934. doi: 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Y., Wang M., Zuo Z., Fan C., Ye F., Cai Z., Wang Y., Cui H., Pan K., Xu A. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int. J. Infect. Dis. 2020;94:49–52. doi: 10.1016/j.ijid.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C., Cai J.P., Chan J.M., Chik T.S., Lau D.P., Choi C.Y., Chen L.L., Chan W.M., Chan K.H., Ip J.D., Ng A.C., Poon R.W., Luo C.T., Cheng V.C., Chan J.F., Hung I.F., Chen Z., Chen H., Yuen K.Y. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FDA, In Vitro Diagnostics EUAs - Serology and Other Adaptive Immune Response Tests for SARS-CoV-2. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-serology-and-other-adaptive-immune-response-tests-sars-cov-2 (Accessed 2023/01/06).
- 30.Suhandynata R.T., Hoffman M.A., Kelner M.J., McLawhon R.W., Reed S.L., Fitzgerald R.L. Multi-Platform Comparison of SARS-CoV-2 Serology Assays for the Detection of COVID-19. J. Appl. Labor. Med. 2020;5(6):1324–1336. doi: 10.1093/jalm/jfaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suhandynata R.T., Hoffman M.A., Kelner M.J., McLawhon R.W., Reed S.L., Fitzgerald R.L. Longitudinal Monitoring of SARS-CoV-2 IgM and IgG Seropositivity to Detect COVID-19. J. Appl. Labor. Med. 2020;5(5):908–920. doi: 10.1093/jalm/jfaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang M.S., Hock K.G., Logsdon N.M., Hayes J.E., Gronowski A.M., Anderson N.W., Farnsworth C.W. Clinical Performance of Two SARS-CoV-2 Serologic Assays. Clin. Chem. 2020;66(8):1055–1062. doi: 10.1093/clinchem/hvaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theel E.S., Harring J., Hilgart H., Granger D. Performance Characteristics of Four High-Throughput Immunoassays for Detection of IgG Antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;58(8) doi: 10.1128/JCM.01243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Association of Public Health Laboratories. Public Health Considerations: Serologic Testing for COVID-19. https://www.aphl.org/Pages/default.aspx. (Accessed 2022-08-25).
- 35.Infectious Diseases Society of America. COVID-19 Antibody Testing Primer. https://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsa-covid-19-antibody-testing-primer.pdf. (Accessed 2022/08/25) 2021).
- 36.American Medical Association. Serological testing for SARS-CoV-2 antibodies. https://www.ama-assn.org/delivering-care/public-health/serological-testing-sars-cov-2-antibodies. (Accessed 2022-08-25).
- 37.CDC, Interim Guidelines for COVID-19 Antibody Testing, 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html. (Accessed 2022/08/25).
- 38.Beavis K.G., Matushek S.M., Abeleda A.P.F., Bethel C., Hunt C., Gillen S., Moran A., Tesic V. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for detection of IgA and IgG antibodies. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan C.W., Parker K., Tesic V., Baldwin A., Tang N.Y., van Wijk X.M.R., Yeo K.-T.-J. Analytical and clinical evaluation of the automated elecsys Anti–SARS-CoV-2 antibody assay on the roche cobas e602 analyzer. Am. J. Clin. Pathol. 2020;154(5):620–626. doi: 10.1093/ajcp/aqaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egger M., Bundschuh C., Wiesinger K., Gabriel C., Clodi M., Mueller T., Dieplinger B. Comparison of the Elecsys® Anti-SARS-CoV-2 immunoassay with the EDI™ enzyme linked immunosorbent assays for the detection of SARS-CoV-2 antibodies in human plasma. Clin. Chim. Acta. 2020;509:18–21. doi: 10.1016/j.cca.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farnsworth C.W., Anderson N.W. SARS-CoV-2 serology: much hype. Little Data, Clin. Chem. 2020;66(7):875–877. doi: 10.1093/clinchem/hvaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Favresse J., Eucher C., Elsen M., Tré-Hardy M., Dogné J.-M., Douxfils J. Clinical performance of the elecsys electrochemiluminescent immunoassay for the detection of SARS-CoV-2 total antibodies. Clin. Chem. 2020;66(8):1104–1106. doi: 10.1093/clinchem/hvaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harb R., Remaley A.T., Sacks D.B. Evaluation of three commercial automated assays for the detection of anti-SARS-CoV-2 antibodies. Clin. Chem. 2020;66(10):1351–1353. doi: 10.1093/clinchem/hvaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krammer F., Simon V. Serology assays to manage COVID-19. Science. 2020;368(6495):1060–1061. doi: 10.1126/science.abc1227. [DOI] [PubMed] [Google Scholar]
- 45.Merrill A.E., Jackson J.B., Ehlers A., Voss D., Krasowski M.D. Head-to-head comparison of two SARS-CoV-2 serology assays. J. Appl. Labor. Med. 2020;5(6):1351–1357. doi: 10.1093/jalm/jfaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padoan A., Bonfante F., Pagliari M., Bortolami A., Negrini D., Zuin S., Bozzato D., Cosma C., Sciacovelli L., Plebani M. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine. 2020;62 doi: 10.1016/j.ebiom.2020.103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perkmann T., Perkmann-Nagele N., Breyer M.-K., Breyer-Kohansal R., Burghuber O.C., Hartl S., Aletaha D., Sieghart D., Quehenberger P., Marculescu R., Mucher P., Strassl R., Wagner O.F., Binder C.J., Haslacher H. Side-by-Side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin. Chem. 2020;66(11):1405–1413. doi: 10.1093/clinchem/hvaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.A. Bal, B. Pozzetto, M.-A. Trabaud, V. Escuret, M. Rabilloud, C. Langlois-Jacques, A. Paul, N. Guibert, C. D’Aubarède-Frieh, A. Massardier-Pilonchery, N. Fabien, D. Goncalves, A. Boibieux, F. Morfin-Sherpa, V. Pitiot, F. Gueyffier, B. Lina, J.-B. Fassier, S. Trouillet-Assant, C.S.S. Group, Evaluation of High-Throughput SARS-CoV-2 Serological Assays in a Longitudinal Cohort of Patients with Mild COVID-19: Clinical Sensitivity, Specificity, and Association with Virus Neutralization Test, Clinical Chemistry 67(5) (2021) 742-752. [DOI] [PMC free article] [PubMed]
- 49.Hubbard J.A., Geno K.A., Khan J., Szczepiorkowski Z.M., de Gijsel D., Ovalle A.A., AlSalman A.S., Gallagher T.L., Johnston A.A., Tibbetts A.R., Vital S.E., Cervinski M.A., Nerenz R.D. Comparison of two automated immunoassays for the detection of SARS-CoV-2 nucleocapsid antibodies. J. Appl. Labor. Med. 2021;6(2):429–440. doi: 10.1093/jalm/jfaa175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bond K.A., Williams E., Nicholson S., Lim S., Johnson D., Cox B., Putland M., Gardiner E., Tippett E., Graham M., Mordant F., Catton M., Lewin S.R., Subbarao K., Howden B.P., Williamson D.A. Longitudinal evaluation of laboratory-based serological assays for SARS-CoV-2 antibody detection. Pathology. 2021;53(6):773–779. doi: 10.1016/j.pathol.2021.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kittel M., Muth M.C., Zahn I., Roth H.J., Thiaucourt M., Gerhards C., Haselmann V., Neumaier M., Findeisen P. Clinical evaluation of commercial automated SARS-CoV-2 immunoassays, International journal of infectious diseases : IJID : official publication of the International Society for. Infect. Dis. 2021;103:590–596. doi: 10.1016/j.ijid.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekirov I., Barakauskas V.E., Simons J., Cook D., Bates B., Burns L., Masud S., Charles M., McLennan M., Mak A., Chahil N., Vijh R., Hayden A., Goldfarb D., Levett P.N., Krajden M., Morshed M. SARS-CoV-2 serology: Validation of high-throughput chemiluminescent immunoassay (CLIA) platforms and a field study in British Columbia. J. Clin. Virol. 2021;142 doi: 10.1016/j.jcv.2021.104914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irsara C., Egger A.E., Prokop W., Nairz M., Loacker L., Sahanic S., Pizzini A., Sonnweber T., Mayer W., Schennach H., Loeffler-Ragg J., Bellmann-Weiler R., Tancevski I., Weiss G., Anliker M., Griesmacher A., Hoermann G. Evaluation of four commercial, fully automated SARS-CoV-2 antibody tests suggests a revision of the Siemens SARS-CoV-2 IgG assay. Clin. Chem. Lab. Med. 2021;59(6):1143–1154. doi: 10.1515/cclm-2020-1758. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y.V., Wiencek J., Meng Q.H., Theel E.S., Babic N., Sepiashvili L., Pecora N.D., Slev P., Cameron A., Konforte D. AACC practical recommendations for implementing and interpreting SARS-CoV-2 emergency use authorization and laboratory-developed test serologic testing in clinical laboratories. Clin. Chem. 2021;67(9):1188–1200. doi: 10.1093/clinchem/hvab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.M. DeSimone, D.P. Simmons, N. Tolan, S. Melanson, A. Petrides, M. Tanasijevic, P. Schur, Clinical correlations of SARS-CoV-2 antibody responses in patients with COVID-19 infection, medRxiv (2020) 2020.10.22.20213207.
- 56.FDA, Convalescent plasma EUA letter of authorization 06032021, 2021. https://www.fda.gov/media/141477/download. (Accessed 2021/10/08).
- 57.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H., He X., Martinez D.R., Rutten L., Bos R., van Manen D., Vellinga J., Custers J., Langedijk J.P., Kwaks T., Bakkers M.J.G., Zuijdgeest D., Rosendahl Huber S.K., Atyeo C., Fischinger S., Burke J.S., Feldman J., Hauser B.M., Caradonna T.M., Bondzie E.A., Dagotto G., Gebre M.S., Hoffman E., Jacob-Dolan C., Kirilova M., Li Z., Lin Z., Mahrokhian S.H., Maxfield L.F., Nampanya F., Nityanandam R., Nkolola J.P., Patel S., Ventura J.D., Verrington K., Wan H., Pessaint L., Van Ry A., Blade K., Strasbaugh A., Cabus M., Brown R., Cook A., Zouantchangadou S., Teow E., Andersen H., Lewis M.G., Cai Y., Chen B., Schmidt A.G., Reeves R.K., Baric R.S., Lauffenburger D.A., Alter G., Stoffels P., Mammen M., Van Hoof J., Schuitemaker H., Barouch D.H. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sherman A.C., Desjardins M., Cheng C.A., Bausk B., Izaguirre N., Zhou G., Krauss J., Tolan N., Walt D.R., Soiffer R., Ho V.T., Issa N.C., Baden L.R. SARS-CoV-2 mRNA vaccines in allogeneic hematopoietic stem cell transplant recipients: immunogenicity and reactogenicity. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.E.A. Melgoza-Gonzalez, D. Hinojosa-Trujillo, M. Resendiz-Sandoval, V. Mata-Haro, S. Hernandez-Valenzuela, M. Garcia-Vega, M. Bravo-Parra, A.A. Arvizu-Flores, O. Valenzuela, E. Velazquez, A. Soto-Gaxiola, M.B. Gomez-Meza, F. Perez-Jacobo, L. Villela, J. Hernandez, Analysis of IgG, IgA and IgM antibodies against SARS-CoV-2 spike protein S1 in convalescent and vaccinated patients with the Pfizer-BioNTech and CanSinoBio vaccines, Transbound Emerg Dis 69(4) (2022) e734-e745. [DOI] [PMC free article] [PubMed]
- 60.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claer L., Quentric P., Fadlallah J., Devilliers H., Ghillani P., Gunn C., Hockett R., Mudumba S., Guihot A., Luyt C.E., Mayaux J., Beurton A., Fourati S., Bruel T., Schwartz O., Lacorte J.M., Yssel H., Parizot C., Dorgham K., Charneau P., Amoura Z., Gorochov G. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021;13(577) doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.NIH, COVID-19 Treatment Guidelines; COVID-19 Convalescent Plasma, https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/covid-19-convalescent-plasma/.
- 62.Padoan A., Dall'Olmo L., Rocca F.D., Barbaro F., Cosma C., Basso D., Cattelan A., Cianci V., Plebani M. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin. Chim. Acta. 2021;519:60–63. doi: 10.1016/j.cca.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.J. Favresse, J.L. Bayart, F. Mullier, J.M. Dogne, M. Closset, J. Douxfils, Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2), Clin Microbiol Infect 27(9) (2021) 1351 e5-1351 e7. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.