Abstract
In lung cancer, tumor‐associated macrophages (TAMs), especially M2‐like TAMs, represent the main tumor progression components in the tumor microenvironment (TME). Therefore, M2‐like TAMs may serve as a therapeutic target. The purpose of this study was to investigate the effect of M2‐like TAM depletion in the TME on tumor growth and chemotherapy response in lung cancer. The levels of secreted monocyte chemoattractant protein (MCP‐1) and prostaglandin E2 (PGE2) in the supernatants of lung cancer cell lines A549 and LLC were evaluated via ELISA. Cell migration assays were performed to assess the recruitment ability of macrophage cell lines THP‐1 and J774‐1 cells. Differentiation of macrophages was assessed via flow cytometry. Immunohistochemical staining was performed to visualize M2‐like TAMs in transplanted lung cancer in mouse. We used the COX‐2 inhibitor nimesulide to inhibit the secretion of MCP‐1 and PGE2, which promotes macrophage migration and M2‐like differentiation. Nimesulide treatment decreased the secretion of MCP‐1 and PGE2 from lung cancer cells. Nimesulide treatment suppressed the migration of macrophages by blocking MCP‐1. Lung cancer supernatant induced the differentiation of macrophages toward the M2‐like phenotype, and nimesulide treatment inhibited M2‐like differentiation by blocking MCP‐1 and PGE2. In the lung cancer mouse model, treatment with nimesulide depleted M2‐like TAMs in the TME and enhanced the tumor inhibitory effect of cisplatin. Our results indicated that blocking the secretion of MCP‐1 and PGE2 from tumor cells depleted M2‐like TAMs in the TME and the combination therapy with cisplatin considerably suppressed tumor growth in the LLC mouse model.
Keywords: lung cancer, M2‐like tumor‐associated macrophages, monocyte chemoattractant protein‐1, prostaglandin E2, tumor microenvironment
Nimesulide blocked the secretion of MCP‐1 and PGE2 from tumor cells, resulting in the depletion of M2 tumor‐associated macrophages (TAMs) in the tumor microenvironment (TME). Combination therapy of nimesulide with cisplatin considerably suppressed tumor growth in the LLC mouse model.
Abbreviations
- CSF‐1
colony‐stimulating factor‐1
- MCP‐1
monocyte chemoattractant protein‐1
- PGE2
prostaglandin E2
- PSA
penicillin‐streptomycin‐amphotericin B
- TAM
tumor‐associated macrophage
- TBS‐T
tris‐buffered saline with Tween 20
- TME
tumor microenvironment
1. INTRODUCTION
Lung cancer is the leading cause of cancer‐related mortality worldwide. 1 Despite the availability of molecular targeted therapies, immunotherapies, and other anticancer agents for patients with lung cancer, recurrence and disease progression due to drug resistance are inevitable. 2 Lung cancer cells can interact with immune cells and alter immune cell phenotypes that may contribute to the establishment of a tumor‐supporting environment, which indicates resistance to cancer therapy. 3 As one of the main regulatory components in the tumor microenvironment (TME), tumor‐associated macrophages (TAMs) are especially important in lung cancer, given their significant correlation with cancer growth, metastasis, patient prognosis, 4 , 5 , 6 and chemotherapy resistance. 7
Previous studies have suggested that TAMs can be functionally subtyped according to polarization status, namely M1‐like and M2‐like, and further demonstrated that M2‐like TAMs play a tumor‐supportive role in lung cancer. 4 As the decrease in M2‐like TAM population in the TME has been reported to block lung cancer growth and metastasis, 4 , 8 , 9 M2‐like TAMs have a potential as a clinical therapeutic target. 10 However, despite the conclusive functional correlation between M2‐like TAMs and lung cancer progression, treatment targeting M2‐like TAMs has not been established in clinical setting.
Monocytes and macrophages can be recruited into tumor sites via the secretion of various mediators by tumor cells, such as monocyte chemoattractant protein‐1 (MCP‐1) and colony‐stimulating factor‐1 (CSF‐1), and can be polarized to M2 TAMs via the secretion of MCP‐1, prostaglandin E2 (PGE2), CSF‐1, and CCN3 (also known as nephroblastoma overexpressed [NOV]). 4 We believe that blocking the secretion of these mediators can decrease the population of M2‐like TAMs in the TME.
MCP‐1 is a key factor in the recruitment and differentiation of TAMs in the TME and is mainly produced by tumor cells in the TME. 11 MCP‐1 promotes recruitment of monocytes from the peripheral blood to the TME and differentiation into TAMs. 7 Furthermore, MCP‐1 polarizes TAMs to M2‐like TAMs. 4 The blockade of MCP‐1 has attracted extensive attention owing to its antitumor activity and is currently under investigation in clinical trials for certain cancers other than lung cancer. 12 , 13 Regarding lung cancer, one study has reported the metastasis‐suppressing effect of MCP‐1 inhibition in preclinical experiments; however, the data on the antitumor effect may be insufficient to conduct clinical trials using MCP‐1 inhibitors for lung cancer. 13
PGE2 is produced by tumor cells in the TME and polarizes TAMs into M2‐like TAMs. 4 Furthermore, PGE2 is associated with carcinogenic tumor proliferation, infiltration, metastasis, and angiogenesis. 14 Preclinical studies have shown that cyclooxygenase‐2 (COX‐2) inhibitors show efficacy to a certain extent in treating non–small cell lung cancer. 14 Therefore, six clinical trials evaluated the use of the COX‐2 inhibitors: celecoxib in four trials, rofecoxib in one trial, and apricoxib in combination with chemotherapy in one trial. However, the use of COX‐2 inhibitors did not improve prognosis. 15 We speculated that PGE2 blockade may be insufficient to suppress tumor progression, and we expect that the dual blockade of both MCP‐1 and PGE2 may strongly inhibit the recruitment of TAMs into the TME and differentiation to M2‐like TAMs, thereby suppressing tumor progression.
In this study, we used the COX‐2 inhibitor nimesulide. Nimesulide is commonly used as a nonsteroidal anti‐inflammatory drug in many countries. Unlike celecoxib which is a major COX‐2 inhibitor, nimesulide suppresses the production of not only PGE2 but also MCP‐1. 16 We hypothesized that nimesulide functions as an anticancer agent by blocking both MCP‐1 and PGE2. Finally, we demonstrated the tumor inhibitory effect of nimesulide in mouse lung cancer models via depletion of M2‐like TAMs in the TME. Furthermore, we considered that it was necessary to combine anticancer drugs and regulation of M2‐like TAMs for tumor suppression. In this study, we used cisplatin, which binds cancer cell DNA, subsequently causing cell death via apoptosis, because it is commonly used as chemotherapeutic agent for lung cancer. 17 The combination therapy of M2‐like TAM depletion and cisplatin treatment showed an enhanced tumor inhibitory effect. Collectively, these novel findings revealed that targeting M2‐like TAMs by blocking both MCP‐1 and PGE2 may have a therapeutic potential for lung cancer, and M2‐like TAM depletion may represent one of the strategies to enhance the effect of lung cancer chemotherapy.
2. MATERIALS AND METHODS
2.1. Cell culture
The human lung adenocarcinoma cell line A549, ABC‐1, human lung squamous cell carcinoma cell line LK‐2, EBC‐1, and the murine carcinoma cell line LLC were provided by the cell resource center for biomedical research of Tohoku University. The human lung adenocarcinoma cell lines H522 and H358 were purchased from the American Type Culture Collection (ATCC). The human lung adenocarcinoma cell line RERF‐LC‐MS was provided by the National Institutes of Biomedical Innovation, Health and Nutrition JCRB Cell Bank. These cell lines were maintained in DMEM (Nacalai tesque) supplemented with 10% FBS (Biosera, South Africa origin) and 1% penicillin‐streptomycin‐amphotericin B (PSA; Fujifilm Wako Pure Chemical Corporation) at 37°C in 5% CO2 in a humidified incubator. The human acute monocytic leukemia cell line THP‐1 and the murine macrophage cell line J774‐1 were also provided by the Cell Resource Center for Biomedical Research of Tohoku University. These cell lines were maintained in RPMI (Nacalai tesque) supplemented with 10% FBS and 1% PSA at 37°C in 5% CO2 in a humidified incubator.
2.2. Reagents
Nimesulide, celecoxib, and cisplatin were purchased from Fujifilm Wako Pure Chemical Corporation. MTT solution (cell titer) was purchased from Promega. Recombinant human MCP‐1 and recombinant mouse MCP‐1 were purchased from R&D systems. PGE2 was purchased from Cayman Chemical. Anti‐human and anti‐mouse IgG polyclonal neutralization antibodies against MCP‐1 and isotype‐matched IgG control were purchased from R&D systems. APC‐conjugated anti‐human and anti‐mouse CD206 antibodies for flow cytometry were purchased from R&D systems. Anti‐mouse and anti‐human CD206 antibodies, anti‐mouse F4/80 antibodies, and anti‐human CD68 antibodies for immunohistochemistry were purchased from Abcam.
2.3. ELISA
A549, H522, H358, ABC‐1, LK‐2, EBC‐1 (5 × 105 cells), RERF‐LC‐MS, and LLC (1 × 105 cells) cells were seeded on a 6‐cm dish and cultured in 10% FBS and 1% PSA‐containing DMEM with nimesulide ranging from 0 to 100 μM for 48 hours. Similarly, A549 (5 × 105 cells), RERF‐LC‐MS (1 × 105 cells), and LLC (1 × 105 cells) cells were seeded on a 6‐cm dish and cultured in 10% FBS and 1% PSA‐containing DMEM with celecoxib ranging from 0 to 10 μM for 48 hours. The supernatants were collected, and the levels of secreted MCP‐1, PGE2, CSF‐1, and CCN3 were determined using ELISA (MCP‐1 kit, R&D systems; PGE2 kit, Cayman Chemical; CSF‐1 kit, R&D systems; CCN3 kit, Boster) according to the manufacturer's instructions.
2.4. MTT assay
A549, RERF‐LC‐MS, LLC, THP‐1, and J774‐1 (1 × 104 cells) cells were seeded into 96‐well plates and treated with nimesulide (0‐100 μM) or celecoxib (0‐10 μM) for 48 hours. Subsequently, 10 μl of MTT solution was added. After 4 hours of incubation, the absorbance values at 492 nm were measured using a microplate reader (TECAN; Infinite M200).
2.5. Cell migration assay
Cell migration assays were performed using 24‐well transwell systems. We used 5‐μm pore transwell inserts (Chemotaxicell; Kurabo) as the upper chamber and 24‐well plates as the lower chamber (Corning). Human THP‐1 (1 × 105 cells) or mouse J774‐1 (1 × 105 cells) cells were placed in the upper chamber containing 200 μl 10% FBS and 1% PSA‐containing RPMI. Human A549 (5 × 105 cells) cells, RERF‐LC‐MS (1 × 105 cells) cells or mouse LLC (1 × 105 cells) cells were seeded on a 6‐cm dish and cultured with nimesulide (0 or 100 μM) in 10% FBS and 1% PSA‐containing RPMI for 48 hours. These supernatants were collected and centrifuged at 530 g to remove the debris and were subsequently referred to as A549, RERF‐LC‐MS, or LLC‐conditioned medium. Subsequently, 500 μl of conditioned medium was placed in the lower chamber. After incubation at 37°C for 24 hours, the cells migrating to the lower surface of the membrane were fixed with 4% paraformaldehyde for 2 minutes and stained with 0.5% crystal violet solution for 2 minutes at room temperature. The migrating cells were counted in 10 randomly photographed fields using a microscope at ×200 magnification. To confirm the association between the migration ability and MCP‐1, we added 1 ng/ml recombinant MCP‐1 to conditioned medium containing nimesulide and assessed the migration of cells. Furthermore, we added 100 ng/ml of MCP‐1 neutralization antibody or isotype‐matched IgG control to the conditioned medium and assessed the migration of cells.
2.6. Macrophage differentiation
Differentiation of THP‐1 monocytes (1 × 106 cells/well) into macrophages was induced via treatment with 100 ng/mL PMA (Fujifilm Wako Pure Chemical Corporation) for 24 hours. These macrophages were referred to as PMA‐treated THP‐1 cells. 18 , 19 A549 (2 × 105 cells), RERF‐LC‐MS (2 × 105 cells), or LLC (2 × 105 cells) cells were seeded on a 10‐cm dish and cultured with nimesulide (0 or 100 μM) in 10% FBS and 1% PSA‐containing RPMI for 48 hours. The supernatant was collected and used as A549 or LLC‐conditioned medium after centrifugation at 1500 rpm to remove the debris. M2‐like differentiation of PMA‐treated THP‐1 (1 × 106 cells/well) or J774‐1 (1 × 106 cells/well) cells was induced by cultivating the cells in A549, RERF‐LC‐MS, or LLC‐conditioned medium for 24 hours, respectively.
2.7. Flow cytometry
CD206 antibodies were used as M2‐like TAM markers. PMA‐treated THP‐1 and J774‐1 cells were collected using a scraper and washed with PBS. Subsequently, the cells were stained with 2 μl APC‐conjugated CD206 antibodies for 30 minutes in 20 μl of FACS buffer (PBS containing 2% FBS and 0.1% sodium azide). After 30 minutes, the cells were washed twice with PBS and resuspended in 500 μl FACS buffer for flow cytometric analysis. Data were analyzed using the FACSCanto flow cytometer (BD biosciences).
2.8. Animals
Female C57BL/6J mice aged 6 weeks were purchased from Japan SLC. The mice were maintained under specific pathogen‐free conditions. All mouse experiments were performed in compliance with the Guidelines for Animal Experimentation from Shiga University of Medical Science (approval number: 2021‐5‐3). The mice were subcutaneously injected in the right flank with 1 × 105 LLC cells on day 0, and palpable tumors developed on day 7.
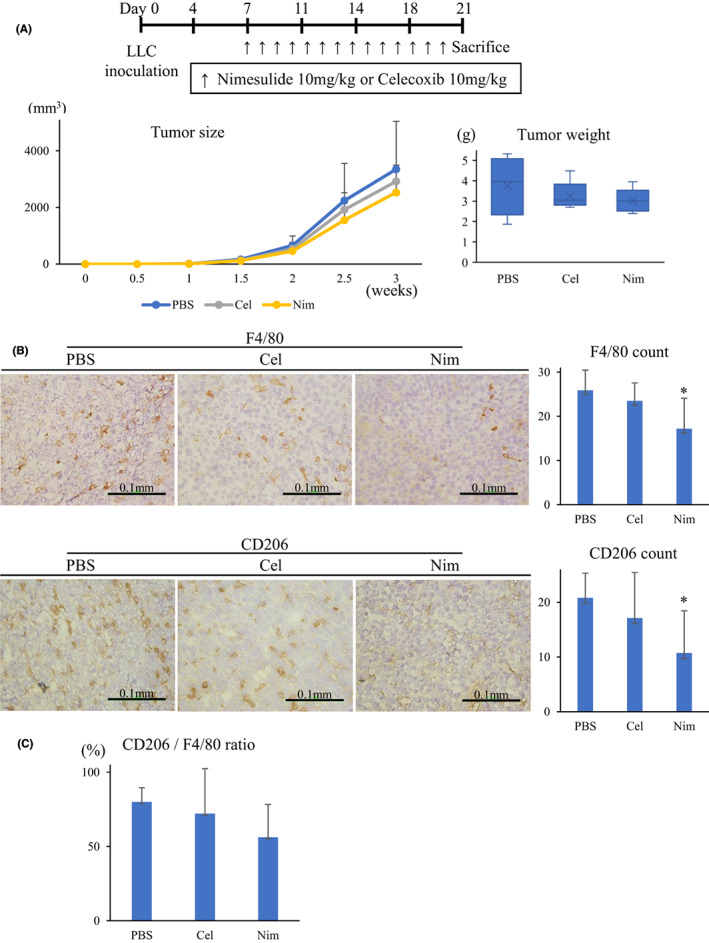
The TAM population‐decreasing and tumor‐inhibitory effects of nimesulide were compared with another COX‐2 inhibitor celecoxib, which is mainly used in many clinical trials for patients with lung cancer. 15 After LLC implantation at day 7, the mice were randomly assigned to control, celecoxib, and nimesulide groups (five mice in each group; Figure 4A). In the control group, 0.1 ml PBS was administered to mice via oral gavage. In the celecoxib and nimesulide groups, 10 mg/kg celecoxib or nimesulide suspended in 0.1 ml PBS was administered to mice via oral gavage. The oral gavage was performed daily between day 7 and day 20. The tumor dimensions were measured twice a week using a caliper, and tumor volumes were calculated using the following formula: a × b 2/2, where a is the largest diameter and b is the smallest diameter. At day 21, all mice were euthanized and the tumor tissue samples were collected, snap‐frozen in liquid nitrogen, and then stored at −80°C until analysis.
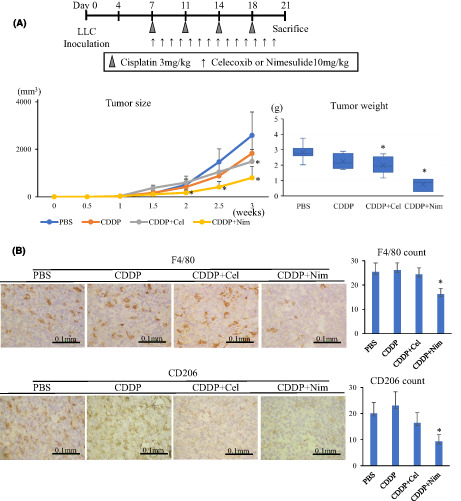
FIGURE 4.
Treatment with nimesulide depletes M2‐like macrophages in the tumor microenvironment. A, Nim treatment showed a minor antitumor effect (n = 5 in each group). B, In the immunohistochemical analysis of the tumor samples, Nim administration decreased F4/80 and CD206 macrophages. C, Nim treatment decreased the ratio of CD206 macrophages. Nim, nimesulide; Cel, celecoxib
The association of TAM population with the effect of anticancer drugs was investigated using cisplatin, which is mainly used as the first‐line treatment for patients with advanced lung cancer. 17 The mice were randomly assigned to the following groups: control, cisplatin, celecoxib+cisplatin, and nimesulide+cisplatin groups (seven mice in each group). In the control group, 0.1 ml PBS was administered to mice via oral gavage and 0.3 ml PBS by intraperitoneal administration. In the cisplatin group, 0.1 ml PBS was administered to mice via oral gavage followed by 3 mg/kg cisplatin dissolved in 0.3 ml PBS. In the celecoxib/nimesulide+cisplatin group, both celecoxib/nimesulide and cisplatin were administered in the same manner as mentioned above. The intraperitoneal administration of cisplatin was performed on days 7, 11, 14, and 18. All mice were monitored for survival and tumor volume as mentioned above. Mice were euthanized on ethical grounds when the tumor diameter exceeded 2.5 cm, and these cases were regarded as deaths.
2.9. Immunohistochemistry of transplanted LLC tissues
Immunohistochemical staining was performed to visualize total TAMs by F4/80 and M2‐like TAMs by CD206 in transplanted LLC tissues. LLC tissues were cut into 5‐μm sections. The frozen sections were air‐dried at room temperature and then acetone fixed for 2 minutes. After rinsing with TBS containing Tween 20 (TBS‐T; Fujifilm Wako Pure Chemical Corporation), the slides were treated with 3% H2O2 to inactivate endogenous peroxidases. Subsequently, the slides were rinsed with TBS‐T and immunostained using a rabbit polyclonal anti‐mouse F4/80 antibody (0.96 μg/ml) and a rabbit polyclonal anti‐mouse CD206 antibody (0.01 μg/ml) and incubated overnight at 4°C. Next, the slides were rinsed with TBS‐T and exposed to secondary antibodies for 60 minutes. After rinsing with TBS‐T, the slides were stained with 3, 3′diamino‐benzidine. Hematoxylin was used as a counter stain. F4/80‐ or CD206‐positive cells were counted in three randomly photographed fields using a microscope at ×400 magnification.
2.10. Tumor tissue samples and immunohistochemistry
Tumor tissue samples were obtained from 63 patients (Table 1) with invasive lung adenocarcinoma who underwent surgery at Shiga University of Medical Science Hospital between January 2014 and October 2015. This study was approved by the Institutional Review Board at Shiga University of Medical Science (R2021‐070). Sections of formalin‐fixed paraffin‐embedded tumor tissues (4 μm) were stained with anti‐human CD68 monoclonal antibody (0.34 μg/ml) and anti‐human CD206 polyclonal antibody (1.0 μg/ml) using the EnVision kit (Dako) according to the manufacturer's instructions. Slides were then treated with substrate/chromogen (Dako), followed by counterstaining with hematoxylin. CD68‐ or CD206‐positive cells in the stroma were counted in three randomly photographed fields using a microscope at 200× magnification. We regarded CD206 expression as high at >3 positively stained cells and CD68 expression as high at >15 positively stained cells. We regarded the ratio as high if CD206/CD68 was ≥20%.
TABLE 1.
Correlation between CD68 or CD206 expression and clinicopathological characteristics of patients with invasive lung adenocarcinoma
| Variables | N | High expression of CD68 N (%) | P value | High expression of CD206 N (%) | P value |
|---|---|---|---|---|---|
| Total | 63 | 26 (41) | – | 21 (33) | – |
| Age (years) | |||||
| ≧65 | 40 | 18 (41) | 0.428 | 13 (33) | 0.853 |
| <65 | 23 | 8 (35) | 8 (35) | ||
| Sex | |||||
| Male | 38 | 21 (55) | 0.005 | 17 (45) | 0.018 |
| Female | 25 | 5 (20) | 4 (16) | ||
| Smoking states | |||||
| Never | 25 | 8 (32) | 0.225 | 6 (24) | 0.202 |
| Smoker | 38 | 18 (47) | 15 (39) | ||
| p‐TNM stage | |||||
| I | 48 | 17 (35) | 0.086 | 14 (29) | 0.209 |
| II | 2 | 2 (100) | 1 (50) | ||
| III | 13 | 7 (54) | 6 (46) | ||
| Histological subtype | |||||
| Mixed subtype | 52 | 22 (42) | 0.398 | 18 (35) | 0.099 |
| Papillary | 5 | 2 (40) | 0 (0) | ||
| Acinar | 3 | 1 (33) | 1 (33) | ||
| Lepidic | 3 | 1 (33) | 1 (33) | ||
| Poorly differentiated | 1 | 0 (0) | 1 (100) | ||
| Tumor recurrence | |||||
| Yes | 16 | 7 (44) | 0.816 | 9 (56) | 0.024 |
| No | 47 | 19 (40) | 12 (26) | ||
2.11. Statistical analysis
Statistical analysis was performed using SPSS Statistics for Windows, version 25 (IBM). Significant differences between groups were determined using Student's t test. The association between variables was analyzed by the χ 2 test. Survival curves were prepared using the Kaplan‐Meier method and compared by the log‐rank test. P values of <0.05 were considered significant.
3. RESULTS
3.1. Nimesulide treatment decreased the secretion of MCP‐1 and PGE2 from lung cancer cells
We think that MCP‐1 is the key mediator that increases M2‐like TAMs in the TME. First, we investigated lung cancer cell lines secreting MCP‐1, namely, five human adenocarcinoma cell lines (A549, RERF‐LC‐MS, H522, H358, ABC‐1) and two human squamous cell carcinoma cell lines (LK‐2, EBC‐1). A549 and RERF‐LC‐MS showed considerable MCP‐1 secretion into the supernatant by ELISA, while the others did not (Figure 1A). Next, we investigated A549 and RERF‐LC‐MS secretion levels of PGE2 (Figure 1B,C), CSF‐1, and CCN3 (Figure S1), which were previously reported as major mediators to increase M2‐like TAMs by promoting migration and M2‐like differentiation. 4 The supernatants contained considerable amounts of PGE2 and moderate to small amounts of CSF‐1 and CCN3. Furthermore, nimesulide treatment at 100 μM decreased the MCP‐1 and PGE2 concentrations in the supernatants of A549 and RERF‐LC‐MS cells (Figure 1B,C), but CSF‐1 and CCN3 did not (Figure S1). Celecoxib treatment at 10 μM did not decrease the MCP‐1 concentrations but decreased the PGE2 concentrations in A549 and RERF‐LC‐MS supernatants (Figure 1B,C). In addition, the murine lung carcinoma cell line LLC secreted considerable amounts of MCP‐1 and PGE2 in the supernatants, and nimesulide treatment decreased the MCP‐1 and PGE2 concentrations (Figure 1D) just as in human A549 and RERF‐LC‐MS cells.
FIGURE 1.
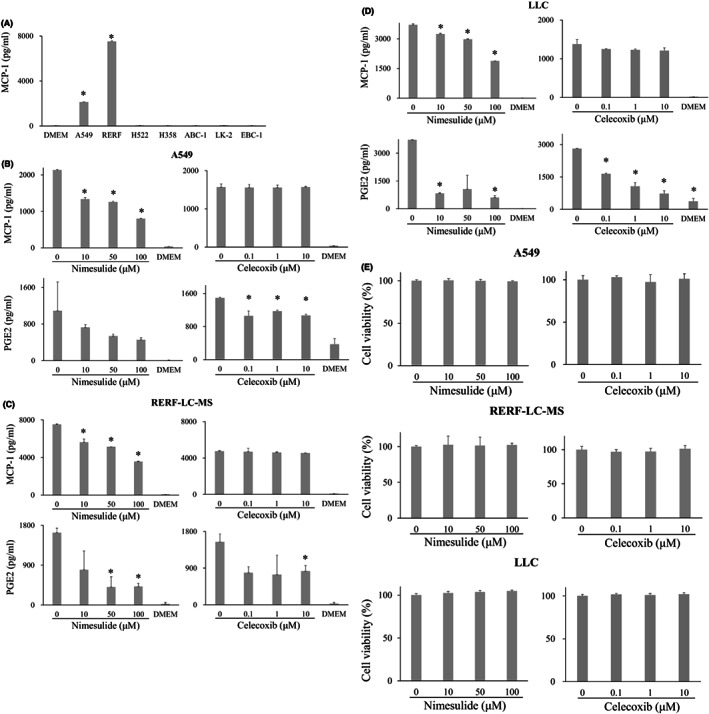
MCP‐1 and PGE2 concentration in the supernatant of lung cancer cell lines. (A), MCP‐1 concentration in the supernatant of A549, RERF‐LC‐MS, H522, H358, ABC‐1, LK‐2, and EBC‐1 cell lines. Treatment of A549(B), RERF‐LC‐MS (C), and LLC cells (D) with 100 μM nimesulide decreased the MCP‐1 and PGE2 concentrations in the supernatant. Celecoxib treatment at 10 μM did not decrease the MCP‐1 concentrations but decreased PGE2 concentration in the supernatant of these cell lines. (E), No significant inhibition of cell growth was observed after treatment with 100 μM nimesulide and 10 μM celecoxib. MCP‐1, monocyte chemoattractant protein‐1; PGE2, prostaglandin E2
As nimesulide is cytotoxic, we analyzed the effect on lung cancer cell proliferation by treating A549, RERF‐LC‐MS, and LLC cells with nimesulide in concentrations ranging from 0 to 100 μM. No significant inhibition of cell growth was observed within this concentration range (Figure 1E). Nimesulide treatment also did not affect THP‐1 and J774‐1 cell proliferation in concentrations ranging from 0 to 100 μM (data not shown). Similarly, we demonstrated that celecoxib treatment did not affect A549, RERF‐LC‐MS, LLC (Figure 1E), THP‐1, or J774‐1 (data not shown) cell proliferation in concentrations ranging from 0 to 10 μM.
3.2. Nimesulide treatment suppressed the migration or chemoattractant ability of monocytes and macrophages via MCP‐1
To clarify whether nimesulide treatment inhibited the migration ability of monocytes and macrophages via MCP‐1, we performed transwell chamber assays using conditioned medium. First, we confirmed that PGE2 blockade did not affect the migration ability of THP‐1 (Figure S2A) and J774‐1 (Figure S2B) cells. The migration of THP‐1 cells decreased when we cultured A549 or RERF‐LC‐MS cells with nimesulide (Figure 2A,B). Similarly, when LLC cells were cultured with nimesulide, the migration of J774‐1 cells was reduced (Figure 2C). Furthermore, recombinant MCP‐1 treatment recovered the migration ability in both cases. We also confirmed that nimesulide treatment did not directly suppress the migration ability of THP‐1 (Figure S3A) and J774‐1 (Figure S3B) cells. These results suggest that nimesulide treatment reduced THP‐1 monocyte and J774‐1 macrophage migration by suppressing MCP‐1 secretion by A549 and LLC cells.
FIGURE 2.
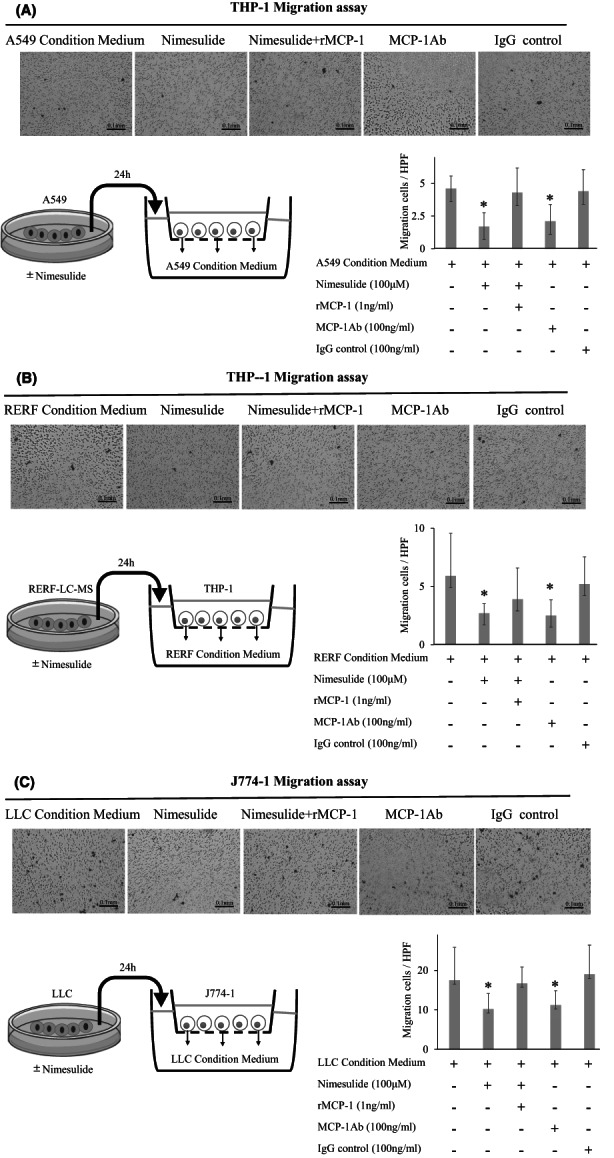
Change in migration ability of monocytes and macrophages based on transwell assays. A, B, The migration of THP‐1 cells decreased A549 or RERF‐LC‐MS cells cultured with nimesulide. Recombinant MCP‐1 treatment recovered the migration ability. C, Similarly, when LLC cells were cultured with nimesulide, the migration of J774‐1 cells was reduced. Recombinant MCP‐1 treatment recovered the migration ability. HPF, high–power field (×200); MCP‐1, monocyte chemoattractant protein‐1
3.3. Lung cancer cells induced the differentiation of macrophages toward the M2‐like phenotype
To confirm that lung cancer induces the differentiation of macrophages toward the M2‐like phenotype, we cultured macrophages with lung cancer cell–conditioned medium. Treatment with A549 or RERF‐LC‐MS–conditioned medium increased the expression of CD206 on PMA‐treated THP‐1 cells (Figure 3A,B). Treatment with LLC‐conditioned medium increased the expression of CD206 on J774‐1 cells (Figure 3C).
FIGURE 3.
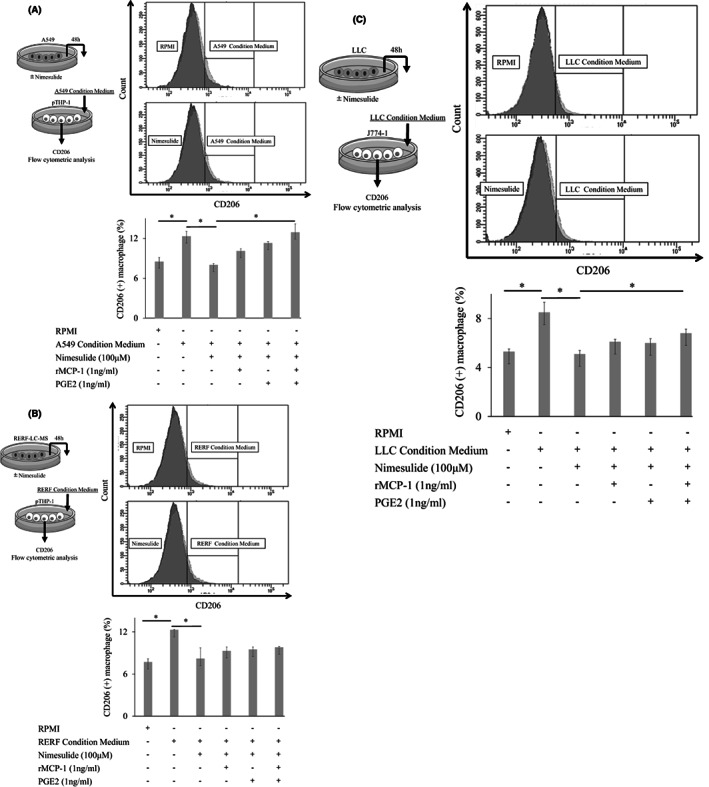
Differentiation of macrophages into M2‐like phenotype. A, B, Treatment with A549 or RERF‐LC‐MS‐conditioned medium increased the expression of CD206 on PMA‐treated THP‐1 cells. When A549 or RERF‐LC‐MS cells were cultured with nimesulide, the conditioned medium did not increase CD206 expression. Furthermore, recombinant MCP‐1 and PGE2 treatment recovered CD206 expression. C, Similarly, LLC‐conditioned medium increased the expression of CD206 on J774‐1 cells. When LLC cells were cultured with nimesulide, the conditioned medium did not increase CD206 expression. Furthermore, recombinant MCP‐1 and PGE2 treatment recovered CD206 expression. rMCP‐1, recombinant monocyte chemoattractant protein‐1; PGE2, prostaglandin E2
3.4. Nimesulide treatment inhibited the differentiation of macrophages into M2‐like phenotype via MCP‐1 and PGE2
As the inhibition of differentiation of macrophages into M2‐like phenotype may represent a therapeutic target for lung cancer, we investigated whether nimesulide treatment actually inhibited differentiation. First, we confirmed that recombinant MCP‐1 and PGE2 treatment increased the expression of CD206 on PMA‐treated THP‐1 and J774‐1 cells (Figure S4A,B). Next, we assessed the inhibitory effect of nimesulide on the differentiation of M2‐like macrophages by blocking MCP‐1 and PGE2 production derived from tumor cells. When we cultured A549 or RERF‐LC‐MS cells with nimesulide, treatment with conditioned medium did not increase the expression of CD206 on PMA‐treated THP‐1 cells (Figure 3A,B). Furthermore, treatment with recombinant human MCP‐1 and PGE2 recovered the expression of CD206 (Figure 3A,B). When LLC cells were cultured with nimesulide, treatment with conditioned medium did not increase the expression of CD206 on J774‐1 cells (Figure 3C). Furthermore, treatment with recombinant mouse MCP‐1 and PGE2 recovered the expression of CD206 (Figure 3C). These results suggested that nimesulide treatment decreased MCP‐1 and PGE2 release from lung cancer cells, resulting in inhibition of differentiation of macrophages into M2‐like TAMs.
3.5. Treatment with nimesulide decreased M2‐like macrophage population and enhanced the tumor inhibitory effect of cisplatin
We investigated whether oral administration of nimesulide could deplete M2‐like TAMs and suppress tumor growth using mouse lung cancer models. Furthermore, we investigated the antitumor effect of combination therapy with cisplatin. First, we evaluated the minor antitumor effect of nimesulide, which was found to be stronger than that of celecoxib (Figure 4A). Immunohistochemical analysis of tumor samples showed that the administration of nimesulide consistently contributed to a significant decrease in F4/80 macrophages (Figures 4B and 5B) and the ratio of CD206 macrophages (Figures 4C and 5C).
FIGURE 5.
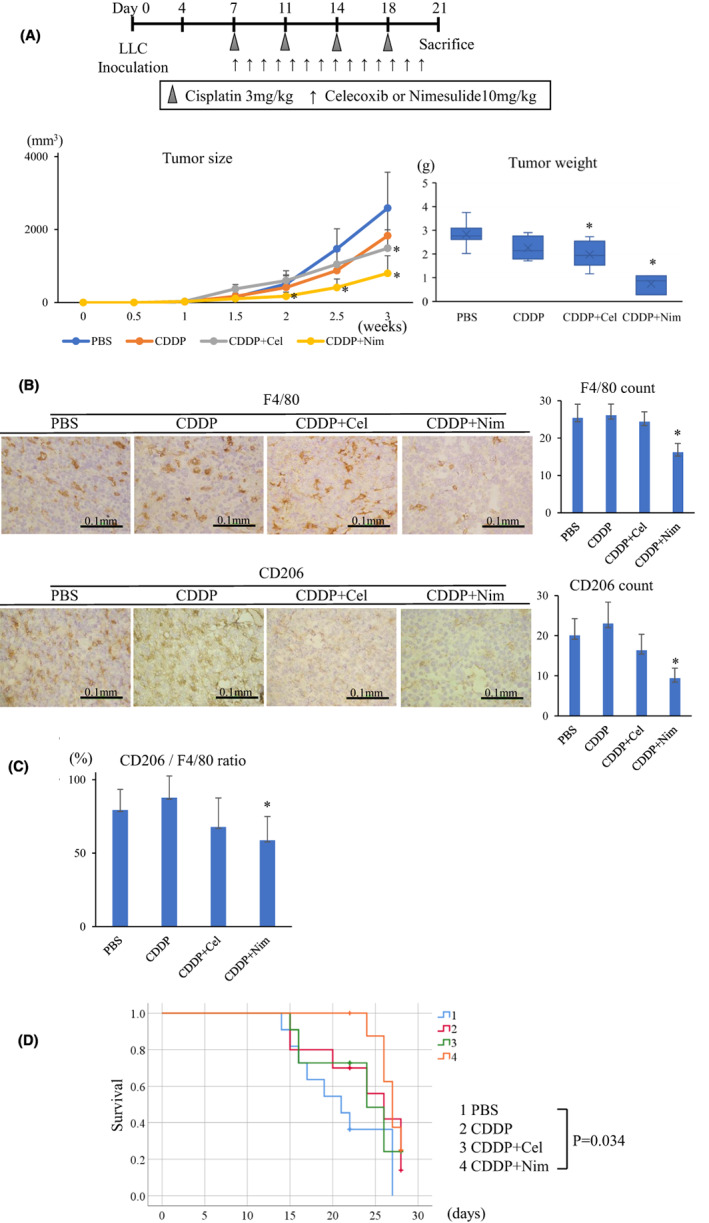
Treatment with nimesulide enhances the tumor inhibitory effect of CDDP. A, The combination therapy of Nim and CDDP had a stronger antitumor effect than that of CDDP monotherapy (n = 7 in each group). B, In the immunohistochemical analysis of the tumor samples, nimesulide administration decreased F4/80 and CD206 macrophages. C, Nim treatment decreased the ratio of CD206 macrophages. D, The combination therapy of Nim and CDDP prolonged survival after LLC inoculation (n = 11 in the PBS group; n = 10 in the CDDP group; n = 11 in the CDDP+Cel group; n = 9 in the CDDP+Nim group). Nim, nimesulide; CDDP, cisplatin; Cel, celecoxib
Next, we assessed the synergistic effect of nimesulide and cisplatin, which is commonly used as a chemotherapeutic agent for lung cancer. 17 Monotherapy of cisplatin suppressed tumor growth compared with the control group. Notably, the combination therapy of nimesulide and cisplatin showed a stronger antitumor effect than cisplatin monotherapy. The tumor burden was the lowest in the combination treatment group compared with the other treatment groups (Figure 5A). Furthermore, the combination therapy of nimesulide and cisplatin significantly prolonged the survival after LLC inoculation (Figure 5D).
3.6. Prognostic significance of CD206‐positive TAMs in human lung adenocarcinoma
To evaluate the association between M2‐like TAMs and prognosis, a series of 63 invasive adenocarcinoma specimens were examined by immunohistochemistry for total TAMs by CD68 and M2‐like TAMs by CD206 (Table 1). First, we evaluated CD68 as a pan‐macrophage marker, and it was expressed on all cancer tissues (Figure 6A) at an average of 15.3 counts (1‐44) per photographed field. CD206 as an M2‐like TAM marker was expressed in 44 cases (70%; Figure 6A) at an average of 2.7 counts (0‐19) per photographed field. The average frequency of M2‐like TAMs in the total macrophages was 18% (0%‐86%). Univariate analysis showed that high expression of CD206 was significantly associated with tumor recurrence after surgery (p = 0.024; Table 1). The expression of CD68 was not associated with prognosis (Figure 6B); however, the high expression of CD206 and the high M2 ratio (CD206/CD68) led to a significantly lower overall survival than that of groups with low CD206 expression and M2 ratios (CD206: Figure 6C; p < 0.002, M2 ratio: Figure 6D; p < 0.038).
FIGURE 6.
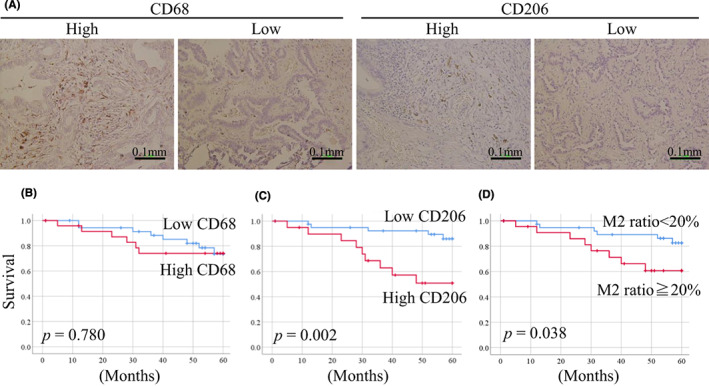
Immunohistochemical staining of human invasive lung adenocarcinoma with CD68 and CD206 antibodies. (A), Cases of high and low staining of CD68 and CD206. (B), Patients with high expression of CD68‐positive TAMs showed no difference in prognosis. (C), Patients with high expression of CD206‐positive M2‐like TAMs and (D) patients with high M2 ratio (100 × CD206/CD68) had poor prognosis
4. DISCUSSION
In lung cancer, TAMs infiltrate into the TME and promote tumorigenesis by interacting with tumor cells. M2‐polarized TAMs or M2‐like TAMs represent important mediators, as they are one of the key contributors to cancer growth and metastasis via angiogenesis and epithelial‐mesenchymal transition, leading to poor prognosis in cancer patients. 4 Presently, M2‐like TAMs are considered therapeutic targets for lung cancer; however, this has not been applied to a clinical setting at present.
We used CD206 as an M2 TAM marker. M2 TAM markers have not been established; however, several studies define M2 TAMs as CD206‐positive and have demonstrated that CD206‐positive TAMs are associated with poor prognosis in patients with lung cancer. 9 , 20 We also showed that the high expression of CD206 led to tumor recurrence and low overall survival in patients with lung adenocarcinoma (Figure 6C,D). In this study, LLC tumor‐bearing C57BL/6J mice treated with nimesulide showed decreased CD206‐positive TAMs in the TME, which was accompanied with reduced tumor growth (Figure 4A). Two studies have reported that the depletion of CD206‐positive TAMs leads to inhibition of LLC growth in vivo. Qin et al. demonstrated that administration of P2X7 inhibitor, which is highly expressed in CD206‐positive TAMs, depletes CD206‐positive TAMs in the TME and reduces tumor growth. 8 Lu et al. demonstrated that administration of Oct‐4 inhibitor, which is a cancer stemness marker, reduces the secretion of CSF‐1 from tumor cells and depletes CD206‐positive TAMs in the TME. Consequently, tumor growth is inhibited. 9 In contrast to these two studies, we achieved the depletion of M2‐like TAMs and reduction of tumor growth using the approved drug nimesulide via oral administration.
Lung cancer cells recruit monocytes to the TME via the secretion of various mediators, such as MCP‐1 21 and CSF‐1, 9 and induce their polarization to M2‐like TAMs via secretion of MCP‐1, PGE2, CSF‐1, and CCN3. 4 Blocking these mediators can deplete M2‐like TAMs in the TME. However, the single blockade of these mediators has been found to be insufficient to suppress tumor growth and has not been applied in clinical therapy. In this study, we used nimesulide as a dual blocker of both MCP‐1 and PGE2.
MCP‐1 functions as an important mediator of macrophage recruitment into the TME and of M2 polarization. Tumor cells secrete MCP‐1 to mobilize bone marrow‐derived monocytes 22 in the peripheral circulation into the TME. 13 These recruited monocytes are named as TAMs and are polarized into M2‐like TAMs by MCP‐1, contributing to tumor cell survival. 22 Treatment with MCP‐1 blockade depletes TAMs in a mouse model with pancreatic ductal adenocarcinoma, 13 breast cancer, 12 and lung cancer. 23 At present, phase 1 clinical trials are ongoing to evaluate the safety and efficacy of MCP‐1 blockade for patients with pancreatic cancer, 24 breast cancer, 25 and colorectal cancer. 26 MCP‐1 blockade‐related adverse events include grade 1‐2 fatigue (9%), nausea (7%), headache (7%), vomiting (5%), and pruritus (5%), and they are judged as well tolerated. 26 In lung cancer, as the overexpression of MCP‐1 in patients with lung adenocarcinoma is associated with poor survival, 27 MCP‐1 blockade may represent a suitable strategy to deplete TAMs in the TME. In the present study, we showed that nimesulide treatment blocked the secretion of MCP‐1 from lung cancer cells (Figure 1B–D). Furthermore, nimesulide treatment reduced human monocyte and mouse macrophage migration (Figure 2A–C) and prevented the differentiation of macrophages into M2 TAMs (Figure 3A,B,D) by suppressing MCP‐1 secretion from lung cancer cells.
PGE2 functions as an important mediator of M2‐like polarization in macrophages. Several studies have suggested that PGE2 induces macrophage polarization toward M2 type, 28 , 29 and treatment of macrophages via PGE2 blockade reduces the expression of CD206 on TAMs in glioblastoma. 30 In the present study, we showed that nimesulide treatment blocked the production of PGE2 from lung cancer cells (Figure 1B,C,D) and prevented the differentiation of macrophages into M2‐like TAMs (3A, 3B, and 3D). Taken together, our data suggest that in the TME, monocytes or macrophages are recruited by the chemoattractant MCP‐1 (Figure 2A,B); subsequently, MCP‐1 and PGE2 induce the differentiation into M2‐like macrophages (Figure 3A,B,D). Thus, MCP‐1 and PGE2 may contribute to the development of an M2‐like TAM‐dominant TME in lung cancer.
Preclinical studies have shown that inhibiting PGE2 activity using COX‐2 inhibitors is effective to a certain extent against non–small cell lung cancer. 14 Based on these findings, clinical trials using COX‐2 inhibitors, mainly celecoxib, have been conducted. However, celecoxib treatment has been found to not improve prognosis. 15 PGE2 blockade may be insufficient in mediating the depletion of M2 TAMs. We demonstrated that in addition to PGE2 blockade, MCP‐1 blockade considerably depleted M2‐like TAMs in the TME compared with that obtained via PGE2 blockade alone, consequently suppressing tumor progression (Figure 4A). These results may also indicate that MCP‐1 is a key mediator for increasing the population of M2‐like TAMs and lung cancer growth. In the future, clinical trials should evaluate the tumor inhibitory effect of nimesulide treatment.
We considered the necessity to combine anticancer drugs and the regulation of M2‐like TAMs for tumor suppression. In the present study, M2‐like TAMs were depleted by blocking MCP‐1 and PGE2 secretion; however, this was not enough to suppress tumor growth (Figure 4A). The depletion of M2‐like TAMs enhanced the tumor inhibitory effect of cisplatin (Figure 5A). The TME changes considerably upon receiving chemotherapy. A previous study on lung cancer showed that neoadjuvant cisplatin treatment is associated with an altered TME with increasing population of TAMs, 31 and a large number of TAMs is associated with poor prognosis. 32 In an experimental study, the repeated exposure of cisplatin to lung cancer cell lines A549 and H460 has been shown to increase the levels of their oncogenic markers, Src and CD155. The cisplatin‐exposed cell lines promote M2‐like polarization of TAMs to a greater extent than that in control cell lines by increasing pro‐M2 cytokine levels such as MCP‐1. The generation of M2‐like TAMs may subsequently facilitate tumor growth even under chemotherapy. Therefore, we expect that depleting M2‐like TAMs may enhance the tumor inhibitory effect of cisplatin. In the present study, we demonstrated that the combination therapy of nimesulide and cisplatin considerably suppressed tumor growth compared with that obtained using cisplatin alone (Figure 5A) and also prolonged survival (Figure 5D). In the future, the depletion of M2‐like TAMs may represent one of the strategies to overcome cisplatin resistance in lung cancer. Furthermore, we expect that many experiments will demonstrate the synergistic effect of M2‐like TAM depletion on not only cisplatin activity but also that of other anticancer drugs.
One of the original aims of this study was to use approved drugs in clinically acceptable doses. Nimesulide is currently administered clinically as pain medication, and our results indicated its ability to inhibit cancer growth. We administered nimesulide at 10 mg/kg daily to mice, which is almost the same as the human administration dose per kg body weight. The nimesulide plasma concentration in mice at the same dose of single administration has been reported to be 77 μM. 33 Furthermore, Li et al. reported no toxicity in mice at a dose of 62.5 mg/kg. 34 We administered celecoxib at a dose of 10 mg/kg daily to mice, which is also almost the same as the human administration dose per kg body weight. The celecoxib plasma level in mice at the same dose has been reported to be 10 μM. 35 Thus, these results indicate that it may be possible to use nimesulide and celecoxib for cancer suppression at a clinically acceptable dose range.
As a study limitation, we examined MCP‐1, PGE2, CSF‐1, and CCN3 as major M2‐like differentiation mediators in this study; however, many other mediators may have the potential to promote the polarization of macrophages into the M2‐like phenotype. We should recognize the possibility that nimesulide also affects other mediators of M2‐like differentiation.
In conclusion, our experimental results provided evidence that MCP‐1 functions as a key mediator for increasing the population of M2‐like TAMs, and the dual inhibition of MCP‐1 and PGE2 by nimesulide treatment led to considerable depletion of M2‐like TAMs in the TME. Finally, our in vivo results demonstrated that nimesulide treatment suppressed tumor growth and the combination therapy with cisplatin considerably suppressed the tumor growth in an LLC mouse model. These results indicated that targeting M2‐like TAM therapy relieved immunosuppression and improved the chemotherapeutic response. Therefore, our findings offer an additional strategy for therapeutic development, as we focus on both tumor cells and their microenvironment.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: All mouse experiments were performed in compliance with the Guidelines for Animal Experimentation from Shiga University of Medical Science (Approval number: 2021‐5‐3).
Supporting information
Figure S1
Figure S2
Figure S3
Figure S4
ACKNOWLEDGMENTS
This study was supported by the YOKOYAMA Foundation for Clinical Pharmacology (YRY‐2005).
Kawaguchi Y, Ohshio Y, Watanabe A, et al. Depletion of tumor‐associated macrophages inhibits lung cancer growth and enhances the antitumor effect of cisplatin. Cancer Sci. 2023;114:750‐763. doi: 10.1111/cas.15671
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Rothschild SI, Gautschi O, Haura EB, Johnson FM. Src inhibitors in lung cancer: current status and future directions. Clin Lung Cancer. 2010;11:238‐242. [DOI] [PubMed] [Google Scholar]
- 3. Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782‐795. [DOI] [PubMed] [Google Scholar]
- 4. Lin Y, Xu J, Lan H. Tumor‐associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hwang I, Kim JW, Ylaya K, et al. Tumor‐associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non‐small cell lung cancer patients. J Transl Med. 2020;18:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liang ZW, Ge XX, Xu MD, et al. Tumor‐associated macrophages promote the metastasis and growth of non‐small‐cell lung cancer cells through NF‐κB/PP2Ac‐positive feedback loop. Cancer Sci. 2021;112:2140‐2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hao Q, Vadgama JV, Wang P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun Signal. 2020;18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qin J, Zhang X, Tan B, et al. Blocking P2X7‐mediated macrophage polarization overcomes treatment resistance in lung cancer. Cancer Immunol Res. 2020;8:1426‐1439. [DOI] [PubMed] [Google Scholar]
- 9. Lu CS, Shiau AL, Su BH, et al. Oct4 promotes M2 macrophage polarization through upregulation of macrophage colony‐stimulating factor in lung cancer. J Hematol Oncol. 2020;13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin Y, Wei C, Liu Y, Qiu Y, Liu C, Guo F. Selective ablation of tumor‐associated macrophages suppresses metastasis and angiogenesis. Cancer Sci. 2013;104:1217‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshimura T. The production of monocyte chemoattractant protein‐1 (MCP‐1)/CCL2 in tumor microenvironments. Cytokine. 2017;98:71‐78. [DOI] [PubMed] [Google Scholar]
- 12. Yumimoto K, Akiyoshi S, Ueo H, et al. F‐box protein FBXW7 inhibits cancer metastasis in a non‐cell‐autonomous manner. J Clin Invest. 2015;125:621‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yumimoto K, Sugiyama S, Mimori K, Nakayama KI. Potentials of C‐C motif chemokine 2‐C‐C chemokine receptor type 2 blockers including propagermanium as anticancer agents. Cancer Sci. 2019;110:2090‐2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yokouchi H, Kanazawa K. Revisiting the role of COX‐2 inhibitor for non‐small cell lung cancer. Transl Lung Cancer Res. 2015;4:660‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dai P, Li J, Ma XP, Huang J, Meng JJ, Gong P. Efficacy and safety of COX‐2 inhibitors for advanced non‐small‐cell lung cancer with chemotherapy: a meta‐analysis. Onco Targets Ther. 2018;11:721‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Genç S, Attar E, Gürdöl F, Kendigelen S, Bilir A, Serdaroğlu H. The effect of COX‐2 inhibitor, nimesulide, on angiogenetic factors in primary endometrial carcinoma cell culture. Clin Exp Med. 2007;7:6‐10. [DOI] [PubMed] [Google Scholar]
- 17. Rossi A, Chiodini P, Sun JM, et al. Six versus fewer planned cycles of first‐line platinum‐based chemotherapy for non‐small‐cell lung cancer: a systematic review and meta‐analysis of individual patient data. Lancet Oncol. 2014;15:1254‐1262. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Du W, Chen Z, Xiang C. Upregulation of PD‐L1 by SPP1 mediates macrophage polarization and facilitates immune escape in lung adenocarcinoma. Exp Cell Res. 2017;359:449‐457. [DOI] [PubMed] [Google Scholar]
- 19. Auwerx J. The human leukemia cell line, THP‐1: a multifacetted model for the study of monocyte‐macrophage differentiation. Experientia. 1991;47:22‐31. [DOI] [PubMed] [Google Scholar]
- 20. Zhang B, Yao G, Zhang Y, Gao J, Yang B, Rao Z. M2‐polarized tumor‐associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics (Sao Paulo). 2011;66:1879‐1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lim SY, Yuzhalin AE, Gordon‐Weeks AN, Muschel RJ. Targeting the CCL2‐CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697‐28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast‐tumour metastasis. Nature. 2011;475:222‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Porrello A, Leslie PL, Harrison EB, et al. Factor XIIIA‐expressing inflammatory monocytes promote lung squamous cancer through fibrin cross‐linking. Nat Commun. 2018;9:1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noel M, O'Reilly EM, Wolpin BM, et al. Phase 1b study of a small molecule antagonist of human chemokine (C‐C motif) receptor 2 (PF‐04136309) in combination with nab‐paclitaxel/gemcitabine in first‐line treatment of metastatic pancreatic ductal adenocarcinoma. Invest New Drugs. 2020;38:800‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Masuda T, Noda M, Kogawa T, et al. Phase I dose‐escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 2020;111:924‐931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sandhu SK, Papadopoulos K, Fong PC, et al. A first‐in‐human, first‐in‐class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC‐chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:1041‐1050. [DOI] [PubMed] [Google Scholar]
- 27. Li L, Liu YD, Zhan YT, et al. High levels of CCL2 or CCL4 in the tumor microenvironment predict unfavorable survival in lung adenocarcinoma. Thorac Cancer. 2018;9:775‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luan B, Yoon YS, Le Lay J, Kaestner KH, Hedrick S, Montminy M. CREB pathway links PGE2 signaling with macrophage polarization. Proc Natl Acad Sci USA. 2015;112:15642‐15647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE. Sci Rep. 2016;6:38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yin J, Kim SS, Choi E, et al. ARS2/MAGL signaling in glioblastoma stem cells promotes self‐renewal and M2‐like polarization of tumor‐associated macrophages. Nat Commun. 2020;11:2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parra ER, Villalobos P, Behrens C, et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non‐small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer. 2018;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng PH, Yu CT, Wu CY, et al. Tumor‐associated macrophages in stage IIIA pN2 non‐small cell lung cancer after neoadjuvant chemotherapy and surgery. Am J Transl Res. 2014;6:593‐603. [PMC free article] [PubMed] [Google Scholar]
- 33. Sliva J, Dolezal T, Sykora D, Vosmanska M, Krsiak M. Guaifenesin enhances the analgesic potency of ibuprofen, nimesulide and celecoxib in mice. Neuro Endocrinol Lett. 2009;30:352‐356. [PubMed] [Google Scholar]
- 34. Li W, Zhang HH, Xu RJ, Zhuo GC, Hu YQ, Li J. Effects of a selective cyclooxygenase‐2 inhibitor, nimesulide, on the growth of ovarian carcinoma in vivo. Med Oncol. 2008;25:172‐177. [DOI] [PubMed] [Google Scholar]
- 35. Raje AA, Mahajan V, Pathade VV, et al. Capillary microsampling in mice: effective way to move from sparse sampling to serial sampling in pharmacokinetics profiling. Xenobiotica. 2020;50:663‐669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Figure S4


