Abstract
Background:
Both catechin polyphenols and caffeine have been shown to have beneficial effects on weight control in the adult population. However, the influence of tea or coffee supplementation on body weight in adolescents has never been tested. The aim of the present study was to investigate the effect of tea and coffee consumption on body weight and body fat in adolescents with obesity.
Methods:
Randomized clinical trial comparing three weight-loss interventions composed of similar family-based counseling sessions on nutritional education with coffee (2 cups per day, total amount 160 mg caffeine), green tea (3 cups per day, total amount 252 mg catechin and 96 mg caffeine), or herbal tea (as placebo, 3 cups per day). Nutritional intake, BMI, and fat percentage, as measured by bioelectrical impedance, were compared between the groups at 3 and 6 months.
Results:
Forty-eight children were included in the final analysis: 18 in the coffee arm, 17 in the green tea arm, and 13 in the placebo arm. Nineteen (39.6%) children were males, with a median (interquartile range) age of 13 (11–14) years. There were no significant group differences in age, sex, and BMI (absolute number and percent of the 95th percentile) upon study entry. Comparison between the three interventions in total change in BMI from baseline revealed a significant advantage for coffee consumption compared with green tea and placebo (−9.2% change in BMI in the coffee group compared with −2.3% and 0.76% in the green tea and placebo group, respectively, p = 0.002).
Conclusions:
Dietary recommendations combined with coffee intake and, to a lesser extent, tea catechins may be associated with reduced weight and adiposity among adolescents.
Clinical trial registration number: NCT05181176
Keywords: adolescence, coffee, obesity, tea
Introduction
Obesity has become a major public health problem of global significance.1 The adverse effects of excess adiposity on health outcomes are widely recognized; however, conventional weight management (dietary intervention, physical activity, and behavioral modification) has shown limited long-term effectiveness, with most studies on weight maintenance demonstrating undesired weight regain,2–5 thus calling for alternative weight reduction strategies. Tea and coffee are the most popular beverages worldwide. Tea has been categorized into three main types on the basis of processing during manufacturing. While most of the tea produced worldwide is black tea extracts, 20% is green tea.6 Green tea contains two major active ingredients. One is catechin polyphenol (epicatechin, epicatechin gallate, epigallocatechin, and the most pharmacologically active, epigallocatechin gallate [EGCG]), which inhibits the action of catechol-o-methyl-transferase, resulting in a prolonged action of catecholamines. The other is caffeine, which inhibits the phosphodiesterase-induced degradation of intracellular cyclic adenosine monophosphate (cAMP), leading to an increase in norepinephrine release.7 The net result is an elevated cellular concentration of cAMP, a critical intracellular mediator for the action of catecholamines on thermogenesis.7–9 Furthermore, brain catecholamines may play a major role in energy intake and satiety.10
Both catechin polyphenols and caffeine (in coffee or in green tea) may be effective promoters of thermogenesis and fat oxidation, resulting in the reduction of body weight.7–21 Indeed, several observational and interventional studies in adults demonstrated a significant effect of tea or coffee consumption on body weight.11–16 Chantre and Lairon demonstrated a decrease of 4.6% of body weight and a 4.6% decrease in waist circumference after 3 months of consumption of green tea extract (containing 270 mg EGCG and 150 mg caffeine).11 In a randomized controlled trial involving 60 adults with obesity, consumption of green tea capsules induced a significant weight reduction by the 8th and 12th weeks of the study, which was accompanied by a significant increase in resting energy expenditure.12 In their interventional study of 10 healthy men, Dulloo et al. reported a 3.5% increase in energy expenditure and a 27 gram/24 hour increase in fat oxidation with green tea consumption.7 Rudelle et al. reported a 4%–6% increase in 24-hour energy expenditure following consumption of green tea extract and caffeine.14 Gregersen et al. examined 15 normal weight males who received capsules containing placebo, caffeine (150 mg), or caffeine plus catechin (600 mg). Those authors documented an increase of 250 kJ (about 2%) in energy expenditure in the caffeine plus catechin group compared with the placebo and caffeine groups.15 Other studies have also demonstrated a reduction in energy intake during the consumption of tea catechin, caffeine, or a combination of both. In a recent review by Schubert et al., caffeine consumption was found to decrease single-meal energy intake by 430 kJ.16 Westerterp-Plantenga et al. observed a lower energy intake after 300 mg of caffeine administration in adult males.19 Conversely, in the study by Wan et al.,21 frequent tea drinkers wound up having a higher BMI, elevated triglyceride (TG) levels, and elevated fasting glucose levels. As far as we know, there have been no interventional studies that aimed to examine the influence of tea or coffee supplementation on body weight in the pediatric population. Therefore, the aim of the present study was to investigate the effect of tea and coffee consumption on body weight and body fat in a population of adolescents with obesity.
Materials and Methods
Patient Population
The participants were recruited at the Obesity Clinic of the Pediatric Gastroenterology Institute, “Dana Dwek” Children's Hospital, from January 2018 to December 2020. No participant was recruited during the COVID pandemic.
The Obesity Clinic is a tertiary referral center for children and adolescents with obesity and related complications. Patients were recruited for the study during the initial clinic session. All adolescents aged 12–17 years with a BMI in the 95th percentile or higher were eligible for study participation. Excluded from the study were adolescents with major medical conditions that could affect body composition (such as neurological conditions or inflammatory diseases), who chronically used medications that could affect study outcomes and body composition (such as corticosteroids and insulin), and who consumed tea or coffee on a regular basis. Adolescents with obesity-derived comorbidities were not excluded.
Study Design
This randomized clinical trial compared three weight-loss interventions composed of the consumption of coffee (coffee group), green tea (tea group), or herbal tea (control group). The randomization was performed using a computer software (Random.org). The study consisted of a 2-week run-in period, followed by a 24-week period of consumption of the assigned beverage.
Intervention
The three groups received similar weight-reducing interventions composed of diets that differed only with regard to the recommended coffee, green tea, or herbal tea consumption. The intervention included twice weekly family-based counseling sessions on nutritional education (low glycemic index diet), behavioral counseling, and recommendation for physical activity. The coffee group was instructed to consume two cups of coffee daily, which is the amount that had been described as beneficial in epidemiological studies and as being safe for children and adolescents.20 Each 250 mL cup of coffee contained ∼80 mg of caffeine. The participants were instructed to drink the coffee in the morning and shortly after lunch to avoid sleep disturbance at night. The green tea group was instructed to drink three cups (230 mL) of Chinese green tea (Wissotzky Tea, Israel Ltd.). Each tea bag contained 500 grams of fine dried herb parts, and each cup contained 84 mg total catechin and 32 mg caffeine. The members of this group were instructed to leave the teabag in the hot water for 2 minutes before drinking. The control group was instructed to consume three daily cups of the Wissotzky Kid Drink. The Wissotzky Kid Drink (Wissotzky Tea, Israel Ltd.) is a beverage that contains an infusion of fruits and plants, with each bag containing 2.7 grams of plant parts free of polyphenols and caffeine. The subjects were allowed to add milk and sweeten the drinks with artificial sweeteners. All the tea products were provided to the participants. Adherence to all products was monitored by a 3-day dietary questionnaire, filled before each visit and by collecting empty boxes at each monthly visit.
Clinical and Demographic Variables
Information retrieved from the medical files of the study participants included:
-
1.
Sociodemographic characteristics: age, sex.
-
2.
Medical history: perinatal characteristics (birth weight, gestational age), medications, and family history of cardiometabolic diseases (diabetes, hypertension, dyslipidemia, cardiovascular disease, and cerebrovascular episodes) among first- and second-degree relatives.
-
3.
Physical examination: systolic and diastolic blood pressure (BP) and anthropometric measurements (height, weight, calculated BMI, and body fat).
-
4.
Screening for obesity-related comorbidities: laboratory metabolic workup, abdominal ultrasonography (steatohepatitis) findings, and polysomnographic findings (obstructive sleep apnea).
Outcomes
The primary outcomes of the study were a decrease in the BMI, BMI percent of the 95th percentile, and body fat at 3 and 6 months into the intervention. Weight and height were assessed at baseline, monthly for the ensuing 3 months, and again after 6 months since the baseline. Body weight and fat percentage were measured indirectly by a Tanita Body Composition Analyzer (BIA; Tanita DC-360 S and GMON Professional Software), which has been clinically verified to be accurate and reliable and to provide highly reproducible results.21 The GMON software provides the BIA data adjusted for sex, age, height, and race (Caucasian and Asian) according to reference ranges.22 The BMI Z score and the BMI percentile were calculated by reference data for sex and age.23 Metabolic parameters documented at study entry included glucose, insulin, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol, TG, alanine transaminase, aspartate transaminase, and C reactive protein levels. Fatty liver and fibrosis were assessed by ultrasonography.
Definition of Study Variables
Anthropometric data (at baseline and after the dietary intervention) were reported in accordance to the recent editorial by Ryder et al.24 BMI and percent of the 95th percentile were calculated with PediTools Electronic Growth Chart Calculators based on CDC growth charts.23
Glucose intolerance was defined as fasting glucose ≥100 mg/dL (5.5 mmol/L); elevated BP was defined as systolic and/or diastolic BP ≥90th percentile for sex, age, and height25; hypertriglyceridemia was defined as TG levels ≥110 mg/dL (1.24 mmol/L) and HDL-c ≤40 mg/dL (1.03 mmol/L).26
Obesity-related comorbidities were compiled as follows. Insulin resistance was the calculated by Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).27,28 A provisional diagnosis of nonalcoholic fatty liver disease was made by excluding other causes of liver disease through a focused history, physical examination, laboratory evaluation, and an abdominal ultrasound showing increased echogenicity suggestive of fatty liver.29 Obstructive sleep apnea was defined by recurrent events of partial or complete upper airway obstruction during sleep as detected by polysomnography performed in patients with a history of persistent snoring and/or recurrent awakenings.30 Pseudotumor cerebri was diagnosed according to the modified Dandy criteria: symptoms and signs of increased intracranial pressure (e.g., headache, transient visual obscurations, papilledema, loss of vision), no other neurological abnormalities, elevated intracranial pressure with normal cerebrospinal fluid composition, and a neuroimaging study that showed no etiology for intracranial hypertension.31
Statistical Analyses
SPSS (IBM Corp. Released 2016; IBM SPSS Statistics for Windows, Version 27.0; IBM Corp., Armonk, NY, USA) was used for all statistical analyses. All statistical tests were two-sided. The Kolmogorov–Smirnov test and the Shapiro–Wilk test were applied to assess the normality of continuous data. The data are expressed as means ± standard deviations for normally distributed variables, and as medians and interquartile ranges (IQR) for skewed distribution. Pearson's chi-square test was performed to compare the distribution of categorical variables between the three intervention groups. To evaluate the effect of diet on the four measures (BMI, Z score, percentile, and fat) for each measure, the percent change from baseline was calculated at 3 and 6 months. The Kruskal–Wallis test followed by Dunn's post hoc test was used to compare the differences between coffee, tea, and placebo for continuous variables. Paired sample T test was used to compare nutritional intake at baseline and after dietary intervention in the three intervention groups. The changes in BMI over time were compared for each arm separately by means of Friedman's test for paired data, followed by Dunn's post hoc test. A p-value of ≤0.05 was considered significant.
Ethical Considerations
The study was approved by the institutional review board of Tel Aviv Medical Center (TLV-18-0166). All parents provided written informed consent before enrollment (MOH_2018-06-10_002438).
Results
Study Population
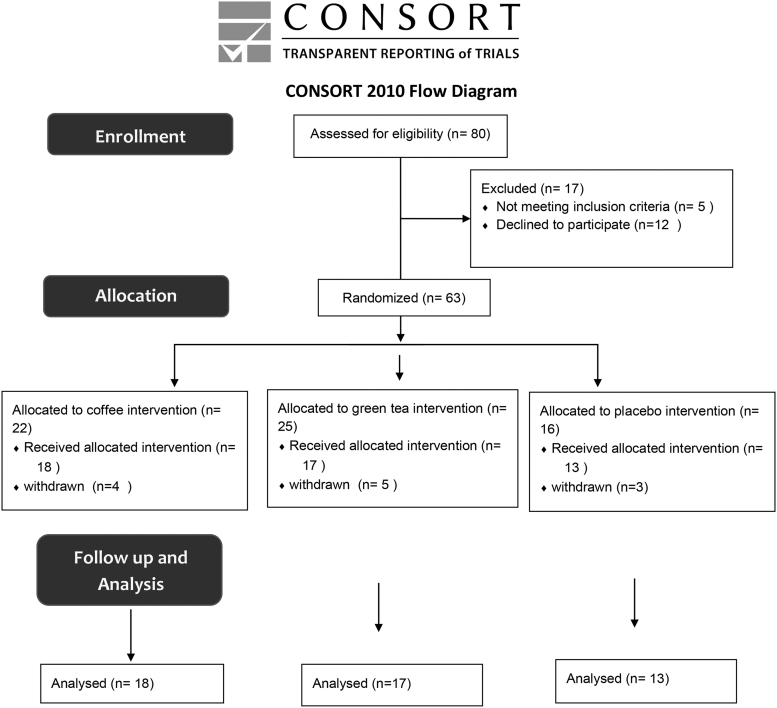
The final study sample is displayed in Figure 1. Overall, 80 adolescents with obesity were assessed for eligibility. Five were excluded (1 due to chronic kidney disease, 1 due to celiac disease, and 3 due to regular consumption of coffee), and 12 adolescent and their parents declined to participate. Sixty-three adolescents were randomized to the study, of whom 22 were assigned to the coffee arm, 22 to the green tea arm, and 16 to the herbal tea (control) arm. Twelve of them withdrew from the study due to difficulty in maintaining dietary recommendations regardless of their beverage assignment, and 3 were excluded due to missing data, leaving 48 adolescents in the final analysis: 18 in the coffee arm, 17 in the green tea arm, and 13 in the placebo arm. The study cohort consisted of 19 (39.6%) males and 29 (60.4%) females, with a median (IQR) age of 13 (12–14) years at recruitment. There were no significant group differences in the parameters of age, sex, BMI, fat percentage, and metabolic complications. There was a significant difference in the TG serum level between the placebo group and the two other groups. The demographic and clinical data of the patients are presented in Table 1
Figure 1.
Determination study participants.
Table 1.
Demographic and Clinical Characteristics of the Study Cohort
| Variable | Coffee (N = 18) | Green tea (N = 17) | Herbal tea (N = 13) | p |
|---|---|---|---|---|
| Age, years | 12 (12, 15.5) | 15 (12, 15) | 14 (12, 14) | 0.45 |
| Sex, male | 5 (27.8) | 7 (41.2) | 6 (46.1) | 0.45 |
| BMI | 34.86 (30–45.5) | 38.33 (31–61) | 35.72 (30–52.8) | |
| Percent of the 95th percentile | 121.5 (99.2, 151.2) | 153 (124, 175) | 140.5 (99, 156.2) | 0.33 |
| Fat percentage | 37.4 (32.6, 42.9) | 45 (36.2, 54.6) | 44.2 (40.9, 46) | 0.30 |
| LDL-c, mg/dL | 116 (84, 118) | 121.7 (85, 171.5) | 99 (88.5, 117) | 0.74 |
| TGs, mg/dL | 164 (114, 176.5) | 123 (86, 261) | 81 (63.2, 134) | 0.04 |
| TG:HDL-c ratio | 3.78 (2.33, 5.67) | 3.05 (2.09, 7.94) | 1.46 (1.35, 2.6) | 0.11 |
| Glucose, mg/dL | 88.5 (81.5, 93.5) | 89 (88, 92.5) | 93 (88, 96) | 0.45 |
| Hemoglobin A1c, % | 5.5 (3.5, 5.5) | 5.3 (4.5, 5.6) | 5.5 (5.5, 5.7) | 0.41 |
| HOMA-IR | 9.20 (7.40, 9.70) | 7.30 (6.85, 8.50) | 7.90 (7.30, 9.32) | 0.10 |
| NAFLD | 8 (66.7) | 13 (86.7) | 3 (42.9) | 0.23 |
| OSA | 2 (7.7) | 2 (11.8) | 2 (15) | 0.24 |
| PTC | 0 | 1 (5.9) | 0 | 0.48 |
| PCOS | 3 (20) | 0 | 1 (7) | 0.34 |
Values are given as median (interquartile range) or n (%). Bold values denote statistical significance at p ≤ 0.05.
HDL-c, high-density lipoprotein cholesterol; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; LDL-c, low-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; OSA, obstructive sleep apnea; PCOS, polycystic ovary syndrome; PTC, pseudotumor cerebri; TG, triglyceride.
Table 2 shows the average of the participants' self-reported dietary intake before the nutritional intervention and at the 3-month midpoint of the intervention. The average preintervention caloric consumption was 1752 ± 388.43 kcal/day, of which 48% was derived from carbohydrates, 20% from proteins, and 32% from fat. At the 3-month midpoint of the intervention, the average caloric consumption was 1356.67 ± 294.33 kcal/day, and it consisted of 39% energy from carbohydrates, 25% from proteins, and 35% from fat. All groups demonstrated significant decrease in total calories and carbohydrates, with no significant differences in energy and dietary intake between the groups.
Table 2.
Self-Reported Dietary Intake Before and After the Nutritional Intervention
| Variable | Coffee |
Green tea |
Herbal tea (placebo) |
p-value between the group |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After dietary intervention | p | Baseline | After dietary intervention | p | Baseline | After dietary intervention | p | Baseline | After dietary intervention | |
| Total energy (kcal) | 1894.75 ± 443.29 | 1431.25 ± 322.91 | 0.007 | 1705.83 ± 468.58 | 1319.67 ± 324.63 | 0.03 | 1695.00 ± 280.37 | 1341.40 ± 254.54 | 0.01 | 0.72 | 0.85 |
| Carbohydrates, g/day (% of energy) | 219.75 ± 92.1 (58%) | 115.25 ± 19.22 (40%) | 0.08 | 186.83 ± 58.85 (55%) | 129.5 ± 38.35 (41%) | 0.01 | 186.8 ± 18.37 (45%) | 100.6 ± 29.67 (38%) | 0.01 | 0.65 | 0.58 |
| Protein, g/day (% of energy) | 75.75 ± 21.79 (20%) | 80.25 ± 47.47 (22%) | 0.86 | 78.0 ± 19.03 (17%) | 95.33 ± 34.2 (23%) | 0.06 | 91.6 ± 31.28 (21%) | 90 ± 38.44 (26%) | 0.88 | 0.60 | 0.58 |
| Fat, g/day (% of energy) | 49.25 ± 19.93 (22%) | 60.5 ± 12.93 (38%) | 0.63 | 64.67 ± 21.43 (28%) | 66.17 ± 18.74 (36%) | 0.85 | 59.4 ± 11.32 (34%) | 59.4 ± 19.8 (36%) | 0.165 | 0.40 | 0.64 |
| Saturated fat, g/day (% of energy) | 20.5 ± 9.25 (12%) | 18 ± 10.03 (11%) | 0.06 | 17.50 ± 10.05 (8%) | 17.5 ± 7.9 (9%) | 0.34 | 14.2 ± 2.77 (10%) | 13.6 ± 5.07 (9%) | 0.64 | 0.52 | 0.63 |
| Sodium (mg/day) | 2967.75 ± 1267.85 | 2256.17 ± 891.74 | 0.29 | 2372.17 ± 820.8 | 2722.83 ± 532.08 | 0.27 | 2911 ± 1015.45 | 2604 ± 677.94 | 0.68 | 0.58 | 0.52 |
Values are given ± standard deviation; bold values denote statistical significance at p ≤ 0.05.
Effect of Tea or Coffee Consumption on Weight Reduction
A significant decrease in the BMI was noted after 3 and 6 months of green tea consumption (from a BMI of 38.33 to 37.76 at 3 months and to 37.45 at 6 months, p = 0.03). A significant reduction was also noted in the BMI after 3 and 6 months of coffee consumption (from a BMI of 34.86 to 33.47 at 3 months and to 31.73 at 6 months, p = 0.02), accompanied by a significant reduction in fat percentage (from 37.4% to 32.5%, p = 0.02). There was no significant weight reduction after herbal tea consumption (from a BMI of 35.72 to 35.67 at 3 months and 35.92 at 6 months).
Comparison Between the Three Interventions
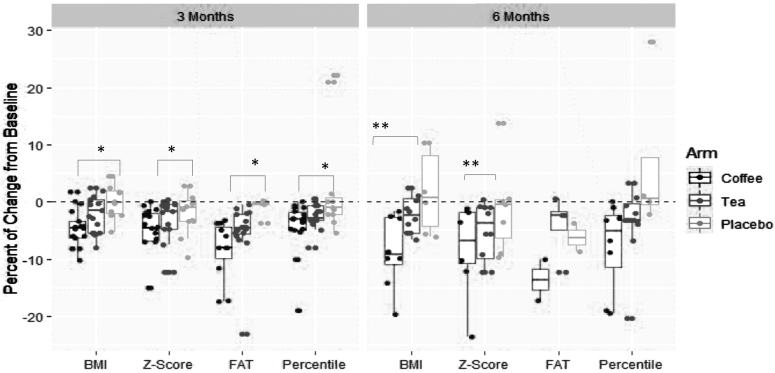
Table 3 and Figure 2 depict the percent change from baseline in BMI and fat over 3 and 6 months of dietary interventions. At 3 months, a significant advantage of coffee consumption compared with placebo was documented in weight and fat reduction. The median change in BMI was −4.58% (−6.24, −3.33) for the coffee group, −1.51% (−5.36, 0.32) for the green tea group, and −0.14% (−1.93, 1.89) for the herbal tea (control) group (p = 0.003). The weight reduction was accompanied by a significant reduction in fat percentage in the coffee group compared with placebo. The median change in fat was −7.98% in the coffee group compared with −4.6% and −0.2% in the green tea and placebo, respectively (p = 0.002). The percent change in BMI at 6 months compared with baseline demonstrated a significant advantage for coffee compared with both tea and control. The median change in BMI was −9.26% (−10.9, −2.7) for the coffee group, −2.32% (−5.36, 0.49) for the green tea group, and 0.76 (−4.23, 8.17) for the control group (p = 0.02).
Table 3.
Comparison of Percent Changes from Baseline
| Variable | Coffee | Green tea | Herbal tea (placebo) | p | |
|---|---|---|---|---|---|
| 3 Months | BMI | −4.58 (−6.24, −3.33) | −1.51 (−5.36, 0.32) | −0.14 (−1.93, 1.89) | 0.003* |
| Body fat | −7.98 (−9.82, −4.37) | −4.64 (−5.55, −2.12) | −0.22 (−0.45, 0.00) | 0.002* | |
| BMI percent of the 95th percentile | −3.09 (−4.91, −1.78) | −2.90 (−3.23, −0.68) | −1.03 (−2.21, 0.71) | 0.03* | |
| 6 Months | BMI | −9.26 (−10.92, −2.77) | −2.32 (−5.36, 0.49) | 0.76 (−4.23, 8.17) | 0.02** |
| Body fat | −13.64 (−15.46, −11.82) | −2.43 (−4.91, −1.68) | −6.21 (−7.43, −5.00) | NS | |
| BMI percent of the 95th percentile | −5.02 (−11.38, −2.35) | −3.19 (−3.42, −0.31) | 0.51 (−0.54, 7.76) | NS |
Values are given as median (interquartile range); bold values denote statistical significance at p ≤ 0.05.
Significant change between coffee and placebo.
Significant change between coffee and both green tea and placebo.
Figure 2.
Change from baseline in BMI and body fat over 3 and 6 months of coffee, tea, and placebo interventions. *Significant change between coffee and placebo. **Significant change between coffee and both green tea and placebo. Percentile, percent of the 95th percentile.
Discussion
We conducted a randomized clinical trial to evaluate the addition of coffee, green tea, or herbal tea consumption to standard dietary recommendations for weight loss among adolescents with obesity. The results of this 6-month trial demonstrated a significant decrease in the BMI Z score for both coffee and green tea consumption compared with placebo consumption. A comparison between the three interventions revealed a significant advantage for coffee over both tea and placebo.
Despite growing recognition of the problem, the obesity epidemic continues to rise, and obesity rates are increasing worldwide.1 The epidemic of obesity arose gradually over time, apparently from a small consistent degree of positive energy balance, which in itself may be very small but can amount to several kilograms of fat over the course of months to years.32 Moreover, any reduction of weight elicits a physiological increase in hunger and a decrease in energy expenditure.33 These changes lead to the common phenomenon of weight regain with its accompanying deleterious metabolic effects.2–5,32,33 Therefore, any tool that will help lower appetite or balance energy expenditure may be beneficial in the process of weight loss over time.
Both caffeine (found in both coffee and tea) and catechins (found in green tea) have been suggested as being capable of eliciting an increase in diet-induced thermogenesis, fat oxidation (and thereby daily energy expenditure), and increased satiety.7–21,34
Interestingly, Gavrieli et al.'s study on 33 adults (16 normal weight and 17 obese) who consumed a standard breakfast along with 200 mL of either coffee (with 3 or 6 mg caffeine/kg body weight) or water demonstrated lower energy intake after the high-dose caffeine consumption only in the overweight/obese individuals.34 EGCG was also found to control appetite and to decrease voluntary food intake, feeding frequency, and meal size in high-fat diet-fed mice by regulating the expression of key appetite-related neuropeptide genes and key circadian genes in the hypothalamus.35 The caffeine group in our human study consumed 160 mg of caffeine daily, whereas the green tea group consumed 252 mg of catechins and 96 mg of caffeine. Although all the groups demonstrated reductions in energy and carbohydrates intake, no significant differences in those parameters were noted between the groups. As such, the significant weight loss that was demonstrated in the green tea and caffeine groups may be related to an increase in energy expenditure and not to energy intake. The significant decrease in fat mass that was demonstrated in the coffee group may imply the involvement of fat oxidation in the increase in energy expenditure.
Of note, although a large body of evidence demonstrated antiobesity properties for tea or coffee consumption, those studies varied both in the dose of caffeine, catechins, or their combinations, as well as in the volume of the treatment beverages. Such large discrepancies between the studies precluded our discerning any clear pattern of dose response. In the present study, both coffee and tea consumption showed significant effects on weight reduction in an adolescent population. A comparison between the three interventions revealed a significant advantage for coffee over tea and placebo, which may imply that the effect of a higher dose of caffeine is more beneficial the addition of tea catechins. Similarly, Gavrieli et al. also demonstrated an advantage for a higher dose of caffeine intake (6 mg/kg body weight) compared with a lower dose (3 mg/kg body weight) in reducing energy intake.34 Westerterp-Plantenga et al. examined the effect of a green tea: caffeine mixture on weight maintenance after body weight loss in adults with obesity and observed that green tea reduced body weight and increased energy expenditure among individuals with habitual low caffeine intake. However, no added value was demonstrated with the consumption of the mixture among individuals with habitual high caffeine intake.17
A large body of evidence suggests that consumption of caffeinated coffee does not increase the risk of cardiovascular diseases and cancers as had been believed in the past.16,36,37 Moreover, the daily consumption of three to five standard cups of coffee has been consistently associated with numerous health benefits for adults, such as decreased risk of heart disease, lower levels of inflammation and endothelial dysfunction, reduced incidence of diabetes, and improved mental health and well-being.38–41 In the present study, we used a total amount of 3.0 mg/kg of body weight, which had been described as safe for children and adolescents,16 and no adverse events, including sleep disturbance, were noted throughout the study period.
As far as we know this is the first study to assess the effect of tea and caffeine consumption in adolescents with obesity and to suggest that coffee can be introduced to them as a means of maintaining weight loss over time. The strengths of this study include its randomized design and the inclusion of a body composition analysis. The limitations of the study are its small sample size and reliance upon self-reporting for dietary assessment, recognizing the well-documented potential biases associated with recall and social acceptability. Moreover, our results may be influenced by other biologically active ingredients that are found in coffee, such as chlorogenic acid and lignans, that were also shown to modulate glucose and fat metabolism,41 and by artificial sweeteners that were used to increase adherence, that was demonstrated to increase adiposity.42 Therefore, any research findings for coffee and other dietary sources of caffeine should be interpreted with some caution since effects may not be due solely to caffeine.
In conclusion, the findings of the present study demonstrated that the consumption of caffeine or green tea in the context of a weight-reducing diet may have a small but beneficial effect on body weight reduction among adolescents with obesity. Future larger scale investigations to test feasibility and effectiveness over the long term are warranted.
Acknowledgment
We want to thank Wissotzky tea Ltd. for the products provided during the trial.
Funding Information
No funding was received.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. J Am Med Assoc 2014;311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tobias DK, Chen M, Manson JE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: A systematic review and meta-analysis. Crit Rev Food Sci Nutr 2015;55:939–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wadden TA, Stunkard AJ, Liebschutz J. Three-year follow-up of the treatment of obesity by very low calorie diet, behavior therapy, and their combination. J Consult Clin Psychol 1988;56:925–928. [DOI] [PubMed] [Google Scholar]
- 4. Kramer FM, Jeffery RW, Forster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: Patterns of weight regain among men and women. Int J Obes 1989;13:123–136. [PubMed] [Google Scholar]
- 5. Pasman WJ, Westerterp-Plantenga MS, Muls E, et al. The effectiveness of long-term fibre supplementation on weight maintenance in weight-reduced women. Int J Obes Relat Metab Disord 1997;21:548–555. [DOI] [PubMed] [Google Scholar]
- 6. Mukhtar H, Ahmad N. Tea polyphenols: Prevention of cancer and optimizing health. Am J Clin Nutr 2000;71(6 Suppl):1698S–1702S. [DOI] [PubMed] [Google Scholar]
- 7. Dulloo AG, Duret C, Rohrer D, Girardier L. Efficacy of a Green tea extract rich in catechin polyphenols and caffeine in increasing 24h energy expenditure and fat oxidation in human. Am J Clin Nutr 1999;70:1040–1045. [DOI] [PubMed] [Google Scholar]
- 8. Diepvens K, Westerterp KR, Westerterp-Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin and green tea. Am J Physiol Regul Integr Comp Physiol 2007;292:77–85. [DOI] [PubMed] [Google Scholar]
- 9. Pourmasoumi M, Hadi A, Marx W, et al. The effect of green coffee bean extract on cardiovascular risk factors: A systematic review and meta-analysis. Adv Exp Med Biol 2021;1328:323–345. [DOI] [PubMed] [Google Scholar]
- 10. Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 2000;141:980–987. [DOI] [PubMed] [Google Scholar]
- 11. Chantre P, Lairon D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine 2002;9:3–8. [DOI] [PubMed] [Google Scholar]
- 12. Lopez-Garcia E, van Dam RM, Rajpathak S, et al. Changes in caffeine intake and long-term weight change in men and women. Am J Clin Nutr 2006;83:674–680. [DOI] [PubMed] [Google Scholar]
- 13. Kolesárová A. The anti-obesity and health-promoting effects of tea and coffee. Physiol Res 2021;70:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rudelle S, Ferruzzi MG, Cristiani I, et al. Effect of a thermogenic beverage on 24-hour energy metabolism in humans. Obesity (Silver Spring) 2007;15:349–355. [DOI] [PubMed] [Google Scholar]
- 15. Gregersen NT, Bitz C, Krog-Mikkelsen I, et al. Effect of moderate intakes of different tea catechins and caffeine on acute measures of energy metabolism under sedentary conditions. Br J Nutr 2009;102:1187–1194. [DOI] [PubMed] [Google Scholar]
- 16. Schubert MM, Irwin C, Seay RF, et al. Caffeine, coffee, and appetite control: A review. Int J Food Sci Nutr 2017;68:901–912. [DOI] [PubMed] [Google Scholar]
- 17. Westerterp-Plantenga M, Diepvens K, Joosen AM, et al. Metabolic effects of spices, teas, and caffeine. Physiol Behav 2006;89:85–91. [DOI] [PubMed] [Google Scholar]
- 18. Wawrzyniak N, Skrypnik K, Suliburska J. Dietary supplements in therapy to support weight reduction in obese patients. Acta Sci Pol Technol Aliment 2022;21:67–80. [DOI] [PubMed] [Google Scholar]
- 19. Wan CJ, Lin LY, Yu TH, Sheu WH. Metabolic syndrome associated with habitual indulgence and dietary behavior in middle-aged health-care professionals. J Diabetes Investig 2010;1:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higdon JV, Frei B. Coffee and health: A review of recent human research. Crit Rev Food Sci Nutr 2006;46:101–123. [DOI] [PubMed] [Google Scholar]
- 21. Brener A, Peleg I, Rosenfeld T, et al. Beyond body mass index—Body composition assessment by bioimpedance in Routine Endocrine Practice. Endocr Pract 2021;27:419–425. [DOI] [PubMed] [Google Scholar]
- 22. Shypailo RJ, Motil KJ. The use of bioimpedance in pediatric health, nutrition, and disease. J Pediatr Gastroenterol Nutr 2018;67:435–436. [DOI] [PubMed] [Google Scholar]
- 23. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: Methods and development. Vital Heal Stat 2002;11:1–190. [PubMed] [Google Scholar]
- 24. Ryder JR, Kelly AS, Freedman DS. Metrics matter: Toward consensus reporting of BMI and weight-related outcomes in pediatric obesity clinical trials. Obesity (Silver Spring) 2022;30:571–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017;140:1–74. [DOI] [PubMed] [Google Scholar]
- 26. Vardi P, Shahaf-Alkalai K, Sprecher E, et al. Components of the metabolic syndrome (MTS), hyperinsulinemia, and insulin resistance in obese Israeli children and adolescents. Diabetes Metab Syndr Clin Res Rev 2007;1:97–103. [Google Scholar]
- 27. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 28. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:13–28. [DOI] [PubMed] [Google Scholar]
- 29. Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 2017;64:319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bitners AC, Arens R. Evaluation and management of children with obstructive sleep apnea syndrome. Lung 2020;198:257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedman D, Jacobson D. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002;59:1492–1495. [DOI] [PubMed] [Google Scholar]
- 32. Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: Where do we go from here? Science 2003;299:853–855. [DOI] [PubMed] [Google Scholar]
- 33. Melby CL, Paris HL, Foright RM, Peth J. Attenuating the biologic drive for weight regain following weight loss: Must what goes down always go back up? Nutrients 2017;9:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gavrieli A, Karfopoulou E, Kardatou E, et al. Effect of different amounts of coffee on dietary intake and appetite of normal-weight and overweight/obese individuals. Obesity (Silver Spring) 2013;21:1127–1132. [DOI] [PubMed] [Google Scholar]
- 35. Li H, Kek HC, Lim J, et al. Green tea (−)-epigallocatechin-3-gallate counteracts daytime overeating induced by high-fat diet in mice. Mol Nutr Food Res 2016;60:2565–2575. [DOI] [PubMed] [Google Scholar]
- 36. Higdon JV, Frei B. Coffee and health: A review of recent human research. Crit Rev Food Sci Nutr 2006;46:101–123. [DOI] [PubMed] [Google Scholar]
- 37. van Dam RM, Hu FB, Willett WC. Coffee, caffeine, and health. N Engl J Med 2020;383:369–378. [DOI] [PubMed] [Google Scholar]
- 38. Andersen LF, Jacobs DR Jr, Carlsen MH, Blomhoff R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women's Health Study. Am J Clin Nutr 2006;83:1039–1046. [DOI] [PubMed] [Google Scholar]
- 39. Ding M, Bhupathiraju SN, Chen M, et al. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care 2014;37:569–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Gelder BM, Buijsse B, Tijhuis M, et al. Coffee consumption is inversely associated with cognitive decline in elderly European men: The FINE Study. Eur J Clin Nutr 2007;61:226–232 [DOI] [PubMed] [Google Scholar]
- 41. Bhandarkar NS, Mouatt P, Majzoub ME, et al. Coffee pulp, a by-product of coffee production, modulates gut microbiota and improves metabolic syndrome in high-carbohydrate, high-fat diet-fed rats. Pathogens 2021;10:1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514:181–186. [DOI] [PubMed] [Google Scholar]