Abstract
Chemotherapy‐induced alopecia is frequently induced by various regimens of chemotherapy and has a significant impact on mental health and quality of life. However, the effect of available current treatment for chemotherapy‐induced alopecia is not sufficient. This study aimed to clarify the therapeutic effects and mechanism of skin cooling and the antioxidant α‐lipoic acid derivative on chemotherapy‐induced alopecia. We developed a chemotherapy‐induced alopecia model of cyclophosphamide (120 μg/g) using Institute of Cancer Research mice. We used cooling therapy and α‐lipoic acid derivative application as the treatments. We compared the alopecia score, hair bulb diameter, insulin‐like growth factor‐1 level, vascular permeability, and apoptosis between the control and treatment groups. The alopecia score significantly improved in each treatment group compared with that in the cyclophosphamide group. Hair bulb diameter significantly improved in the cyclophosphamide + cooling group compared with that in the cyclophosphamide group. The insulin‐like growth factor‐1 level and vascular permeability level was significantly retained and suppressed, respectively, in each treatment group compared with that in the cyclophosphamide group. The number of apoptotic cells in the vascular endothelium significantly decreased in the cyclophosphamide + α‐lipoic acid derivative group compared with that in the cyclophosphamide group. In conclusion, cooling therapy and α‐lipoic acid derivative facilitated recovery from chemotherapy‐induced alopecia caused by cyclophosphamide through decreasing vascular permeability.
Keywords: alopecia, antioxidants, chemotherapy, cooling therapy, cyclophosphamide
Chemotherapy‐induced alopecia is frequently induced by various regimens of chemotherapy and has a significant impact on mental health and quality of life. In this study, we demonstrated that cooling therapy and a‐lipoic acid derivative facilitated recovery from chemotherapy‐induced alopecia caused by cyclophosphamide through decreasing vascular permeability.
Abbreviations
- ALAD
α‐lipoic acid derivative
- AS
alopecia score
- CIA
chemotherapy‐induced alopecia
- CYP
cyclophosphamide
- ICR
Institute of Cancer Research
- OCT
optimal cutting temperature
- QOL
quality of life
- TRITC
tetramethylrhodamine
1. INTRODUCTION
With recent advances in medicine, an increasing number of patients lead active social lives while continuing cancer treatment. Therefore, changes in appearance due to treatment side effects have a significant impact on mental health and QOL for cancer patients. Among these, alopecia is a side effect that occurs frequently in various regimens of chemotherapy. 1 The degree of alopecia varies with the type of anticancer drug. Cyclophosphamide, anthracyclines, and taxanes cause severe alopecia with hair loss of ≥50%, ≥60%, 80%–90% and 100%, respectively. 2 , 3 , 4 , 5 Hair loss of ≥50% occurred in 96% of breast cancer patients receiving the standard adjuvant chemotherapy combination of epirubicin/paclitaxel/cyclophosphamide. 6
Clinical applications of scalp cooling have proven effective to some extent for preventing and reducing the severity of CIA, making it the recommended treatment for CIA in patients with breast cancer. 7 Randomized controlled trials have shown that the use of a scalp cooling system results in a 50% success rate of hair loss prevention, where hair loss is defined as ≤50%. 8 , 9 , 10 Scalp cooling is presumed to attenuate the influence of anticancer drugs on hair bulbs by constricting scalp blood vessels and reducing blood flow. However, details of this hypothesis as a preventive mechanism are not well understood. To prevent hair loss completely and promote recovery, the development of novel treatments is needed.
The ALAD DHLHisZn is a potent antioxidant, which is stable even in air. 11 , 12 Its transdermal application was found to inhibit hair loss in a CIA model, and histological findings showed that it reduced inflammatory cell infiltration of the skin and destruction of hair bulbs and hair shaft structures caused by anticancer drugs. 12 According to clinical features of chemotherapy‐induced hair loss, 13 we have demonstrated the promotion of recovery after CIA by transdermal administration of ALADs. 14 Our previous study also revealed that the regulation of vascular permeability, oxidative stress, and apoptosis occurring around skin hair bulbs may be a key factor in the prevention and treatment of CIA, 15 but the treatment effects and mechanisms of ALAD for CIA remain to be elucidated. Therefore, this study aimed to examine the therapeutic effects and mechanism of scalp cooling and ALAD for CIA in an animal model.
2. MATERIALS AND METHODS
2.1. Agents
Cyclophosphamide was selected as the anticancer agent for use in this study. This was due to CYPs being widely used in breast cancer treatment in practice and causing high rates of CIA. Moreover, CYP has been shown to induce follicular apoptosis and CIA in mice, including in our previous study. 15 , 16 Cyclophosphamide (030–12,953, FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was used as the anticancer agent to induce CIA. The applied antioxidant was an ALAD, a modified version of alpha‐lipoic acid, which is a potent antioxidant that is more stable in air.
2.2. Animals
Mice have long been used in CIA research, and their short hair cycle is evident. Therefore, it is considered that mice are an established alternative model for CIA in humans. This study utilized 6‐week‐old female ICR mice. The mice were kept in an air conditioning‐controlled facility under a 12‐h light/dark cycle, with free access to food and water. This study was approved by the Animal Care and Use Committee of Oita University (approval number: 202003A).
2.3. Induction of anagen and alopecia
The anagen phase was induced by removing the hair on the dorsal area of the mice. First, the backs of the mice were shaved using a clipper. Next, a dissolved wax/rosin mixture was applied on the backs of the mice under general anesthesia to remove their hair. On the 9th day after hair removal, all hair bulbs were in the anagen phase and thereby suitable as a model of the human hair cycle. As CYP is administered in one dose on the 9th day, anagen is converted to catagen on the 16th day, catagen is converted to telogen on the 24th day, and telogen is converted to anagen on the 30th day. 17 Visible alopecia is completed on the 16th day, and recovery from CIA begins around the 25th day. 16 , 18 Therefore, we defined the period until the 15th day as the early phase, the period from the 16th day to the 29th day as the middle phase, and the period after the 30th day as the late phase. Alopecia was induced by intraperitoneal administration of 120 μg/g of CYP dissolved in PBS. 15 , 16
2.4. Treatments
We used two treatments: DHLHisZn (DHLHZn, 01702, Iwaki & Co., Ltd.), an ALAD, and cooling therapy. ALAD was mixed with white Vaseline® to a concentration of 1% and gently applied at a transdermal dosage of 3 g to the hair‐removed skin on the backs of the mice with a spatula daily. This was performed in the morning, from the 8th day until the mice were sacrificed according to our previous study. 12 During cooling therapy, the backs of the mice were cooled under general anesthesia. A spiral‐formed silicone tube was connected to a chiller (CF302L, Yamato Scientific Co., Ltd), and the back skin of the mice was cooled. Tap water cooled to 4°C was used as the circulating fluid. The required temperature of circulating refrigerant was 4°C, in order to lower the mouse skin temperature to 19°C using this cooling system in the preliminary experiment. The mouse skin in contact with the fluid was set to 19°C and cooling was conducted for 2 h. As there were no existing reports of cooling experiments using mice in CIA, cooling time was set in accordance with human scalp cooling therapy. We allocated five mice each to the following six groups: control group, ALAD group, cooling group, CYP group, CYP + ALAD group and CYP + cooling group. We administered CYP and conducted cooling therapy on the 9th day following hair removal. For the CYP + cooling group, we continuously conducted 30 min of cooling, CYP administration, and then 1.5 h of cooling, for a total of 2 h of cooling. This was in accordance with the human scalp cooling therapy as there were no reported cooling experiments using mice with CIA. For the ALAD and CYP + ALAD groups, the DHLHZn mixture described above was applied once a day from the 8th day after hair removal. Two researchers examined the AS of each mouse every week starting on the 16th day. These were scored from 0 to 4 according to the following scoring criteria; score 0 (0% hair coverage), 1 (approximately 25% hair coverage), 2 (approximately 50% hair coverage), 3 (approximately 75% hair coverage), and 4 (100% hair coverage). 12 The mice were sacrificed on the 10th day (1 day after treatment, early phase), 16th day (7 days after treatment, middle phase), and 37th day (4 weeks after treatment, late phase), it was also confirmed that each mouse was growing appropriately, after which evaluations were conducted.
2.5. Insulin‐like growth factor‐1 (IGF‐1) measurement
We used the dorsal skin of mice collected in the early, middle, and late phases and stored them at −80°C. The skins were sufficiently homogenized, after which the protein concentrations were adjusted to 250 μg/ml and measured using an IGF ELISA kit (ELISA kit, KA0493, Abnova).
2.6. Two‐photon microscopy
In the early phase (10th day; 24 h after anticancer drug administration), we conducted observations by creating a skin flap from the back skin of the mice under general anesthesia. First, we injected 100 μg/100 μl Texas‐Red®‐labeled Lycopersicon esculentum lectin (TL‐1176, Vector Laboratories) into the tail vein of the mice. We excised approximately 2 × 3 cm of skin from the mice under general anesthesia, and the skin on the lateral side was set to enable observation using an upright two‐photon excitation fluorescence microscopy system (A1RMP, Nikon Corporation) from the inside by inverting it while still attached to the body. We injected 3.75 mg of TRITC‐conjugated 70‐kDa dextran (D‐1819, Life Technologies Corporation) and 1.25 mg of FITC‐conjugated 150‐kDa dextran (FD150S‐1G, Sigma‐Aldrich) that were dissolved in 50 μl PBS into the mice eyeball and conducted observations. 15 We randomly imaged and evaluated three locations from each mouse to reduce selection bias. Analyses were conducted using NIS‐Elements (Nikon, Tokyo, Japan). The blood vessel section was extracted, and the fluorescent intensity of the section outside the blood vessel was measured using Texas‐Red®‐drawn images. Similarly, the fluorescence intensity of the section outside the blood vessel was measured in the composite TRITC and FITC images. The fluorescence intensity of the composite image was compared based on the fluorescent intensity of the Texas‐Red®‐drawn images.
2.7. Immunofluorescence and TUNEL staining
The dorsal skin of mice from each phase was collected and cryopreserved in a −80°C OCT compound, from which 9‐μm sections were made. Apoptosis was evaluated using a TUNEL staining kit (In Situ Cell Death Detection Kit; Roche Diagnostics), and staining was conducted according to the manufacturer's protocol. Counterstaining was conducted using DAPI (Thermo Fisher Scientific). Staining of the endothelium of the blood vessels was conducted using rabbit polyclonal anti‐CD31 antibodies (1:40 dilution, ab28364, Abcam plc) and Alexa 594 donkey antirabbit IgG (1:400 dilution, 2,066,086, Life Technologies). Two researchers randomly selected three capillaries near the hair bulb from the skin samples of each mouse. The capillaries were identified by CD31 staining. The number of TUNEL‐positive cells in the area was counted by two researchers in a blind fashion, and the average number of TUNEL‐positive cells in each group was compared.
2.8. Statistical analysis
The mean and standard error values are shown for the data. Results were analyzed using SPSS for Mac version 28 (IBM Corp). Statistical significance was determined using Student's t‐test, with a p‐value less than 0.05 considered statistically significant.
3. RESULTS
3.1. Assessment of alopecia score
Two researchers evaluated the extent of AS of each mouse on the 16th, 23rd, 30th, and 37th day and the mean score of the five mice in each group is shown (Figure 1). All groups of mice grew adequately in terms of body weight and visible nutritional status. On the 16th day, the CYP group had a significantly lower AS than the control, ALAD, and cooling groups. Similarly, on the 23rd and 30th day, the CYP group had a significantly lower AS than the control, ALAD, and cooling groups. Meanwhile, on the 23rd and 30th days, the CYP + ALAD and CYP + cooling groups showed improvements in AS compared with that in the CYP group. On the 37th day, no significant differences were noted in AS among the all groups (Figure 1).
FIGURE 1.
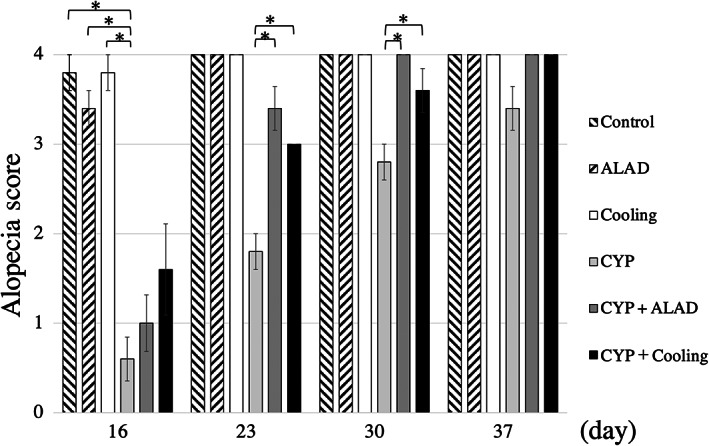
Group alopecia scores by week. The alopecia score is a 5‐point scale where 0 means complete alopecia and 4 means complete hair growth. Means and standard errors of the alopecia score are shown as vertical and error bars, respectively. Statistical analysis was performed using Student's t‐test (*p < 0.05)
3.2. Histological changes in hair bulb diameter
We measured hair bulb diameter of three subcutaneous tissues from a section of the dorsal skin from each mouse (Figure 2A). In the early phase, the hair bulb diameter was significantly shorter in the CYP group than in the control group (p < 0.05; Figure 2B). Meanwhile, no significant shortening of hair bulb diameter was avoided in the CYP + cooling groups compared with that in the CYP group (p < 0.05; Figure 2B). In the middle and late phases, no significant differences were noted in the hair bulb diameter of the subcutaneous tissue (Figure 2C,D).
FIGURE 2.
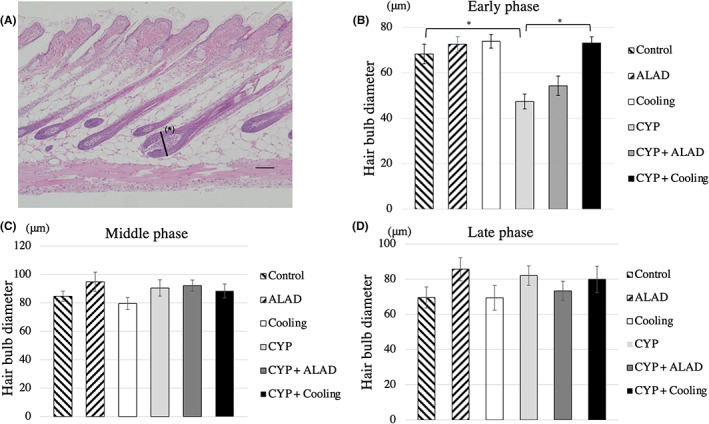
Subcutaneous tissue measurements. A, Subcutaneous histology at the early phase in the control group. We measured the hair bulb diameter of the largest split surface (*). Scale bars, 100 μm. B–D, Comparison of the hair bulb diameter in the early phase, in the middle phase, and in the late phase. Means and standard errors of the hair bulb diameter are shown as vertical and the error bars, respectively. Statistical analysis was performed using Student's t‐test (*p < 0.05)
3.3. IGF‐1 measurement in skin tissue
In the early phase, there were no significant differences in the IGF‐1 level among the groups (Figure 3A). However, in the middle phase, the IGF‐1 level was significantly lower in the CYP group than in the control group and significantly higher in the CYP + ALAD and CYP + cooling groups than in the CYP group (p < 0.05; Figure 3B). In the late phase, there were no significant differences in the IGF‐1 level among the groups (Figure 3C).
FIGURE 3.
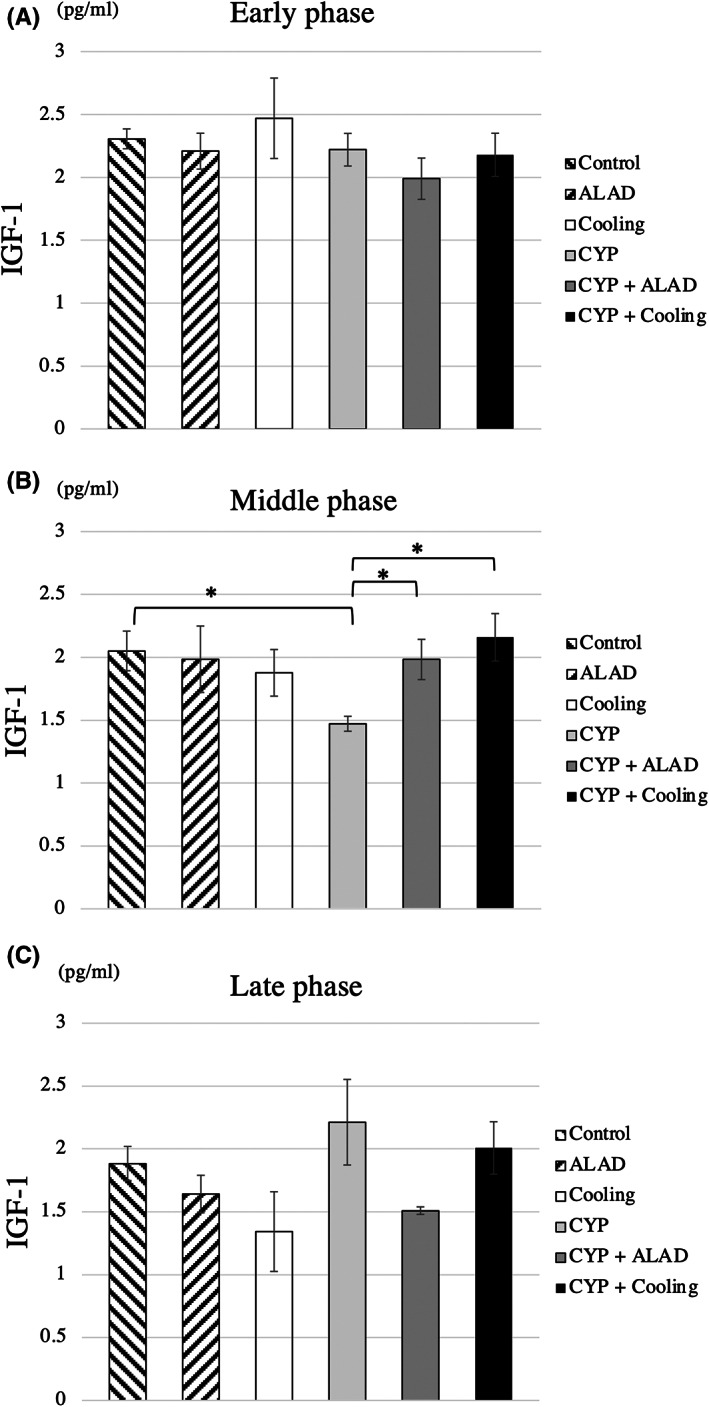
IGF‐1 measurements. Comparison of IGF‐1 in the early (A), middle (B), and late phases (C). Means and standard errors of IGF‐1 are shown as the vertical and the error bars, respectively. Statistical analysis was performed using Student's t‐test (*p < 0.05)
3.4. Visualization of vascular permeability
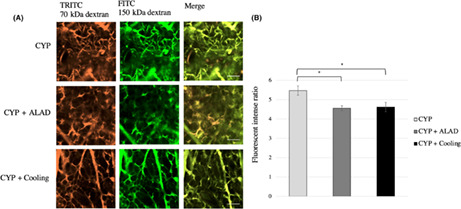
Changes in vascular permeability in the early phase were evaluated using two‐photon microscopy (Figure 4A). We obtained images combining TRITC‐conjugated 70‐kDa dextran and FITC‐conjugated 150‐kDa dextran (Figure 4A). There was a significant decrease in the fluorescent intensity ratio in the CYP + ALAD and CYP + cooling groups compared with that in the CYP group (p < 0.05; Figure 4B).
FIGURE 4.
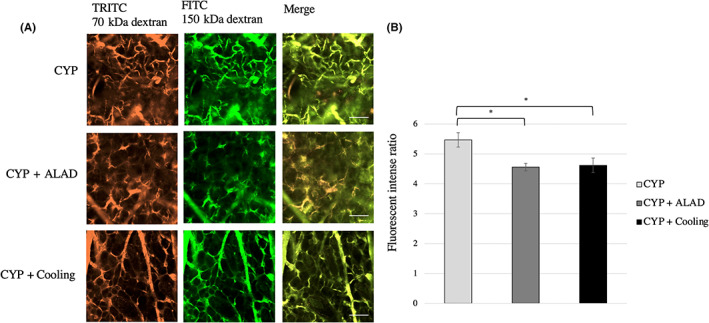
Visualization of vascular permeability. A, Changes in vascular permeability caused by CYP 120 μg/g and detected by two‐photon microscopy. Approximately 24 h after the injection of CYP, two substances of different molecular weights were injected and evaluated by two‐photon microscopy. TRITC fluorescence is bound to the 70‐kDa dextran and FITC is bound to the 150‐kDa dextran. The scale bars represent 100 μm. B, Comparison of the fluorescent intense ratio in the early phase. Means and standard errors of the luminance relative to the Texas‐Red® are shown as the vertical and the error bars, respectively. Statistical analysis was performed using Student's t‐test (*p < 0.05)
3.5. Apoptotic changes of vascular endothelium in skin tissue by treatment
Changes in apoptosis of vascular endothelial cells were compared by fluorescent immunostaining. The results of fluorescent immunostaining in the early stage are shown (Figure 5A), and the number of apoptotic cells in vascular endothelium during the early phase are shown (Figure 5B). We counted apoptotic cells in three capillaries from each mouse. In the early phase, the number of apoptotic cells in capillaries was reduced in the CYP + ALAD group compared with that in the CYP group (p < 0.05; Figure 5B).
FIGURE 5.
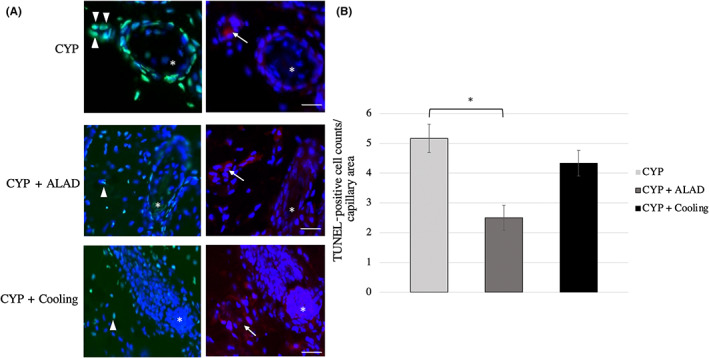
Apoptotic changes of vascular endothelium in skin tissue by treatment. A, Fluorescent immunostaining image of subcutaneous tissue including hair bulb (*) in the early stage. White arrowheads and white arrows indicate TUNEL‐positive cells and vascular endothelium cells, respectively. TUNEL, green; CD31, red; and DAPI, blue. Scale bars, 50 μm. B, Comparison of the vascular endothelium apoptosis in the early phase. Means and standard errors of the TUNEL‐positive cell counts/capillary area are shown as the vertical and the error bars, respectively. Statistical analysis was performed using Student's t‐test (*p < 0.05)
4. DISCUSSION
This study investigated the efficacy of different treatments for CIA and found that each studied intervention promoted early recovery of CIA, including ALAD and cooling therapy. The shortening of the hair bulb diameter and the decrease in the IGF‐1 level due to CYP administration were thought to be maintained by the regulation of vascular permeability, exhibiting an early recovery from alopecia.
In AS, we found that each treatment group accelerated recovery from CIA. Yoneda et al. showed that by maintaining IGF‐1 expression at the 16th day following hair removal in mice, the extent of CIA was reduced. 19 Therefore, we focused on IGF‐1 as a factor in recovery from CIA. 20 IGF‐1 is expressed in the epidermis stratum granulosum, fibroblasts, and dermal papilla cells. 21 It has been reported that IGF‐1 acts as both an autocrine and a paracrine factor. 19 , 22 As both cooling therapy and ALAD are topical treatments, we assumed that IGF‐1 acts as a paracrine factor in hair loss through secretion from skin cells. It also affects hair bulb growth, hair growth cycle, and hair bulb differentiation. 23 Reportedly, IGF‐1 plays a key role in maintaining hair bulbs in the anagen phase, 21 and its expression in dermal papilla cells increases rapidly in the catagen and at the beginning of telogen phases of the hair growth cycle, promoting the transition to the anagen phase. 17 Neonatal mice usually transition from the catagen to telogen phase and exhibit a rapid increase in the IGF‐1 level in the second to third week after birth. 17 While an adult mouse model was used in this study, it has been reported that by aligning the growth phase through hair removal, the growth phase shifts from mature anagen to catagen by the 15th day, and enters the mature anagen phase again after the 30th day. 16 , 17 In a previous study, we also reported that changes occurred in the perifollicular area 24 h after anticancer drug administration. 15 Therefore, in this study, the 10th day, 24 h after administration of CYP was evaluated as early phase, the 16th day as middle phase, and the 37th day as late phase. In this study, IGF‐1 expression in the middle phase was lower in the CYP group than in the control group, and it took time for levels to recover. Meanwhile, IGF‐1 expression in the treatment group was maintained at the same level as that in the control group, and there is the possibility of reduced damage to IGF‐1‐producing cells, maintenance of the anagen phase, and promotion of hair bulb transition and hair recovery.
In this study, two‐photon microscopy investigation showed that increased vascular permeability caused by the anticancer drugs was suppressed by each treatment. CYP has been reported to have a high sensitivity to vascular endothelial cells and to induce apoptosis of vascular endothelial cells. 24 Previously, we have also reported that the pathological condition of increased vascular permeability is potentially due to the induction of apoptosis in those vascular endothelial cells. 15 We demonstrated that the suppression of this pathological condition may promote hair recovery, and that the effectiveness of these treatments may be due to the regulation of this pathological condition. The effect of cooling therapy on hair loss has been clinically applied by reducing the distribution of anticancer drugs to the scalp due to its vasoconstricting action. In this study, we investigated the effects of cooling therapy on CIA in an in vivo model for the first time, in which we showed its alopecia‐suppressing effects. A previous study on mice reported that stimulation by skin cooling stimulated α2 receptors in vascular smooth muscle, constricted blood vessels, and reduced blood flow. 25 Various substances, such as nitric oxide and vasopressin, are involved in vasoconstriction and decreased blood flow due to skin cooling. 24 , 26 In this study, the model skin was cooled to a set temperature of 19°C, referencing the effective set temperature used in clinical practice. Increased vascular permeability was suppressed by the 24th hour after administration of the anticancer drug, which was likely to be the result of a cascade of events caused by cooling, such as stimulation of vascular smooth muscle, vasoconstriction, and decreased blood flow. While hair matrix cells were not investigated in this study, the results suggest that apoptosis of the hair matrix cells was reduced, and recovery was promoted as a result of the decrease in the distribution amount of the anticancer drug in the tissues around the hair bulb due to the suppression of the vascular permeability caused by the anticancer drugs.
ALAD also suppressed CYP‐induced vascular permeability. ALAD is an antioxidant that is stable even in air and has been shown to not only suppress skin inflammation by anticancer drugs but also have in vivo antioxidant and antiapoptotic effects. 11 , 12 CIA is reportedly caused by increased active oxygen production, also known as oxidative stress, around the hair bulbs and the subsequent induction of apoptosis of the hair matrix cells, against which antioxidants have been shown to be useful. 19 , 27 , 28 Our current immunostaining study confirmed that the apoptotic cells in the vascular endothelium decreased in the ALAD‐administered group, suggesting that ALAD administration before and after anticancer drug administration affected the vascular endothelium, enhanced its stability, and controlled its permeability. This may have suppressed the leakage of anticancer drugs around the hair bulbs and the subsequent oxidative stress in a similar manner as that noted in cooling therapy. Furthermore, ALAD application may be less invasive to the body compared with cooling therapy, and continuous administration is possible, so it is expected that the oxidative stress and apoptosis that occur in the late stages of anticancer drug administration can also be regulated. In this study, effective ALAD treatment was achieved through 4 weeks of continuous administration however, in reality, vascular permeability was regulated even by the 24th hour, and the effect of promoted recovery was shown from an early stage (1 week after anticancer drug administration). Therefore, future studies should focus on investigating the effective application period.
There are several limitations in this study. First, this study used only a single anticancer drug (CYP), and we will need to conduct further studies on the effects of interventional treatment using other anticancer drugs that cause alopecia with high frequency, such as taxanes and anthracyclines. Second, this study used mice, and differences between mice and humans are not denied in terms of the hair cycle. Third, the effect of alpha‐lipoic acid self‐production in vivo by cooling, and the effect of self‐produced lipoic acid on alopecia are unknown. Therefore, the influence of lipoic acid in vivo on the study results cannot be completely excluded.
CIA is commonly perceived as a serious problem that needs to be solved; therefore, there is a need for novel potential solutions. We showed that cooling therapy and ALAD each had the effect of promoting recovery from CIA in CYP‐induced CIA mouse models. In the future, clinical studies are needed to evaluate the effect of these treatments in humans.
AUTHOR CONTRIBUTIONS
Takayuki Aiba, Yoko Kawano, and Yusuke Oshima conducted the experiments. Takayuki Aiba, Yohei Kono, and Yusuke Oshima drafted the manuscript with support from Tsuyoshi Etoh. Takayuki Aiba and Yohei Kono analyzed the data. Masafumi Inomata supervised this project. All authors discussed the results and contributed to the final manuscript.
FUNDING INFORMATION
This study is founded by JSPS KAKENHI grant number JP19K18031, the Grant for Joint Research Project of The Institute of Medical Science, The University of Tokyo, and Aderans Co., Ltd. (project number: C213F01015).
CONFLICT OF INTEREST
Yohei Kono and Masafumi Inomata belong to Department of Advanced Medical Research and Development for Cancer and Hair [Aderans], which is supported by donation from Aderans Co., Ltd. The other authors have no conflicts of interest.
ETHICAL APPROVAL
Approval of the research protocol by an Institutional Reviewer Board: This study was approved by the Animal Care and Use Committee of Oita University (approval number: 202003A). Informed Consent: N/A. Registry and the Registration No. of the study/trial: N/A. Animal Studies: Yes.
ACKNOWLEDGMENTS
We thank Ms. Nozomi Ito, Ms. Yoko Kudo, Ms. Mayumi Wada, Mr. Masaaki Utsugi and Ms. Chie Kondo for their valuable assistance, Dr. Noriko Sagawa for valuable advice and Dr. Yoshinori Shirasaka and Dr. Masahiro Kitagawa for their support in animal management. Furthermore, we thank Prof. Mutsuhiro Takekawa and Dr. Noriko Tokai‐Nishizumi for their productive discussions and technical support with two‐photon microscopy in the Imaging Core Laboratory, The Institute of Medical Science, The University of Tokyo.
Aiba T, Kono Y, Etoh T, Kawano Y, Oshima Y, Inomata M. Efficacy of cooling therapy and α‐lipoic acid derivative against chemotherapy‐induced alopecia in an animal model. Cancer Sci. 2023;114:1007‐1014. doi: 10.1111/cas.15639
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Nozawa K, Shimizu C, Kakimoto M, et al. Quantitative assessment of appearance changes and related distress in cancer patients. Psychooncology. 2013;22(9):2140‐2147. doi: 10.1002/pon.3268 [DOI] [PubMed] [Google Scholar]
- 2. Hesketh P, Batchelor D, Golant M, Lyman G, Rhodes N, Yardley D. Chemotherapy‐induced alopecia: psychosocial impact and therapeutic approaches. Support Care Cancer. 2004;12(8):543‐549. doi: 10.1007/s00520-003-0562-5 [DOI] [PubMed] [Google Scholar]
- 3. Freites‐Martinez A, Shapiro J, Goldfarb S, et al. Hair disorders in patients with cancer. J Am Acad Dermatol. 2019;80(5):1179‐1196. doi: 10.1016/j.jaad.2018.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trueb RM. Chemotherapy‐induced alopecia. Semin Cutan Med Surg. 2009;28(1):11‐14. doi: 10.1016/j.sder.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 5. Giaccone G, Di Giulio F, Morandini MP, Calciati A. Scalp hypothermia in the prevention of doxorubicin‐induced hair loss. Cancer Nurs. 1988;11(3):170‐173. [PubMed] [Google Scholar]
- 6. Untch M, Möbus V, Kuhn W, et al. Intensive dose‐dense compared with conventionally scheduled preoperative chemotherapy for high‐risk primary breast cancer. J Clin Oncol. 2009;27(18):2938‐2945. doi: 10.1200/JCO.2008.20.3133 [DOI] [PubMed] [Google Scholar]
- 7. Cancer JAoSCi . Clinical Guideline for Appearance Care in Cancer Treatment. KANEHARA & Co., LTD; 2021:2021. [Google Scholar]
- 8. Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. Jama. 2017;317(6):596‐605. doi: 10.1001/jama.2016.20939 [DOI] [PubMed] [Google Scholar]
- 9. Smetanay K, Junio P, Feißt M, et al. COOLHAIR: a prospective randomized trial to investigate the efficacy and tolerability of scalp cooling in patients undergoing (neo)adjuvant chemotherapy for early breast cancer. Breast Cancer Res Treat. 2019;173(1):135‐143. doi: 10.1007/s10549-018-4983-8 [DOI] [PubMed] [Google Scholar]
- 10. Bajpai J, Kagwade S, Chandrasekharan A, et al. Randomised controlled trial of scalp cooling for the prevention of chemotherapy induced alopecia. Breast. 2020;49:187‐193. doi: 10.1016/j.breast.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masuda T, Iwashita Y, Hagiwara S, et al. Dihydrolipoyl histidinate zinc complex, a new antioxidant, attenuates hepatic ischemia‐reperfusion injury in rats. J Gastroenterol Hepatol. 2011;26(11):1652‐1658. doi: 10.1111/j.1440-1746.2011.06773.x [DOI] [PubMed] [Google Scholar]
- 12. Hagiwara S, Uchida T, Koga H, et al. The alpha‐lipoic acid derivative sodium zinc dihydrolipoylhistidinate reduces chemotherapy‐induced alopecia in a rat model: a pilot study. Surg Today. 2011;41(5):693‐697. doi: 10.1007/s00595-010-4481-z [DOI] [PubMed] [Google Scholar]
- 13. Watanabe T, Yagata H, Saito M, et al. A multicenter survey of temporal changes in chemotherapy‐induced hair loss in breast cancer patients. PLoS One. 2019;14(1):e0208118. doi: 10.1371/journal.pone.0208118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sagawa N, Ohno S, Hiratsuka T, et al. The utility of DHL‐HisZnNa, a novel antioxidant, against anticancer agent‐induced alopecia in breast cancer patients: a multicenter phase II clinical trial. Breast Cancer Res Treat. 2019;176(3):625‐630. doi: 10.1007/s10549-019-05164-5 [DOI] [PubMed] [Google Scholar]
- 15. Sagawa N, Oshima Y, Hiratsuka T, Kono Y, Etoh T, Inomata M. Role of increased vascular permeability in chemotherapy‐induced alopecia: In vivo imaging of the hair follicular microenvironment in mice. Cancer Sci. 2020;111(6):2146‐2155. doi: 10.1111/cas.14396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hendrix S, Handjiski B, Peters EM, Paus R. A guide to assessing damage response pathways of the hair bulb: lessons from cyclophosphamide‐induced alopecia in mice. J Invest Dermatol. 2005;125(1):42‐51. doi: 10.1111/j.0022-202X.2005.23787.x [DOI] [PubMed] [Google Scholar]
- 17. Schlake T, Beibel M, Weger N, Boehm T. Major shifts in genomic activity accompany progression through different stages of the hair cycle. Gene Expr Patterns. 2004;4(2):141‐152. doi: 10.1016/j.modgep.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 18. Paus R, Handjiski B, Eichmuller S, Czarnetzki BM. Chemotherapy‐induced alopecia in mice. Induction by cyclophosphamide, inhibition by cyclosporine a, and modulation by dexamethasone. Am J Pathol. 1994;144(4):719‐734. [PMC free article] [PubMed] [Google Scholar]
- 19. Yoneda K, Fujii M, Imaoka A, et al. Preventive effect of edaravone ointment on cyclophosphamide‐chemotherapy induced alopecia. Support Care Cancer. 2021;29(10):6127‐6134. doi: 10.1007/s00520-021-06189-7 [DOI] [PubMed] [Google Scholar]
- 20. Philpott MP, Sanders DA, Kealey T. Effects of insulin and insulin‐like growth factors on cultured human hair bulbs: IGF‐I at physiologic concentrations is an important regulator of hair bulb growth in vitro. J Invest Dermatol. 1994;102(6):857‐861. doi: 10.1111/1523-1747.ep12382494 [DOI] [PubMed] [Google Scholar]
- 21. Cook JJ, Haynes KM, Werther GA. Mitogenic effects of growth hormone in cultured human fibroblasts. Evidence for action via local insulin‐like growth factor I production. J Clin Invest. 1988;81(1):206‐212. doi: 10.1172/JCI113296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rudman SM, Philpott MP, Thomas GA, Kealey T. The role of IGF‐I in human skin and its appendages: morphogen as well as mitogen? J Invest Dermatol. 1997;109(6):770‐777. doi: 10.1111/1523-1747.ep12340934 [DOI] [PubMed] [Google Scholar]
- 23. Weger N, Schlake T. Igf‐I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol. 2005;125(5):873‐882. doi: 10.1111/j.0022-202X.2005.23946.x [DOI] [PubMed] [Google Scholar]
- 24. Hodges GJ, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J Physiol. 2006;574(Pt 3):849‐857. doi: 10.1113/jphysiol.2006.109884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishikawa T. In vivo analysis of skin microcirculation in rats and mice. Folia Pharmacol Jpn. 2008;132(2):79‐82. doi: 10.1254/fpj.132.79 [DOI] [PubMed] [Google Scholar]
- 26. García‐Villalón AL, Padilla J, Monge L, et al. Effects of vasopressin on the sympathetic contraction of rabbit ear artery during cooling. Br J Pharmacol. 1999;126(3):785‐793. doi: 10.1038/sj.bjp.0702345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiménez JJ, Huang HS, Yunis AA. Treatment with ImuVert/N‐acetylcysteine protects rats from cyclophosphamide/cytarabine‐induced alopecia. Cancer Invest. 1992;10(4):271‐276. doi: 10.3109/07357909209032751 [DOI] [PubMed] [Google Scholar]
- 28. Dunnill CJ, Al‐Tameemi W, Collett A, Haslam IS, Georgopoulos NT. A clinical and biological guide for understanding chemotherapy‐induced alopecia and its prevention. Oncologist. 2018;23(1):84‐96. doi: 10.1634/theoncologist.2017-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


