Abstract
Site-selective functionalization is a core synthetic strategy that has broad implications in organic synthesis. Particularly, exploiting chiral catalysis to control site selectivity in complex carbohydrate functionalizations has emerged as a leading method to unravel unprecedented routes into biologically relevant glycosides. However, robust catalytic systems available to overcome multiple facets of stereoselectivity challenges to this end still remain scarce. Here we report a synergistic chiral Rh(I)- and organoboron-catalysed protocol, which enables access into synthetically challenging but biologically relevant arylnaphthalene glycosides. Our method depicts the employment of chiral Rh(I) catalysis in site-selective carbohydrate functionalization and showcases the utility of boronic acid as a compatible co-catalyst. Crucial to the success of our method is the judicious choice of a suitable organoboron catalyst. We also determine that exquisite multiple aspects of stereocontrol, including enantio-, diastereo-, regio- and anomeric control and dynamic kinetic resolution, are concomitantly operative.

Subject terms: Asymmetric catalysis, Carbohydrate chemistry, Synthetic chemistry methodology, Synthetic chemistry methodology
Asymmetric systems for catalytic carbohydrate functionalization are mostly limited to chiral copper complexes and organocatalysts. Now, a synergistic chiral Rh(I)- and organoboron-catalysed site-selective functionalization of carbohydrate polyols has been developed, giving stereocontrolled access to biologically relevant arylhydronaphthalene glycosides. Enantio-, diastereo-, regio- and anomeric control and dynamic kinetic resolution were found to be concomitantly operative.
Main
Site-selective functionalization has emerged as a powerful strategy at the forefront of stereoselective methods1,2, largely due to its capability in enabling discrimination of chemically equivalent functionalities in different stereochemical environments. Compared to the more commonly addressed challenge of enantioselectivity, which uses chiral information on catalysts to generate new stereocentres in prochiral substrates, site- or regio-selective methods that enforce regiocontrol have broader relevance to biological reactions. These reactions often involve substrates endowed with dense stereogenic information. However, this endeavour poses a formidable degree of challenge as the overpowering substrate control arising from the inherent stereogenic information must be surmounted by the catalyst.
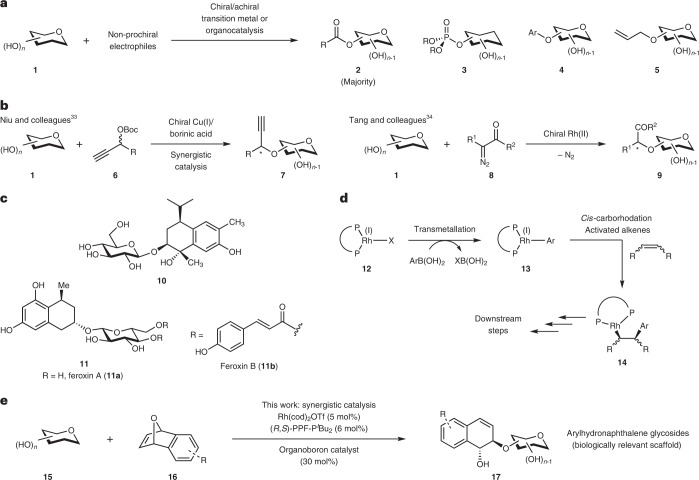
Carbohydrates are a key natural class of biomolecules implicated in this challenge. Despite the importance of carbohydrates in a multitude of physiological processes3, universal stereoselective access to carbohydrates has largely remained elusive due to the stereochemical complexity of sugars4. Hence, chiral catalytic strategies for site-selective functionalizations of carbohydrates provide a highly promising avenue to access hitherto unachievable glycosidic scaffolds5–8. An unresolved gap in this field pertains to the majority of currently developed chiral catalytic systems for this purpose being still limited to chiral copper complexes and organocatalysis (Fig. 1a)9–28, despite the overwhelming number of examples of asymmetric transition-metal complexes available for enantioselective catalysis. This severely limits selective bond-forming opportunities for carbohydrate functionalizations. The known usage of achiral transition-metal complexes for site-selectivity control, while proved to be useful to a certain extent29–32, does not allow the simultaneous enantiocontrol of newly generated chiral centres. A fundamental drawback of such protocols lies in the high reliance on conventional substrate control by the carbohydrate, and the lack of chiral information on these catalysts precludes the exploitation of versatile catalyst control in numerous facets of stereoselection. Thus, this significantly constricts the scope to functionalizations that do not involve chirality generation from prochiral electrophiles.
Fig. 1. Prior reports of site-selective functionalizations, the conventional role of organoboron reagents in Rh(I) catalysis and current work.
a, Site-selective functionalizations with non-prochiral electrophiles. b, Rare examples of site-selective glycofunctionalizations with concomitant external chirality generation. Asterisks denote new stereogenic centres formed with simultaneous regio- and enantio-control by the participating catalysts. c, Bioactive arylnaphthalene glycosides. Compound 10 inhibits Eppstein-Barr Virus Early Antigen (EBV-EA) activation and possesses potent anti-tumour promoting activity. Feroxins A and B possess cathartic properties. d, Established transmetallating role of boronic acids in Rh(I) catalysis. e, Current work of synergistically employing Rh(I) and organoboron catalysis to achieve multiple stereocontrol in synthesizing arylhydronaphthalene glycosides. Boc, tert-butyloxycarbonyl.
To the best of our understanding, there are only two rare instances in the literature that have sought to tackle the multiple stereoselectivity issues of chirality generation of the external prochiral electrophile concurrently with site-selective carbohydrate functionalization. These included a copper-catalysed site-selective propargylation reported by Niu and colleagues using synergistic copper and borinic acid catalysis33 and a Rh(II)-catalysed site-selective alkylation by carbenoid insertion by Tang and colleagues (Fig. 1b)34. These examples are currently limited to the exogenous formation of only one single chirality centre, and additional formation of further chiral centres would unavoidably require the surmounting of an additional diastereoselectivity issue by the catalytic system. To the best of our understanding, the generation of more than one chirality centre in site-selective carbohydrate functionalization is not yet known. The current lack of quality catalytic strategies beyond these mentioned cases for concomitant stereocontrol constitutes a major impediment for the applicability of chiral catalysis in constructing the vast array of complex biologically relevant glycosides with stereoprecision.
As part of our ongoing research efforts in expediting access to synthetic glycosides with biological relevance35–37, we were intrigued by a class of carbohydrate scaffolds known as arylhydronaphthalene glycosides38. These constitute an important class of natural products in plants such as cotton or Aloe species (Fig. 1c). In particular, analogue 10 displays important anti-tumour activity39 and feroxins A and B 11 show cathartic properties40. However, any conceivable stereoselective catalytic strategy towards related scaffolds would involve a challenging blend of enantio-, diastereo- and regio-control, as well as chirality generation from an external electrophile. This has hampered a direct stereoselective entry into the arylhydronaphthalene glycoside scaffold to date.
We then targeted chiral Rh(I) catalysis41–45. This is not yet explored as a synthetic tool in site-selective carbohydrate functionalizations, despite enjoying significant success in enantioselective catalysis41,44–46, such as the Rh(I)-catalysed asymmetric ring-opening reaction (ARO)47–51. However, the central question of whether the chiral information endowed in such chiral Rh(I) complexes could also be harnessed to achieve the challenging site-selective control, as well as multiple stereocontrol in complex carbohydrate polyols, still remains unanswered.
Moreover, while organoboron compounds such as boronic acids are widely exploited in Rh(I) catalysis based on excellent pioneering work by Miyaura, Hayashi and colleagues52–54, they are currently only known to function as transmetallating agents involving cis-carborhodation (Fig. 1d)50. Lately, the alternate utility of organoboron reagents as catalysts in glycofunctionalization is gaining traction55–63. Hence, assimilating organoboron catalysis synergistically with chiral Rh(I) catalysis would pave a direct route into hydronaphthalene glycoside motifs. However, this non-trivial endeavour requires orchestrating a challenging mechanistic manoeuvre that bypasses the boron to Rh(I) transmetallation pathway.
In this Article, we report a multi-stereocontrolled access to biologically interesting arylhydronaphthalene glycosides by harnessing the power of synergistic chiral Rh(I) and organoboron catalysis64,65, with the contemporaneous formation of two external stereogenic centres on a prochiral meso-oxanorbornadiene with diastereo-, enantio-, regio- and anomeric control (Fig. 1e). This method employs chiral Rh(I) catalysis in site-selective carbohydrate functionalization. The cooperative effect of rhodium and boronic acid catalysis collectively enforces fourfold stereocontrol in (Supplementary Note 13): (1) site-selective functionalization on a broad range of carbohydrate polyols, (2) enantiocontrol on the Rh(I) oxidative addition step of a bridgehead C–O bond in meso-oxanorbornadienes, (3) a dynamic kinetic resolution-type process in anomeric oxygen functionalization and (4) trans-diastereocontrol on the hydronaphthalene scaffold. Our protocol introduces a co-catalytic role that boronic acids can serve in the domain of Rh(I) catalysis, taking precedence over the conventional transmetallation pathway. Moreover, the trans-diastereoselectivity observed in our methodology is in marked contrast to the cis-selectivity when boronic acid nucleophiles were employed in ARO reactions50. Fine tuning of the boronic acid catalyst enabled us to identify an unexplored cyclohexylvinylboronic acid as the optimal catalyst. Our studies also revealed that the ligand choice had a pivotal influence in determining both the stereoselectivity and the reaction pathway. Further kinetic studies and computations provide further evidence that the resting states of both the Rh and the boronic acid catalysts are actively involved in the rate-limiting step of the mechanism.
Results and discussion
Establishment of the synergistic catalysis methodology
We initiated our investigation by exploring a series of rhodium complexes/chiral ligand combinations for the site-selective functionalization of a mannosyl triol derivative 15a with oxanorbornadiene 16a, in the absence of boronic acid. It became apparent in our early experiments that unlike simple alcohols48,51, the carbohydrate polyol substrate performed poorly as a naked nucleophile. When neutral dimeric rhodium complexes such as [Rh(cod)Cl]2 (cod is 1,5-cyclooctadiene) were employed under conventional ARO conditions, negligible yields of hydronaphthalene product were observed (Supplementary Table 1). When 5 mol% of cationic rhodium complex Rh(cod)2OTf was used, an encouraging combined 15% yield (regiomeric ratio (r.r.) 8.3:1, 17a:18a) was obtained, although the overall reaction outcome was still unsatisfactory.
Promising results were only obtained when a panel of boronic acids was evaluated as potential co-catalysts (Table 1). While aryl-based boronic acids were more commonly employed as catalysts in cases involving carbohydrate diol complexations62,63,66, we observed that in our instance vinylboronic acid performed comparatively much better as a catalyst, while aliphatic boronic acids 22 and 23 were deleterious in the reaction. Density functional theory (DFT) studies (Supplementary Section ‘Computational Details’) suggested that fine-tuning electron-donating and conjugation effects on the boronate substituent is pivotal to increase nucleophilicity and also to provide stability to the negatively charged boronate resting-state species. In particular, we have identified the cyclohexylvinylboronic acid 26 (see below) possessing the suitable balance of the above mentioned effects as the optimal co-catalyst candidate. This generated the C3-functionalized hydronaphthalene mannoside 17a with 92% yield and excellent regio-, diastereo- and enantio-control. Changing the cyclohexyl moiety in 26 to an n-octyl moiety also gave reasonably good NMR yields of 17a at 84% with >20:1 r.r. (17a:18a). We also used instead the borinic acid 27 under the same conditions. This also gave reasonably good NMR yields of 17a at 85% with slightly diminished regioselectivity (15:1 r.r., 17a:18a). No by-products arising from the transmetallation pathway were detected by meticulous analysis of the NMR of our crude reaction mixtures.
Table 1.
Selected optimization by screening influences of boronic acid catalysts and chiral ligands
Conditions: [a] Polyol 15a (0.1 mmol), 16a (0.2 mmol), Rh(cod)2OTf (5 mol%), ligand (6 mol%), organoboron catalyst (30 mol%), in THF (1 ml), argon, 50 °C, 24 h. Screening of boronic acid catalyst achieved with L12 and screening of ligand achieved with boronic acid 26. [b] Yields and r.r. (C3:C2) ratio were determined by analysis of the crude 1H NMR spectra obtained using 1,3,5-trimethoxybenzene as an internal standard. [c] Polyol 15a (0.2 mmol), 16a (0.4 mmol), Rh(cod)2OTf (5 mol%), ligand (6 mol%), organoboron catalyst (30 mol%), in THF (2 ml), argon, 50 °C, 24 h. [d] Isolated yields. cod, 1,5-cyclooctadiene; TBS, tert-butyldimethylsilyl.
Subsequently, we studied a range of chiral bisphosphine ligands across various ligand families and discovered that the chiral ligand choice and its corresponding stereogenic information had a clear influence on stereoselectivity (r.r. and diastereomeric ratio (d.r.)) and in steering the mechanistic pathway of the reaction. When planar chiral ligands based on the ferroceno backbone were screened (Table 1, L1–L3 and L10–L13), such as those belonging to the Walphos, Mandyphos and Josiphos families, we observed generally good to excellent combined yields of both C3 and C2 regio and diastereoisomers with a range of regioselectivities. A fine balance between the steric hindrance and electronics of these ligands appeared to be pivotal for optimal yields and selectivity.
Bisphosphine ligands with axial chirality such as those based on the Garphos (L4), Segphos (L5, L8, L9), BINAP (L6) and BIPHEP (L7) families were also tested and found to have a diverse influence on the Rh(I) catalysis outcomes. While L4 gave 95% yield of a mixture of C3 and C2 regioisomers (5:1 r.r.), L8 and L9 gave a more complicated mixture containing the C3,C2 regioisomers 17–19a and a cyclodimerized product 21 that was previously observed by Hayashi and colleagues67. Notably, when chiral ligands containing the xylyl substituent such as in L5–L7 were used, the synergistic catalysis pathway was significantly suppressed and the cyclodimerization pathway towards 21 was instead favoured.
Overall, the (R,S)-PPF-PtBu2 (L12) ligand of the Josiphos family in the presence of Rh(cod)2OTf provided the most optimal conditions for synergistic catalysis, yielding the C3 regioisomer with excellent yields and selectivity (92%, >20:1 r.r.), while other variants of the same family such as L10 and L11 showed inferior performance. When other cationic rhodium complexes bearing norbornadiene (nbd) ligands such as Rh(nbd)2OTf and Rh(ndb)2BF4 were employed under similar conditions, the regioselectivities obtained were also excellent (>20:1 r.r.), although yields were slightly decreased to 81–83% (Supplementary Table 4). Further, when we employed the enantiomeric (S,R)-PPF-PtBu2 Josiphos ligand (L13), significantly diminished C3,C2 selectivity (6:1 r.r.) was obtained, albeit with similar yields. Our optimization data suggest that a matching ligand–polyol pairing was pivotal in the regioselective control.
Substrate scope
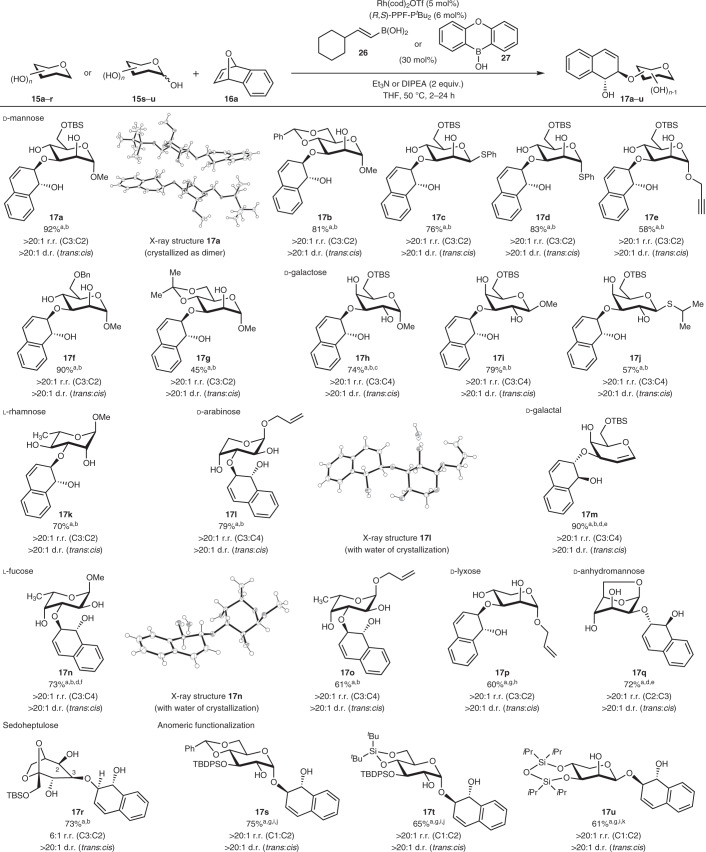
With these optimal conditions in hand, we proceeded to evaluate the substrate scope of the reaction, and tested a wide range of carbohydrate polyols including mannose, galactose, rhamnose, arabinose, galactal, fucose, lyxose, 1,6-anhydromannose, sedoheptulose and also, significantly, anomeric unprotected saccharides (Table 2).
Table 2.
Substrate scope with respect to the carbohydrate polyol
Conditions: [a] Polyol 15a–p (0.2 mmol), 16a (0.4 mmol), Rh(cod)2OTf (5 mol%), (R,S)-PPF-PtBu2 (6 mol%), organoboron catalyst (30 mol%), in THF (2 ml), argon, 50 °C, 24 h. r.r. and d.r. were determined by analysis of the crude 1H NMR spectra. [b] 26 was used as the catalyst. [c] 48 h reaction time. [d] 0.5 ml THF was used. [e] (S,R)-PPF-PtBu2 was used instead. [f] 12 h reaction time. [g] 27 was used. [h] 6% of C3,C2-disubstituted product was isolated (Supplementary Note 14), 1.5 equiv. 16a (0.3 mmol) was used. [i] DIPEA was used as the base instead. [j] 2 h reaction time. [k] 13 h reaction time. Bn, benzyl; cod,1,5-cyclooctadiene; TBS, tert-butyldimethylsilyl; TBDPS, tert-butyldiphenylsilyl. For X-ray structures, thermal ellipsoids shown at 50% probability.
These were very well tolerated in this synergistic catalysis protocol to yield generally trans-hydronaphthalene glycosides with excellent regio- and diastereo-selectivity. Both the α- and β-anomers were accommodated in multiple cases, for instance the thiomannosides 17c and 17d and the methoxygalactosides 17h–i. When lyxose 15p was employed, an organoboron reagent switch from the vinylboronic acid 26 to borinic acid catalyst 27 was essential to tolerate this substrate. Interestingly, when galactal and 1,6-anhydromannose 15m and 15q were employed, the (S,R)-PPF-PtBu2 Josiphos ligand provided instead the matching conditions for excellent regiocontrol to generate 17m and 17q, respectively.
We further embarked on the immense challenge of functionalizing the anomeric oxygen of anomeric unprotected saccharides 15s–u, more commonly found in bioactive arylnaphthalene glycosides. Such substrates uniquely contain a non-static hemiacetal moiety that enables dynamic equilibration between the α- and β-anomers, thus elevating the stereoselectivity challenge. Delightfully, by applying catalyst 27 and N,N-diisopropylethylamine (DIPEA) as a base68,69, we were able to functionalize 15s–u cleanly with concomitant enantio-, diastereo-, site- and anomeric selectivity to yield 1,2-cis 17s–u. Hence, this unravelled a deeper level of catalytic intricacy: a dynamic kinetic resolution-type control by the organoboron catalyst can also be activated by our strategy to control anomeric selectivity.
In all, our scope suggested that the chirality and identity of the ligand, the three-dimensional structure of the carbohydrate polyol and the electronic effect of the organoboron reagent were collectively contributing to the resulting stereoselectivity and yields. By selecting representative polyol substrates from each of our monosaccharide family of sugars and subjecting them to the opposite enantiomeric chiral ligand under exact conditions, we noted varying carbohydrate substrate influences on yields and regioselectivity (Supplementary Table 30). While deleterious effects were generally observed with mismatching combinations, in cases when mannose, galactal, arabinose, lyxose and 1,6-anhydromannose substrates were employed, the mismatching influence on regioselectivities in these cases was substantial. On the other hand, when galactose, fucose and sedoheptulose substrates were employed, the mismatching combinations resulted instead in significantly diminished yields with marginal influence on regioselectivity. The only exception was l-rhamnose 15k, where employment of the (S,R)-PPF-PtBu2 ligand generated the other diastereomer 19k with good stereoselectivity and yields.
Taking into consideration structural elucidation ambiguities that could arise from subtle diastereomeric or regioisomeric variations of our products, further confidence in our stereogenic assignments beyond NMR analysis was paramount. Hence, single crystal X-ray crystallography was conducted on derivatives 17a, 17l and 17n (Table 2). Furthermore, we also subjected isolated 17a, 17s, 17u, 19a and 20a (19a and 20a obtained using (S,R)-PPF-PtBu2 ligand, Table 1) to vibrational circular dichroism (VCD) analysis70 and compared the measured spectroscopic data with the DFT-calculated spectra (Supplementary Figs. 1, 2, 4 and 5). Both X-ray crystallography and VCD analysis unambiguously confirmed the absolute and regioisomeric configurations of our hydronaphthalene glycosides that were obtained using both enantiomers of the PPF-PtBu2 Josiphos ligands. The regiomeric identities of other derivatives were accordingly assigned by analogy and counterchecked with 2-dimensional hetereonuclear multiple bond correlation (HMBC) NMR spectroscopy (Supplementary Section ‘Structural elucidation with the help of HMBC’). The anomeric configurations of 17s, 17t and 17u were further confirmed with the diagnostic 1JC–H coupling constant and 2D-nuclear Overhauser effect spectroscopy (NOESY) (Supplementary Note 15 and Supplementary Figs. 409–414).
To assess the scope of our method with respect to the electrophile, we tested a range of meso-oxanorbornadienes 16b–i bearing a variety of electron-donating and electron-withdrawing moieties (Table 3). Methyl, methoxyl and dioxolane groups in different aromatic positions on the oxanorbornadienes were accommodated to generate the hydronaphthalene glycosides with excellent stereocontrol and yields. Bridgehead oxanorbornadienes can also be employed to yield 17ae efficiently. In addition, electron-withdrawing halogens such as fluorines and bromines can be incorporated to yield 17af and 17ag with very good yields and stereoselectivities. The additional fusion of a benzene ring in oxabicycle 16i was also well tolerated to yield 17ah effectively.
Table 3.
Expanded substrate scope with respect to the meso-oxanorbornadiene
Conditions: Polyol 15a (0.2 mmol), 16aa–ah (0.4 mmol), Rh(cod)2OTf (5 mol%), (R,S)-PPF-PtBu2 (6 mol%), organoboron catalyst 26 (30 mol%), in THF (2 ml), argon, 50 °C, 24 h. r.r. and d.r. were determined by analysis of the crude 1H NMR spectra. TBS, tert-butyldimethylsilyl.
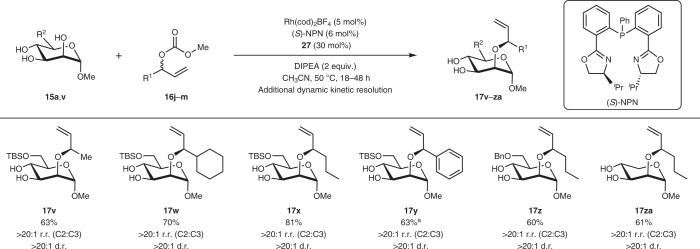
Taking our methodology a step further, we sought to evaluate if this synergistic concept could also be extended to the structurally related allylic carbonate substrates 16j–m (Table 4). While ligand and solvent modifications were found to be essential71, we noted that the reaction still proceeded smoothly to yield the corresponding functionalized products 17v–za with concomitant regio and diastereoselectivity. We further observed that the allylic substitution can be extended to allylic carbonates with sterically and electronically differing substituents ranging from aliphatic and cyclic to aromatic moieties. Significantly, since a racemic mixture of the allylic carbonate 16j–m was employed, an additional facet of dynamic kinetic resolution by the Rh(I) catalyst on the electrophile is operative in such systems, further confirming the rich multifaceted stereocontrol through our strategy. The absolute configuration of 17v and its mismatched congener 20v was further ascertained using VCD analysis and the other derivatives were assigned by analogy (Supplementary Fig. 3). Further, the regioselectivity of all allylic substitution products was double checked with 2-dimensional HMBC NMR spectroscopy (Supplementary Section ‘Structural elucidation with the help of HMBC’).
Table 4.
Expanded substrate scope using allylic carbonates as electrophiles
Conditions: Polyol 15a (0.2 mmol), 16i (0.24 mmol), Rh(cod)2BF4 (5 mol%), (S)-NPN (6 mol%), organoboron catalyst 27 (30 mol%), in CH3CN (1 ml), argon, 50 °C, 18-48 h. r.r. and d.r. were determined by analysis of the crude 1H NMR spectra. [a] 10 mol% Rh(cod)2BF4, 12 mol% (S)-NPN, 70 °C. cod,1,5-cyclooctadiene; TBS, tert-butyldimethylsilyl.
Mechanistic study
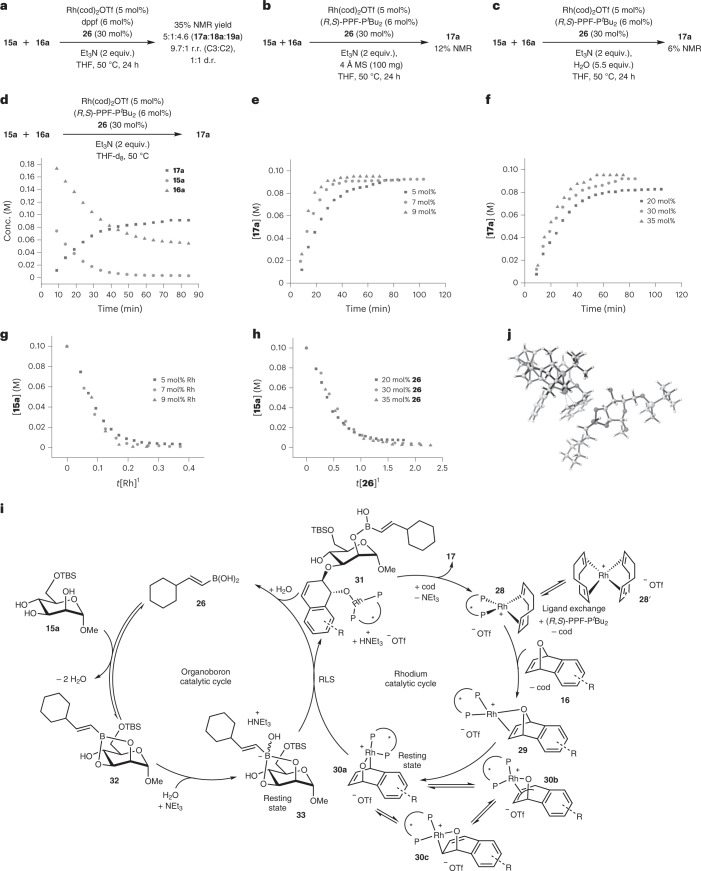
To shed further light on the mechanism, we conducted a series of control experiments. First, we employed the 1,1′-bis(diphenylphosphino)ferrocene (dppf) achiral ligand instead of (R,S)-PPF-PtBu2 with our optimized standard conditions (Fig. 2a). This ligand permutation resulted in a concurrent depletion of yields, regio- and diastereo-selectivity. Furthermore, the depletion of yields and stereocontrol in a separate control experiment in the absence of boronic acid (15% yield, 8.3:1 r.r. (C3:C2), >20:1 d.r., see Supplementary Section ‘Control Experiment 1’) under standard conditions corroborate the pivotal role both catalysts play in the exquisite stereocontrol of the reaction mechanism.
Fig. 2. Mechanistic insights into the synergistic catalysis through control experiments, kinetic investigation and DFT computations.
a, Control experiment using achiral dppf as the ligand revealed depletion of yields and stereoselectivity. b, Control experiment adding 4 Å molecular sieves (MS) to sequester H2O was deleterious to product yield. c, Control experiment adding 5.5 equiv. H2O under standard conditions gave trace product yield. d, Temporal kinetic profile by in situ NMR monitoring. e, Influence of rhodium catalyst concentration on the kinetic profile. f, Influence of boronic acid catalyst 26 on the kinetic profile. g, Burés overlaid plot with respect to rhodium catalyst concentration. h, Burés overlaid plot with respect to boronic acid 26 concentration. i, Proposed mechanism of the site-selective functionalization by Rh/organoboron synergistic catalysis. The two interlocking catalytic cycles are comprised of the organoboron catalytic cycle (left) and the rhodium catalytic cycle (right). The reversible covalent complexation of the boronic acid 26 and the polyol 15a forms eventually the resting-state organoboronate 33. Similarly, the Rh(I)/Rh(III) redox couple forms the resting-state bridged Rh(III) intermediate 30a. Compounds 33 and 30a then react in the rate-limiting step (RLS) through multiple stereocontrol to generate stereoselectively 17. j, Computed transition state with Gibbs free energy barrier of 18.3 kcal mol–1 of the rate-limiting step at the ωB97M-V/def2-QZVPP/CPCM(THF)//r2SCAN-3c/CPCM(THF) level of theory.
The crucial role of water in the mechanism is worthy of special mention. We accordingly performed two control experiments to understand the role of water in the catalytic cycle (Fig. 2b,c). Our data suggest that fine-balancing of the catalytic amount of water liberated from the condensation of the boronic acid 26 on a cis-diol motif was pivotal. Addition of 4 Å molecular sieves to sequester the liberated water was deleterious and suppressed the reaction. Conversely, addition of 5.5 equiv. H2O into the reaction only gave trace amounts of product, as detected by 1H NMR. These observations suggest that the turnover of liberated catalytic water at equilibrium during the in situ formation of the boronic ester from the polyol is absolutely essential in the overall mechanism.
We then conducted in situ NMR monitoring of the model reaction between 15a and 16a under standard conditions to obtain the temporal kinetic profile of this synergistic protocol (Fig. 2d). To evaluate the influence of the catalysts on the kinetic profile, in situ NMR monitoring was then performed at different catalyst concentrations. The overlay of these profiles revealed a positive kinetic correlation with respect to both Rh and boronic acid catalyst concentrations (Fig. 2e,f). This supports the hypothesis that the synergistic actions of these two catalysts are vital in the rate-limiting step of the reaction. By employing the graphical method reported by Burés72, we further determined a first-order kinetic dependence with respect to Rh, as well as a first-order kinetic dependence with respect to boronic acid (Fig. 2g,h).
Additionally, we noted that the kinetic profiles overlapped well and were rather invariant to changes in concentrations of both the NEt3 base and oxabicycle (Supplementary Figs. 22 and 26), indicating that they were not actively involved in the rate-limiting step. Interestingly, we noticed a negative kinetic correlation with respect to the increase of concentration of carbohydrate polyol 15a (Supplementary Fig. 18), which suggested the presence of off-cycle secondary effects in the reaction.
Based on observations from our previous work on non-covalent catalysed glycosylations37,73, as well as literature reports documenting the formation of hydrogen-bonded aggregates between carbohydrates74, we surmised that this observed negative order could be accounted for by the formation of intermolecular hydrogen-bonded carbohydrate clusters at higher concentrations, which may lower the reactivity of the carbohydrate polyols, hence retarding the formation of the boronic ester. We propose a synergistic mechanism consisting of two interlocking catalytic cycles (Fig. 2i). In the rhodium catalytic cycle, the cationic Rh(cod)2OTf complex 28′ will first undergo ligand exchange with the (R,S)-PPF-PtBu2 ligand to yield complex 28, followed by the departure of the first cod ligand. Compound 28 will further undergo ligand exchange with oxanorbornadiene 16 with the concomitant expulsion of the second cod ligand to form the exo-coordinated intermediate 29. Subsequently, a Rh(I) oxidative addition will yield the Rh(III) intermediate 30a, which we propose is the resting state of the rhodium catalyst. Based on previous reports75, 30a can also exist in equilibrium with other resonance structures such as the rhodium-π-allyl complex 30b and the rhodaoxetane 30c. Simultaneously, the organoboron catalytic cycle (Fig. 2i, left) is also operative. We postulate that cyclohexylvinylboronic acid 26 will first undergo a reversible condensation with the vicinal cis-diol motif on the carbohydrate polyol 15 to generate the boronic ester 32. Two molecules of water would be liberated in this conversion, which will be consumed in subsequent elementary steps. Compound 32 will then react with a molecule of liberated water in the presence of the NEt3 base to yield the more nucleophilic boronate complex 33, which we propose as the resting state of the organoboron catalyst.
In the rate-limiting step, the reaction between the resting states 30a and 33 of the rhodium and organoboron catalysts enabled a highly stereocontrolled site-selective outer-sphere attack of the equatorial O3 of 33 on the endo face of 30a in an SN2′ fashion. A subsequent reductive elimination led to the generation of boronic acid hemiester 31 and the ammonium hydrotriflate by-product. To gain further insights into the proposed boronate resting-state intermediate 33 and the feasibility of the pivotal rate-limiting step connecting both catalytic cycles, we conducted DFT calculations using ORCA 76 at the ωB97M-V/def2-QZVPP/CPCM(THF)//r2SCAN-3c/CPCM(THF) level of theory77,78 to model the reaction path of the rate-limiting step. Delightfully, we successfully located the modelled transition state connecting 33, 30a and 31 (Fig. 2j and Supplementary Section ‘Computational Details’). Furthermore, we computed a kinetically feasible 18.3 kcal mol–1 Gibbs free energy barrier for this elementary step. Finally, hydrolysis with a second molecule of liberated water, a proton transfer from the ammonium hydrotriflate salt and a ligand exchange with a molecule of cod will regenerate both catalysts 28 and 26, thereafter restarting both synergistic catalytic cycles.
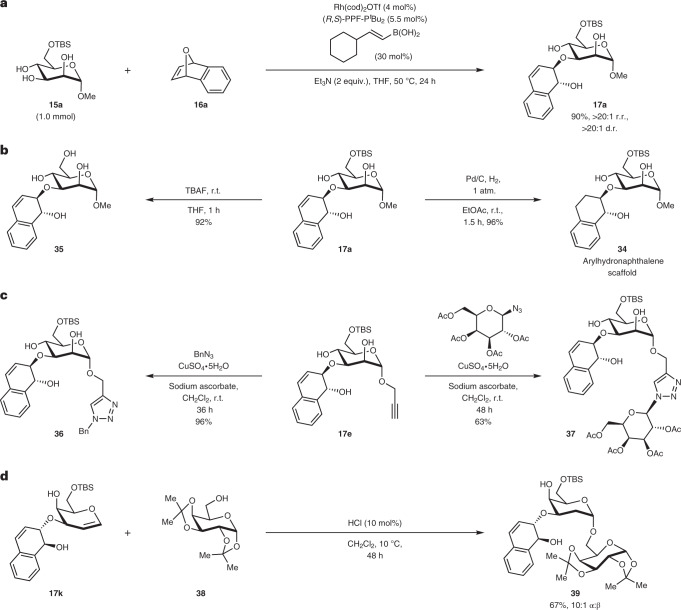
To evaluate the upscaling potential of our protocol, we conducted a 1-mmol-scale reaction at reduced catalyst loading (4 mol% Rh) and obtained 17a with an almost identical result (90% yield, >20:1 r.r.) as for the 0.2 mmol reaction (Fig. 3a). Further derivatizations also proceeded smoothly (Fig. 3b). Hydrogenation of 17a catalysed by palladium on charcoal under ambient conditions allowed entry into arylhydronaphthalene glycoside 34 with quantitative yield. The tert-butyldimethylsilyl (TBS) group on the O6 of 17a can also be deprotected using tetra-n-butylammonium fluoride (TBAF) in a facile fashion to generate 35 with 92% yield. Employing glycosyl substrates with a propargyl moiety tethered to the anomeric carbon enabled the incorporation of ‘click’ chemistry79, allowing facile access into click derivatives such as 36 or a synthetic disaccharide 37 that expands the synthetic utility of these hydronaphthalene glycosides (Fig. 3c). Lastly, the galactal derivative 17k can be subjected to an acid-catalysed 2-deoxyglycosylation with 38 as the glycosyl acceptor to generate disaccharide 39 in a highly α-selective fashion (Fig. 3d), enabling compatibility of our products with the biologically relevant glycosidic linkage formation80.
Fig. 3. Upscaling example, further derivatizations and transformations.
a, Larger-scale reaction using 1 mmol of 15a at reduced catalyst loadings. b, Functional group interconversion of the methodology product through TBS deprotection (left) or alkene hydrogenation using palladium on a charcoal catalyst (right). c, Copper-catalysed click functionalizations on propargyl derivative 17e that enables facile attachment of Bn (left) or saccharide (right) moieties. d, Acid-catalysed 2-deoxyglycosylation of galactal-containing products to access disaccharide 39. Bn, benzyl; r.t., room temperature.
Conclusion
In conclusion, we have demonstrated the employment of chiral Rh(I) catalysis synergistically with boronic acid catalysis in site-selective carbohydrate functionalizations,81 surmounting the challenge of multiple regio-, diastereo-, enantio- and anomeric control within a single bond-forming step. Thus, this method paves the way for stereoselective access into biologically relevant arylnaphthalene glycosides. An additional dimension of dynamic kinetic resolution by the catalytic system can also be observed for substrates such as anomeric unprotected saccharides or racemic allylic carbonates. Furthermore, the successful execution of this strategy required a challenging evasion of the decades-long established boron to Rh(I) transmetallation pathway. Hence, our strategy defines a compatible co-catalytic role that organoboron reagents can play in the realm of Rh(I) catalysis, thereby opening up future site-selective opportunities that could assimilate the repertoire of chiral Rh(I)-catalysed functionalizations in complex carbohydrate synthesis. Furthermore, kinetic and DFT studies provide further evidence that the resting states of the Rh and the organoboron catalysts are actively participating in the rate-limiting step. We anticipate that this protocol would inspire further work in the development of chiral transition catalytic systems for challenging site-selective functionalizations of carbohydrates with prochiral electrophiles—an endeavour that will potentially unravel novel synthetic solutions towards hitherto inaccessible but biologically important glycosides.
Methods
General procedure for the synergistic Rh(I)/organoboron-catalysed site-selective functionalization
An oven-dried dram vial purged with an argon balloon was charged with (R,S)-PPF-PtBu2 Josiphos ligand (6.51 mg, 0.012 mmol, 6 mol%), organoboron 26 or 27 (0.06 mmol, 30 mol%), oxabicycle 16 (0.4 mmol, 2 equiv.), polyol 15 (0.2 mmol, 1 equiv.) and Rh(cod)2OTf (4.68 mg, 0.01 mmol, 5 mol%) and then dry THF (2 ml) and NEt3 or DIPEA (0.4 mmol, 2 equiv.) were added. The dram vial was sealed and the mixture was immersed in a 50 °C oil bath and stirred for 24–48 h. Upon completion of the reaction, the reaction mixture was filtered over a short silica plug and flushed with 250–300 ml ethyl acetate. The filtrate was then evaporated and the determination of the regiomeric ratio (r.r.) was then performed using 1H NMR analysis of this concentrated crude mixture with 1,3,5-trimethoxybenzene as the internal standard. The crude mixture was subsequently dry-loaded onto silica gel and subjected to flash column chromatography for purification.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41557-022-01110-z.
Supplementary information
Supplementary Figs. 1–414, Tables 1–52 and Notes 1–16.
Cartesian coordinates of structures from VCD calculations.
Crystallographic data for compound 17a; CCDC reference 2097738.
Crystallographic data for compound 17l; CCDC reference 2097739.
Crystallographic data for compound 17n; CCDC reference 2097740.
Cartesian coordinates (.xyz) of all DFT-computed structures for the mechanistic understanding.
Acknowledgements
We are grateful to Fonds der Chemischen Industrie for generous funding to C.C.J.L. through a Liebig fellowship. Both C.C.J.L. and C.M. thank the Boehringer Ingelheim Foundation for the very generous funding support through the Plus 3 Perspectives Programme. C.M. acknowledges funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy (EXC-2033; project no. 390677874) and the DFG’s Heisenberg programme (ME 4267/5-1; project no. 418661145). V.U.B.R. acknowledges the Max Planck Society for postdoctoral funding. D.P.D. acknowledges financial support through a postdoctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Alexander von Humboldt foundation. We are grateful for infrastructural support from the Technical University of Dortmund and the Max Planck Institut für Molekulare Physiologie, Dortmund. B. Griewel is gratefully acknowledged for NMR expertise and generous assistance with the in situ NMR monitoring experiments. H. Waldmann is acknowledged for his generous assistance and infrastructural support. The NMR department of the faculty of Chemistry and Chemical Biology of the Technical University of Dortmund is gratefully acknowledged for providing NMR timeslots for the kinetic and NMR monitoring experiments. Umicore AG and Solvias AG are gratefully acknowledged for their generous donation of rhodium complexes and chiral ligands and for their support for young academics. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author contributions
C.C.J.L conceived the idea and supervised the project. V.U.B.R. and C.W. conducted the majority of the experimental work. C.C.J.L, V.U.B.R. and C.W. prepared this manuscript. D.P.D., C.G. and C.M. carried out the VCD measurements and the corresponding DFT analysis of the spectra. C.C.J.L. conducted the DFT and theoretical calculations, as well as theoretical analysis for the mechanistic component. F.O. and C.S. contributed to X-ray crystallography measurements and analysis. C.C.J.L, V.U.B.R., C.W., D.P.D., C.G., C.M., F.O. and C.S. commented on the manuscript.
Peer review
Peer review information
Nature Chemistry thanks Vincent Gandon, Qian Wan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Funding
Open access funding provided by the Max Planck Society
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information files. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2097738 (17a), CCDC 2097739 (17l) and CCDC 2097740 (17n). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: V. U. Bhaskara Rao, Caiming Wang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41557-022-01110-z.
References
- 1.Huang Z, Dong G. Site-selectivity control in organic reactions: a quest to differentiate reactivity among the same kind of functional groups. Acc. Chem. Res. 2017;50:465–471. doi: 10.1021/acs.accounts.6b00476. [DOI] [PubMed] [Google Scholar]
- 2.Toste FD, Sigman MS, Miller SJ. Pursuit of noncovalent interactions for strategic site-selective catalysis. Acc. Chem. Res. 2017;50:609–615. doi: 10.1021/acs.accounts.6b00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dwek RA. Glycobiology: toward understanding the function of sugars. Chem. Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 4.Boltje TJ, Buskas T, Boons G-J. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nat. Chem. 2009;1:611. doi: 10.1038/nchem.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song W, Zheng N. Chiral catalyst-directed site-selective functionalization of hydroxyl groups in carbohydrates. J. Carbohydr. Chem. 2017;36:143–161. doi: 10.1080/07328303.2017.1390575. [DOI] [Google Scholar]
- 6.Dimakos V, Taylor MS. Site-selective functionalization of hydroxyl groups in carbohydrate derivatives. Chem. Rev. 2018;118:11457–11517. doi: 10.1021/acs.chemrev.8b00442. [DOI] [PubMed] [Google Scholar]
- 7.Blaszczyk SA, Homan TC, Tang W. Recent advances in site-selective functionalization of carbohydrates mediated by organocatalysts. Carbohydr. Res. 2019;471:64–77. doi: 10.1016/j.carres.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang W, He B, Niu D. Ligand-controlled, transition-metal catalyzed site-selective modification of glycosides. Carbohydr. Res. 2019;474:16–33. doi: 10.1016/j.carres.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Fiori KW, Puchlopek ALA, Miller SJ. Enantioselective sulfonylation reactions mediated by a tetrapeptide catalyst. Nat. Chem. 2009;1:630–634. doi: 10.1038/nchem.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griswold KS, Miller SJ. A peptide-based catalyst approach to regioselective functionalization of carbohydrates. Tetrahedron. 2003;59:8869–8875. doi: 10.1016/j.tet.2003.05.002. [DOI] [Google Scholar]
- 11.Kawabata T, Muramatsu W, Nishio T, Shibata T, Schedel H. A catalytic one-step process for the chemo- and regioselective acylation of monosaccharides. J. Am. Chem. Soc. 2007;129:12890–12895. doi: 10.1021/ja074882e. [DOI] [PubMed] [Google Scholar]
- 12.Sculimbrene BR, Miller SJ. Discovery of a catalytic asymmetric phosphorylation through selection of a minimal kinase mimic: a concise total synthesis of d-myo-inositol-1-phosphate. J. Am. Chem. Soc. 2001;123:10125–10126. doi: 10.1021/ja016779+. [DOI] [PubMed] [Google Scholar]
- 13.Sculimbrene BR, Morgan AJ, Miller SJ. Enantiodivergence in small-molecule catalysis of asymmetric phosphorylation: concise total syntheses of the enantiomeric d-myo-inositol-1-phosphate and d-myo-inositol-3-phosphate. J. Am. Chem. Soc. 2002;124:11653–11656. doi: 10.1021/ja027402m. [DOI] [PubMed] [Google Scholar]
- 14.Sculimbrene, B. R., Morgan, A. J. & Miller, S. J. Nonenzymatic peptide-based catalytic asymmetric phosphorylation of inositol derivatives. Chem. Commun., 1781–1785 (2003). [DOI] [PubMed]
- 15.Allen CL, Miller SJ. Chiral copper(II) complex-catalyzed reactions of partially protected carbohydrates. Org. Lett. 2013;15:6178–6181. doi: 10.1021/ol4033072. [DOI] [PubMed] [Google Scholar]
- 16.Chen I-H, Kou KGM, Le DN, Rathbun CM, Dong VM. Recognition and site-selective transformation of monosaccharides by using copper(II) catalysis. Chem. Eur. J. 2014;20:5013–5018. doi: 10.1002/chem.201400133. [DOI] [PubMed] [Google Scholar]
- 17.Matsumura Y, Maki T, Tsurumaki K, Onomura O. Kinetic resolution of d,l-myo-inositol derivatives catalyzed by chiral Cu(II) complex. Tetrahedron Lett. 2004;45:9131–9134. doi: 10.1016/j.tetlet.2004.10.019. [DOI] [Google Scholar]
- 18.Shang W, et al. Site-selective O-arylation of glycosides. Angew. Chem. Int. Ed. 2018;57:314–318. doi: 10.1002/anie.201710310. [DOI] [PubMed] [Google Scholar]
- 19.Blaisdell TP, Lee S, Kasaplar P, Sun X, Tan KL. Practical silyl protection of ribonucleosides. Org. Lett. 2013;15:4710–4713. doi: 10.1021/ol402023c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun X, Lee H, Lee S, Tan KL. Catalyst recognition of cis-1,2-diols enables site-selective functionalization of complex molecules. Nat. Chem. 2013;5:790–795. doi: 10.1038/nchem.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T, et al. Catalytic regioselective benzoylation of 1,2-trans-diols in carbohydrates with benzoyl cyanide: the axial oxy group effect and the action of achiral and chiral amine catalysts. ACS Catal. 2020;10:11406–11416. doi: 10.1021/acscatal.0c02112. [DOI] [Google Scholar]
- 22.Matsumura Y, Maki T, Murakami S, Onomura O. Copper ion-induced activation and asymmetric benzoylation of 1,2-diols: kinetic chiral molecular recognition. J. Am. Chem. Soc. 2003;125:2052–2053. doi: 10.1021/ja0289402. [DOI] [PubMed] [Google Scholar]
- 23.Xiao G, et al. Catalytic site-selective acylation of carbohydrates directed by cation–n interaction. J. Am. Chem. Soc. 2017;139:4346–4349. doi: 10.1021/jacs.7b01412. [DOI] [PubMed] [Google Scholar]
- 24.Mensah E, Camasso N, Kaplan W, Nagorny P. Chiral phosphoric acid directed regioselective acetalization of carbohydrate-derived 1,2-diols. Angew. Chem. Int. Ed. 2013;52:12932–12936. doi: 10.1002/anie.201304298. [DOI] [PubMed] [Google Scholar]
- 25.Tay J-H, et al. Regiodivergent glycosylations of 6-deoxy-erythronolide B and oleandomycin-derived macrolactones enabled by chiral acid catalysis. J. Am. Chem. Soc. 2017;139:8570–8578. doi: 10.1021/jacs.7b03198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S, et al. Studies of catalyst-controlled regioselective acetalization and its application to single-pot synthesis of differentially protected saccharides. J. Am. Chem. Soc. 2021;143:18592–18604. doi: 10.1021/jacs.1c08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv W-X, et al. Programmable selective acylation of saccharides mediated by carbene and boronic acid. Chem. 2022;8:1518–1534. doi: 10.1016/j.chempr.2022.04.019. [DOI] [Google Scholar]
- 28.Li Q, Levi SM, Wagen C, Wendlandt AE, Jacobsen EN. Site-selective, stereocontrolled glycosylation of minimally protected sugars. Nature. 2022;608:74–79. doi: 10.1038/s41586-022-04958-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimakos V, et al. Site-selective redox isomerizations of furanosides using a combined arylboronic acid/photoredox catalyst system. Chem. Sci. 2020;11:1531–1537. doi: 10.1039/C9SC05173B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimakos V, Garrett GE, Taylor MS. Site-selective, copper-mediated O-arylation of carbohydrate derivatives. J. Am. Chem. Soc. 2017;139:15515–15521. doi: 10.1021/jacs.7b09420. [DOI] [PubMed] [Google Scholar]
- 31.Bajaj SO, Sharif EU, Akhmedov NG, O’Doherty GA. Denovo asymmetric synthesis of the mezzettiaside family of natural products via the iterative use of a dual B-/Pd-catalyzed glycosylation. Chem. Sci. 2014;5:2230–2234. doi: 10.1039/C4SC00593G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang H, et al. Site-switchable mono-O-allylation of polyols. Nat. Commun. 2020;11:5681. doi: 10.1038/s41467-020-19348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R-Z, et al. Site-divergent delivery of terminal propargyls to carbohydrates by synergistic catalysis. Chem. 2017;3:834–845. doi: 10.1016/j.chempr.2017.09.007. [DOI] [Google Scholar]
- 34.Wu J, et al. Site-selective and stereoselective O-alkylation of glycosides by Rh(II)-catalyzed carbenoid insertion. J. Am. Chem. Soc. 2019;141:19902–19910. doi: 10.1021/jacs.9b11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu C, Loh CCJ. An ultra-low thiourea catalyzed strain-release glycosylation and a multicatalytic diversification strategy. Nat. Commun. 2018;9:4057. doi: 10.1038/s41467-018-06329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu C, Loh CCJ. A multistage halogen bond catalyzed strain-release glycosylation unravels new hedgehog signaling inhibitors. J. Am. Chem. Soc. 2019;141:5381–5391. doi: 10.1021/jacs.9b00040. [DOI] [PubMed] [Google Scholar]
- 37.Xu C, Rao VUB, Weigen J, Loh CCJ. A robust and tunable halogen bond organocatalyzed 2-deoxyglycosylation involving quantum tunneling. Nat. Commun. 2020;11:4911. doi: 10.1038/s41467-020-18595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, et al. Update on naturally occurring novel arylnaphthalenes from plants. Phytochem. Rev. 2020;19:337–403. doi: 10.1007/s11101-020-09668-7. [DOI] [Google Scholar]
- 39.Nagatsu A, et al. Tyrosinase inhibitory and anti-tumor promoting activities of compounds isolated from safflower (Carthamus tinctorius L.) and cotton (Gossypium hirsutum L.) oil cakes. Nat. Prod. Lett. 2000;14:153–158. doi: 10.1080/10575630008041225. [DOI] [Google Scholar]
- 40.Speranza G, Manitto P, Monti D, Pezzuto D. Studies on Aloe, part 10. Feroxins A and B, two O-glucosylated 1-methyltetralins from Cape Aloe. J. Nat. Prod. 1992;55:723–729. doi: 10.1021/np50084a003. [DOI] [Google Scholar]
- 41.Fagnou K, Lautens M. Rhodium-catalyzed carbon−carbon bond forming reactions of organometallic compounds. Chem. Rev. 2003;103:169–196. doi: 10.1021/cr020007u. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi T, Yamasaki K. Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions. Chem. Rev. 2003;103:2829–2844. doi: 10.1021/cr020022z. [DOI] [PubMed] [Google Scholar]
- 43.Koschker P, Breit B. Branching out: rhodium-catalyzed allylation with alkynes and allenes. Acc. Chem. Res. 2016;49:1524–1536. doi: 10.1021/acs.accounts.6b00252. [DOI] [PubMed] [Google Scholar]
- 44.Thoke MB, Kang Q. Rhodium-catalyzed allylation reactions. Synthesis. 2019;51:2585–2631. doi: 10.1055/s-0037-1611784. [DOI] [Google Scholar]
- 45.Turnbull BWH, Evans PA. Asymmetric rhodium-catalyzed allylic substitution reactions: discovery, development and applications to target-directed synthesis. J. Org. Chem. 2018;83:11463–11479. doi: 10.1021/acs.joc.8b00583. [DOI] [PubMed] [Google Scholar]
- 46.Lautens M, Fagnou K, Hiebert S. Transition metal-catalyzed enantioselective ring-opening reactions of oxabicyclic alkenes. Acc. Chem. Res. 2003;36:48–58. doi: 10.1021/ar010112a. [DOI] [PubMed] [Google Scholar]
- 47.Vivek Kumar S, Yen A, Lautens M, Guiry PJ. Catalytic asymmetric transformations of oxa- and azabicyclic alkenes. Chem. Soc. Rev. 2021;50:3013–3093. doi: 10.1039/D0CS00702A. [DOI] [PubMed] [Google Scholar]
- 48.Lautens M, Fagnou K, Rovis T. Rhodium-catalyzed asymmetric alcoholysis and aminolysis of oxabenzonorbornadiene: a new enantioselective carbon−heteroatom bond forming process. J. Am. Chem. Soc. 2000;122:5650–5651. doi: 10.1021/ja000134c. [DOI] [Google Scholar]
- 49.Lautens M, Fagnou K. Effects of halide ligands and protic additives on enantioselectivity and reactivity in rhodium-catalyzed asymmetric ring-opening reactions. J. Am. Chem. Soc. 2001;123:7170–7171. doi: 10.1021/ja010262g. [DOI] [PubMed] [Google Scholar]
- 50.Lautens M, Dockendorff C, Fagnou K, Malicki A. Rhodium-catalyzed asymmetric ring opening of oxabicyclic alkenes with organoboronic acids. Org. Lett. 2002;4:1311–1314. doi: 10.1021/ol0256062. [DOI] [PubMed] [Google Scholar]
- 51.Lautens M, Fagnou K, Yang D. Rhodium-catalyzed asymmetric ring opening reactions of oxabicyclic alkenes: application of halide effects in the development of a general process. J. Am. Chem. Soc. 2003;125:14884–14892. doi: 10.1021/ja034845x. [DOI] [PubMed] [Google Scholar]
- 52.Sakai M, Hayashi H, Miyaura N. Rhodium-catalyzed conjugate addition of aryl- or 1-alkenylboronic acids to enones. Organometallics. 1997;16:4229–4231. doi: 10.1021/om9705113. [DOI] [Google Scholar]
- 53.Sakai M, Ueda M, Miyaura N. Rhodium-catalyzed addition of organoboronic acids to aldehydes. Angew. Chem. Int. Ed. 1998;37:3279–3281. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3279::AID-ANIE3279>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 54.Takaya Y, Ogasawara M, Hayashi T, Sakai M, Miyaura N. Rhodium-catalyzed asymmetric 1,4-addition of aryl- and alkenylboronic acids to enones. J. Am. Chem. Soc. 1998;120:5579–5580. doi: 10.1021/ja980666h. [DOI] [Google Scholar]
- 55.Hall DG. Boronic acid catalysis. Chem. Soc. Rev. 2019;48:3475–3496. doi: 10.1039/C9CS00191C. [DOI] [PubMed] [Google Scholar]
- 56.Taylor MS. Catalysis based on reversible covalent interactions of organoboron compounds. Acc. Chem. Res. 2015;48:295–305. doi: 10.1021/ar500371z. [DOI] [PubMed] [Google Scholar]
- 57.Lee D, Taylor MS. Borinic acid-catalyzed regioselective acylation of carbohydrate derivatives. J. Am. Chem. Soc. 2011;133:3724–3727. doi: 10.1021/ja110332r. [DOI] [PubMed] [Google Scholar]
- 58.Lee D, Williamson CL, Chan L, Taylor MS. Regioselective, borinic acid-catalyzed monoacylation, sulfonylation and alkylation of diols and carbohydrates: expansion of substrate scope and mechanistic studies. J. Am. Chem. Soc. 2012;134:8260–8267. doi: 10.1021/ja302549c. [DOI] [PubMed] [Google Scholar]
- 59.D’Angelo KA, Taylor MS. Borinic acid catalyzed stereo- and regioselective couplings of glycosyl methanesulfonates. J. Am. Chem. Soc. 2016;138:11058–11066. doi: 10.1021/jacs.6b06943. [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa A, Tanaka M, Hanamura S, Takahashi D, Toshima K. Regioselective and 1,2-cis-α-stereoselective glycosylation utilizing glycosyl-acceptor-derived boronic ester catalyst. Angew. Chem. Int. Ed. 2015;54:10935–10939. doi: 10.1002/anie.201504182. [DOI] [PubMed] [Google Scholar]
- 61.Nishi N, et al. Stereospecific β-l-rhamnopyranosylation through an SNi-type mechanism by using organoboron reagents. Angew. Chem. Int. Ed. 2018;57:13858–13862. doi: 10.1002/anie.201808045. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka M, et al. Boronic-acid-catalyzed regioselective and 1,2-cis-stereoselective glycosylation of unprotected sugar acceptors via SNi-type mechanism. J. Am. Chem. Soc. 2018;140:3644–3651. doi: 10.1021/jacs.7b12108. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka M, et al. Diastereoselective desymmetric 1,2-cis-glycosylation of meso-diols via chirality transfer from a glycosyl donor. Nat. Commun. 2020;11:2431. doi: 10.1038/s41467-020-16365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meng L, et al. Glycosylation enabled by successive rhodium(II) and Brønsted acid catalysis. J. Am. Chem. Soc. 2019;141:11775–11780. doi: 10.1021/jacs.9b04619. [DOI] [PubMed] [Google Scholar]
- 65.Allen AE, MacMillan DWC. Synergistic catalysis: a powerful synthetic strategy for new reaction development. Chem. Sci. 2012;3:633–658. doi: 10.1039/c2sc00907b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimada N, et al. Boronic acid-catalyzed regioselective Koenigs–Knorr-type glycosylation. J. Org. Chem. 2021;86:5973–5982. doi: 10.1021/acs.joc.1c00130. [DOI] [PubMed] [Google Scholar]
- 67.Nishimura T, Kawamoto T, Sasaki K, Tsurumaki E, Hayashi T. Rhodium-catalyzed asymmetric cyclodimerization of oxa- and azabicyclic alkenes. J. Am. Chem. Soc. 2007;129:1492–1493. doi: 10.1021/ja068488c. [DOI] [PubMed] [Google Scholar]
- 68.Izumi S, Kobayashi Y, Takemoto Y. Regio- and stereoselective synthesis of 1,2-cis-glycosides by anomeric O-alkylation with organoboron catalysis. Org. Lett. 2019;21:665–670. doi: 10.1021/acs.orglett.8b03823. [DOI] [PubMed] [Google Scholar]
- 69.Izumi S, Kobayashi Y, Takemoto Y. Stereoselective synthesis of 1,1′-disacharides by organoboron catalysis. Angew. Chem. Int. Ed. 2020;59:14054–14059. doi: 10.1002/anie.202004476. [DOI] [PubMed] [Google Scholar]
- 70.Merten C, Golub TP, Kreienborg NM. Absolute configurations of synthetic molecular scaffolds from vibrational CD spectroscopy. J. Org. Chem. 2019;84:8797–8814. doi: 10.1021/acs.joc.9b00466. [DOI] [PubMed] [Google Scholar]
- 71.Xu W-B, Ghorai S, Huang W, Li C. Rh(I)/bisoxazolinephosphine-catalyzed regio- and enantioselective allylic substitutions. ACS Catal. 2020;10:4491–4496. doi: 10.1021/acscatal.0c00712. [DOI] [Google Scholar]
- 72.Burés J. A simple graphical method to determine the order in catalyst. Angew. Chem. Int. Ed. 2016;55:2028–2031. doi: 10.1002/anie.201508983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loh CCJ. Exploiting non-covalent interactions in selective carbohydrate synthesis. Nat. Rev. Chem. 2021;5:792–815. doi: 10.1038/s41570-021-00324-y. [DOI] [PubMed] [Google Scholar]
- 74.Kononov LO. Chemical reactivity and solution structure: on the way to a paradigm shift? RSC Adv. 2015;5:46718–46734. doi: 10.1039/C4RA17257D. [DOI] [Google Scholar]
- 75.Qi Z-H, et al. Mechanism, reactivity, and regioselectivity in rhodium-catalyzed asymmetric ring-opening reactions of oxabicyclic alkenes: a DFT Investigation. Sci Rep. 2017;7:40491. doi: 10.1038/srep40491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nesse F. Software update: the ORCA program system, version 5.0. WIREs Comput. Mol. Sci. 2022;12:e1606. [Google Scholar]
- 77.Grimme S, et al. r2SCAN-3c: a ‘Swiss army knife’ composite electronic-structure method. J. Chem. Phys. 2021;154:064103. doi: 10.1063/5.0040021. [DOI] [PubMed] [Google Scholar]
- 78.Mardirossian N, Head-Gorden M. ωB97M-V: a combinatorially optimized, range-separated hybrid, meta-GGA density functional with VV10 nonlocal correlation. J. Chem. Phys. 2016;144:214110. doi: 10.1063/1.4952647. [DOI] [PubMed] [Google Scholar]
- 79.Agrahari AK, et al. Cu(I)-catalyzed click chemistry in glycoscience and their diverse applications. Chem. Rev. 2021;121:7638–7956. doi: 10.1021/acs.chemrev.0c00920. [DOI] [PubMed] [Google Scholar]
- 80.Bennett CS, Galan MC. Methods for 2-deoxyglycoside synthesis. Chem. Rev. 2018;118:7931–7985. doi: 10.1021/acs.chemrev.7b00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Witte MD, Minnaard AJ. Site-selective modification of (oligo)saccharides. ACS Catal. 2022;12:12195–12205. doi: 10.1021/acscatal.2c03876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–414, Tables 1–52 and Notes 1–16.
Cartesian coordinates of structures from VCD calculations.
Crystallographic data for compound 17a; CCDC reference 2097738.
Crystallographic data for compound 17l; CCDC reference 2097739.
Crystallographic data for compound 17n; CCDC reference 2097740.
Cartesian coordinates (.xyz) of all DFT-computed structures for the mechanistic understanding.
Data Availability Statement
The data supporting the findings of this study are available within the Article and its Supplementary Information files. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2097738 (17a), CCDC 2097739 (17l) and CCDC 2097740 (17n). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.