Abstract
The advent of Corona virus Disease 2019 (COVID-19) distorted health systems of many countries. Efforts have been made to either develop new treatment solutions such as vaccines or repurpose previously adopted drugs. Challenges in accessing available treatment, inadequate, non-existent, or overstretched healthcare facilities, long COVID disease, cultural practices and beliefs about vaccination, vaccine hesitancy, availability, accessibility and perceived safety of herbal supplements seem to be major factors propelling individuals to use herbal supplements. Published reports advocating for clinical development of herbal supplements for COVID-19 and other emerging and re-emerging viral diseases are sparse. This paper aims to review the pathogenesis of COVID-19, use of herbal products during the pandemic and make case for clinical development of herbal supplements through the adoption of modern and acceptable technologies and research processes.
This was a scoping review. Database searches of Google Scholar, PubMed and ResearchGate among others were performed using related keywords to identify relevant journals and lists of primary articles. Clinical trial databases:-Clinicaltrial.gov, Pan African Clinical Trial Registry (PACTR) and WHO international clinical trial registry (ICTRP) were reviewed to extract data.
The use of herbal supplements during COVID-19 was not only peculiar to individuals living in Sub-Saharan Africa, but a global practice. Herbal supplements recommended to manage COVID-19 have not been validated using clinical trials. Available data showed that the number of herbal supplements undergoing clinical trial for COVID-19 indication in Africa was low.
The availability of medicinal plants in Sub-Saharan Africa if well explored has great potentials to address various emerging and re-emerging viral diseases confronting the region. The economic potential of clinically validated herbal supplements are huge, and tapping into this opportunity created by preference of population to herbal supplement could increase export of herbal supplement and gross domestic product (GDP) of respective countries in Africa.
Keywords: Herbal Supplements, Herbal Products, COVID-19, Clinical Development, Herbal Medicine, SARS-CoV-2
Introduction
The Corona virus Disease 2019 (COVID-19) since reported in late 2019 has placed a significant health burden across countries. The disease which the World Health Organization (WHO) declared a pandemic has resulted in several mortalities across countries; and has had significant social, medical and economic impacts globally [1]. In an effort to control the spread of the disease caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2), various measures including non-pharmacological approaches have been adopted with a view to curtailing its spread, reduce person to person transmission, mortality and morbidity associated with the disease. Since the pandemic began, Health systems across the world have been stretched; and in poorer nations, the gaps have been widened [[1], [2], [3]].
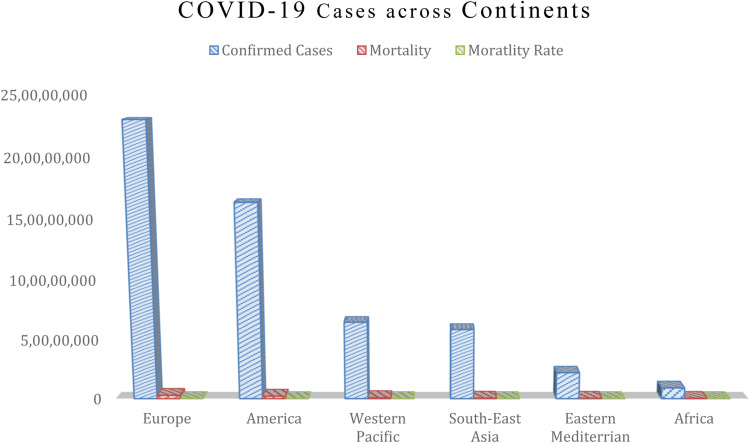
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) which is a novel beta coronavirus can cause symptoms ranging from mild to severe. However, a good number of infected individuals may be asymptomatic, but can also transmit the infection to other individuals [1]. As at the time of documenting this report, the collective number of confirmed cases globally was 594,367,247, while the number of COVID-19 related deaths was 6,451,016 [4]. Nonetheless, due to limited resources available to most countries in Africa, there were limitations assessing available and approved testing diagnostics for massive diagnosis and identification of populations at risk. This precipitated the underdiagnosis reported within the continent especially among individuals who are asymptomatic Fig. 1 [2,3].
Fig. 1.
COVID-19 confirmed cases, mortality and mortality ratio across various continents. Source of Data: WHO COVID 19 Dashboard. Available https://covid19.who.int/table. Accessed 7 July 2022
COVID‐19 transmission has been found to be mainly enabled through proximal contact with air droplets and physical contact from infected persons, in addition to the exposure to aerosol in enclosed spaces [5]. Since the first incident of the disease was reported from Wuhan city in China, several other cases have been reported from all parts of the world and caused millions of deaths [6]. SARS‐CoV‐2 causes a wide variety of respiratory symptoms which range from those similar to that of the common cold to more severe illness like pneumonia, and its major route of transmission is through droplet spread [7], [8], [9]. Patients who are infected with SARS-CoV-2 usually have a lengthy course of infections and are at increased risk of death [10].
Five different types of SARS-COV-2 with their composite molecular genetics have been identified (Table 1 ). They comprised of the alpha variant [B.1.1.7] (first documented in the UK in September 2020 and designated a variant of concern on 18 December 2020); the beta variant [B.1.351] (first documented in South Africa in May 2020, and designated a variant of concern on 18 December 2020); the Gamma variant [P.1] (first documented in Brazil in November 2020, and designated a variant of concern in 11 January 2021). Other identified variants were, the delta variant [B.1.617.2] (first documented in India in October 2020 and designated a variant of concern 11 May 2021), and the omicron variant (first documented in multiple countries in November 2021 and designated a variant of concern on November 26 2021). [11,12].
Table 1.
Previous and currently circulating COVID-19 variants. Source of Data: World Health Organization. (2022). Historical working definitions and primary actions for SARS-CoV-2 variants. https://www.who.int/docs/default-source/coronaviruse/annex2_previous_vocs_and_definitions.pdf.
| WHO label | Pango lineage⁎ |
GISAID clade | Nextstrain clade | Earliest documented samples |
Date of designation |
|---|---|---|---|---|---|
| Alpha | B.1.1.7 | GRY | 20I (V1) | United Kingdom, Sep-2020 |
VOC: 18-Dec-2020 Previous VOC: 09-Mar-2022 |
| Beta | B.1.351 | GH/501Y.V2 | 20H (V2) | South Africa, May-2020 |
VOC: 18-Dec-2020 Previous VOC: 09-Mar-2022 |
| Gamma | P.1 | GR/501Y.V3 | 20J (V3) | Brazil, Nov-2020 |
VOC: 11-Jan-2021 Previous VOC: 09-Mar-2022 |
| Delta | B.1.617.2 | G/478K.V1 | 21A, 21I, 21J | India, Oct-2020 |
VOI: 4-Apr-2021 VOC: 11-May-2021 Previous VOC: 7-Jun-2022 |
| Omicron⁎⁎ | B.1.1.529 | GR/484A | 21K, 21L, 21M, 22A, 22B, 22C, 22D |
+S:R346K +S:L452X +S:F486V |
Multiple countries, Nov-2021 |
Includes all descendent lineages.
Includes BA.1, BA.2, BA.3, BA.4, BA.5 and descendent lineages. It also includes BA.1/BA.2 circulating recombinant forms such as XE. WHO emphasizes that these descendant lineages should be monitored as distinct lineages by public health authorities and comparative assessments of their virus characteristics should be undertaken.
Pathogenesis of COVID-19
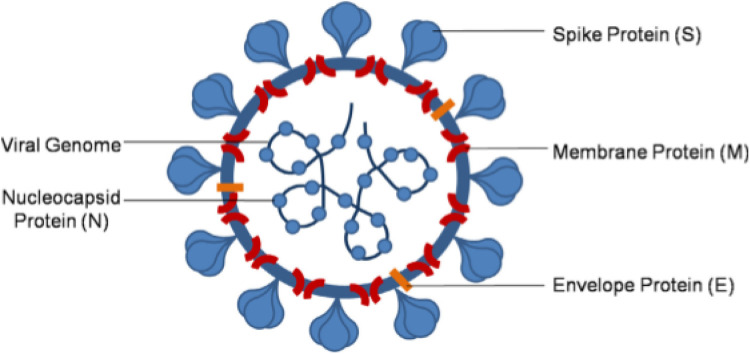
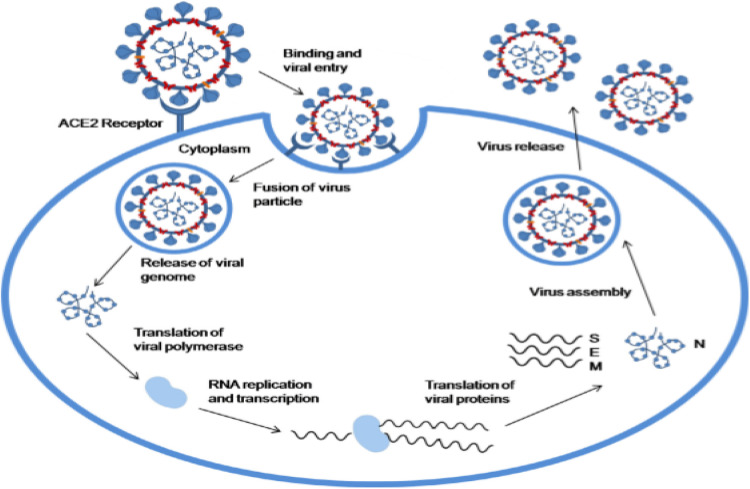
The SARS-CoV-2 expresses a precise spike glycoprotein (Fig. 2 ) which has a strong binding affinity for angiotensin converting enzyme 2 (ACE2) receptors. Studies have shown that pathogenesis of SARS-CoV-2 and that of SARS-CoV-1 might look similar; but that the affinity which SARS-CoV- 2 has for S-Protein is higher than that for SARS-CoV-1. This property confers more pathogenicity to the virus [8,13]. This also implies that human organs which express higher concentrations of ACE2 are more prone to destructive tendencies of the SARS-CoV-2. Priming of the viral spike protein is accomplished by transmembrane protease serine 2 (TMPSS2) [6]. The fusion of the virus to the host cell takes place through various cleavage steps and finally cell entry (Fig. 3) [14]. The coronavirus then expresses and replicates their genomic RNA which is then incorporated into new viral particles [14]. After unrestrained replications of the virus, which multiplies the number of infected epithelial cells and cellular debris, large quantities of cytokines are released, followed by serious inflammation, which reduce the number of CD4+ memory T helper cells and amplifies the cytotoxic activity of CD8 [15,16]. Infection with this virus stimulates a pro-thrombotic and pro-inflammatory reaction which may enhance the risk of severe thrombotic disorders [17]. Many studies propose instant apparent increases in both arterial (largely Myocardial Infarction and stroke), and venous thromboembolic events (VTEs) [18], [19], [20], [21], [22].
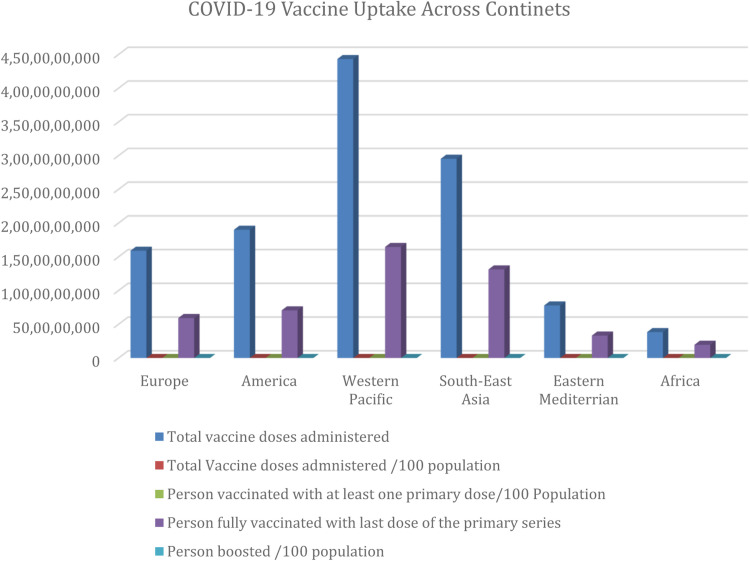
Fig. 3.
COVID-19 Vaccination Analysis across continents. Source of Data: WHO COVID 19 Dashboard. Available https://covid19.who.int/table. Accessed 7 July 2022.
Graph shows that uptake of COVID-19 Vaccines is lowest among populations in Africa.
Fig. 2.
Structure of SAR-CoV-2. Adopted from Yashika et al., [13].
Available COVID-19 Treatment Options and Challenges to using available treatment
Various treatment options for COVID-19 have been developed and various approaches adopted for treating and reducing mortalities associated with the disease. Among such treatment options are vaccines (Table 2 ) and other repurposed drugs (Table 3 ). In spite of availability of vaccines for managing COVID-19, uptake of such vaccines remains low in Africa (Fig. 4 ) [23]. Another challenge to deploying vaccines for managing COVID-19 are reported cases of viral escape and long COVID-19 among individuals who have been vaccinated [24], [25], [26]. Low uptake of available COVID-19 vaccines has been attributed to vaccine hesitancy, cultural and religious beliefs and fear of health complications and possible death following vaccination [27]. It has been reported that about 10-20% of individuals who previously suffered from COVID-19 were estimated to suffer long COVID. Such individuals exhibit over 200 symptoms some of which are fatigue, breathlessness and mental health issues which persisted months after they had recovered from the disease. Researchers are yet to unravel the mechanisms behind this disorder and possible solution to limiting a worldwide estimate of over 145 million of individuals who are prone to this disorder [28,25].
Table 2.
Vaccines approved in the high-income countries and selected vaccines of global relevance [29].
| Manufacturer | Vaccine type | Dosage Overall | Dosage Overall | efficacy Current approvals* | |
|---|---|---|---|---|---|
| mRNA-1273 | Moderna (USA) | mRNA | Two doses 28 days apart | 94·1% 14 days after second dose | The USA, Europe, and the UK |
| BNT162b2 | Pfizer–BioNTech (USA) | mRNA | Two doses 21 days apart | 52% after one dose; 94·6% 7 days after the second dose | The USA, Europe, and the UK |
| Ad26.COV2.S | Johnson & Johnson (USA) |
Viral vector | One dose | Vaccine efficacy against COVID-19 is 66·1%; vaccine efficacy against severe COVID-19 is 85·4% (at 28 days) | The USA and Europe |
| ChAdOx1 nCoV-19 (AZD1222) |
Oxford– AstraZeneca (UK) |
Viral vector | Two doses 28 days apart (intervals of >12 weeks studied) | Overall vaccine efficacy is 70·4% at 14 days or more after second dose | WHO and COVAX, the UK, Europe, the USA, India, and Mexico |
| NVX-CoV2373 | Novavax (USA) | Protein subunit | Two doses | 89·7% in the UK after two doses | Emergency use authorisation* application planned |
| Gam-COVID-Vac (Sputnik V) |
Gamaleya National Research Center for Epidemiology and Microbiology (Russia) |
Viral vector | Two doses (first, rAd26; second, rAd5) 21 days apart | 91·6% at 21 days after first dose (day of dose two) | Russia, Belarus, Argentina, Serbia, UAE, Algeria, Palestine, and Egypt |
| CoronaVac | Sinovac Biontech (China) |
Inactivated virus | Two doses 14 days apart | 83·5% at 14 days or more after dose two | China, Brazil, Columbia, Bolivia, Chile, Uruguay, Turkey, Indonesia, and Azerbaijan |
| BBIBP-CorV | Sinopharm ½ (China) | Inactivated virus | Two doses 21 days apart | 78·1% or more after dose two | China, UAE, Bahrain, Serbia, Peru, and Zimbabwe |
Table 3.
Immunomodulatory therapies recommended by the Infectious Diseases Society of America (IDSA) and/or FDA for COVID-19 treatment [30].
| Therapeutic | Adult patient population | Dosing | Potential adverse reactions |
Certainty of recommendation |
|---|---|---|---|---|
| Glucocorticoids (Dexamethasone preferred) |
Hospitalized and/or severe COVID-19 disease. |
Dexamethasone 6 mg IV or PO × 10 days or until discharge | Hyperglycemia, neurological side effects (agitation/confusion), adrenal suppression, risk of bacterial or fungal infection | Moderate |
| Tocilizumab | Hospitalized with severe COVID-19 disease, elevated inflammatory markers, requiring supplemental oxygen, NIMV, IMV, or ECMO. |
Weight < 30 kg: 12 mg/kg IV over 60 min. Weight > 30 kg: 8 mg/kg IV over 60 min. (Maximum dose 800 mg) |
Increased risk of infection, gastrointestinal perforation (seen in non-COVID settings) |
Low |
| Sarilumab | Hospitalized who meet criteria for tocilizumab, but it is not available. |
400 mg IV over 60 min. | Increased risk of infection | Very low |
| Baricitinib | Hospitalized with severe COVID-19 disease and elevated inflammatory markers, requiring supplemental oxygen, NIMV, IMV, or ECMO. Also indicated for use with remdesivir when corticosteroid contraindicated. |
Baricitinib 4 mg PO daily × 14 days or until discharge. |
Increased risk of infection, bowel perforation, thromboembolism, ischemic colitis, elevated transaminases, seizure |
Moderate |
| Convalescent plasma (high-titer antibody) |
Outpatient or hospitalized, with immunosuppressive disease or receiving immunosuppressive treatment, early in disease course |
NA | Circulatory overload, transfusion-associated lung injury, allergic transfusion reaction, thromboembolism |
Low |
Certainty of Recommendation Grades (based on data from clinical trials, and the risk of bias, inconsistency, indirectness, imprecision, and publication bias noted in the studies):
High: Based on data from clinical trials, the true effect lies close to that of the estimate of the effect.
Moderate: Based on data from clinical trials, the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low Certainty: Based on data from clinical trials, the true effect may be substantially different from the estimate of the effect.
Very Low Certainty: Based on data from clinical trials, the true effect is likely to be substantially different from the estimate of the effect.
IV, intravenous; PO, oral; NIMV, non-invasive mechanical ventilation; IMV, invasive mechanical ventilation; ECMO, extracorporeal membrane oxygenation; NA, not applicable.
Fig. 4.
Pathogenesis of SAR-COV-2. Adopted from Yashika et al., [13].
In vitro studies have revealed that the omicron variant evades antibody neutralization in persons who were previously infected with or who were vaccinated against SARS-CoV-2 [24,26]. Data from epidemiological studies proposed that vaccine effectiveness was decreased [31] and the rates of reinfection were much higher for the omicron variant than for the beta (B.1.351) and delta variants [32]. Pre-delta variant studies showed that vaccines had high effectiveness [33,34,35] which remained largely intact for up to 6 months [36]. Early research on the delta variant suggested only a modest difference in vaccine effectiveness [7] but subsequent reports suggested protection against delta infection might be somewhat lower than previous variants [18,19,17,37,21]. A US study found vaccine effectiveness fell from 92% to 80% as delta cases rose, although vaccine effectiveness against hospitalization remained at 90% [22].
Concern about waning COVID-19 vaccine effectiveness has prompted intense public discussion about the need for booster vaccinations and the introduction of herbal remedies. Furthermore, in spite of the availability of vaccines, several countries are still reporting COVID-19-related deaths because of lack of immunization following vaccination and occurrence of different variants which might precipitate viral vaccine escape [38,39]. Also, the persistence of long COVID (for which no new treatment options seem hypothesized and investigated to manage the symptoms) among individuals who recovered from COVID following either vaccination or deployment of other available treatment remains a global concern [25]. Since perceived gaps still exist regarding unmet demands for improving care for serious and critically ill COVID-19 patients, and other observed challenges to accessing validated treatment options such as: cost, distribution challenges, low vaccine uptake and vaccine hesitancy across Africa, the need to explore the use of herbal remedies as treatment options for COVID-19 are being proposed not just for COVID-19, but for other emerging viruses of global health significance.
Use of herbal supplements for COVID-19
As COVID-19 ravaged the world, Epidemiologists have projected that based on poor health system in Africa, mortality from the disease within the region might likely be on the increase. In fact, some opinion from the West has projected that human bodies would be found across major streets in Africa if the continent did not put in place urgent measures to curtail the spread of the disease through community infection [40]. Unfortunately, Africa recorded the least mortalities from COVID-19 (Fig. 1). Till date, the world has been hypothesizing the reasons responsible for the low mortalities recorded in Africa. While several reasons have been hypothesized, strong evidences suggest that Africans practically survived COVID-19 based on the use of herbal supplements which are available within the region. Investigations into mostly used herbal supplements during COVID-19 showed that garlic, ginger, lemon, turmeric, onions, Negro pepper, and black pepper among other items were widely consumed primarily as immune boosters to limit the infection [40].
For centuries, herbal supplements have been used to manage different medical conditions including viral infection. The advent of COVID-19 distorted the health systems of many countries and there was urgency to research and develop quick treatment solutions which would help reduce mortalities associated with the disease. Challenges in accessing available treatment options made countries seek for alternative treatment options including the use of herbal medicine. Inadequate, non-existent, or overstretched healthcare facilities, cultural practices and beliefs in Africa were reported as possible reasons for resorting to using herbal remedies as alternatives to prevent or alleviate the symptoms of COVID-19 [27]. Due to their availability, accessibility, affordability, and the belief that they are effective and safe, herbal preparations and alternative curative and preventive approaches have been explored in managing various diseases in Africa [41]. Antwi-Baffour et al., [42] reported that approximately 80% of the African population used herbs to manage various diseases. Since use of herbs is a documented practice among Africans, the advent of the pandemic and the exploration of herbs to manage diseases was not a new phenomenon since medicinal plants are usually the primary source of healthcare in many communities in Africa.
In an effort to introduce herbal product to manage COVID-19, Madagascar came up with herbal tonic code named COVID-Organics. The product was reported to contain Artemisia annua, Cinnamomum camphora and other phytochemicals such as essential oils, flavonoids, coumarins, polysaccharides, saponins, tannins, and pentacyclic triterpenes [43,44]. However, its acceptance by population within the Africa region was with mixed feelings since the herbal product was not validated using clinical trials. Furthermore, in the same effort to provide herbal solution for COVID-19, the National Institute for Pharmaceutical Research and Development Nigeria (NIPRD), repurposed NIPRIMUNE as an adjunct therapy for COVID-19. It is worthy of note that the repurposed herbal product for treating COVID-19 has not been validated using clinical trials. The herbal-based supplement was first developed in 2018 for managing patients with HIV/AIDS [45]. In another development, the Ghana Center for Awareness repurposed a herbal mixture for COVID-19 . This was code named CoA mixture. This herbal supplement was reported to contain about 160 phytochemicals with immune supporting properties [46].
Exploring the use of herbal supplement to manage COVID-19 among infected individuals in Nigeria, a group of researchers formulated a herbal supplement code named Combi-5. This supplement comprised of Zingiber officinale (Ginger), Curcuma longa (Tumeric), Piper guineense (black pepper), Allium sativum (Garlic) and Xylopia aethiopica (Negro pepper). The researchers have published a case report and case series showing how successful the herbal supplement was used to manage COVID-19 infected subjects with mild to moderate symptoms [47,48].
A recent review by Chikowe et al. [49] identified 30 Malawian plants with various activities against COVID-19 (Table 4 ). The plants were repurposed to manage pneumonia, fever, cough, asthma, or breathing problems which are typical symptoms of COVID-19. The fear of COVID-19 was reported to cause many Ugandans to resort to herbal remedies such as ginger and lemon as preventive measures and immune boosting benefits against COVID-19 [50].
Table 4.
Summary of some herbal remedies used for COVID-19 from different continents.
| Continent | Medicinal plants for COVID-19 | Conditions managed in COVID-19 | Bioactive compounds | Proposed action | References |
|---|---|---|---|---|---|
| Africa | Sclerocarya birrea, Pyrenacantha kaurabassana, Moringa oleifera, Azadirachta indica, Curcuma longa, Piper guineense, Eucalyptus globulus, Thymus maroccanus, Zingiber officinale, Allium cepa, Olea europaea, Allium sativum | Pneumonia, fever, cough, asthma, or breathing problems | Phenolic moieties, Enzymes, | Antioxidant, anti-inflammatory | [49,51,27]; |
| Americas and the Caribbean | Spondias mombin, Plectranthus amboinicus, Ocimum gratissimum, Libidibia ferrea, Dysphania ambrosioides, Citrus limon, Bixa orellana, Alpinia zerumbet, Erythroxylum coca, Matricaria recutita, Piper aduncum, Allium sativum, Zingiber officinale, Eucalyptus globulus, Salvia rosmarinus, Morus alba, Eucalyptus spp, Cúrcuma longa, Coriandrum sativum, Cinchona pubescens, Azadirachta indica |
Fever, breathing, immune boosters, cough | Flavonoids, phenols, polyphenols, carotenoids, | Antiviral, anti-inflammatory, anti-immunomodulatory, antioxidant | [52,53,54,55,56] |
| Asia and the Middle East | Lianhua qingwen, Jinhua qinggan, Xuebijing liquoric, Scutellaria baicalensis, Pinellia rhizome, Forsythia suspensa, Prunus armeniaca, Peganum harmala, Camellia sinensis, Nigella sativa, Pimpinella anisum, Trigonella foenum-graecum Ocimum sanctum, Curcuma longa, Zingiber officinale, Tinospora cordifolia |
fever, cough, fatigue, acute upper respiratory, tract infection, febrile diseases, Forsythaside, amygdalin | Glycyrrhetinic acid, glycyrrhizin, baicalin, baicalein, Forsythaside, amygdalin, |
Anti-inflammation, antitussive, expectant effects, scavenging free radicals |
[57,58,59,60,61] |
| Australia | Eucalyptus globulus | Improves breathing, nasal congestion, asthma | Eucalyptol and Jensenone | Anti-inflammatory, immunomodulatory | [55], [70] |
| Europe | Echinacea purpurea, Andrographis paniculate | Cough, fever, flu, pharyngitis | Alkylamides, caffeic acids, polysaccharides, and chicoric acids | Antiviral, immunomodulatory, and anti-inflammatory | [62,63] |
In Morocco, El Alami et al., [51] reported that Eucalyptus globulus, Thymus maroccanus, Zingiber officinale, Allium cepa, Olea europaea, and Allium sativum were commonly used herbal remedies among Moroccans against COVID-19.
Similarly, In Nigeria, Orisakwe et al., [27] reported plants with anti-inflammatory and antioxidant properties, such as garlic, guava, ginger, neem, and papaya, as commonly acclaimed herbal remedies for COVID-19. A few research institutes in Africa have sought approval for herbal remedies to treat COVID-19. The low death rates recorded in Africa due to COVID-19 have been attributed partly to the use of herbal remedies which are available within the continent. Many of these herbal remedies have been reported to possess immunoprotective, free radical scavenging and antioxidant properties [40]. The use of herbal supplement to manage COVID-19 was not only peculiar to Africans, but also was a common practice among individuals in various continents (Table 4).
Mechanism of action of herbal-based products on SARS-CoV-2
The pathogenesis of COVID-19 infection is multifaceted. In severe infection, there is accelerated release of inflammatory cytokines resulting in cytokine storm and dysregulation of individual's immune system [64]. Release of cytokine storm precipitates respiratory distress, multiple organ failure, disseminated intravascular coagulation (DIC) and possibly death [64].
There are basically two key crucial pathways required for COVID-19 pathogenesis. The first is inhibition of the virus from attaching to host cell and the second is limiting the virus from replicating within the host cell [13]. The likely effects of herbal products to exert their effect have been premised on ability to limit either or all of these essential processes.
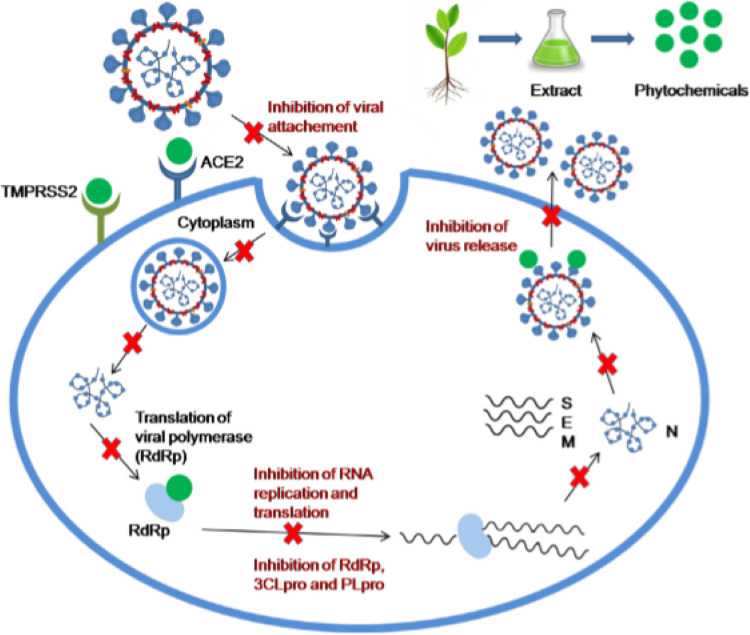
Many herbal supplements have been identified to achieve their benefits by preventing the fusion of SARS-COV-2 to host cells, inhibition of entry into human cells, inhibition of viral RNA transcription and translation, decrease intracellular acidity thereby limiting viral replication, viral transport within the host cell and inhibit the release of proinflammatory cytokines thus preventing cytokine storm and death (Fig. 5 ) [64,13]. The ability of herbal-based products to suppress the progression of COVID-19 is attributed to multiple array of phytochemicals:–Tannins, Flavonoids, Terpenes, Glycosides, Carbohydrates and Saponins among others. All of these chemicals have been reported to limit oxidative stress, improve immunity, possess antiviral, anti-inflammatory and antibacterial properties [47].
Fig. 5.
Schematic representation of key areas herbal supplements play inhibitory roles in the COVID-19 pathogenesis. Adopted from [13]. Angiotensin converting enzyme2 (ACE2); 3-chymotrypsin-like protease (3CLpro); Transmembrane protease serine 2 (TMPRSS2); papain-like protease (PLpro); RNA-dependent RNA polymerase (RdRp).
Discussion
The use of herbal supplements has persisted for years. Populations across the globe have relied on herbal remedies to meet basic health needs. Increased use of herbal products is precipitated by high cost of conventional drugs worsened by out-of-pocket spending for healthcare which is very common in many countries in Africa. As demand for herbal-based products increase, there is an urgent need to upscale the clinical development of such products with a view to ascertaining their clinical usefulness in managing various diseases including COVID-19 and other emerging and re-emerging viral threats. As new viral diseases and microorganisms which are highly resistant to validated and available treatment solutions emerge, there is need for Scientists in Africa to accelerate the search, identification, isolation and clinical development of new treatment options sourced from herbal-based products. The efforts of World Health Organization (WHO), African Center for Disease Control (ACDC) and other respective agencies in Africa towards accelerating the clinical development of novel treatment options using resources which are indigenous to the people cannot be overemphasized. Inability of African countries to manufacture the drugs her citizens consume has been a big challenge. Currently, Africa contributes only about 3% of total drugs produced globally, but about 95% of medicine consumed in the continent are imported [65]. Accelerating the local production of clinically validated herbal-based products could help in changing the paradigm.
The WHO has recognized the critical role of herbal medicine in meeting global health needs especially among individuals living in Africa [23]. This is premised on the long history of use of herbal medicine as means of providing care for individuals seeking healthcare [64]. The organization however recommended that applicable evidence based approach should be adopted for validating the safety and efficacy of herbal products targeted for population use through the application of rigorous clinical trial process [23]. The WHO in advocating for accelerated clinical development of herbal-based solutions came up with various suggestions which if implemented would help to achieve the desired goal. Some of these recommendations include: encouraging countries to upscale the implementation of policies centered on the use of herbal medicine, integration of herbal medicine into the training curriculum of healthcare professionals and advocating for more clinical development of herbal-based products using evidence based approach [66]. While some of these efforts have been implemented in part by some countries in Africa, more efforts are still required across countries within the continent [66] with a view to achieving deliverables for universal health coverage.
An effective universal health coverage supports accessing health care services which are economical, effective, accessible and preventive. While out of pocket expenditure for healthcare is common in Africa, the need to develop herbal based products which are safe, accessible cost effective with scientifically proven efficacy seem to be the most likely alternative for achieving sustainable healthcare especially among individuals living in resource limited economies .
Clinical trial investigates the safety and efficacy of an investigational new drug (IND) on human subjects. Is often designed to generate new evidences using validated scientific approach which will help improve health outcomes. While it is the gold standard for assessing the efficacy and safety of herbal medicine, evidence has shown that few of such trials for COVID-19 and other related emerging viral infections are conducted in sub-Saharan Africa. Available data from clinicaltrial.gov showed that the number of herbal supplements undergoing clinical trial in Africa for either curative or supportive care among COVID-19 infected subjects was scanty when compared with that conducted in Asia. As of the time of documenting this manuscript, a search on clinical trial.gov showed that a total of 20 herbal supplement clinical trial was on going for COVID-19 indication in Asia and Africa. Of this number, only one (1) is ongoing in Africa as against 19 in Asia. Search on other databases such as Pan African Clinical Trial Registry (PACTR) and WHO international clinical trial registry (ICTRP) showed no result. This data show there still exists wide gap in conducting COVID-19 related clinical trial using herbal supplements within the region. That more clinical trial on herbal supplements for COVID-19 was on going in Asia cannot be over emphasized. Many Countries in Asia including China have adopted the use of clinically validated herbal medicine as one of the main streams for achieving quality healthcare; and have integrated complimentary and integrative medicine into its national policy for health. Considering the various health challenges confronting Africa compounded by emerging of deadly viral threats such as Ebola, Monkey pox, Lassa virus among others, high cost of available treatment for diseases caused by these viral threats, a responsibility is placed on African leaders, and health agencies to advocate for massive clinical development of herbal-based supplement.
The economic potentials inherent in clinical development of herbal-based products are enormous; and different countries in Africa should be able to harvest from it through making polices and providing conducive research and regulatory environments to ‘cut from the cake’.
According to report from Procedure Research, the global market size of clinical trial is estimated to worth around US$ 84.43 billion by 2030 from US$ 48.4 billion in 2020, growing at a compound average growth rate (CAGR) of 5.7% from 2021 to 2030 (Procedure Research. Available at https://www.globenewswire.com/news-release/2022/01/27/2374515/0/en/Clinical-Trials-Market-Size-Worth-Around-US-84-43-Bn-by-2030.html).
On the other hand, a report from Global plant Extract Report projected that the global market price for herbal based products would worth over US$ 47.42 Billion by 2028. (Global Plant Extracts Market Report and Market analysis. Available at https://www.globenewswire.com/en/news-release/2022/08/04/2491986/28124/en/Global-Plant-Extracts-Market-Report-to-Reach-47-42-Billion-by-2028.html).
Furthermore, Data Bridge Market Research (DBMR) analyses showed that herbal extracts market was valued at US$ 8.26 billion in 2021 and was expected to reach US$15.23 billion by 2029, at a CAGR of 7.95% within the forecast period of 2022 to 2029. (Data Bridge Market Research on Global Phytomedicines and Herbal Extracts Market-Industry Trends and Forecast. Available at https://www.databridgemarketresearch.com/reports/global-phytomedicines-and-herbal-extracts-market)
Some major factors that will be responsible for driving this increase have been identified. These are: increase in demand for clinically validated herbal products, high inclination of respective populations towards herbal-based products, application of modern and advanced technology to improve the process of plant extraction, increase regulatory requirements for approvals and use of high-tech manufacturing equipment and chemical apparatus to accelerate the clinical development process (Data Bridge Market Research on Global Phytomedicines and Herbal Extracts Market-Industry Trends and Forecast. Available at https://www.databridgemarketresearch.com/reports/global-phytomedicines-and-herbal-extracts-market)
In spite of this huge economic potentials of herbal product, available report suggests that ethical and regulatory restrictions towards approval of herbal-based protocols and products still exist in some countries within the continent; and this has been identified as a challenge (Global Plant Extracts Market Research and Market analysis. Available at https://www.globenewswire.com/en/news-release/2022/08/04/2491986/28124/en/Global-Plant-Extracts-Market-Report-to-Reach-47-42-Billion-by-2028.html.).
For effective clinical trial of herbal medicines, ethical and regulatory frameworks which will support the conduct of such trials should be provided. Previous studies have reported that ethical committee in some countries within the continent used between 8-72 weeks to review herbal medicine clinical trial protocol [67,68,69]. Other issues which have been identified were insufficient proof of efficacy and safety pre-clinical data, standardization and quality assurance [68]. Strengthening the capacities of regulatory agencies, researchers and ethical review committees through training could be helpful.
Following these reports, there are supporting evidences that preference of individuals to herbal- based products is on the increase. Based on this, Research Scientists should invest more efforts towards taking herbal-based products to clinical trial. This is with a view to bringing to the market products which are validated using evidence base approach. The Alma declaration of 1978 strongly supported the development of treatment solutions which are indigenous to the people using resources available and accessible within communities [4].
For effective and accelerated clinical development of herbal-based supplements, the need to address infrastructural challenges for herbal clinical trial becomes critical. Prior to subjecting herbal supplements to clinical trial, extensive pre-clinical studies are required. These studies include toxicity and safety evaluation of herbal supplements which should be performed using modern technologies. Premised on the Organization and Economic Cooperation Development (OECD) recommendations, safety evaluation studies should be performed in a good laboratory practice (GLP) approved facility. In many research institutions within the sub-Saharan Africa, facilities needed for carrying out robust pre-clinical studies may not be available; and where they are available may not be sufficiently equipped to handle robust testing. Furthermore, accepting data emanating from such laboratories might have questionable quality issues since the laboratory generating the data may lack GLP approval. Some testing facilities may lack storage capacities for preserving under approved temperature and environmental conditions human cell lines which might be used for pre-clinical studies. Modern equipment for performing toxicogenomic evaluation (Micro array), product characterization (Liquid Chromatography Mass Spectrometry (LCMS) and identification (Nuclear Magnetic Resonance (NMR) and Fourier Transform Infrared Spectroscopy (FTIRS)) prior to taking identified, potential and effective molecules to clinical trials remain another huge gaps. There is need for respective countries in Africa to provide pre-clinical research laboratories under one room which shall be equipped to handle all types of pre-clinical evaluations of herbal remedies. Africa is blessed with lots of medicinal plants with potentials to address various diseases confronting the region. Developing these into acceptable treatment options using clinical trials will not just help the region meet her ever increasing health needs, but also result in massive export of such products thus adding to the continent's gross domestic product (GDP). Health systems across countries within the continent should be strengthened to enable the region attract a higher percentage of clinical trials.
Conclusion
The use of herbal supplements in Africa has been a long practice. Emerging and re-emerging viral threats if not curtailed using validated and multiple approach treatment solutions could put more pressure on health systems within the region. As new viruses emerge with their constantly changing genomic compositions, it becomes increasingly difficult to use a single treatment option to manage the associated crisis. The need to deploy clinically validated herbal supplements which are known to be holistic, complimentary and integrative should strongly be considered. Providing solution to numerous infectious diseases confronting Africans lies within the continent. While research is business and funders always fund studies in the area of their interest, organizations, institutions and health agencies within the continent should accelerate funding of herbal medicine clinical trial with a view to developing treatment solutions which are cheaper, indigenous and acceptable to the people. The economic potentials of herbal-based products are huge; and tapping into this opportunity created by preference of populations to herbal-based products could increase drug export and the GDP of respective countries in Africa.
Funding
The Authors received no funding for this work.
CRediT authorship contribution statement
Augustine Anayochukwu Onyeaghala: Conceptualization, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Arinze Favour Anyiam: Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Danladi Chiroma Husaini: Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Emmanuella Ogechi Onyeaghala: Data curation, Writing – review & editing. Ejeatuluchukwu Obi: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor name: DR B Gyampoh
References
- 1.Hu B., Guo H., Zhou P.S.Z. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Sadr W.M., Justman J. Africa in the path of Covid-19. N. Engl. J. Med. 2020;383(3):e11. doi: 10.1056/NEJMp2008193. [DOI] [PubMed] [Google Scholar]
- 3.Nachega J., Seydi M., Zumla A. The late arrival of coronavirus disease 2019 (COVID-19) in Africa: mitigating pan-continental spread. Clin. Infect. Dis. 2020;71(15):875–878. doi: 10.1093/cid/ciaa353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization, Declaration of alma-ata. (1978). Available at: https://www.who.int/teams/social-determinants-of-health/declaration-of-alma-ata. Retrieved September 30, 2022.
- 5.Mattey-Mora P.P., Begle C.A., Owusu C.K., Chen C., Parker M.A. Hospitalised versus outpatient COVID-19 patients’ background characteristics and comorbidities: a systematic review and meta-analysis. Rev. Med. Virol. 2022;32(3) doi: 10.1002/rmv.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dastenae Z.H., Bahadori A., Dehghani M., Asadi-samani M., Izadi I., Shahraki H.R. Comparison of the effect of intravenous dexamethasone and methylprednisolone on the treatment of hospitalized patients with COVID-19 : a randomized clinical trial R. Int. J. Infect. Dis. 2022;122:659–664. doi: 10.1016/j.ijid.2022.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haleem A., Javaid M., Vaishya R. Effects of COVID 19 pandemic in daily life. Curr. Med. Res. Pract. 2020 doi: 10.1016/j.cmrp.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Intawong K., Olson D., Chariyalertsak S. Application technology to fight the COVID-19 pandemic: lessons learned in Thailand. Biochem. Biophys. Res. Commun. 2021;538:231–237. doi: 10.1016/j.bbrc.2021.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (2020). Coronavirus disease (COVID-19) advice for the public. Avaiable at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed 16th August, 2022.
- 10.Chari A., Samur M.K., Martinez-Lopez J., et al. Clinical features associated with COVID-19 outcome in multiple myeloma: first results from the international Myeloma society data set. Blood. 2020;136:3033–3040. doi: 10.1182/blood.2020008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). (2022). Johns Hopkins coronavirus resource center. https://coronavirus.jhu.edu/map.html. Accessed 20th August, 2022.
- 12.Goga A., Bekker L., Garrett N., Reddy T., Yende-Zuma N., Fairall L., Moultrie H., Takalani A., Trivella V., Faesen M., Bailey V., Seocharan I.G.G. Breakthrough SARSCoV-2 infections during periods of delta and omicron predominance, South Africa. Lancet. 2022;400:269–271. doi: 10.1016/S0140-6736(22)01190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yashika G., Sujeet K., Mishra’ H.R., Jyotika G., Ravi K., Shakya SK., Vipin K.J., Babu G., Arjun G., Ravindra S., Rabinarayan S., Vijay K A. Phytomedicines explored under in vitro and in-silico studies against coronavirus: An opportunity to develop traditional medicines. S. Afr. J. Bot. 2022;16:31. doi: 10.1016/j.sajb.2022.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matarese A., Gambardella J., Sardu C.S.G. miR-98 regulates TMPRSS2 expression in human endothelial cells: key implications for COVID-19. Biomedicines. 2020;8:462. doi: 10.3390/biomedicines8110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loo J., Spittle D.A. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76:412–420. doi: 10.1136/thoraxjnl-2020-216243. [DOI] [PubMed] [Google Scholar]
- 18.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hippisley-Cox J., Patone M., Mei X.W., et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modin D., Claggett B., Sindet-Pedersen C., et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142:2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nannoni S., de Groot R., Bell S., Markus H.S. Stroke in COVID-19: a systematic review and meta-analysis. Int. J. Stroke. 2020;16(2):137–149. doi: 10.1177/1747493020972922. 2021 FebEpub 2020 Nov 11. PMID: 33103610; PMICID: PMC7859578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan B.K., Mainbourg S., Friggeri A., et al. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax. 2021;76:970–979. doi: 10.1136/thoraxjnl-2020-215383. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Tracking SARS-Cov-2 variants). Available at: https://www.who.int/activities/tracking-SARS-Cov-2-variants. Accessed on August 24, 2022
- 24.Callaway E. Omicron likely to weaken COVID vaccine protection. Sci. Am. 2021;600(7889):367–368. doi: 10.1038/d41586-021-03672-3. [DOI] [PubMed] [Google Scholar]; Available at: https://www.scientificamerican.com/article/heavilymutated-omicron-variant-puts-scientists-onalert/ Accessed 24th August, 2022.
- 25.T. Isabelle (2022). Long COVID is still raising more questions than answers, say researchers. Available at: https://www.sciencealert.com/long-covid-is-still-raising-more-questions-than-answers-say-researchers. Accessed 20th September, 2022.
- 26.Wilhelm A., Widera M., Grikscheit K., et al. MedRxiv; 2021. Reduced Neutralization of SARS-CoV-2 Omicron Variant by Vaccine Sera and Monoclonal Antibodies. [DOI] [Google Scholar]
- 27.Orisakwe O.E., Orish C.N., Nwanaforo E.O. Vol. 10. 2020. Coronavirus disease (COVID-19) and Africa: acclaimed home remedies; p. e00620. (Sci. Afr.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute for Health Metrics and Evaluation (2022). WHO: At least 17 million people in the WHO European Region experienced long COVID in the first two years of the pandemic; millions may have to live with it for years to come. Available at https://www.healthdata.org/news-release/who-least-17-million-people-who-european-region-experienced-long-covid-first-two-years. Accessed 29th September, 2022.
- 29.Ludwig H., Sonneveld P., Boccadoro M., Terpos E. Covid-19 and Myeloma. HemaSphere. 2022;6:5–6. doi: 10.1097/01.hs9.0000829540.80501.f8. [DOI] [Google Scholar]
- 30.P. Mathur & S. Kottilil (2022). Immunomodulatory therapies for COVID-19. 19:1–10. 10.3389/fmed.2022.921452 [DOI] [PMC free article] [PubMed]
- 31.Collie S., Champion J., Moultrie H., Bekker L.G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N. Engl. J. Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulliam J., van Schalkwyk C., Govender N., et al. MedRxiv; 2021. Increased Risk of SARS-CoV-2 Reinfection Associated With Emergence of the Omicron Variant in South Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Zaks T. Efficacy and safety of the mrna-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young-Xu Y., Korves C., Roberts J., et al. Coverage and estimated effectiveness of mRNA COVID-19 vaccines among US veterans. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas S.J., Moreira E.D., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N. Engl. J. Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyerowitz E.A, Richterman A., Gandhi R.T.S.P. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann. Intern Med. 2021;174(1):69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mallapaty S. Delta threatens rural regions that dodged earlier COVID waves. Nature. 2021;596:325–326. doi: 10.1038/d41586-021-02146-w. [DOI] [PubMed] [Google Scholar]
- 39.Schaefer G.O., Leland R.J. Making vaccines available to other countries before offering domestic booster vaccinations. JAMA. 2021;326:903–904. doi: 10.1001/jama.2021.13226. [DOI] [PubMed] [Google Scholar]
- 40.M. Chukwuma (2022). Nigeria's slow, tedious road to herbal medicine development. The Guradian Newapaper available at https://guardian.ng/features/nigerias-slow-tedious-road-to-herbal-medicine-development/. Accessed on 10th September.
- 41.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;10(4):177. doi: 10.3389/fphar.2013.00177. PMID: 24454289; PMCID: PMC3887317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antwi-Baffour S.S. The place of traditional medicine in the African Society: the science, acceptance and support. Am. J. Health Res. 2014;2(2):49. doi: 10.11648/j.ajhr.20140202.13. [DOI] [Google Scholar]
- 43.Nordling L. Science Magazine; 2020. Unproven herbal remedy against COVID-19 could fuel drug resistant malaria, scientists warn.https://www.sciencemag.org/news/2020/05/unproven-herbalremedy-againstcovid-19-could-fuel-drug-resistant-malaria-scientists May 6 2020. Available at. Accessed 16 July, 2022. [Google Scholar]
- 44.Wikipedia. Covid-organics. 2020. [Available from: https://en.wikipedia.org/wiki/Covid-Organics. Accessed: 6 July, 2022
- 45.Xinhuanet News. Nigeria unveils drugs for treatment of Ebola, malaria 2018.Available at: http://www.xinhuanet.com/english/2018-01/20/c_1369092 97.htm. Accessed: 10 August, 2022
- 46.The Center of Awareness Food Supplement. CoA mixture 2020. Available at: https://www.coadrugs.org/coamixture/. Accessed: 12 July, 2022.
- 47.Onyeaghala A.A., Onyeaghala C.P.B.E.O., Olubiyi O.O, Attah F.A. Management of mild and moderate symptoms of COVID-19 in infected subjects using combi-5 herbal supplement: case series. J. Med. Lab. Sci. 2022;32(1) 56-6. [Google Scholar]
- 48.Onyeaghala A.A., Onyeaghala E.O., Babalola C.P., Aina O.O., Jelpe D. Herbal supplement (combi-5) in the management of COVID 19 individual with mild to moderate symptoms: A case report. J. Complement. Integr. Med. 2021;18(4):865–867. doi: 10.1515/jcim-2020-0430. [DOI] [PubMed] [Google Scholar]
- 49.Chikowe I., Mtewa A.G., Tembo D., Smith D., Ibrahim E., Mwamatope B., Nkhungulu J., Kumpalume P., Maroyi A. Potential of Malawi's medicinal plants in COVID-19 disease management: a review. Malawi Med. J. J. Med. Assoc. Malawi. 2021;33(2):85–107. doi: 10.4314/mmj.v33i2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musoke P., Nantaayi B., Ndawula Kato R., Wannyana B., Ssewante N., Wekha G., Olum R., Nakyagaba L., Rhoda Nassozi D., Nabukeera G., Marvin Kanyike A., Ojilong D., Madut Akech G., Kajjimu J., Kiwumulo J., Agira D., Okot J., Bongomin F. Fear of covid-19 and the media influence on herbal medication use in Uganda: a cross-sectional study. Risk Manag. Healthc. Policy. 2021;14:3965–3975. doi: 10.2147/rmhp.s332325. Volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El Alami A., Fattah A., Abderrahman C. Medicinal plants used for the prevention purposes during the COVID-19 pandemic in Morocco. J. Anal. Sci. Appl. Biotechnol. 2020;2 doi: 10.48402/IMIST.PRSM/jasab-v2i1.21056. [DOI] [Google Scholar]
- 52.Bendezu-Quispe G., Benites-Meza J.K., Urrunaga-Pastor D., Herrera-Añazco P., Uyen-Cateriano A., Rodriguez-Morales A.J., Toro-Huamanchumo C.J., Hernandez A.V., Benites-Zapata V.A. Consumption of herbal supplements or homeopathic remedies to prevent COVID-19 and intention of vaccination for covid-19 in Latin America and the Caribbean. Trop. Med. Infect. Dis. 2022;7(6):95. doi: 10.3390/tropicalmed7060095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomes P., Martins L., Gomes E., Muribeca A., Pamplona S., Komesu A., Bichara C., Rai M., Silva C., Silva M. Antiviral plants from Marajó Island, Brazilian amazon: a narrative review. Molecules. 2022;27(5):1542. doi: 10.3390/molecules27051542. Basel, Switzerland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Husaini D.C., Orisakwe O.E., Mphuthi D.D., Garba M.S., Obasi C.N., Nwachukwu I.E. Phytotherapies for COVID-19 in Latin America and the Caribbean (LAC): implications for present and future pandemics. Arab. Gulf J. Sci. Res. 2023 doi: 10.1108/AGJSR-08-2022-0144. 2023[Epub ahead of print] [DOI] [Google Scholar]
- 55.Manzano-Santana P.I., Peñarreta Tivillin J.P., Chóez-Guaranda I.A., Barragán Lucas A.D., Orellana-Manzano A.K., Rastrelli L. Potential bioactive compounds of medicinal plants against new coronavirus (SARS-CoV-2): a Review. Bionatura. 2021;6(1):1653–1658. doi: 10.21931/rb/2021.06.01.30. [DOI] [Google Scholar]
- 56.Villena-Tejada M., Vera-Ferchau I., Cardona-Rivero A., Zamalloa-Cornejo R., Quispe-Florez M., Frisancho-Triveño Z., et al. Use of medicinal plants for COVID-19 prevention and respiratory symptom treatment during the pandemic in Cusco, Peru: a cross-sectional survey. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0257165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.AlNajrany S.M., Asiri Y., Sales I., AlRuthia Y. The commonly utilized natural products during the COVID-19 pandemic in Saudi Arabia: a cross-sectional online survey. Int. J. Environ. Res. Public Health. 2021;18(9):4688. doi: 10.3390/ijerph18094688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C., Wang L., Ren L. Antiviral mechanisms of candidate chemical medicines and traditional Chinese medicines for SARS-CoV-2 infection. Virus Res. 2020;286 doi: 10.1016/j.virusres.2020.198073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rastogi S., Pandey D.N., Singh R.H. COVID-19 pandemic: a pragmatic plan for ayurveda intervention. J. Ayurveda Integr. Med. 2020 doi: 10.1016/j.jaim.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh N.A., Kumar P., Kumar N. Spices and herbs: potential antiviral preventives and immunity boosters during COVID-19. Phytother. Res. PTR. 2021;35(5):2745–2757. doi: 10.1002/ptr.7019. Jyoti. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiong X., Wang P., Su K., Cho W.C., Xing Y. Chinese herbal medicine for coronavirus disease 2019: A systematic review and meta-analysis. Pharmacological research. 2020;160 doi: 10.1016/j.phrs.2020.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nugraha R.V., Ridwansyah H., Ghozali M., Khairani A.F., Atik N. Traditional herbal medicine candidates as complementary treatments for covid-19: a review of their mechanisms, pros and cons. Evid. Based Complement. Altern. Med. 2020;2020:1–12. doi: 10.1155/2020/2560645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paudyal V., Sun S., Hussain R., Abutaleb M.H., Hedima E.W. Complementary and alternative medicines use in COVID-19: a global perspective on practice, policy and research. Res. Soc. Adm. Pharm.: RSAP. 2022;18(3):2524–2528. doi: 10.1016/j.sapharm.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demeke C.A., Woldeyohanins A.E., Kifle Z.D. Herbal medicine use for the management of COVID 19: a review article. Metab. Open. 2021;12 doi: 10.1016/j.metop.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O.C. Kurrian Expanding pharmaceutical local production in Africa: an idea whose time has come? Health express. 10 April. 2019. Available at: https://www.orfonline.org/expert-speak/expanding-pharmaceutical-local-production-in-africa-an-idea-whose-time-has-come-49805/. Accessed: 22 August, 2022.
- 66.Word Health Organization, (2013). WHO traditional medicine strategy: 2014-2023. https://www.who.int/publications/i/item/9789241506096 (accessed August, 24, 2022)
- 67.Siegfried N.L., Hughes G. Herbal medicine, randomized controlled trials and global core competencies. S. Afr. Med. J. 2021;102(12):912. doi: 10.7196/samj.6392. [DOI] [PubMed] [Google Scholar]
- 68.Tsitsi G.M., Charles C.M., Gene D.M., Charles F.B.N. Capacity for ethical and regulatory review of herbal trials in developing countries: a case study of Moringa oleifera research in HIV-infected patients. J. Pharm. Policy Pract. 2017;10(9):1–5. doi: 10.1186/s40545-017-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willcox M., Siegfried N., Johnson Q. Capacity for clinical research on herbal medicines in Africa. J. Altern. Complement. Med. 2012;18(6):622–628. doi: 10.1089/acm.2011.0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.A.D. Sharma & I. Kaur (2021). Eucalyptus essential oil bioactive molecules from against SARS-CoV-2 spike protein: & NBSP; insights from computational studies. 10.21203/rs.3.rs-140069/v1. [DOI]