Abstract
Suboptimal adherence to antiretroviral therapy (ART) in people with HIV, even during sustained viral suppression, is associated with persistent inflammation, immune activation, and coagulopathy. Persistently low CD4-CD8 Ratio has been also associated with residual inflammation, is a good predictor of increased risk of death and more widely available than inflammatory biomarkers. We tested the hypothesis that the CD4-CD8 Ratio is associated with ART adherence during periods of complete viral suppression. We used the Medication Possession Ratio based in pharmacy registries as measure of adherence and time-varying, routine care CD4 and CD8 measurements as outcome. We used a linear mixed model for longitudinal data, including fixed effects for sex, age, education, date of ART initiation, AIDS-related conditions, and baseline CD4 to model the outcome. In 988 adults with a median follow-up of 4.13 years, higher ART adherence was independently associated with a modest increase in CD4-CD8. For each increasing percentage point in adherence, the CD4-CD8 Ratio increased 0.000857 (95% confidence interval [CI] −0.000494 to 0.002209, p = .213731) in the first year after achieving viral suppression; 0.001057 (95% CI 0.000262–0.001853, p = .009160) in years 1 to 3; 0.000323 (95% CI −0.000448 to 0.001095, p = .411441) in years 3 to 5; and 0.000850 (95% CI 0.000272–0.001429, p = .003946) 5–10 years after achieving viral suppression. The magnitude of the effect of adherence over CD4-CD8 Ratios varied over time and by baseline CD4 count, with increasing adherence having a larger effect early after ART initiation in people with higher baseline CD4 (>500 cells/μL) and in later years in people with lower baseline CD4 count (≥200 cells/μL). Our findings expand on previous evidence suggesting that the benefits of optimal adherence to modern ART regimens goes beyond maintaining viral suppression. These results highlight the importance of including objective measurements of adherence as part of routine care, even in patients with complete HIV suppression over long-term follow-up.
Keywords: sustained virologic response, adult, pharmacy, linear mixed models, cohort studies, acquired immunodeficiency syndrome
Introduction
Persistent systemic inflammation and immune cellular activation are the hallmarks of HIV infection.1,2 Heightened immune activation and broad systemic inflammation persist even in patients with complete HIV suppression receiving effective combined antiretroviral therapies (ARTs), particularly in those failing to achieve immune restoration after ART initiation.3 For example, persons with HIV (PWH) and suppressed viral load on ART have been consistently shown have higher circulating inflammatory biomarkers, such as interleukin 6 (IL-6) and C-reactive protein (CRP),4 among others. Increased inflammatory biomarkers are associated with an increased risk of death and serious non-AIDS diseases, in particular cardiovascular events, when compared with PWH.5 The mechanisms behind this heightened inflammation are only partially known.
Most patients initiating ART with current regimens achieve and maintain viral suppression in their first regimen up to 3 to 5 years after ART initiation.6–8 Furthermore, the potency and efficacy of modern ART do not require 95% or higher adherence to maintain viral suppression in most people as previously estimated.9 Adherence levels of 75% to 78% might be enough to achieve viral suppression in 90% of people starting on integrase strand inhibitor- or non-nucleoside reverse transcriptase inhibitor-based regimens.10 Nonetheless, incomplete ART adherence (even if enough to achieve and sustained viral suppression) has emerged as one of the potential factors that could drive heightened inflammation, immune activation, and coagulopathy in PWH receiving ART.11–18
Similar to several biomarkers of inflammation and immune activation, the CD4-CD8 Ratio has also been used as a marker of disease stage in PWH.19,20 For example, a persistently low CD4-CD8 Ratio has been associated with residual inflammation and is a good predictor of increased risk of death.21 CD4-CD8 Ratio however, is more widely available than other inflammatory biomarkers and is part of routine clinical care when monitoring CD4 in many clinical settings. To date, the mechanism(s) behind a persistently low CD4-CD8 Ratio in PWH remain unknown. In particular, the potential impact of ART adherence in the CD4-CD8 Ratio has not been studied. Thus, we aimed to evaluate whether suboptimal ART adherence in PWH and viral suppression is associated with a decreased CD4-CD8 Ratio over time.
Materials and Methods
Study design and population
The source population for this analysis was adult PWH receiving medical care at the HIV/AIDS Clinic at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ). The INCMNSZ is a national, tertiary care, university-affiliated, referral center located in Mexico City that provides medical care to adults from all around the country.
Patients included in this analysis were those diagnosed with HIV infection that achieved viral suppression after starting their first ART regimen between January 2005 and September 2017, and followed up for at least 6 months, after viral suppression. We followed patients exclusively during uninterrupted periods of HIV suppression. Thus, time in follow-up started the date of the first HIV RNA measurement <200 copies/mL after having started ART. Follow-up ended the date of a confirmed HIV RNA measurement ≥400 copies/mL, a period longer than 6 months without ART provision, last medical visit, 10 years of follow-up, death, or cohort administrative censoring (September 28, 2017). Accordingly, any patient enrolled in care between April and September 2017 was excluded, as they would not have completed the minimum 6-month follow-up for eligibility.
The time of observation was partitioned in four periods after achieving viral suppression on ART: 0–1, 1–3, 3–5, and 5–10 years; under the assumption that adherence would vary by time on ART virally suppressed (e.g., adherence in people in their 10th year of viral suppression on ART might be different than that in the first year of viral suppression). Patients could contribute to one or more of the partitions established according to the time spent on virological suppression during individual follow-up.
Data sources and data collection
We retrieved data from the HIV/AIDS clinic and pharmacy databases. We prospectively collected information on all PWH receiving care in our clinic as part of our collaboration with the Caribbean, Central, and South America network for HIV Epidemiology (CCASAnet), which has been reviewed and approved by the Ethics Committee on Human Subjects at the INCMNSZ.22 The Ethics Committee waived the need to obtain informed consent for the study. The database contains patients' demographic, clinical, and laboratory characteristics, including CD4 and CD8 measurements at baseline (enrollment) and during follow-up visits. Data are collected at each visit and entered immediately into the database. As a clinical HIV care center receiving funds for ART from Federal Government's Ministry of Health, our clinic provides free-of-charge ART prescribed by the physicians in the clinic.23 Patients refill their medications monthly or bimonthly, and the pharmacy utilizes electronic records to register ART provision to patients.
The pharmacy registers the refill date and when 30- or 60-day provision of ART is delivered to each patient. Following the Mexican guidelines for ART management, we routinely measure absolute CD4+ and CD8+ T lymphocyte counts (cells/μL) by flow cytometry two to three times a year in patients with viral suppression on ART. The median annual number of visits for CD4+ and CD8+ T lymphocyte counts in our clinic is around 2.6 (percentile 25 and 75 range: 2.2–3.1).24
Definition of variables
The outcome was the CD4-CD8 Ratio over time as a function of ART adherence. We measured adherence using the medication possession ratio (MPR)25 based on pharmacy registries. We divided the 30-day doses of ART provided for a period of one month by the total number of days that the patient took to refill their next prescription to calculate the percentage of days that patients have ART on their possession. All surplus ART was taken into account in case the patient had picked up a previous prescription before 30 days. For example, if a patient filled her/his prescription at day 25, she/he was considered to have a 100% adherence for that month, and we carried forward the extra 5-day provision to the next month estimated MPR.25 The MPR moving average of the last 4 months, preceding each CD4-CD8 measurement over the total time in follow-up, was taken as the measure of adherence for the statistical analysis.26 Adherence was capped at 100%.
We defined AIDS-related conditions as the presence of any of the conditions listed in the Centers for Disease Control and Prevention 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults diagnosed before or at enrollment in care in our clinic.
Statistical analyses
The hypothesis that the CD4-CD8 Ratio was associated with ART adherence was tested using linear mixed models (LMMs) for longitudinal data.27,28 The dependent variable was the logarithm transformed CD4-CD8 Ratio considering two cases. In both cases, random effects for the intercept and time were included, assuming a Gaussian spatial correlation structure for the time. First, we analyzed the CD4-CD8 Ratio measurements using the explanatory covariates ART adherence and time. We paired each CD4-CD8 ratio measurement with the adherence measurement data of the preceding 4 months. Hence, the model predicts changes in CD4-CD8 ratios based on the ART adherence measurement moving average of the preceding 4 months; adjusting for potential confounders and stratified in four longer term periods of observation in viral suppression receiving ART.
In this LMM, we included the ART adherence as a continuous variable measured in a range of 0%–100% and a three-order polynomial to account for the time effect. In addition, in each period of observation (0–1, 1–3, 3–5, and 5–10 years), data were stratified according to baseline CD4+ counts, and for each partition (namely the four time periods and three categories of baseline CD4 counts), the same LMM was estimated. Then, to explore for other factors associated with CD4-CD8 Ratio changes over time and control its potential confounding effects, we fit an LMM, including fixed effects of sex (male vs. female), age in years at ART initiation, education (categorized in two groups with a cutoff at 9 years of lower, corresponding to the median years of education for the Mexican population), and date of ART initiation as a categorical variable in four groups (2005–2007, 2008–2010, 2011–2013, and 2014–2017). We selected these calendar-year categories to correspond with shifts in clinical practice in our center (and more broadly changes in ART in Mexico).
All ART-naive patients started treatment with zidovudine, lamivudine, and efavirenz (EFV) regimens up to 2007. In 2008, a shift was made to start all patients with tenofovir fumarate (TDF) and emtricitabine (FTC) in single-tablet presentation in addition to EFV in a separated tablet. In 2010, there was a change to start ART with TDF/FTC/EFV single-tablet regimens. During these periods, ART initiation was guided by CD4 counts, starting when patients had 350 cells/μL or lower. In 2014, the CD4 threshold to start ART was increased to 500 cells/mm3, following the WHO recommendations. The model also included AIDS-related conditions previous to enrollment in care (yes or no), CD4+ count at the beginning of follow-up (in three categories: >500, 201–500, or ≤200 cells/mm3). ART adherence was included in the models as a continuous variable measured in a range of 0%–100%, with a four-order polynomial to account for the time effect. For each time period the same LMM was estimated.
The Akaike information criterion and Bayesian information criterion were used to choose the proposed models considering different options to describe the time effect, the ART adherence effect and possible interactions between time and ART adherence.
Correction terms were applied to obtain the estimations in the original scale from the logarithm transformation.29 In addition, we analyzed the data using age–period–cohort (APC) and generalized estimating equations (GEEs) during the peer-review process. All statistical analyses were performed using packages “nlme” and “ggplot2” of software R version 3.4.1, and we considered a p value <.05 to be statistically significant.
Results
Descriptive analysis
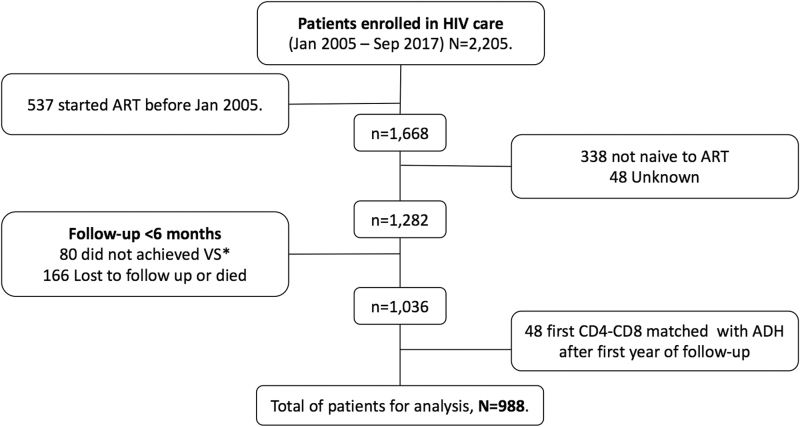
We included longitudinal data on 988 PWH (Fig. 1). We present the characteristics of the study population by period of observation in Table 1. The overall trajectories of CD4+ and CD8+ counts are shown in Supplementary Figure S1. At baseline, 100 patients (10.1%) had a CD4-CD8 ratio ≥1. The proportion of patients that achieved a CD4-CD8 ratio ≥1 over time, among those with ratio ≤8, increased to reach 4.5% (95% confidence interval [CI] 3.1–6.0) at the first year of viral suppression, 16.9% (95% CI 14.1–19.7) at the third, 27.9% (95% CI 24.1–31.5) at the fifth, and 47.3% (95% CI 40.9–53.0) at the 10th year of follow-up during viral suppression (Supplementary Fig. S2).
FIG. 1.
Patient selection for analysis. *VS = HIV viral load suppression.
Table 1.
Characteristics of Participants in the Cohort by Period of Observation After Having Achieved Virological Suppression on Antiretroviral Therapy
| Characteristics | Period of observation after achieving viral suppression |
|||
|---|---|---|---|---|
| 0–1 years N = 988 | 1–3 years N = 858 | 3–5 years N = 618 | 5–10 years N = 427 | |
| Baseline CD4-CD8 ratioa | 0.3291 (0.1897–0.5345) | 0.4787 (0.3058–0.7183) | 0.6253 (0.4581–0.8584) | 0.7096 (0.5022–0.9633) |
| Time of follow-up in years | 0.766 (0.660–0.879) | 2.705 (2.472–2.845) | 4.683 (4.371–4.830) | 7.688 (6.329–9.257) |
| Male | 894 (90.48%) | 773 (90.09%) | 557 (90.13%) | 387 (90.63%) |
| Age in years at ART initiationb | 33.34 (28.04–41.17) | 33.35 (28.15–41.14) | 34.43 (28.90–41.43) | 34.86 (28.97–41.30) |
| >9 Years of educationb | 780 (78.95%) | 672 (78.32%) | 471 (76.21%) | 325 (76.11%) |
| Period of ART initiationb | ||||
| 2005–2007 | 157 (15.89%) | 146 (17.02%) | 125 (20.23%) | 122 (28.57%) |
| 2008–2010 | 265 (26.82%) | 254 (29.60%) | 238 (38.51%) | 221 (51.76%) |
| 2011–2013 | 294 (29.76%) | 272 (31.70%) | 235 (38.02%) | 84 (19.67%) |
| 2014–2017 | 272 (27.53%) | 186 (21.68%) | 20 (3.24%) | 0 (0%) |
| Previous AIDS-defining eventc | 287 (29.05%) | 264 (30.77%) | 229 (37.05%) | 197 (46.13%) |
| Baseline CD4+ counts (cells/μL)b | ||||
| >500 | 178 (18.02%) | 136 (15.85%) | 71 (11.49%) | 40 (9.37%) |
| 201–500 | 469 (47.47%) | 429 (50.00%) | 322 (52.10%) | 216 (50.58%) |
| ≤200 | 341 (34.51%) | 293 (34.15%) | 225 (36.41%) | 171 (40.05%) |
| Percentage of ART adherenced | 99 (96–100) | 99 (95–100) | 98 (94–100) | 98 (92–100) |
Summary of cases (%) or median (interquartile range).
At the beginning of follow-up in each period of observation.
At the beginning of follow-up.
Condition previous to enrollment in care.
At the end of follow-up in each period of observation.
ART, antiretroviral therapy.
Effect of adherence in CD4-CD8 ratio
In the first analysis, we show that the predicted changes in CD4-CD8 Ratio increases as a function of ART adherence for different baseline CD4+ counts (Fig. 2). The association of adherence with CD4-CD8 Ratio changes over time with greater CD4-CD8 Ratios increases with increasing adherence early after achieving viral suppression (year 1) for patients with higher baseline CD4+ counts (Fig. 2A); and very late (years 5 and 10) after achieving viral suppression for patients with lower baseline CD4+ counts (Fig. 2D).
FIG. 2.
Unadjusted predicted values in CD4-CD8 Ratio by ART adherence for different baseline CD4+ counts (colored lines in all panels) and periods of time after achieving viral suppression (1, 3, 5, and 10 years, in A–D). Footnote: Lines represent mean predictions and shades their 95% CI. Each fitted line and its 95% CI were independently estimated for each time partition and baseline CD4+ count category using the first LMM. Only data for adherence over 60% are plotted since these encompassed 98% of the ART adherence data. ART, antiretroviral therapy; CI, confidence interval; LMM, linear mixed model.
In the second analysis, after adjusting for age, sex, baseline CD4+ counts, education, the presence of AIDS-defining conditions before ART initiation, and date of ART initiation, we observed that increasing ART adherence was independently associated with an overall increased CD4-CD8 Ratio. The model predicts a mean decrease in CD4-CD8 Ratio of 0.002165 for every 3 days off ART per month in the first year after achieving viral suppression on ART. In every period of observation there is a clear inverse association between the magnitude of suboptimal adherence per month and the predicted decrease in CD4-CD8 Ratio. To ease interpretation, in Figure 3, we exemplify the predicted effect on CD4-CD8 Ratio, for every 3% cumulative decrease in ART adherence (equivalent to approximately one missed day of ART in a month).
FIG. 3.
CD4-CD8 Ratio predicted changes by different values of suboptimal ART adherence on each period of observation after achieving viral suppression. The estimates have been obtained by using reference categories of male, the mean age of 34 at ART initiation, education equal or lower than 9 years, ART initiation in 2005–2007, no AIDS previous to enrollment in care, baseline CD4+ counts ≤200, and at the end of each period (1, 3, 5, and 10 years, respectively). Each line was independently estimated for each time partition.
We also observed that the magnitude and significance of the effect of adherence over CD4-CD8 Ratios varies over time, with increasing adherence having a larger effect early after ART initiation. Figure 4 shows that the predicted changes in CD4-CD8 Ratio increases as a function of ART adherence in virally suppressed adults for different time points after ART initiation (at the end of each period, i.e., 1, 3, 5, and 10 years) after adjusting for sex, age, education, calendar-year of ART initiation, and AIDS-related conditions previous to enrollment in care, according to baseline CD4+ count.
FIG. 4.
Adjusted 1 predicted values in CD4-CD8 Ratio by ART adherence for different baseline CD4+ counts (colored lines in all panels) and periods of time after achieving viral suppression (1, 3, 5, and 10 years, in A–D). Adjusted for sex at birth, age at ART initiation, education, and period of ART initiation. Each plot was independently obtained by estimating the second LMM for each time partition (at the end of each period, i.e., 1, 3, 5, and 10 years), using reference categories of male, the mean age of 34 at ART initiation, education equal or lower than 9 years, ART initiation in 2005–2007, and no AIDS previous to enrollment in care. Lines represent mean predictions and shades their 95% CI. Only data for adherence over 60% are plotted since these encompassed 98% of the ART adherence data.
The effect of adherence over CD4-CD8 Ratio changes over time with greater increases with increasing adherence early (year 1) after achieving viral suppression on ART. The results from the LMM show that for each increasing percentage point in adherence, the CD4-CD8 Ratio increased 0.000857 (95% CI −0.000494 to 0.002209, p = .213731) in the first year after achieving viral suppression; 0.001057 (95% CI 0.000262–0.001853, p = .009160) in years 1 to 3 after, 0.000323 (95% CI −0.000448 to 0.001095, p = .411441) in years 3–5; and 0.000850 (95% CI 0.000272–0.001429, p = .003946) in 5–10 years later (Supplementary Tables S1–S4).
Factors associated with changes in CD4-CD8 Ratio
In the second analysis, we observed that women and people with higher baseline CD4+ count had a higher mean CD4-CD8 Ratio increase in all periods of observation. Younger age at ART initiation was positively associated with mean CD4-CD8 Ratio increase in the first 5 years after viral suppression but not later. Lower education status, and the presence of an AIDS-defining illness before ART initiation, were negatively associated with mean CD4-CD8 Ratio increase only in the first year after viral suppression. We observed no differences in the mean CD4-CD8 Ratio increase by date of ART initiation in any period of observation. See details in Supplementary Tables S1–S4 and Supplementary Figures S2–S6 in Supplementary Material.
We obtained similar estimates when we repeated the analysis using the APC and the GEE models (data not shown).
Discussion
In this retrospective, observational cohort study of PWH, we observed that after adjusting for sex, age, calendar-year at ART initiation, educational status, previous AIDS-defining events, and baseline CD4+ count, incomplete ART adherence was associated with reduced mean CD4-CD8 Ratio recovery. The magnitude of the difference in mean CD4-CD8 Ratios between complete and incomplete adherence is more evident early after initial viral suppression, particularly among patients that started ART with CD4+ count higher than 500 cells/μL. While previous studies have demonstrated that suboptimal ART adherence is associated with heightened residual inflammation, immune activation, and coagulopathy despite being sufficient to achieve and maintain viral suppression,11–18 to our knowledge this is the first study that has evaluated its association with CD4-CD8 Ratio. Our analysis allows us to understand the change in CD4-CD8 ratio per unit change in adherence and provides framework to assess what would be the expected change in the ratio during a significant period of suboptimal adherence.
Given the association of an impaired CD4-CD8 Ratio recovery with mortality and serious non-AIDS events,19,21 our findings highlight the potential role that incomplete ART adherence could have on clinical outcomes beyond viral suppression in PWH.
The association between incomplete ART adherence and lower CD4-CD8 Ratios over time could be explained by low-level viremia (below the limit of quantification using ultra-sensible polymerase chain reaction for HIV RNA) in people with incomplete adherence who are deemed to be virally suppressed using clinically available HIV-RNA assays.30 In people with low-level viremia, higher concentrations of ultra-sensitive C-reactive protein, IL-6, Erythrocyte Sedimentation Rate, IL-2, and other inflammatory markers have been observed.11–18 Higher residual inflammation in people with HIV has been associated with poor immune recovery as measured by CD4-CD8 Ratios.21
The clinical implications of our study, while potentially manifold, should be cautiously considered. We acknowledge that the magnitude of this association makes it difficult to attribute to any clinical significance, nonetheless our results are consistent with widely accepted models of HIV pathogenesis and can be coherently interpreted within broader evidence suggesting that the benefits of optimal adherence to modern ART regimens goes beyond maintaining measurable viral suppression. Our findings support the growing notion that incomplete ART adherence, even if sufficient to achieve and maintain viral suppression, could have deleterious clinical consequences that may not be immediately recognizable. Second, these and other previously established associations,11–18 could lead to additional discussions between patients and clinical providers regarding the importance of aiming toward the highest possible adherence even in the setting of suppressed HIV RNA.
This is of particular importance given the potency of currently available ART medications, which can translate in viral suppression when overall adherence is 80%–85%, or even lower in people starting integrase strand transfer inhibitors or non-nucleoside reverse transcriptase inhibitors-based regimens,10,31–34 making HIV viral load an imperfect adherence measure. Last, these findings open the field to the possibility of modifying ART adherence as a potential clinical intervention that could lead to a reduction in the residual inflammation observed in virally suppressed PWH.
Among the strengths of our study are that we were able to follow up of a relatively large number of patients during prolonged periods of viral suppression and heterogeneous levels of adherence. In addition, we used robust analytical methods, which allowed us to analyze the potentially time-varying magnitude of association between adherence and CD4-CD8 Ratios. Third, we focused on a clinically available biomarker (CD4-CD8 Ratio) that can be monitored in most clinical settings, instead of a specialized set of biomarkers that are limited to research settings. Despite these strengths, there are several limitations to our study. First, the effect of adherence over CD4-CD8 Ratio appears to be minor, even when statistically significant. Nonetheless, our results contribute to growing evidence that suboptimal ART adherence during complete HIV suppression might have deleterious effects11,17,18 as CD4-CD8 Ratio are related to adverse clinical outcomes in PWH.19 Second, adherence to ART is difficult to measure accurately.35
We used the MPR built from electronic records from our pharmacy, which assumes that all filled prescriptions are ingested by patients and might be subject to high variability.35 While this is an imperfect measure, MPR has been widely used,9,10,33,35 and previous evidence has shown a good correlation with drug concentrations measured in dried blood spots,36 which are reliable and objective measures of cumulative ART adherence over the preceding 6–8 weeks.36 In addition, we did not include in the analysis any adjustment for ART class regimen, which might have had an effect on immune reconstitution patterns. Nonetheless, we used calendar-year to adjust for changes in potential confounders over time. We think this is a reasonable approximation since the calendar-year categories correspond with shifts in clinical practice in and ART use in Mexico. Moreover, during the studied period most of our patients were started on EFV-based regimens according to the Mexican ART guidelines (which changed the first-line regimen to integrase inhibitors during 2018).37
The latter is another limitation of our study, since modern ART regimens appear to provide greater forgiveness for nonadherence, future studies should focus on integrase inhibitors.9,10
Conclusion
Incomplete ART adherence is associated with CD4-CD8 ratio recovery in PWH who are virally suppressed to <200 copies/mL. These findings should be considered exploratory and interpreted only as part of broader evidence suggesting that the benefits of optimal adherence to modern ART regimens goes beyond maintaining viral suppression. In addition, this research highlights the importance of including objective measurements of adherence as part of routine care, even in patients with complete HIV suppression over long-term follow-up.
Supplementary Material
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors' Contributions
P.F.B.-Z. contributed to the study conception and design, acquisition of data, analysis and interpretation of data. L.N. contributed to the study design, performed the statistical analysis and contributed to interpretation of data. Y.C.-V. contributed to the study design, acquisition of data, statistical analysis, and interpretation of data. J.R.C.-M. contributed to the study conception and design, analysis, and interpretation of data. A.C.-Z. contributed to the analysis. R.F.G. contributed to the statistical analysis and interpretation of data. B.E.C.-R. contributed to the study conception, acquisition of data, and interpretation of data. J.G.S.-M. contributed to the study conception, acquisition of data, and interpretation of data. All authors were involved in drafting the article or revising it critically for important intellectual content. All authors have read and approved the final article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the NIH-funded Caribbean, Central and South America network for HIV epidemiology (CCASAnet), a member cohort of the International Epidemiologic Databases to Evaluate AIDS (leDEA) (U01AI069923). This award is funded by the following institutes: the National Institute of Allergy and Infectious Diseases (NIAID); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD); the National Cancer Institute (NCI), the National Institute of Mental Health (NIMH); the National Institute on Drug Abuse (NIDA); the National Heart, Lung, and Blood Institute (NHLBI); the National Institute on Alcohol Abuse and Alcoholism (NIAAA); the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); the Fogarty International Center (FIC); and the National Library of Medicine (NLM). L.N. and R.F.-G. have been, in part, supported by UNAM-DGAPA-PAPIIT, Mexico (Project IN118720). J.R.C.-M. is supported by NIH/NIAID R01AI145453.
Supplementary Material
References
- 1. Younas M, Psomas C, Reynes J, et al. Immune activation in the course of HIV-1 infection: Causes, phenotypes and persistence under therapy. HIV Med 2016;17(2): 89–105. [DOI] [PubMed] [Google Scholar]
- 2. Lederman M, Funderburg N, Sekaly F, et al. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013;119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lederman M, Calabrese L, Funderburg N, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 2011;204(8): 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neuhaus J, Jacobs Jr DR, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010;201(12):1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013;382(9903):1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wohl DA, Yazdanpanah Y, Baumgarten A, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: Week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV 2019;6(6):e355–e363; doi: 10.1016/S2352-3018(19)30077-3 [DOI] [PubMed] [Google Scholar]
- 7. Calmy A, Tovar Sanchez T, Kouanfack C, et al. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): Week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV 2020;7(10):e677–e687; doi: 10.1016/S2352-3018(20)30238-1 [DOI] [PubMed] [Google Scholar]
- 8. Caro-Vega Y, Belaunzarán-Zamudio PF, Crabtree-Ramírez BE, et al. Durability of Efavirenz compared with boosted protease inhibitor-based regimens in antiretroviral-naïve patients in the Caribbean and Central and South America. Open Forum Infect Dis 2018;5(3):ofy004; doi: 10.1093/ofid/ofy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133(1):21–30. [DOI] [PubMed] [Google Scholar]
- 10. Byrd KK, Hou JG, Hazen R. Antiretroviral adherence level necessary for HIV viral suppression using real-world data. J Acquir Immune DeficSyndr 2019;82(3):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castillo-Mancilla JR, Brown TT, Erlandson KM, et al. Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis 2016;63(12):1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castillo-Mancilla JR, Brown TT, PalellaJr FJ, et al. Partial normalization of biomarkers of inflammation and immune activation among virally suppressed men with HIV infection and high ART adherence. Open Forum Infect Dis 2020;7(4):ofaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castillo-Mancilla JR, Morrow M, Boum Y, et al. Higher ART adherence is associated with lower systemic inflammation in treatment-naïve Ugandans who achieve virologic suppression. J Acquir Immune Defic Syndr 2018;77(5);507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castillo-Mancilla JR, Phillips AN, Neaton JD, et al. Association of suboptimal antiretroviral therapy adherence with inflammation in virologically suppressed individuals enrolled in the SMART study. Open Forum Infect Dis 2018;5(1);ofx275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castillo-Mancilla JR, Phillips AN, Neaton JD, et al. Incomplete ART adherence is associated with higher inflammation in individuals who achieved virologic suppression in the START study. J Int AIDS Soc 2019;22(6):e25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Musinguzi NM, Castillo-Mancilla JR, Morrow MM, et al. Antiretroviral therapy adherence interruptions are associated with systemic inflammation among Ugandans who achieved viral suppression. J Acquire Immune Defic Synd 2018. 82(4):386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falasca F, Di Carlo D, De Vito C, et al. Evaluation of HIV-DNA and inflammatory markers in HIV-infected individuals with different viral load patterns. BMC Infect Dis 2017;17:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bastard JP, Soulié C, Fellahi S, et al. Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir Ther 2012;17(5):915–919. [DOI] [PubMed] [Google Scholar]
- 19. Han W, Apornpong T, Kerr S, et al. CD4/CD8 ratio normalization rates and low ratio as prognostic marker for non-AIDS defining events among long-term virologically suppressed people living with HIV. AIDS Res Ther 2018;15(1):13; doi: 10.1186/s12981-018-0200-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu W, Mehraj V, Vyboh K, et al. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc 2015;18(1):20052; doi: 10.7448/IAS.18.1.20052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bruno G, Saracino A, Monno L, et al. The revival of an “Old” marker: CD4/CD8 ratio. AIDS Rev 2017;19(2):81–88. [PubMed] [Google Scholar]
- 22. Rebeiro P, Cesar C, Shepherd B, et al. Assessing the HIV care continuum in Latin America: Progress in clinical retention, cART use and viral suppression. J Int AIDS Soc 2016;19(1):20636; doi: 10.7448/IAS.19.1.20636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sierra-Madero J, Belaunzaran-Zamudio PF, Crabtree-Ramírez B, et al. Mexico's fragmented health system as a barrier to HIV care. Lancet HIV 2019;6(2):e74–e75; doi: 10.1016/S2352-3018(18)30356-4 [DOI] [PubMed] [Google Scholar]
- 24. Belaunzarán-Zamudio PF, Caro-Vega Y, Shepherd B, et al. Monitoring of HIV treatment in seven countries in WHO Region of the Americas. Bull World Health Organ 2015;93(8):529–539; doi: 10.2471/BLT.14.147447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McMahon J, Jordan M, Kelley K, et al. Pharmacy adherence measures to assess adherence to antiretroviral therapy: Review of the literature and implications for treatment monitoring. Clin Infect Dis 2011;52(4):493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boussari O, Subtil F, Genolini C, et al. Impact of variability in adherence to HIV antiretroviral therapy on the immunovirological response and mortality. BMC Med Res Methodol 2015;15:10; doi: 10.1186/1471-2288-15-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verbeke G, Molengerhs G.. Linear Mixed Models for Longitudinal Data. Springer: New York, NY, USA; 2000. [Google Scholar]
- 28. Galecki A, Burzykowski T.. Linear Mixed-Effects Models Using R. A Step-by-Step Approach. Springer: New York, NY, USA; 2013. [Google Scholar]
- 29. Olsson U. Confidence intervals for the mean of a log-normal distribution. J Stat Educ 2005;13(1):1; doi: jse.amstat.org/v13n1/olsson.html [Google Scholar]
- 30. Li JZ, Gallien S, Ribaudo H, et al. Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS 2014;28(2):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep 2017;14(3):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Viswanathan S, Detels R, Mehta SH, et al. Level of adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART). AIDS Behav 2015;19(4):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gordon LL, Gharibian D, Chong K, et al. Comparison of HIV virologic failure rates between patients with variable adherence to three antiretroviral regimen types. AIDS Patient Care STDS 2015;29(7):384–388. [DOI] [PubMed] [Google Scholar]
- 34. Rosenblum M, Deeks SG, Van der Laan M, et al. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One 2009;4(9):e7196; doi: 10.1371/journal.pone.0007196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spinelli MA, Haberer JE, Chai PR, et al. Approaches to objectively measure antiretroviral medication adherence and drive adherence interventions. Curr HIV/AIDS Rep 2020;17(4):301–314; doi: 10.1007/s11904-020-00502-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castillo-Mancilla JR, Searls K, Caraway P, et al. Tenofovir di-phosphate in dried blood spots as an objective measure of adherence in HIV-infected women. AIDS Res Hum Retroviruses 2015. Apr;31(4):428–432; doi: 10.1089/AID.2014.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guideliness on Antiretroviral Use for People with HIV, 8th ed. Mexico City, Mexico: The guidelines are endorsed and published by the Consejo Nacional para la Prevenciœn y Control del Sida (Conasida). (National Council for AIDS Prevention and Control). 2018; 185p. Available from: https://www.gob.mx/cms/uploads/attachment/file/326631/Gu_a_ARV_2018.pdf [Last accessed: January 17, 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.