Abstract
The growth arrest and DNA damage-inducible protein, GADD34, was identified by its interaction with human inhibitor 1 (I-1), a protein kinase A (PKA)-activated inhibitor of type 1 protein serine/threonine phosphatase (PP1), in a yeast two-hybrid screen of a human brain cDNA library. Recombinant GADD34 (amino acids 233 to 674) bound both PKA-phosphorylated and unphosphorylated I-1(1–171). Serial truncations mapped the C terminus of I-1 (amino acids 142 to 171) as essential for GADD34 binding. In contrast, PKA phosphorylation was required for PP1 binding and inhibition by the N-terminal I-1(1–80) fragment. Pulldowns of GADD34 proteins expressed in HEK293T cells showed that I-1 bound the central domain of GADD34 (amino acids 180 to 483). By comparison, affinity isolation of cellular GADD34/PP1 complexes showed that PP1 bound near the C terminus of GADD34 (amino acids 483 to 619), a region that shows sequence homology with the virulence factors ICP34.5 of herpes simplex virus and NL-S of avian sarcoma virus. While GADD34 inhibited PP1-catalyzed dephosphorylation of phosphorylase a, the GADD34-bound PP1 was an active eIF-2α phosphatase. In brain extracts from active ground squirrels, GADD34 bound both I-1 and PP1 and eIF-2α was largely dephosphorylated. In contrast, the I-1/GADD34 and PP1/GADD34 interactions were disrupted in brain from hibernating animals, in which eIF-2α was highly phosphorylated at serine-51 and protein synthesis was inhibited. These studies suggested that modification of the I-1/GADD34/PP1 signaling complex regulates the initiation of protein translation in mammalian tissues.
The type 1 protein serine/threonine phosphatase, first identified as a regulator of glycogen metabolism in skeletal muscle, is expressed in all eukaryotic cells and implicated in many physiological events, including muscle contraction (15), ion permeability (18), cell division (67), and infection by viruses (35). These PP1 functions are regulated by the association of a highly conserved catalytic subunit (four PP1 isoforms are encoded by three human genes) with a growing number of protein regulators or “targeting subunits” that localize the phosphatase to specific subcellular compartments and dictate its substrate specificity. This paradigm, first established with the skeletal muscle glycogen synthase phosphatase (63), led to the identification of more than 30 different PP1-binding proteins that may function as PP1 regulators (2).
Physiological studies established that hormones and growth factors control PP1 activity in mammalian tissues (6, 60). The thermostable protein inhibitor 1 (I-1), was the first mechanism identified for hormonal control of rabbit skeletal muscle PP1 activity (39). Protein kinase A (PKA) phosphorylation on a specific threonine converted I-1 into a potent PP1 inhibitor (54). By coordinating PP1 inhibition with the activation of PKA, I-1 broadened and amplified cyclic AMP (cAMP) signals to control glycogen metabolism (28, 29, 45). However, I-1 is widely expressed in mammalian tissues, where it most likely controls other PP1 functions (17, 36, 50). Recent studies showed that I-1 regulates proliferation in some cells (26). In the rat hippocampus, I-1 activation prolongs the autophosphorylation of CaMKII required for long-term potentiation (5, 8). However, the full scope of I-1's actions in mammalian tissue remains to be explored.
Biochemical studies show that I-1 is a more effective inhibitor of the isolated PP1 catalytic subunit than PP1 holoenzymes containing regulatory or targeting subunits. In skeletal muscle, I-1 phosphorylation is coordinated with that of GM, the glycogen-targeting subunit that tethers PP1 to glycogen (41, 42). As PKA phosphorylates GM within the PP1 docking site, it results in the displacement of PP1 from glycogen, which in turn promotes PP1 inhibition by the phosphorylated I-1. Expression of human I-1 in the budding yeast Saccharomyces cerevisiae, which contains an I-1-sensitive PP1 catalytic subunit (12), resulted in only modest deficits in glycogen accumulation and suggested that I-1 was more effective in regulating other physiological processes, such as gene transcription and mitosis (68). Whether the disruption of PP1 holoenzymes regulating these events is also necessary for their inhibition by I-1 remains unclear.
Biochemical studies showed that the N-terminal 54 amino acids of I-1 show significant homology to DARPP-32, another PKA-activated PP1 inhibitor (17), and are sufficient to inhibit PP1 activity in vitro (19) and in vivo (31). However, analysis of yeast strains dependent on human I-1 for growth and viability (50) suggested that most of the I-1 protein is required for PP1 regulation in cells (S. Li and S. Shenolikar, unpublished data). In this respect, the C terminus of I-1 shows little or no homology to DARPP-32 and may direct its unique functions in the mammalian brain (53) and kidney (37), where both PP1 inhibitors are expressed. Understanding of the function(s) of the unique C-terminal sequences may also unravel the distinct phenotypes of mice with disruptions in the I-1 (3, 57) and DARPP-32 genes (24, 25).
Using a protein interaction screen, we identified GADD34 as a novel I-1-interacting protein that associates with the C terminus of human I-1. GADD34, whose expression in mammalian cells is elevated by growth arrest, DNA damage, and other forms of cell stress, has structural homology to a region of the herpes simplex virus (HSV-1) neurovirulence factor ICP-345, previously shown to bind PP1 (34). We also established that GADD34 associates with the PP1 catalytic subunit. Structure-function studies defined the unique regions of I-1 and GADD34 that associate with each other and with the PP1 catalytic subunit. This suggested the formation of a novel heterotrimeric signaling complex that contained multiple PP1 regulators. Biochemical and cellular studies suggested that the PP1/GADD34/I-1 complex regulates the dephosphorylation of the eukaryotic initiation factor eIF-2α and may control protein translation in the mammalian brain in response to cell stress.
MATERIALS AND METHODS
Materials.
Phosphorylase b was purchased from Calzyme, and phosphorylase kinase was purchased from Sigma. CNBr-activated Sepharose was purchased from Pharmacia, and Ni-nitrilotriacetic acid (NTA)-agarose was from Qiagen. Lipofectamine was purchased from Gibco-BRL. Anti-GAL4 and anti-GADD34 antibodies were purchased from Santa Cruz, and anti-PP1 antibody was obtained from Transduction Laboratories. All other chemicals were obtained from Sigma. Anti-phospho-eIF-2α (serine-51) antibody was obtained from New England Biolabs.
Mammalian GADD34 expression constructs were kindly provided by D. C. Tkachuk (University of Washington, Seattle, Wash.). Recombinant eIF-2α and hemin-controlled regulator (HCR) were provided by S. Kimball and L. S. Jefferson (Pennsylvania State University College of Medicine, Hershey, Pa.). Anti-phospho-DARPP-32 antibody was a kind gift from Gretchen Snyder (Laboratory for Molecular and Cellular Neuroscience, Rockefeller University, New York, N.Y.). Glutathione-S-transferase (GST)-GM(1–240), the PP1-binding fragment of the skeletal muscle glycogen-targeting subunits, was provided by David L. Brautigan (University of Virginia, Charlottesville, Va.). Microcystin-LR-Sepharose was made as described (11).
Two-hybrid screen for I-1-binding proteins.
The cDNA encoding the entire coding sequence for human I-1 was inserted into the pAS1 vector to yield an in-frame fusion of I-1 with the GAL4 DNA-binding domain. pAS1-I-1 was transformed into S. cerevisiae PJ4A (44) and analyzed for expression of the GAL4–I-1 fusion protein by immunoblotting the yeast extracts with an anti-GAL4 antibody (Santa Cruz). The I-1–GAL4-expressing yeast strain was used to screen a human brain cDNA library in pGBKT7 (Clontech), a GAL4 activation domain-containing vector. Positive clones were isolated by growth of yeast cells on synthetic medium lacking histidine and adenine and a filter lift assay that estimated GAL4-driven expression of the β-galactosidase (lacZ) reporter gene visualized by the colorimetric substrate X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (44). β-Galactosidase activity was also more accurately quantified using a liquid assay (12, 13). Plasmid DNAs were isolated from lacZ-positive colonies and transformed into Escherichia coli DH5α by electroporation. The isolated plasmids were digested with restriction enzymes to establish the presence of inserts in the pGBKT7 vector and subjected to DNA sequencing at the Duke University DNA sequence facility.
Expression of recombinant I-1 and GADD34 proteins.
The cDNAs encoding the full-length I-1 (171 amino acids) and N-terminal 80, 123, and 153 amino acids of human I-1 were subcloned into pRSETB (Invitrogen). The plasmids were transformed into E. coli BLR(pLYSs) cells to express hexahistidine-tagged I-1 proteins. Briefly, bacteria were grown in Luria-Bertani (LB) medium containing ampicillin (50 μg/ml) at 30°C until the optical density of the culture at 600 nm (OD600) was approximately 0.6. Expression of recombinant I-1 proteins was induced by addition of 0.5 mM IPTG (isopropylthiogalactopyranoside) to the culture medium and continued growth for 4 h at 30°C. The bacteria were sedimented at 3,000 × g for 10 min and lysed by sonication. Bacterial lysates were cleared of cell debris by centrifugation at 20,000 × g for 30 min, and the cleared lysates were gently shaken with Ni-NTA-agarose beads (Qiagen) for 1 h at 4°C. The nickel beads were washed five times with phosphate-buffered saline (PBS) (10 mM potassium phosphate [pH 7.5] containing 150 mM NaCl) containing 20 mM imidazole, and the His-tagged proteins were eluted with PBS containing 150 mM imidazole.
The cDNA encoding a human GADD34 fragment (amino acids 233 to 674) was excised from pGBKT7 using BglII and subcloned into pRSETB for expression in E. coli BL21(DE3) cells. As described above, the pRSETB-transformed bacteria were grown in LB medium containing ampicillin at 30°C until the culture OD600 was 0.6. Protein expression was initiated by addition of 0.1 mM IPTG and growth for a further 6 to 12 h at 21°C. The His-tagged GADD34 protein was purified using Ni-NTA-agarose as described above.
GADD34 and PP1 binding to immobilized I-1.
Full-length untagged I-1 (10) and His-tagged I-1 peptides (0.25 mg of total protein) were coupled to CNBr-activated Sepharose (1 ml) according to the manufacturer's (Pharmacia) instructions. Aliquots of the immobilized I-1 peptides were phosphorylated by addition of purified bovine heart PKA catalytic subunit (50 U) and Mg-ATP (100 μM ATP and 1 mM MgCl2) and incubated for 8 h at room temperature (20°C) with gentle rocking. Before use, all I-1-Sepharose beads were washed four times with TBS (10 mM Tris-HCl [pH 7.5] containing 150 mM NaCl) by repeated centrifugation at 1,000 × g for 10 min.
For the PP1- and GADD34-binding assays, 20 μl (bed volume) of the I-1-Sepharose beads were incubated with 100 U of purified rabbit skeletal muscle PP1 catalytic subunit or 2 μg of recombinant GADD34 per ml expressed in E. coli, respectively. The mixture was shaken gently in 1 ml of TBS at 4°C for 1 h and washed four times with 1 ml each of TBS. The I-1-Sepharose was then resuspended in 25 μl of sodium dodecyl sulfate (SDS) sample buffer, and the eluted proteins were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) on a 10% (wt/vol) polyacrylamide gel. The gels were electrophoretically transferred to polyvinylidene difluoride membranes and blotted with anti-PP1 or anti-GADD34 antibody.
GADD34 expression in mammalian cells.
Human embryonic kidney HEK293T cells (106) were grown in six-well dishes containing Dulbecco's modified Eagle's medium (DMEM) with 10% (vol/vol) fetal bovine serum (FBS), penicillin (10 U/ml), and streptomycin (10 μg/ml). The cells were transfected with the GADD34 expression vectors using 6 μl of Lipofectamine per μg of plasmid in DMEM without serum or antibiotics as recommended by the manufacturer (Gibco-BRL). Approximately 4 h following the transfections, DMEM and 20% (vol/vol) FBS were added, and the cells were grown for an additional 12 h prior to analysis.
Preparation of squirrel brain extracts.
Brains of active and hibernating ground squirrels (Spermophilus tridecemlineatus) were rapidly frozen in liquid nitrogen. The brain tissue was homogenized in 4 volumes of postmitochondrial supernatant (PMS) buffer (30) using a Dounce homogenizer. The brain homogenates were cleared of cell debris by centrifugation at 15,000 × g for 20 min and stored at −80°C.
Protein phosphatase assays.
Protein phosphatase assays using [32P]phosphorylase a as the substrate were carried out as previously described (61). Briefly, purified rabbit skeletal muscle PP1 catalytic subunit (0.02 U) was preincubated with increasing concentrations of recombinant GADD34 for 5 min at 37°C. The enzyme assay was initiated by the addition of [32P]phosphorylase a. In the competition experiments, fixed concentrations of PP1 and GADD34 were preincubated as above, and increasing amounts of I-1 were added immediately prior to initiation of the assay with [32P]phosphorylase a.
Recombinant eIF-2α was phosphorylated in vitro with HCR, the reticulocyte eIF-2α kinase, and [γ-32P]ATP as previously described (46). 32P-labeled eIF-2α (10 μg) was incubated with purified rabbit skeletal muscle PP1 catalytic subunit (0.2 U) in a total reaction volume of 30 μl containing increasing concentrations of recombinant GADD34. The reactions were terminated by addition of SDS sample buffer, and aliquots were subjected to SDS-PAGE on 10% (wt/vol) polyacrylamide gels, which were analyzed by phosphorimager.
Isolation of cellular PP1 complexes.
Squirrel brain extract (2 mg total protein) or extracts of HEK293T cells expressing FLAG-tagged GADD34 (200 μg of total protein) were incubated with 20 μl of packed microcystin-LR-Sepharose. Purified PP1 catalytic subunit (100 U) was used as the control. The mixtures were rocked gently at 4°C for 1 h, the beads were washed five times with TBS, and the bound proteins were eluted with SDS sample buffer. The eluted proteins were then analyzed by SDS-PAGE and Western immunoblotting as described above.
Immunoprecipitation of I-1.
For immunoprecipitation of I-1, squirrel brain extracts (100 μg total protein) were incubated with affinity-purified anti-human I-1 antibody (1 μg total protein) at 4°C for 1 h. The immunocomplexes were sedimented using protein A-Sepharose (Pharmacia) and analyzed for the presence of GADD34 by Western immunoblotting using the anti-GADD34 antibodies according to the supplier's instructions.
RESULTS
Isolation of I-1-binding proteins.
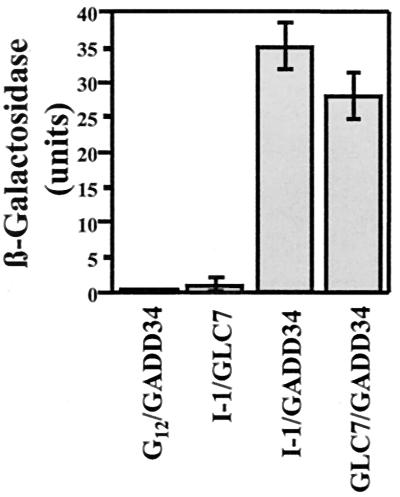
The two-hybrid screen developed by James et al. (44) with growth selection on both adenine and histidine was used to identify I-1-binding proteins from a human brain cDNA library using human I-1 as the bait. We screened approximately 100,000 individual clones and isolated more than 40 positive colonies that together yielded eight distinct cDNAs encoding proteins that interacted with I-1 in the LacZ filter-lift assay. To exclude the possibility that these interactions were mediated through the yeast PP1 catalytic subunit, which has 87% sequence identity with human PP1 and binds mammalian PP1 regulators (12, 13), we rescreened all I-1 interactors for their binding to yeast PP1 (GLC7). One clone, termed clone A2, interacted with both I-1 and PP1 (Fig. 1). Thus, the yeast strain containing pACTII-A2 and pAS-I-1, pAS-GLC7, or pAS-Gi12 α-subunit (used as the control) grew normally on nonselective medium at 24°C, but only the yeast strain containing pACTII-A2 and pAS-I-1 or pAS-GLC7 grew effectively on the double selection medium (data not shown). This established that the protein encoded by the A2 cDNA bound both I-1 and PP1 but did not associate with the α-subunit of the heterotrimeric GTP-binding protein Gi12. Quantitation of the protein-protein interactions using a liquid assay for β-galactosidase demonstrated that both I-1 and PP1 bound avidly to the A2 gene product (Fig. 1), exceeding the binding of PP1 to I-1, a known regulator, in the same assay 25- to 30-fold. Sequence analysis established that the A2 cDNA encoded a fragment of the human growth arrest and DNA damage-inducible protein GADD34.
FIG. 1.
Identification of GADD34 as a protein interacting with PP1 and I-1. Yeast strains containing pACTII-GADD34 and either pASI-I-1, pAS-GLC7, or control vector pAS-Gi12 were grown on nonselective medium lacking Trp and Leu and selective medium lacking Trp, Leu, Ade, and His as described in the text. The figure shows the strength of the protein-protein interactions as estimated by a liquid β-galactosidase assay, with standard errors.
Association of GADD34 with I-1.
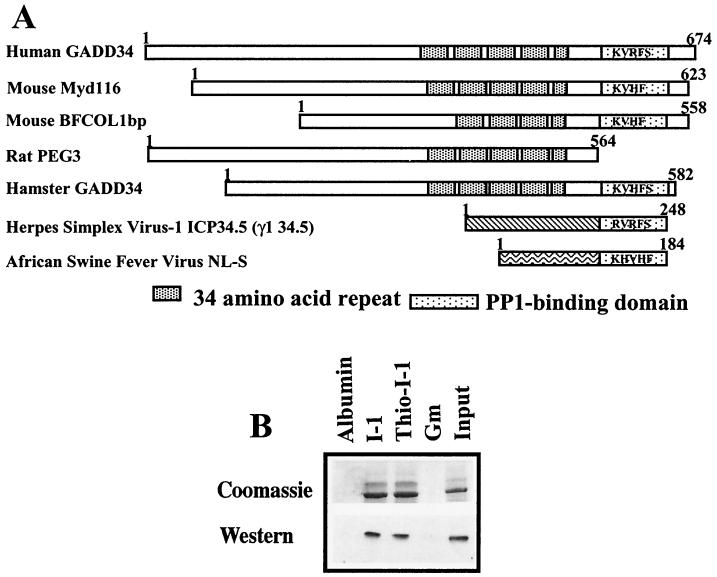
GADD34 was originally identified as an mRNA induced in a hamster cell line by DNA-damaging agents (27). Since then, several other GADD34-related gene products have been identified (Fig. 2A). The full-length GADD34 contains a highly basic N terminus, a central domain containing four and a half repeats of approximately 34 amino acids in length (shown as striped boxes in Fig. 2A), which encompass three predicted PEST sequences, and a C-terminal region of 60 to 80 amino acids that shows sequence homology to the HSV-1 neurovirulence factor ICP34.5 (dotted box in Fig. 2A). ICP34.5 and mouse MYD116 were previously shown to bind PP1 (35). The cDNA identified in our screen represented amino acids 233 to 674 of human GADD34 and contains KVRF, the proposed PP1-binding sequence.
FIG. 2.
I-1 associates directly with GADD34. (A) GADD34-related proteins, shown schematically with regions of structural and functional homology. These proteins also show structural homology with two viral proteins, HSV-1 ICP34.5 and ASV NL-S, specifically in the C-terminal domains containing putative KIXF PP1-binding motifs. (B) Association of recombinant His-GADD34(230–674) with thiophosphorylated (thio-I-1) and unphosphorylated I-1 covalently linked to Sepharose. Controls included albumin and GST-GM(1–240), the PP1-binding fragment of the skeletal muscle glycogen-targeting subunit, which were also covalently linked to Sepharose. Input shows that almost all GADD34 was bound by immobilized I-1. GADD34 binding was visualized using both Coomassie blue protein stain and Western immunoblotting with anti-GADD34 antibody.
To establish whether PP1 was required to recruit I-1 to GADD34 or whether the two proteins bound independently to GADD34, we expressed hexahistidine-tagged GADD34(233–674) and analyzed its association in vitro with recombinant human I-1 coupled to CNBr-activated Sepharose. In a cosedimentation assay, GADD34(233–674) bound equally well to both thiophosphorylated (active) and unphosphorylated (inactive) forms of human I-1 (Fig. 2B). No GADD34 association was seen with immobilized bovine serum albumin or GST-GM(1–240), the PP1-binding fragment of the skeletal muscle glycogen-targeting subunit. This demonstrated that GADD34 directly bound I-1 and the conformation change that results from I-1 phosphorylation by PKA to produce a potent PP1 inhibitor does not influence I-1's association with GADD34.
Mapping GADD34 binding to I-1.
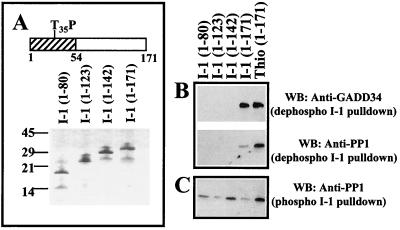
To map the region of I-1 that binds GADD34, serial truncations were made to produce I-1 polypeptides representing the N-terminal 80, 123, and 142 amino acids and full-length I-1 (171 amino acids) as His-tagged proteins (Fig. 3A). Following their purification on Ni-NTA-agarose, the I-1 peptides were covalently coupled to CNBr-activated Sepharose as described above. As anticipated from the above experiments, GADD34(233–674) bound equally well to both thiophosphorylated and unphosphorylated forms of full-length His-tagged I-1 (Fig. 3B). In contrast, GADD34 did not bind I-1(1–80), I-1(1–123), or I-1(1–143). This suggested that the C terminus of I-1, between amino acids 143 and 171, was essential for GADD34 binding in vitro.
FIG. 3.
Mapping GADD34 binding to I-1. (A) Schematic of I-1 structure, with the N-terminal 54 residues (cross-hatched) previously shown to bind PP1 and the PKA-phosphorylated threonine (T35P) required for PP1 inhibition marked. Polypeptides representing C-terminal truncations of I-1 were expressed in bacteria as hexahistidine-tagged proteins and purified by affinity chromatography on Ni-NTA-agarose. Purity of I-1 proteins is shown by SDS-PAGE on a 10% (wt/vol) polyacrylamide gel stained with Coomassie blue. The I-1 proteins were covalently linked to CNBr-activated Sepharose and used in pulldowns of recombinant His-GADD34(230–674) (B). Pulldowns of PP1 catalytic subunits purified from rabbit skeletal muscle were also undertaken using the immobilized unphosphorylated (B) and phosphorylated I-1 (C). The results are compared with thiophosphorylated I-1 immobilized to Sepharose. I-1-bound proteins were analyzed by SDS-PAGE and Western immunoblotting (WB) with anti-GADD34 and anti-PP1 antibodies.
Interestingly, compared to the thiophosphorylated I-1, PP1 bound weakly to the unphosphorylated full-length I-1. However, none of the other unphosphorylated I-1 peptides bound PP1 in parallel assays (Fig. 3B). As we have shown previously (11, 20), PP1 binding was greatly increased by phosphorylation of I-1. Indeed, following their phosphorylation by PKA, all the immobilized I-1 peptides bound PP1 (Fig. 3C). Some variability in PP1 binding to individual phosphopeptides was noted and may reflect differences in their relative phosphorylation by PKA and/or their dephosphorylation by I-1-insensitive phosphatases contaminating preparations of the skeletal muscle PP1 catalytic subunit. Earlier experiments showed that the I-1 phosphopeptide containing the N-terminal 54 amino acids inhibited PP1 activity with a 50% inhibitory concentration (IC50) similar to wild-type (WT) full-length I-1 (19). This suggested not only that the weak PP1 interaction seen with full-length unphosphorylated I-1 was further strengthened by I-1 phosphorylation, but also that PKA phosphorylation generates a stable PP1-binding site within an N-terminal domain that includes the KIQF PP1-binding sequence and the PKA-phosphorylated threonine-35.
Mapping I-1 and PP1 binding in GADD34.
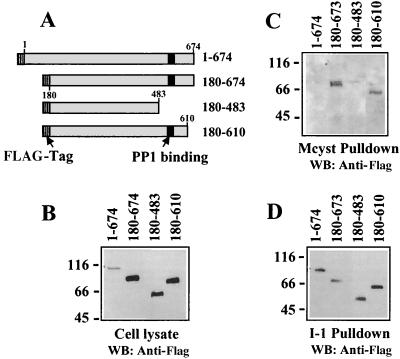
To define the region of GADD34 required for PP1 binding instead of inferring this information from sequence conservation between Myd116 and ICP34.5 and ability of the conserved Myd116 region to complement the loss of function of the γ134.5 gene, which encodes ICP34.5, in HSV-1 (34, 35), we expressed several HA-tagged GADD34 proteins in HEK293T cells (Fig. 4A). This also addressed the potential requirement for covalent modification of GADD34 for its association with I-1 and PP1. Full-length GADD34, which possesses significant apoptotic activity (1), was poorly expressed in HEK293T cells. By comparison, the three truncated proteins, GADD34(180–674), GADD34(180–483), and GADD34(180–610), were well expressed in HEK293T cells (Fig. 4B).
FIG. 4.
Mapping PP1 and I-1 binding to GADD34. FLAG-tagged GADD34 proteins (schematically shown in panel A with FLAG tag and PP1-binding sites highlighted) were expressed in HEK293T cells. Immunoblotting of total cell extracts with anti-FLAG antibody established relative expression of the GADD34 polypeptides in cells (B). HEK293T cell extracts were incubated with microcystin-LR-Sepharose (labeled Mcyst pulldown) to isolate cellular PP1/GADD34 complexes, and GADD34 was detected by Western immunoblotting (WB) (C). I-1-Sepharose was used to isolate GADD34 from HEK293T cell extracts, and the bound proteins in the “I-1 pulldown” were analyzed by immunoblotting with the anti-HA antibody (D).
Affinity isolation of endogenous PP1 complexes on the immobilized cyclic peptide microcystin-LR, a potent phosphatase inhibitor, has been used previously to identify PP1-associated proteins (14). Extracts from HEK293T cells expressing the GADD34 polypeptides were adsorbed to microcystin-LR-Sepharose. Immunoblotting with an anti-FLAG antibody showed that the affinity chromatography successfully isolated PP1 complexes containing GADD34(180–674) and GADD34(180–610) (Fig. 4C). However, PP1 complexes associated with either full-length GADD34 or GADD34(180–483) were not observed The lack of PP1 association with full-length GADD34 was somewhat unexpected and may reflect its low expression or weaker affinity for PP1 due to covalent modification, as GADD34 is phosphorylated in HEK293T cells (J. H. Connor and S. Shenolikar, unpublished observation). The data suggested that PP1 bound GADD34 in the HEK293T cells through the ICP34.5 homology domain (amino acids 483 to 610), which contained the proposed PP1-binding motif, KVRF.
As HEK293T cells express no detectable levels of I-1, the presence of a cellular GADD34/I-1 complex could not be analyzed. Thus, we undertook pulldowns of GADD34 from extracts of transfected HEK293T cells using human I-1 immobilized to Sepharose, as in Fig. 2. Immunoblotting the I-1-bound proteins with an anti-FLAG antibody established that all GADD34 polypeptides, including the full-length protein, were concentrated by the I-1 beads (Fig. 4D). This defined the I-1 binding to the common central region between amino acids 180 and 483 present in all the GADD34 peptides. The relative concentration of full-length GADD34 by the I-1 beads compared to other GADD34 peptides suggested that WT GADD34 possessed a higher affinity for I-1 than the shorter GADD34 peptides that lacked the highly basic N-terminal 180 amino acids. This provided support for an I-1/GADD34 complex in mammalian cells and also suggested that I-1 binding was not sensitive to potential cellular modifications that may have prevented PP1 binding to full-length GADD34 in HEK293T cells. The presence of independent, nonoverlapping sites in GADD34 for I-1 and PP1 argued for a potential heterotrimeric complex that contains both PP1 and I-1 bound to GADD34.
Functional effects of GADD34 on PP1 activity.
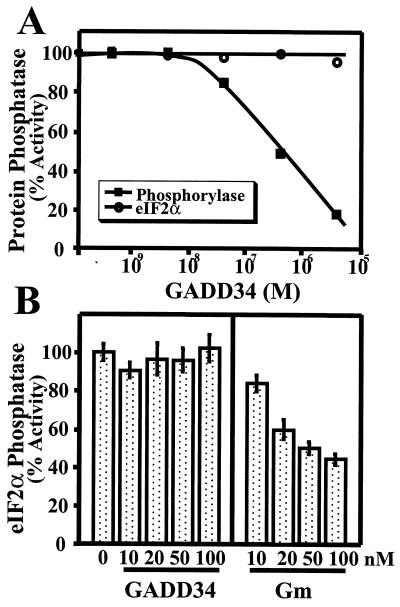
Many PP1-binding proteins modify substrate recognition by the PP1 catalytic subunit. This has been assessed in vitro as the inhibition of phosphorylase phosphatase activity of PP1 by PP1-binding proteins, such as the smooth muscle myosin-targeting subunit MYPT1 (24), the neuronal PP1-binding proteins neurabin I (51) and neurofilament-L (65), and the PKA-anchoring protein AKAP220 (56). To assess whether GADD34 also displayed this property, we analyzed the in vitro dephosphorylation of phosphorylase a by PP1 catalytic subunit isolated from rabbit skeletal muscle in the presence of increasing concentrations of recombinant GADD34(230–674). The GADD34 peptide inhibited PP1 activity with an IC50 of approximately 200 nM (Fig. 5A), demonstrating a potency equal to or better than that of many known PP1-regulatory subunits.
FIG. 5.
Modulation of PP1 activity by GADD34. (A) PP1 catalytic subunit isolated from rabbit skeletal muscle was assayed using 32P-labeled phosphorylase a and eIF-2α as substrates in the presence of increasing concentrations of recombinant His-tagged GADD34(230–674). A representative assay from three independent experiments that varied by less than 5% is shown. (B) Effects of increasing concentrations of recombinant human GADD34(230–674) and human GST-GM(1–240) on the in vitro dephosphorylation of eIF-2α by skeletal muscle PP1 catalytic subunit are shown, with standard error bars. The results represent the sum of three independent experiments carried out in duplicate.
Myd116 and structurally related GADD34 proteins show limited structural homology with HSV-1 ICP34.5, which binds PP1 and generates a phosphatase complex that preferentially dephosphorylates the eukaryotic translation initiation factor eIF-2α (34, 35). Thus, we also analyzed the effects of GADD34 on the PP1-catalyzed dephosphorylation of recombinant eIF-2α. 32P-labeled eIF-2α, phosphorylated on the regulatory site, serine-51, using recombinant HCR, was dephosphorylated by PP1 catalytic subunit in a time- and concentration-dependent manner. In contrast to the inhibition of phosphorylase a phosphatase activity, increasing concentrations of GADD34(230–674) up to 6 μM had no deleterious effect on eIF-2α dephosphorylation by PP1 (Fig. 5A). Based on similar studies with the GM-bound glycogen synthase phosphatase (38, 63) and the myosin-bound phosphatase (15), this suggests that the GADD34-bound PP1 may function as an eIF-2α phosphatase in mammalian tissues.
Interestingly, when the effects of His-GADD34(230–674) on eIF-2α dephosphorylation were compared with those of GST-GM(1–240), a known regulator of PP1 activity as a glycogen synthase phosphatase (38, 63), eIF-2α dephosphorylation, which was unaffected by concentrations of GADD34(230–674) up to 100 nM, was inhibited by GST-GM(1–240) (Fig. 5B). This clearly demonstrated that the correct combination of substrate (eIF-2α) and regulator (GADD34) is required to generate an effective eIF-2α phosphatase.
GADD34 does not impair PP1 inhibition by I-1.
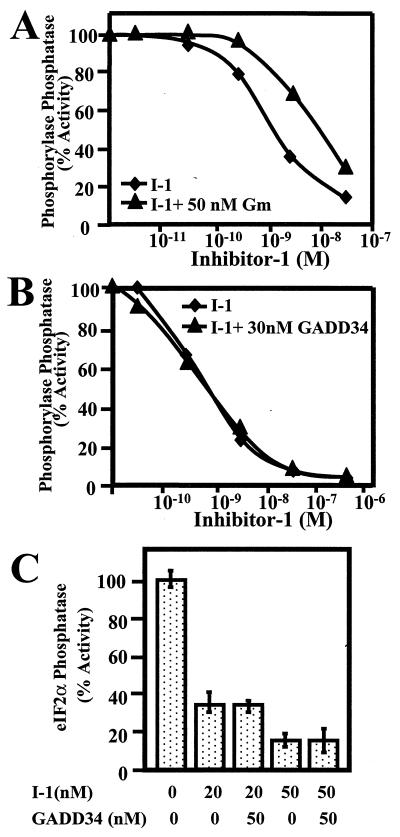
Structure-function studies showed that the KIQF PP1-binding sequence in I-1 was essential for its efficacy as a PP1 inhibitor (15). However, both I-1 and GADD34 contained a highly homologous PP1-binding motif. This predicted that two PP1 regulators might compete for association at the hydrophobic site on the PP1 catalytic subunit that accommodates the KIXF peptide. Thus, the GADD34-bound PP1, like the glycogen synthase phosphatase formed by PP1 binding to GM, the skeletal muscle glycogen-targeting subunit, might be more resistant to inhibition by I-1 than the isolated PP1 catalytic subunit (38, 63). On the other hand, GADD34 contains independent docking sites for both PP1 and I-1 and may recruit the two proteins to permit or even facilitate PP1 regulation by I-1.
To analyze the role of GADD34 on PP1 regulation by I-1, we compared PP1 inhibition by thiophosphorylated I-1 in the presence of GST-GM(1–240), which binds PP1 (see Fig. 2), and His-GADD34(230–674), which binds both PP1 and I-1. At high concentrations, I-1 displaced GADD34 from PP1 bound to microcystin-LR-Sepharose (data not shown), perhaps favoring the formation of dimeric I-1/GADD34 and/or I-1/PP1 complexes. However, at the lower concentrations of the PP1-binding proteins used in a standard phosphorylase phosphatase assay, 50 nM GST-GM (1–240) by itself had no effect on PP1 activity but caused an 8- to 10-fold right shift in dose-response for PP1 inhibition by I-1 (Fig. 6A). By comparison, similar concentrations of His-GADD34(230–674) had no effect on PP1 activity or its inhibition by I-1 (Fig. 6B). The phosphorylase phosphatase activity of rabbit skeletal muscle PP1 catalytic subunit was inhibited by I-1 with an IC50 of 1 nM in the presence and absence of 30 nM His-GADD34(230–674). As the PP1 concentration in the standard phosphatase assay is approximately 1 nM, this suggested that the thiophosphorylated I-1 was a very effective inhibitor of the His-GADD34(230–674)-bound PP1. Similar results were obtained using eIF-2α as the substrate (Fig. 6C). The presence of excess His-GADD34(230–674) had little or no effect on the inhibition of eIF-2α dephosphorylation by PP1 at two different concentrations of I-1. This suggested that hormones that elevate cAMP and activate I-1 may effectively regulate the phosphatase activity associated with the I-1/GADD34/PP1 complex.
FIG. 6.
Effect of GADD34 on PP1 inhibition by I-1. The dose-dependent inhibition of phosphorylase phosphatase activity of the skeletal muscle PP1 catalytic subunit was analyzed in the presence (triangles) and absence (diamonds) of 50 nM recombinant GST-GM(1–240) (A) and 30 nM His-GADD34(230–674) (B). Representative curves from three independent experiments that varied by less than 5% are shown. (C) Inhibition of eIF-2α phosphatase activity of the PP1 catalytic subunit by I-1 in the absence and presence of 50 nM His-GADD34(230–674). Results are the sum of three different experiments carried out in duplicate and are shown with standard error bars.
Physiological regulation of the I-1/GADD34/PP1 complex.
Our in vitro studies suggested that PP1 bound to GADD34, encoded by a human brain cDNA, functions as an eIF-2α phosphatase. In this regard, cell stress, such as ischemia and nutrient starvation, has been linked to reduction in eIF-2α phosphatase activity that is sensitive to PP1 inhibitors (52). Thus, ischemia increased eIF-2α phosphorylation and inhibited protein synthesis in nerve growth factor-differentiated PC12 cells (52). In contrast to such tissue culture models, hibernating ground squirrels provide an excellent model for analyzing the reversible biochemical changes associated with ischemia. Protein synthesis in the brains of hibernating squirrels is essentially shut off due in part to the persistent phosphorylation of eIF-2α (30). It was speculated that hibernation might result in changes in eIF-2α kinase activity or diminished eIF-2α phosphatase. In any case, this provided an excellent and stable setting in which to analyze the role of the I-1/GADD34/PP1 complex in regulating protein synthesis.
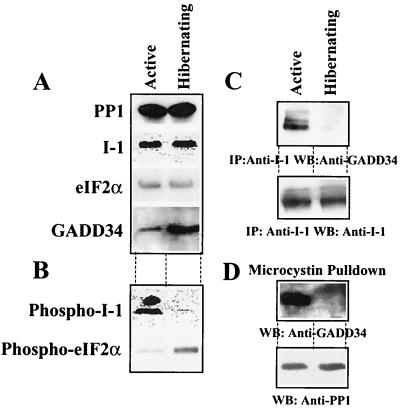
We first compared the steady-state levels of PP1, I-1, eIF-2α and GADD34 in brain extracts from active and hibernating squirrels by Western immunoblotting (Fig. 7A). This showed that the levels of PP1, I-1, and eIF-2α were not altered in the squirrel brain tissue by hibernation or activity. Consistent with cell stress such as serum starvation, UV irradiation, and DNA damage, which induced GADD34 in tissue culture cells, hibernation resulted in an 8- to 10-fold increase in brain GADD34 levels. Using phosphospecific antibodies, we observed that the reduced metabolic activity in brain tissue from hibernating animals resulted in nearly complete elimination of phosphorylated I-1 (Fig. 7B). As reported previously (30), eIF-2α phosphorylation was increased in the brain tissue from hibernating squirrels.
FIG. 7.
Physiological regulation of PP1/GADD34 and I-1/GADD34 complexes. (A) Comparison of total amounts of PP1 catalytic subunit, I-1, eIF-2α, and GADD34 in extracts from brains of active and hibernating squirrel assessed by Western immunoblotting with the appropriate antibodies. (B) Levels of I-1 phosphorylated on threonine-35 and eIF-2α phosphorylated on serine-51 in brain extracts by immunoblotting with the relevant phosphospecific antibodies. (C) I-1 was immunoprecipitated from squirrel brain extracts using a polyclonal anti-human I-1 antibody. The immunoprecipitates were subjected to SDS-PAGE and blotted with an anti-GADD34 antibody. (D) Microcystin-LR-Sepharose was used to isolate PP1 complexes from squirrel brain extracts. The presence of PP1 and GADD34 in microcystin-LR-bound complexes from brain extracts from active (left lanes) and hibernating (right lanes) ground squirrels was analyzed by immunoblotting with anti-PP1 and anti-GADD34 antibodies, respectively.
To address whether the hyperphosphorylation of eIF-2α in the hibernating squirrel brain arose from modifications to the proposed eIF-2α phosphatase complex, we analyzed the association of GADD34 with I-1 in brain extracts by coimmunoprecipitation (Fig. 7C). Equivalent amounts of I-1 were immunoprecipitated from active and hibernating brain extracts using a polyclonal anti-human I-1 antibody. Western blotting showed that GADD34 was associated with I-1 in active brain samples, but despite greatly elevated levels of GADD34 in the hibernating brain samples, no detectable GADD34 association with I-1 was observed. As the anti-human GADD34 antibody failed to immunoprecipitate squirrel GADD34, we were unable to analyze the interaction of I-1 (or PP1) with GADD34 in the reverse direction.
To analyze the GADD34/PP1 complex in active versus hibernating brain samples, we used affinity isolation of PP1 complexes from equivalent amounts of brain extracts using microcystin-LR-Sepharose. Western immunoblotting of the microcystin-LR-bound proteins showed that equivalent amounts of PP1 catalytic subunits were adsorbed from active and hibernating brain extracts (Fig. 7D). Moreover, immunoblotting the microcystin-LR-bound PP1 complexes with an anti-GADD34 antibody demonstrated the presence of a GADD34/PP1 complex in brains of active animals. However, no detectable association of GADD34 with PP1 was observed in the brain extracts from hibernating squirrels. All efforts to detect I-1 in neuronal PP1 complexes from several species by affinity chromatography on microcystin-LR-Sepharose have been unsuccessful (S. Endo and S. Shenolikar, unpublished studies). This may imply that a small subset of GADD34/PP1 complexes in active brain extracts binds I-1 or that affinity isolation of cellular PP1 complexes on microcystin-LR excludes PP1 complexes containing I-1. Microcystin-LR has been shown to compete with I-1 and other endogenous inhibitors for binding to the PP1 catalytic subunit (11). Finally, the reduced affinity of the anti-human GADD34, PP1, and I-1 antibodies for relevant squirrel proteins may also contribute to this result. In any case, the studies provided the first experimental evidence for the reversible association of I-1 and PP1 with GADD34 in mammalian tissues and suggested that, in addition to changes in I-1 phosphorylation/activity, the assembly and disassembly of the proposed eIF-2α phosphatase complex may represent a physiological mechanism for regulating eIF-2α phosphorylation and protein synthesis in the mammalian brain.
DISCUSSION
The known mammalian PP1 regulators fall into two categories, targeting subunits and inhibitor proteins. Many members of these two groups of proteins possess a conserved KIXF motif, a pivotal binding site for the PP1 catalytic subunit (16). Consistent with the competition among these protein regulators for binding the KIXF docking pocket in the PP1 catalytic subunit, PP1 bound to the prototypic PP1-targeting subunit GM and its structural homologue PTG (protein targeting to glycogen) and shows decreased sensitivity to the inhibitors I-1 (38, 63) and DARPP-32 (7), and dissociation of the PP1 catalytic subunit from these targeting subunits enhances PP1 regulation by these inhibitors. Thus, endogenous PP1 inhibitors such as CPI-17 (48), which lacks a KIXF motif, are considered more viable mechanisms for regulating PP1 holoenzymes, such as the smooth muscle myosin phosphatase (58). However, recent studies (23) have identified two PP1 holoenzyme inhibitors (PHIs). PHI-I, although structurally related to CPI-17, contains a putative RVXF motif and inhibits the glycogen-bound and myosin-bound PP1 complexes. This suggests novel mechanisms of PP1 regulation that can circumvent the potential competition between targeting subunits and inhibitors for the KIXF binding pocket on the PP1 catalytic subunit.
I-1 activity as a PP1 inhibitor is increased more than 25,000-fold following its phosphorylation by PKA (19), making I-1 a remarkable signal transduction switch or gate that amplifies cAMP signals in many mammalian cells. Structure-function studies of I-1 (19) and its structural and functional homologue DARPP-32 have established that the key elements for PP1 regulation are localized in the highly homologous N-terminal 50 amino acids, which contain not only the PKA-phosphorylated threonine but also the KIXF motif required for PP1 inhibition by these proteins. The role of their unique C-terminal sequences remains largely unknown. The finding that I-1 forms a stable association, at least in vitro, with the growth arrest and DNA damage-inducible protein GADD34 provided the first evidence for a novel function of the I-1 C terminus, namely, to recruit cellular proteins that modulate the PP1 catalytic subunit. Thus, GADD34 established a new regulatory paradigm that brings two distinct KIXF-containing PP1 regulators, a targeting subunit and an inhibitor, to the same PP1 complex.
Structure-function analysis of I-1 showed that the C-terminal 30 amino acids of I-1 were not essential for PP1 binding or inhibition but were required for GADD34 binding. Conversely, analysis of GADD34 suggested that PP1 and I-1 may bind different regions of this protein, with PP1 binding to a C-terminal domain that is conserved in the viral proteins HSV-1 ICP34.5 and avian sarcoma virus (ASV) NL-S, which harbor a KIXF PP1-binding motif (34), and I-1 binding to the central domain of GADD34, which contains multiple 34-amino-acid repeats. Finally, enzymatic studies showed that, unlike PP1 bound to GM, the prototypic PP1 targeting subunit, which displays a reduced sensitivity to I-1 (38, 63), the presence of excess GADD34 did not preclude PP1 inhibition by PKA-phosphorylated I-1. Together, these studies pointed to independent interactions of I-1, PP1, and GADD34 through adjacent but separable sites on the individual proteins, with the potential to form a heterotrimeric I-1/GADD34/PP1 complex. This raised the intriguing possibility that by scaffolding of PP1 and I-1 together, the requirement for the KIQF sequence in I-1 for PP1 inhibition may be eliminated, as the two key elements necessary for PP1 inhibition (19), the phosphorylated threonine and the KIXF motif, are provided by two different closely associated PP1 regulators, I-1 and GADD34, respectively. Alternately, the GADD34-bound I-1 may be subject to the physiological phosphorylation at serine-67 catalyzed by the neuronal protein kinase Cdk5 (4, 40), which regulates neuronal differentiation and development.
Early studies used reticulocyte lysates as a model system for protein synthesis and showed that phosphorylated I-1 (and I-2, another PP1-specific inhibitor) inhibited protein synthesis, specifically the formation of the translation initiation complex, by increasing eIF-2α phosphorylation (22). As PP1 inhibitors did not alter eIF-2α kinase activity (55), this identified eIF-2α as the PP1 substrate. Genetic antagonism between GLC7, the gene encoding the yeast PP1 catalytic subunit, and GCN2, the eIF-2α kinase, also pointed to a conserved function for PP1 as an eIF-2α phosphatase (66). In reticulocyte lysates (21, 47), increases in cAMP activated PKA and inhibited the assembly of the translation initiation complex. PKA did not directly phosphorylate eIF-2α or hemin-regulated inhibitor (also known as HCR), the eIF-2α kinase present in reticulocytes and other cells. Thus, it was speculated that cAMP may mediate its effects through a reduction in eIF-2α phosphatase activity. However, the precise mechanism by which cAMP inhibited eIF-2α phosphatase has not been resolved. Thus, it is tempting to speculate that PKA phosphorylates the GADD34-bound I-1 to suppress the eIF-2α phosphatase activity of the PP1 catalytic subunit bound at the neighboring site on GADD34 and represents a mechanism for cAMP inhibition of protein synthesis in mammalian tissues.
In pursuing the physiological role of GADD34, overexpression of full-length GADD34 (1) has been shown to promote apoptosis in tissue culture cells. Similarly, nutrient deprivation, UV and gamma irradiation, anticancer drugs (43), and other inducers of GADD34 transcription have varied and damaging effects on cultured cells. Thus, we used the experimental model of hibernating ground squirrels, which demonstrate a reversible shutoff of global protein synthesis associated with hyperphosphorylation of eIF-2α (30). Our studies showed that hibernation is another cell stress that induces GADD34 levels in brain tissue. While the reduced metabolic activity and blood flow in the hibernating squirrel brain resulted in dephosphorylation of threonine-35 and inactivated I-1, eIF-2α phosphorylation was greatly increased. Analysis of I-1/GADD34 and PP1/GADD34 interactions showed that both PP1 and I-1 bound GADD34 in brain tissue from active animals, which demonstrated low steady-state phosphorylation of eIF-2α. In contrast, despite the elevated expression of GADD34 in hibernating brain tissue, its associations with both PP1 and I-1 were disrupted. Coincident with the loss of these protein-protein interactions, eIF-2α phosphorylation was elevated and protein synthesis was inhibited in the hibernating squirrel brain. This argued that the GADD34-bound phosphatase complex played a key role in the control of protein translation and suggested that changes in I-1 phosphorylation/activity, altered GADD34 expression, and coordinated recruitment of I-1 and PP1 to the GADD34 protein may represent distinct mechanisms for controlling protein synthesis in the mammalian brain following various forms of cell stress.
Studies of the mechanisms underlying host cell infection by HSV-1 pointed to a key role for ICP34.5, the product of the γ134.5 gene, in viral infectivity of neuronal cells. Analysis of extracts from cells infected with HSV-1 expressing a functional γ134.5 gene showed that its gene product, ICP34.5, bound PP1 and increased eIF-2α phosphatase activity by nearly 3,000-fold (35) compared to uninfected cells or cells infected with HSV-1 containing a mutant γ134.5 gene. Thus, the expression of ICP34.5 represented a mechanism by which HSV-1 precluded host cell-mediated shutoff of protein synthesis. Structural homology between HSV-1 ICP34.5 and the C-terminal region of GADD34 and the ability of this region of the mouse GADD34 homologue, Myd116 (49), to complement the loss of the viral γ34.5 gene argued that PP1 bound to the GADD34 C terminus may also function as an eIF-2α phosphatase. In this regard, our biochemical studies established that GADD34(230–674) had the hallmarks of a regulatory or targeting subunit that reduced or abolished PP1 activity against phosphorylase a but was an active eIF-2α phosphatase. This provided experimental support for the notion that the cellular GADD34/PP1 complex, like the ICP34.5/PP1 complex, may also regulate protein synthesis.
However, ICP34.5 differs from GADD34 in that it does not induce apoptosis but rather favors cell survival in mammalian cells. Whether this represents differences in the recruitment of other proteins by the viral and cellular PP1-binding proteins or differences in biochemical properties of the eIF-2α phosphatases assembled by these proteins is currently unknown. For example, the C-terminal domain of ICP34.5 that recruits PP1 also binds the cell cycle protein PCNA (9). In contrast, PCNA binding to GADD34 has not been demonstrated. Interestingly, ICP34.5 expression inhibits apoptosis induced by GADD34 (59), possibly suggesting that they compete for common cellular targets. In this regard, differences in PP1 binding and regulation by ICP34.5 and GADD34, which may contain both positive and negative regulatory elements, may also account for their different cellular effects. While increases in eIF-2α phosphorylation have been linked to programmed cell death (62), it is also possible that the apoptotic effects of GADD34 reflect other protein interactions or differences in turnover of eIF-2α phosphorylation at serine-51 by the GADD34-bound PP1 compared to the ICP34.5-bound PP1. A clear distinction between these two proteins is the unique ability of GADD34 to recruit I-1, a known PP1 regulator. Understanding the functional interactions between PP1 and I-1 within the GADD34 signaling complex and their regulation by physiological signals should yield new insights into the physiological role of the complex in the control of protein synthesis and the choice between apoptosis and cell survival.
In addition to PP1 and I-1, several other GADD34-binding proteins have been identified, including the transcription factor BFCOL1, which binds to the promoter of the gene encoding the p21cdk inhibitor (32), KIF1A kinesin (33) and the product of the HRX gene that is translocated in many human leukemias (1). All of these proteins associate with the central region of GADD34 that also binds I-1. This raises the possibility that I-1 also competes with cellular proteins for GADD34 binding and suggests a complex regulation of the GADD34-bound phosphatase. As HRX association attenuates GADD34's ability to induce apoptosis, and it may displace I-1 or other GADD34-bound proteins that participate in apoptotic signaling. Finally, rat cell lines express a proliferation marker, PEG3, with sequence homology to the N terminus and central domain of GADD34 but does not contain a PP1-binding domain (64). Whether PEG3 is expressed in other species and contributes to regulation of the I-1/GADD34/PP1 complex requires further investigation.
Identification of numerous GADD34-binding proteins suggests a wider role for GADD34 than simply the regulation of protein translation. In this regard, analysis of genetically modified mice, such as the I-1-null mice (3), which display a complex neuronal phenotype, may provide insights into the specific contribution of I-1, and perhaps PP1, in eIF-2α dephosphorylation and protein translation. In summary, we have identified the first PP1 complex that may contain more than one PP1 regulator, and the challenge for future studies is to elucidate the cell signaling events that modify the assembly and activity of this signaling complex to control protein synthesis, cell survival, and apoptosis.
ACKNOWLEDGMENTS
This work was supported by NIH grant DK52054 (to S.S.). J.H.C. is a Terry and Frances Seelinger Fellow in Cancer at Duke University.
We thank Ryan T. Terry-Lorenzo and Carey J. Oliver for helpful comments on the manuscript.
REFERENCES
- 1.Adler H T, Chinery R, Wu D Y, Kussick S J, Payne J M, Fornace A J, Tkachuk D C. Leukemic HRX fusion proteins inhibit GADD34-induced apoptosis and associate with the GADD34 and hSNF5/INI1 proteins. Mol Cell Biol. 1999;19:7050–7060. doi: 10.1128/mcb.19.10.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggen J B, Nairn A C, Chamberlin R. Regulation of protein phosphatase-1. Chem Biol. 2000;7:13–23. doi: 10.1016/s1074-5521(00)00069-7. [DOI] [PubMed] [Google Scholar]
- 3.Allen P B, Hvalby O, Jensen V, Errington M L, Ramsay M, Chaudhry F A, Bliss T V, Storm-Mathisen J, Morris R G, Andersen P, Greengard P. Protein phosphatase-1 regulation in the induction of long-term potentiation: heterogeneous molecular mechanisms. J Neurosci. 2000;20:3537–3543. doi: 10.1523/JNEUROSCI.20-10-03537.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibb J A, Nishi A, O'Callaghan J P, Ule J, Lan M, Snyder G L, Horiuchi A, Saito T, Hisanaga S, Czernik A J, Nairn A C, Greengard P. Phosphorylation of protein phosphatase inhibitor-1 by Cdk5 J. Biol Chem. 2001;276:14490–14497. doi: 10.1074/jbc.M007197200. [DOI] [PubMed] [Google Scholar]
- 5.Blitzer R D, Connor J H, Brown G P, Wong T, Shenolikar S, Iyengar R, Landau E M. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science. 1998;280:1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- 6.Bollen M, Stalmans W. The structure, role, and regulation of type 1 protein phosphatases. Crit Rev Biochem Mol Biol. 1992;27:227–281. doi: 10.3109/10409239209082564. [DOI] [PubMed] [Google Scholar]
- 7.Brady M J, Printen J A, Mastick C C, Saltiel A R. Role of protein targeting to glycogen (PTG) in the regulation of protein phosphatase-1 activity. J Biol Chem. 1997;272:20198–20204. doi: 10.1074/jbc.272.32.20198. [DOI] [PubMed] [Google Scholar]
- 8.Brown G P, Blitzer R D, Connor J H, Wong T, Shenolikar S, Iyengar R, Landau E M. Long-term potentiation induced by theta frequency stimulation is regulated by a protein phosphatase-1-operated gate. J Neurosci. 2000;20:7880–7887. doi: 10.1523/JNEUROSCI.20-21-07880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown S M, MacLean A R, McKie E A, Harland J. The herpes simplex virus virulence factor ICP34.5 and the cellular protein MyD116 complex with proliferating cell nuclear antigen through the 63-amino-acid domain conserved in ICP34.5, MyD116, and GADD34. J Virol. 1997;71:9442–9449. doi: 10.1128/jvi.71.12.9442-9449.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor J H, Quan H, Oliver C, Shenolikar S. Inhibitor-1, a regulator of protein phosphatase 1 function. Methods Mol Biol. 1998;93:41–58. doi: 10.1385/0-89603-468-2:41. [DOI] [PubMed] [Google Scholar]
- 11.Connor J H, Kleeman T, Barik S, Honkanen R E, Shenolikar S. Importance of the beta12-beta13 loop in protein phosphatase-1 catalytic subunit for inhibition by toxins and mammalian protein inhibitors. J Biol Chem. 1999;274:22366–22372. doi: 10.1074/jbc.274.32.22366. [DOI] [PubMed] [Google Scholar]
- 12.Connor J H, Quan H N, Ramaswamy N T, Zhang L, Barik S, Zheng J, Cannon J F, Lee E Y, Shenolikar S. Inhibitor-1 interaction domain that mediates the inhibition of protein phosphatase-1. J Biol Chem. 1998;273:27716–27724. doi: 10.1074/jbc.273.42.27716. [DOI] [PubMed] [Google Scholar]
- 13.Connor J H, Frederick D, Huang H B, Yang J, Helps N R, Cohen P T, Nairn A C, DePaoli-Roach A, Tatchell K, Shenolikar S. Cellular mechanisms regulating protein phosphatase-1. A key functional interaction between inhibitor-2 and the type 1 protein phosphatase catalytic subunit. J Biol Chem. 2000;275:18670–18675. doi: 10.1074/jbc.M909312199. [DOI] [PubMed] [Google Scholar]
- 14.Damer C K, Partridge J, Pearson W R, Haystead T A. Rapid identification of protein phosphatase 1-binding proteins by mixed peptide sequencing and data base searching. Characterization of a novel holoenzymic form of protein phosphatase 1. J Biol Chem. 1998;273:24396–24405. doi: 10.1074/jbc.273.38.24396. [DOI] [PubMed] [Google Scholar]
- 15.Dent P, MacDougall L K, MacKintosh C, Campbell D G, Cohen P. A myofibrillar protein phosphatase from rabbit skeletal muscle contains the beta isoform of protein phosphatase-1 complexed to a regulatory subunit which greatly enhances the dephosphorylation of myosin. Eur J Biochem. 1992;210:1037–1044. doi: 10.1111/j.1432-1033.1992.tb17509.x. [DOI] [PubMed] [Google Scholar]
- 16.Egloff M P, Johnson D F, Moorhead G, Cohen P T, Cohen P, Barford D. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 1997;16:1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbrecht A, DiRenzo J, Smith R G, Shenolikar S. Molecular cloning of protein phosphatase inhibitor-1 and its expression in rat and rabbit tissues. J Biol Chem. 1990;265:13415–13418. [PubMed] [Google Scholar]
- 18.Endo S, Critz S D, Byrne J H, Shenolikar S. Protein phosphatase-1 regulates outward K+ currents in sensory neurons of Aplysia californica. J Neurochem. 1995;64:1833–1840. doi: 10.1046/j.1471-4159.1995.64041833.x. [DOI] [PubMed] [Google Scholar]
- 19.Endo S, Zhou X, Connor J, Wang B, Shenolikar S. Multiple structural elements define the specificity of recombinant human inhibitor-1 as a protein phosphatase-1 inhibitor. Biochemistry. 1996;35:5220–5228. doi: 10.1021/bi952940f. [DOI] [PubMed] [Google Scholar]
- 20.Endo S, Connor J H, Forney B, Zhang L, Ingebritsen T S, Lee E Y, Shenolikar S. Conversion of protein phosphatase 1 catalytic subunit to a Mn2+-dependent enzyme impairs its regulation by inhibitor 1. Biochemistry. 1997;36:6986–6992. doi: 10.1021/bi970418i. [DOI] [PubMed] [Google Scholar]
- 21.Ernst V, Levin D H, Ranu R S, London I M. Control of protein synthesis in reticulocyte lysates: effects of 3′:5′- cyclic AMP, ATP, and GTP on inhibitions induced by heme deficiency, double-stranded RNA, and a reticulocyte translational inhibitor. Proc Natl Acad Sci USA. 1976;73:1112–1116. doi: 10.1073/pnas.73.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernst V, Levin D H, Foulkes J G, London I M. Effects of skeletal muscle protein phosphatase inhibitor-2 on protein synthesis and protein phosphorylation in rabbit reticulocyte lysates. Proc Natl Acad Sci USA. 1982;79:7092–7096. doi: 10.1073/pnas.79.23.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eto M, Karginov A, Brautigan D L. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry. 1999;38:16952–16957. doi: 10.1021/bi992030o. [DOI] [PubMed] [Google Scholar]
- 24.Fienberg A A, Hiroi N, Mermelstein P G, Song W, Snyder G L, Nishi A, Cheramy A, O'Callaghan J P, Miller D B, Cole D G, Corbett R, Haile C N, Cooper D C, Onn S P, Grace A A, Ouimet C C, White F J, Hyman S E, Surmeier D J, Girault J, Nestler E J, Greengard P. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 25.Fienberg A A, Greengard P. The DARPP-32 knockout mouse. Brain Res Rev. 2000;31:313–319. doi: 10.1016/s0165-0173(99)00047-8. [DOI] [PubMed] [Google Scholar]
- 26.Florio T, Perrino B A, Stork P J. Cyclic 3,5 adenoise monophosphate and cyclosporin A inhibit cellular proliferation and serine/threonine protein phosphatase activity in pituitary cells. Endocrinology. 1996;137:4409–4418. doi: 10.1210/endo.137.10.8828502. [DOI] [PubMed] [Google Scholar]
- 27.Fornace A J, Jr, Nebert D W, Hollander M C, Luethy J D, Papathanasiou M, Fargnoli J, Holbrook N J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989;9:4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foulkes J G, Cohen P. The hormonal control of glycogen metabolism: phosphorylation of protein phosphatase inhibitor-1 in vivo in response to adrenaline. Eur J Biochem. 1979;97:251–256. doi: 10.1111/j.1432-1033.1979.tb13109.x. [DOI] [PubMed] [Google Scholar]
- 29.Foulkes J G, Cohen P, Strada S J, Everson W V, Jefferson L S. Antagonistic effects of insulin and beta-adrenergic agonists on the activity of protein phosphatase inhibitor-1 in skeletal muscle of the perfused rat hemicorpus. J Biol Chem. 1982;257:12493–12496. [PubMed] [Google Scholar]
- 30.Frerichs K U, Smith C B, Brenner M, DeGracia D J, Krause G S, Marrone L, Dever T E, Hallenbeck J M. Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc Natl Acad Sci USA. 1998;95:14511–14516. doi: 10.1073/pnas.95.24.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992;70:105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa T, Xiao H, Isobe K. Cloning of a GADD34-like gene that interacts with the zinc-finger transcription factor which binds to the p21(WAF) promoter. Biochem Biophys Res Commun. 1999;256:249–254. doi: 10.1006/bbrc.1999.0275. [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa T, Yagi A, Isobe K. Interaction between GADD34 and kinesin superfamily, KIF3A. Biochem Biophys Res Commun. 2000;267:593–596. doi: 10.1006/bbrc.1999.1991. [DOI] [PubMed] [Google Scholar]
- 34.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He B, Gross M, Roizman B. The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J Biol Chem. 1998;273:20737–20743. doi: 10.1074/jbc.273.33.20737. [DOI] [PubMed] [Google Scholar]
- 36.Hemmings H C, Girault J A, Nairn A C, Bertuzzi G, Greengard P. Distribution of protein phosphatase inhibitor-1 in brain and peripheral tissues of various species: comparison with DARPP-32. J Neurochem. 1992;59:1053–1061. doi: 10.1111/j.1471-4159.1992.tb08347.x. [DOI] [PubMed] [Google Scholar]
- 37.Higuchi E, Nishi A, Higashi H, Ito Y, Kato H. Phosphorylation of protein phosphatase-1 inhibitors, inhibitor-1 and DARPP-32, in renal medulla. Eur J Pharmacol. 2000;408:107–116. doi: 10.1016/s0014-2999(00)00767-6. [DOI] [PubMed] [Google Scholar]
- 38.Hiraga A, Cohen P. Phosphorylation of the glycogen-binding subunit of protein phosphatase-1G by cyclic-AMP-dependent protein kinase promotes translocation of the phosphatase from glycogen to cytosol in rabbit skeletal muscle. Eur J Biochem. 1986;161:763–769. doi: 10.1111/j.1432-1033.1986.tb10505.x. [DOI] [PubMed] [Google Scholar]
- 39.Huang F L, Glinsmann W H. Inactivation of rabbit muscle phosphorylase phosphatase by cyclic AMP-dependent kinase. Proc Natl Acad Sci USA. 1975;72:3004–3008. doi: 10.1073/pnas.72.8.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang K X, Paudel H K. Ser67-phosphorylated inhibitor 1 is a potent protein phosphatase 1 inhibitor. Proc Natl Acad Sci USA. 2000;97:5824–5829. doi: 10.1073/pnas.100460897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hubbard M J, Cohen P. Regulation of protein phosphatase-1G from rabbit skeletal muscle. 2. Catalytic subunit translocation is a mechanism for reversible inhibition of activity toward glycogen-bound substrates. Eur J Biochem. 1989;186:711–716. doi: 10.1111/j.1432-1033.1989.tb15264.x. [DOI] [PubMed] [Google Scholar]
- 42.Hubbard M J, Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993;18:172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- 43.Jackman J, Alamo I, Jr, Fornace A J. Genotoxic stress confers preferential and coordinate messenger RNA stability on the five GADD genes. Cancer Res. 1994;54:5656–5662. [PubMed] [Google Scholar]
- 44.James P, Halladay J, Craig E A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khatra B S, Chiasson J L, Shikama H, Exton J H, Soderling T R. Effect of epinephrine and insulin on the phosphorylation of phosphorylase phosphatase inhibitor 1 in perfused rat skeletal muscle. FEBS Lett. 1980;114:253–256. doi: 10.1016/0014-5793(80)81127-6. [DOI] [PubMed] [Google Scholar]
- 46.Kimball S R, Horetsky R L, Jagus R, Jefferson L S. Expression and purification of the alpha-subunit of eukaryotic initiation factor eIF-2: use as a kinase substrate. Protein Expr Purif. 1998;12:415–419. doi: 10.1006/prep.1998.0863. [DOI] [PubMed] [Google Scholar]
- 47.Levin D, Ernst V, London I M. Effects of the catalytic subunit of cAMP-dependent protein kinase (type II) from reticulocytes and bovine heart muscle on protein phosphorylation and protein synthesis in reticulocyte lysates. J Biol Chem. 1979;254:7935–7941. [PubMed] [Google Scholar]
- 48.Li L, Eto M, Lee M R, Morita F, Yazawa M, Kitazawa T. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. J Physiol (London) 1998;508:871–881. doi: 10.1111/j.1469-7793.1998.871bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lord K A, Hoffman-Liebermann B, Liebermann D A. Complexity of the immediate early response of myeloid cells to terminal differentiation and growth arrest includes ICAM-1, Jun-B and histone variants. Oncogene. 1990;5:387–396. [PubMed] [Google Scholar]
- 50.MacDougall L K, Campbell D G, Hubbard M J, Cohen P. Partial structure and hormonal regulation of rabbit liver inhibitor-1; distribution of inhibitor-1 and inhibitor-2 in rabbit and rat tissues. Biochim Biophys Acta. 1989;1010:218–226. doi: 10.1016/0167-4889(89)90164-x. [DOI] [PubMed] [Google Scholar]
- 51.McAvoy T, Allen P B, Obaishi H, Nakanishi H, Takai Y, Greengard P, Nairn A C, Hemmings H C. Regulation of neurabin I interaction with protein phosphatase 1 by phosphorylation. Biochemistry. 1999;38:12943–12949. doi: 10.1021/bi991227d. [DOI] [PubMed] [Google Scholar]
- 52.Munoz F, Martin M E, Tomico J M, Berlanga J, Salinas M, Fando J L. Ischemia-induced phosphorylation of initiation factor 2 in differentiated PC12 cells: role for initiation factor 2 phosphatase. J Neurochem. 2000;75:2335–2345. doi: 10.1046/j.1471-4159.2000.0752335.x. [DOI] [PubMed] [Google Scholar]
- 53.Nairn A C, Hemmings H C, Walaas S I, Greengard P. DARPP-32 and phosphatase inhibitor-1, two structurally related inhibitors of protein phosphatase-1, are both present in striatonigral neurons. J Neurochem. 1988;50:257–262. doi: 10.1111/j.1471-4159.1988.tb13258.x. [DOI] [PubMed] [Google Scholar]
- 54.Nimmo G A, Cohen P. The regulation of glycogen metabolism. Purification and characterisation of protein phosphatase inhibitor-1 from rabbit skeletal muscle. Eur J Biochem. 1978;87:341–351. doi: 10.1111/j.1432-1033.1978.tb12383.x. [DOI] [PubMed] [Google Scholar]
- 55.Petryshyn R, Levin D H, London I M. Regulation of double-stranded RNA-activated eukaryotic initiation factor 2 alpha kinase by type 2 protein phosphatase in reticulocyte lysates. Proc Natl Acad Sci USA. 1982;79:6512–6516. doi: 10.1073/pnas.79.21.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schillace R V, Voltz J W, Shenolikar S, Scott J D. Multiple interactions within the AKAP220 signaling complex contribute to protein phosphatase 1 regulation. J Biol Chem. 2001;276:12128–12134. doi: 10.1074/jbc.M010398200. [DOI] [PubMed] [Google Scholar]
- 57.Scrimgeour A G, Allen P B, Fienberg A A, Greengard P, Lawrence J C. Inhibitor-1 is not required for the activation of glycogen synthase by insulin in skeletal muscle. J Biol Chem. 1999;274:20949–20952. doi: 10.1074/jbc.274.30.20949. [DOI] [PubMed] [Google Scholar]
- 58.Senba S, Eto M, Yazawa M. Identification of trimeric myosin phosphatase (PP1M) as a target for a novel PKC-potentiated protein phosphatase-1 inhibitory protein (CPI17) in porcine aorta smooth muscle. J Biochem. 1999;125:354–362. doi: 10.1093/oxfordjournals.jbchem.a022294. [DOI] [PubMed] [Google Scholar]
- 59.Sheikh M S, Fornace A J. Regulation of translation initiation following stress. Oncogene. 1999;18:6121–6128. doi: 10.1038/sj.onc.1203131. [DOI] [PubMed] [Google Scholar]
- 60.Shenolikar S. Protein serine/threonine phosphatases—new avenues for cell regulation. Annu Rev Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- 61.Shenolikar S, Ingebritsen T S. Protein (serine and threonine) phosphate phosphatases. Methods Enzymol. 1984;107:102–129. doi: 10.1016/0076-6879(84)07007-5. [DOI] [PubMed] [Google Scholar]
- 62.Srivastava S P, Kumar K U, Kaufman R J. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 63.Stralfors P, Hiraga A, Cohen P. The protein phosphatases involved in cellular regulation. Purification and characterisation of the glycogen-bound form of protein phosphatase-1 from rabbit skeletal muscle. Eur J Biochem. 1985;149:295–303. doi: 10.1111/j.1432-1033.1985.tb08926.x. [DOI] [PubMed] [Google Scholar]
- 64.Su Z Z, Shi Y, Fisher P B. Subtraction hybridization identifies a transformation progression-associated gene PEG-3 with sequence homology to a growth arrest and DNA damage-inducible gene. Proc Natl Acad Sci USA. 1997;94:9125–9130. doi: 10.1073/pnas.94.17.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terry-Lorenzo R T, Inoue M, Connor J H, Haystead T A, Armbruster B N, Gupta R P, Oliver C J, Shenolikar S. Neurofilament-L is a protein phosphatase-1-binding protein associated with neuronal plasma membrane and postsynaptic density. J Biol Chem. 2000;275:2439–2446. doi: 10.1074/jbc.275.4.2439. [DOI] [PubMed] [Google Scholar]
- 66.Wek R C, Cannon J F, Dever T E, Hinnebusch A G. Truncated protein phosphatase GLC7 restores translational activation of GCN4 expression in yeast mutants defective for the eIF-2α kinase GCN2. Mol Cell Biol. 1992;12:5700–5710. doi: 10.1128/mcb.12.12.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yanagida M, Kinoshita N, Stone E M, Yamano H. Protein phosphatases and cell division cycle control. CIBA Found Symp. 1992;170:130–140. doi: 10.1002/9780470514320.ch9. [DOI] [PubMed] [Google Scholar]
- 68.Zheng J, Khalil M, Cannon J F. Glc7p protein phosphatase inhibits expression of glutamine-fructose-6-phosphate transaminase from GFA1. J Biol Chem. 2000;275:18070–18078. doi: 10.1074/jbc.M000918200. [DOI] [PubMed] [Google Scholar]