Abstract
Adverse intrauterine environments can cause persistent changes in epigenetic profiles of stem cells, increasing susceptibility of the offspring to developing metabolic diseases later in life. Effective approaches to restore the epigenetic landscape and function of stem cells remain to be determined. In this study, we investigated the effects of pharmaceutical activation of AMP-activated protein kinase (AMPK), an essential regulator of energy metabolism, on mitochondrial programming of Wharton's Jelly mesenchymal stem cells (WJ-MSCs) from women with diabetes during pregnancy. Induction of myogenic differentiation of WJ-MSCs was associated with increased proliferator-activated receptor-γ coactivator-1α (PGC-1α) expression and mitochondrial DNA (mtDNA) abundance. Inhibition of DNA methylation by 5 Azacytidine significantly increased PGC-1α expression and mtDNA abundance in WJ-MSCs, which were abolished by AMPK inhibitor Compound C (CC), suggesting an AMPK-dependent role of DNA demethylation in regulating mitochondrial biogenesis in WJ-MSCs. Furthermore, activation of AMPK in diabetic WJ-MSCs by AICAR or metformin decreased the level of PGC-1α promoter methylation and increased PGC-1α expression. Notably, decreased PGC-1α promoter methylation by transient treatment of AMPK activators persisted after myogenic differentiation. This was associated with enhanced myogenic differentiation capacity of human WJ-MSCs and increased mitochondrial function. Taken together, our findings revealed an important role for AMPK activators in epigenetic regulation of mitochondrial biogenesis and myogenesis in WJ-MSCs, which could lead to potential therapeutics for preventing fetal mitochondrial programming and long-term adverse outcome in offspring of women with diabetes during pregnancy.
Keywords: stem cells, AMPK, DNA methylation, PGC-1α, mitochondria
Introduction
Mesenchymal stem cells from umbilical cord Wharton's Jelly (WJ-MSCs) are multipotent stem cells that maintain high multilineage differentiation capacity into adipocytes, osteocytes, and myocytes upon in vitro induction [1,2]. Recent evidence suggests that maternal overnutrition, including diabetes and obesity during pregnancy, impacts the metabolic characteristic and differentiation capacity of human WJ-MSCs, which are strongly associated with infant and offspring metabolic dysfunction [3–6]. For example, maternal obesity reduces human WJ-MSCs mitochondrial fatty acid oxidation upon myogenesis, which is linked to higher infant adiposity [4,7]. However, molecular mechanisms regulating metabolic activity and differentiation commitment of WJ-MSCs in response to maternal overnutrition remain not fully known.
Epigenetic processes, including DNA methylation and histone modification, play a key role in determining stem cells lineage commitment and properties without affecting DNA sequence [8]. Initiation of myogenesis requires an activation of mitochondrial biogenesis and increased oxidative phosphorylation [9]. Maternal obesity alters the methylome and transcriptome of WJ-MSCs, including hypermethylation of genes regulating mitochondrial fatty acid oxidation during myogenesis [7,10]. Similar to maternal obesity, WJ-MSCs from gestational diabetes mellitus (GDM) display mitochondrial dysfunction and premature aging [11].
Of note, expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), the central regulator of mitochondrial metabolism and biogenesis, is decreased in GDM WJ-MSCs [11]. We have demonstrated that maternal diabetes increases PGC-1α promoter methylation, which is associated with decreased PGC-1α and mitochondrial content in placenta [12]. It is unknown whether epigenetic modification regulates PGC-1α/mitochondrial programming in WJ-MSCs in response to maternal overnutrition and whether pharmaceutical interventions can reverse those alterations.
AMP-activated protein kinase (AMPK) is an energy sensor that plays a central role in regulating mitochondrial function and maintaining cellular metabolic homeostasis [13,14]. Emerging evidence identifies AMPK as an important epigenetic regulator, involving both DNA methylation and histone modifications [12,15]. Multiple pathways mediate the epigenetic roles of AMPK, such as inhibition of DNA methyltransferase 1 (DNMT1) and phosphorylation of DNA demethylation enzyme TET2 16–18. Prior studies by our group and others showed that activities of AMPK in WJ-MSCs [7], human umbilical vein endothelial cells (HUVECs) [19], and placenta [12] are suppressed by maternal diabetes or obesity. In this study, we demonstrated pharmaceutical AMPK activators exerted an epigenetic role in regulating PGC-1α expression in diabetic WJ-MSCs, which programs the stem cells for enhanced myogenic differentiation capacity and improved mitochondrial function.
Materials and Methods
Human WJ-MSCs isolation and differentiation
Human WJ-MSCs were obtained from umbilical cords of patients with diabetes during pregnancy (including three patients with GDM and one patient with T2D) after delivery by Cesarean section at term. The protocol was approved by the Institutional Review Board of the University of Oklahoma Health Science Center. Mothers included in this study do not have other medical conditions, such as type 1 diabetes, pre-eclampsia, chronic hypertension, renal disorders nor a smoking history of more than five cigarettes per day. They do not have fever or infection at the time of birth. WJ-MSCs were isolated using the “explant culture” method as previously described with modification [5,20]. In brief, the vein and arteries were removed from the umbilical cord.
The excised cord tissue with Wharton's jelly was then cut into smaller (3–5 mm) pieces and placed in tissue culture dish and cultured in medium composed of Dulbecco's modified Eagle medium (DMEM) (1 g/L glucose) with 2 mM l-glutamine, supplemented with 10% fetal bovine serum (FBS), and 100 U penicillin/streptomycin. At confluence, WJ-MSCs were harvested for cryogenic storage or experimentations. For myogenic differentiation, WJ-MSCs at passage 3 or 4 were induced in differentiation medium (DMEM with 10% FBS, 5% horse serum, 0.1 μM dexamethasone, and 50 μM hydrocortisone) and changed with fresh differentiation medium every 2–3 days until experimental analysis. For manipulation AMPK activity or DNA methylation, WJ-MSCs were treated with AICAR (2 mM) or metformin (2 mg/mL), or 5 Azacytidine (AZA, 5 μM), or AZA with compound C (CC 10 μM) for 1 or 2 days prior differentiation as indicated in the figure legends.
RNA extraction and real-time PCR
Total RNA was extracted with RNA isolation kit (miRNeasy, Qiagen) and converted to complementary DNA (cDNA) with high-capacity cDNA reverse transcription Kit (ThermoFisher Scientific) according to the manufacturer's instructions. Real-time qPCR was performed in triplicate using SYBR green qPCR master mix and single amplification product was confirmed by single peak of melting curve. Human PGC-1α mRNA were quantified using primers: Forward: CCAAAGGATGCGCTCTCGTTCA, Reverse: CGGTGTCTGTAGTGGCTTGACT. Human desmin mRNA were quantified using primers: Forward: ATTCCCTGATGAGGCAGATG, Reverse: CTTCAGGGAGCAGTGAGGAC. Human β-actin mRNA was quantified using primers: Forward: GATCGGCGGCTCCATCCTG, Reverse: GACTCGTCATACTCCTGCTTGC. Results were calculated using the 2−ΔΔCt method and normalized to β-actin.
mitochondrial DNA copy number measurement
DNA was isolated from cells using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma, St. Louis, MO) according to the manufacturer's instructions. Mitochondrial DNA (mtDNA) copy number was estimated by comparing the abundance of the mitochondrial transfer RNA (Mt-tRNA, by real-time q-PCR, primers Forward: CACCCAAGAACAGGGTTTGT; Reverse: TGGCCATGGGTATGTTGTTA) and with that of the nuclear β2-microglobulin (B2M, Forward: TGCTGTCTCCATGTTTGATGTATCT; Reverse: TCTCTGCTCCCCACCTCTAAGT).
Western blot analysis
Western blot analysis was performed as previously described [12]. Cells were lysed in RIPA buffer in the presence of protease and phosphatase inhibitor cocktail (Pierce Biotechnology, Rockford, IL). Protein concentrations were determined by BCA assay (Pierce, Rockford, IL). Equal amounts of protein lysate were reduced in sample buffer (Sigma), and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The blots were incubated with antibodies specific for Myogenin, myosin HC, P(T172)-AMPK, P(S97)-ACC, or β-actin (Cell Signaling Technology, Danvers, MA), and detected by enhanced chemiluminescence (Pierce). The blots were quantified and analyzed by imaging densitometry with Image Lab Software (Bio-Rad, Hercules, CA).
Promoter methylation
Methylated DNA was analyzed with the EpiJET DNA Methylation Analysis kit (Msp1/Hpa11; ThermoFisher Scientific) as previously described [12]. The proportion of DNA methylation of human PGC-1α was quantified by qPCR using primers as previously described [17]: Forward: AAAACGCAAACTACACAACCC, Reverse: AGGCTCCCAGAAAACAAGTG.
Measurement of mitochondrial respiration
Oxygen consumption rates (OCRs) in differentiated cells were measured by using a Seahorse extracellular flux analyzer (XFe96; Agilent Seahorse). OCR was measured overtime and after sequential injections of modulators (Seahorse XF cell mito stress test kit; Agilent) including oligomycin (Olig, ATP synthase inhibitor, 1 μM), phenylhydrazone (FCCP, uncoupler, 1 μM), and Antimycin A & Rotenone (mitochondrial respiration inhibitor, 0.5 μM). Indices including baseline OCR, proton leak, maximal respiration, and ATP production were determined after the treatment.
Flow cytometry
Undifferentiated WJ-MSCs at passage #3 from all subjects were pooled and 2 × 105 cells were used for staining with antibodies specific for CD105-APC (BioLegend), CD90-PE (Miltenyi Biotec), CD73-BV421 (BioLegend), CD45-BB515 (BD Biosciences), CD-34-PE (BioLegend) CD-19-BV711 (BioLegend), and the corresponding isotype controls separately. Samples were acquired on the Stratedigm-4 Flow Cytometer.
Statistical analysis
Comparison between AICAR, metformin, or 5-AZA treated groups with the corresponding vehicle controls were assessed using Student's t-test for continuous measures. Comparisons among the groups of different days of differentiation were done by performing one-way analysis of variance tests followed by post hoc analysis with Dunnett's multiple-comparison test. Differences were considered significant at P < 0.05. Data are presented as mean ± standard deviation.
Results
WJ-MSC characterization
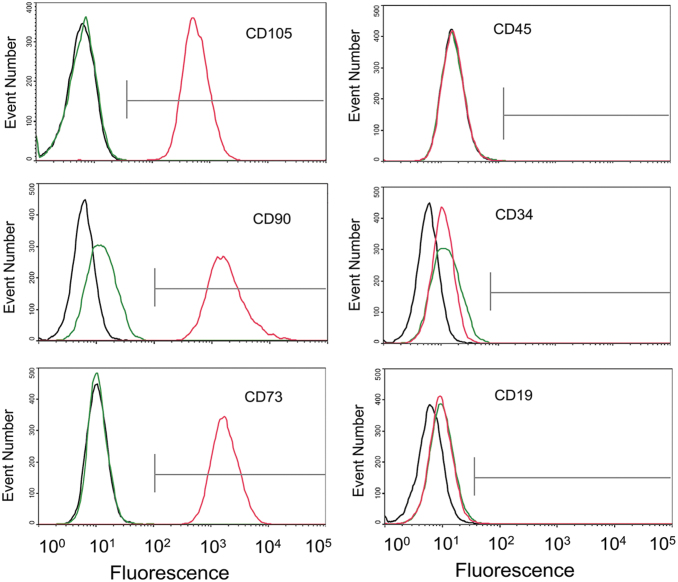
The WJ-MSC identity of the cord cells used in this study was characterized based on the guidelines and recommendations of the International Society for Cellular Therapy for the characterization of MSCs [21]. As the results shown in Fig. 1, Wharton's Jelly-derived MSCs strongly expressed MSC markers CD105 (>99%), CD90 (>99%), and CD73 (>99%), and lack expression of hematopoietic stem cell or lymphocyte markers CD45 (<1%), CD34 (<1%), or CD19 (<1%), meeting the criteria defining human MSC.
FIG. 1.
Flow cytometry histogram of WJ-MSCs. Undifferentiated WJ-MSCs at passage #3 were pooled (from all four subjects) and stained for indicated stem cell markers. Red lines indicate staining with CD105, CD90, CD73, CD34, CD45, and CD19 antibodies; green lines indicate corresponding isotype controls; black lines indicate unstained background fluorescence. WJ-MSC, Wharton's Jelly mesenchymal stem cell.
Increased PGC-1α expression and mtDNA content after induction of myogenic differentiation of human MSCs
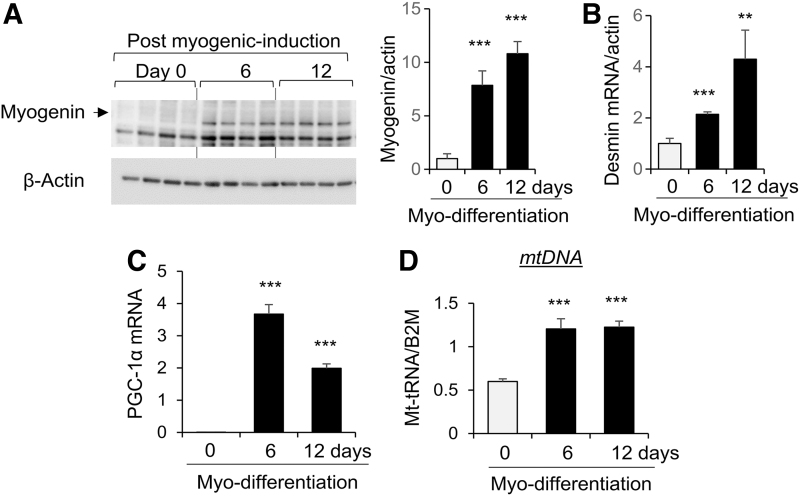
WJ-MSCs were induced for myogenic differentiation in vitro. Levels of myogenin and desmin, markers of myogenesis [22], were increased at 6 and 12 days after induction of myo-differentiation (Fig. 2A, B). Concomitantly, expression of PGC-1α was significantly increased after myo-differentiation with highest abundance at 6 days (Fig. 2C). Also, mtDNA copy number was increased at 6 and 12 days after myogenic differentiation (Fig. 2D).
FIG. 2.
Induction of myogenic differentiation of human WJ-MSCs is associated with increased PGC-1α and mitochondrial content. WJ-MSCs were induced for myogenic differentiation. At day 0 (before differentiation), 6, and 12 after differentiation, the levels of Myogenin protein (A), desmin mRNA (B), PGC-1α mRNA (C), and MT-tRNA (mtDNA, D) were measured by western blot analysis or real-time PCR and normalized to β-actin protein, β-actin mRNA, or B2M gene, respectively. Bar graphs were presented as mean ± SD, n = 4 in each group, **P < 0.01; ***P < 0.001 compared with day 0 groups. B2m, β2-microglobulin; mtDNA, mitochondrial DNA; PGC-1α, proliferator-activated receptor-γ coactivator-1α; SD, standard deviation.
DNA methylation and AMPK regulate PGC-1α expression and mitochondrial biogenesis of WJ-MSCs
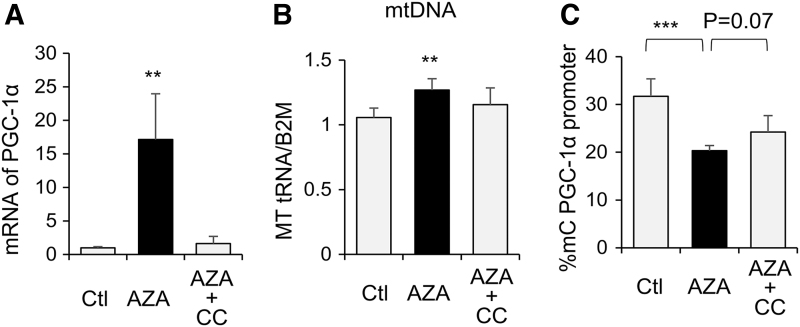
Treating WJ-MSCs with the DNA methylation transferase (DNMT) inhibitor, 5 Azacytidine (AZA), resulted in significant increase in PGC-1α expression (Fig. 3A) and mtDNA abundance (Fig. 3B), as well as decreased PGC-1α promoter methylation (Fig. 3C). These results suggest a role of DNA methylation in regulating PGC-1α/mitochondrial biogenesis in human WJ-MSCs, likely involving PGC-1α promoter methylation, which can negatively regulate PGC-1α expression. In the presence of AMPK inhibitor, Compound C (CC), the effects of AZA on PGC-1α expression and DNA methylation were suppressed (Fig. 3A–C), which suggests AMPK activation is required for DNA demethylation-induced increase in mitochondrial biogenesis of WJ-MSCs.
FIG. 3.
DNA demethylation significantly increased PGC-1α expression and mitochondrial content in WJ-MSCs, which requires AMPK. WJ-MSCs were treated with the 5 Azacytidine (AZA, 5 μM), or AZA with compound C (AZA 5 μM+CC 10 μM) for 2 days. (A) Total RNA was extracted and PGC-1α expression was measured by RT-PCR and normalized to β-actin mRNA. (B) DNA was extracted and mtDNA abundance was determined by measuring Mt-tRNA with RT-PCR and normalized to B2M. (C) PGC-1α promoter methylation was measured. Bar graphs were presented as mean ± SD, n = 4 in each group, **P < 0.01; ***P < 0.001. AMPK, AMP-activated protein kinase; CC, Compound C; MT-tRNA, mitochondrial transfer RNA.
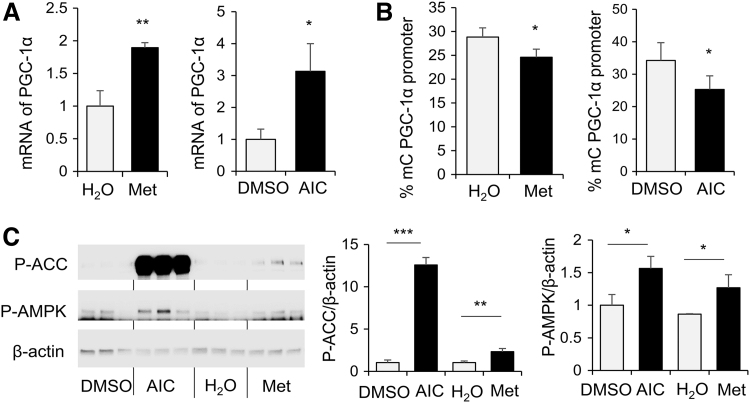
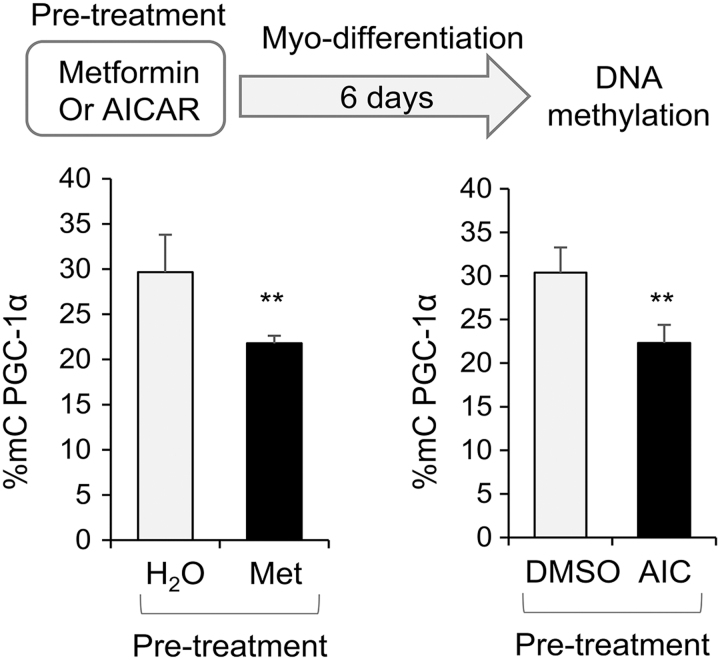
AMPK activation increased PGC-1α mRNA expression in human MSCs of women with diabetes during pregnancy, concomitant with decreased level of PGC-1α promoter methylation
To determine the roles of AMPK, WJ-MSCs from patients with diabetes during pregnancy were treated with AMPK activators, metformin or AICAR. As results shown in Fig. 4, metformin and AICAR treatment significantly increased PGC-1α expression in WJ-MSCs (Fig. 4A), which was associated with significant decreases in DNA methylation of PGC-1α promoter (Fig. 4B). Activation of AMPK by AICAR or metformin in WJ-MSCs was demonstrated by increased ACC and AMPK phosphorylation (Fig. 4C).
FIG. 4.
AMPK activation increased PGC-1α mRNA expression in human MSCs of women with diabetes during pregnancy, concomitant with decreased level of PGC-1α promoter methylation. Diabetic WJ-MSCs were treated with metformin (Met, 2 mg/mL), AICAR (AIC, 2 mM), or vehicle controls for 1 day. (A) Total RNA was extracted and PGC-1α expression was measured by RT-PCR and normalized to β-actin mRNA. (B) DNA was extracted and PGC-1α promoter methylation was measured. (C) Protein extracts were subjected to western blot analysis and levels of P-(Ser79)-ACC, P-(Thr172)-AMPK, and β-actin. Were measured. Bar graphs were presented as mean ± SD, n = 4 in each group, *P < 0.05, **P < 0.01, ***P < 0.001. Results of WJ-MSCs from one diabetic subject were shown. Similar results were obtained in WJ-MSCs from three individuals with diabetes during pregnancy.
AMPK-induced decrease of PGC-1α promoter methylation lasts after differentiation
To determine whether AMPK activation-induced alteration in PGC-1α promoter methylation in WJ-MSC persists after differentiation, WJ-MSCs were treated with metformin or AICAR for 1 day prior differentiation and myo-differentiation was induced after withdrawal of the treatment. Metformin or AICAR pretreatment resulted in decreased DNA methylation of PGC-1α promoter at 6 days after induction of myogenesis (Fig. 5).
FIG. 5.
Decreased PGC-1α promoter methylation by prior-differentiation treatment of AMPK activators persisted after myogenic differentiation. Diabetic WJ-MSCs were treated with metformin (Met, 2 mg/mL), AICAR (AIC, 2 mM), or vehicle controls. The treatments were withdrawn after 2 days and myogenic differentiation was induced. DNA was extracted and PGC-1α promoter methylation was measured at 6 days after differentiation. Bar graphs were presented as mean ± SD, n = 4 in each group, **P < 0.01. Results of WJ-MSCs from 1 diabetic subject were shown. Similar results were obtained in WJ-MSCs from three individuals with diabetes during pregnancy.
AMPK activation before differentiation programs WJ-MSCs for increased myogenic differentiation and mitochondrial function
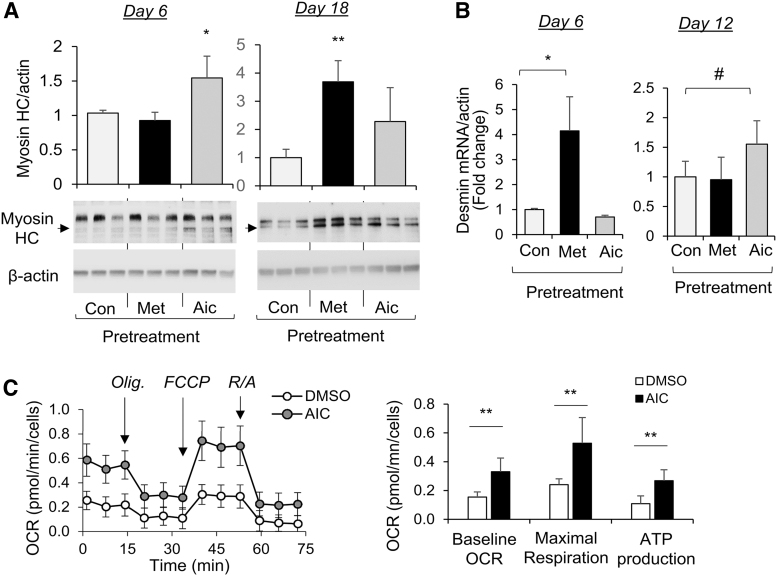
We next determined how AMPK activation in WJ-MSCs impacts the myogenic differentiation capacity. As results shown in Fig. 6A, treatment of WJ-MSCs with AICAR or metformin prior differentiation significantly increased the abundance of myosin heavy chain (myosin HC) at 6 days (AICAR) and 18 days (metformin) of myogenic differentiation. Also, WJ-MSC pretreatment with metformin or AICAR significantly increased desmin expression at 6 or 12 days after induction of myogenic differentiation, respectively (Fig. 6B).
FIG. 6.
Treatment with an AMPK activator, AICAR, enhanced the differentiation capacity of human WJ-MSCs into myocytes. WJ-MSCs were treated with metformin (Met, 2 mg/mL) or AICAR (AIC, 2 mM) for 2 days and myogenic differentiation was induced after withdrawing the treatment. (A) Myosin heavy chain levels at 6 and 18 days after myo-differentiation were shown. (B) Desmin mRNA levels at 6 and 12 days after myo-differentiation were measured by real-time PCR and normalized to β-actin. Bar graphs were presented as mean ± SD, n = 3–4 in each group, *P < 0.05; **P < 0.01; #P < 0.05 (one-tail). (C) At 4 days after myo-differentiation, OCR was measured over time and after stress treatment of Olig (1 μM), FCCP (1 μM), and Ant.A&Rot (0.5 μM). Indices of baseline OCR, maximal respiration, and ATP production were calculated according to altered OCR after the treatment. Mean ± SD, n = 8 in each group, **P < 0.01. OCR, oxygen consumption rate.
To determine how AMPK activation before differentiation impacts mitochondrial bioenergetics after myogenesis, the Seahorse Extracellular Flux analyzer was used to measure oxygen consumption (OCR) overtime and after sequential injections of modulators (Seahorse XF cell mito stress test kit; Agilent), including oligomycin (Olig, ATP synthase inhibitor, 1 μM), phenylhydrazone (FCCP, uncoupler, 1 μM), and Antimycin A & Rotenone (mitochondrial respiration inhibitor, 0.5 μM). As results shown in Fig. 6C, the baseline and maximal respiration, and ATP-linked OCR were significantly increased in AICAR pretreated MSCs upon myogenic differentiation (Fig. 6C). Together, those results suggest that “priming” of WJ-MSCs with AMPK activator enhanced their myogenic differentiation capacity and mitochondrial function.
Discussion
Through studying WJ-MSCs from women with diabetes during pregnancy, this study revealed a role of DNA methylation in regulating mitochondrial biogenesis in WJ-MSCs, and demonstrated the capability of pharmaceutical AMPK activators to regulate epigenetic programming of mitochondrial biogenesis in human WJ-MSCs, promoting their myogenic capacity and mitochondrial function.
It has been increasingly recognized that an adverse intrauterine environment can induce fetal programming of metabolic diseases later in life [23–27]. However, the underlying mechanisms and effective treatments remain largely unknown in humans, partly due to limited accessibility of human fetal tissues. As perinatal stem cells, human WJ-MSCs are a valuable tool to unravel the cell type-specific mechanisms of fetal programming and explore potential therapeutic approaches in humans as they have multilineage differentiation capacity, a relatively homogeneous population, and a close association of their in vitro differentiation capacity with infant and offspring metabolism [1,3–5]. In this study, we used the WJ-MSCs from patients with diabetes during pregnancy to determine whether pharmaceutical AMPK activators can restore the relevant alterations in WJ-MSCs exposed to in utero diabetic environment.
Human WJ-MSCs affected by GDM display mitochondrial dysfunction and reduced expression of PGC-1α, the central regulator of mitochondrial biogenesis [11]. In addition, expression of PGC-1α is decreased in skeletal muscle of adult offspring of women with GDM, which is significantly associated with offspring insulin resistance [29]. Those studies point to PGC-1α as an important component in fetal programming, which is affected by intrauterine exposure to the diabetic milieu and may impact skeletal muscle insulin sensitivity later in life. We demonstrated that WJ-MSCs can be induced in vitro to differentiate into myocyte lineage. Such myogenic induction was associated with increased PGC-1α expression, as well as increased mtDNA abundance.
Mitochondrial biogenesis and remodeling upon differentiation of stem cells into myocytes are critical to meet the increased energy demand [9,28]. Epigenetic programming is a critical process during fetal development and stem cells differentiation [1,30]. We found that inhibition of DNA methylation by AZA decreased PGC-1α promoter methylation in WJ-MSCs, concomitant with increased PGC-1α expression and mtDNA content, which was abolished in the presence of AMPK inhibitor Compound C. This suggests that DNA methylation plays a key role in inhibiting PGC-1α expression and mitochondrial content in WJ-MSCs, and restoration of such inhibition requires AMPK. Increased PGC-1α promoter methylation is observed in skeletal muscle of human subjects exposed to an unfavorable intrauterine environment, including maternal high-fat diet [31,32].
As DNA methylation can be retained throughout life, the decreased expression of PGC-1α in skeletal muscle of adult offspring of women with GDM as reported by Kelstrup et al. [29] can be explained by increased PGC-1α DNA methylation as a result of fetal exposure to the diabetic milieu during pregnancy. However, Kelstrup et al. did not find a direct association between PGC-1α expression and promoter methylation in skeletal muscle [29]. Mixed cell types and the epigenetic heterogeneity in the muscle samples may explain their inability to make this association [33]. Cell type-specific DNA methylation and gene expression analysis warrant future studies.
As an energy sensor, AMPK has been widely studied in metabolic active tissues, such as liver and muscle [13,14,34]. Much less is known about the roles of AMPK in fetal programming of metabolic diseases. AMPK activity during gestation is suppressed by maternal overnutrition, including obesity and diabetes [7,11,12,19]. We showed that pharmaceutical treatments, including metformin and AICAR, can activate AMPK in WJ-MSCs from women with diabetes. Increasing evidence demonstrates that AMPK plays a critical role in epigenetic regulations, including DNA methylation and histone modification [12,15–17]. We demonstrated that AMPK activators significantly reduced PGC-1α promoter methylation, which was associated with increased PGC-1α mRNA expression in diabetic WJ-MSCs.
This is consistent with our previous findings on human placenta [12] and vascular cells [17]. Of note, AMPK activation-induced PGC-1α promoter demethylation in WJ-MSCs of diabetic patients persisted after induction of myogenesis. We further demonstrated that preconditioning of WJ-MSCs with AMPK activators program WJ-MSCs to increased myogenic differentiation capacity and enhanced mitochondrial function. Mitochondria and energy management play crucial roles in myogenic stem cell fate and function [9,28]. In particular, increased oxidative metabolism enhances myogenic capabilities and skeletal muscle insulin sensitivity [35]. Thus, early-life AMPK activation has the potential to restore the altered PGC-1α/mitochondrial programming due to intrauterine exposure to maternal diabetes.
The strengths of this study include using human WJ-MSCs from individuals with diabetes during pregnancy, demonstration of epigenetic regulation of PGC-1α/mitochondrial content, and providing AMPK as a promising early therapeutic target for offspring metabolic disease prevention. Limitation includes small patient sample size. The main findings of AMPK epigenetic effects were consistent in WJ-MSCs from three individuals with diabetes during pregnancy. However, larger scale studies are needed to determine potential subject-specific responses to AMPK activators.
In conclusion, this study revealed that epigenetics plays a role in regulating PGC-1α/mitochondrial content and transient AMPK activation in stem cells can result in lasting epigenetic changes in PGC-1α, enhancing myogenic differentiation capacity and mitochondrial function. AMPK activity is suppressed by maternal overnutrition, including obesity and diabetes [7,11,12,19]. Thus, restoration of AMPK activity in maternal overnutrition during early life may have the potential to “reprogram” PGC-1α/mitochondria signaling to attenuate the long-term consequences of adverse maternal environment on offspring.
Acknowledgments
Collection of human WJ-MSCs are supported by NIH K23 award (PI: J. Tryggestad-K23DK106533). The XFe96 seahorse equipment provided by the Cancer Functional Genomics core was supported partly by the National Institute of General Medical Sciences Grant P20GM103639 and National Cancer Institute Grant P30CA225520 of the National Institutes of Health, Stephenson Cancer Functional Genomics Core, the University of Oklahoma. We thank the Institutional Research Core Facility at OUHSC for providing the Flow Cytometry service. We thank Dr. Steven D Chernausek for help and comments.
Author Disclosure Statement
The authors have no conflict of interests to disclose.
Funding Information
This study is supported by OK INBRE (5P20GM103447; Research project PI: S. Jiang) and the CMRI Metabolic Research Program in section of Pediatric Endocrinology and Diabetes, the University of Oklahoma.
References
- 1. Todtenhaupt P, van Pel M, Roest AAW and Heijmans BT. (2022). Mesenchymal stromal cells as a tool to unravel the developmental origins of disease. Trends Endocrinol Metab 33:614–627. [DOI] [PubMed] [Google Scholar]
- 2. Batsali AK, Kastrinaki MC, Papadaki HA and Pontikoglou C. (2013). Mesenchymal stem cells derived from Wharton's Jelly of the umbilical cord: biological properties and emerging clinical applications. Curr Stem Cell Res Ther 8:144–155. [DOI] [PubMed] [Google Scholar]
- 3. Baker PR, 2nd, Patinkin ZW, Shapiro ALB, de la Houssaye BA, Janssen RC, Vanderlinden LA, Dabelea D and Friedman JE. (2017). Altered gene expression and metabolism in fetal umbilical cord mesenchymal stem cells correspond with differences in 5-month-old infant adiposity gain. Sci Rep 7:18095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erickson ML, Patinkin ZW, Duensing AM, Dabelea D, Redman LM and Boyle KE. (2021). Maternal metabolic health drives mesenchymal stem cell metabolism and infant fat mass at birth. JCI Insight 6:e146606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyle KE, Patinkin ZW, Shapiro AL, Baker PR, 2nd, Dabelea D and Friedman JE. (2016). Mesenchymal stem cells from infants born to obese mothers exhibit greater potential for adipogenesis: the Healthy Start BabyBUMP Project. Diabetes 65:647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wajid N, Naseem R, Anwar SS, Awan SJ, Ali M, Javed S and Ali F. (2015). The effect of gestational diabetes on proliferation capacity and viability of human umbilical cord-derived stromal cells. Cell Tissue Bank 16:389–397. [DOI] [PubMed] [Google Scholar]
- 7. Boyle KE, Patinkin ZW, Shapiro ALB, Bader C, Vanderlinden L, Kechris K, Janssen RC, Ford RJ, Smith BK, et al. (2017). Maternal obesity alters fatty acid oxidation, AMPK activity, and associated DNA methylation in mesenchymal stem cells from human infants. Mol Metab 6:1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atlasi Y and Stunnenberg HG. (2017). The interplay of epigenetic marks during stem cell differentiation and development. Nat Rev Genet 18:643–658. [DOI] [PubMed] [Google Scholar]
- 9. Bhattacharya D and Scime A. (2020). Mitochondrial function in muscle stem cell fates. Front Cell Dev Biol 8:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Badraiq H, Cvoro A, Galleu A, Simon M, Miere C, Hobbs C, Schulz R, Siow R, Dazzi F and Ilic D. (2017). Effects of maternal obesity on Wharton's Jelly mesenchymal stromal cells. Sci Rep 7:17595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim J, Piao Y, Pak YK, Chung D, Han YM, Hong JS, Jun EJ, Shim JY, Choi J and Kim CJ. (2015). Umbilical cord mesenchymal stromal cells affected by gestational diabetes mellitus display premature aging and mitochondrial dysfunction. Stem Cells Dev 24:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang S, Teague AM, Tryggestad JB, Jensen ME and Chernausek SD. (2020). Role of metformin in epigenetic regulation of placental mitochondrial biogenesis in maternal diabetes. Sci Rep 10:8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Neill HM, Holloway GP and Steinberg GR. (2013). AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity. Mol Cell Endocrinol 366:135–151. [DOI] [PubMed] [Google Scholar]
- 14. Hardie DG. (2011). AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 25:1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gongol B, Sari I, Bryant T, Rosete G and Marin T. (2018). AMPK: an epigenetic landscape modulator. Int J Mol Sci 19:3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kusuyama J, Alves-Wagner AB, Conlin RH, Makarewicz NS, Albertson BG, Prince NB, Kobayashi S, Kozuka C, Moller M, et al. (2021). Placental superoxide dismutase 3 mediates benefits of maternal exercise on offspring health. Cell Metab 33:939–956 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marin TL, Gongol B, Zhang F, Martin M, Johnson DA, Xiao H, Wang Y, Subramaniam S, Chien S and Shyy JY. (2017). AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci Signal 10:eaaf7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Q, Liang X, Sun X, Zhang L, Fu X, Rogers CJ, Berim A, Zhang S, Wang S, et al. (2016). AMPK/alpha-ketoglutarate axis dynamically mediates DNA demethylation in the Prdm16 promoter and brown adipogenesis. Cell Metab 24:542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tryggestad JB, Vishwanath A, Jiang S, Mallappa A, Teague AM, Takahashi Y, Thompson DM and Chernausek SD. (2016). Influence of gestational diabetes mellitus on human umbilical vein endothelial cell miRNA. Clin Sci (Lond) 130:1955–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goyal U, Jaiswal C and Ta M. (2018). Isolation and establishment of mesenchymal stem cells from Wharton's Jelly of human umbilical cord. Bio Protoc 8:e2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. International Society for Cellular Therapy position statement. Cytotherapy 8:315–317. [DOI] [PubMed] [Google Scholar]
- 22. Li H, Choudhary SK, Milner DJ, Munir MI, Kuisk IR and Capetanaki Y. (1994). Inhibition of desmin expression blocks myoblast fusion and interferes with the myogenic regulators MyoD and myogenin. J Cell Biol 124:827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fetita LS, Sobngwi E, Serradas P, Calvo F and Gautier JF. (2006). Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab 91:3718–3724. [DOI] [PubMed] [Google Scholar]
- 24. Dabelea D and Crume T. (2011). Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes 60:1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER and Clausen TD. (2016). Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia 59:1396–1399. [DOI] [PubMed] [Google Scholar]
- 26. Friedman JE. (2018). Developmental programming of obesity and diabetes in mouse, monkey, and man in 2018: where are we headed? Diabetes 67:2137–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanli E and Kabaran S. (2019). Maternal obesity, maternal overnutrition and fetal programming: effects of epigenetic mechanisms on the development of metabolic disorders. Curr Genomics 20:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wagatsuma A and Sakuma K. (2013). Mitochondria as a potential regulator of myogenesis. Sci World J 2013:593267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelstrup L, Hjort L, Houshmand-Oeregaard A, Clausen TD, Hansen NS, Broholm C, Borch-Johnsen L, Mathiesen ER, Vaag AA and Damm P. (2016). Gene expression and DNA methylation of PPARGC1A in muscle and adipose tissue from adult offspring of women with diabetes in pregnancy. Diabetes 65:2900–2910. [DOI] [PubMed] [Google Scholar]
- 30. Wu H and Sun YE. (2006). Epigenetic regulation of stem cell differentiation. Pediatr Res 59:21R–25R. [DOI] [PubMed] [Google Scholar]
- 31. Brons C, Jacobsen S, Nilsson E, Ronn T, Jensen CB, Storgaard H, Poulsen P, Groop L, Ling C, Astrup A and Vaag A. (2010). Deoxyribonucleic acid methylation and gene expression of PPARGC1A in human muscle is influenced by high-fat overfeeding in a birth-weight-dependent manner. J Clin Endocrinol Metab 95:3048–3056. [DOI] [PubMed] [Google Scholar]
- 32. Laker RC, Lillard TS, Okutsu M, Zhang M, Hoehn KL, Connelly JJ and Yan Z. (2014). Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1alpha gene and age-dependent metabolic dysfunction in the offspring. Diabetes 63:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bengtsen M, Winje IM, Eftestol E, Landskron J, Sun C, Nygard K, Domanska D, Millay DP, Meza-Zepeda LA and Gundersen K. (2021). Comparing the epigenetic landscape in myonuclei purified with a PCM1 antibody from a fast/glycolytic and a slow/oxidative muscle. PLoS Genet 17:e1009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herzig S and Shaw RJ. (2018). AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19:121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Belli R, Bonato A, De Angelis L, Mirabilii S, Ricciardi MR, Tafuri A, Molfino A, Gorini S, Leigheb M, et al. (2019). Metabolic reprogramming promotes myogenesis during aging. Front Physiol 10:897. [DOI] [PMC free article] [PubMed] [Google Scholar]