Abstract
Background
High on-treatment platelet reactivity has been reported in 30% of patients on clopidogrel and 50% in elderly patients; however, little is known about the mechanisms of this biological resistance. One hypothesis is an age-related impaired hepatic metabolism of the prodrug clopidogrel, leading to a lower formation of its active metabolite (clopidogrel-AM).
Objectives
To compare the levels of clopidogrel-AM formed in vitro using “old” and “young” human liver microsomes (HLMs) and their consequences on platelet functions.
Methods
We developed an in vitro model using “old” (73.6 ± 2.3 years) and “young” (51.2 ± 8.5 years) HLMs, added to platelet-rich plasma from 21 healthy donors with or without clopidogrel (50 μM) and incubated at 37 °C for 30 (T30) and 45 minutes (T45). Clopidogrel-AM was quantified by liquid chromatography–mass spectrometry/mass spectrometry method. Platelet aggregation was performed by light transmission aggregometry.
Results
The generation of clopidogrel-AM increased over time and reached concentrations comparable with those reported in treated patients. At T30, mean clopidogrel-AM concentrations were significantly higher with “young” (8.56 μg/L; 95% CI, 5.87-11.24) than with “old” HLMs (7.64 μg/L; 95% CI, 5.14-10.14; P = .002); and at T45, 11.40 μg/L; 95% CI (7.57-15.22) vs 10.63 μg/L, 95% CI (7.10-14.15), P = .02 (n = 21). Despite a significant inhibition of platelet aggregation, no significant difference was found in light transmission aggregometry (adenosine diphosphate, 10 μM) after clopidogrel metabolism by “old” or “young” HLMs, probably because of low sensitivity of the method to small variations of clopidogrel-AM.
Conclusion
In this original model combining metabolic and functional approaches, less clopidogrel-AM was produced with HLMs from older patients. This provides support for a decreased CYP450 activity that may contribute to high on-treatment platelet reactivity in elderly patients.
KeyWords: aged, clopidogrel, microsomes, platelet aggregation, prodrugs
Essentials
-
•
Causes for clopidogrel higher on-treatment platelet reactivity in the elderly are unknown.
-
•
In vitro clopidogrel metabolism using “old” and “young” human liver microsomes, was compared.
-
•
Less clopidogrel-active metabolite was produced with older than younger microsomes.
-
•
A decreased CYP450 activity could contribute to clopidogrel resistance in elderly patients.
1. Introduction
Clopidogrel is an antiplatelet prodrug that requires activation via 2 successive oxidation steps of hepatic metabolism involving cytochrome P450 (CYP), in particular CYP3A4, CYP2C19, CYP1A2, and CYP2B6 [1,2] and a reduction step to form clopidogrel-active metabolite (clopidogrel-AM) [[1], [2], [3], [4], [5]], which specifically and irreversibly binds to the adenosine diphosphate (ADP) platelet receptor P2Y12.
Approximately 30% of patients treated with clopidogrel present high on-treatment platelet reactivity (HTPR) [6], characterized by a low platelet inhibition under well-conducted treatment. These poor responders have an increased risk of cardiovascular ischemic events (odds ration [OR] = 8.0 [95% CI, 3.4-19.0]) [7,8]. HTPR was also associated with worse clinical outcomes after lower limb endovascular intervention for peripheral arterial disease [9]. Several risk factors of HTPR have been identified, such as diabetes mellitus, obesity, age, acute coronary syndrome, renal failure, inflammation, drug–drug interactions, and CYP450 or P2Y12 gene polymorphisms [[10], [11], [12], [13]].
Although elderly patients (>70-years old) are the main users of antiplatelet agents, they are 2 to 3 times more likely to present with HTPR than younger patients [14,15]. In this specific population, at high risk for both bleeding and ischemic events, clopidogrel remains a treatment of first choice, notably, since the POPular AGE study found that in patients with non-ST-elevation acute coronary syndrome, clopidogrel is not inferior to ticagrelor and prasugrel with fewer bleeding events [16]. The reasons for the higher resistance to clopidogrel in the elderly have not been investigated. We hypothesized an age-related alteration of CYP450 activity, which would result in a lower formation of clopidogrel-AM [5,15,17]. The aim of this work was, therefore, to assess the effects of age on the hepatic metabolism of clopidogrel via the development of an in vitro model using “young” (ie, <70 years old) and “old” (ie, ≥70 years old) human liver microsomes (HLMs). The primary objective was to compare the levels of the active metabolite formed after activation of clopidogrel by human “old” and “young” microsomes, and the secondary objective was to compare the effects of the metabolism products on platelet functions assessed by ADP-induced platelet aggregation.
2. Methods
2.1. Study design
2.1.1. Platelets
Whole blood was obtained from healthy donors of the blood bank of Rennes (Etablissement Français du Sang, convention 19/2019–2022) after ethical approval. It was collected on citrate tubes (BD Vacutainer sodium citrate 0.105M, tubes of 2.7 mL). The donors were aged <65 years and were not treated by antiplatelet agents or nonsteroidal antiinflammatory drugs.
Platelet-rich plasma (PRP) was obtained after 10 minutes of centrifugation at 150 g with no breaks and platelet-poor plasma after 15 minutes of centrifugation at 2500 g.
2.1.2. In vitro clopidogrel metabolism
Pooled HLMs 20 mg proteins/mL (5 donors, nongenotyped and selected on age) were from Tebu-Bio. HLMs are US Food and Drug Administration-approved for in vitro metabolism- and transporter-mediated drug–drug interaction studies. They consisted of HLMs from donors aged 71 to 77 years (mean = 73.6 ± 2.3) and 37 to 58 years (mean = 51.2 ± 8.5), called “old” and “young” HLMs, respectively. They were stored at −80 °C and thawed for 1-minute incubation at 37 °C before use. One batch of young and 1 batch of old HLMs were used.
β-nicotinamide adenine dinucleotide phosphate (NADP)+ hydrate, D-glucose-6-phosphate (G6P), recombinant glucose-6-phosphate dehydrogenase (G6PD) from Leuconostoc mesenteroides and magnesium chloride (MgCl2), were all from Sigma–Aldrich. β-NADP+ hydrate and MgCl2 were reconstituted in distilled water (Fresenius Kabi), and G6P and G6PD were in solution. All reagents were stored at −20 °C, according to the supplier’s recommendations. Clopidogrel hydrogen sulfate (Alsachim) was reconstituted in acetonitrile (10 mg/mL) and stored at −20 °C.
The incubation mixture was extemporaneously prepared at 37 °C in a potassium-phosphate buffer (K2HPO4 + KH2PO4, 0.1 M pH 7.4) at the following final concentrations: β-NADP+ (1 mM), G6P (15 mM), and MgCl2 (5 mM).
Samples were prepared as follows: 212 μL of incubation mixture, 3 μL of HLMs (final concentration of 0.2 mg protein/mL), 10 μL of clopidogrel (final concentration of 50 μM, ie, 20.5 μg/mL), and 225 μL of PRP. The reaction was initiated by the addition of 1 μL of G6PD (final concentration 4 UI/mL), and incubated at 37 °C for 30 (T30) and 45 minutes (T45), with no agitation, according to preliminary established optimal conditions. In each experiment, a sample with “young” and one with “old” HLMs were prepared in parallel.
Control samples consisting of the same mix with 10 μL acetonitrile without clopidogrel were also prepared with “young” and with “old” HLMs and incubated at 37 °C for 30 and 45 minutes.
After 30 and 45 minutes of incubation, samples were separated into 2 parts, one was handled for clopidogrel-AM quantification and the other part was for platelet aggregation.
2.1.3. Clopidogrel-AM quantification
At T30 and T45, 25 μL of 2-bromo-3′-methoxyacetophenone 500 mM (Sigma–Aldrich) and acetonitrile 2:1 were added to 200 μL of the mixture before being stored at −80 °C until analysis.
Clopidogrel-AM quantification was performed by ultra-performance liquid chromatography–mass spectrometry/mass spectrometry (Waters) in the Laboratory of Pharmacology, Toxicology and Blood Gazes of the University Hospital of St Etienne, according to a validated and previously described method [18]. The lower limit of quantification was 0.8 ng/mL.
2.1.4. Platelet aggregation
At T30 and T45, platelet aggregation was immediately performed on the incubation mixture–PRP mix, by light transmission aggregometry on an optical platelet aggregometer (PAP-8 Bio/Data). Maximal aggregation and area under the curve (AUC) at 5 minutes induced by ADP (Sigma–Aldrich) of 10 μM final concentration, were analyzed. ADP 5 μM was also tested and showed similar response to clopidogrel-AM.
A PRP sample and a PRP sample spiked with commercialized clopidogrel-AM (Alsachim) and incubated at 37 °C for 30 and 45 minutes were systematically run in parallel to test each platelet preparation reactivity to ADP 10 μM and platelet sensitivity to clopidogrel-AM.
Inhibition of platelet aggregation (IPA) was calculated for each condition on AUC according to the following formula:
IPA for maximal aggregation was also calculated, based on the same formula.
2.1.5. Statistics
The normality of the different results was evaluated by the Shapiro–Wilk test, with an alpha level of 5%.
The 2 HLM groups were compared with Student’s t-test for paired samples in case of a normally distributed difference between the 2 samples or the Wilcoxon signed-rank test if the difference was not normally distributed. Correlations were evaluated by simple linear regression. The effect sizes were provided in addition to the P value. They were calculated using Cohen’s formula and an effect ≥0.2 was considered noticeable [19].
3. Results and Discussion
3.1. Effect of age on the in vitro formation of clopidogrel-AM by HLMs
Before testing “young” and “old” HLMs, we performed a preliminary study, based on previous studies [20,21], designed to set up the best experimental conditions to generate in vitro active metabolite, without any deleterious effect on platelets contained in the reagent mixture, such as cell membrane lysis or spontaneous activation.
Then, blood from 21 healthy blood donors was used. The sex ratio was 15:6 (male:female), and the median age was 45 years (IQR, 37-55). The mean platelet count was 304 G/L in whole blood (SD = 56 G/L) and 392 G/L in PRP (SD = 81 G/L).
Twenty-one experiments were performed, each of them using both “young” and “old” HLMs at the 2-incubation time points (T30 and T45), resulting in 84 clopidogrel-AM measurements (Figure 1).
Figure 1.
Concentrations of clopidogrel-active metabolite after metabolism by <70 (young) and ≥70 (old) years old human liver microsomes after 30 minutes (T30) or 45 minutes (T45) of incubation. n = 21 in each group. ∗∗: P < .01 ∗∗∗: P < .001. HLM,
Interestingly, clopidogrel-AM levels generated in our in vitro conditions were of the same order of magnitude as those observed in patients treated with clopidogrel 75 mg/d at a steady state [18,22,23].
The generation of clopidogrel-AM increased over time between T30 and T45 in both HLMs groups (P < .001 and d > 0.8 in each group) (Figure 1), in accordance with the few data from the literature on the subject [20]. We limited the maximal incubation time to T45 to avoid the toxicity of the HLM incubation medium on platelets. However, at both incubation times, we bring the first evidence that “young” HLMs generate higher concentrations of clopidogrel-AM than older ones. Indeed, at T30 the mean production of active metabolite was significantly higher with “young” HLMs (8.56 μg/L; 95%CI, 5.87-11.24) than with “old” HLMs (7.64 μg/L; 95% CI, 5.14-10.14) (P = .002, d = 0.35), corresponding to a 10.7% decrease in metabolite production in “old” compared with “young“ HLMs. Similar results were found at T45: mean production of clopidogrel-AM was 11.40 μg/L (95% CI, 7.57-15.22) with “young” HLM vs 10.63 μg/L (95% CI, 7.10-14.15), with “old” HLMs (P = .02, d = 0.21).
These significantly higher concentrations of clopidogrel-AM produced with younger HLMs, under strictly identical experimental conditions, suggest an age-related lower hepatic metabolism of clopidogrel, especially because it has been described that the CYP450 activity is influenced by age [24,25]. Given that (i) the difference of age between the 2 groups of HLMs we tested was small (22.4 years) and (ii) that the “old” HLMs had only a mean age of 73.6 years, one can expect that the impaired generation of clopidogrel-AM we found, would be even much greater in patients aged >80 years who present HTPR. Unfortunately, HLMs from subjects aged >75 years are not commercially available. These in vitro results, therefore, support the hypothesis of a decreased CYP450 activity in elderly patients that might contribute to HTPR. An increased esterase activity and increased basal platelet activity because of age [5,15,17] have also been proposed as potential causes of HTPR in the elderly. Our study was not designed to address these issues.
Nevertheless, as far as we know, this is the first study using microsomes to explore age-related clopidogrel metabolism. This approach could be applied to other prodrugs as an easy tool to evaluate age-related drug metabolism instead or in addition to in vivo studies.
Finally, we checked that neither age, gender, nor PRP-platelet count of blood donors correlated with the concentrations of clopidogrel-AM (data not shown).
3.2. Effects of clopidogrel bioactivation with “young” and “old” HLMs on platelet functions
IPA induced by 10 μM ADP was assessed on 8 and 13 different experiments out of 21, in each group of the HLMs, at T30 and T45.
HLMs, in the incubation mixture in the absence of clopidogrel, did not impair platelet aggregation in PRP, with respective mean maximum aggregations of 78% (95% CI, 71-85) and 79% (95% CI, 73-85) (P = .52) in young and old HLMs, respectively. Moreover, aggregations were >72% in all experiments (whatever the time point), showing the absence of any toxicity on platelet suspension or spontaneous activation.
Median IPA at T30 and T45 with young and old microsomes in the presence of clopidogrel are presented in Table. Surprisingly, no significant difference was found between the 2 groups of HLMs. Neither age, gender, nor PRP-platelet count of blood donors were correlated to IPA.
Table.
Area under the curve and maximal aggregation inhibition of platelet aggregation after 30 (T30) and 45 (T45) minutes of incubation of clopidogrel with either old or young human liver microsomes.
| Aggregation parameters | T30 |
T45 |
||||
|---|---|---|---|---|---|---|
| Old HLMs | Young HLMs | P | Old HLMs | Young HLMs | P | |
| AUC IPA (%) |
50.6 (17.9-95.5) | 25.9 (11.3-80.6) | .25 | 33.1 (10.9-94.3) | 25.4 (9.32-90.5) | .37 |
| Maximal aggregation IPA (%) |
46.8 (15.4-87.9) | 17 (5.15-67.9) | .11 | 17.9 (7.52-86.1) | 22.7 (3.01-81.4) | .61 |
Results are expressed as median [interquartile ratio].
AUC, area under the curve; HLMs, human liver microsomes; IPA, inhibition of platelet aggregation; IQR, interquartile range.
This absence of significant difference in IPA after clopidogrel metabolism by “young” or “old” HLMs is likely due to a lack of sensitivity of platelet aggregation to detect significant but small variations in the amount of active metabolite, especially in this range of concentrations. Indeed, few studies [20,26] have shown an association between IPA and concentration of active metabolite; however, they evaluated widest ranges of active metabolite than in the present one. In these studies, as in the present one, the variability of IPA in response to clopidogrel-AM was high at concentrations similar to ours [20]. It cannot be excluded that the number of tests performed (8 and 13) does not bring sufficient power to the study.
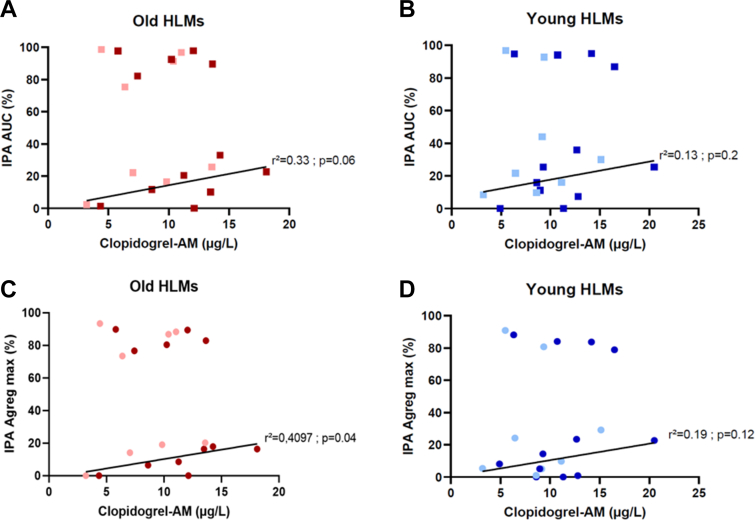
There was no significant relationship between IPA and clopidogrel-AM concentration in the whole sample population. This may be due to the narrow range of clopidogrel-AM concentrations, in comparison with other studies [26]. Nonetheless, we observed that all IPA were either >70% or <45%, irrespective of the metabolite concentration (Figure 2) and whatever the incubation times. Focusing on samples with IPA <45%, we found a relationship between IPA and clopidogrel-AM concentration, whereas there was no relationship when IPA were >70%. This “on–off effect” at low concentrations of active metabolite could be in favor of an individual platelet sensitivity to clopidogrel with good and poor responders to clopidogrel-AM. In good responders, whatever the age, the concentration of clopidogrel-AM generated is probably in saturating concentration with a strong platelet inhibition. In contrast, in low responders, the inhibition depends on the clopidogrel-AM concentration. These results are not age-related but dependent on platelet donors, showing that HTPR is multifactorial.
Figure 2.
Relationship between concentration of clopidogrel-active metabolite (μg/L) and inhibition of platelet aggregation (IPA). AUC-IPA with old HLMs (A), AUC-IPA with young HLMs (B), maximal aggregation-IPA with old HLMs (C), and maximal aggregation-IPA with young HLMs (D). Light red or blue squares stand for T30 incubation (n = 8), and dark red and blue squares stand for T45 incubation (n = 13). AUC, area under the curve; clopidogrel-AM, clopidogrel-active metabolite; HLMs, human liver microsomes; IPA, inhibition of platelet aggregation.
4. Limitations
This study has limitations. First, HLMs were not genotyped and thus an effect of CYP450 polymorphism cannot be excluded but it is unlikely because they consisted of a mixture of pooled HLMs with no donor of Asian origin. Then, the results of an in vitro study cannot be overestimated, especially in terms of clinical outcomes and need to be validated in patients. To further unravel the mechanisms underlying HTPR in the elderly, in addition to the present in vitro study, we are currently conducting a prospective multicenter study designed to assess the relationship between the clopidogrel-AM (pharmacokinetics) and the platelet reactivity (pharmacodynamics) according to age in patients treated with clopidogrel, with a clinical follow-up (NCT04990596).
5. Conclusions
To our knowledge, this study is the first one to compare clopidogrel metabolism with HLMs of 2 age ranges, associating the quantification of the generated active metabolite and the assessment of the effect of the metabolism compounds on platelet functions.
We set up experimental conditions to generate clopidogrel-AM concentrations consistent with those observed in patients under clopidogrel at steady state, without any toxic effect on platelets. We found significantly higher concentrations of active metabolite generated with “young” than with “old” HLMs, suggesting an age-related lower hepatic metabolism of clopidogrel; however, with no functional effect in our experimental conditions. Nonetheless, the IPA with “young” and “old” HLMs displayed profiles that deserve further investigation.
Overall, our study supports a decrease in CYP450 activity that may contribute to HTPR in the elderly patients.
Acknowledgments
Funding
This is an academic study with institutional funding from the University Hospital of Rennes, France.
Author contributions
A.P., I.G.T, P.G., C.B.L., and X.D. designed the study. A.P. set up the study model. A.P. and A.A. performed the experiments. M.C.V. contributed to set up the in vitro model using microsomes. P.G., F.N.G., and C.B.L. contributed to the platelet function tests. S.H. measured the clopidogrel-active metabolite. A.P. and I.G.T. wrote the manuscript. P.G. and C.B.L. contributed to the writing.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Y Senis
References
- 1.Kazui M., Nishiya Y., Ishizuka T., Hagihara K., Farid N.A., Okazaki O., et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 2.Dansette P.M., Rosi J., Bertho G., Mansuy D. Cytochromes P450 catalyze both steps of the major pathway of clopidogrel bioactivation, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer. Chem Res Toxicol. 2012;25:348–356. doi: 10.1021/tx2004085. [DOI] [PubMed] [Google Scholar]
- 3.Pereillo J.M., Maftouh M., Andrieu A., Uzabiaga M.F., Fedeli O., Savi P., et al. Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab Dispos. 2002;30:1288–1295. doi: 10.1124/dmd.30.11.1288. [DOI] [PubMed] [Google Scholar]
- 4.Farid N.A., Kurihara A., Wrighton S.A. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126–142. doi: 10.1177/0091270009343005. [DOI] [PubMed] [Google Scholar]
- 5.Dansette P.M., Libraire J., Bertho G., Mansuy D. Metabolic oxidative cleavage of thioesters: evidence for the formation of sulfenic acid intermediates in the bioactivation of the antithrombotic prodrugs ticlopidine and clopidogrel. Chem Res Toxicol. 2009;22:369–373. doi: 10.1021/tx8004828. [DOI] [PubMed] [Google Scholar]
- 6.Siller-Matula J.M., Trenk D., Schrör K., Gawaz M., Kristensen S.D., Storey R.F., et al. Response variability to P2Y12 receptor inhibitors: expectations and reality. JACC Cardiovasc Interv. 2013;6:1111–1128. doi: 10.1016/j.jcin.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Snoep J.D., Hovens M.M.C., Eikenboom J.C.J., van der Bom J.G., Jukema J.W., Huisman M.V. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007;154:221–231. doi: 10.1016/j.ahj.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Sibbing D., Schulz S., Braun S., Morath T., Stegherr J., Mehilli J., et al. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost. 2010;8:250–256. doi: 10.1111/j.1538-7836.2009.03709.x. [DOI] [PubMed] [Google Scholar]
- 9.Zlatanovic P., Wong K.H.F., Kakkos S.K., Twine C.P. A systematic review and meta-analysis on the impact of high on-treatment platelet reactivity on clinical outcomes for patients taking ADP receptor inhibitors following lower limb arterial endovascular intervention. Eur J Vasc Endovasc Surg. 2022;63:91–101. doi: 10.1016/j.ejvs.2021.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Siller-Matula J.M., Trenk D., Schröer K., Gawaz M., Kristensen S.D., Storey R.F., et al. How to improve the concept of individualised antiplatelet therapy with P2Y12 receptor inhibitors–is an algorithm the answer? Thromb Haemost. 2015;113:37–52. doi: 10.1160/TH14-03-0238. [DOI] [PubMed] [Google Scholar]
- 11.Bura A., Bachelot-Loza C., Ali F.D., Aiach M., Gaussem P. Role of the P2Y12 gene polymorphism in platelet responsiveness to clopidogrel in healthy subjects. J Thromb Haemost. 2006;4:2096–2097. doi: 10.1111/j.1538-7836.2006.02113.x. [DOI] [PubMed] [Google Scholar]
- 12.Hulot J.S., Bura A., Villard E., Azizi M., Remones V., Goyenvalle C., et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 13.Shuldiner A.R., O’Connell J.R., Bliden K.P., Gandhi A., Ryan K., Horenstein R.B., et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silvain J., Cayla G., Hulot J.S., Finzi J., Kerneis M., O’Connor S.A., et al. High on-thienopyridine platelet reactivity in elderly coronary patients: the SENIOR-PLATELET study. Eur Heart J. 2012;33:1241–1249. doi: 10.1093/eurheartj/ehr407. [DOI] [PubMed] [Google Scholar]
- 15.Verdoia M., Pergolini P., Rolla R., Nardin M., Schaffer A., Barbieri L., et al. Advanced age and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. J Thromb Haemost. 2016;14:57–64. doi: 10.1111/jth.13177. [DOI] [PubMed] [Google Scholar]
- 16.Gimbel M., Qaderdan K., Willemsen L., Hermanides R., Bergmeijer T., de Vrey E., et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. 2020;395:1374–1381. doi: 10.1016/S0140-6736(20)30325-1. [DOI] [PubMed] [Google Scholar]
- 17.Capodanno D., Angiolillo D.J. Antithrombotic therapy in the elderly. J Am Coll Cardiol. 2010;56:1683–1692. doi: 10.1016/j.jacc.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 18.Delavenne X., Basset T., Zufferey P., Malouk N., Laporte S., Mismetti P. Ultra-performance LC MS/MS method for quantification of clopidogrel active metabolite. J Sep Sci. 2010;33:1968–1972. doi: 10.1002/jssc.201000115. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 20.Zahno A., Brecht K., Bodmer M., Bur D., Tsakiris D.A., Krähenbühl S. Effects of drug interactions on biotransformation and antiplatelet effect of clopidogrel in vitro. Br J Pharmacol. 2010;161:393–404. doi: 10.1111/j.1476-5381.2010.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abell L.M., Liu E.C.K. Dissecting the activation of thienopyridines by cytochromes p450 using a pharmacodynamic assay in vitro. J Pharmacol Exp Ther. 2011;339:589–596. doi: 10.1124/jpet.111.184895. [DOI] [PubMed] [Google Scholar]
- 22.Wallentin L., Becker R.C., Budaj A., Cannon C.P., Emanuelsson H., Held C., et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 23.Liu C., Chen Z., Zhong K., Li L., Zhu W., Chen X., et al. Human liver cytochrome P450 enzymes and microsomal thiol methyltransferase are involved in the stereoselective formation and methylation of the pharmacologically active metabolite of clopidogrel. Drug Metab Dispos. 2015;43:1632–1641. doi: 10.1124/dmd.115.064949. [DOI] [PubMed] [Google Scholar]
- 24.Yang X., Zhang B., Molony C., Chudin E., Hao K., Zhu J., et al. Systematic genetic and genomic analysis of cytochrome P450 enzyme activities in human liver. Genome Res. 2010;20:1020–1036. doi: 10.1101/gr.103341.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bebia Z., Buch S.C., Wilson J.W., Frye R., Romkes M., Cecchetti A., et al. Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther. 2004;76:618–627. doi: 10.1016/j.clpt.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Delavenne X., Mallouk N., Piot M., Mismetti P., Laporte S. Is there really a relationship between the plasma concentration of the active metabolite of clopidogrel and the results of platelet function tests? J Thromb Haemost. 2010;8:2334–2338. doi: 10.1111/j.1538-7836.2010.04004.x. [DOI] [PubMed] [Google Scholar]