Summary
Background
COVID-19 has exacerbated existing ethnic inequalities in health. Little is known about whether inequalities in severe disease and deaths, observed globally among minoritised ethnic groups, relates to greater infection risk, poorer prognosis, or both. We analysed global data on COVID-19 clinical outcomes examining inequalities between people from minoritised ethnic groups compared to the ethnic majority group.
Methods
Databases (MEDLINE, EMBASE, EMCARE, CINAHL, Cochrane Library) were searched from 1st December 2019 to 3rd October 2022, for studies reporting original clinical data for COVID-19 outcomes disaggregated by ethnicity: infection, hospitalisation, intensive care unit (ICU) admission, and mortality. We assessed inequalities in incidence and prognosis using random-effects meta-analyses, with Grading of Recommendations Assessment, Development, and Evaluation (GRADE) use to assess certainty of findings. Meta-regressions explored the impact of region and time-frame (vaccine roll-out) on heterogeneity. PROSPERO: CRD42021284981.
Findings
77 studies comprising over 200,000,000 participants were included. Compared with White majority populations, we observed an increased risk of testing positive for infection for people from Black (adjusted Risk Ratio [aRR]:1.78, 95% CI:1.59–1.99, I2 = 99.1), South Asian (aRR:3.00, 95% CI:1.59–5.66, I2 = 99.1), Mixed (aRR:1.64, 95% CI:1.02–1.67, I2 = 93.2) and Other ethnic groups (aRR:1.36, 95% CI:1.01–1.82, I2 = 85.6). Black, Hispanic, and South Asian people were more likely to be seropositive. Among population-based studies, Black and Hispanic ethnic groups and Indigenous peoples had an increased risk of hospitalisation; Black, Hispanic, South Asian, East Asian and Mixed ethnic groups and Indigenous peoples had an increased risk of ICU admission. Mortality risk was increased for Hispanic, Mixed, and Indigenous groups. Smaller differences were seen for prognosis following infection. Following hospitalisation, South Asian, East Asian, Black and Mixed ethnic groups had an increased risk of ICU admission, and mortality risk was greater in Mixed ethnic groups. Certainty of evidence ranged from very low to moderate.
Interpretation
Our study suggests that systematic ethnic inequalities in COVID-19 health outcomes exist, with large differences in exposure risk and some differences in prognosis following hospitalisation. Response and recovery interventions must focus on tackling drivers of ethnic inequalities which increase exposure risk and vulnerabilities to severe disease, including structural racism and racial discrimination.
Funding
ESRC:ES/W000849/1.
Keywords: Meta-analysis, Ethnicity, COVID-19, SARS-CoV-2, Systematic review, Prognosis
Research in context.
Evidence before this study
We searched PROSPERO for existing systematic reviews with ‘ethnic’ or ‘race’ in the title, from 1st December 2019 to 30th August 2022. Previous systematic reviews, conducted early in the pandemic, synthesised emerging evidence primarily from the United Kingdom (UK) and United States of America (USA), identifying an increased risk of infection, severe disease, and death amongst minoritised ethnic groups. However, it is not known whether ethnic inequalities in severe disease and deaths, seen in many countries, relates to greater infection risk, poorer prognosis, or both. Broad ethnic groups described in previous meta-analyses may have also obscured heterogeneity in risks of infection, severe disease and death between different minoritised ethnic groups.
Added value of this study
We searched MEDLINE, EMBASE, EMCARE, CINAHL, and the Cochrane Library from 1st December 2019 to 3rd October 2022. We assessed inequalities in both incidence and prognosis by separating population-based studies from studies including only COVID-19 cases. Over 200 million participants were included from 77 studies. Compared with White majority groups, we identified an increased risk of testing positive for infection for Black, South Asian, Mixed, and Other ethnic groups; an increased risk of infection for Hispanic people was observed only in seroprevalence studies. In population-based studies, Black and Hispanic ethnic groups and Indigenous peoples had an increased risk of hospitalisation; most minoritised ethnic groups and Indigenous peoples had an increased risk of ICU admission; and Hispanic, Mixed, and Indigenous groups had an increased risk of mortality. Following hospitalisation, South Asian, East Asian, Black and Mixed ethnic groups had an increased risk of ICU admission, and the risk of mortality was greater in Mixed ethnic groups.
Implications of all the available evidence
We present the most comprehensive summary of ethnic inequalities in a range of outcomes relating to COVID-19, during the first few years of the pandemic, before widespread immunity. We demonstrate the presence of systematic ethnic inequalities relating to both COVID-19 infection and severe disease, but to varying extents across minoritised ethnic groups. In particular, we observed large differences in infection risk for minoritised ethnic groups. Our findings highlight the need for policy interventions to address ethnic inequalities in exposure to the virus. Ethnic inequalities in prognosis following hospitalisation were also noted, which may reflect poorer healthcare quality for minoritised ethnic groups or differential vulnerability to severe disease. Our findings are of vital public health importance and should inform strategic response and recovery policies.
Introduction
Minoritised ethnic groups have suffered disproportionately from the COVID-19 pandemic, with higher rates of SARS-CoV-2 infection, hospitalisation, intensive care unit (ICU) admission, and death, compared to the ethnic majority group in a given population.1, 2, 3, 4, 5, 6, 7 It is not known whether the inequalities in severe disease and deaths, seen in many countries among minoritised groups, relates to greater infection risk, poorer prognosis, or both.8, 9, 10, 11, 12
Previous systematic reviews conducted early in the pandemic have demonstrated and quantified some of these risks.13, 14, 15, 16, 17 However, a new review is warranted for several reasons: first, seroprevalence studies were lacking despite providing the best estimates of infection; second, broad ethnic categorisations were analysed and may hide important heterogeneity in risks between different ethnic groups; and third, now that COVID-19 has spread across the world and remains endemic in many areas, a much larger body of literature is available to address these issues.13, 14, 15, 16, 17
We therefore sought to perform a systematic review and meta-analysis examining clinical outcomes in COVID-19, comparing people from minoritised ethnic groups to the ethnic majority group, incorporating data from lower- and middle-income countries that were not available in previous meta-analyses.
Methods
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,18 and was registered with PROSPERO (CRD420212824981). Ethical approval and informed consent of participants were not required for this meta-analysis as no new data were collected.
Search strategy
The search strategy was developed and conducted by an academic librarian (PD), using the databases MEDLINE, EMBASE, EMCARE, CINAHL, and the Cochrane Library (supplementary materials: search strategy). Peer-reviewed publications were searched for between 1st December 2019 and 3rd October 2022.
Eligibility criteria
The relevant outcomes were SARS-CoV-2 infection (laboratory confirmed infection through polymerase chain reaction [PCR] or evidence of previous infection through laboratory confirmed IgG/IgM for SARS-CoV-2 [seroprevalence]), hospitalisation, ICU admission, and mortality. Studies were eligible if they reported original clinical data (cross-sectional, case–control, cohort studies) on the relevant outcomes, disaggregated by ethnicity or closely related indicators of ethnicity (i.e., Indigenous status, race, country of birth, migrant status) (see Table S1 for detailed inclusion and exclusion criteria). Although we did not set out to make comparisons by country of birth or migrant status, these indicators were included as ethnicity data are not collected in some countries.19 Population-based studies (individuals with and without confirmed SARS-CoV-2 infection) were eligible, as well as studies which examined prognosis (individuals with confirmed SARS-CoV-2 infection only). Ecological studies, prognostic modelling studies, animal studies, qualitative studies, and pre-prints, were excluded. Studies were also excluded if participants were recruited based on a specific physical or mental health condition or healthcare utilisation (other than for COVID-19), as were studies specifically in children (under the age of 16) and religious groups.
Study selection
Two reviewers independently screened titles, abstracts, and full texts (PI assessing all articles, with DK, HT, SVK, LB, DP and SS each assessing a proportion). Where there were disagreements in title and abstract screening, articles identified as potentially relevant by one reviewer were included for full text screening. Disagreements in full texts were resolved by discussion or consultation with the review team. The software, Covidence,20 was used for screening following automatic de-duplication. To minimise the inclusion of duplicate data (i.e., participants from the same population assessing the same outcome), predefined criteria were used to determine which dataset to include (supplementary materials: criteria to minimise inclusion of duplicate data).
Data extraction
One reviewer (PI) completed 100% of the data extraction from each eligible article, all of which were independently checked by additional reviewers (DK, HT, DP, SS). Data on study and participant characteristics, ethnicity, outcomes, and covariates, were extracted to an Excel file. Ethnicity measures were extracted and evaluated to determine the comparison group for meta-analyses. When describing ethnic groups, we have used terminology from the included studies. Where US studies reported both race and ethnicity, we chose the categories that were most amenable to meta-analysis (i.e., comparable to other studies).
Risk of bias and conceptualisation of ethnicity
Two reviewers (PI completing 100% and DK, HT, LB, DP and SS a proportion) independently assessed the risk of bias of each included study, using an adapted Joanna Briggs Institute (JBI) tool21 (supplementary materials: adapted JBI critical appraisal tool). Scores were calculated as a total out of the maximum number of applicable questions, then standardised. Studies with a score of 80–100% were considered low risk of bias, 60–79% medium risk of bias, and 0–59% high risk of bias. One item from the JBI, regarding the description of study participants, was adapted to critically evaluate how ethnicity was conceptualised and measured. Studies which measured ethnicity through self-report or another reliable indicator (e.g., country of birth registered at birth or migration) and used disaggregated descriptions of ethnicity scored positively on this item of the risk of bias tool. No studies were excluded based on critical appraisal scores.
Data synthesis
Meta-analysis
To determine ethnic inequalities in COVID-19 health outcomes, we prioritised extracting age- and sex-adjusted results which are important confounders of ethnic inequalities,22 but considered other variables to be likely mediators and therefore should not be adjusted for.8 We contacted authors to request data, if unavailable (supplementary material: data manipulation methods). Unadjusted or over-adjusted models were extracted if age- and sex-adjusted models were not available (highlighted in forest plots and reported in Table 1).
Table 1.
Description of studies by outcome, period of data collection, comorbidities considered, covariates adjusted for, study quality, and sample sizes for the ethnic majority group and minoritised ethnic groups.
| First author | Outcomes | Country | Period of data collection | Comorbidities considered | Covariates adjusted for | Study quality % | Majority N | Minority N |
|---|---|---|---|---|---|---|---|---|
| High-income countries | ||||||||
| Song | Hospital admission; ICU admission | USA | 1st Mar–10th Aug 20 | Respiratory failure, myocardial infarction, stroke, pulmonary hypertension, embolism, thrombosis, acute renal failure | N/A | 50% | 465,565 (White) | 182,637 |
| Acosta | Mortality | USA | 1st Mar 20–28th Feb 21 | NR | Age | 88% | 63,981 (White) | 79,361 |
| Adeji | Mortality | USA | 1st Jul 21–30th Jun 22 | NR | N/A | 81% | 207,926 (White) | 80,218 |
| Lindsay | Seropositivity | USA | 20th Feb–10th Jul 20 | Charlson Comorbidity Index | N/A | 75% | 1,092,652 (White) | 269,941 |
| Luo | Mortality | USA | 4th Mar–23rd Jun 20 | Cardiovascular disease, obesity, diabetes | N/A | 25% | 7,300,959 (White) | 3,321,251 |
| Metra | ICU admission | USA | 20th Jan–30th Sep 20 | Hypertension, diabetes, obesity, COPD, cerebral infarction, systemic connective tissue disorders, reduced mobility, pregnancy, neoplasm, nicotine dependence | Age, sex, diabetes, hypertension, obesity, homelessness, neoplasms | 50% | 157,049 (White) | 50,376 |
| Zerbo | Hospital admission; ICU admission; Mortality | USA | 1st Nov 20–27th Jul 21 | BMI, diabetes, hypertension, renal disease, asthma, heart disease, COPD, pneumonia, cerebral infarction, Alzheimers, Parkinsons, movement disorders, demyelinating disorders, epilepsy | Age, comorbidities | 75% | 1,891,899 (White) | 1,300,923 |
| Bennett | Hospital admission; Mortality | USA | 1st Jan 20–7th Dec 20 | Diabetes, renal disease, heart failure, chronic pulmonary disease, peripheral vascular disease, stroke, cancer, dementia, myocardial infarction, liver disease, rheumatologic disease, hemiplegia, peptic ulcer disase | N/A | 69% | 1,251,401 (White) | 675,298 |
| Chang | Infection; Hospital admission | USA | 1st Jan 20–31st Dec 20 | 27 conditions | N/A | 88% | 23,769,184 (White) | 7,859,910 |
| Egede | ICU admission | USA | 1st Mar 20–10th Jul 20 | Comorbidity count | N/A | 81% | 23,788 (White) | 7761 |
| Feldman | Mortality | USA | 1st Jan 20–24th Feb 21 | NR | Age | 69% | 227,532 (White) | 148,593 |
| Ioannou | Infection | USA | 1st Feb 20–31st Mar 21 | Charlson Comorbidity Index | Age, sex, region, location, BMI, comorbidities | 93% | 5,887,349 (White) | 3,240,324 |
| Jones | Seroprevalence | USA | 1st Jul 20–31st May 21 | NR | N/A | 63% | 1,226,745 (White) | 216,774 |
| Young | Hospital admission | USA | 1st Jan 20–31st Dec 20 | Presence of comorbidities | Age, sex, service, rank, education, occupation, region, comorbidities | 93% | 374,525 (White | 622,726 |
| Thomas 1 | ICU admission; Mortality | UK | 1st Mar 20–31st May 20 | NR | N/A | 81% | 13,092 (White) | 697 |
| Gray | Mortality | UK | 1st Mar 20–31st Mar 21 | Charlson Comorbidity Index | Age, sex, deprivation, comorbidities | 93% | 359,160 (White) | 15,084 |
| Knight | Hospital admission | UK | 1st Jan 20–7th Dec 20 | Number of diagnoses | N/A | 93% | 36,131,134 (White) | 8,833,352 |
| Thomas 2 | Infection | UK | 21st Nov 20–22nd Dec 20 | NR | Age, deprivation, catchment area, smoking status, place of work, key worker | 88% | 2729 (White British or Irish) | 88 |
| Martin | Infection | UK | Dec 20–Mar 21 | Diabetes, immunosuppressed | Age, sex, migration, religiosity, deprivation, household factors | 93% | 7583 (White) | 8276 |
| Talaei | Seroprevalence | UK | 1st May 20–2nd Nov 20 | Arterial disease, asthma, autoimmune disease, cancer, COPD, diabetes, heart disease, hypertension, immunodeficiency, kidney disease, major neurological conditions | Age, sex | 93% | 10,651 (White) | 479 |
| Mathur | Infection; Hospital admission; ICU admission; Mortality | UK | 1st Feb 20 to 31st Dec 20 | Hypertension, asthma, respiratory disease, heart disease, diabetes, cancer, liver disease, stroke, dementia, neurological disease, kidney disease, immunosuppression | Age, sex | 100% | 10,877,978 (White) | 6,410,554 |
| Hippisley Cox | Hospital admission; Mortality | UK | 8th Dec 20–15th Jun 21 | Kidney disease, chemotherapy, diabetes, other health conditions | Age, sex, BMI, vaccination dose, background infection rate | 75% | 4,781,050 (White) | 903,979 |
| Ward | Seropositivity | UK | 20th Jun 20–13th July 20 | NR | Age, sex, region | 94% | 92,737 (White) | 6667 |
| Farrell | ICU admission; Mortality | Ireland | 13th Mar 20–1st May 20 | Charlson Comorbidity Index | Age | 69% | 208 (White Irish and White Other) | 49 |
| Allen | Seroprevalence | Ireland | 19th Apr 21–28th Apr 21 | NR | N/A | 81% | 3798 (White Irish) | 1287 |
| Chu | Hospital admission | Canada | 1st Jen 20–30th Sep 20 | Charlson Comorbidity Index | N/A | 75% | 42,547 (General) | 4645 |
| Saeed | Seropositivity | Canada | 9th May 20–21st Jul 20 | NR | Age, sex, region, deprivation, blood type | 81% | 52,852 (White) | 10,695 |
| Passos-Castilho | Hospital admission | Canada | 1st Jan 20–30th Sep 20 | Charlson Comorbidity Index | N/A | 88% | 42,547 (Canadian born) | 4645 |
| Islamoska | Hospital admission | Denmark | 1st Feb 20–30th Jun 20 | Charlson Comorbidity Index | Age, sex | 88% | 433,539 (Danish) | 60,114 |
| Guijarro | Infection | Spain | 1st Jan 20–14th Mar 20 | NR | Age, sex | 100% | 131,599 (Spain) | 20,419 |
| Ramos-Rincon | ICU admission; Mortality | Spain | 1st Mar 20–31st Dec 21 | Charlson Comorbidity Index | N/A | 81% | 20,599 (European) | 3354 |
| Rostila | Mortality | Sweden | 31st Jan 20–4th May 20 | NR | Age, sex | 94% | 11,232,511 (Sweden) | 541,119 |
| Nwaru | Hospital admission; ICU admission | Sweden | 1st Jan 20–28th Feb 21 | Hypertension, diabetes, obesity, stroke, pneumonia, COPD, asthma, psychiatric conditions | N/A | 88% | 266,848 (Swedish born) | 59,204 |
| Stralin | ICU admission; Mortality | Sweden | 1st Mar 20–30th Sep 20 | Hypertension, diabetes, chronic lung disease, cancer, ischaemic disease, neuromuscular disease, stroke, obesity, dementia | Age, sex, comorbidities, care dependency, healthcare region | 88% | 9973 (Sweden) | 6243 |
| Gustafsson | Hospital admission; Mortality | Sweden | 4th Apr 20–14th Sep 20 | Charlson Comorbidity Index | Age, sex, comorbidities, housing, family structure, civil status, region, education, income | 100% | 52,201 (Sweden) | 20,527 |
| Consolazio | Infection | Italy | 20th Feb 20–3rd May 20 | NR | Age, sex, comorbidities | 88% | 2,856,202 (Italy) | 469,473 |
| Lombardi | Seropositivity | Italy | 27th Apr 20–12th Jun 20 | Hypertension, diabetes, cardiac, respiratory, renal chronic diseases | Age, sex, occupation, frontline area, BMI, smoking | 81% | 3869 (Italian) | 186 |
| Fabiani | Hospital admission; ICU admission; Mortality | Italy | 20th Feb 20–19th Jul 20 | Oncologic, cardiovascular, respiratory, diabetes/metabolic diseases | Age, sex, geographical macro-area of diagnosis, level of urbanisation of place of residence, comorbidities, calendar period of diagnosis, random effect due to regional contextual differences | 88% | 197,206 (Italian) | 15,974 |
| Cacciani | Hospital admission; ICU admission | Italy | 22nd Feb 21–2nd Jul 21 | NR | N/A | 88% | 45,580 (Italian) | 2513 |
| DiGirolamo | Mortality | Italy | 22nd Feb 21–16th Jul 21 | NR | N/A | 81% | 34,370,041 (Italian) | 4,006,808 |
| Pagani | Seroprevalence | Italy | 23rd Dec 20–19th Feb 21 | NR | N/A | 88% | 1572 (Italian) | 472 |
| Coyer 1 | Hospital admission | Netherlands | 29th Feb 20–31st May 20 | NR | Age | 88% | 386,521 (Ethnic Dutch) | 486,534 |
| Collard | Mortality | Netherlands | 18th Feb 20–30th Jan 21 | Hypertension, asthma/COPD, chronic kidney disease, diabetes, malignancy, chronic cardiac disase, obesity, BMI | N/A | 69% | 763 (Dutch) | 415 |
| Coyer 2 | Seropositivity | Netherlands | 24th Jun 20–9th Oct 20 | Diabetes, high blood pressure | N/A | 88% | 503 (Dutch) | 1994 |
| Vos | Seropositivity | Netherlands | 31st Mar 20–11th May 20 | NR | Weighted to population | 88% | 2306 (Dutch) | 331 |
| Indseth | Infection; Hospital admission; Mortality | Norway | 1st Mar 20–18th Oct 20 | NR | N/A | 81% | 450,801 (Non-Immigrants) | 869,442 |
| Labberton | Infection; Hospital admission | Norway | 15th Jun 20–21st Mar 21 | 14 medical conditions | N/A | 100% | 53,890 (Norwegian) | 28,642 |
| Jefferies | Infection | New Zealand | 2nd Feb 20–13th May 20 | NR | N/A | 69% | 1091 (European) | 412 |
| Ishii | Infection | Japan | 16th Jul 20 – NR | NR | N/A | 75% | 3242 (Japanese) | 298 |
| Saidel Odes | Infection | Israel | 8th Sep 20–31st Dec 20 | NR | Age, sex | 81% | 6381 (Jewish) | 2137 |
| Al Awaidy | Hospital admission; Mortality | Oman | 14th Feb 20–23rd Jul 20 | Hypertension, cardiovascular disease, lung disease | N/A | 75% | 40,859 (Omani) | 28,523 |
| Al Abri | Seropositivity | Oman | 1st Jul 20–30th Nov 20 | Presence of comorbidities | Selection bias | 81% | 11,582 (Omani) | 5875 |
| Abu Ruz | Mortality | United Arab Emirates | 1st Mar 20–30th May 20 | Diabetes, hypertension, anaemia, vitamin D deficiency, dyslipidaemia, chronic kidney disease, asthma, cancer, COPD | N/A | 75% | 486 (Middle Eastern) | 2810 |
| Al Zahmi | ICU admission | United Arab Emirates | 26th Feb 20–31st May 20 | Cardiac disease, hypertension, chronic lung disease, asthma, chronic kidney disease, diabetes, cancer, immunosuppression, HIV, medication | N/A | 75% | 73 (Emirati) | 4,87 |
| Hamadah | ICU admission; Mortality | Kuwait | 24th Feb 20–20th Apr 20 | Diabetes, hypertension, cardiovascular, asthma, cerebrovascular, hepatitis, cancer, hypothyroidism, renal disease, immunodeficiency, recent surgery | Age, smoking, comorbidities | 88% | 294 (Kuwait) | 829 |
| Al Kuwari | Infection | Qatar | 18th Feb 20–18th Apr 20 | Hypertension, diabetes, cardiovascular disease, lung disease, kidney disease, malignancy, tuberculosis, liver disease, immunodeficiency | N/A | 88% | 497 (Qatari National) | 5188 |
| Shaikh | ICU admission; Mortality | Saudi Arabia | 1st May 20–1st Aug 20 | Hypertension, obesity, diabetes, cardiovascular disease, respiratory diseases, malignancies, other chronic conditions | Age, BMI, comorbidities, ICU admission (for mortality only) | 69% | 131 (Saudi National) | 434 |
| Nasif | Mortality | Saudi Arabia | Nov 20–Jun 21 | NR | N/A | 50% | 953 (Saudi National) | 1664 |
| Low- and middle-income countries | ||||||||
| Horta | Seropositivity | Brazil | 14th May 21–24th Jun 20 | NR | Age, sex | 100% | 2064 (White) | 32,383 |
| Da Silva | Infection | Brazil | 27th Oct 20–11th Dec 20 | NR | N/A | 56% | 2945 (White) | 3039 |
| Rodrigues | Mortality | Brazil | 22nd Feb 20–10th May 21 | Cardiovascular disease, diabetes, haematological disease, down syndrome, obesity, pulmonary disease, liver disease, renal disease, neurological disease | N/A | 50% | 435,144 (White) | 405,057 |
| Sansone | Mortality | Brazil | 22nd Feb 20–4th Apr 21 | Cardiopathy, haematological disorder, down syndrome, Hepatic disorder, asthma, diabetes, neurological disorder, chronic respiratory disease, immunosuppressive disorder, renal disease, obesity, other comorbidities | N/A | 88% | 309,646 (White) | 276,009 |
| Silva | ICU admission | Brazil | 26th Feb 20–9th Oct 20 | Obesity, cardiovascular disease | Age | 50% | 73,464 (White) | 86,399 |
| Ibarra-Nava | ICU admission | Mexico | 28th Feb 20–3rd Aug 20 | Diabetes, COPD, high blood pressure, chronic kidney disease | N/A | 94% | 412,368 (Non-Indigenous) | 3487 |
| Servan-Mori | Hospital admission; Mortality | Mexico | 1st Mar 20–28th Feb 21 | Diabetes, obesity, hypertension, COPD, asthma, health problems during pregnancy | Age, sex, comorbidities, timing of presentation to care, social deprivation | 88% | 787,856 (Non-Indigenous) | 8022 |
| Bojorquez-Chapela | Seroprevalence | Mexico | 1st Nov 20–30th Apr 21 | NR | N/A | 75% | 121 (Mexican-born) | 227 |
| Dahal | Mortality | Mexico | 19th Feb 20–25th Mar 22 | Pneumonia, diabetes, COPD, asthma, other disease, cardiovascular disease, obesity, chronic kidney failure | Age, sex | 100% | 2,151,140 (Non-Indigenous) | 21,896 |
| Ramli | Infection | Malaysia | 1st Jan 21–31st Oct 21 | Presence of comorbidities | N/A | 56% | 110 (Malay) | 10 |
| Utulu | Infection | Nigeria | Nov 20–Dec 20 | Hypertension, diabetes | N/A | 63% | 157 (Other) | 1337 |
| Cifuentes | Mortality | Colombia | 2nd Mar 20–26th Oct 20 | NR | Age, sex, area of residence, health insurance, socioeconomic status | 81% | 971,078 (White) | 62,140 |
| Concha | Infection | Colombia | 28th Mar 21–26th Apr 21 | NR | Sex, village | 81% | 72 (Colombian) | 380 |
| Sultanoglu | Mortality | Cyprus | 9th Mar 20–4th May 20 | NR | N/A | 13% | 76 (Native) | 32 |
| Sacoto | Mortality | Ecuador | 21st Feb 20–31st Dec 20 | Presence of comorbidities | N/A | 50% | 205,718 (Mestizo) | 46,047 |
| Kadyrova | Infection; Seroprevalence | Kazakhstan | 1st Apr 21–30th May 21 | Presence of comorbidities | N/A | 38% | 55 (Kazakh) | 45 |
| Ikram | Infection | South Africa | NR | NR | N/A | 69% | 8 (White) | 228 |
| Jugwanth | Infection | South Africa | Nov 20–Dec 20 | Diabetes, hypertension, HIV, tuberculosis, chronic kidney disease, heart disease, asthma/COPD, liver disease, cancer, pregnancy | N/A | 44% | 275 (White) | 255 |
| Stead | Infection | South Africa | 1st Jan 20–14th Mar 20 | Diabetes, hypertension, HIV | Sex, education, smoking status, profession, COVID-19 exposure, PPE training, public transport use, BMI, diabetes, hypertension, HIV | 93% | 114 (White) | 1181 |
We conducted meta-analyses on age- and sex adjusted data to determine the risk of each outcome across disaggregated minoritised ethnic groups (guided by the critical appraisal) compared to the White majority. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to evaluate the certainty of the overall evidence for adjusted analyses.23 The overall certainty estimates were categorised as high, moderate, low, or very low. In keeping with GRADE guidance for prognostic studies, observational studies started as high certainty evidence and were rated down for risk of bias, imprecision, inconsistency, indirectness, and publication bias (supplementary materials: GRADE criteria).23
To disentangle ethnic inequalities in risk of testing positive for infection from SARS-CoV-2, from ethnic inequalities in prognosis once infected (i.e., hospital admission, ICU admission, mortality), we separated studies by their denominator in the following way: (1) general population studies including individuals with and without SARS-CoV-2 infection; (2) individuals with confirmed SARS-CoV-2 infection; and (3) individuals hospitalised with COVID-19.
As some studies used country of birth or migrant status as an indicator of ethnicity, and the majority ethnic group was not White in some studies, we conducted unadjusted meta-analyses to identify the risk of each outcome for minoritised ethnic groups (combined within each study) compared with the ethnic majority group, which varied depending on which ethnic group was the majority in the study country. Crude numbers were used to calculate unadjusted risk ratios (RR) for minoritised ethnic groups versus the ethnic majority group. We conceptualised majority groups in terms of power and privilege, and minority if the ethnic or Indigenous group meets one or more of the following criteria: the group is numerically smaller than the rest of the population; it is not in a social, economic, or politically dominant position; and it has a culture, language, religion or ethnicity that is distinct from that of the majority. This guided our decisions when selecting the ethnic majority group (for the unadjusted analyses), as we did not always use the reference group of the study or the statistical majority. This analysis is reported in supplementary materials.
We performed meta-analyses using the DerSimonian and Laird random effects model24 to determine the pooled effect sizes with corresponding 95% Confidence Intervals (CIs). Levels of statistical heterogeneity were determined using the I2 statistic.25 Where there were sufficient data (n > 10), meta-regressions were used to explore whether region (low- and middle-income countries [LMIC]) versus high-income countries [HIC]) and time frame (before widespread vaccine roll-out versus after widespread vaccine roll-out) were drivers of heterogeneity. Publication bias was explored through visual funnel plots, Egger's test of asymmetry for meta-analyses with 10 or more studies.26 All analyses were conducted using Stata SE 15.27
Synthesis without meta-analysis (SWiM)
Where data were not amenable to meta-analyses, for example, where it was not possible to extract or calculate effect sizes, we provide synthesis without meta-analysis (SWiM) in supplementary materials. Effect direction plots and sign tests were conducted to assess evidence of associations. The quantitative synthesis is reported in line with the SWiM guidelines.28
Sensitivity analyses
As several studies reported country of birth, nationality, or migrant status, as an indicator of ethnicity, sensitivity analyses were conducted, replicating the unadjusted analyses with these studies excluded. To further explore differences across regions (LMIC versus HIC), the adjusted meta-analyses were stratified by region, where there were sufficient data (n > 10). Additional sensitivity analyses were conducted, excluding studies with a high risk of bias (as determined using the JBI), to explore the impact on the main findings. The findings of the sensitivity analyses are presented in supplementary materials.
Role of the funding source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All authors had access to the data and critically reviewed and approved the manuscript as submitted.
Results
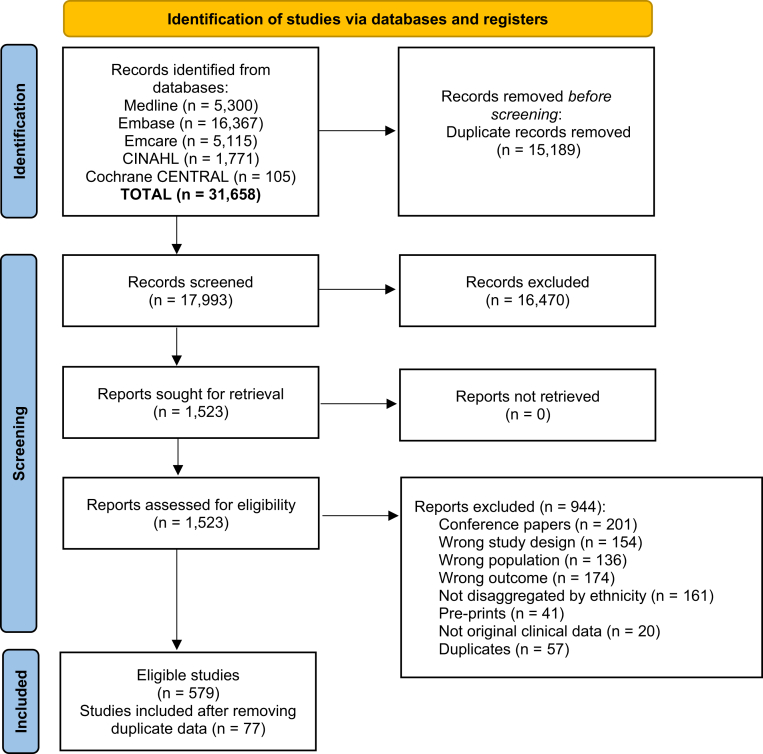
After removing 15,189 duplicates, 17,993 records were screened against eligibility criteria, resulting in 579 eligible studies (Fig. 1). After excluding studies which contained duplicate patient data, 77 studies comprising over 200,000,000 confirmed COVID-19 cases were included.4,29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135 Thirty studies were conducted in Europe (11 in the UK and Ireland), 21 in North America (14 in USA, four in Mexico, three in Canada), eight in South America, 13 in Asia, four in Africa, and one in Oceania (Aotearoa/New Zealand) (Fig. S1). Most study designs were cohort (N = 50, 65%) or cross-sectional (N = 23, 30%) (Table S2). Details of the outcomes, comorbidities, and covariates included in each study are reported in Table 1.
Fig. 1.
PRISMA flow diagram indicating the identification of studies via databases and registers. (∗) unadjusted risk ratio used (adjOR) adjusted odds ratio used (unadjOR) unadjusted odds ratio used. R: Risk Ratio. ROB: Risk of Bias.
The summary of scores for the JBI risk of bias items are presented in supplementary materials (Fig. S2) and the standardised scores for each study are presented in Table S2. Whist an indicator for ethnicity was reported in all 77 studies, only 29 (38%) studies measured ethnicity (or closely related indicators) in a valid and reliable way, and it was unclear how ethnicity was measured in 17 (22%) studies. These 29 studies also disaggregated broad ethnic groups, which we considered important to determine whether there was heterogeneity in COVID-19 outcomes when using a more granular categorisation of minoritised ethnic groups (e.g., South Asian and East Asian, as opposed to an aggregated Asian ethnic group).
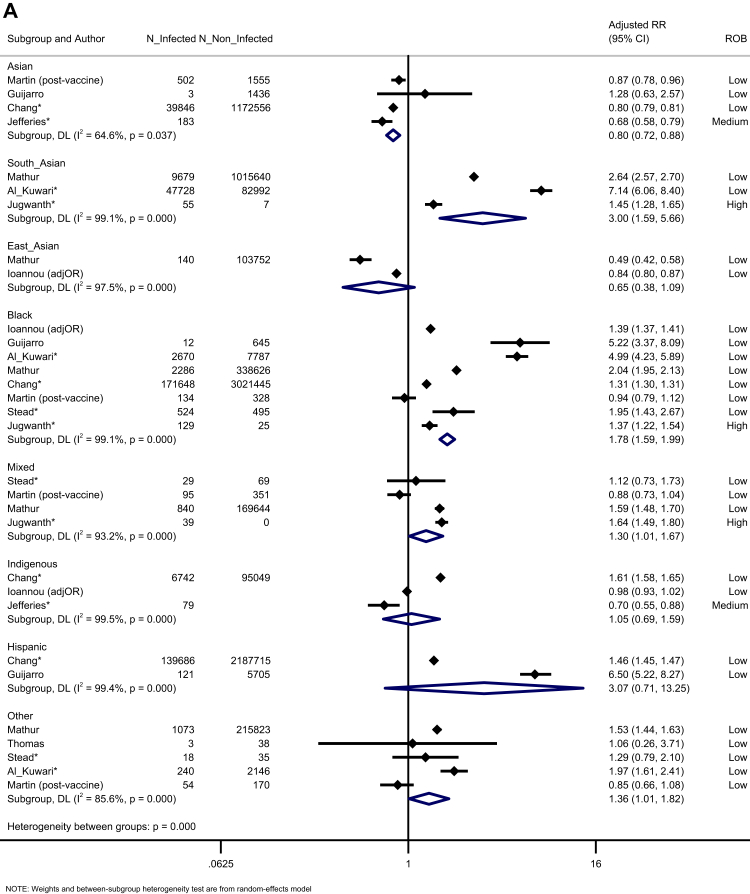
Ten studies reported the risk of testing positive for infection across disaggregated ethnic groups. Compared to White majority people, Black people were more likely to be infected (K = 8, adjusted risk ratio [aRR] = 1.78, 95% CI = 1.59 to 1.99, I2 = 99.1), as were South Asian people (K = 3, aRR = 3.00, 95% CI:1.59 to 5.66, I2 = 99.1), Mixed people (K = 4, aRR = 1.64, 95% CI:1.02 to 1.67, I2 = 93.2), and those from Other ethnic backgrounds (K = 5, aRR = 1.36, 95% CI:1.01 to 1.82, I2 = 85.6). Studies using an aggregated Asian ethnic group showed a reduced likelihood of testing positive. There was no difference in the risk of infection for East Asian, Indigenous, and Hispanic people, compared to the White majority (Fig. 2A). Evidence was rated as moderate certainty (Table S3). Egger's test showed no evidence of publication bias (p = 0.706, Fig. S18).
Fig. 2.
Forest plot showing the pooled effect sizes for the risk of infection (compared to White ethnicity) for each ethnic group (A) and the pooled effect sizes for the risk of seropositivity (compared to majority White ethnic group) for each minoritised ethnic group (B). (∗) unadjusted risk ratio used (adjOR) adjusted odds ratio used (unadjOR) unadjusted odds ratio used. R: Risk Ratio. ROB: Risk of Bias.
Seven studies investigated seroprevalence (due to infection rather than vaccination). Compared to White majority participants, Black and Hispanic ethnic groups were more likely to be seropositive (Black: K = 6, aRR = 1.81, 95% CI: 1.10–2.97, I2 = 99.6; Hispanic: K = 2, aRR = 1.86, 95% CI: 1.40–2.48, I2 = 95.9). South Asian people were more likely to be seropositive (K = 1, aRR = 1.52, 95% CI: 1.11–2.08), though this was not seen for studies which only reported outcomes for aggregated Asian ethnic groups (including both South and East Asian people) (K = 6, aRR = 1.34, 95% CI: 0.84–2.14, I2 = 99.1). People from Other ethnic groups were also more likely to be seropositive (K = 3, aRR = 1.46, 95% CI: 1.23–1.72, I2 = 0.00). There was no difference in the risk of seropositivity for Mixed ethnic groups or Indigenous people, compared to White majority participants (Fig. 2B). The certainty of evidence was moderate (Table S3).
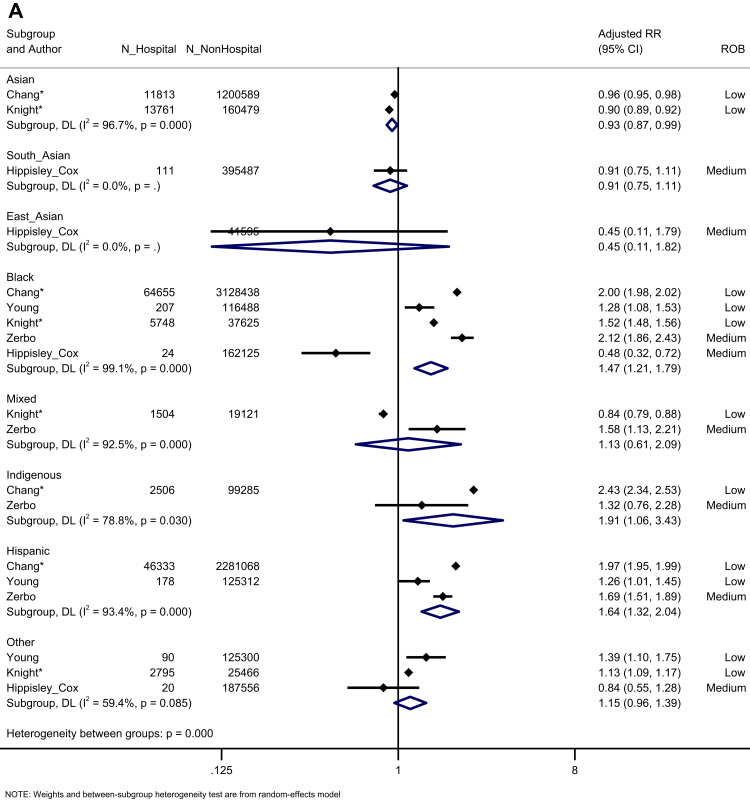
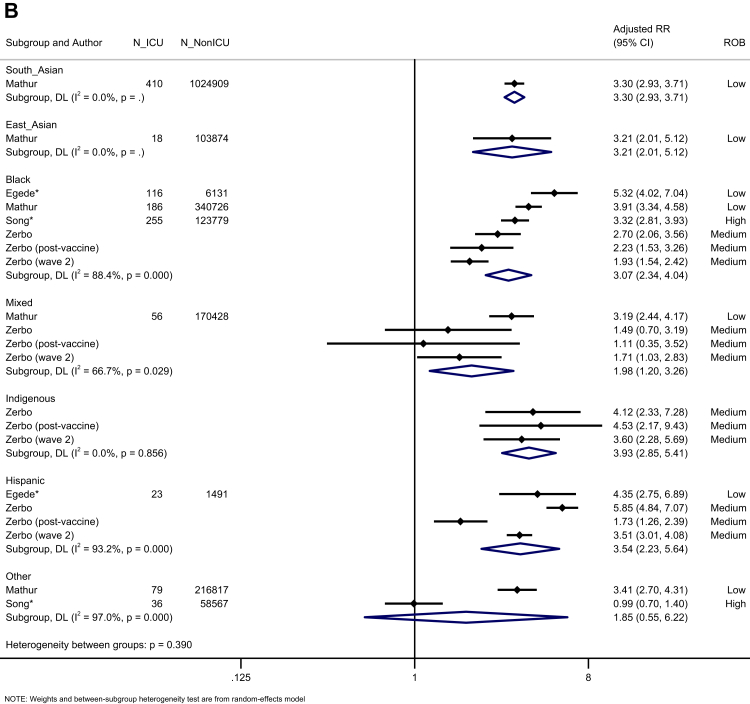
Five population-based studies investigated the risk of hospitalisation (Fig. 3A). Black, Hispanic, and Indigenous people had an increased risk of hospitalisation (Black: K = 4, aRR = 1.71, 95% CI: 1.40–2.08, I2 = 99.1; Hispanic: K = 3, aRR = 1.64, 95% CI: 1.32–2.04, I2 = 93.4; Indigenous: K = 2, aRR = 1.91, 95% CI: 1.06–1.64, I2 = 78.8) (moderate certainty). Six studies reported the risk of ICU admission in the general population (Fig. 3B). We identified an increased risk of ICU admission for South Asian, East Asian, Black, Mixed, Indigenous, and Hispanic people, but not other ethnic groups, compared to the White majority (very low certainty). Five studies investigated the risk of mortality in the general population (Fig. 3C). We observed an increased risk of mortality for Mixed, Hispanic, and Indigenous people, compared to the White majority (Mixed: K = 2, aRR = 1.43, 95% CI: 1.13–1.82, I2 = 0.0; Hispanic: K = 2, aRR = 1.30, 95% CI: 1.12–1.52, I2 = 17.0; Indigenous: K = 2, aRR = 2.14, 95% CI: 1.99–2.31, I2 = 0.0), but not Asian, Black or Other ethnic groups (very low certainty).
Fig. 3.
Forest plot showing the pooled effect sizes for the risk of hospital admission (compared to White majority ethnic group) in the general population (A); the pooled effect sizes for the risk of ICU admission (compared to White majority ethnic group) in the general population (B) and the pooled effect sizes for the risk of mortality (compared to White majority ethnic group) in the general population (C). (∗) unadjusted risk ratio used (adjOR) adjusted odds ratio used (unadjOR) unadjusted odds ratio used. R: Risk Ratio. ROB: Risk of Bias.
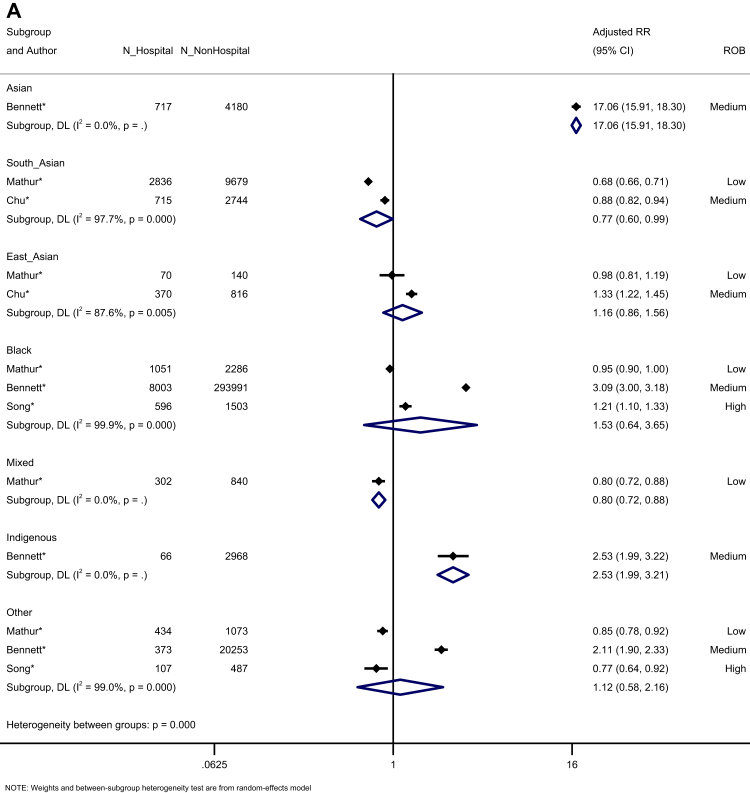
We assessed prognosis following infection. Four studies reported the risk of hospitalisation among confirmed COVID-19 cases (Fig. 4A). Hospitalisation risk was increased for Asian and Indigenous people, compared to White majority participants (Asian: K = 1, aRR = 17.06, 95% CI: 15.91–18.30; Indigenous: K = 1, aRR = 2.53, 95% CI: 1.99–3.21), and reduced for South Asian and Mixed ethnicity people. People from East Asian and Other ethnic groups showed no difference in the risk of hospitalisation, following infection, compared to White ethnic groups. Four studies assessed the risk of ICU admission among confirmed COVID-19 cases, demonstrating a trend towards an increased risk for Black people (aRR = 1.53, 95% CI: 0.99–2.35, I2 = 99.0), with no other differences across ethnic groups (Fig. 4B). However, three of the four studies had a high risk of bias. The risk of death among confirmed COVID-19 cases was reported in four studies, identifying no increased risk for any minoritised ethnic group, compared to the White majority, observing reduced risks for South Asian, East Asian, Mixed, and Other ethnic groups (Fig. 4C).
Fig. 4.
Forest plot showing the pooled effect sizes for the risk of hospitalisation (compared to White majority ethnic group) in confirmed COVID-19 cases (A); the pooled effect sizes for the risk of ICU admission (compared to White majority ethnic group) in confirmed COVID-19 cases (B); and the pooled effect sizes for the risk of mortality (compared to White majority ethnic group) in confirmed COVID-19 cases (C). (∗) unadjusted risk ratio used (adjOR) adjusted odds ratio used (unadjOR) unadjusted odds ratio used. R: Risk Ratio. ROB: Risk of Bias.
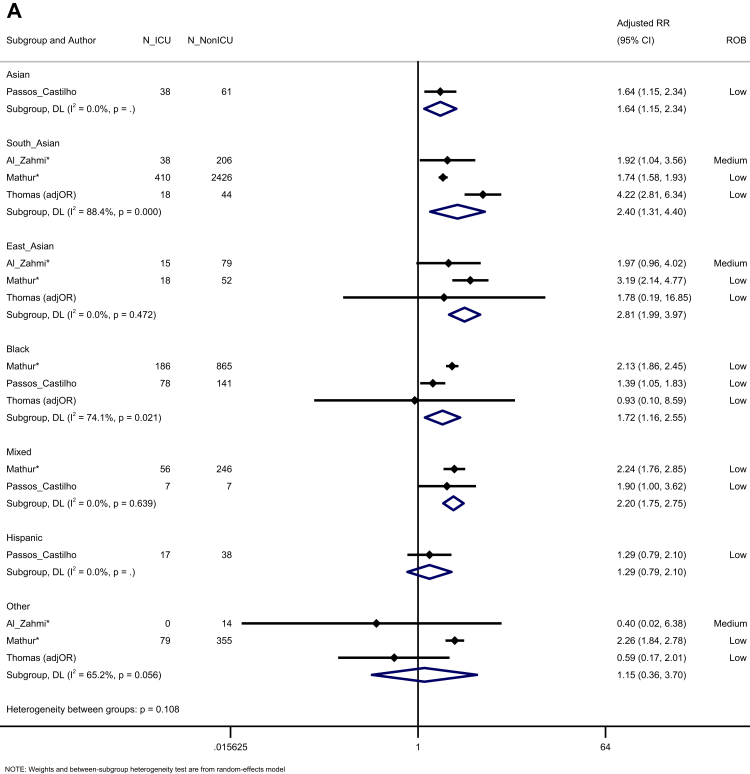
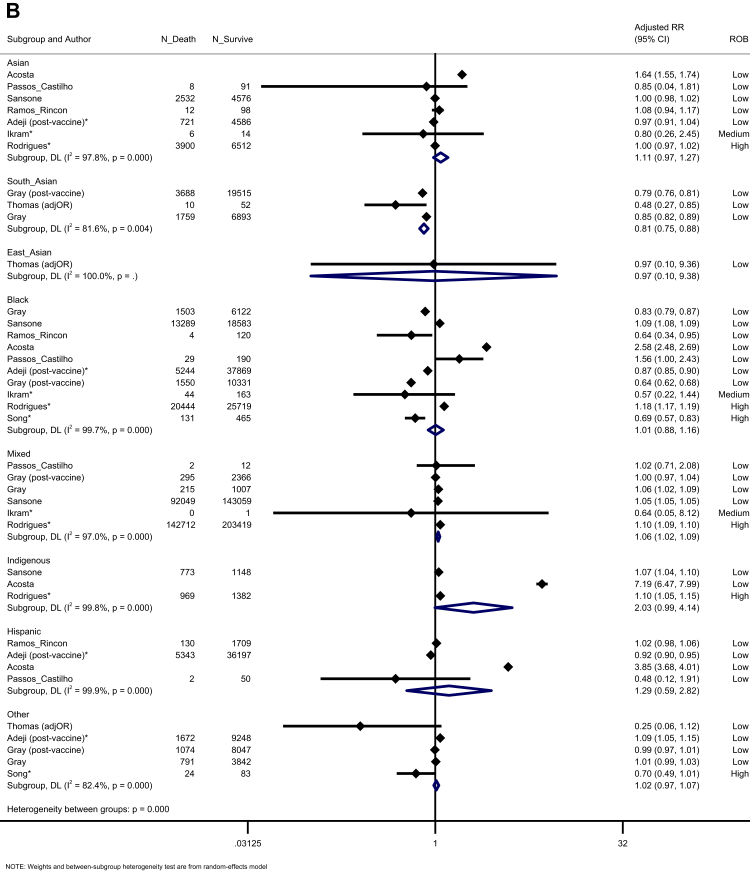
When assessing prognosis following hospitalisation, we noted that South Asian, East Asian, Asian (aggregated in studies, including South and East Asian people), Black, and Mixed groups were all more likely to be admitted to ICU compared to White majority participants. Those from Hispanic and Other ethnic groups were not at an increased risk, across four studies (Fig. 5A). In a synthesis of 11 studies, we observed an increased risk of mortality for Mixed ethnic groups (K = 6, aRR = 1.06, 95% CI: 1.02–1.09, I2 = 97.0), compared to the White majority, with trends towards an increased risk for Indigenous peoples (K = 3, aRR = 2.03, 95% CI: 0.99–4.14, I2 = 99.8) (Fig. 5B). Egger's test indicated no evidence of publication bias (p = 0.626).
Fig. 5.
Forest plot showing the pooled effect sizes for the risk of ICU admission (compared to White majority ethnic group) in hospitalised patients with COVID-19 (A); the pooled effect sizes for the risk of mortality (compared to White majority ethnic group) in hospitalised patients with COVID-19 (B). (∗) unadjusted risk ratio used (adjOR) adjusted odds ratio used (unadjOR) unadjusted odds ratio used. R: Risk Ratio. ROB: Risk of Bias.
The findings of the unadjusted analyses are reported in supplementary materials. Minoritised ethnic groups were found to have an increased risk of infection, seropositivity, hospital admission (population-based studies only), ICU admission (population-based studies and studies of hospitalised patients with COVID-19 only), but not mortality (Figs. S3-S7). Sensitivity analyses replicated the unadjusted analyses, excluding studies which reported country of birth, nationality, and migrant status. In these analyses, minoritised ethnic groups were not at an increased risk of infection or hospital admission, though all other original findings remained the same (Figs. S8–S12).
Meta-regressions explored whether region (LMIC versus HIC) was associated with heterogeneity, where there were sufficient data (n > 10). Region did not explain heterogeneity for the pooled unadjusted or adjusted risk of infection (unadjusted: β = 0.91, 95% CI: 0.38–2.22, p = 0.838, I2 = 99.95; adjusted: β = 0.98, 95% CI: 0.52–1.84, p = 0.950, I2 = 99.77), unadjusted risk of seropositivity (β = 1.70, 95% CI: 0.57–5.06, p = 0.291, I2 = 99.02), or the unadjusted risk of hospital admission (among studies of confirmed COVID-19 cases only, β = 2.22, 95% CI: 0.48–10.33, p = 0.250, I2 = 99.93). Region was significantly associated with variance in the unadjusted risk of ICU admission, for studies of hospitalised COVID-19 cases (β = 3.40, 95% CI: 1.98–5.84, p = 0.001, I2 = 92.97), whereby the unadjusted risk of ICU admission was greater in LMIC than in HIC (Fig. S20). In the unadjusted analyses, for mortality, region was significantly associated with heterogeneity among studies of confirmed COVID-19 cases (β = 3.57, 95% CI: 1.73 to 7.35, p = 0.004, I2 = 98.75, Fig. S21), and among studies of hospitalised COVID-19 cases (β = 1.75, 95% CI: 1.02–3.02, p = 0.044, I2 = 98.74, Fig. S22), whereby the risk of mortality was greater in LMIC than in HIC. However, region did not explain heterogeneity for the pooled adjusted risk of mortality among studies of hospitalised COVID-19 cases (β = 0.94, 95% CI: 0.63–1.40, p = 0.745, I2 = 99.59). The findings of the meta-analyses, stratified by region, are presented in supplementary materials (Figs. S23–S26).
Meta-regressions were also used to explore whether time-frame (before vaccine roll-out versus after vaccine roll-out) was associated with heterogeneity, where there was sufficient data. Time-frame did not explain heterogeneity for the pooled unadjusted or adjusted risk of infection (unadjusted: β = 0.63, 95% CI: 0.21–1.92, p = 0.384, I2 = 99.95; adjusted: β = 0.56, 95% CI: 0.28–1.12, p = 0.096, I2 = 99.77). However, when examining Fig. 2A, the study conducted post-vaccine roll-out shows no difference in the adjusted risk of infection for each ethnic group, compared to the White majority, contrasting most other effect sizes. Time-frame was not associated with heterogeneity in the unadjusted risk of seropositivity (β = 1.09, 95% CI: 0.49–2.36, p = 0.827, I2 = 96.80). Time-frame did not explain heterogeneity for the unadjusted or adjusted risk of mortality among hospitalised cases (unadjusted: β = 1.23, 95% CI: 0.63–2.43, p = 0.522, I2 = 99.37; adjusted: β = 0.79, 95% CI: 0.54–1.18, p = 0.245, I2 = 99.55).
We provide SWiM for the findings of studies that were not amenable to meta-analysis. Across all outcomes, 16 studies were excluded from the unadjusted analyses, and 37 studies that either contained certain ethnic groups that were not included (i.e., if only one study reported an effect for that ethnic group), or could not be included at all (i.e., if the reference group was not White). The SWiM reports mixed findings, which may reflect the heterogeneity of the studies (supplementary materials: Tables S4–S6).
Discussion
We identified systematic inequalities experienced by minoritised ethnic groups, but to a varying extent across ethnic and Indigenous groups. We found that Black, South Asian, Mixed, and Other ethnic groups had a greater risk of testing positive for infection. The findings demonstrate large differences in exposure risk, which may be driving ethnic inequalities in severe outcomes. Almost all minoritised ethnic groups were at an increased risk of hospital admission and ICU admission, in population-based studies, yet these findings attenuated when examining outcomes among confirmed COVID-19 cases only. Additionally, Hispanic people were more likely to be seropositive, compared to the White majority. Seropositivity to any SARS-CoV-2 protein within populations that have not yet been vaccinated highlights a history of past infection; therefore, it may be that Hispanic groups had reduced access to testing early in the pandemic.
We also observed differences in prognosis following hospitalisation, with South and East Asian, Black and Mixed ethnic groups being more at risk of ICU admission, and Mixed ethnic groups being more likely to die from COVID-19. Finally, we found that the unadjusted risk of severe disease (ICU admission and mortality) among hospitalised COVID-19 cases was greater for minoritised ethnic groups (versus the majority ethnic group) in LMIC compared to HIC. This could be due to HIC having more universal health care systems (excluding the USA). Our work is the most comprehensive summary of risk for minoritised ethnic groups globally, by using multiple markers for key clinical outcomes (molecular testing and serology for infection, and hospitalisation, ICU admission, and death for severe disease). Future work will likely include cohorts with differing levels of immunity (from previous infections, or heterogenous vaccine regimens) to different SARS-CoV-2 variants.
In agreement with previous meta-analyses, our data clearly demonstrates that the COVID-19 pandemic has exacerbated existing socioeconomic inequalities that disproportionately affect the health of minoritised ethnic and Indigenous groups.7,13,14,16,17,136 Structural racism (discrimination embedded within systems) drives socioeconomic inequalities that increase the risk of exposure to COVID-19 infection.137 During the pandemic, when multiple countries implemented strict lockdown measures, minoritised ethnic groups were more likely to be employed in sectors with increased exposure and were less likely to be able to self-isolate or work from home, due to economic precarity.8,138,139 Minoritised ethnic groups were also more likely to live in overcrowded households with reduced access to open spaces (a consequence of racism and socioeconomic inequality), which could lead to frequent and prolonged exposure to airborne pathogens.140 Among population-based studies, we observed an increased risk of severe disease for Black, Hispanic, South Asian, East Asian, Mixed and Indigenous ethnic groups, but this attenuated once we examined prognosis following infection, demonstrating that differences in exposure risk may have driven the greater number of people experiencing severe disease. When assessing prognosis following hospitalisation, we observed ethnic inequalities in ICU admission and mortality, which potentially reflect poorer healthcare quality, or barriers to adequate healthcare (e.g., language barriers, migrant status, mistrust, disparities resulting from highly marketised healthcare in the US),141 resulting from institutional racism.136 Going forwards, unless significant effort is made to address these inequalities, it is likely that minoritised ethnic groups will continue to have increased exposure to respiratory viruses as the world learns to live with COVID-19.
Despite the importance of racism in relation to clinical outcomes, as captured by ethnicity, we found that the quality of ethnicity recording in studies to be suboptimal. Approximately a quarter of studies did not describe how they recorded ethnicity, despite investigating its relation to clinical outcomes. The most common method was using routinely recorded data, yet evidence shows that ethnicity is often miscoded, and this affects minoritised ethnic groups disproportionately.142 Poor data may obscure the true extent of ethnic inequalities in COVID-19 health outcomes. In contrast to previous systematic reviews,13,14,16,17 when we further disaggregated Asian groups, we found that South Asian people were at increased risk of infection, whereas East Asian people were not, and studies using an aggregated Asian group observed a decreased risk of infection. Our analysis demonstrates the importance of granularity in collecting ethnic categories to describe the extent and drivers of ethnic inequalities in COVID-19 outcomes. If we only examined ‘Asian’ as one ethnic group, we may have missed the increased risk of infection among South Asian people, since the decreased risk of infection in all Asian people may have nullified this effect. We call for health systems to make a concerted effort to record self-identified ethnicity and for research to use disaggregated ethnic groups.143
Our systematic review has limitations. There was a large decrease in the number of studies and participants when we excluded studies with duplicate data, which was necessary to ensure rigour. This meant that in some analyses, estimates for certain ethnic groups were obtained from a small number of studies. Furthermore, although we set out to conduct a global synthesis, there were regions with no or limited data (e.g., Australia). Although there was some indication of publication bias in the unadjusted analyses, there was no evidence of publication bias in the adjusted analyses. Relatedly, the certainty of evidence for each outcome ranged from moderate to very low, mainly due to inconsistency within ethnic groups across studies. Additionally, heterogeneity between studies was high. However, we note that this is common with observational studies as I2 is calculated as the proportion of total variation which is attributable to between-study variation, meaning studies with large sample sizes (i.e., small within-study variation), are likely to show inflated heterogeneity.144 These limitations may result from the inclusion of observational studies from a range of regions. Nevertheless, we sought to present the most comprehensive work illustrating the disproportionate impact COVID-19 has had on minoritised ethnic groups. Clearly, this will have implications for future pandemics, especially if future pathogens have similar transmission dynamics and structural determinants.
In conclusion, we found clear evidence of systematic inequalities in COVID-19 health outcomes, experienced by minoritised ethnic groups, but to varying extents across ethnic groups during the first two years of the pandemic. We highlight the need to recognise and determine that pathways that lead to differing risks to COVID-19, before and after vaccine rollout periods. We observed large differences in exposure risk, particularly for Black, South Asian, Mixed, and Other ethnic groups. Hispanic groups may have had limited access to molecular testing early on in the pandemic, as reflected by the greater risk of seropositivity. Almost all minoritised ethnic groups being at an increased risk of severe outcomes among population-based studies (which attenuated when assessing outcomes only in confirmed cases), demonstrating the need for policy interventions to reduce exposure to infection. The differences in prognosis following hospitalisation may reflect poorer healthcare quality, illustrating the need for services and clinicians to ensure equitable care.145 The COVID-19 pandemic has exposed and exacerbated ethnic inequalities in health, therefore response and recovery should focus on tackling the drivers of inequalities, including structural racism and racial discrimination.146
Contributors
PI, DK, LB, HT, SA, and SVK drafted the study protocol. DP, SS, PD, LJG, LBN, and MP provided critical feedback on the protocol. PD conducted the literature searches. PI screened all records, and DK, DP, SS, SK, LB, HT, and SVK contributed to the screening process and selection of included studies. PI initially extracted data, which were subsequently verified by a second reviewer (DK, DP, SS, SVK, HT) who also completed independent risk of bias scores. PI completed the data analysis, and all authors had access to the data. EK created the visual map and both EK and LJG supported the analyses. All authors critically reviewed and approved the manuscript as submitted.
Data sharing statement
The study protocol is published on PROSPERO: CRD42021284981. All extracted data and analytical codes are available from the corresponding author are available upon request.
Declaration of interests
SVK was co-chair of the Scottish Government's Expert Reference Group on Ethnicity and COVID-19 and a member of the Scientific Advisory Group on Emergencies (SAGE) subgroup on ethnicity. MP reports grants from Sanofi and Gilead Sciences and personal fees from QIAGEN, outside the submitted work.
Acknowledgements
This work was supported by the Economic and Social Research Council (ESRC; grant number ES/W000849/1). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. LG is supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration East Midlands (ARC EM). DP is supported by an NIHR Doctoral Research Fellowship (NIHR302338). SA and EK are supported by Medical Research Council (MC_UU_00022/2) and the Scottish Government Chief Scientist Office (SPHSU17). MP is supported by a NIHR Development and Skills Enhancement Award and by the NIHR Leicester Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. SVK acknowledges funding from a NHS Research Scotland Senior Clinical Fellowship (SCAF/15/02), the Medical Research Council (MC_UU_00022/2) and the Scottish Government Chief Scientist Office (SPHSU17). LBN is supported by an Academy of Medical Sciences Springboard Award (SBF005/1047).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101877.
Appendix A. Supplementary data
References
- 1.Patel P., Hiam L., Sowemimo A., Devakumar D., McKee M. Ethnicity and covid-19. BMJ. 2020;369:m2282. doi: 10.1136/bmj.m2282. [DOI] [PubMed] [Google Scholar]
- 2.Pareek M., Bangash M.N., Pareek N., et al. Ethnicity and COVID-19: an urgent public health research priority. Lancet. 2020;395(10234):1421–1422. doi: 10.1016/S0140-6736(20)30922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khunti K., Singh A.K., Pareek M., Hanif W. Is ethnicity linked to incidence or outcomes of COVID-19? BMJ. 2020;369:m1548. doi: 10.1136/bmj.m1548. [DOI] [PubMed] [Google Scholar]
- 4.Mathur R., Rentsch C.T., Morton C.E., et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021;397(10286):1711–1724. doi: 10.1016/S0140-6736(21)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Public Health England . 2020. Disparities in the risk and outcomes of COVID-19. [Google Scholar]
- 6.Office for National Statistics . 2021. Updating ethnic contrasts in deaths involving the coronavirus (COVID-19), England: 24 January 2020 to 31 March 2021. [Google Scholar]
- 7.Nazroo J., Bécares L. Evidence for ethnic inequalities in mortality related to COVID-19 infections: findings from an ecological analysis of England. BMJ Open. 2020;10(12) doi: 10.1136/bmjopen-2020-041750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katikireddi S.V., Lal S., Carrol E.D., et al. Unequal impact of the COVID-19 crisis on minority ethnic groups: a framework for understanding and addressing inequalities. J Epidemiol Community Health. 2021;75(10):970–974. doi: 10.1136/jech-2020-216061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan D., Sze S., Martin C.A., et al. Covid-19 and ethnicity: we must seek to understand the drivers of higher transmission. BMJ. 2021;375:n2709. doi: 10.1136/bmj.n2709. [DOI] [PubMed] [Google Scholar]
- 10.Bambra C., Riordan R., Ford J., Matthews F. The COVID-19 pandemic and health inequalities. J Epidemiol Community Health. 2020;74(11):964–968. doi: 10.1136/jech-2020-214401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocha R., Atun R., Massuda A., et al. Effect of socioeconomic inequalities and vulnerabilities on health-system preparedness and response to COVID-19 in Brazil: a comprehensive analysis. Lancet Glob Health. 2021;9(6):e782–e792. doi: 10.1016/S2214-109X(21)00081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agyemang C., Richters A., Jolani S., et al. Ethnic minority status as social determinant for COVID-19 infection, hospitalisation, severity, ICU admission and deaths in the early phase of the pandemic: a meta-analysis. BMJ Glob Health. 2021;6(11) doi: 10.1136/bmjgh-2021-007433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sze S., Pan D., Nevill C.R., et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. eClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan D., Sze S., Minhas J.S., et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. eClinicalMedicine. 2020;23 doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raharja A., Tamara A., Kok L.T. Association between ethnicity and severe COVID-19 disease: a systematic review and meta-analysis. J Racial Ethn Health Disparities. 2020;8:1–10. doi: 10.1007/s40615-020-00921-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magesh S., John D., Li W.T., et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4(11):e2134147. doi: 10.1001/jamanetworkopen.2021.34147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanijahani A., Iezadi S., Gholipour K., Azami-Aghdash S., Naghibi D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health. 2021;20(1):1–30. doi: 10.1186/s12939-021-01582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balestra C., Fleischer L. 2018. Diversity statistics in the OECD: how do OECD countries collect data on ethnic, racial and indigenous identity? [Google Scholar]
- 20.2022. Covidence systematic review software.www.covidence.org [Google Scholar]
- 21.Moola S., Munn Z., Tufanaru C., et al. In: JBI manual for evidence synthesis. Aromataris E., Munn Z., editors. 2020. Chapter 7: systematic reviews of etiology and risk. [Google Scholar]
- 22.GOV.UK . GOV.UK; 2020. Ethnicity facts and figures.https://www.ethnicity-facts-figures.service.gov.uk/ [Google Scholar]
- 23.Iorio A., Spencer F.A., Falavigna M., et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Harbord R.M., Egger M., Sterne J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 27.StataCorp . StataCorp LLC; College Station: TX: 2017. Stata statistical software: release 15. [Google Scholar]
- 28.Campbell M., McKenzie J.E., Sowden A., et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368 doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva I., Faria NCd, Ferreira Á.R.S., Anastácio L.R., Ferreira L.G. Risk factors for critical illness and death among adult Brazilians with COVID-19. Rev Soc Bras Med Trop. 2021;54:e0014. doi: 10.1590/0037-8682-0014-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horta B.L., Silveira M.F., Barros A.J., et al. Prevalence of antibodies against SARS-CoV-2 according to socioeconomic and ethnic status in a nationwide Brazilian survey. Rev Panam Salud Pública. 2020;44:e135. doi: 10.26633/RPSP.2020.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu J.Y., Kaliwal Y., Koh M., et al. COVID-19 and its cardiac and neurological complications among ontario visible minorities. Can J Neurol Sci. 2021;49:1–10. doi: 10.1017/cjn.2021.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeed S., Drews S.J., Pambrun C., Yi Q.L., Osmond L., O'Brien S.F. SARS-CoV-2 seroprevalence among blood donors after the first COVID-19 wave in Canada. Transfusion. 2021;61(3):862–872. doi: 10.1111/trf.16296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Consolazio D., Murtas R., Tunesi S., Gervasi F., Benassi D., Russo A.G. Assessing the impact of individual characteristics and neighborhood socioeconomic status during the COVID-19 pandemic in the provinces of Milan and Lodi. Int J Health Serv. 2021;51(3):311–324. doi: 10.1177/0020731421994842. [DOI] [PubMed] [Google Scholar]
- 34.Fabiani M., Mateo-Urdiales A., Andrianou X., et al. Epidemiological characteristics of COVID-19 cases in non-Italian nationals notified to the Italian surveillance system. Eur J Public Health. 2021;31(1):37–44. doi: 10.1093/eurpub/ckaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombardi A., Mangioni D., Consonni D., et al. Seroprevalence of anti-SARS-CoV-2 IgG among healthcare workers of a large university hospital in Milan, Lombardy, Italy: a cross-sectional study. BMJ Open. 2021;11(2) doi: 10.1136/bmjopen-2020-047216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibarra-Nava I., Flores-Rodriguez K.G., Ruiz-Herrera V., et al. Ethnic disparities in COVID-19 mortality in Mexico: a cross-sectional study based on national data. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0239168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serván-Mori E., Seiglie J.A., Gómez-Dantés O., Wirtz V.J. Hospitalisation and mortality from COVID-19 in Mexican indigenous people: a cross-sectional observational study. J Epidemiol Community Health. 2022;76(1):16–23. doi: 10.1136/jech-2020-216129. [DOI] [PubMed] [Google Scholar]
- 38.Coyer L., Wynberg E., Buster M., et al. Hospitalisation rates differed by city district and ethnicity during the first wave of COVID-19 in Amsterdam, The Netherlands. BMC Public Health. 2021;21(1):1–9. doi: 10.1186/s12889-021-11782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rostila M., Cederström A., Wallace M., Brandén M., Malmberg B., Andersson G. Disparities in coronavirus disease 2019 mortality by country of birth in stockholm, Sweden: a total-population–based cohort study. Am J Epidemiol. 2021;190(8):1510–1518. doi: 10.1093/aje/kwab057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward H., Atchison C., Whitaker M., et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Commun. 2021;12(1):1–8. doi: 10.1038/s41467-021-21237-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farrell R.J., O'Regan R., O'Neill E., et al. Sociodemographic variables as predictors of adverse outcome in SARS-CoV-2 infection: an Irish hospital experience. Ir J Med Sci. 2021;190(3):893–903. doi: 10.1007/s11845-020-02407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hippisley-Cox J., Coupland C.A., Mehta N., et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374:n2244. doi: 10.1136/bmj.n2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas D.R., Orife O., Plimmer A., et al. Ethnic variation in outcome of people hospitalised during the first COVID-19 epidemic wave in Wales (UK): an analysis of national surveillance data using Onomap, a name-based ethnicity classification tool. BMJ Open. 2021;11(8) doi: 10.1136/bmjopen-2020-048335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song R.J., Ho Y.-L., Schubert P., et al. Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the veterans health administration million veteran program. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0251651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo J., Jeyapalina S., Stoddard G.J., Kwok A.C., Agarwal J.P. Coronavirus disease 2019 in veterans receiving care at veterans health administration facilities. Ann Epidemiol. 2021;55:10–14. doi: 10.1016/j.annepidem.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metra B., Summer R., Brooks S.E., George G., Sundaram B. Racial disparities in COVID-19 associated pulmonary embolism: a multicenter cohort study. Thromb Res. 2021;205:84–91. doi: 10.1016/j.thromres.2021.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zerbo O., Lewis N., Fireman B., et al. Population-based assessment of risks for severe COVID-19 disease outcomes. Influenza Other Respir Viruses. 2022;16(1):159–165. doi: 10.1111/irv.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindsay L., Secrest M.H., Rizzo S., Keebler D.S., Yang F., Tsai L. Factors associated with COVID-19 viral and antibody test positivity and assessment of test concordance: a retrospective cohort study using electronic health records from the USA. BMJ Open. 2021;11(10) doi: 10.1136/bmjopen-2021-051707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishii T., Kushimoto S., Katori Y., et al. Predictors of SARS-CoV-2 positivity based on RT-PCR swab tests at a drive-through outpatient clinic for COVID-19 screening in Japan. Tohoku J Exp Med. 2021;253(2):101–108. doi: 10.1620/tjem.253.101. [DOI] [PubMed] [Google Scholar]
- 50.Hamadah H., Alahmad B., Behbehani M., et al. COVID-19 clinical outcomes and nationality: results from a Nationwide registry in Kuwait. BMC Public Health. 2020;20(1):1–9. doi: 10.1186/s12889-020-09490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jefferies S., French N., Gilkison C., et al. COVID-19 in New Zealand and the impact of the national response: a descriptive epidemiological study. Lancet Public Health. 2020;5(11):e612–e623. doi: 10.1016/S2468-2667(20)30225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Indseth T., Grøsland M., Arnesen T., et al. COVID-19 among immigrants in Norway, notified infections, related hospitalizations and associated mortality: a register-based study. Scand J Public Health. 2021;49(1):48–56. doi: 10.1177/1403494820984026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al Awaidy S.T., Khamis F., Al Rashidi B., Al Wahaibi A.H., Albahri A., Mahomed O. Epidemiological characteristics of 69,382 COVID-19 patients in Oman. J Epidemiol Glob Health. 2021;11(4):326–337. doi: 10.1007/s44197-021-00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaikh F.S., Aldhafferi N., Buker A., et al. Comorbidities and risk factors for severe outcomes in COVID-19 patients in Saudi Arabia: a retrospective cohort study. J Multidiscip Healthc. 2021;14:2169. doi: 10.2147/JMDH.S317884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.da Silva Júnior A.E., de Lima Macena M., Pureza IRdOM., et al. Association between COVID-19 diagnosis and economic class, race/skin color and social distancing in Brazilian university students. Medicina. 2021;54(4) [Google Scholar]
- 56.Rodrigues W., da Costa Frizzera H., de Queiroz Trevisan D.M., Prata D., Reis G.R., Resende R.A. Social, economic, and regional determinants of mortality in hospitalized patients with COVID-19 in Brazil. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.856137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sansone N.M., Boschiero M.N., Valencise F.E., Palamim C.V., Marson F.A. Characterization of demographic data, clinical signs, comorbidities, and outcomes according to the race in hospitalized individuals with COVID-19 in Brazil: an observational study. J Glob Health. 2022;12 doi: 10.7189/jogh.12.05027. :05027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Passos-Castilho A.M., Labbé A.-C., Barkati S., et al. Outcomes of hospitalized COVID-19 patients in Canada: impact of ethnicity, migration status and country of birth. J Travel Med. 2022;29(6) doi: 10.1093/jtm/taac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cifuentes M.P., Rodriguez-Villamizar L.A., Rojas-Botero M.L., Alvarez-Moreno C.A., Fernández-Niño J.A. Socioeconomic inequalities associated with mortality for COVID-19 in Colombia: a cohort nationwide study. J Epidemiol Community Health. 2021;75(7):610–615. doi: 10.1136/jech-2020-216275. [DOI] [PubMed] [Google Scholar]
- 60.Concha G., Frickmann H., Oey A., Strengert M., Kreienbrock L., Kann S. Direct and indirect proof of SARS-CoV-2 infections in indigenous wiwa communities in North-eastern Colombia—a cross-sectional assessment providing preliminary surveillance data. Vaccines. 2021;9(10):1120. doi: 10.3390/vaccines9101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sultanoglu N., Baddal B., Suer K., Sanlidag T. Current situation of COVID-19 in northern Cyprus. East Mediterr Health J. 2020;26(6):641–645. doi: 10.26719/emhj.20.070. [DOI] [PubMed] [Google Scholar]
- 62.Islamoska S., Petersen J.H., Benfield T., Norredam M. Socioeconomic and demographic risk factors in COVID-19 hospitalization among immigrants and ethnic minorities. Eur J Public Health. 2022;32(2):302–310. doi: 10.1093/eurpub/ckab186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flores Sacoto K.M., Sanchez Del Hierro G.A., Moreno-Piedrahita Hernández F.G., Jarrin Estupiñan J.X. Case fatality rate of COVID-19 and its relationship to sociodemographic characteristics in Ecuador, 2020. Int J Public Health. 2022;67 doi: 10.3389/ijph.2022.1604768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allen N., Brady M., Ni Riain U., et al. Prevalence of antibodies to SARS-CoV-2 following natural infection and vaccination in Irish hospital healthcare workers: changing epidemiology as the pandemic progresses. Front Med. 2022;8:3133. doi: 10.3389/fmed.2021.758118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saidel-Odes L., Shafat T., Nativ R., Borer A., Nesher L. SARS-CoV-2 universal screening upon adult hospital admission in Southern Israel. J Hosp Infect. 2021;114:167–170. doi: 10.1016/j.jhin.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cacciani L., Calandrini E., Cascini S., et al. Hospital assistance for COVID-19: a comparison between non-Italian and Italian resident population in five Italian Regions since the beginning of the pandemic until June 2021. Epidemiol Prev. 2022;46(4):49–58. doi: 10.19191/EP22.4S1.056. [DOI] [PubMed] [Google Scholar]
- 67.Di Girolamo C., Bartolini L., Allotta A.V., et al. Mortality and impact of COVID-19 by citizenship in seven Italian Regions from the beginning of the pandemic to mid-July 2021. Epidemiol Prev. 2022;46(4):59–69. doi: 10.19191/EP22.4S1.057. [DOI] [PubMed] [Google Scholar]
- 68.Pagani G., Conti F., Giacomelli A., et al. Differences in the Prevalence of SARS-CoV-2 infection and access to care between Italians and non-Italians in a social-housing neighbourhood of Milan, Italy. Int J Environ Res Public Health. 2021;18(20) doi: 10.3390/ijerph182010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kadyrova I., Yegorov S., Negmetzhanov B., et al. High SARS-CoV-2 seroprevalence in Karaganda, Kazakhstan before the launch of COVID-19 vaccination. PLoS One. 2022;17(7) doi: 10.1371/journal.pone.0272008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramli N.S., Fauzi M.F.M., Moktar N.M.A., Hajib N., Nawi A.M. Prevalence, characteristics, and predictors of healthcare workers with COVID-19 infection in an urban district in Malaysia. Pan Afr Med J. 2022;41:243. doi: 10.11604/pamj.2022.41.243.33300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bojorquez-Chapela I., Strathdee S.A., Garfein R.S., et al. The impact of the COVID-19 pandemic among migrants in shelters in Tijuana, Baja California, Mexico. BMJ Glob Health. 2022;7(3) doi: 10.1136/bmjgh-2021-007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dahal S., Mamelund S.-E., Luo R., Sattenspiel L., Self-Brown S., Chowell G. Investigating COVID-19 transmission and mortality differences between indigenous and non-indigenous populations in Mexico. Int J Infect Dis. 2022;122:910–920. doi: 10.1016/j.ijid.2022.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collard D., Stronks K., Harris V., et al. Ethnic differences in coronavirus disease 2019 hospitalization and hospital outcomes in a multiethnic population in the Netherlands. Open Forum Infect Dis. 2022;9:ofac257. doi: 10.1093/ofid/ofac257. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coyer L., Boyd A., Schinkel J., et al. SARS-CoV-2 antibody prevalence and correlates of six ethnic groups living in Amsterdam, The Netherlands: a population-based cross-sectional study, June–October 2020. BMJ Open. 2022;12(1) doi: 10.1136/bmjopen-2021-052752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vos E.R., Den Hartog G., Schepp R.M., et al. Nationwide seroprevalence of SARS-CoV-2 and identification of risk factors in the general population of The Netherlands during the first epidemic wave. J Epidemiol Community Health. 2021;75(6):489–495. doi: 10.1136/jech-2020-215678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Utulu R., Ajayi I.O., Bello S., et al. Risk factors for COVID-19 infection and disease severity in Nigeria: a case-control study. Pan Afr Med J. 2022;41:317. doi: 10.11604/pamj.2022.41.317.34307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Labberton A.S., Godøy A., Elgersma I.H., et al. SARS-CoV-2 infections and hospitalisations among immigrants in Norway-significance of occupation, household crowding, education, household income and medical risk: a nationwide register study. Scand J Public Health. 2022;50 doi: 10.1177/14034948221075029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Abri S.S., Al-Wahaibi A., Al-Kindi H., et al. Seroprevalence of SARS-CoV-2 antibodies in the general population of Oman: results from four successive nationwide sero-epidemiological surveys. Int J Infect Dis. 2021;112:269–277. doi: 10.1016/j.ijid.2021.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Kuwari M.G., Al-Nuaimi A.A., Abdulmajeed J., et al. COVID-19 infection across workplace settings in Qatar: a comparison of COVID-19 positivity rates of screened workers from March 1st until July 31st, 2020. J Occup Med Toxicol. 2021;16(1):1–9. doi: 10.1186/s12995-021-00311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nasif W.A., Ali A.S., Khogeer A.A., et al. Emphasizing the link between blood types in multi-ethnic disparities and COVID-19 infection in Makkah, Saudi Arabia. Saudi Med J. 2022;43(2):177. doi: 10.15537/smj.2022.43.2.20210847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ikram A.S., Pillay S. Admission vital signs as predictors of COVID-19 mortality: a retrospective cross-sectional study. BMC Emerg Med. 2022;22(1):1–10. doi: 10.1186/s12873-022-00631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jugwanth S., Gededzha M.P., Mampeule N., et al. Performance of the Abbott SARS-CoV-2 IgG serological assay in South African 2 patients. PLoS One. 2022;17(2) doi: 10.1371/journal.pone.0262442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stead D., Adeniyi O.V., Singata-Madliki M., et al. Cumulative incidence of SARS-CoV-2 and associated risk factors among healthcare workers: a cross-sectional study in the Eastern Cape, South Africa. BMJ Open. 2022;12(3) doi: 10.1136/bmjopen-2021-058761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guijarro C., Pérez-Fernández E., González-Piñeiro B., et al. Differential risk for COVID-19 in the first wave of the disease among Spaniards and migrants from different areas of the world living in Spain. Rev Clín Esp. 2021;221(5):264–273. doi: 10.1016/j.rceng.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 85.Ramos-Rincon J.-M., Cobos-Palacios L., López-Sampalo A., et al. Ethnicity and clinical outcomes in patients hospitalized for COVID-19 in Spain: results from the multicenter SEMI-COVID-19 registry. J Clin Med. 2022;11(7):1949. doi: 10.3390/jcm11071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nwaru C.A., Santosa A., Franzén S., Nyberg F. Occupation and COVID-19 diagnosis, hospitalisation and ICU admission among foreign-born and Swedish-born employees: a register-based study. J Epidemiol Community Health. 2022;76(5):440–447. doi: 10.1136/jech-2021-218278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strålin K., Wahlström E., Walther S., et al. Mortality trends among hospitalised COVID-19 patients in Sweden: a nationwide observational cohort study. Lancet Reg Health Eur. 2021;4 doi: 10.1016/j.lanepe.2021.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gustafsson P.E., San Sebastian M., Fonseca-Rodriguez O., Connolly A.-M.F. Inequitable impact of infection: social gradients in severe COVID-19 outcomes among all confirmed SARS-CoV-2 cases during the first pandemic wave in Sweden. J Epidemiol Community Health. 2022;76(3):261–267. doi: 10.1136/jech-2021-216778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gray W.K., Navaratnam A.V., Day J., Wendon J., Briggs T.W. COVID-19 hospital activity and in-hospital mortality during the first and second waves of the pandemic in England: an observational study. Thorax. 2022;77(11):1113–1120. doi: 10.1136/thoraxjnl-2021-218025. [DOI] [PubMed] [Google Scholar]
- 90.Knight R., Walker V., Ip S., et al. Association of COVID-19 with arterial and venous vascular diseases: a population-wide cohort study of 48 million adults in England and Wales. Circulation. 2021;146(12):892–906. doi: 10.1161/CIRCULATIONAHA.122.060785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas D.R., Fina L.H., Adamson J.P., et al. Social, demographic and behavioural determinants of SARS-CoV-2 infection: a case-control study carried out during mass community testing of asymptomatic individuals in South Wales, December 2020. Epidemiol Infect. 2022;150:e115. doi: 10.1017/S0950268822000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin C.A., Pan D., Melbourne C., et al. Risk factors associated with SARS-CoV-2 infection in a multiethnic cohort of United Kingdom healthcare workers (UK-REACH): a cross-sectional analysis. PLoS Med. 2022;19(5) doi: 10.1371/journal.pmed.1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Talaei M., Faustini S., Holt H., et al. Determinants of pre-vaccination antibody responses to SARS-CoV-2: a population-based longitudinal study (COVIDENCE UK) BMC Med. 2022;20(1):1–18. doi: 10.1186/s12916-022-02286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.AbuRuz S., Al-Azayzih A., ZainAlAbdin S., Beiram R., Al Hajjar M. Clinical characteristics and risk factors for mortality among COVID-19 hospitalized patients in UAE: does ethnic origin have an impact. PLoS One. 2022;17(3) doi: 10.1371/journal.pone.0264547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al Zahmi F., Habuza T., Awawdeh R., et al. Ethnicity-specific features of COVID-19 among arabs, africans, South asians, East asians, and caucasians in the United Arab Emirates. Front Cell Infect Microbiol. 2022;11:1241. doi: 10.3389/fcimb.2021.773141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Acosta A.M., Garg S., Pham H., et al. Racial and ethnic disparities in rates of COVID-19–associated hospitalization, intensive care unit admission, and in-hospital death in the United States from March 2020 to February 2021. JAMA Netw Open. 2021;4(10):e2130479–e. doi: 10.1001/jamanetworkopen.2021.30479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adjei S., Hong K., Molinari N.M., et al. Mortality risk among patients hospitalized primarily for COVID-19 during the omicron and delta variant pandemic periods—United States, april 2020–June 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1182–1189. doi: 10.15585/mmwr.mm7137a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bennett T.D., Moffitt R.A., Hajagos J.G., et al. Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US National COVID Cohort Collaborative. JAMA Netw Open. 2021;4(7):e2116901–e. doi: 10.1001/jamanetworkopen.2021.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang M.H., Moonesinghe R., Truman B.I. Racial and ethnic differences in COVID-19 hospitalisations by metropolitan status among Medicare beneficiaries, 1 January-31 December 2020. J Public Health. 2022;44(2):e211–e220. doi: 10.1093/pubmed/fdab355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Egede L.E., Walker R.J., Garacci E., Raymond Sr JR. Racial/Ethnic differences in COVID-19 screening, hospitalisation, and mortality in Southeast Wisconsin: study examines racial/ethnic differences in COVID-19 screening, symptom presentation, hospitalisation, and mortality among 31,549 adults tested for COVID-19 in Wisconsin. Health Aff. 2020;39(11):1926–1934. doi: 10.1377/hlthaff.2020.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feldman J.M., Bassett M.T. Variation in COVID-19 mortality in the US by race and ethnicity and educational attainment. JAMA Netw Open. 2021;4(11):e2135967–e. doi: 10.1001/jamanetworkopen.2021.35967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ioannou G.N., Ferguson J.M., O'Hare A.M., et al. Changes in the associations of race and rurality with SARS-CoV-2 infection, mortality, and case fatality in the United States from February 2020 to March 2021: a population-based cohort study. PLoS Med. 2021;18(10) doi: 10.1371/journal.pmed.1003807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jones J.M., Stone M., Sulaeman H., et al. Estimated US infection-and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA. 2021;326(14):1400–1409. doi: 10.1001/jama.2021.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Young J.M., Stahlman S.L., Clausen S.S., Bova M.L., Mancuso J.D. Racial and ethnic disparities in COVID-19 infection and hospitalization in the active component US military. Am J Public Health. 2021;111(2):2194–2201. doi: 10.2105/AJPH.2021.306527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deeb A., Khawaja K., Sakrani N., et al. Impact of ethnicity and underlying comorbidity on COVID-19 inhospital mortality: an observational study in Abu Dhabi, UAE. Biomed Res Int. 2021;2021 doi: 10.1155/2021/6695707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Al Kuwari H.M., Rahim H.F.A., Abu-Raddad L.J., et al. Epidemiological investigation of the first 5685 cases of SARS-CoV-2 infection in Qatar, 28 February–18 April 2020. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carbó A.V., del Castillo J.G., Miró O., et al. Increased severity in SARS-CoV-2 infection of minorities in Spain. Rev Esp Quimioter. 2021;34(6):664. doi: 10.37201/req/099.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soares RdCM., Mattos L.R., Raposo L.M. Risk factors for hospitalization and mortality due to COVID-19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 2020;103(3):1184. doi: 10.4269/ajtmh.20-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zimmermann I.R., Sanchez M.N., Frio G.S., et al. Trends in COVID-19 case-fatality rates in Brazilian public hospitals: a longitudinal cohort of 398,063 hospital admissions from 1st March to 3rd October 2020. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0254633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martin C.A., Patel P., Goss C., et al. Demographic and occupational determinants of anti-SARS-CoV-2 IgG seropositivity in hospital staff. J Public Health. 2020;44(2):234–245. doi: 10.1093/pubmed/fdaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patel M., Nair M., Pirozzoli E., Cienfuegos M.C., Aitken E. Prevalence and socio-demographic factors of SARS-CoV-2 antibody in multi-ethnic healthcare workers. Clin Med. 2021;21(1):e5. doi: 10.7861/clinmed.2020-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shorten R.J., Haslam S., Hurley M.A., et al. Seroprevalence of SARS-CoV-2 infection in healthcare workers in a large teaching hospital in the North West of England: a period prevalence survey. BMJ Open. 2021;11(3) doi: 10.1136/bmjopen-2020-045384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanrath Aidan T., van der Loeff Ina S., Lendrem Dennis W., et al. SARS-CoV-2 testing of 11,884 healthcare workers at an acute NHS hospital trust in england: a retrospective analysis. Front Med. 2021;8 doi: 10.3389/fmed.2021.636160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lumley S.F., Wei J., O'Donnell D., et al. The duration, dynamics, and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clin Infect Dis. 2021;73(3):e699–e709. doi: 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ken-Dror G., Wade C., Sharma S.S., et al. SARS-CoV-2 antibody seroprevalence in NHS healthcare workers in a large double-sited UK hospital. Clin Med. 2021;21(3) doi: 10.7861/clinmed.2020-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Razvi S., Oliver R., Moore J., Beeby A. Exposure of hospital healthcare workers to the novel coronavirus (SARS-CoV-2) Clin Med. 2020;20(6):e238. doi: 10.7861/clinmed.2020-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choudhry N., Drysdale K., Usai C., et al. Disparities of SARS-CoV-2 nucleoprotein-specific IgG in healthcare workers in East London, UK. Front Med. 2021;8 doi: 10.3389/fmed.2021.642723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shields A., Faustini S., Kristunas C., et al. COVID-19: seroprevalence and vaccine responses in UK dental care professionals. J Dent Res. 2021;100 doi: 10.1177/00220345211020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Valdes A.M., Moon J.C., Vijay A., et al. Longitudinal assessment of symptoms and risk of SARS-CoV-2 infection in healthcare workers across 5 hospitals to understand ethnic differences in infection risk. eClinicalMedicine. 2021;34 doi: 10.1016/j.eclinm.2021.100835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shields A.M., Faustini S.E., Perez-Toledo M., et al. Serological responses to SARS-CoV-2 following non-hospitalised infection: clinical and ethnodemographic features associated with the magnitude of the antibody response. BMJ Open Respir Res. 2021;8(1) doi: 10.1136/bmjresp-2020-000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nafilyan V., Islam N., Mathur R., et al. Ethnic differences in COVID-19 mortality during the first two waves of the Coronavirus Pandemic: a nationwide cohort study of 29 million adults in England. Eur J Epidemiol. 2021;36(6):605–617. doi: 10.1007/s10654-021-00765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bassett M.T., Chen J.T., Krieger N. Variation in racial/ethnic disparities in COVID-19 mortality by age in the United States: a cross-sectional study. PLoS Med. 2020;17(10) doi: 10.1371/journal.pmed.1003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaufman H.W., Niles J.K., Nash D.B. Disparities in SARS-CoV-2 positivity rates: associations with race and ethnicity. Popul Health Manag. 2021;24(1):20–26. doi: 10.1089/pop.2020.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao Z., Salerno S., Shi X., Lee S., Mukherjee B., Fritsche L.G. Understanding the patterns of serological testing for COVID-19 pre-and post-vaccination rollout in Michigan. J Clin Med. 2021;10(19):4341. doi: 10.3390/jcm10194341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Allan-Blitz L.-T., Goldbeck C., Hertlein F., Turner I., Klausner J.D. Association of lower socioeconomic status and SARS-CoV-2 positivity in Los Angeles, California. J Prev Med Public Health. 2021;54(3):161. doi: 10.3961/jpmph.21.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sullivan P.S., Siegler A.J., Shioda K., et al. Severe acute respiratory syndrome coronavirus 2 cumulative incidence, United States, August 2020–December 2020. Clin Infect Dis. 2022;74(7):1141–1150. doi: 10.1093/cid/ciab626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bruckner T.A., Parker D.M., Bartell S.M., et al. Estimated seroprevalence of SARS-CoV-2 antibodies among adults in Orange County, California. Sci Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-82662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Santarelli A., Lalitsasivimol D., Bartholomew N., et al. The seroprevalence of SARS-CoV-2 in a rural southwest community. J Osteopath Med. 2021;121(2):199–210. doi: 10.1515/jom-2020-0287. [DOI] [PubMed] [Google Scholar]
- 129.Parrott J.C., Maleki A.N., Vassor V.E., et al. Prevalence of SARS-CoV-2 antibodies in New York City adults, June–October 2020: a population-based survey. J Infect Dis. 2021;224(2):188–195. doi: 10.1093/infdis/jiab296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xavier C., Rasu R.S. Health disparities of coronavirus disease 2019 in Texas, March–July 2020. South Med J. 2021;114(10):649. doi: 10.14423/SMJ.0000000000001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fitzpatrick V., Rivelli A., Blair C., Copeland K., Richards J. Disparities in seroprevalence of SARS-CoV-2 immunoglobulin antibodies in a large midwestern health care system. Public Health Rep. 2021;136(3):361–367. doi: 10.1177/0033354921999168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Feehan A.K., Velasco C., Fort D., et al. Racial and workplace disparities in seroprevalence of SARS-CoV-2, Baton Rouge, Louisiana, USA. Emerg Infect Dis. 2021;27(1):314. doi: 10.3201/eid2701.203808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ioannou G.N., Locke E., Green P., et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9):e2022310–e. doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Esaryk E.E., Wesson P., Fields J., et al. Variation in SARS-CoV-2 infection risk and socioeconomic disadvantage among a Mayan-Latinx population in Oakland, California. JAMA Netw Open. 2021;4(5):e2110789–e. doi: 10.1001/jamanetworkopen.2021.10789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bigelow B.F., Saxton R.E., Flores-Miller A., et al. Community testing and SARS-CoV-2 rates for Latinxs in Baltimore. Am J Prev Med. 2021;60(6):e281–e286. doi: 10.1016/j.amepre.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 136.Kapadia D., Bradby H. Routledge international handbook of critical issues in health and illness. Routledge; 2021. Ethnicity and health; pp. 183–196. [Google Scholar]
- 137.Nazroo J., Bécares L. Runnymede Trust; 2021. Ethnic Inequalities in COVID-19 mortality: a consequence of persistent racism.https://www.runnymedetrust.org/publications/ethnic-inequalities-in-covid-19-mortality-a-consequence-of-persistent-racism [Google Scholar]
- 138.Hawkins D. Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. Am J Ind Med. 2020;63(9):817–820. doi: 10.1002/ajim.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mutambudzi M., Niedzwiedz C., Macdonald E.B., et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2021;78(5):307–314. doi: 10.1136/oemed-2020-106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Soltan M.A., Varney J., Sutton B., et al. COVID-19 admission risk tools should include multiethnic age structures, multimorbidity and deprivation metrics for air pollution, household overcrowding, housing quality and adult skills. BMJ Open Respir Res. 2021;8(1) doi: 10.1136/bmjresp-2021-000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Germain S., Yong A. COVID-19 highlighting inequalities in access to healthcare in England: a case study of ethnic minority and migrant women. Fem Leg Stud. 2020;28(3):301–310. doi: 10.1007/s10691-020-09437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scobie S., Spencer J., Raleigh V. 2021. Ethnicity coding in English health service datasets. [Google Scholar]
- 143.Routen A., Akbari A., Banerjee A., et al. Strategies to record and use ethnicity information in routine health data. Nat Med. 2022;28:1–4. doi: 10.1038/s41591-022-01842-y. [DOI] [PubMed] [Google Scholar]
- 144.Coory M.D. Comment on: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39(3):932. doi: 10.1093/ije/dyp157. [DOI] [PubMed] [Google Scholar]
- 145.Kapadia D., Zhang J., Salway S., et al. NHS Race and Health Observatory; 2022. Ethnic inequalities in healthcare: a rapid evidence review.https://www.nhsrho.org/publications/ethnic-inequalities-in-healthcare-a-rapid-evidence-review/ [Google Scholar]
- 146.Linos N., Bassett M.T., Salemi A., et al. Opportunities to tackle structural racism and ethnicity-based discrimination in recovering and rebuilding from the COVID-19 pandemic. Nat Commun. 2022;13(1):1–4. doi: 10.1038/s41467-022-30791-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.