Abstract
Identifying and exploiting cost-effective and green methods of metal recovery from natural and contaminated aqueous systems is widely recognized as necessary to supplement the supply of critical elements, decrease the environmental impacts associated with hardrock mining, and remediate metal-contaminated waters. This research examines a novel approach based on rhamnolipid-facilitated chemical precipitation of metals. Three techniques were assessed to remove the rhamnolipid:metal complex from solution: mixing only, and mixing following by filtration or centrifugation. Recent advances in the ability to synthetically produce rhamnolipid surfactants allowed investigation of a variety of rhamnolipid structures. Rhamnolipids differing in the length and number of hydrophobic tails were assessed to remove Pb, La, and Mg from single metal solutions. In general, removal increased with increased rhamnolipid hydrophobicity and with the addition of an active removal step (filtration or centrifugation). Filtration removed up to 96% of all metals while centrifugation removed up to 97% for Pb and La and 60% for Mg. Results suggest tailoring the rhamnolipid structure and removal methods may enable selective metal removal to achieve specific outcomes. Future studies in mixed-metal and real-world solutions will be needed to confirm the viability of these techniques in complex systems.
Keywords: Rhamnolipid, glycolipid, remediation, water treatment, metals
Graphical Abstract
1. Introduction
The 2015 Paris Agreement set forth an essential shift towards carbon neutral technologies such as solar power, wind power, and electric vehicles to combat climate change [1]. These technologies require rare earth elements (REE) and other metals, also known as critical materials, due to their economic importance and high supply risk [2]. To facilitate the transition to green energy technologies, REE consumption is expected to increase up to 9% annually [3]. This rapid increase in demand, coupled with a push for REE resources to be produced domestically to ensure national security and economic prosperity, is driving the expansion of traditional hardrock mining for REE [4,5]. This expansion is accompanied by negative environmental impacts such as high greenhouse gas emissions, energy and water consumption, and disruption of natural resources [6–8]. One way to offset these environmental impacts, supplement future supply, and reduce the reliance on hardrock mining is to identify and exploit alternative sources of REE.
Many aqueous waste systems (acid mine drainage, industrial waste streams, produced waters from oil and gas production, and saltwater brines) contain REE and other valuable elements [8], but they are not currently exploited as a resource because selective, cost-competitive metal-recovery technology is not yet available. Chemical precipitation, specifically hydroxide and sulfide precipitation, is one of the most widely used methods to remove metals from aqueous systems for remediation purposes as it is simple and effective [9]. This process involves the addition of a chemical agent to bind metal ions creating an insoluble solid particle which can then be isolated by sedimentation or filtration [9,10]. Disadvantages of chemical precipitation include the fact that it is non-selective, produces a large amount of sludge (that needs subsequent disposal), and can create toxic by-products [9]. Commercially available chelating precipitants for heavy metals (e.g., trimercaptotriazine, potassium/sodiumthiocarbonate) have been developed as a treatment option, but many of these products offer weak binding and pose environmental hazards [11]. Thus, there is a need for chemical precipitants that are effective, have high selectivity for target metals, and are environmentally compatible [9].
The biosurfactant rhamnolipid (Fig. 1A) exhibits several characteristics indicating they have potential as effective and green chemical precipitation agents [12,13]. These include biodegradability, low toxicity [14,15], and the ability to complex metals in solution [16,17]. In particular, monorhamnolipids have been previously shown to selectively bind REE in comparison to common soil and water cations (e.g., magnesium, calcium) with conditional stability constants ranging up to seven orders of magnitude higher for REE [13,16]. One model of the metal binding exhibited by monorhamnolipid shows a “pocket” in the structure that coordinates with the metal through the carboxyl moiety and two sugar hydroxyl groups (Fig. 1C) [18,19]. Metal selectivity is believed to be the result of preferential fit into this metal-binding pocket [12,17]; as shown previously, there is a correlation between the strength of metal binding and an ion’s charge and size [12]. Like In these previous studies, the monorhamnolipid examined was produced biologically as a complex mixture of congeners that vary in tail length. The most abundant congener (75–85%) in this mixture is a Rha-C10-C10 (rhamnosyl-β-hydroxydecanoyl-β-hydroxydecanoate) (Fig. 1A) [20,21]. Recent advancements in the synthetic production of rhamnolipids has enabled the production of high-purity single congeners that can be “tailored” to meet the requirements of specific applications (Fig. 1B) [22,23].
Figure 1.

Structure of (A) biosynthetic rhamnolipid where ‘n’ and ‘m’ vary from 4 to 12 and (B) synthetic rhamnolipid-C10-C10 showing the three tailorable glycolipid structure moieties: hydrophilic sugar (red), linkage (green), and hydrophobic tail(s) (blue). (C) A model of one potential binding mechanism between monorhamnolipid (Rha-C10-C10) and a representative metal within the oxygen-rich cavity of the rhamnolipid structure [18].
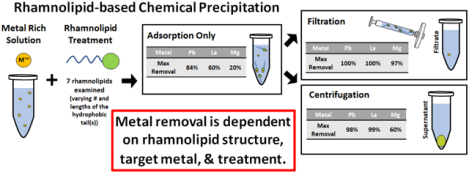
The objective of this research is to evaluate synthetic rhamnolipids as chemical precipitants and to determine how variations in molecular structure affect their performance. To this end, seven synthetic rhamnolipids with tail groups varying by number and length were tested for their ability to precipitate three model metals: lead (Pb2+), lanthanum (La3+), and magnesium (Mg2+). Pb2+ was examined because it is an element of environmental concern with a high rhamnolipid-metal conditional stability constant. La3+ was chosen because it is a critical element that is representative of other REEs with a high rhamnolipid-metal conditional stability constant. Mg2+ was selected to represent common water cations with a relatively small conditional stability constant, and because it would usually not be the specific target for typical remediation or recovery applications. Previously reported conditional stability constants for these metals with biosynthetic monorhamnolipid are 9.13 for Pb2+, 9.29 for La3+, and 2.66 for Mg2+ [12]. The effects of molecular structure and metal:rhamnolipid molar ratio were investigated for each rhamnolipid and metal pair at three molar ratios. Following complexation, separation of the rhamnolipid-metal precipitate from solution was evaluated without further treatment or with an additional treatment step using either filtration or centrifugation to aid in removal of the precipitate.
2. Experimental
2.1. Surfactants
Synthetic rhamnolipids were used in this study (GlycoSurf Inc., Salt Lake City, >95% purity). Seven different structural variants were selected to examine the effect of the number and length of the tail group(s) on rhamnolipid as a chemical precipitant (Table 1): rhamnosyl-β-hydroxyhexanoate, rhamnosyl-β-hydroxydecanoate, rhamnosyl-β-hydroxytetradecanoate, rhamnosyl-β- hydroxyoctadecanoate, rhamnosyl-β-hydroxydecanoyl-β-hydroxydecanoate, rhamnosyl-β-hydroxydodecanoyl-β-hydroxydodecanoate, and rhamnosyl-β-hydroxytetradecanoyl-β-hydroxytetradecanoate. Solutions (10 mM) were made using nanopure water (≥18 MΩ-cm) then adjusted to a pH of 6.90 ± 0.05 with HNO3 and NaOH. These solutions were made in advance, stored at 4°C for up to one month, and brought to room temperature (~25°C) before each experiment.
Table 1.
The rhamnolipids used in this study.
| Rhamnolipid | Abbreviation | Molecular Weight | Chemical Formula | Structure |
|---|---|---|---|---|
| rhamnosyl-β-hydroxyhexanoate | Rha-C6 | 278.3 | C12H22O7 |

|
| rhamnosyl-β-hydroxydecanoate | Rha-C10 | 334.4 | C16H30O7 |

|
| rhamnosyl-β-hydroxytetradecanoate | Rha-C14 | 390.5 | C20H38O7 |

|
| rhamnosyl-β-hydroxyoctadecanoate | Rha-C18 | 446.6 | C24H46O7 |
|
| rhamnosyl-β-hydroxydecanoyl-β-hydroxydecanoate | Rha-C10-C10 | 504.7 | C26H48O9 |

|
| rhamnosyl-β-hydroxydodecanoyl-β-hydroxydodecanoate | Rha-C12-C12 | 560.8 | C30H56O9 |

|
| rhamnosyl-β-hydroxytetradecanoyl-β-hydroxytetradecanoate | Rha-C14-C14 | 616.9 | C34H64O9 |

|
2.2. Metals
Three metals were tested for binding with the rhamnolipids: Pb2+ as Pb(NO3)2 (Fisher Scientific, Waltham, MA, 99% purity), La3+ as La(NO3)3 6(H2O) (Sigma-Aldrich, St. Louis, MO, 99.99% purity), and Mg2+ as Mg(NO3)2 6(H2O) (Sigma-Aldrich, St. Louis, MO, 99% purity). Metal solutions (5 mM) were made using nanopure water (≥18 MΩ-cm) and were not pH balanced. The pH conditions for all experiments in this study ranged from 5.8 to 6.2. A Visual MINTEQ v3.1 analysis of metal speciation at pH 6 showed that all metals used in this study were present as the soluble species La3+ (100%), Mg2+ (100%), and Pb2+ (97%) [24].
2.3. Experimental Methods
Each metal (Pb2+, La3+, and Mg2+) was evaluated separately with each rhamnolipid. For every experiment, a metal solution was mixed with a rhamnolipid solution in a 1.7 mL polypropylene centrifuge tube at varying molar ratios (rhamnolipid:metal): 0:1 (rhamnolipid-free control), 2:1, 5:1, and 10:1; concentrations are provided in Tables 2–4. The reactions were mixed in the order of nanopure water (≥18 MΩ-cm), rhamnolipid solution, then metal solution. The metal and rhamnolipid solutions were continuously stirred as they were aliquoted into the reaction tubes. All experiments were performed in triplicate.
Following set up, the reaction tubes were rotated overnight (16 to 24 h) and then three samples were collected from each tube to measure metal removal from solution: (1) no further treatment; (2) an additional filtration step; and (3) an additional centrifugation step. A flow chart delineating the sampling process can be found in Fig. 2. Briefly, for the rhamnolipid no treatment samples all tubes were vortexed for approximately 5 sec immediately before sampling. After mixing, 0.1 mL of the reaction solution was aliquoted into a dilution tube of 2% trace-metal grade nitric acid for analysis. This sample measures the loss of metal due to the formation of immiscible hydrophobic scums (observed in Fig. 3a and 3b) or surface adsorbed complexes not attributable to the subsequent filtration or centrifugation treatments.
Figure 2.

Experimental sampling process flow chart
Figure 3.

Reaction tubes from the Pb-Rha-C18 experiment. The top row shows the tubes after the rhamnolipid adsorption treatment (prior to vortexing). The soap scums are observed in pictures a and b while a cloudy/ dispersed precipitate can be observed in picture c. The bottom row shows the tubes after the rhamnolipid and centrifugation treatment. Pellets formed during centrifugation can be observed in pictures e and f. The percent of metal removed (R) is displayed at the bottom of the pictures for each treatment and ratio.
Samples for the filtration treatment were vortexed for 5 sec, then a 0.6 mL aliquot was collected with a 1 mL syringe. A GHP acrodisc disk filter (hydrophilic polypropylene membrane, 0.45 μm pore size, 25 mm disc diameter) from Pall Life Sciences (Port Washington, New York) was placed onto the end of the syringe and approximately 0.1 mL of filtrate was collected into a dilution tube for analysis. The centrifuge sample was collected by centrifuging the remaining reaction solution at 10,000 RCF for 15 min. Approximately 0.1 mL of the supernatant was aliquoted into a dilution tube for analysis. Centrifugation conditions were selected to achieve optimal recovery results in this system (preliminary data not shown), and these conditions are likely application specific. Following collection, all samples were analyzed at the Arizona Laboratory for Emerging Contaminants at the University of Arizona using inductively coupled plasma mass spectrometry (ICP-MS).
2.4. Calculations
The efficiency of metal removal (R) was determined for each of the three treatments: Rnt for the rhamnolipid with no additional treatment, Rf for the rhamnolipid and filtration treatment, and Rc for the rhamnolipid and centrifugation treatment. R was determined by comparing the solution metal concentration of the treatment sample (Cnt for the rhamnolipid adsorption sample, Cf for the rhamnolipid and filtration sample, and Cc for the rhamnolipid and centrifugation sample) to the solution metal concentration of a rhamnolipid-free control (Ci) that underwent the same handling and processing steps.
The equations for these calculations are shown below:
| Eq. 1 |
| Eq. 2 |
| Eq. 3 |
3. Results
3.1. Metal recovery without further treatment
The samples that received no further treatment following the addition of rhamnolipid were visually observed, and aqueous metal concentrations were measured to evaluate the chemical precipitation of Pb2+, La3+, and Mg2+. Depending on the treatment (the rhamnolipid, the metal, and/or the rhamnolipid:metal molar ratio tested) different complexation behaviors were observed. In some cases, hydrophobic soap scums formed. In other cases, smaller complexes were observed adsorbed to the centrifugation tube surfaces. In yet other instances, the solution became cloudy, or no changes were observed.
In general, the rhamnolipids precipitated metals more strongly as scums or adsorbed complexes in the order Pb2+ > La3+ >> Mg2+. For lead, the Rha-C18 removed the highest amount (83.9%) of Pb2+ from solution followed by Rha-C10-C10 with 60.1% (Table 2). No other rhamnolipid removed more than 30% of Pb2+ from solution. The surfactant-Pb2+ interaction was dependent on the rhamnolipid:Pb2+ molar ratio. This was visually observable; examination of the Pb-Rha-C18 reactions showed precipitate formation on the walls of the reaction tube after mixing at the 2:1 and 5:1 ratios while the 10:1 solution remained uniformly cloudy, as shown in Fig. 3. Correspondingly, the Rha-C18 exhibited better removal at molar ratios that showed precipitate formation with a removal order of 5:1 > 2:1 >> 10:1 (83.9%, 54.8%, and 6.2% removal, respectively). The molar ratio effect was not consistent among the rhamnolipid structures tested. For example, in contrast to the Rha-C18, the Rha-C10-C10 exhibited a removal order of 2:1 >> 10:1 ~ 5:1 with 60.1%, 1.9%, and 0.5% removal, respectively.
Table 2.
Metal concentrations (mmol/L) of lead in the reaction tube initially and after the three treatments.
| Rhamnolipid | Molar Ratio | Ci mmol/L |
Cnt mmol/L Rnt |
Cf mmol/L Rf |
Cc mmol/L Rc |
|---|---|---|---|---|---|
| Rha-C6 | 2 to 1 | 0.321 ± 0.008 | 0.323 ± 0.005 (NR) |
0.115 ± 0.022 (64.0%) |
0.313 ± 0.003 (2.2%) |
| 5 to 1 | 0.329 ± 0.014 (NR) |
0.046
± 0.017 (85.8%) |
0.322 ± 0.004 (NR) |
||
| 10 to 1 | 0.316 ± 0.008 (1.4%) |
0.119 ± 0.018 (62.7%) |
0.323 ± 0.005 (NR) |
||
| Rha-C10 | 2 to 1 | 0.329 ± 0.003 | 0.320 ± 0.012 (2.7%) |
0.037
± 0.048 (88.8%) |
0.320 ± 0.045 (2.5%) |
| 5 to 1 | 0.293 ± 0.006 (10.7%) |
0.028
± 0.031 (91.6%) |
0.288 ± 0.002 (12.2%) |
||
| 10 to 1 | 0.232 ± 0.009 (29.3%) |
0.001
± 0.000 (99.8%) |
0.231 ± 0.003 (29.7%) |
||
| Rha-C14 | 2 to 1 | 0.321 ± 0.008 | 0.278 ± 0.011 (13.3%) |
0.031
± 0.030 (90.2%) |
0.194 ± 0.006 (39.4%) |
| 5 to 1 | 0.315 ± 0.004 (1.6%) |
0.000
± 0.000 (100.0%) |
0.315 ± 0.004 (1.8%) |
||
| 10 to 1 | 0.311 ± 0.008 (3.0%) |
0.001
± 0.001 (99.7%) |
0.327 ± 0.001 (NR) |
||
| Rha-C18 | 2 to 1 | 0.272 ± 0.010 | 0.123 ± 0.002 (54.8%) |
0.027
± 0.001 (90.1%) |
0.132 ± 0.001 (51.6%) |
| 5 to 1 | 0.044
± 0.008 (83.9%) |
0.000
± 0.002 (99.9%) |
0.005
± 0.002 (98.1%) |
||
| 10 to 1 | 0.255 ± 0.015 (6.2%) |
0.000
± 0.001 (99.9%) |
0.014
± 0.001 (94.7%) |
||
| Rha-C10-C10 | 2 to 1 | 0.272 ± 0.010 | 0.109 ± 0.006 (60.1%) |
0.017
± 0.007 (93.8%) |
0.091 ± 0.003 (66.6%) |
| 5 to 1 | 0.271 ± 0.013 (0.5%) |
0.002 ± 0.002 (99.2%) | 0.005
± 0.000 (98.1%) |
||
| 10 to 1 | 0.267 ± 0.005 (1.9%) |
0.083 ± 0.009 (69.7%) |
0.214 ± 0.004 (21.4%) |
||
| Rha-C12-C12 | 2 to 1 | 0.272 ± 0.010 | 0.211 ± 0.010 (22.4%) |
0.069
±0.018 (74.8%) |
0.146 ± 0.008 (46.4%) |
| 5 to 1 | 0.204 ± 0.004 (25.0%) |
0.049
± 0.021 (81.8%) |
0.047
± 0.003 (82.8%) |
||
| 10 to 1 | 0.249 ± 0.10 (8.6%) |
0.059
± 0.028 (78.3%) |
0.129 ± 0.002 (52.8%) |
||
| Rha-C14-C14 | 2 to 1 | 0.272 ± 0.010 | 0.213 ± 0.003 (21.4%) |
0.094 ± 0.010 (65.6%) |
0.141 ± 0.002 (48.1%) |
| 5 to 1 | 0.243 ± 0.022 (10.8%) |
0.016 ± 0.001 (94.1%) | 0.042
± 0.001 (84.4%) |
||
| 10 to 1 | 0.241 ± 0.011 (11.3%) |
0.005
± 0.001 (98.2%) |
0.008
± 0.000 (97.1%) |
Ci stands for the initial metal concentration; Cnt, the metal concentration after the rhamnolipid and no additional treatment; Cf, the metal concentration after the rhamnolipid and filtration treatment; and Cc, the metal concentration after the rhamnolipid and centrifugation treatment. The efficiency of removal is presented as a percent (calculated in Eqs. 1–3) below the concentrations. ’NR’ represents no removal. Colored cells indicate removals > 70% (yellow), > 85% (light green), and > 95% (dark green).
Lanthanum removal was also highest with Rha-C18 (59.4%) (Table 3). The Rha-C10-C10 and Rha-C14-C14 performed comparably with each removing approximately 47% of the La3+. As observed for Pb2+, removals for La3+ did not follow any trends related to rhamnolipid:metal molar ratio. Rha-C18 exhibited an order of 5:1 >> 2:1 >> 10:1 with removals of 59.4%, 16.5%, and no removal, respectively. Both Rha-C10-C10 and Rha-C14-C14 had their lowest removals at the 10:1 molar ratio, but Rha-C10-C10 exhibited the best removal at the 2:1 ratio while Rha-C14-C14 did so at 5:1. Similar to data for Pb2+, maximal removals by other rhamnolipid treatments were limited to 32% or less.
Table 3.
Metal concentrations (mmol/L) of lanthanum in the reaction tube initially and after the three treatments.
| Rhamnolipid | Molar Ratio | Ci mmol/L |
Cnt mmol/L Rnt |
Cf mmol/L Rf |
Cc mmol/L Rc |
|---|---|---|---|---|---|
| Rha-C6 | 2 to 1 | 0.298 ± 0.005 | 0.325 ± 0.026 (NR) | 0.143 ± 0.062 (51.8%) | 0.324 ± 0.018 (NR) |
| 5 to 1 | 0.282 ± 0.026 (5.2%) | 0.106 ± 0.061 (64.4%) | 0.320 ± 0.024 (NR) | ||
| 10 to 1 | 0.332 ± 0.040 (NR) | 0.114 ± 0.064 (61.6%) | 0.294 ± 0.010 (1.1%) | ||
| Rha-C10 | 2 to 1 | 0.280 ± 0.009 | 0.282 ± 0.009 (NR) | 0.096 ± 0.025 (65.7%) | 0.228 ± 0.013 (18.6%) |
| 5 to 1 | 0.246 ± 0.016 (12.3%) | 0.015 ± 0.010 (94.6%) | 0.088 ± 0.003 (68.7%) | ||
| 10 to 1 | 0.190 ± 0.004 (32.2%) | 0.022 ± 0.028 (92.1%) | 0.051 ± 0.010 (81.7%) | ||
| Rha-C14 | 2 to 1 | 0.298 ± 0.005 | 0.247 ± 0.034 (16.9%) | 0.164 ± 0.058 (44.9%) | 0.221 ± 0.014 (25.8%) |
| 5 to 1 | 0.215 ± 0.024 (27.7%) | 0.044 ± 0.007 (85.3%) | 0.094 ± 0.002 (68.5%) | ||
| 10 to 1 | 0.314 ± 0.016 (NR) | 0.000 ± 0.000 (99.9%) | 0.006 ± 0.001 (98.0%) | ||
| Rha-C18 | 2 to 1 | 0.298 ± 0.005 | 0.249 ± 0.009 (16.5%) | 0.116 ± 0.029 (61.1%) | 0.228 ± 0.013 (23.4%) |
| 5 to 1 | 0.121 ± 0.009 (59.4%) | 0.001 ± 0.002 (99.5%) | 0.088 ± 0.003 (70.6%) | ||
| 10 to 1 | 0.331 ± 0.024 (NR) | 0.004 ± 0.001 (98.8%) | 0.051 ± 0.010 (82.8%) | ||
| Rha-C10-C10 | 2 to 1 | 0.268 ± 0.002 | 0.144 ± 0.006 (46.4%) | 0.085 ± 0.005 (68.5%) | 0.155 ± 0.002 (42.1%) |
| 5 to 1 | 0.219 ± 0.007 (18.4%) | 0.001 ± 0.000 (99.7%) | 0.003 ± 0.000 (98.9%) | ||
| 10 to 1 | 0.257 ± 0.004 (4.0%) | 0.030 ± 0.002 (88.7%) | 0.035 ± 0.003 (86.9%) | ||
| Rha-C12-C12 | 2 to 1 | 0.268 ± 0.002 | 0.184 ± 0.014 (31.5%) | 0.119 ± 0.007 (55.7%) | 0.164 ± 0.013 (38.7%) |
| 5 to 1 | 0.197 ± 0.006 (26.4%) | 0.044 ± 0.009 (83.7%) | 0.074 ± 0.003 (72.4%) | ||
| 10 to 1 | 0.263 ± 0.007 (2.0%) | 0.035 ± 0.001 (86.9%) | 0.057 ± 0.004 (78.7%) | ||
| Rha-C14-C14 | 2 to 1 | 0.268 ± 0.002 | 0.158 ± 0.016 (41.1%) | 0.105 ± 0.013 (60.7%) | 0.160 ± 0.002 (40.3%) |
| 5 to 1 | 0.143 ± 0.005 (46.7%) | 0.022 ± 0.004 (92.0%) | 0.060 ± 0.003 (77.5%) | ||
| 10 to 1 | 0.246 ± 0.014 (8.1%) | 0.031 ± 0.008 (88.3%) | 0.029 ± 0.003 (89.1%) |
Ci stands for the initial metal concentration; Cnt, the metal concentration after the rhamnolipid and no additional treatment; Cf, the metal concentration after the rhamnolipid and filtration treatment; and Cc, the metal concentration after the rhamnolipid and centrifugation treatment. The efficiency of removal is presented as a percent (calculated in Eqs. 1–3) below the concentrations. ’NR’ represents no removal. Colored cells indicate removals > 70% (yellow), > 85% (light green), and > 95% (dark green).
Finally, removal of Mg2+ was much lower than for Pb2+ and La3+ in all cases (Table 4). The best performing rhamnolipid (Rha-C18) only removed 19.5% of the Mg2+ followed by the Rha-C6 which removed 7.6%. For Mg2+, four of the rhamnolipids, including all of the two chain molecules, removed no metal.
Table 4.
Metal concentrations (mmol/L) of magnesium in the reaction tube initially and after the three treatments.
| Rhamnolipid | Molar Ratio | Ci mmol/L |
Cnt mmol/L Rnt |
Cf mmol/L Rf |
Cc mmol/L Rc |
|---|---|---|---|---|---|
| Rha-C6 | 2 to 1 | 0.268 ± 0.008 | 0.247 ± 0.014 (7.6%) |
0.202 ± 0.016 (24.5%) |
0.265 ± 0.010 (0.9%) |
| 5 to 1 | 0.248 ± 0.013 (7.3%) |
0.178 ± 0.020 (33.4%) |
0.255 ± 0.001 (4.9%) |
||
| 10 to 1 | 0.258 ± 0.011 (3.6%) |
0.165 ± 0.001 (38.2%) |
0.272 ± 0.016 (NR) |
||
| Rha-C10 | 2 to 1 | 0.501 ± 0.009 | 0.486 ± 0.021 (3.1%) |
0.328 ± 0.036 (34.6%) |
0.497 ± 0.014 (0.8%) |
| 5 to 1 | 0.492 ± 0.007 (1.8%) |
0.283 ± 0.059 (43.6%) | 0.500 ± 0.008 (0.2%) |
||
| 10 to 1 | 0.503 ± 0.004 (NR) |
0.317 ± 0.073 (36.7%) |
0.490 ± 0.004 (2.2%) |
||
| Rha-C14 | 2 to 1 | 0.268 ± 0.008 | 0.291 ± 0.005 (NR) |
0.147 ± 0.045 (45.0%) |
0.292 ± 0.008 (NR) |
| 5 to 1 | 0.300 ± 0.018 (NR) |
0.088 ± 0.042 (66.9%) |
0.279 ± 0.016 (NR) |
||
| 10 to 1 | 0.302 ± 0.017 (NR) |
0.009
± 0.004 (96.6%) |
0.219 ± 0.021 (NR) |
||
| Rha-C18 | 2 to 1 | 0.268 ± 0.008 | 0.265 ± 0.002 (12.1%) |
0.169 ± 0.015 (37.0%) |
0.213 ± 0.002 (20.5%) |
| 5 to 1 | 0.215 ± 0.002 (19.5%) |
0.056
± 0.012 (79.0%) |
0.141 ± 0.001 (47.4%) |
||
| 10 to 1 | 0.304 ± 0.001 (NR) |
0.017
± 0.002 (93.7%) |
0.107 ± 0.004 (60.1%) |
||
| Rha-C10-C10 | 2 to 1 | 0.268 ± 0.008 | 0.297 ± 0.001 (NR) |
0.127 ± 0.007 (52.4%) |
0.292 ± 0.002 (NR) |
| 5 to 1 | 0.282 ± 0.023 (NR) |
0.023
± 0.003 (91.4%) |
0.300 ± 0.002 (NR) |
||
| 10 to 1 | 0.308 ± 0.004 (NR) |
0.008
± 0.002 (97.1%) |
0282 ± 0.004 (NR) |
||
| Rha-C12-C12 | 2 to 1 | 0.268 ± 0.008 | 0.281 ± 0.002 (NR) |
0.182 ± 0.055 (32.1%) |
0.276 ± 0.005 (NR) |
| 5 to 1 | 0.285 ± 0.005 (NR) |
0.135 ± 0.074 (49.4%) |
0.273 ± 0.014 (NR) |
||
| 10 to 1 | 0.285 ± 0.012 (NR) |
0.008
± 0.004 (97.1%) |
0.266 ± 0.012 (0.4%) |
||
| Rha-C14-C14 | 2 to 1 | 0.268 ± 0.008 | 0.279 ± 0.002 (NR) |
0.152 ± 0.077 (43.2%) |
0.272 ± 0.004 (NR) |
| 5 to 1 | 0.270 ± 0.006 (NR) |
0.170 ± 0.098 (36.4%) |
0.269 ± 0.013 (NR) |
||
| 10 to 1 | 0.275 ± 0.009 (NR) |
0.009
± 0.006 (96.8%) |
0.264 ± 0.010 (1.5%) |
Ci stands for the initial metal concentration; Cnt, the metal concentration after the rhamnolipid and no additional treatment; Cf, the metal concentration after the rhamnolipid and filtration treatment; and Cc, the metal concentration after the rhamnolipid and centrifugation treatment. The efficiency of removal is presented as a percent (calculated in Eqs. 1–3) below the concentrations. ’NR’ represents no removal. Colored cells indicate removals > 70% (yellow), > 85% (light green), and > 95% (dark green).
3.2. Rhamnolipid and Filtration Treatment
The next set of samples evaluated the ability of an added filtration step to increase removal of metals from solution. Filtration removed metals in the order of Pb2+ > La3+ > Mg2+ and metal removal were uniformly higher with the added filtration step. All seven rhamnolipids tested removed > 81.8%, > 64.4%, and > 38.2% of Pb2+, La3+, and Mg2+, respectively (Table 2–4). This can be compared to the samples that received no further treatment for which removals were generally < 60% for Pb2+, < 50% for La3+, and < 10% for Mg2+.
Lead removals of >97% were achieved with five of the seven rhamnolipids tested: Rha-C10, -C14, -C18, -C10-C10, and -C14-C14 (Table 2) while the other two rhamnolipids (Rha-C6 and -C12-C12) removed 85.8% and 81.8%, respectively. All three rhamnolipid-metal molar ratios showed relatively good removal with lead with slightly better performance by the 5:1 and 10:1 ratios in most cases. The Rha-C10, -C14, and -C18 exhibited >89% removals at all three ratios with the 2:1 exhibiting the lowest removal. For the Rha-C10-C10, the least effective ratio was the 10:1 ratio which removed 69.7% while for Rha-C14-C14 the 2:1 molar ratio had the lowest removal at 65.6%.
For lanthanum, highest removals were observed in the Rha-C14, -C18, and -C10-C10 reactions with each achieving > 99% removal for at least one of the molar ratios tested (Table 3). The next best rhamnolipids were the Rha-C10 and Rha-C14-C14 with removals exceeding 90%. Finally, the Rha-C6 and -C12-C12 removed 64.4% and 86.9%, respectively. For this metal, all of the 2:1 ratio treatments were the least effective while the 5:1 and 10:1 ratios showed similar removals in most cases.
Magnesium removal was generally lower than removal of lead or lanthanum. Best removal performance for magnesium exceeded 93% at the 10:1 molar ratio for five rhamnolipids (Rha-C14, -C18, -C10-C10, -C12-C12, and -C14-C14). For the other molar ratios with these five rhamnolipids, removals ranged from 32.1% to 79.0%, except Rha-C10-C10 at the 5:1 ratio which removed 91.4%. For the remaining two rhamnolipids (Rha-C6, Rha-C10) removals were poor, ranging from 24.5% to 43.6%. In general, among all rhamnolipids, the removal percentages increased in the order of 2:1 < 5:1 < 10:1.
Overall, the addition of a filtration step improved the recovery of all three metals. Several rhamnolipids removed over 97% from solution for each metal tested: five for Pb2+, three for La3+, and four for Mg2+. Generally, the best overall performing structures were Rha-C14, -C18 and -C10-C10, each achieving > 94% removal for all three metals tested. The most effective rhamnolipid-metal molar ratios were 10:1 and 5:1.
3.3. Rhamnolipid and Centrifugation Treatment
As an alternative to filtration, we evaluated a centrifugation step following the addition of rhamnolipid. Similar to the no further treatment, the centrifugation treatment could be visually observed alongside the aqueous metal concentrations. The type of precipitation and pellet depended on the treatment (the rhamnolipid structure and concentration). A variety of pellet characteristics were observed ranging from “cloudy” unconsolidated pellets (Fig. 3f) to consolidated pellets (Fig 3e). In some cases, the metal-rhamnolipid precipitate that formed on the sides of the reaction tube during mixing was unable to be removed under the centrifugal force used in this experiment (Fig 3d).
When examining the aqueous metal concentrations, three rhamnolipids (Rha-C18, -C10-C10, -C14-C14) removed > 97% of lead, the Rha-C12-C12 removed 82.8%, and the three remaining structures (Rha-C6, -C10, -C14) had poor lead removals ranging from 2.2% to 39.4% (Table 2). In terms of the rhamnolipid:metal molar ratio, performance was generally in the order 5:1 > 10:1 > 2:1.
For La3+, two rhamnolipids (Rha-C14 and -C10-C10), showed > 98% removal, four rhamnolipids (Rha-C10, -C18, -C12-C12, and -C14-C14) showed maximum removals ranging from 78.7% to 89.1%, and the remaining Rha-C6 rhamnolipid had poor removal <2% (Table 3). In terms of the rhamnolipid:metal molar ratio, performance was generally in the order 10:1 > 5:1 > 2:1.
Centrifugation did not remove magnesium (< 5%) for any rhamnolipid tested except for Rha-C18 which removed 21%, 47% and 60% at the 2:1, 5:1, and 10:1 molar ratios, respectively (Table 4).
Overall, the addition of a centrifugation step improved metal removal for all the metals tested, but centrifugation effectiveness was more variable across surfactant structure and molar ratio than filtration.
4. Discussion
Based on the known ability of biologically-produced monorhamnolipid to selectively bind cationic metals, here we investigated whether rhamnolipid can be used as a chemical precipitant for metals and the effect of structural variation in the hydrophobic tail moiety on the precipitation reaction. The results of this study followed rhamnolipid-metal binding trends expected by results from Ochoa-Loza et al. [13], and subsequent studies [12,25]. For example, as evidenced by generalized recovery observations, the rhamnolipids tested herein appear to interact more strongly with the high stability constant metals (lead and lanthanum), while interactions with the lower stability constant metal (magnesium) exhibited weaker interactions, i.e., lower recovery percentages. While the affinity of rhamnolipid for the target metal appears to follow the same trend across studies regardless of the separation method employed, the optimal conditions do not. Several studies which focused on rhamnolipid-based metal removal techniques concluded that two important factors determining removal efficiencies were the target metal and the initial surfactant concentration [16,25–27]. Similar findings are shown in the current study, and results show that removal approach also impacts removal efficiency. While the optimal conditions for removal varied between filtration and centrifugation, both approaches were able to achieve high removal rates (over 99%) for metals with high rhamnolipid-binding constants (Pb2+ and La3+).
4.1. Effect of Treatment on Metal Recovery
Seven structurally distinct synthetic monorhamnolipids that varied in the length (C6 to C18) and number (one or two) of tail moieties were tested. Results show that precipitation reactions differ among the rhamnolipids tested and this was visually observed immediately upon mixing of rhamnolipid with metals. In some cases, these mixtures formed visible scums or precipitates on the surfaces of the reaction tube (Fig. 3a and 3b) and metal removal ranged as high as 84% in one case even without subsequent filtration or centrifugation. However, the addition of an active removal step by either filtration or centrifugation substantially increased the removal for all metals tested. Variations in rhamnolipid structure, such as the number and length of the hydrophobic tail(s), influenced the formation of precipitates and the ability to recover those precipitates by either filtration or centrifugation. In general, filtration was the best approach for all metals with high removal for most metal and rhamnolipid combinations. Centrifugation also demonstrated high removal efficiencies for each metal, but results were more dependent on specific conditions related to structure, rhamnolipid:metal molar ratio, and the metal being tested.
4.2. The Effect of Rhamnolipid Structure on Precipitation
This study identified several trends relating to the structure-function relationship of the rhamnolipids examined. The short-tailed Rha-C6 generally performed poorly while long-tailed Rha-C14 and Rha-C18 and the double chain molecules typically performed the best. The longer chain and two chain molecules may perform better because they are more hydrophobic and produce precipitates that more readily aggregate into larger and easier to collect products. Alternatively, these larger molecules may also simply provide more mass per complex that improves separation via filtration and centrifugation. Further examination of these types of trends may allow the design of molecules with improved metal precipitation characteristics. This result, combined with recent advances in synthetic production of rhamnolipids [22], has led to the real possibility of improving metal binding through structural modification.
4.3. Rhamnolipid Precipitant Potential
Based on these observations, it is clear that rhamnolipids have potential for use as chemical precipitants in metal removal from aqueous sources as a remediation or metal recovery strategy. The different metal performance achieved by varying rhamnolipid structure, rhamnolipid:metal molar ratio and use of either filtration or centrifugation suggest that there is opportunity to achieve specific remediation aims in complex real-world solutions. For example, metal removal using filtration exhibited high removals for each metal at most of the tested molar ratios. Thus, this approach may be effective at removing a broad range of metals from solution simultaneously. On the other hand, a more selective metal removal approach may be possible with centrifugation. Under the conditions of this study, centrifugation exhibited some differential removal behaviors that could enable some degree of selectivity under specific reaction conditions. For example, Rha-C14 at the 10:1 ratio removed > 98% of La3+ while neither Pb2+ nor Mg2+ were removed under the same conditions (Tables 2–4). Under slightly different conditions, Rha-C10-C10 at the 5:1 ratio removed > 98% of both Pb2+ and La3+ but removed no Mg2+ (Tables 2–4). To further explore these possible applications, the next step will be to perform mixed-metal studies in both simple and complex solution matrices.
Furthermore, the fact that rhamnolipids preferably bind metals like REE suggests that such metals can be selectively recovered from complex aqueous solutions. Further studies are needed to better understand the fundamental interaction of rhamnolipids with metals to improve metal binding, selectivity, and recovery. This includes understanding complex stoichiometries, precipitate crystal structure, and the metal/rhamnolipid interaction mechanics. Other practical considerations also need to be evaluated, such as the ability for filtration and centrifugation approaches to be implemented in real-world scenarios where engineering challenges may occur due to processing large volumes or continuous operations.
4.4. Comparison to Other Rhamnolipid-Based Approaches
Precipitation of metals with rhamnolipid and other glycolipid biosurfactants has been observed previously [16,28–30]. For example, Luna et al. [28] noted precipitation between a sophorolipid biosurfactant and both Cd2+ and Pb2+ in solution. Similarly, Hogan et al. [16], observed the formation of a rhamnolipid-La3+ scum on the sides of the flotation apparatus under specific conditions similar to those studied herein. While these studies note the formation of rhamnolipid/metal precipitates during the investigation of other rhamnolipid/metal properties and removal technologies, the current study reports rhamnolipid-based chemical precipitation as a direct approach for removal of metals from aqueous systems. In addition to this chemical precipitation approach, rhamnolipids have also been examined for the recovery of metals from aqueous sources using techniques such as ion flotation and micellar enhanced ultrafiltration (MEUF). Comparison of these rhamnolipid-based approaches shows that each has potential to achieve high metal recovery efficiencies, but each technology has operational parameters and characteristics that dictate the conditions where it is useful, appropriate, and/or practical.
Ion flotation utilizes air bubbles and surfactants to separate the dissolved metals from aqueous solutions by concentrating metals in a collectable foam at the solution surface [9]. This process can involve the addition of a chemical agent such as ferrous sulfate to bind with the dissolved metals before adding the surfactant to aid in the flotation of the metal complex [16,17,31] or a single chemical agent, such as rhamnolipid, which can aid in both the complexation and removal of the metal [32,33]. Hogan et al., utilize this approach with rhamnolipid as the binder and collector with simple, model solutions [16] and real-world groundwater [17]. In model solutions, 98% of Cd2+ and La3+ were removed from single metal solutions, and removals were achieved under similar solution conditions and rhamnolipid:metal molar ratios as those utilized in this study. When ion flotation was utilized in uranium contaminated groundwater, a removal of 93% was achieved, but the process required a higher molar ratio and lower pH conditions compared to this study. While the overall removal efficiencies achieved using ion flotation are comparable to the results above, chemical precipitation’s major advantage over ion flotation is that it is a relatively simple set up and process compared to ion flotation which requires multiple steps and specialized flotation apparatus. Furthermore, the concentration of surfactant used during ion flotation may be limited to enable high removal efficiencies; high surfactant concentrations can reduce flotation efficiency due to excess water entrainment in the foam or the formation of surfactant aggregates (e.g., micelles, vesicles) that reduce the transport efficiency of metals from the bulk phase into the collectable foam [16]. The precipitation approach, on the other hand, is only limited by the solubility of the surfactant itself, and this can be tailored during synthesis.
MEUF is a second surfactant-based method utilized to remove metals from aqueous systems. This technique involves the addition of the surfactant at concentrations exceeding their critical micelle concentration (CMC)—the concentration at which micelles and other multimolecular aggregates form in solution. Metal ions are bound by the surfactant aggregates, and the solution is passed through membrane filters with pore sizes smaller than the aggregates. As a result, metals are retained and concentrated with the aggregate retentate while the filtrate is purified water [25,26]. Verma et al. [26], utilized rhamnolipid with MEUF and achieved a high removal of Cd2+ (96%) from industrial wastewater [26]. Similarly, El Zeftawy and Mulligan [25] used a mixture of mono- and dirhamnolipids to remove >99% of cadmium, lead, zinc, nickel, and copper from model solutions, and subsequently treated six wastewaters from a metal refining plant sufficiently to meet regulatory requirements. While this technique is comparable to both ion flotation and chemical precipitation in terms of metal removal efficiencies, MEUF requires surfactants be present above their CMC regardless of metal concentration and specialized membrane filters/systems that are readily fouled [9,25,26]. The precipitation approach foregoes the need for specialized equipment and filters, has no specific surfactant concentration requirements, and can utilize a variety of separation strategies as shown above.
5. Conclusion
Many metals that are either environmentally hazardous (e.g., cadmium, lead) or valuable (e.g., REEs) are present in aqueous resources such as seawater, desalination brines, produced waters from oil and gas production, geothermal aquifers, and mining influenced waters. To effectively extract these materials, there is a need for green, selective, and efficient technologies to replace the current approaches that are cost prohibitive or non-selective [8]. In this study, rhamnolipid-based chemical precipitation was shown to effectively remove Pb2+, La3+, and Mg2+ from solution when combined with a filtration or centrifugation step. The efficacy of the precipitation step as well as the additional filtration or centrifugation step was dependent on the metal being recovered, the rhamnolipid:metal molar ratio, and importantly, the molecular structure of the rhamnolipid.
There is an extensive body of research indicating the utility and advantages of rhamnolipids as green chemicals for remediation and other important applications. Chemical and physical properties have been described for biosynthetic rhamnolipids mainly as complex undefined mixtures. In contrast, limited data exist for synthetic rhamnolipids which are produced as single defined congeners and have only recently been available for study. Future research on synthetic rhamnolipids is needed to characterize their physicochemical properties (e.g., solubility, critical micelle concentration, minimum surface tension), metal-binding mechanism(s), sensitivity to environmental factors (e.g., pH, ionic strength, solution constituents), and resultant utility in diverse surfactant applications. The present study suggests that the use of such characterization in combination with structure-function analysis could be used to optimize the performance of synthetically produced rhamnolipids for metal remediation applications.
Environmental Implication.
Metal contaminants are in aqueous solutions from a variety of industries. Human and environmental risks from hazardous metals (e.g., Pb) are well established, and because metals are not biodegradable, they pose accumulation risks in environmental resources. Rhamnolipids are non-toxic biodegradable materials that have demonstrated utility in a variety of metal remediation applications. This work describes direct metal precipitation by rhamnolipids as a stand-alone technology.
Due to the use of unique synthetic structures, this data highlights the role of rhamnolipid structure in metal remediation efficacy which may improve previously described rhamnolipid-based metal remediation technologies.
Highlights.
Synthetic rhamnolipids effectively complex metals in solution.
Removal depends on rhamnolipid structure, target metal, and treatment.
Synthetic and biosynthetic rhamnolipids behave similarly.
Increased rhamnolipid hydrophobicity improves metal precipitation.
Results suggest rhamnolipid structures can be tuned to increase metal removal.
Acknowledgements
This work was supported by National Institute of Environmental Health Sciences Superfund Research Program (grant No. P42ES004940) and Small Business Innovation Research program (grant No. R44ES031897), as well as the University of Arizona Center for Environmentally Sustainable Mining.
One author of this paper (RMM) has equity ownership in GlycoSurf, Inc., which is developing products related to the research being reported. DEH has also been employed by GlycoSurf, Inc. The terms of this arrangement have been reviewed and approved by the University of Arizona in accordance with its policy on objectivity in research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement
Ida A McCawley: Conceptualization, Methodology, Formal Analysis, Investigation, Writing – Original Draft, Visualization Raina M. Maier: Writing – Review and Editing, Supervision, Project Administration, Funding Acquisition David E. Hogan: Conceptualization, Methodology, Writing – Original Draft, Writing – Review and Editing, Supervision, Project Administration, Funding Acquisition
Declaration of Competing Interest
David E. Hogan reports equipment, drugs, or supplies was provided by GlycoSurf, Inc. Raina M. Maier reports equipment, drugs, or supplies was provided by GlycoSurf, Inc. Raina M. Maier reports a relationship with GlycoSurf, Inc. that includes: board membership and equity or stocks. David E. Hogan reports a relationship with GlycoSurf, Inc. that includes: employment. David E. Hogan has patent Separation of Metal Ions from a Sample Using Glycolipids pending to Arizona Board of Regents of University of Arizona. Raina M. Maier has patent Separation of Metal Ions from a Sample Using Glycolipids pending to Arizona Board of Regents of University of Arizona. N/A
References
- [1].Rogelj J, den Elzen M, Hohne N, Fransen T, Fekete H, Winkler H, Schaeffer R, Sha F, Riahi K, Meinshausen M, Paris Agreement climate proposals need a boost to keep warming well below 2C, Nature. 534 (2016) 631–639. https://doi.org/dio: 10.1038/nature18307. [DOI] [PubMed] [Google Scholar]
- [2].Watari T, Nansai K, Nakajima K, Review of critical metal dynamics to 2050 for 48 elements, Resour Conserv Recycl. 155 (2020). https://doi.org/ 10.1016/j.resconrec.2019.104669. [DOI] [Google Scholar]
- [3].Swain N, Mishra S, A review on the recovery and separation of rare earths and transition metals from secondary resources, J Clean Prod. 220 (2019) 884–898. 10.1016/j.jclepro.2019.02.094. [DOI] [Google Scholar]
- [4].Barakos G, Mischo H, Gutzmer J, A forward look into the US rare-earth industry; How potential mines can connect to the global REE market, Min Eng. (2018) 30–37. www.miningengineeringmagazine.com. [Google Scholar]
- [5].Biden J, EO 14017: America’s Supply Chain, Fed Regist. 86 (2021) 11849–11854. [Google Scholar]
- [6].Haque N, Hughes A, Lim S, Vernon C, Rare earth elements: Overview of mining, mineralogy, uses, sustainability and environmental impact, Resources. 3 (2014) 614–635. 10.3390/RESOURCES3040614. [DOI] [Google Scholar]
- [7].Burns C, Church J, It’s complicated … Mining and climate change, Canadian Mining Journal. (2019) 6–7. [Google Scholar]
- [8].Can Sener SE, Thomas VM, Hogan DE, Maier RM, Carbajales-Dale M, Barton MD, Karanfil T, Crittenden JC, Amy GL, Recovery of Critical Metals from Aqueous Sources, ACS Sustain Chem Eng. 9 (2021) 11616–11634. 10.1021/acssuschemeng.1c03005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fu F, Wang Q, Removal of heavy metal ions from wastewaters: A review, J Environ Manage. 92 (2011) 407–418. 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- [10].Peng H, Guo J, Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review, Environ Chem Lett. 18 (2020) 2055–2068. 10.1007/s10311-020-01058-x. [DOI] [Google Scholar]
- [11].Matlock MM, Henke KR, Atwood DA, Effectiveness of commercial reagents for heavy metal removal from water with new insights for future chelate designs, J Hazard Mater. 92 (2002) 129–142. [DOI] [PubMed] [Google Scholar]
- [12].Hogan DE, Curry JE, Pemberton JE, Maier RM, Rhamnolipid biosurfactant complexation of rare earth elements, J Hazard Mater. 340 (2017) 171–178. 10.1016/j.jhazmat.2017.06.056. [DOI] [PubMed] [Google Scholar]
- [13].Ochoa-Loza FJ, Artiola JF, Maier RM, Stability Constants for the Complexation of Various Metals with a Rhamnolipid Biosurfactant, J Environ Qual. 30 (2001) 479–485. 10.2134/jeq2001.302479x. [DOI] [PubMed] [Google Scholar]
- [14].Hogan DE, Tian F, Malm SW, Kegel LL, Szabó LZ, Hunjan AS, Pemberton JE, Klimecki WT, Polt R, Maier RM, Biodegradability and Toxicity of Cellobiosides and Melibiosides, J Surfactants Deterg. 23 (2020) 715–724. 10.1002/jsde.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hogan DE, Tian F, Malm SW, Olivares C, Palos Pacheco R, Simonich MT, Hunjan AS, Tanguay RL, Klimecki WT, Polt R, Pemberton JE, Curry JE, Maier RM, Biodegradability and toxicity of monorhamnolipid biosurfactant diastereomers, J Hazard Mater. 364 (2019) 600–607. 10.1016/j.jhazmat.2018.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hogan DE, Curry JE, Maier RM, Ion flotation of la3+, cd2+, and cs+ using monorhamnolipid collector, Colloids and Interfaces. 2 (2018). 10.3390/colloids2040043. [DOI] [Google Scholar]
- [17].Hogan DE, Stolley RM, Boxley C, Amistadi MK, Maier RM, Removal of uranium from contaminated groundwater using monorhamnolipids and ion flotation, J Environ Manage. 301 (2022). 10.1016/j.jenvman.2021.113835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schalnat TA, Metal Complexation and Interfacial Behavior of the Microbially Produced Surfactant Monorhamnolipid by Pseudomonas Aeruginosa ATCC 9027, University of Arizona, 2013. http://hdl.handle.net/10150/268577. [Google Scholar]
- [19].Hogan DE, Biosurfactant (Monorhamnolipid) Complexation of Metals and Applications for Aqueous Metalliferous Waste Remedation, The University of Arizona, 2016. [Google Scholar]
- [20].Lebron-Paler A, Solution and Interfacial Characterization of Rhamnolipid Biosurfactant from P.aeruginosa ATCC 9027, The University of Arizona, 2008. http://hdl.handle.net/10150/193778. [Google Scholar]
- [21].Zhang L, Pemberton JE, Maier RM, Effect of fatty acid substrate chain length on Pseudomonas aeruginosa ATCC 9027 monorhamnolipid yield and congener distribution, Process Biochemistry. 49 (2014) 989–995. 10.1016/j.procbio.2014.03.003. [DOI] [Google Scholar]
- [22].Palos Pacheco R, Eismin RJ, Coss CS, Wang H, Maier RM, Polt R, Pemberton JE, Synthesis and Characterization of Four Diastereomers of Monorhamnolipids, J Am Chem Soc. 139 (2017) 5125–5132. 10.1021/jacs.7b00427. [DOI] [PubMed] [Google Scholar]
- [23].Pemberton J, Polt R, Maier R, Coss C, Synthesis of Carbohydrate-Based Surfactants., US9499575B2, 2016. [Google Scholar]
- [24].Gustafsson JP, Visual MINTEQ v.3.1, (2013). https://vminteq.lwr.kth.se/. [Google Scholar]
- [25].el Zeftawy MAM, Mulligan CN, Use of rhamnolipid to remove heavy metals from wastewater by micellar-enhanced ultrafiltration (MEUF), Sep Purif Technol. 77 (2011) 120–127. 10.1016/j.seppur.2010.11.030. [DOI] [Google Scholar]
- [26].Verma SP, Sarkar B, Use of rhamnolipid in micellar-enhanced ultrafiltration for simultaneous removal of Cd+2 and crystal violet from aqueous solution, Asia-Pacific Journal of Chemical Engineering. 14 (2019). 10.1002/apj.2315. [DOI] [Google Scholar]
- [27].Shetty S, Chernyshova I. v., Ponnurangam S, Foam flotation of rare earth elements by conventional and green surfactants, Miner Eng. 158 (2020). 10.1016/j.mineng.2020.106585. [DOI] [Google Scholar]
- [28].Luna JM, Rufino RD, Sarubbo LA, Biosurfactant from Candida sphaerica UCP0995 exhibiting heavy metal remediation properties, Process Safety and Environmental Protection. 102 (2016) 558–566. 10.1016/j.psep.2016.05.010. [DOI] [Google Scholar]
- [29].Das P, Mukherjee S, Sen R, Biosurfactant of marine origin exhibiting heavy metal remediation properties, Bioresour Technol. 100 (2009) 4887–4890. 10.1016/j.biortech.2009.05.028. [DOI] [PubMed] [Google Scholar]
- [30].Santos DKF, Resende AHM, de Almeida DG, da Silva R. de C.F.S., Rufino RD, Luna JM, Banat IM, Sarubbo LA, Candida lipolytica UCP0988 biosurfactant: Potential as a bioremediation agent and in formulating a commercial related product, Front Microbiol. 8 (2017). 10.3389/fmicb.2017.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bodagh A, Khoshdast H, Sharafi H, Shahbani Zahiri H, Akbari Noghabi K, Removal of cadmium(II) from aqueous solution by ion flotation using rhamnolipid biosurfactant as an ion collector, Ind Eng Chem Res. 52 (2013) 3910–3917. 10.1021/ie400085t. [DOI] [Google Scholar]
- [32].Salmani Abyaneh A, Fazaelipoor MH, Evaluation of rhamnolipid (RL) as a biosurfactant for the removal of chromium from aqueous solutions by precipitate flotation, J Environ Manage. 165 (2016) 184–187. 10.1016/j.jenvman.2015.09.034. [DOI] [PubMed] [Google Scholar]
- [33].Shojaei V, Khoshdast H, Efficient chromium removal from aqueous solutions by precipitate flotation using rhamnolipid biosurfactants, Physicochemical Problems of Mineral Processing. 54 (2018) 1014–1025. 10.5277/ppmp18103. [DOI] [Google Scholar]


