Abstract
Background
Immune tolerance induction (ITI) aims to eradicate anti-factor VIII (FVIII) antibodies (inhibitors) in persons with hemophilia A. However, this burdensome treatment fails in 10% to 40%. To estimate the chance of ITI success in clinical decision making, it is important to identify the predictors of ITI success.
Objectives
We performed a systematic review and meta-analysis to summarize the current evidence on determinants of ITI outcome in persons with hemophilia A.
Methods
A literature search was conducted to identify randomized controlled trials, cohort, or case-control studies reporting on the predictors for ITI outcome in persons with hemophilia A. The main outcome was ITI success. Methodological quality was assessed using an adapted Joanna Briggs Institute checklist, rating as high if ≥11 of 13 criteria were met. Pooled odds ratios (ORs) for ITI success were calculated for each determinant. ITI success was defined as negative inhibitor titer (<0.6 BU/mL), FVIII recovery ≥66% of expected, and FVIII half-life ≥6 hours in 16 (59.3%) studies.
Results
We included 27 studies, involving 1,734 participants. Methodological quality of 6 (22.2%) studies (418 participants) was rated as high. Twenty different determinants were assessed. Historical peak titer ≤100 BU/mL (compared with >100 BU/mL, OR, 1.7; 95% CI, 1.4-2.1), pre-ITI titer ≤10 BU/mL (compared with >10 BU/mL, OR, 1.8; 95% CI, 1.4-2.3), and peak titer during ITI ≤100 BU/mL (compared with >100 BU/mL, OR, 2.7; 95% CI, 1.9-3.8) were associated with a higher chance of ITI success.
Conclusion
Our results suggest that determinants related to the inhibitor titer are associated with ITI success.
KeyWords: hemophilia A, factor VIII, antibodies, immune tolerance, systematic review
Essentials
-
•
Knowledge on determinants of immune tolerance induction (ITI) outcome may guide clinical decision making.
-
•
In this systematic review we calculated pooled odds ratios for ITI success for each determinant.
-
•
Historical peak titer, pre-ITI titer, and peak titer during ITI were associated with ITI outcome.
-
•
Patient- and treatment-related determinants were not clearly associated with ITI outcome.
1. Introduction
Hemophilia A is a congenital deficiency of factor VIII (FVIII), causing muscle and joint bleeds that can lead to severe disability [1]. It occurs due to mutations in the FVIII coding gene (F8), located at position Xq28 [2]. Bleeds in persons with hemophilia A are effectively treated with intravenous administration of plasma-derived (pdFVIII) or recombinant FVIII (rFVIII) [3]. However, a major complication of treatment is the development of neutralizing alloantibodies (inhibitors) against FVIII [4]. When this occurs, replacement therapy is less effective, resulting in a more challenging management of bleeding episodes with possible consequent worsening of musculoskeletal damage, health-related quality of life, and life expectancy [4]. Inhibitors occur in 5% to 10% of persons with non-severe hemophilia A, and in 25% to 35% of previously untreated persons with severe hemophilia A [5,6].
Bypassing agents, such as recombinant activated factor VII (rFVIIa) and activated prothrombin complex concentrate (aPCC) have been used to control bleeding in persons with hemophilia A and inhibitors [[7], [8], [9]] However, the hemostatic effectiveness of bypassing agents is lower than that of replacement therapy, and their use as prophylactic agents has only led to partial success [10]. Recently, a humanized bispecific monoclonal antibody, emicizumab, which bridges activated factor IX and factor X, therewith mimicking FVIII activity, entered therapeutic armamentarium to prevent bleeds in people with hemophilia A, with and without inhibitors [11,12]. Emicizumab has been shown to be highly effective as a prophylactic agent administered subcutaneously according to standard dosing schedules across all ages and body weights [13,14]. However, it does not eliminate the need for treatment with FVIII during bleeding or surgery. In such settings, bypassing agents are still required with the additional caveat of increased thrombotic risk if aPCC is administered in setting of emicizumab [11,15,16]. Therefore, eradication of inhibitors to restore the hemostatic effectiveness of FVIII represents a desirable treatment goal [[17], [18], [19]].
The recommended treatment strategy to eradicate inhibitors is immune tolerance induction (ITI), which consists of frequent administration of FVIII concentrate [20]. ITI regimens vary between low-dose consisting of 25–50 international units (IU)/kilogram (kg) three times a week to high-dose of ≥200 IU/kg/d [21]. Successful ITI eradicates the inhibitor and restores the hemostatic activity of FVIII in approximately 70% of cases with the advantage that bleedings are responsive to FVIII concentrates again [21,22]. However, ITI is an expensive treatment with estimated costs up to US$ 11,388.00/kg/year for low-dose ITI regimen [23]. Moreover, ITI is a burdensome treatment for patients and caregivers [17]. In this light, it is of utmost importance to determine the predictors for ITI outcome to help clinicians identifying those at high risk of ITI failure upfront and thus enabling shared decision making on withholding ITI and choose alternative treatments, such as emicizumab, in such patients.
Previous studies have reported several patient-, treatment-, and inhibitor-related determinants of ITI outcome. Large F8 gene deletions have been related to a poorer ITI outcome [24]. Furthermore, low pre-ITI (<10 Bethesda units [BU]/milliliter [mL]), low historical peak titer (<200 BU/mL), young age at start of ITI, and a short time interval between inhibitor detection as well as start of ITI have been associated with a favorable ITI outcome [25,26]. However, these studies reported inconsistent results, and were predominantly conducted in small populations.
Therefore, we performed a systematic review to summarize the currently available evidence on the association between patient-, treatment-, and inhibitor-related determinants and ITI success in persons with hemophilia A and inhibitors. Additionally, we performed a meta-analysis to pool the data to obtain more precise estimates of these associations with ITI success.
2. Methods
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [27]. Inclusion and methodological quality criteria were specified and published in a protocol in advance in the International Prospective Register of Systematic Reviews (PROSPERO)(registration number CRD42020219062). Methods on data analysis were drafted in this protocol but were adjusted to an appropriate model for the extracted data.
2.1. Study eligibility criteria
2.1.1. Studies
Randomized controlled trials, cohort, and case-control studies reporting on the determinants for ITI outcome in persons with hemophilia A, published as a peer-reviewed full paper, were eligible for inclusion without any restriction on publication date. Only studies written in English were included.
2.1.2. Participants
Studies including adult and pediatric persons with mild (FVIII 0.05-0.40 IU/mL), moderate (FVIII 0.01-0.05 IU/mL), and severe (FVIII <0.01 IU/mL) congenital hemophilia A with inhibitors who underwent ITI were eligible for inclusion. Any type of treatment intended to eradicate inhibitors was considered as ITI, regardless of ITI product, FVIII dose, or infusion frequency. The first ITI trial for persons with hemophilia A and inhibitor was defined as primary ITI. Any ITI trial after the first one was defined as rescue ITI.
2.1.3. Endpoints
The primary outcome was complete ITI success, defined according to the definitions of the investigators of the original studies. Non-complete ITI response included partial response and ITI failure, both defined according to the definitions used in the original studies.
2.1.4. Determinants
Any patient-, treatment-, or inhibitor-related determinant reported in included studies was evaluated. Ethnicity of participants was categorized as White or Non-White, or as African-American, or Non-African-American [28]. F8 variants were categorized into high-risk and low-risk for our analysis [29]. High-risk mutations include intron 22 inversions, intron 1 inversions, large deletions, and nonsense mutations [29]. Low-risk mutations include small deletions or insertions, missense mutations, or splice site mutations [29]. ITI dosing was categorized into low- (≤50 IU/kg/d), intermediate- (50-200 IU/kg/d), and high-dose ITI (≥200 IU/kg/d) [21]. Adjuvant therapy included intravenous immunoglobulin, steroids, cyclophosphamide, or rituximab [30]. Inhibitor response was categorized into high-, or low-responding inhibitors, if the lifelong peak titers were ≥5 BU/mL or <5 BU/mL, respectively [31]. Historical peak titer was defined as the highest measured inhibitor titer before ITI. Pre-ITI titer was defined as the most recent inhibitor titer measured before ITI start. Peak titer during ITI was defined as the highest measured inhibitor titer during ITI treatment.
2.1.5. Information sources
Studies were identified by searching MEDLINE (<1946-present) and Embase (<1947-present).
2.2. Search
We used the following search terms: “hemophilia” and “immune tolerance.” The full search for each database is listed in Supplementary Tables S1 and S2. The search was designed and supervised by an information expert (R. Spijker, Amsterdam UMC, Amsterdam, The Netherlands). The first search was conducted on February 15, 2021. An updated search was run on December 29, 2021.
2.3. Study selection
Title and abstracts were examined by 2 independent reviewers (IO and RMC) to identify potentially relevant studies. Full texts of selected articles were reviewed to assess their eligibility for inclusion, by the same 2 independent reviewers. Any doubt or disagreement between the 2 independent reviewers was discussed with a methodological expert (SCG).
2.4. Data collection process
Duplicate articles were identified and excluded by checking the authors’ names, affiliations, and catchment areas. If studies included overlapping patient cohorts evaluated during the same period, we included the most recent study, the study with the largest number of participants, or the study with the highest methodological quality.
2.5. Data extraction and management
Data extraction was performed by two investigators (IO and RMC) using a structured electronic data collection form. We contacted all corresponding authors of the studies with missing data by email among which 2 out of 7 responded to our request. The requested data included ethnicity, F8 genotypes, age at inhibitor development or ITI start, interval between inhibitor development and ITI start, ITI product and regimen, and inhibitor titers (historical peak titer, pre-ITI titer, and peak titer during ITI).
2.6. Data items
The following data were extracted from each included study: study characteristics (name of the first author, year of publication, number of persons included, country, study design, inclusion criteria, and outcome definitions); patient characteristics (age, hemophilia severity, ethnicity, and F8 genotype); treatment characteristics (ITI regimen, including dose, frequency, and product, age at inhibitor development, interval between inhibitor detection and ITI start, age at ITI start, and ITI outcome definitions); inhibitor characteristics (number of exposure days before inhibitor development, historical peak titer, pre-ITI titer, and peak titer during ITI); and the cumulative incidence of ITI success as well as partial response or failure.
2.7. Summary measures
Treatment characteristics were described using median and IQR or range for continuous variables and numbers and proportions for categorical variables. Numbers and proportions were summarized for complete ITI success. Odds ratios (ORs) were extracted from the reports or calculated from the reported data, as we aimed to use most of the available data. Odds ratios (ORs) with 95% CI were calculated for each determinant of ITI success. Relative risk (RR) with 95% CI, was also calculated to report the most precise estimates because OR, can substantially overestimate RR [32]. Exact 95% CI, were calculated using an online tool for the analysis of epidemiologic data (http://epitools.ausvet.com.au). No missing data were imputed. The OR, with 95% CI, for each determinant were plotted in forest plots. Results were presented for primary and rescue ITI separately.
2.8. Data exploration
To explore the within-study and between-study variability (heterogeneity), we visually assessed the extent of overlap in 95% CI, of the OR, in forest plots. In addition, we estimated τ2 which is an estimate of between-study variance [33].
2.9. Data synthesis
As clinical and methodological heterogeneity was limited, we calculated the pooled OR, for each determinant. Conventional methods for meta-analysis can be biased when determinants are rare in the study population and when continuity corrections are used [34]. Therefore, we applied the hypergeometric-normal model for the meta-analysis of predictors for ITI outcome [35]. The meta-analysis was performed in R (version 4.0.3), using the metaphor package for random effects [35].
The pooled OR, with 95% CI, of each determinant for ITI outcome were plotted in a summary plot.
2.10. Data evaluation
2.10.1. Small study data trends
We evaluated whether small study data trends were present by visually assessing the asymmetry of forest plots after arranging the studies by study sample size [36].
2.10.2. Sensitivity analysis
We performed the following sensitivity analyses: the first sub analysis only included studies among persons with severe hemophilia A; the second only included studies with more than 20 participants, the third only included studies in which complete ITI success was defined in accordance to the definition used in the International ITI study [21], the fourth only included intermediate- or high-quality studies, and in the fifth sub analysis ITI success was alternatively defined as both partial and complete success, and compared with failures.
2.11. Quality assessment
The critical appraisal of studies was assessed by 2 reviewers independently (IO and RMC). The Joanna Briggs Institute (JBI) checklists for cohort and cross-sectional studies were combined and adapted to assess the methodological quality of each included study (Supplementary Tables S3 and S4) [[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]] (https://jbi.global/critical-appraisal-tools). Studies were classified as high quality if ≥11 of 13 criteria and intermediate quality if ≥8/13 criteria were met. A score of ≤7 was considered as low quality.
3. Results
3.1. Study selection
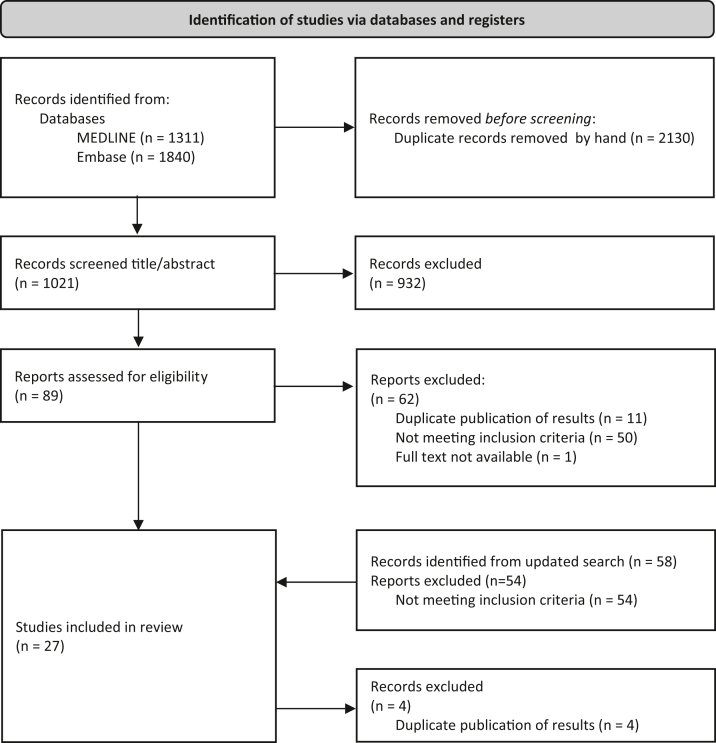
The process of study selection is depicted in Figure 1. Our literature search yielded 1,021 unique references. After screening titles and abstracts, 89 reports were potentially eligible for inclusion. After reading full texts and applying the inclusion criteria, 27 studies were included. Reasons for exclusion of studies after full text screening included duplicate publications of results (n = 11), not meeting the inclusion criteria (n = 50), or full text not available (n = 1). Our updated search yielded another 58 reports, of which 4 were eligible for inclusion. We excluded 4 originally included studies because these cohorts overlapped with the studies included after the search update. Finally, 27 studies were selected. Supplementary Table S5 summarizes the list of studies that appeared to meet the inclusion criteria but were excluded after further inspection.
Figure 1.
Flow chart of study selection. The first literature search was conducted on February 15, 2021. After study selection, 27 studies were eligible for inclusion. The search was updated on December 29, 2021. Four studies were eligible for inclusion; however, these cohorts overlapped with previously included studies. Therefore, we had to exclude 4 studies. In total, 27 studies were included in this systematic review and meta-analysis.
3.2. Included studies
The characteristics of the included studies are summarized in Table 1 [[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]]. Eleven studies were conducted in Europe; 5 in the USA; 4 in Asia; 2 in Africa; 1 in Canada; 1 in USA and Canada; 1 in Europe and Canada; 1 in Europe, USA, Canada, and Australia; and the origin was unknown in one study. One study was a randomized controlled trial, and the other 26 (96%) were cohort studies.
Table 1.
Study characteristics
| Source | Year | N | Country | Study cohort | Inclusion criteria | Severe % | Ethnicity % |
F8 genotype % |
ITI outcome definitions |
Quality | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete success | Partial success | Failure | ||||||||||
| Barnes [37] | 2006 | 32 | Canada | MC | † | 94 | 66 white, 9 African American, 16 Asian, 9 others | na | (A) ‡ (n = 12) (B) (i) Negative inhibitor; (ii) normal recovery (n = 4); (C) Negative inhibitor (n = 6); (D) No criteria (n = 1) |
na | na | +/- |
| Batorova[38] | 2016 | 19 | Slovakia | MC | Children (≤18 y) | 100 | na | 32 Int22Inv, 5 Int1Inv, 21 small deletion, 26 missense mutation, 16 nonsense mutation | ‡ | (i) Negative inhibitor; (ii) Inability to normalize FVIII recovery or half-life; (iii) Clinical response to FVIII therapy without an anamnestic increase | (A) Inability to eradicate inhibitor; (B) Inability to install an effective prophylaxis within 36 mo of ITI | +/- |
| Callaghan[39] | 2011 | 31 | US | SC | Children (≤18 y) | 100 | 26 white, 61 African American, 13 others | na | ‡ | na | (A) Inability to achieve CS in 22 mo; (B) ≤20% decrease inhibitor titer over a 6-mo period after 3 mo ITI | + |
| DiMichele[25] | 2002 | 164 | US, Canada | MC | † | 93 | 63 white, 13 Asian, 24 others | na | ‡ | na | (A) ineffectiveness ITI; (B) self desire to terminate ITI; (C) CVAD complications; (D) adverse reaction to therapy; (E) loss of medical insurance; (F) patient relocation; (G) death from unrelated causes; (H) poor patient compliance; (I) enrollment in a BPA study | +/- |
| Di Minno[40] | 2021 | 137 | Italy | MC | HR inhibitors | 100 | na | 53 Int1/22Inv, 7 small deletion, 8 small insertion, 9 large deletion, 3 missense, 8 nonsense, 3 splice site | ‡ | (A) Inhibitor <5 BU/mL; (B) Inability to normalize FVIII recovery or half-life | (A) Inability to achieve CS in 33 mo; (B) ≤20% decrease inhibitor titer over a 6-mo period after 3 mo ITI | +/- |
| Dou[41] | 2021 | 110 | China | MC | † | na | na | na | Negative inhibitor (<0.6 BU/mL) | na | na | - |
| El Alfy[42] | 2000 | 10 | Egypt | SC | Children (<18 y) | 100 | na | na | (i) Inhibitor <2 BU/mL; (ii) FVIII recovery ≥60%; (iii) absence of anamnestic response on subsequent FVIII exposure | na | No decrease inhibitor titer over a 6-mo period | +/- |
| Elafly[43] | 2021 | 20 | Egypt | MC | Children (2-18 y); HR inhibitors |
100 | other | na | (i) Negative inhibitor (<0.6 BU/mL); (ii) FVIII recovery >66% | (i) Inhibitor <5 BU/mL; (ii) FVIII recovery <66% | Inability to achieve CS/PS in 24 mo | +/- |
| Escobar[44] | 2020 | 13 | US | MC | † | 92 | 15 white, 46 African American, 8 Asian, 31 others |
31 Int22Inv, 15 small deletion | ‡ | (i) Inhibitor <5 BU/mL; (ii) Inability to normalize FVIII recovery or half-life; (iii) Clinical response to FVIII therapy without an anamnestic increase | Inability to achieve CS/PS | +/- |
| Greninger[45] | 2008 | 11 | US | SC | HR inhibitors; treatment (≥1) with Alphanate | 100 | 46 white, 9 African American, 9 Asian, 36 others | 36 Int22Inv, 9 small deletion, 9 missense, 18 nonsense, 9 splice site | ‡ | (i) Negative inhibitor (<0.6 BU/mL); (ii) Inability to normalize FVIII recovery or half-life | Inability to achieve CS/PS | +/- |
| Hay[21] | 2012 | 115 | na | MC | Children (<8 y); Peak inhibitor titer ≥5 - ≤200; Pre-ITI titer ≤10 BU/mL |
100 | 51 white, 8 African American, 20 Asian, 20 others | na | ‡ | (i) Negative inhibitor (<0.6 BU/mL); (ii) Inability to normalize FVIII recovery or half-life; (iii) Clinical response to FVIII therapy without an anamnestic increase | (A) Inability to achieve CS in 33 mo; (B) ≤20% decrease inhibitor titer over a 6-mo period after 3 mo ITI; (C) Withdrawal from study | + |
| Haya[46] | 2001 | 42 | Spain | MC | † | 83 | na | na | (i) Negative inhibitor; (ii) FVIII half-life ≥8 hours | (i) Immune response shifted from a high- to low responder (<10 BU/mL) | No decrease inhibitor titer | +/- |
| Kreuz[47] | 2016 | 48 | Croatia, Germany, Poland, Portugal, Russia, Slovakia, Slovenia, Spain | MC | † | 83 | 96 white | na | ‡ | (i) Negative inhibitor (<0.6 BU/mL); (ii) Inability to normalize FVIII recovery or half-life | (A) Inability to achieve CS/PS in 36 mo; (B) Withdrawal from study | + |
| Kurth[30] | 2011 | 33 | US | MC | HR inhibitors; Treatment with pdFVIII/VWF |
100 | 54 white, 24 African American, 6 Asian, 15 others | na | ‡ | (i) Inhibitor titer <5 BU/mL; (ii) Clinical response to FVIII therapy without an anamnestic increase | (A) Inability to achieve CS in 33 mo; (B) ≤20% decrease inhibitor titer over a 6-mo period after 3 mo ITI |
+/- |
| Lapalud[48] | 2015 | 15 | France | MC | Children (≤6 y); HR inhibitors; Follow-up ≥33 mo |
100 | na | na | ‡ | na | Inability to achieve CS/PS | +/- |
| Lenk[49] | 2000 | 140 | Germany | MC | † | 87 | na | na | (i) Therapy completed; (ii) Normal FVIII recovery; (iii) Normal FVIII half-life | (i) Inhibitor ≤2 BU/mL; and/or (ii) Inability to normalize FVIII recovery or half-life | Inability to achieve CS/PS (lack of compliance, additional diseases or death included) | - |
| Lin [50] | 2011 | 29 | Taiwan | SC | † | 93 | na | na | (i) Negative inhibitor (<0.6 BU/mL); (ii) Absence of anamnestic response on subsequent FVIII exposure | (i) >50% reduction of inhibitor titer; (ii) Clinical response to FVIII therapy; (iii) No need for bypass therapy | No decrease inhibitor titer | + |
| Mariani[51] | 2001 | 314 | Australia, Canada, Europe, Japan, US | MC | † | 100 | na | na | ‡ | Immune response shifted from a high- to low responder | No decrease inhibitor titer | +/- |
| Nakar[52] | 2015 | 58 | US | MC | † | 95 | 85 white, 2 African American, 2 Asian, 10 others | 48 Int22Inv, 2 Int1Inv, 10 small deletion, 2 small insertion, 16 missense, 3 nonsense, 3 frame shift | (i) Negative inhibitor; (ii) Ability to use FVIII concentrate for treatment of bleeding | (i) Inhibitor titer 1-5 BU/mL; (ii) Ability to use FVIII concentrate for treatment of bleeding | (A) Inability to achieve CS/PS in 36 mo; (B) Withdrawal from study | + |
| Nogami[31] | 2018 | 155 | Japan | MC | † | 90 | Asian | na | Negative inhibitor | na | No decrease inhibitor titer under threshold | +/- |
| Oldenburg[53] | 2014 | 60 | Germany, Italy, Spain | MC | Treatment with high-purity pdFVIII/VWF. | na | 88 white, 12 others | na | ‡ | (i) Negative inhibitor (<5 BU/mL); (ii) Inability to normalize FVIII recovery or half-life; (iii) Clinical response to FVIII therapy without an anamnestic increase | Inability to achieve CS/PS | +/- |
| Rivard[54] | 2013 | 32 | Canada, France, Greece, Italy, Spain | MC | Children (≤8 y); Treatment with rFVIII-FS; HR inhibitors; Treatment ≥9 mo |
100 | 78 white, 6 African American, 6 Asian, 9 others | na | (A) (i) Negative inhibitor; (ii) Normal FVIII recovery (n = 9); (B) Negative inhibitor (n = 8); (C) ‡ (n = 5) |
na | na | +/- |
| Rocino[55] | 2016 | 71 | France, Germany, Italy, Netherlands, Portugal, Spain, Sweden | MC | Follow-up ≥12 mo | 100 | na | 61 Int22Inv, 3 Int1Inv, 9 small deletion, 5 large deletion, 6 nonsense, 6 splice site | ‡ | (i) Negative inhibitor (<0.6 BU/mL); (ii) Inability to normalize FVIII recovery or half-life; (iii) Clinical response to FVIII therapy without an anamnestic increase | Inability to achieve CS/PS within 9 mo follow-up | +/- |
| Ryu[56] | 2015 | 17 | Korea | SC | Historical titer >5 BU/mL; Pre-ITI titer <10 BU/mL |
100 | Asian | 35 Int1Inv, 12 small deletion, 29 nonsense, 24 frame shift | ‡ | (i) Immune response shifted from a high- to low responder; (ii) Ability to use FVIII concentrate for treatment of bleeding | No decrease inhibitor titer for 10 wk | +/- |
| Salviato[24] | 2007 | 16 | Italy | MC | † | 100 | na | 31 Int22Inv, 19 small deletion, 13 large deletion, 31 nonsense, 6 splice site | na | na | na | - |
| Ter Avest[57] | 2010 | 21 | Netherlands | SC | Children (<6 y) Low dose ITI treatment |
100 | White | 52 Int22Inv, 14 small deletion, 29 large deletion | ‡ | (i) Negative inhibitor (<0.6 BU/mL); (ii) Inability to normalize FVIII recovery or half-life; (iii) Clinical response to FVIII therapy without an anamnestic increase | (A) No decrease inhibitor titer for ≥ 26 wk ITI treatment; (B) Switch to a high dose regimen | +/- |
| Unuvar[58] | 2008 | 21 | Turkey | MC | Children (≤20 y); HR inhibitors | 95 | na | na | ‡ | (i) Inhibitor titer <5 BU/mL; (ii) Clinical response to FVIII therapy without an anamnestic increase | (A) Inability to achieve CS/PS within 6-36 mo follow-up; (B) No decrease inhibitor titer <5 BU/mL | +/- |
Abbreviations: na = not available, N = number of participants, ITI = immune tolerance induction, SC = single center, MC = multicenter, HR inhibitors = High-responding inhibitor ≥5 BU/mL, Int22Inv = intron 22 inversion, Int1Inv = intron 1 inversion, Int1/22Inv = Intron 1 or Intron 22 inversion, y = year, CS = complete success, PS = partial success, BU = Bethesda Unit, rFVIII-FS = recombinant FVIII formulated with sucrose, pdFVIII/VWF = plasma-derived FVIII containing von Willebrand Factor.
Study quality scored according to the JBI checklist as follows: + high-quality, +/- intermediate- quality, - low quality.
inclusion criteria (i) hemophilia A; (ii) inhibitor; (iii) ITI treatment.
ITI success defined according to the International ITI study: (i) Negative inhibitor (<0.6 BU/mL); (ii) FVIII recovery >66%; (iii) FVIII half-life >6 hours; ((iv) Absence of anamnestic response on subsequent FVIII exposure).[21]
3.2.1. Participants
The number of subjects per study varied from 10 to 314. The studies included a total of 1,734 persons with hemophilia A, of whom 1,496 (86.3%) had severe hemophilia A and 68 (3.9%) had non-severe hemophilia A, and 170 (9.8%) participants had an unknown severity. Most studies (63.0%) involved both adults and children with hemophilia A, and another nine (33.3%) studies included only children less than 18 years (one study defined children aged less than 20 years [58]). The age of study participants was unknown in 1 (3.7%) study [24]. Studies described mainly primary ITI courses administered to 1,679 (96.8%) participants, whereas rescue ITI represented only 4.4% of cases (77 participants). For 22 (1.2%) participants, data for both primary and rescue ITI were available [31]. Almost a quarter of the participants self-identified as White (n = 410; 23.6%), 49 (2.8%) as African-American, 228 (13.1%) as Asian, and 118 (6.8%) as being of other ethnicities. The ethnicity was unknown in 929 (53.6%) persons. Nine studies (33.3%) reported the persons’ F8 genotypes.
3.2.2. Primary ITI treatment characteristics
In 24 (88.9%) studies, information on FVIII product for ITI was given, with similar distribution among participants (pdFVIII for 41.1%; rFVIII for 43.8%, Table 2). A quarter of persons (435 of 1,679, 25.9%) received low-dose ITI, 455 (27.1%) persons intermediate-dose ITI, and 245 (14.6%) participants high-dose ITI. A combination of these regimens was given in 19.5% (328 of 1,679) of cases. The dose and infusion frequency were unknown in 12.9% of primary ITI treatments.
Table 2.
Treatment characteristics for primary ITI
| Source | No. of participants | ITI regimen (%) | ITI Product (%) |
Age at inhibitor detection (mo) | Interval ID - ITI start (mo) | Age at ITI start, (mo) | No. of exposure d before inhibitor administration | Pre-ITI titer | Historical peak titer | Peak titer on ITI | Outcome (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pd | r | pd+r | CS | PS | F | ||||||||||
| Barnes [37] | 32 | 50 IU/kg 3-4x/wk (28); 100 IU/kg/d (72) |
6 | 88 | 6 | 25.2 (14.4-55.2) | 3.5 (0.3-19.2) | na | na | 3.3 (1.5-7.8) | 8.5 (4.3-67.8) | 13.5 (0-126.5) | 79.3 | na | 20.7 |
| Batorova [38] | 19 | 50 IU/kg 2x/d (for 2-3w), 50 IU/kg 3-4x/wk (42); 100 IU/kg 2x/d (58) |
68 | 32 | 0 | 22.8 (16.8-156) | na | 60 (18-180) | 25 (15-32) | 1.2 (0.6-3.4) | 7.8 (2.8-20) | 3.6 (1.9-4.6) | 78.9 | 10.5 | 5.3f |
| Callaghan [39] | 31 | 50 IU/kg 3-4x/wk (74); 50-250 IU/kg/d (26) |
26 | 68 | 6 | 13 (2-141b) | 13.3 (7.3-36.5) | 56.5 (16.4-139.7) | 7.5 (4.5-13.8) | 4.4 (1.3-22) | 34 (7.1-153) | 22 (2-139) | 71.0 | na | 29.0 |
| DiMichele [25] | 164 | Alternate day dosing (21); ≥100 IU/kg/d (47); ≥200 IU/kg/d (7) Unspecified (25) |
25 | 75 | 0 | na | 55.8e (0-256b) | 111.6e (1.2-768b) | na | CS/19.5 (0-230) F/61.3 (0-523) |
CS/130 (5-4833) F/571 (6-9999.5) |
CS/154 (0-1770) F/619 (34-3500) |
70.1 | na | 29.9 |
| Di Minno [40] | 137 | 50 IU/kg 3x/wk (24); 100 IU/kg/d (42); 200 IU/kg/d (34) |
27 | 73 | 0 | 30 (1-576) | 19 (<1-332) | 57.6 (6-702) | 15 (3->150) | 4.5 (0-200) | 64 (6-920) | na | 51.1 | 15.3 | 33.6 |
| Dou [41] | 110 | 100 IU/kg/d (7); 50 IU/kg/d (21); 50 IU/kg/ 1x/2d (72) |
82 | 16 | 3 | na | na | 72 (36-114) | na | na | na | na | 64.9 | na | 35.1 |
| El Alfy [42] | 10 | 25 IU/kg 3-4x/wk (40); 50 IU/kg 3-4x/wk (60) |
na | na | na | na | na | 66 (27-126) | 32 (18.8-95) | 43 (19.5-61.8) | 44.5 (25.5-62.5) | na | 80 | na | 20 |
| Elalfy [43] | 20 | 50 IU/kg 3x/wk | 100 | 0 | 0 | na | 8.5 (0.5-33) | 60 (36-144) | na | 36.5 (12-169) | 62 (2-412) | 41 (8-320) | 60 | 20 | 20 |
| Escobar [44] | 6 | 100 IU/kg/d | 100 | 0 | 0 | 84 (63-384) | NA | 402 (318-495) | na | 1.0 (0.6-13.5) | 33.4 (1.2-118.6) | na | 83.3 | na | 16.7 |
| Greninger [45] | 7 | ≤700 IU/kg/wk (57); 1400 IU/kg/wk (43); |
100 | 0 | 0 | 30 (12-97.5) | 16 (0-207.5) | 97.5 (28-264) | na | 5 (1.4-27) | 89 (5-200) | 10 (5-46) | 71.4 | 28.6 | 0 |
| Hay [21] | 115 | 50 IU/kg 3x/wk (50)††† 200 IU/kg/d (50)‡‡‡ |
11 | 89 | 0 | na | na | 15.6 (10.7-23.2)††† 14.4 (11.4-25.3)‡‡‡ |
na | 5.9 (3.3-7.3)††† 5.1 (3-7.4)‡‡‡ |
21.7 (13.4-52.5)††† 22.4 (12.5-50)‡‡‡ |
40.1 (7.6-150)††† 33 (1.5-205)‡‡‡ |
69.7 | 4.5 | 25.8 |
| Haya [46] | 42 | 140 IU/kg/d | 87 | 13 | 0 | na | 25 (12-78) | 84 (48.0-177) | 29 (3-250b) | 11 (5.5-30) | 76 (29.8-340) | na | 68.4 | 5.3 | 26.3 |
| Kreuz [47] | 48 | 50-100 IU/kg/d (13) 100 IU/kg 2x/d (88) |
100 | 0 | 0 | 37.2 (8.4-298.8b) | na | 69.6 (9.6-337.2b) | na | 12.5 (0.6-9736) | na | na | 70.8 | 8.3 | 20.8 |
| Kurth [30] | 8 | 50 IU/kg/d (13) 100-200 IU/kg/d (88) |
100 | 0 | 0 | 32 (17-41) | na | 41 (28.8-78.8) | na | 24.5 (1.8-73.5) | 250.5 (49.5-735.3) | na | 37.5 | 37.5 | 25 |
| Lapalud [48] | 15 | ≤450 IU/kg/wk (47) 700-1400 IU/kg/wk (53) |
27 | 73 | 0 | na | 7.1 (0.8-10.9) | 22.3 (14.6-46.2) | na | 10 (2.3-15) | 30 (12.5-181.3) | 241 (70-1334) | 46.7 | na | 53.3 |
| Lenk [49] | 140 | na | na | na | na | 132e | na | 154.8e | na | na | na | na | 78.6 | 8.7 | 12.7 |
| Lin [50] | 29 | 30-100 IU/kg/d to 3-4x/wk | 0 | 100 | 0 | na | HR 36 (12.5-49) | 204 (7.2-540) 306 (19.2-540) |
na | 0.85 (0.6-1.6)e 5 (2.8-367.5) |
40 (16-49) | 1.0 (0.7-3.3)e 15 (5.8-442) |
84.6 | 11.5 | 3.8 |
| Mariani [51] | 314 | <50 IU/kg/d (25) 50-99 IU/kg/d (23) 100-199 IU/kg/d (20) ≥200 IU/kg/d (32) |
88 | 12 | 0 | 48 (<12-768b) | 17 (<1-379b) | na | na | 7 (0-720b) | 54 (1-25,000b) | na | 44.6 | 7.3 | 21.0g |
| Nakar [52] | 58 | ≥100 IU/kg/d | 26 | 74 | 0 | 18 (1.3-603.6b) | na | na | na | na | na | na | 88 | na | 12 |
| Nogami [31] | 155 | ≤75 IU/kg 3x/wk (52); ≥90 IU/kg/d (12); Other (30) Unclassified (5) |
13 | 87 | 0 | 14.4 (10.8-25.2) | 7.2 (2.4-26.4) | na | NA | 3.9 (1.7-7.9) | 14 (3.7-46) | na | 60.6 | na | 24.5h |
| Oldenburg [53] | 32c 9d |
<100 IU/kg/d (22)a ≥100 IU/kg/d (78)a |
100 | 0 | 0 | na | na | 20.4 (0.7-206.4b)c 422.4 (277.2-638.4b)d |
na | 5.7 (0.7-831b)c 7.4 (1.0-109b)d |
20 (1.7-6842b)c 73 (11.2-737b)d |
na | 63.4 | 24.4 | 12.2 |
| Rivard [54] | 32 | <85 IU/kg/d (34) ≥85 IU/kg/d (66) |
0 | 100 | 0 | 12 (0-48b) | 8.7 (0.2-38.9b) | 24 (0-60b) | 13.5 | na | na | na | 68.8 | na | 31.2 |
| Rocino [55] | 71 | 50-≥200 IU/kg/d to 3-4x/wk | 17 | 83 | 0 | 20.4 (2.6-492b) | na | 45.6 (5.3-492.2b) | 15 (2-43b) | 4 (0-165b) | 18.5 (0.8-704b) | 43 (0.5-16,384b) | 71.8 | 12.7 | 15.5 |
| Ryu [56] | 17 | <25 IU/kg 3x/wk (6); <50 IU/kg/d (6); 75-100 IU/kg 3x/wk (65); 90-110 IU/kg/d (24) |
65 | 24 | 12 | 24 (12-36) | 57.6 (29.4-109.8) | 69.6 (44.4-148.8) | 16 (12-47) | 3.7 (2.1-5.9) | 30.8 (12.6-56.8) | 14.4 (3.3-39.6) | 82.4 | 5.9 | 11.8 |
| Salviato [24] | 16 | na | na | na | na | na | na | na | na | 4.4 (1.5-8) | 300 (72-976) | na | 68.8 | na | 31.3 |
| Ter Avest [57] | 21 | 25-50 IU/kg 2-4x/wk (86); From LD to HD (14) |
48 | 52 | 0 | 19 (13.5-28) | na | na | 17 (10.5-35) | 4.5 (2.5-14.8) | na | 4.6 (1.7-16.4) | 85.7 | na | 14.3 |
| Unuvar [58] | 21 | 20-50 IU/kg 2x/wk (57); 50 IU/kg 3x/wk (5); 50 IU/kg 3x/wk; HD VWF/FVIII (5); 25 IU/kg 3x/wk (10); 30 IU/kg 3x/wk (19); Dutch protocol (5) |
100 | 0 | 0 | na | 2.5 (0.3-60b) | 108 (42-138) | 25 (14-64.5) | 19.2 (3.6-515b) | 80 (6-517b) | 27 (2.5-517b) | 26.3 | 36.8 | 36.8 |
Numbers are reported as median (IQR), unless states otherwise.
CS, complete success; F, failure; H, high dose; HR, high-responding inhibitor ≥5 BU/mL; IU, international unit; ITI, immune tolerance induction; kg, kilogram; L, low dose; LR, low-responding inhibitor <5 BU/mL; na, not available; PS, partial success; pd, plasma-derived FVIII; r, recombinant FVIII; pd+r, plasma-derived and recombinant FVIII.
Both primary and rescue ITI.
Range.
Children.
Adults.
Reported numbers of the largest group.
One person died while on ITI, therefore the ITI outcome is not available in 5.3%.
Data was insufficient to evaluate ITI outcome in 37 persons (11.8%) and ITI was ongoing in 48 persons (15.3%).
ITI was ongoing in 23 patients (14.8%).
3.2.3. Rescue ITI treatment characteristics
In total, 5 studies reported on rescue ITI, involving 77 persons with hemophilia A (Table 3). Fifty-eight (75.3%) participants received pdFVIII, and 19 (24.7%) received rFVIII. The ITI regimens were different among all studies.
Table 3.
Treatment characteristics for rescue ITI
| Source | No. of participants | ITI regimen (%) | ITI Product (%) |
Age at inhibitor detection (m) | Interval ID - ITI start (m) | Age at ITI start, (m) | No. of exposure d before inhibitor administration | Pre-ITI titer | Historical peak titer | Peak titer on ITI | Outcome (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pd | r | pd+r | CS | PS | F | ||||||||||
| Escobar [44] | 7 | 100 IU/kg/d | 100 | 0 | 0 | 24 (12-36) | na | 126 (60-384) | na | 17 (2.4-109) | 104.1 (75.5-265.1) | na | 28.6 | 42.9 | 28.6 |
| Greninger [45] | 4 | 1400 IU/kg/wk | 100 | 0 | 0 | 14 (3.4-17.4) | 3.4 (0.1-52.3) | 18 (5.1-67.5) | na | 7.4 (4.4-197.8) | 47 (11.6-210.8) | 1216 (346-2868.6) | 0 | na | 100 |
| Kurth [30] | 25 | 50 IU/kg/d (8) 100-200 IU/kg/d (92) |
100 | 0 | 0 | 10.5 (7.3-20.5) | na | 54 (35.5-98) | na | 22 (2-76) | 277.5 (122.5-769.5) | na | 32 | 20 | 48 |
| Nogami [31] | 22 | ≤75 IU/kg 3x/wk (52)a; ≥90 IU/kg/d (12)a; Other (30)a |
13 | 87 | 0 | 14.4 (10.8-25.2)a | 7.2 (2.4-26.4)a | na | na | 3.9 (1.7-7.9)a | 14 (3.7-46)a | na | 71.2 | na | 28.8 |
| Oldenburg [53] | 17c 2d |
<100 IU/kg/d (22)a; ≥100 IU/kg/d (78)a |
100 | 0 | 0 | na | na | 92.3 (30-184.8b)c 345.6 (298.8-391.2 b)d |
na | 20.3 (1.6-200 b)c 10 (5-15 b)d |
92 (2.1-4505 b)c 200 (100-300 b)d |
na | 36.8 | 36.8 | 26.3 |
Numbers are reported as median (interquartile range), unless stated otherwise.
CS, complete success; F, failure; IU, international unit; ITI, immune tolerance induction; kg, kilogram; na, not available; PS, partial success; pd, plasma-derived FVIII; r, recombinant FVIII; pd+r, plasma-derived and recombinant FVIII.
Both primary and rescue ITI.
Range.
Children.
Adults.
3.2.4. Complete ITI success definitions
The definition for complete ITI success varied among studies. Sixteen (59.3%) studies used the following definition for complete ITI success
: (i) negative inhibitor titer (<0.6 BU/mL); (ii) FVIII recovery >66%, and (iii) FVIII half-life ≥6 hours. Other definitions used for ITI outcome are presented in Table 1.
3.3. Methodological quality of studies
The methodological quality was high in 6/27 (22.2%) studies, as these met at least 11 criteria from the adapted JBI checklist (Supplementary Table S4) [[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]]. None of the 27 studies met all the criteria. Most frequently, this was because no strategies were described to adjust for potential confounding factors.
3.4. Results of individual studies
The reported and calculated RRs and ORs for associations between patient-, treatment-, and inhibitor-related determinants and ITI success for primary ITI outcome are presented in Supplementary Tables S6–S8. The rescue ITI findings are presented in Supplementary Tables S9–S11. The cumulative incidence of complete ITI success in persons with hemophilia A receiving primary ITI varied from 26.3% to 88% and for rescue ITI from 28.6% to 71.2%. In total, 20 determinants of ITI outcome were reported in the included studies or were re-categorized from raw data reported in the studies.
3.5. Data synthesis
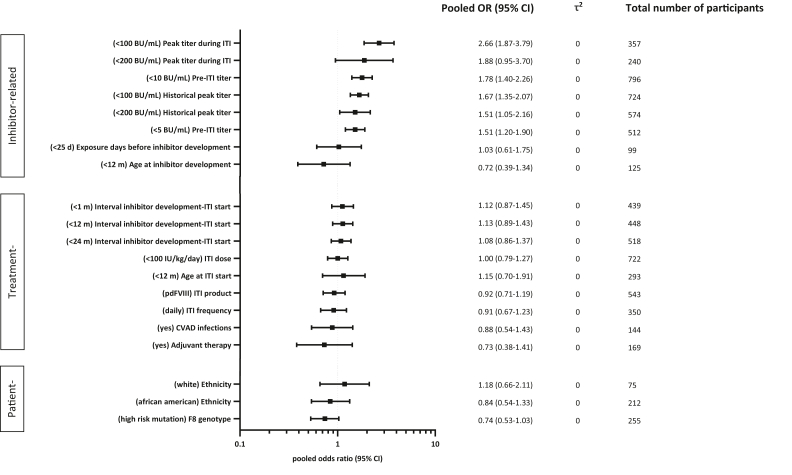
The pooled ORs of each determinant of complete ITI success of primary ITI are presented in Figure 2.
Figure 2.
Pooled odds ratio (OR)s of determinants for complete success in primary ITI (immune tolerance induction). All presented pooled ORs show the chance for complete ITI success. Every determinant is categorized in 2 groups and the reference group is reported. For example, the pooled OR, for peak titer during ITI <100 BU/mL shows a 2.66 increased chance for complete ITI success compared to ≥100 BU/mL. On the other hand, the pooled OR, for age at inhibitor development ≤12 months shows a 0.72 decreased chance for complete ITI success, thus favoring persons older than 12 months.
3.5.1. Patient-related determinants of primary ITI
There was no clear association between ethnicity and ITI outcome. Persons with hemophilia A with high-risk mutations had a 26% lower probability to achieve complete ITI success (pooled OR, 0.7; 95% CI, 0.5-1.0), compared with those with low-risk mutations.
3.5.2. Treatment-related determinants of primary ITI
Eight treatment-related determinants were interrogated for their impact on complete ITI success. Different ITI regimens, including product type, FVIII dose and infusion frequency were not clearly associated with ITI outcome. Neither were the time interval between inhibitor development and ITI start, Central Venous Access Device (CVAD)-infections during ITI, nor adjuvant therapy associated with ITI outcome.
3.5.3. Inhibitor-related determinants of primary ITI
Persons with hemophilia A with historical peak titers ≤100 BU/mL, pre-ITI titer ≤10 BU/mL, and peak titer during ITI ≤100 BU/mL were 1.7 to 2.7 times more likely to achieve complete ITI success than the persons with hemophilia A with respective high inhibitor titers (pooled OR, 1.7; 95% CI, 1.4-2.1; 1.8; 95% CI, 1.4-2.3; and 2.7; 95% CI, 1.9-3.8, respectively). The cumulative number of exposure days to FVIII products and the age of inhibitor development were not associated with ITI outcome.
The data for patient-, treatment-, and inhibitor-related determinants of rescue ITI were not pooled because of the limited studies reported on rescue ITI and the low number of participants included in this sub-cohort.
3.6. Data evaluation
3.6.1. Small study data trends for primary ITI
Forest plots sorted by study sample size did not reveal any small study data trends (Supplementary Fig. S1A–S20A).
3.6.2. Sensitivity analysis for primary ITI
All sensitivity analyses resulted in similar pooled OR, (Supplementary Fig. S1–S20).
4. Discussion
In this systematic review and meta-analysis, we summarized and pooled the data for 1,734 persons with hemophilia A from 27 included studies. The aim was to identify patient-, treatment-, and inhibitor-related determinants of ITI outcome and estimate the effects of these determinants on ITI outcome with more precision than is possible with single studies. We found that historical peak titer below 100 BU/mL, pre-ITI titer below 10 BU/mL, and peak inhibitor titers during ITI below 100 BU/mL were significantly associated with primary ITI success, suggesting that the robustness of anti-FVIII immune response plays a role in ITI outcome, and a strong immune response may be more challenging to be switched-off. Other patient-, treatment-, and inhibitor-related determinants were not clearly related to ITI outcome in primary ITI. For rescue ITI, study findings were summarized; however, we were unable to draw any conclusions regarding the associations between patient-, treatment-, and inhibitor-related determinants and ITI outcome. This was due to the low number of persons with hemophilia A who received rescue ITI included in these studies and the low number of studies reporting on rescue ITI.
Because various studies reported favorable results on ITI outcome in persons with hemophilia A with pre-ITI titers below 10 BU/mL, clinicians attempted to postpone ITI until the inhibitor titer had dropped [21,46,59]. However, the initial level of inhibitor titer is now considered a marker of the immune response strength toward administered FVIII; waiting for the inhibitor to drop below 10 BU/mL is not expected to result in an improved efficacy of inhibitor eradication during ITI. Therefore, it is nowadays recommended to start ITI as soon as possible after a high-titer inhibitor is detected, mainly because of the concern about the potential inhibitor-related morbidity caused by bleeds during the waiting period for inhibitor titer to drop [17,52]. Therefore, we emphasize that pre-ITI titer below 10 BU/mL as a predictor for ITI success should not be interpreted as an indication to wait until titer falls to ≤10 BU/mL before starting ITI.
Presently, the exact mechanisms of achieving tolerance by repeated administration of FVIII is still not completely elucidated [60]. Key players in the immune response to FVIII are FVIII-specific CD4+ T-cells, FVIII-specific B-memory cells, and anti-FVIII antibody producing plasma cells [60]. These cells are the main targets of ITI. Different mechanisms have been described that all contribute to the inhibition or deletion of these FVIII-specific B- and T-cells and thus in inducing immune tolerance: inhibition of B-memory cell differentiation into anti-FVIII antibody secreting plasma cells by high FVIII concentrations, and anergy of effector T-cells induced by exhaustion or overstimulation and emergence of regulatory T cells due to chronic exposure to FVIII in a non-inflammatory state [61,62]
Since the advent of emicizumab, a debate has started on the utility of ITI. Current consensus statements and guidelines still recommend ITI as the mainstay of treatment for persons with hemophilia A with inhibitors. Arguments in favor of ITI are the increased therapeutic options and lower costs when tolerance for FVIII is re-established, as emicizumab is only effective as prophylaxis and not for treatment of bleeding. Patients who are not tolerant and require bypassing agents for the treatment of bleeds can only use doses <100 IU/kg/d for not more than 1 day of aPCC when treatment with rFVIIa fails, as high doses of aPCC in combination with emicizumab may confer a thrombotic risk [11]. In vivo study by Kizilocak et al. [63] reported that a single dose of aPCC was safe for most persons on emicizumab who demonstrated in 66% normal thrombin generation at approved doses of aPCC. Moreover, patients with inhibitors are currently not eligible for gene therapy that offers the chance of a long-term reversal of bleeding phenotype into a mild one without the need for prophylaxis [64]. However, because ITI remains a burdensome and costly treatment, it is of utmost importance to be able to identify which persons are likely to respond to ITI and which persons are not. Knowledge on determinants of ITI outcome can be used as the basis for a prediction algorithm that may estimate the chance of successful ITI to guide clinical decision making.
A strength of this systematic review is large number of participants, yielding the most precise estimates of associations between patient-, treatment-, and inhibitor-related determinants and complete ITI success. We furthermore used an advanced statistical model for our meta-analysis. The number of participants per determinant category was often small, resulting in either 0 or 100% of persons with ITI success in certain subcategories. Because of these “null cells” in the data, results using standard methods for meta-analysis can be severely biased. Therefore, we used a meta-analysis model especially suitable for sparse and unbalanced data: the hypergeometric-normal model [35]. This model has several advantages: it avoids the use of continuity correction and it takes into account the uncertainty in the estimates of standard errors and therewith avoids bias caused by the correlation between estimate and standard error. Another strength is that we have performed several sensitivity analyses, showing that the effect estimates obtained with the meta-analysis are robust.
Our study had several limitations. Firstly, the heterogeneity in ITI protocols, as well as varying ITI outcome definitions across studies made it difficult to compare results. Noteworthy not only the wide variety in definitions for ITI failure, which included failure to achieve tolerance, but also, for example, withdrawal from study, patient relocation, or death made comparison between studies more difficult. We regarded partial ITI success as not complete ITI success, and grouped this into the failure group, as participants with partial success may have not yet reached complete ITI success due to insufficient follow up time. This might have underestimated the ORs. To address this, we performed one sensitivity analysis that grouped complete and partial successes, compared with failures, and another analysis among patients with complete ITI success, as defined in the International ITI study[21], versus failures. This did not result in different pooled OR. Secondly, the data that were reported not precisely according to our used categorization of determinant, were excluded from the current meta-analysis. This could have resulted in underreporting of data. Thirdly, titer at start of ITI is controversial as patients with a titer of ≤10 BU/mL include two types of patients; those who never had a titer >10 BU/mL, and those patients who had peak titers >10 BU/mL but clinicians waited to start ITI after their titer dropped to ≤10 BU/mL. Unfortunately, not all papers reported data on both parameters, not allowing us to analyze the effect on ITI outcome in persons with both historical peak titer ≤10 BU/mL as well as pre-ITI titer ≤10 BU/mL. Fourthly, duplicate inclusion of participants may have occurred, particularly from four large registries [25,46,49,51]. This may have led to slight overestimation of the effect of determinants on ITI outcome, but we expect that duplicate inclusion of participants is limited. Fifthly, we did not include potential immunological factors as predictors of ITI outcome in our study. Evidence on immunological variables affecting ITI outcome is limited. However, it has been suggested that emergence of IgG4 FVIII-specific antibodies during ITI was associated with a poor ITI outcome [65]. Lastly, antibodies directed against the A2-domain were reported to be associated with ITI failure, whereas antibodies directed against the light chain of FVIII were associated with favorable ITI outcome [45,48,66]. Further studies should elaborate on these antibody features to see whether these immunological markers are suitable candidates to predict ITI outcome during the course of ITI.
This study provides a comprehensive overview of patient-, treatment-, and inhibitor-related determinants of ITI outcome. We summarized and pooled the available evidence on associations between potential determinants and ITI outcome. Our findings suggest that determinants especially related to inhibitor titer (pre-ITI and peak titers) are associated with ITI outcome. Future research should elucidate on immunological mechanisms in order to identify biomarkers that could predict ITI outcome, and should further investigate the role for genetic determinants for ITI outcome in a multicenter cohort study with sufficient participants.
Acknowledgments
René Spijker, information expert Amsterdam University Medical Center, provided assistance in designing and conducting the literature search. Barbara L. Kroner, MPH, PhD, RTI International, Research Triangle Park, NC, USA, Liliana R. Preiss, MSA, RTI International, Research Triangle Park, NC, USA, and Donna Di Michele, MD, Courtesy Clinical Professor of Pediatrics, Weill Cornell Medical College, New York, USA, retrieved and reformatted the data from the North American Immune Tolerance Registry.Michael U. Callaghan, MD, Associate Professor of Pediatrics, Children’s Hospital of Michigan, MI, USA, retrieved and reformatted missing data.
Author contributions
I.O., R.M.C., and S.G.: designed study; I.O. and R.M.C.: assessed methodological quality; I.O. and R.M.C.: collected data; I.O. analyzed data; S.C.G. supervised data analysis; I.O., R.M.C., S.C.G. wrote manuscript; all authors critically reviewed manuscript; all authors approved the final version of the manuscript.
Declaration of competing interest statement
R.M.C. received honoraria for participating as a speaker at scientific and educational meetings from Takeda, Hoffman-La Roche, Bayer, and Novo Nordisk, and support for scientific meetings from Takeda, Hoffman-La Roche, and Bayer. I.O. was supported by an unrestricted research grant from Swedish Orphan Biovitrum (SOBI) awarded in 2019, the Heimburger Award 2019 and the Martin Villar Clinical Research Award 2021. S.C.G. received an unrestricted research grant from SOBI. No conflict of interest for other authors.
Footnotes
Funding information I.O. was supported by an unrestricted research grant from Swedish Orphan Biovitrum (SOBI), the Heimburger Award and the Martin Villar research grant. S.C.G. received an unrestricted research grant from SOBI.
Handling Editor: J Mahlangu
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2022.100020
Supplementary material
References
- 1.Gringeri A., Ewenstein B., Reininger A. The burden of bleeding in haemophilia: is one bleed too many? Haemophilia. 2014;20:459–463. doi: 10.1111/hae.12375. [DOI] [PubMed] [Google Scholar]
- 2.Jamil M.A., Sharma A., Nuesgen N., Pezeshkpoor B., Heimbach A., Pavlova A., et al. F8 Inversions at Xq28 causing hemophilia a are associated with specific methylation changes: implication for molecular epigenetic diagnosis. Front Genet. 2019;10:508. doi: 10.3389/fgene.2019.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinchero A., Scholzberg M., Matino D. The evolution of hemophilia care: clinical and laboratory advances, opportunities, and challenges. Hamostaseologie. 2020;40:311–321. doi: 10.1055/a-1175-6530. [DOI] [PubMed] [Google Scholar]
- 4.Ananyeva N.M., Lacroix-Desmazes S., Hauser C.A.E., Shima M., Ovanesov M.V., Khrenov A.V., et al. Inhibitors in hemophilia A: mechanisms of inhibition, management and perspectives. Blood Coagul Fibrinolysis. 2004;15:109–124. doi: 10.1097/00001721-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Wight J., Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9:418–435. doi: 10.1046/j.1365-2516.2003.00780.x. [DOI] [PubMed] [Google Scholar]
- 6.Eckhardt C.L., Loomans J.I., van Velzen A.S., Peters M., Mauser-Bunschoten E., Schwaab R., et al. Inhibitor development and mortality in non-severe hemophilia A. J Thromb Haemost. 2015;13:1217–1225. doi: 10.1111/jth.12990. [DOI] [PubMed] [Google Scholar]
- 7.Meeks S.L., Batsuli G. Hemophilia and inhibitors: current treatment options and potential new therapeutic approaches. Hematology Am Soc Hematol Educ Program. 2016;2016:657–662. doi: 10.1182/asheducation-2016.1.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Fernández M.F., Roca C.A., Álvarez-Román M.T., Hirnyk M.I.C., Mingot-Castellano M.E., et al. Spanish Consensus Guidelines on prophylaxis with bypassing agents in patients with haemophilia and inhibitors. Thromb Haemost. 2016;115:872–895. doi: 10.1160/TH15-07-0568. [DOI] [PubMed] [Google Scholar]
- 9.Barg A.A., Livnat T., Kenet G. Inhibitors in hemophilia: treatment challenges and novel options. Semin Thromb Hemost. 2017;44:544–550. doi: 10.1055/s-0037-1612626. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro A.D., Hedner U. Advances in bypassing agent therapy for hemophilia patients with inhibitors to close care gaps and improve outcomes. Ther Adv Drug Saf. 2011;2:213–225. doi: 10.1177/2042098611415566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oldenburg J., Mahlangu J.N., Kim B., Schmitt C., Callaghan M.U., Young G., et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377:809–818. doi: 10.1056/NEJMoa1703068. [DOI] [PubMed] [Google Scholar]
- 12.Young G., Sidonio R.F., Liesner R., Oldenburg J., Chang T., Uguen M., et al. HAVEN 2 updated analysis: multicenter, open-label, phase 3 study to evaluate efficacy, safety and pharmacokinetics of subcutaneous administration of emicizumab prophylaxis in pediatric patients with hemophilia A with inhibitors. Blood. 2017;130(Suppl 1):85. [Google Scholar]
- 13.Mahlangu J.N., Oldenburg J., Paz-Priel I., Negrier C., Niggli M., Mancuso M.E., et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379:811–822. doi: 10.1056/NEJMoa1803550. [DOI] [PubMed] [Google Scholar]
- 14.Young G., Liesner R., Chang T., Sidonio R.F., Oldenburg J., Jiménez-Yuste V., et al. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134:2127–2138. doi: 10.1182/blood.2019001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castaman G., Santoro C., Coppola A., Mancuso M.E., Santoro R.C., Bernardini S., et al. Emergency management in patients with haemophilia A and inhibitors on prophylaxis with emicizumab: AICE practical guidance in collaboration with SIBioC, SIMEU, SIMEUP, SIPMeL and SISET. Blood Transfus. 2020;18:143–151. doi: 10.2450/2019.0186-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiménez-Yuste V., Rodriguez-Merchan E.C., Matsushita T., Holme P.A. Concomitant use of bypassing agents with emicizumab for people with haemophilia A and inhibitors undergoing surgery. Haemophilia. 2021;27:519–530. doi: 10.1111/hae.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carcao M., Escuriola Ettinghausen C., Santagostino E., Oldenburg J., Liesner R., Nolan B., et al. The changing face of immune tolerance induction in haemophilia A with the advent of emicizumab. Haemophilia. 2019;25:676–684. doi: 10.1111/hae.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart D.P., Alamelu J., Bhatnagar N., Biss T., Collins P.W., Hall G., et al. Immune tolerance induction in severe haemophilia A: A UKHCDO inhibitor and paediatric working party consensus update. Haemophilia. 2021;27:932–937. doi: 10.1111/hae.14381. [DOI] [PubMed] [Google Scholar]
- 19.Holstein K., Le Quellec S., Klamroth R., Batorova A., Holme P.A., Jiménez-Yuste V., et al. Immune tolerance induction in the era of emicizumab – still the first choice for patients with haemophilia A and inhibitors? Haemophilia. 2022;28:215–222. doi: 10.1111/hae.14470. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020;26(Suppl 6):1–158. [DOI] [PubMed]
- 21.Hay C.R., DiMichele D.M., International Immune Tolerance S. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119:1335–1344. doi: 10.1182/blood-2011-08-369132. [DOI] [PubMed] [Google Scholar]
- 22.Santagostino E., Young G., Escuriola Ettinghausen C., Jiménez-Yuste V., Carcao M. Inhibitors: a need for eradication? Acta Haematol. 2019;141:151–155. doi: 10.1159/000495454. [DOI] [PubMed] [Google Scholar]
- 23.Kenet G., Oladapo A., Epstein J., Thompson C., Novack A., Nugent D.J. Estimating the cost savings and cost effectiveness of low dose immune tolerance induction (ITI) therapy with bypassing agent prophylaxis compared with high dose ITI and low dose ITI. Blood. 2017;130:4632. doi: 10.1111/hae.13294. [DOI] [PubMed] [Google Scholar]
- 24.Salviato R., Belvini D., Radossi P., Sartori R., Pierobon F., Zanotto D., et al. F8 gene mutation profile and ITT response in a cohort of Italian haemophilia A patients with inhibitors. Haemophilia. 2007;13:361–372. doi: 10.1111/j.1365-2516.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 25.DiMichele D.M., Kroner B.L., Group N.A.I.T.S. The North American Immune Tolerance Registry: practices, outcomes, outcome predictors. Thromb Haemost. 2002;87:52–57. [PubMed] [Google Scholar]
- 26.Mariani G., Ghirardini A., Bellocco R. Immune tolerance in hemophilia-principal results from the International Registry. Report of the factor VIII and IX Subcommittee. Thromb Haemost. 1994;72:155–158. [PubMed] [Google Scholar]
- 27.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 28.Flanagin A., Frey T., Christiansen S.L., Bachner H. The reporting of race and ethnicity in medical and science journals. JAMA. 2021;325:1049–1052. doi: 10.1001/jama.2021.2104. [DOI] [PubMed] [Google Scholar]
- 29.Coppola A., Margaglione M., Santagostino E., Rocino A., Grandone E., Mannucci P.M., et al. Factor VIII gene (F8) mutations as predictors of outcome in immune tolerance induction of hemophilia A patients with high-responding inhibitors. J Thromb Haemost. 2009;7:1809–1815. doi: 10.1111/j.1538-7836.2009.03615.x. [DOI] [PubMed] [Google Scholar]
- 30.Kurth M., Puetz J., Kouides P., Sanders J., Sexauer C., Bernstein J., et al. The use of a single von Willebrand factor-containing, plasma-derived FVIII product in hemophilia A immune tolerance induction: the US experience. J Thromb Haemost. 2011;9:2229–2234. doi: 10.1111/j.1538-7836.2011.04493.x. [DOI] [PubMed] [Google Scholar]
- 31.Nogami K., Taki M., Matsushita T., Ohga S., Oka T., Horikoshi Y., et al. The Japanese Immune Tolerance Induction (J-ITI) study in haemophilia patients with inhibitor: outcomes and successful predictors of ITI treatment. Haemophilia. 2018;24:e328–e337. doi: 10.1111/hae.13531. [DOI] [PubMed] [Google Scholar]
- 32.Knol M.J., Le Cessie S., Algra A., Vandenbroucke J.P., Groenwold R.H.H. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. CMAJ. 2012;184:895–899. doi: 10.1503/cmaj.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borenstein M., Hedges L.V., Higgings J.P.T., Rothstein H.R. Wiley; 2009. Introduction to Meta-Analysis. [Google Scholar]
- 34.Sweeting M.J., Sutton A.J., Lambert P.C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 35.Stijnen T., Hamza T.H., Özdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29:3046–3067. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 36.Weckmann G., Chenot J.F., Reber K.C. A practical approach to reading and interpreting meta-analyses. Z Allgemeinmed. 2015;91:469–473. [Google Scholar]
- 37.Barnes C., Rivard G.E., Poon M.-C., Teitel J., Pai M., Kern M., et al. Canadian multi-institutional survey of immune tolerance therapy (ITT) - experience with the use of recombinant factor VIII for ITT. Haemophilia. 2006;12:1–6. doi: 10.1111/j.1365-2516.2005.01167.x. [DOI] [PubMed] [Google Scholar]
- 38.Batorova A., Jankovicova D., Morongova A., Bubanska E., Prigancova T., Horakova J., et al. Inhibitors in severe hemophilia A: 25-year experience in Slovakia. Semin Thromb Hemost. 2016;42:550–562. doi: 10.1055/s-0036-1581107. [DOI] [PubMed] [Google Scholar]
- 39.Callaghan M.U., Rajpurkar M., Chitlur M., Warrier I., Lusher J. Immune tolerance induction in 31 children with haemophilia A: is ITI less successful in African Americans? Haemophilia. 2011;17:483–489. doi: 10.1111/j.1365-2516.2010.02429.x. [DOI] [PubMed] [Google Scholar]
- 40.Di Minno G., Coppola A., Margaglione M., Rocino A., Mancuso M.E., Tagliaferri A., et al. Predictors of inhibitor eradication by primary immune tolerance induction in severe haemophilia A with high responding inhibitors. Haemophilia. 2022;28:55–64. doi: 10.1111/hae.14431. [DOI] [PubMed] [Google Scholar]
- 41.Dou X., Liu W., Poon M.-C., Zhang X., Wu J., Zeng X., et al. Patients with haemophilia A with inhibitors in China: a national real-world analysis and follow-up. Br J Haematol. 2021;192:900–908. doi: 10.1111/bjh.17322. [DOI] [PubMed] [Google Scholar]
- 42.El Alfy M.S., Tantawy A.A.G., Ahmed M.H., Abdin I.A. Frequency of inhibitor development in severe haemophilia A children treated with cryoprecipitate and low-dose immune tolerance induction. Haemophilia. 2000;6:635–638. doi: 10.1046/j.1365-2516.2000.00449.x. [DOI] [PubMed] [Google Scholar]
- 43.Elalfy M., Elghamry I., Hassab H., Elalfy O., Andrawes N., El-Ekiaby M. Low-dose immune tolerance induction therapy in children of Arab descent with severe haemophilia A, high inhibitor titres and poor prognostic factors for immune tolerance induction treatment success. Haemophilia. 2022;28:65–72. doi: 10.1111/hae.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escobar M., Shaffer L., Holguin M., McCavit T., Amega N.S., Rajan S.K. Immune tolerance induction with antihaemophilia factor (human) in poor prognosis patients with haemophilia A. Haematologica. 2020;27:e393–e397. doi: 10.1111/hae.14175. [DOI] [PubMed] [Google Scholar]
- 45.Greninger D.A., Saint-Remy J.M., Jacquemin M., Benhida A., DiMichele D.M. The use of factor VIII/von Willebrand factor concentrate for immune tolerance induction in haemophilia A patients with high-titre inhibitors: association of clinical outcome with inhibitor epitope profile. Haemophilia. 2008;14:295–302. doi: 10.1111/j.1365-2516.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 46.Haya S., López M.F., Aznar J.A., Batlle J., Group T.S.I.T. Immune tolerance treatment in haemophilia patients with inhibitors: the Spanish Registry. Haemophilia. 2001;7:154–159. doi: 10.1046/j.1365-2516.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 47.Kreuz W., Escuriola Ettinghausen C., Vdovin V., Zozulya N., Plyushch O., Svirin P., et al. First prospective report on immune tolerance in poor risk haemophilia A inhibitor patients with a single factor VIII/von Willbrand factor concentrate in an observational immune tolerance induction study. Haemophilia. 2016;22:87–95. doi: 10.1111/hae.12774. [DOI] [PubMed] [Google Scholar]
- 48.Lapalud P., Rothschild C., Mathieu-Dupas E., Balicchi J., Gruel Y., Laune D., et al. Anti-A2 and anti-A1 domain antibodies are potential predictors of immune tolerance induction outcome in children with hemophilia A. J Thromb Haemost. 2015;13:540–547. doi: 10.1111/jth.12846. [DOI] [PubMed] [Google Scholar]
- 49.Lenk H., Group tIS. The German Registry of immune tolerance treatment in Hemophilia – 1999 update. Haematologica. 2000;85:45–47. [PubMed] [Google Scholar]
- 50.Lin P.C., Liao Y.M., Tsai S.P., Chang T.T. Immune tolerance induction therapy for patients with hemophilia A and FVIII inhibitors particularly using low-dose regimens. Pediatr Blood Cancer. 2011;57:1029–1033. doi: 10.1002/pbc.23291. [DOI] [PubMed] [Google Scholar]
- 51.Mariani G., Kroner B., (ITSG) ITSG Immune tolerance in hemophilia with factor VIII inhibitors: predictors of success. Haematologica. 2001;86:1186–1193. [PubMed] [Google Scholar]
- 52.Nakar C., Manco-Johnson M.J., Lail A., Donfield S., Maahs J., Chong Y., et al. Prompt immune tolerance induction at inhibitor diagnosis regardless of titre may increase overall success in haemophilia A complicated by inhibitors: experience of two US centres. Haemophilia. 2015;21:365–373. doi: 10.1111/hae.12608. [DOI] [PubMed] [Google Scholar]
- 53.Oldenburg J., Jiménez-Yuste V., Peiró-Jordán R., Aledort L.M., Santagostino E. Primary and rescue immune tolerance induction in children and adults: a multi-centre international study with a VWF-containing plasma-derived FVIII concentrate. Haemophilia. 2014;20:83–91. doi: 10.1111/hae.12263. [DOI] [PubMed] [Google Scholar]
- 54.Rivard G.E., Rothschild C., Toll T., Achilles K. Immune tolerance induction in haemophilia A patients with inhibitors by treatment with recombinant factor VIII: a retrospective non-interventional study. Haemophilia. 2013;19:449–455. doi: 10.1111/hae.12102. [DOI] [PubMed] [Google Scholar]
- 55.Rocino A., Cortesi P.A., Scalone L., Mantovani L.G., Crea R., Gringeri A., (EHTSB) EHTSB Immune tolerance induction in patients with haemophilia a and inhibitors: effectiveness and cost analysis in an European Cohort (The ITER Study) Haemophilia. 2016;22:96–102. doi: 10.1111/hae.12780. [DOI] [PubMed] [Google Scholar]
- 56.Ryu J.E., Park Y.S., Yoo K.Y., Lee K.D., Choi Y.M. Immune tolerance induction in patients with severe hemophilia A with inhibitors. Blood Res. 2015;50:248–253. doi: 10.5045/br.2015.50.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ter Avest P.C., Fischer K., Gouw S.C., Van Dijk K., Mauser-Bunschoten E.P. Successful low dose immune tolerance induction in severe haemophilia A with inhibitors below 40 Bethesda Units. Haemophilia. 2010;16:71–79. doi: 10.1111/j.1365-2516.2010.02225.x. [DOI] [PubMed] [Google Scholar]
- 58.Unuvar A., Kavakli K., Baytan B., Kazanci E., Sayli T., Oren H., et al. Low-dose immune tolerance induction for paediatric haemophilia patients with factor VIII inhibitors. Haemophilia. 2008;14:315–322. doi: 10.1111/j.1365-2516.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 59.DiMichele D.M., Hoots W.K., Pipe S.W., Rivard G.E., Santagostino E. International workshop on immune tolerance induction: consensus recommendations. Haemophilia. 2007;13:1–22. doi: 10.1111/j.1365-2516.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 60.Schep S.J., Schutgens R.E.G., Fischer K., Boes M.L. Review of immune tolerance induction in hemophilia A. Blood Reviews. 2018;32:326–338. doi: 10.1016/j.blre.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Reipert B., Van Helden P.M.W., Schwarz H.P., Hausl C. Mechanisms of action of immune tolerance induction against factor VIII in patients with congenital haemophilia A and factor VIII inhibitors. Br J Haematol. 2006;136:12–25. doi: 10.1111/j.1365-2141.2006.06359.x. [DOI] [PubMed] [Google Scholar]
- 62.Waters B., Lillicrap D. The molecular mechanisms of immunomodulation and tolerance induction to factor VIII. J Thromb Haemost. 2009;7:1446–1456. doi: 10.1111/j.1538-7836.2009.03538.x. [DOI] [PubMed] [Google Scholar]
- 63.Kizilocak H., Marquez-Casas E., Malvar J., Young G. Safety of FEIBA and Emicizumab (SAFE): Dose Escalation Study Evaluating the Safety of in vivo Administration of Activated Prothrombin Complex Concentrate in Hemophilia A Patients with Inhibitors on Emicizumab [abstract] Res Pract Thromb Haemost. 2021;5 doi: 10.1111/hae.14684. [DOI] [PubMed] [Google Scholar]
- 64.Leebeek F.W.G., Miesbach W. Gene therapy for hemophilia: a review on clinical benefit, limitations, and remaining issues. Blood. 2021;138:923–931. doi: 10.1182/blood.2019003777. [DOI] [PubMed] [Google Scholar]
- 65.van Helden P.M.W., Berg van den H.M., Gouw S.C., Kraijen P.H.P., Zuurveld M.G., Mauser-Bunschoten E.P., et al. IgG subclasses of anti-FVIII antibodies during immune tolerance induction in patients with hemophilia A. Br J Haematol. 2008;142:644–652. doi: 10.1111/j.1365-2141.2008.07232.x. [DOI] [PubMed] [Google Scholar]
- 66.Van Helden P.M.W., Kaijen P., Mauser-Bunschoten E., Fischer K., Van den Berg H.M., Voorberg J. Domain specificity of factor VIII inhibitors during immune tolerance induction in patients with haemophilia A. Haemophilia. 2010;16:892–901. doi: 10.1111/j.1365-2516.2010.02272.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.