Abstract
Background
Patients with antiphospholipid syndrome (APS) receive anticoagulant therapy with vitamin K antagonists (VKAs) to prevent recurrent thrombosis. VKA treatment requires strict monitoring with an international normalized ratio (INR). It is known that lupus anticoagulants (LAs) can lead to elevated INR results with point-of-care-testing (POCT) devices, which could result in inadequate adaptation of anticoagulant therapy.
Objective
To determine discrepancies between POCT-INR and laboratory-INR in patients who are LA-positive on VKA therapy.
Methods
Paired INR testing was performed with 1 POCT device (CoaguChek XS) and 2 laboratory assays (Owren and Quick method) in 33 patients with LA-positive APS on VKA in a single-center cross-sectional study. Patients were tested for anti–β2-glycoprotein I, anticardiolipin, and antiphosphatidylserine/prothrombin immunoglobulin (Ig) G and IgM antibodies. Agreement between assays was evaluated with Spearman’s correlation, Lin’s correlation coefficient, and Bland–Altman plots. Agreement limits were considered satisfactory if differences were ≤20% as determined by the Clinical and Laboratory Standards Institute.
Results
We found poor agreement between POCT-INR and laboratory-INR based on Lin’s concordance correlation coefficient (ρc) of 0.42 (95% CI, 0.26-0.55) between POCT-INR and Owren-INR, a ρc of 0.64 (95% CI, 0.47-0.76) between POCT-INR and Quick-INR, and a ρc of 0.77 (95% CI, 0.64-0.85) between Quick-INR and Owren-INR. High anti-β2-glycoprotein I IgG antibody titers correlated with INR disagreement between POCT-INR and laboratory-INR.
Conclusion
There is a disagreement between INR values measured with the CoaguChek XS and laboratory-INR in a proportion of patients with LA. Consequently, laboratory-INR monitoring should be preferred over POCT-INR monitoring in patients with LA-positive APS, especially in patients with high anti-β2-glycoprotein IgG antibody titers.
KeyWords: anticoagulants, international normalized ratio, lupus coagulation inhibitor, point-of-care testing, warfarin
Essentials
-
•
Lupus anticoagulant can interfere with international normalized ratio (INR) results in point-of-care testing (POCT)
-
•
We compared 1 POCT-INR with 2 laboratory INR tests in patients with antiphospholipid syndrome
-
•
We found large disagreement between POCT-INR and laboratory INR
-
•
Anti–β2-glycoprotein I immunoglobulin G antibody titers correlated with INR disagreement
1. Introduction
Antiphospholipid syndrome (APS) is a rare autoimmune disease that is defined as recurrent thrombosis or pregnancy-related complications in combination with the persistent presence of antiphospholipid antibodies [1, 2, 3]. Antiphospholipid antibodies are a heterogeneous but overlapping group of autoantibodies, which include anti-β2-glycoprotein I (β2GPI) and anticardiolipin antibodies, and antibodies that prolong the plasma clotting time in laboratory tests in vitro in a phospholipid-dependent manner, a phenomenon known as lupus anticoagulant (LA) [4].
Considering that antiphospholipid antibodies induce a procoagulant status, the standard treatment for thrombotic APS is anticoagulation for an unspecified duration. Vitamin K antagonists (VKAs) are commonly used for secondary prophylaxis [5]. Because the use of VKAs imposes considerable bleeding risks, it requires strict monitoring using the international normalized ratio (INR) [6,7]. The optimal therapeutic window for VKAs is an INR between 2.0 and 3.0 [6,8]. Measurement of INR is routinely performed using a prothrombin time (PT) with either the Quick or Owren’s method in diagnostic laboratories using a venous blood sample [9,10]. However, because frequent monitoring is required, many patients prefer to monitor their INR with point-of-care (POC) devices using capillary blood derived from a finger stick. It is known that LA interferes with phospholipid-dependent coagulation reactions [11], which can lead to prolonged PT and a falsely elevated INR value [1,12]. Whereas most INR reagents used in diagnostic laboratories are relatively insensitive to interference by LA, there are indications that reagents in POC devices are not [13, 14, 15, 16, 17]. Because INR values are used to adjust the dosage of VKA, accurate INR values are of utmost importance. A falsely elevated INR will lead to a lower dosage of VKA, increasing the risk of thrombotic events in these patients. The interference of LA with reagents in POC devices is thought to be dependent on the type of thromboplastins used. It is known that not only the recombinant thromboplastins that are used in these POC devices but also laboratory assays based on the Quick method, are more sensitive to antiphospholipid antibodies than conventional thromboplastins used with Owren’s method [18]. Moreover, the dilution of plasma used in Owren’s method makes this assay less sensitive to antiphospholipid antibody interference [19,20].
Several studies described the use of POC devices for INR management in patients with APS [13,15, 16, 17,21,22]. Although most of these studies found POC devices more sensitive to antiphospholipid antibody interference than laboratory assays in INR measurements, their conclusions are not uniform [10]. Moreover, these studies have some limitations that are either based on the lack of antiphospholipid antibody specification in their study population [15], the determination of antiphospholipid antibodies and INR on different days [16], or the use of a plasma INR method that is sensitive to antiphospholipid antibody interference [22]. The aim of this study was to investigate whether INR values measured with the most commonly used POC device in the Netherlands, the CoaguChek XS (POCT-INR), are similar to INR values measured in a central diagnostic laboratory using Owren’s (Owren-INR) or Quick (Quick-INR) method in a cohort of patients with LA-positive thrombotic APS on VKAs. Additionally, we wanted to determine which LA-causing antiphospholipid antibodies correlate with the observed INR discrepancies.
2. Methods
2.1. Patient selection
Patients aged ≥18 years with thrombotic APS in accordance with the Sydney classification criteria who were anticoagulated with VKAs for a minimum of 3 months were eligible for inclusion in this study [3]. This study was designed as a single-center cross-sectional observational study and has been approved by the local medical ethical committee of the University Medical Center (UMC) Utrecht in the Netherlands. Written informed consent was obtained from all patients. All medical records were obtained from an electronic database.
2.2. INR determination
Blood samples for point-of-care INR testing (CoaguChek XS) were obtained from a finger stick puncture in accordance with the CoaguChek XS (Roche Diagnostics) manufacturer’s guidelines during a scheduled outpatient clinic visit at the Department of Rheumatology and Clinical Immunology at the UMC Utrecht, the Netherlands. Each blood sample was analyzed with the CoaguChek XS device immediately after the finger stick. Within 1 hour after the finger stick puncture, phlebotomy was performed to draw citrate blood samples (3.2% sodium citrate blood collection tubes, Vacuette, Greiner Bio-One) for determining the INR in the ISO 15189-accredited Central Diagnostic Laboratory of the UMC Utrecht. Plasma was obtained by centrifugation at 3000×g for 5 minutes. The INR was measured on an ACL top 750 LAS coagulation analyzer (Werfen) with a PT based on Owren’s method with rabbit brain–derived thromboplastin (Technoclot PT Owren, Technoclone) and a PT based on the Quick method with a reagent containing recombinant human tissue factor (RecombiPlasTin 2G, Werfen). The local International Sensitivity Index (ISI) was established with certified plasmas (AK verification kit, Technoclone). The INR assay participates in robust internal and external quality assessment schemes, and local performance characteristics were within the predefined limits stated by the manufacturers.
2.3. LA determination
LA testing was performed according to The International Society on Thrombosis and Haemostasis guidelines [23] with HemosIL dilute Russell’s Viper venom time (dRVVT) screen and confirm test reagents (Werfen), and HemosIL silica clotting time (Werfen) LA-sensitive activated partial thromboplastin time reagents. Patient plasma (frozen-thawed) was mixed 1:1 with pooled normal plasma (PNP) to exclude coagulation factor deficiencies and limit the effects of VKA on LA outcome. For 3 patients, plasma was diluted 3 times with PNP (1:2) as INR >3.0. Importantly, LA testing was also performed in all patients before starting with VKA. Normalized LA (nLA) ratios were defined as (screen mix clotting time of patient/screen clotting time of PNP)/(confirm mix clotting time of patient/confirm clotting time of PNP). Samples were considered LA-positive when clotting times determined with screen reagents were prolonged (LA-screen time >99th percentile of time recorded for 40 healthy volunteers) and the nLA-ratio exceeded 1.13 for dRVVT or 1.18 for activated partial thromboplastin time.
2.4. Antiphospholipid immunoglobulin M and immunoglobulin G determination
Quantitative values of anticardiolipin and anti-β2GPI immunoglobulin (Ig) G and IgM were measured with Cardiolipin IgM/IgG ELISA (IBL International IBL International GmbH) or IMTEC β2GPI antibodies IgG/IgM ELISA (Clindia Benelux BV) with a cutoff value for positivity on 12.0 GPL/mL for anticardiolipin IgG, 7.0 MPL/mL for anticardiolipin IgM, 7.0 GPL/mL for anti-β2GPI IgG, and 7.0 MPL/mL for β2GPI IgM, based on the 99th percentile of healthy reference individuals [24]. Quantitative values of antiphosphatidylserine/prothrombin (aPS/PT) IgG and IgM were measured with QUANTA Lite ELISA (Inova Diagnostics) with a cutoff value for positivity on 30 units/mL based on manufacturer’s recommendations.
2.5. Statistical analysis
A statistical power analysis was performed for sample size determination using G∗Power version 3.1.9.2. Sample size calculation was based on a mean POCT-INR (SD) of 3.01 (0.82) and Owren-INR of 2.59 (0.49) as determined in a pilot study of patients with LA-positive APS (N = 16). A sample size of at least 33 patients was required to evaluate the agreement of POCT-INR and Owren-INR with 91% power and a 2-tailed significant level of 0.05. Analyses were performed with IBM SPSS Statistics v27.0 (Armonk) or Graphpad Prism 8.2.0. Data were checked for normality using Shapiro–Wilk and Kolmogorov–Smirnov tests. Spearman’s correlation was performed to assess the correlation between the INR methods. Agreement between INR methods was evaluated with Lin’s concordance correlation coefficient (ρc), which assesses the correlation between 2 measures that fall on the 45° line through the origin [25]. The ρc has a range of −1 to 1, with 1 representing perfect agreement, in which all data points lie on the 45° line. The computation of ρc was performed using a macro in SPSS [26]. In addition, Bland–Altman plots were used to show the level of agreement between INR methods with the INR difference between the tests on the y-axis and the mean INR value of the tests on the x-axis. The limits of agreement were indicated in the Bland–Altman plot and determined using the 2.5th and 97.5th percentiles. In addition, agreement limits described in the Clinical and Laboratory Standards Institute (CLSI) guideline POCT14 were used (INR difference ≤20%) [27]. The effect of antiphospholipid antibody titers and LA strengths on INR agreement was assessed using Mann–Whitney U-tests.
3. Results
Between May 2021 and June 2022, 78 patients were screened for inclusion in this study. Of these patients, 2 patients were not eligible for inclusion because they no longer used VKAs, 2 patients were not willing to participate, 1 patient had no thrombotic APS, 15 patients tested negative for all antiphospholipid antibodies at the time of inclusion and therefore did not meet the APS criteria, 13 patients were negative for LA at inclusion, and 12 patients could not be included because of other reasons. A total of 33 patients were included in this study and the baseline characteristics of these patients are presented in Table 1. All patients tested positive for LA.
Table 1.
Demographic and clinical characteristics of 33 patients with LA-positive APS on VKA.
| Characteristics | Value in patients (N = 33) |
|---|---|
| Age (y)–median (range) | 48 (20-73) |
| Male sex–n (%) | 9 (27) |
| Race/ethnicity–n (%) | |
| European White | 31 (94) |
| Caribbean | 1 (3) |
| Central Asia | 1 (3) |
| Women with obstetric complications–n (%) | 12 (50) |
| Primary APS–n (%) | 16 (48) |
| Type of VKA | |
| Acenocoumarol | 11 |
| Phenprocoumon | 22 |
| aPL profile, n (%) | |
| aβ2GPI IgG | 24 (73) |
| aβ2GPI IgM | 15 (46) |
| aCL IgG | 17 (52) |
| aCL IgM | 8 (24) |
| aPS/PT IgG | 24 (73) |
| aPS/PT IgM | 26 (79) |
| Triple positivea | 16 (49) |
| Tetra positiveb | 16 (49) |
APS, antiphospholipid syndrome; aβ2GPI, anti-β2-glycoprotein I antibody; aCL, anticardiolipin antibody; aPL, antiphospholipid antibody; aPS/PT, antiphosphatidylserine/prothrombin antibody; Ig, immunoglobulin; LA, lupus anticoagulant; VKA, vitamin K antagonists.
Tested positive for LA, anticardiolipin and anti-β2GPI antibodies, same isotype.
Tested positive for LA, anticardiolipin, anti-β2GPI antibodies and antiphosphatidylserine/prothrombin antibodies, same isotype.
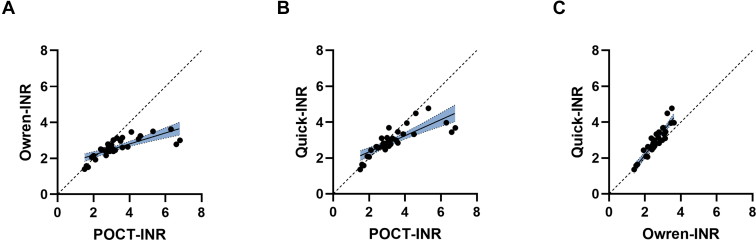
The median INR determined with the CoaguChek XS (POCT-INR) was 3.0 (interquartile range [IQR], 2.7-3.6). Median INR values measured in the laboratory were 2.5 (IQR, 2.4-3.0) for Owren-INR and 2.8 (IQR, 2.6-3.3) for Quick-INR. Twenty-seven patients (77%) had an INR within the therapeutic range (INR, 2.0-3.0) with the Owren reagent, 16 patients (48%) with the Quick reagent, and 20 (61%) with POCT. Overall, POCT-INR values were higher than Owren-INR (P < .001) or Quick-INR (P = .008) values, and differences between these methods became larger when INR values were higher (Figure 1). Furthermore, Quick-INR values were elevated compared with INR values measured with Owren’s method (P < .001, Wilcoxon signed rank test). POCT-INR values correlated well with Owren-INR values (Spearman’s rho [ρ] 0.85; 95% CI, 0.71-0.92). Stronger correlations were observed between POCT-INR and Quick-INR (ρ = 0.88; 95% CI, 0.77-0.94) and Quick-INR and Owren-INR (ρ = 0.90; 95% CI, 0.81-0.95). Because Spearman’s correlation assesses only the strength of a relationship between the INR tests and does not provide information on the agreement between these tests [25], we also determined Lin’s concordance correlation coefficient (ρc). We found a ρc of 0.42 (95% CI, 0.26-0.55) between POCT-INR and Owren-INR, a ρc of 0.64 (95% CI, 0.47-0.76) between POCT-INR and Quick-INR, and a ρc of 0.77 (95% CI, 0.64-0.85) between Quick-INR and Owren-INR. The correlation determined with Lin’s ρc is lower than the correlation observed with Spearman’s ρ showing that although the correlation between tests is high, the agreement between tests is low.
Figure 1.
Scatter plots showing the correlation between the different international normalized ratio (INR) assays. (A) Point-of-care testing (POCT)-INR vs Owren-INR, (B) POCT-INR vs Quick-INR, and (C) Owren-INR vs Quick-INR. Dashed line represents 45° line through origin. Regression line with 95% CI is shown in blue.
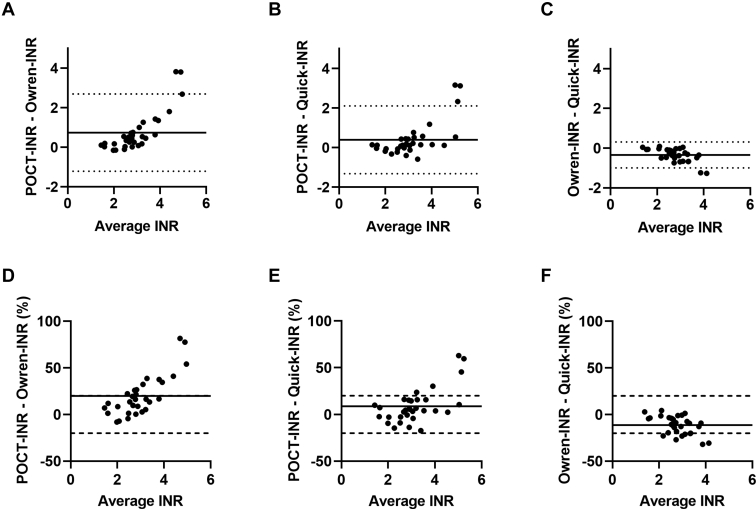
The agreement between POCT-INR and the 2 laboratory assays was also evaluated using a Bland–Altman plot (Figure 2). For the majority of patients, the INR differences fell within the limits of agreement (95% CI). When using the agreement limits that are described in the CLSI guideline (INR difference ≤20%) [27], an agreement between INR measuring methods was still observed in 26 patients using Owren-INR and Quick-INR and 28 patients using POCT-INR and Quick-INR. Less agreement was observed between the POCT-INR and Owren-INR, with a difference of ≤20% observed in 21 patients.
Figure 2.
Bland–Altman plots showing the agreement between different international normalized ratio (INR) assays. Absolute differences (A–C) and relative differences (D–F) between INR assays were plotted on the y-axis and average INR of 2 methods on the x-axis. Horizontal line represents median, dotted line represents limits of agreement (calculated as 2.5th and 97.5th percentile), dashed line represents agreement limits as determined by the Clinical and Laboratory Standards Institute [27].
Next, we evaluated which antiphospholipid antibodies correspond to the observed INR differences. Therefore, we looked at the effect of antiphospholipid antibody titers in patients with or without INR differences exceeding the CLSI agreement limits (Table 2). High nLA-ratios based on the dRVVT were more frequently observed in patients with an INR disagreement between POCT-INR and the Owren-INR or Quick-INR. Elevated anticardiolipin, anti-β2GPI, or aPS/PT IgG antibody titers were also more frequently seen in patients with an INR disagreement between POCT-INR and plasma-INRs of which high anti-β2GPI IgG antibody titers correlated best with the INR disagreement (Table 3). None of the other antiphospholipid antibody titers or LA strengths differed within patients with or without INR agreements. Triple or tetra positivity did not associate with the observed INR differences, P = .11 for POCT-INR versus Owren-INR, P = .13 for POCT-INR vs Quick-INR, and P = .74 for Quick-INR vs Owren-INR (Pearson chi-squared test).
Table 2.
Effect of antiphospholipid antibodies on INR agreement.
| INR method | Assay | nLA-ratio and aPL titers in patients with INR agreement, median (IQR) | nLA-ratio and aPL titers in patients without INR agreement, median (IQR) | P value |
|---|---|---|---|---|
| POCT-INR vs Owren-INR | N = 21 | N = 12 | ||
| LA-dRVVT | 1.41 (1.16-1.69) | 1.81 (1.48-2.47) | .02 | |
| LA-APTT | 1.98 (1.28-2.59) | 2.43 (1.78-3.17) | .15 | |
| aCL IgM | 5.48 (4.41-6.81) | 5.93 (3.76-8.27) | .78 | |
| aCL IgG | 11.31 (5.67-23.88) | 45.06 (8.81-405.52) | .06 | |
| aβ2GPI IgM | 6.65 (6.30-8.13) | 7.00 (6.47-7.94) | .78 | |
| aβ2GPI IgG | 7.97 (6.61-17.17) | 78.21 (20.80-205.05) | .002 | |
| aPS/PT IgM | 120.88 (32.72-308.88) | 225.07 (105.76-257.55) | .78 | |
| aPS/PT IgG | 38.98 (16.01-180.95) | 236.58 (87.89-258.99) | .04 | |
| POCT-INR vs Quick-INR | N = 28 | N = 5 | ||
| LA-dRVVT | 1.48 (1.17-1.87) | 2.36 (1.72-2.57) | .03 | |
| LA-APTT | 2.04 (1.49-2.74) | 2.43 (1.65-3.44) | .45 | |
| aCL IgM | 5.42 (3.99-6.88) | 6.14 (6.00-9.38) | .12 | |
| aCL IgG | 10.86 (5.94-29.44) | 603.80 (207.23-697.39) | .003 | |
| aβ2GPI IgM | 6.62 (6.30-7.98) | 7.46 (7.07-8.93) | .13 | |
| aβ2GPI IgG | 8.93 (6.66-35.04) | 229.44 (180.65-296.03) | <.001 | |
| aPS/PT IgM | 192.03 (32.83-295.22) | 176.76 (50.69-220.46) | .54 | |
| aPS/PT IgG | 113.79 (17.45-225.46) | 221.37 (99.38-259.84) | .14 | |
| Owren-INR vs Quick-INR | N = 26 | N = 7 | ||
| LA-dRVVT | 1.48 (1.18-1.89) | 1.72 (1.46-2.10) | .31 | |
| LA-APTT | 1.96 (1.40-2.56) | 2.55 (1.99-3.03) | .25 | |
| aCL IgM | 5.51 (4.41-6.81) | 5.50 (3.20-10.23) | .95 | |
| aCL IgG | 11.05 (6.20-37.36) | 33.70 (17.14-186.94) | .22 | |
| aβ2GPI IgM | 7.00 (6.44-8.13) | 6.23 (6.12-6.98) | .12 | |
| aβ2GPI IgG | 15.06 (7.09-55.51) | 8.42 (6.52-41.93) | .65 | |
| aPS/PT IgM | 168.79 (32.94-259.29) | 245.69 (124.20-286.60) | .56 | |
| aPS/PT IgG | 113.79 (18.89-204.49) | 256.04 (69.18-273.83) | .16 |
APTT nLA-ratios of 4 patients were not determined as screen clotting time was not prolonged. Statistically significant differences were determined using Mann–Whitney U-test.
APTT, activated partial thromboplastin time; aCL, anticardiolipin antibody; aβ2GPI, anti-β2-glycoprotein I antibody; aPL, antiphospholipid antibody; aPS/PT, antiphosphatidylserine/prothrombin antibody; dRVVT, dilute Russell’s Viper venom time; INR, international normalized ratio; IQR, interquartile range; LA, lupus anticoagulant; nLA-ratio, normalized lupus anticoagulant ratio; POCT, point-of-care testing.
Table 3.
Correlation of antiphospholipid antibody titers with INR differences.
| INR method | Assay | Spearman’s ρ | P value |
|---|---|---|---|
| POCT-INR vs Owren-INR | LA-dRVVT | 0.348 | .03 |
| aβ2GPI IgG | 0.536 | .001 | |
| aPS/PT IgG | 0.290 | .10 | |
| POCT-INR vs Quick-INR | LA-dRVVT | 0.191 | .29 |
| aCL IgG | 0.341 | .05 | |
| aβ2GPI IgG | 0.525 | .002 |
Correlation between antibody titers and INR differences was assessed with Spearman’s correlation coefficient (ρ).
aCL, anticardiolipin antibody; aβ2GPI, anti-β2-glycoprotein I antibody; aPS/PT, antiphosphatidylserine/prothrombin antibody; dRVVT, dilute Russell’s Viper venom time; INR, international normalized ratio; LA, lupus anticoagulant; POCT, point-of-care testing.
4. Discussion
POC devices are used as a reliable alternative for laboratory detection of INR values to monitor anticoagulant therapy in most patients [10]. However, the use of POC devices in INR measurements in patients with LA-positive APS is under debate [10,13, 14, 15, 16, 17]. This is mainly due to the reported interference of antiphospholipid antibodies with thromboplastin reagents, leading to reduced accuracy of certain laboratory INR methods [14,18,20]. Therefore, we aimed to investigate the agreement in INR values determined by the CoaguChek XS as a POC device (POCT-INR) and Owren’s method (Owren-INR) and Quick method (Quick-INR) for laboratory INR measurements. Our study demonstrated disagreement between POCT-INR and Owren-INR or Quick-INR in a large proportion of patients with LA-positive APS. Anti-β2GPI IgG antibody titers correlated with INR disagreement between POCT-INR and the Owren-INR or Quick-INR. Moreover, disagreement between POCT-INR and laboratory assays was especially seen in patients with a POCT-INR >3.0.
We found a large disagreement between POCT-INR and Owren-INR or Quick-INR in patients with LA-positive APS. According to the CLSI agreement limits, 36% of patients with LA-positive APS in our study demonstrated a difference between POCT-INR and Owren-INR. In addition, 15% of patients showed a difference in POCT-INR and Quick-INR, and 21% of patients differed in Quick-INR and Owren-INR. Because POCT-INR values are used to adapt VKA dosage, these falsely elevated POCT-INR values can lead to incorrect lowering of VKA dosage, increasing the risk of thrombotic events. These observed discrepancies can be caused by differences in the sensitivity of the thromboplastin reagents used in these assays. Recombinant thromboplastins, such as those used in the Quick method and POCT, seem to be more sensitive to variation in the presence of LA, which is in accordance with our findings [18]. Moreover, in individuals without APS, INR measurements with recombinant thromboplastin reagents showed to be more susceptible to variation. This is especially seen when INR values are unstable, eg, in the initial phase of VKA therapy. Because the patients enrolled in our study were on VKA for a minimum of 3 months, the observed discrepancies were probably caused by other factors. In addition to the type of thromboplastin used in the assay, the dilution factor of plasma can influence INR results. The dilution factor within Owren’s method is relatively high, making this assay less sensitive to LA interference compared with other methods [19,20].
Our data are in line with other reports on the difference in INR between POC devices and laboratory INR measurements in APS. So, far, no in-depth analysis has been performed to identify the antibodies that correspond with the variability in POCT-INR and laboratory-INRs [10]. Our study focused on the correlation of LA-causing antiphospholipid antibodies with the observed INR disagreement. Of all antiphospholipid antibodies, only anti-β2GPI IgG antibody titers correlated with large INR differences between POCT-INR and the plasma INR methods. We previously showed that anti-β2GPI antibodies cause LA by interfering with the activation of factor V by FXa during the initiation phase of coagulation [11]. We speculate that the difference in sensitivity of POCT-INR, Quick-INR, and Owren-INR for the interference of anti-β2GPI antibodies relies on this mechanism. We assume that the Owren-INR is less sensitive to interference with anti-β2GPI antibodies as the Owren reagent contains additional factor V [18].
One strength of this study is that we only included patients who met the APS criteria according to established guidelines and who were on stable anticoagulant therapy. Moreover, we compared 1 POC device with 2 laboratory assays for INR evaluation and did not only assess correlation with Spearman’s correlation coefficient but also determined agreement with Lin’s concordance correlation coefficient. Furthermore, we included a subanalysis of antiphospholipid antibody titers in patients with or without INR differences. This makes it possible to identify the causal antibody subtype that is responsible for the found INR discrepancies. Finally, the detection of LA and other antiphospholipid antibodies was performed using the same plasma sample as the laboratory INR measurements. This is important because antibody profiles and titers can vary over time. There are also some limitations regarding our results. First, the number of patients with APS in this study was relatively small. However, our sample size was sufficient for analysis based on the power calculation we performed. Second, we did not assess the INR values of individual patients over time. A prospective study with long-term follow-up could provide indications of the clinical implications in patients in which discrepancies between INR measurements are found. Moreover, long-term follow-up data can possibly give information for a POCT-INR/Owren-INR ratio allowing the use of a POC device for INR measurements in these patients. Finally, we only used 1 POC device in our study. Therefore, it should be determined to which end our results can be applied to POC devices other than the CoaguChek XS.
In conclusion, our study demonstrates disagreement of INR values measured with the CoaguChek XS compared with INR values measured with Owren’s or Quick method in a proportion of patients with LA. Consequently, we recommend laboratory monitoring over POCT-monitoring in patients with LA-positive APS, preferably based on Owren’s method because decreased sensitivity for LA. Laboratory monitoring using Owren’s method should especially be performed in patients with LA with anti-β2GPI IgG antibodies and when the POCT-INR >3.0.
Acknowledgments
We thank Ria H.M. Boot and Karin A.L Schrijvers-te Brake for the inclusion of patients in this study.
Funding
This study was funded by a grant from The Netherlands Thrombosis Foundation (2018-03).
Author contributions
T.N, J.J.J., and M.Z. collected the data. T.N. performed the experiments. T.N., R.T.U., M.L., A.H., C.Y.W., and N.M.W. designed the study, analyzed the data, and wrote the manuscript. All authors have read and approved the manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Funding information This study was funded by a grant from The Netherlands Thrombosis Foundation (2018-03).
Handling Editor: A Cihan
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2022.100011
Supporting Information
References
- 1.Keeling D., Mackie I., Moore G.W., Greer I.A., Greaves M. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157:47–58. doi: 10.1111/j.1365-2141.2012.09037.x. [DOI] [PubMed] [Google Scholar]
- 2.Limper M., de Leeuw K., Lely A.T., Westerink J., Teng Y.K.O., Eikenboom J., et al. Diagnosing and treating antiphospholipid syndrome: a consensus paper. Neth J Med. 2019;77:98–108. [PubMed] [Google Scholar]
- 3.Miyakis S., Lockshin M.D., Atsumi T., Branch D.W., Brey R.L., Cervera R., et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 4.Pengo V., Banzato A., Denas G., Jose S.P., Bison E., Hoxha A., et al. Correct laboratory approach to APS diagnosis and monitoring. Autoimmun Rev. 2013;12:832–834. doi: 10.1016/j.autrev.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Tektonidou M.G., Andreoli L., Limper M., Amoura Z., Cervera R., Costedoat-Chalumeau N., et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296–1304. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Irastorza G., Cuadrado M.J., Ruiz-Arruza I., Brey R., Crowther M., Derksen R., et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a Task Force at the 13th International Congress on Antiphospholipid Antibodies. Lupus. 2011;20:206–218. doi: 10.1177/0961203310395803. [DOI] [PubMed] [Google Scholar]
- 7.El-Helou N., Al-Hajje A., Ajrouche R., Awada S., Rachidi S., Zein S., et al. Adverse drug events associated with vitamin K antagonists: factors of therapeutic imbalance. Vasc Health Risk Manag. 2013;9:81–88. doi: 10.2147/VHRM.S41144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsh J., Dalen J.E., Anderson D.R., Poller L., Bussey H., Ansell J., et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119:8S–21S. doi: 10.1378/chest.119.1_suppl.8s. [DOI] [PubMed] [Google Scholar]
- 9.Horsti J. Has the Quick or the Owren prothrombin time method the advantage in harmonization for the international normalized ratio system? Blood Coagul Fibrinolysis. 2002;13:641–646. doi: 10.1097/00001721-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Cohen H., Efthymiou M., Devreese K.M.J. Monitoring of anticoagulation in thrombotic antiphospholipid syndrome. J Thromb Haemost. 2021;19:892–908. doi: 10.1111/jth.15217. [DOI] [PubMed] [Google Scholar]
- 11.Noordermeer T., Molhoek J.E., Schutgens R.E.G., Sebastian S.A.E., Drost-Verhoef S., van Wesel A.C.W., et al. Anti-β2-glycoprotein I and anti-prothrombin antibodies cause lupus anticoagulant through different mechanisms of action. J Thromb Haemost. 2021;19:1018–1028. doi: 10.1111/jth.15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowther M., Crowther M.A. Intensity of warfarin coagulation in the antiphospholipid syndrome. Curr Rheumatol Rep. 2010;12:64–69. doi: 10.1007/s11926-009-0070-9. [DOI] [PubMed] [Google Scholar]
- 13.Isert M., Miesbach W., Schüttfort G., Weil Y., Tirneci V., Kasper A., et al. Monitoring anticoagulant therapy with vitamin K antagonists in patients with antiphospholipid syndrome. Ann Hematol. 2015;94:1291–1299. doi: 10.1007/s00277-015-2374-3. [DOI] [PubMed] [Google Scholar]
- 14.Crowl A., Schullo-Feulner A., Moon J.Y. Warfarin monitoring in antiphospholipid syndrome and lupus anticoagulant. Ann Pharmacother. 2014;48:1479–1483. doi: 10.1177/1060028014546361. [DOI] [PubMed] [Google Scholar]
- 15.Taylor J.R., Richter C., Lindamood C., Liu X., Zumberg M., Fletcher B. Accuracy of CoaguChek XS in patients with antiphospholipid syndrome. Point of Care: The Journal of Near-Patient Testing & Technology. 2017;16:161–163. [Google Scholar]
- 16.Jepsen S.Y., Larsen J.B., Christensen T.D., Grove E.L., Maegaard M., Hvas A.M. Warfarin monitoring and interference by lupus anticoagulant in patients with antiphospholipid syndrome. Thromb Res. 2022;211:127–132. doi: 10.1016/j.thromres.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Perry S.L., Samsa G.P., Ortel T.L. Point-of-care testing of the international normalized ratio in patients with antiphospholipid antibodies. Thromb Haemost. 2005;94:1196–1202. doi: 10.1160/TH05-06-0400. [DOI] [PubMed] [Google Scholar]
- 18.Tripodi A., Chantarangkul V., Clerici M., Negri B., Galli M., Mannucci P.M. Laboratory control of oral anticoagulant treatment by the INR system in patients with the antiphospholipid syndrome and lupus anticoagulant. Results of a collaborative study involving nine commercial thromboplastins. Br J Haematol. 2001;115:672–678. doi: 10.1046/j.1365-2141.2001.03178.x. [DOI] [PubMed] [Google Scholar]
- 19.Horsti J.E. A sensitivity comparison of the Quick and Owren prothrombin time methods in oral anticoagulant therapy. Hematol Rep. 2009;1:15. [Google Scholar]
- 20.Della Valle P., Crippa L., Garlando A.M., Pattarini E., Safa O., Viganò D’angelo S., et al. Interference of lupus anticoagulants in prothrombin time assays: implications for selection of adequate methods to optimize the management of thrombosis in antiphospholipid-antibody syndrome. Haematologica. 1999;84:1065–1074. [PubMed] [Google Scholar]
- 21.Braham S., Novembrino C., Moia M., Torresani E., Tripodi A. Evaluation of a new PT-INR monitoring system in patients with the antiphospholipid syndrome. Int J Lab Hematol. 2016;38:497–504. doi: 10.1111/ijlh.12523. [DOI] [PubMed] [Google Scholar]
- 22.Fonseca M.E.S., Balbi G.G.M., Signorelli F., Gouvea C.P., de Andrade D.C.O. CoaguChek® XS versus standard laboratory prothrombin time for anticoagulant monitoring in patients with antiphospholipid syndrome. Lupus. 2022;31:565–574. doi: 10.1177/09612033221086134. [DOI] [PubMed] [Google Scholar]
- 23.Tripodi A., Cohen H., Devreese K.M.J. Lupus anticoagulant detection in anticoagulated patients. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:1569–1575. doi: 10.1111/jth.14846. [DOI] [PubMed] [Google Scholar]
- 24.Devreese K.M.J., Pierangeli S.S., de Laat B., Tripodi A., Atsumi T., Ortel T.L. Testing for antiphospholipid antibodies with Solid Phase Assays: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:792–795. doi: 10.1111/jth.12537. [DOI] [PubMed] [Google Scholar]
- 25.Morgan C.J., Aban I. Methods for evaluating the agreement between diagnostic tests. J Nucl Cardiol. 2016;23:511–513. doi: 10.1007/s12350-015-0175-7. [DOI] [PubMed] [Google Scholar]
- 26.Nass S.A., Hossain I., Sanyang C., Baldeh B., Pereira D.I.A. SPSS macro for Lin’s concordance correlation coefficient. PLoS One. 2020 doi: 10.1371/journal.pone.0239931.s002. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2004. Point-of-care monitoring of anticoagulation therapy; approved guideline. CLSI document POCT14-A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.