Abstract
Background
Studies evaluating the role of midodrine as an adjunctive therapy to liberate patients with shock from intravenous (IV) vasopressors have yielded mixed results. The aim of our study was to evaluate the efficacy and safety of midodrine as an adjunctive therapy to liberate patients with shock from IV vasopressors.
Methods
Electronic searches of the MEDLINE, EMBASE, and Cochrane databases through April 2022 for randomized controlled trials (RCTs) that evaluated the use of midodrine versus control in patients with shock and a low dose of IV vasopressors. The primary outcome was total IV vasopressor time, while the secondary outcomes included time-to-IV vasopressor discontinuation, IV vasopressor restart, intensive care unit (ICU) length of stay (LOS), hospital LOS, and incidence of bradycardia.
Results
The final analysis included four RCTs with a total of 314 patients: 158 in the midodrine group and 156 in the control group, with a weighted mean age of 64 years (54.2% men). There was no significant difference in the total IV vasopressor time between the midodrine and control groups (standardized mean difference [SMD] − 0.53; 95% confidence interval [CI] − 1.38 to 0.32, p = 0.22; I2 = 92%). Also, there were no significant differences between the two groups in the time-to-IV vasopressor discontinuation (SMD − 0.05; 95% CI − 0.57 to 0.47, p = 0.09), IV vasopressor restart (19.3 vs. 28.3%; risk ratio [RR] 0.74; 95% 0.25–2.20, p = 0.59), ICU LOS (SMD − 0.49; 95% CI − 1.30 to 0.33, p = 0.24), and hospital LOS (SMD 0.01; 95% CI − 0.27 to 0.29, p = 0.92). However, compared with the control group, the midodrine group had a higher risk of bradycardia (15.3 vs. 2.1% RR 5.56; 95% CI 1.54–20.05, p = 0.01).
Conclusions
Among patients with vasopressor-dependent shock, midodrine was not associated with early liberation of vasopressor support or shorter ICU or hospital length of stay. Adding midodrine increased the risk of bradycardia. Further large RCTs are needed to better evaluate the efficacy and safety of midodrine in liberating patients from IV vasopressors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40119-023-00301-0.
Keywords: Midodrine, Vasopressors, Shock, Intensive care unit, ICU, Critical care
Key Summary Points
| There was no significant difference in the total IV vasopressor time among patients in the midodrine versus control groups. |
| There was no difference between either group in the time-to-IV vasopressor discontinuation, IV vasopressor restart, ICU LOS, and hospital LOS. |
| There was a higher risk of bradycardia in patients receiving midodrine compared with control group. |
| Further large RCTs are needed to better evaluate the efficacy and safety of midodrine in liberating patients off IV vasopressors. |
Introduction
Shock is one of the most common causes of intensive care unit (ICU) admissions [1, 2]; and is associated with a significant risk for mortality and morbidity [2, 3]. The management of shock is centered on achieving adequate end-organ perfusion and tissue oxygenation [3]. Intravenous (IV) vasopressors play a major role in treating patients with shock who are fluid-nonresponsive. Vasopressor dosing requirements vary according to the severity of shock [4]. Despite targeted medical therapy and resolution of culprit medical conditions, certain patients with shock remain dependent on low-dose IV vasopressors [5]. The so-called phenomenon vasoplegia has been attributed to loss of vasomotor tone and systemic vasodilatation and has been described in all types of shock [6, 7]. Prolonged use of IV vasopressors has been associated with various side effects, longer ICU stays, and increased burden on health care [1, 8–10].
It has been hypothesized that the use of oral vasopressor agents can help liberate patients from IV vasopressors, and in turn reduce the length of ICU stays. Midodrine is an orally administered peripherally acting alpha receptor stimulant which has been used for the treatment of orthostatic hypotension and is approved by the U.S. Food and Drug Administration (FDA) for that indication [11]. Few randomized controlled trials (RCTs) have evaluated midodrine use in the ICU to liberate patients from IV vasopressors and have showed mixed results [8, 12–14]. Importantly, none of these trials were sufficiently powered to detect clinical outcomes with midodrine. Therefore, we conducted a meta-analysis of randomized trials that evaluated the efficacy and safety of midodrine as an adjunctive therapy to provide hemodynamic support to wean patients from IV vasopressors.
Methods
Data Sources and Search Strategy
An electronic search of MEDLINE, EMBASE, and Cochrane databases through April 2022 was performed without using any language restrictions. We used the terms “midodrine,” “vasopressors,” “length of stay,” “intensive care unit,” or “ICU” separately and in combination to find RCTs that evaluated midodrine as an adjunctive therapy to liberate patients from IV vasopressors compared with control. We also screened prior meta-analyses and ClinicalTrials.gov for relevant studies that were not retrieved from our initial search. This study was reported according to Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines [15], and was submitted to PROSPERO registration for the current systematic review and meta-analysis (ID 328664).
Selection Criteria
We included RCTs that evaluated the role of midodrine among patients with shock and low IV vasopressors requirements (i.e., single agent IV vasopressor) compared with control group (placebo or standard of care). We excluded case–control, cohort, and non-randomized trials.
Data Extraction
Data extraction including different study features, baseline characteristics, and outcomes was performed independently by two investigators (M.H. and S.E.). We resolved any discrepancy among investigators by consensus.
Outcomes
The primary outcome was total IV vasopressor time. Secondary outcomes included: time-to-IV vasopressor discontinuation, IV vasopressor restart, ICU length of stay (LOS), hospital LOS, and incidence of bradycardia. These outcomes were reported for the longest follow-up period during the patients’ hospitalization, and on an intention-to-treat basis.
Assessment of the Quality of the Included Studies
The quality of the included studies was assessed using Cochrane risk assessment for RCTs, which embraces variable criteria; random sequence generation and allocation concealment for selection bias, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias [16]. Studies were then classified to low risk, high risk, or unclear risk of bias depending on the prior mentioned criteria (Supplementary Table 2).
Statistical Analysis
Data were analyzed primarily using a random-effects model as we anticipated heterogeneity among included studies. In order to evaluate statistical heterogeneity between the included studies, I2 statistics was used; values < 25%, 25–50%, and > 50% were considered to be low, moderate, and high degree of heterogeneity, respectively [17]. Sensitivity analyses of the primary outcome were conducted including only studies using midodrine 10 mg TID, and after excluding studies with unclear or high risk of bias. Stepwise sensitivity analysis was also conducted to explore causes of high heterogeneity in the primary study outcome. Publication bias was not assessed as the meta-analysis included a small number of studies. Outcome measures were evaluated using standardized mean difference (SMD) for continuous variables and risk ratios (RR) for categorical variables. Statistical analyses were conducted using RevMan 5.0 software (Cochrane Collaboration, Oxford, UK).
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Included Studies
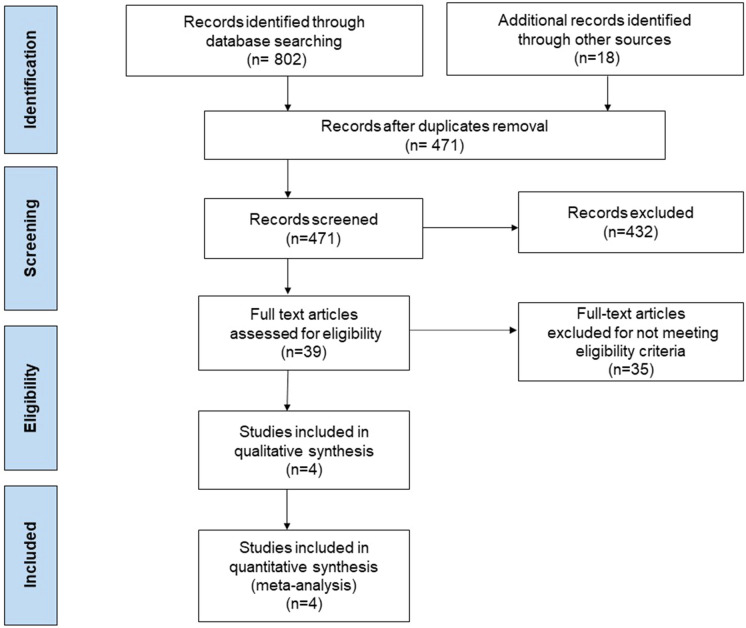
The study selection process is outlined in Fig. 1. Our final analysis included four RCTs with a total of 314 patients: 158 in the midodrine group and 156 in the control group [8, 12–14]. Baseline characteristics of the included RCTs are outlined in Table 1. The weighted mean age was 64 years and the proportion of men was 54.2%. Two studies were single center [8, 12], and two were multicenter [13, 14]. The included RCTs evaluated patients with septic shock [8], spinal shock [12], or any type of circulatory shock [13, 14], and who required low-dose IV vasopressors. The dose of midodrine in all included RCTs was 10 mg TID [8, 12, 14], except for the study by Santer et al., which used a dose of 20 mg TID [13]. The quality of involved studies was outlined in Supplementary Table 2. One study was double-blinded [13], and the other studies were open-label [8, 12, 14]. Otherwise, two studies were considered at low risk of bias [13, 14], and the other two studies were considered to have high risk and unclear risk of bias criteria (Supplementary Table 2) [8, 12].
Fig. 1.
Study flowsheet
Table 1.
Characteristics of the included studies
| Study | Publication year | No. of centers | Group 1 (midodrine) | Group 2 (control) | Type of shock | Definition of hypotension | Type of IV vasopressor | Inclusion criteria | Primary outcome | Midodrine dose |
|---|---|---|---|---|---|---|---|---|---|---|
| Costa-Pinto (MAVERICK) | 2022 | Multi-centered | 32 | 30 | Any type of shock | SBP < 90 mmHg | Norepinephrine (< 10 μg/min) or metaraminol (< 100 μg/min) | Patients with refractory hypotension on single intravenous vasopressor for more than 24 h | The primary physiological efficacy outcome measure was time to cessation of IV vasopressor use from randomization | 10 mg every 8 h |
| El Adly | 2022 | Single center | 30 | 30 | Septic shock | SBP < 90 mmHg, MAP < 65 mmHg | Norepinephrine | Patients with septic shock diagnosis on admission requiring IV vasopressor infusion for > 24 h to maintain their target arterial blood pressure goals | The primary outcome was the duration of IV norepinephrine in hours | 10 mg every 8 h |
| Ali | 2022 | Single center | 30 | 30 | Spinal shock | SBP < 90 mmHg | Norepinephrine (< 8 mcg/min) | Patients diagnosed with spinal shock and in the recovery stage, hemodynamically stable, and have stable blood pressure on single IV vasopressor | The primary outcome was shortening the duration of IV vasopressor requirements | 10 mg every 8 h |
| Santer (MIDAS) | 2020 | Multi-centered | 66 | 66 | Any type of shock | – | Phenylephrine (< 100 mcg/min), norepinephrine (< 8 mcg/min), or metaraminol (< 60 mcg/min) | Hypotensive patients requiring single-agent intravenous vasopressor treatment for at least 24 h and were unable to be liberated from IV vasopressor | The primary outcome was length of time, measured in hours, from study drug initiation until discontinuation of intravenous vasopressors | 20 mg every 8 h |
SBP systolic blood pressure, MAP mean arterial blood pressure, IV Intravenous, h hours
Primary Outcome
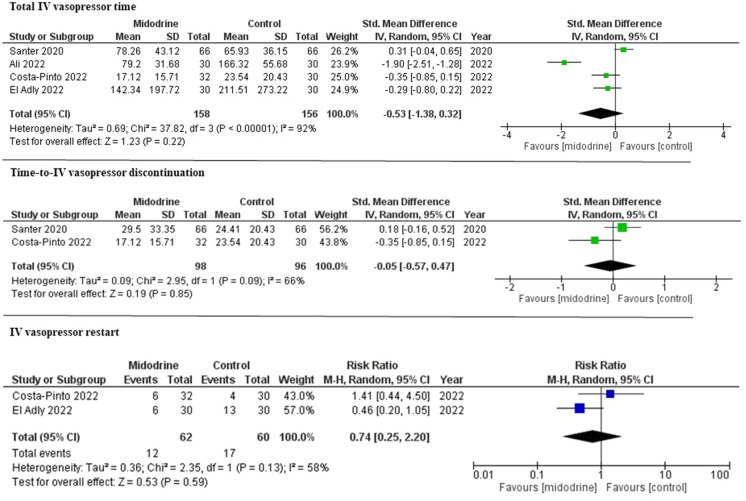
The primary outcome of total IV vasopressor time was reported in all included RCTs [8, 12–14]. Analysis showed no difference in the midodrine group compared with the control group (SMD − 0.53; 95% confidence interval [CI] − 1.38 to 0.32, p = 0.22; I2 = 92%) (Fig. 2). Stepwise sensitivity analyses were conducted to evaluate the source of heterogeneity by excluding one study at a time. After excluding the study with the highest contribution to heterogeneity, similar results were demonstrated (SMD − 0.08; 95% CI − 0.53 to 0.38, p = 0.74; I2 = 68%) (Supplementary Fig. 1). Another sensitivity analysis excluding studies with high risk of bias showed similar results (SMD 0.01; 95% CI − 0.64 to 0.65, p = 0.99; I2 = 78%) (Supplementary Fig. 1). Additional sensitivity analysis including only studies that used midodrine 10 mg TID showed similar results (SMD − 0.83; 95% CI − 1.79 to 0.13, p = 0.09; I2 = 89%) (Supplementary Fig. 1).
Fig. 2.
Forest plot for total IV vasopressor time, time-to-IV vasopressor discontinuation, and IV vasopressor restart between midodrine and control groups
Secondary Outcomes
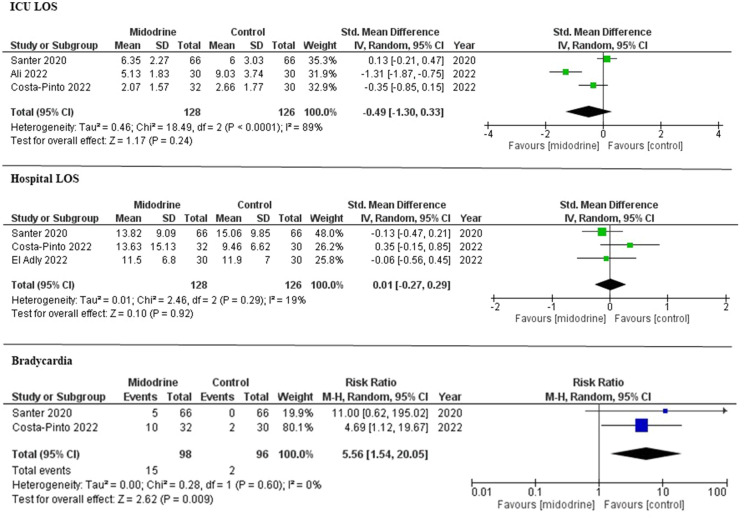
There was no significant difference between the midodrine group and the control group in time-to-IV vasopressor discontinuation (SMD − 0.05; 95% CI − 0.57 to 0.47, p = 0.85; I2 = 66%), IV vasopressor restart (19.3 vs. 28.3%; risk ratio [RR] 0.74; 95% 0.25–2.20, p = 0.59; I2 = 58%), ICU LOS (SMD − 0.49; 95% CI − 1.30 to 0.33, p = 0.24; I2 = 89%), and hospital LOS (SMD 0.01; 95% CI − 0.27 to 0.29, p = 0.92; I2 = 19%). Compared with the control group, the midodrine group had higher risk of bradycardia (15.3 vs. 2.1% RR 5.56; 95% CI 1.54–20.05, p = 0.01; I2 = 0%) (Figs. 2 and 3).
Fig. 3.
Forest plot for ICU LOS, hospital LOS, and bradycardia between midodrine among midodrine versus control groups
Discussion
In this meta-analysis of four RCTs including 314 patients [8, 12–14], we evaluated the efficacy and safety of midodrine as an adjunctive therapy to liberate patients from IV vasopressors. The salient findings of this study are: (1) there was no significant difference in the total IV vasopressor time among patients in the midodrine versus control groups; (2) there was no difference between both groups in the time-to-IV vasopressor discontinuation, IV vasopressor restart, ICU LOS, and hospital LOS; (3) there was a higher risk of bradycardia in patients receiving midodrine compared with the control group (Table 2).
Table 2.
Baseline characteristics of the study population
| Costa-Pinto (MAVERICK) | El Adly | Ali | Santer (MIDAS) | |
|---|---|---|---|---|
| Age in years | ||||
| Midodrine | 61.5 [53, 72]b | 61.8 ± 15.1a | – | 70 ± 12.6a |
| Control | 64 [51, 76.75]b | 59.8 ± 15.1a | – | 66.7 ± 14.7a |
| Male % | ||||
| Midodrine | 68.8 | 50 | – | 54.5 |
| Control | 60 | 43.3 | – | 48.5 |
| Weight (kg) | ||||
| Midodrine | 71 [65, 80]b | 82.9 ± 14.5a | – | 78.6 ± 22.4a |
| Control | 80 [71, 88.75]b | 84.4 ± 15.1a | – | 82.3 ± 23.5a |
| APACHE score | ||||
| Midodrine | 49.5 [41, 56.25]b (APACHE III) | 24 [13–39]b (APACHE II) | – | 14.7 ± 5.2a (APACHE II) |
| Control | 48.5 [38.25, 58]b (APACHE III) | 21.5 [7–39]b (APACHE II) | – | 14.8 ± 5.9a (APACHE II) |
| Diabetes % | ||||
| Midodrine | 6.2 | 53.3 | – | – |
| Control | 0 | 30 | – | – |
| Hypertension % | ||||
| Midodrine | 37.5 | 63.3 | – | – |
| Control | 33.3 | 56.7 | – | – |
| HR (beats per minute) | ||||
| Midodrine | 76 [70, 85]b | 110 [72–130]b | 120.53 ± 11.91a | – |
| Control | 77.5 [65.5, 85]b | 115 [79–148]b | – | – |
| MAP (mmHg) | ||||
| Midodrine | 72 [70, 80]b | 60 [50–75]b | 65.83 ± 20.29a | 75.9 ± 9.4 a |
| Control | 75 [70, 75.75]b | 70 [47–73]b | 62.87 ± 19.21a | 72.8 ± 8.2a |
IQR interquartile range, SD standard deviation, HR heart rate, MAP mean arterial blood pressure, APACHE score Acute Physiology and Chronic Health Evaluation Score
aMean (SD)
bMedian (IQR)
The biggest challenge in the resolution of shock is the persistence state of vasoplegia or vasodilatory shock. Vasoplegia includes activation of intrinsic vasodilatory pathways and decreased vascular responsiveness to vasopressors that could lead to inability to wean patients off pressors [6, 7]. Norepinephrine is still the first-line agent for vasoplegia, but it requires continuous ICU monitoring that leads to prolonged ICU stays [6]. A lot of interest has been directed towards using midodrine, an oral alpha-1 agonist, in patients with resolving shock who remain vasopressor-dependent, with the goal of liberating patients from IV pressors [18–22]. Midodrine is approved for the treatment of a variety of conditions, including orthostatic hypotension, neurogenic hypotension [11], and hypotension caused by carotid artery stenting [23]. However, it has been used lately as an off-label drug in treatment of other conditions such as dialysis induced hypotension, cirrhosis, ascites, and hepatorenal syndrome [4, 24]. Midodrine’s therapeutic effects are mediated via its metabolite desglymidodrine, which acts on stimulating peripheral arterial and venous alfa receptors, causing vasoconstriction, and increasing blood pressure. The most common complication of midodrine therapy is supine hypertension. Its additional side effects include pruritis, urinary retention, and bradycardia. However, these side effects only occur in less than 5% of patients [14, 24, 25]. Midodrine has previously demonstrated significant dose-dependent increases in systolic blood pressure [25], but has not been proven to play a role in the resolution of shock or discontinuing IV vasopressors.
Few randomized trials have been conducted to evaluate the role of midodrine in patients with vasopressor-dependent shock, and these showed conflicting results. Both Santer et al. and Costa-Pinto et al. demonstrated that midodrine failed to show significant benefit on IV vasopressor discontinuation, but also increased the rate of bradycardia [13, 14]. Ali et al. established that oral vasopressors (midodrine and mirinin) reduced IV vasopressor duration and promoted early discharge from the ICU [12]. El Adly et al. showed that midodrine decreased IV vasopressor duration, but did not alter ICU LOS [8]. However, none of these studies had enough power to detect true differences with the use of midodrine. In the current analysis, we pooled the data for available RCTs, and demonstrated no significant difference with midodrine versus control in the primary outcome of total IV vasopressor time. Similarly, the use of midodrine did not impact the time-to-IV vasopressor discontinuation, IV vasopressor restart, or LOS of included patients. Exploratory analysis showed no difference in results according to the dose of midodrine in included studies. Our study results are in accordance with a prior meta-analysis of observational studies by Al-Abdouh et al., who also demonstrated that the use of midodrine was not associated with significant difference in ICU LOS, hospital LOS, mortality, or ICU readmissions [26].
In the current analysis, the lack of efficacy of midodrine in liberating patients from IV vasopressors might be explained by a few factors. First, in the resolution phase of shock, there is inappropriate activation of the sympathetic system that is associated with receptor desensitization, which in turn can alter the efficacy of midodrine [27]. Second, it is plausible that higher and/or more frequent dosing of midodrine might be required to achieve vasomotor effects sufficient to wean IV vasopressors. This is particularly relevant given the short half-life of the active metabolite of midodrine, desglymidodrine, which is approximately 3–4 h [28–30]. However, the observed bradycardia among patients assigned to midodrine, which was more profound in the trial using the 20-mg TID dosing, could be a limitation to using higher or more frequent dosing of midodrine in patients with shock [13].
The current meta-analysis includes the totality of available randomized data evaluating the role of midodrine in liberating patients from IV vasopressors. Our findings demonstrated that the use of adjuvant midodrine therapy with IV vasopressors had no effect on the duration of IV vasopressor use or the length of hospital and ICU stays. However, its use carries a significant risk of bradycardia. Our study findings do not support the routine use of midodrine as an adjunctive agent to liberate patients from IV vasopressors. Nevertheless, given the small number of available trials, further randomized trials with larger sample sizes remain warranted to characterize the role of midodrine in weaning patients off IV vasopressors.
Limitations
This meta-analysis has several limitations. First, despite including totality of randomized data in the study topic, the available trials were small-sized, and hence our study results might still be underpowered for the main study outcomes. Second, there was considerable heterogeneity in some of the study outcomes. However, we have adopted random effects models to mitigate the expected heterogeneity. Furthermore, we conducted exploratory analysis to identify studies contributing to heterogeneity in the primary outcome. Third, the lack of patient-level data precluded more granular analysis.
Conclusions
This meta-analysis of randomized trials demonstrated that among patients with shock and low-dose IV vasopressors, midodrine did not offer benefit in discontinuing IV vasopressors or reducing length of stays, and may even be associated with a higher risk of bradycardia. Further large randomized controlled trials are needed to better characterize the role of midodrine in patients with shock to wean off IV vasopressors.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors Contributions
All authors, including Mohamed Hamed, Sheref A. Elseidy, Ahmed Elkheshen, Jamal Maher, Adel Elmoghrabi, Ahmed Zaghloul, Andrew Panakos, Sidakpal Panaich, Marwan Saad, and Ayman Elbadawi contributed to the study conception and design, material preparation, data collection, statistical analysis, writing the article, critical revision of the article, and final approval of the article.
Disclosures
Mohamed Hamed, Sheref A. Elseidy, Ahmed Elkheshen, Jamal Maher, Adel Elmoghrabi, Ahmed Zaghloul, Andrew Panakos, Sidakpal Panaich, Marwan Saad, and Ayman Elbadawi have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
- 1.Tchen S, Sullivan JB. Clinical utility of midodrine and methylene blue as catecholamine-sparing agents in intensive care unit patients with shock. J Crit Care. 2020;57:148–156. doi: 10.1016/j.jcrc.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Scheeren TWL, Bakker J, De Backer D, et al. Current use of vasopressors in septic shock. Ann Intensive Care. 2019;9(1):20. doi: 10.1186/s13613-019-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 4.Anstey MH, Wibrow B, Thevathasan T, et al. Midodrine as adjunctive support for treatment of refractory hypotension in the intensive care unit: a multicenter, randomized, placebo controlled trial (the MIDAS trial) BMC Anesthesiol. 2017;17(1):47. doi: 10.1186/s12871-017-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine AR, Meyer MJ, Bittner EA, et al. Oral midodrine treatment accelerates the liberation of intensive care unit patients from intravenous vasopressor infusions. J Crit Care. 2013;28(5):756–762. doi: 10.1016/j.jcrc.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Levy B, Fritz C, Tahon E, et al. Vasoplegia treatments: the past, the present, and the future. Crit Care. 2018;22(1):52. doi: 10.1186/s13054-018-1967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimmoun A, Ducrocq N, Levy B. Mechanisms of vascular hyporesponsiveness in septic shock. Curr Vasc Pharmacol. 2013;11(2):139–149. [PubMed] [Google Scholar]
- 8.Adly DHE, Bazan NS, El Borolossy RM, et al. Midodrine improves clinical and economic outcomes in patients with septic shock: a randomized controlled clinical trial. Ir J Med Sci. 2022;191:2785–2795. doi: 10.1007/s11845-021-02903-w. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw SM, Tran DT, Opgenorth D, et al. Assessment of costs of avoidable delays in intensive care unit discharge. JAMA Netw Open. 2020;3(8):e2013913–e2013913. doi: 10.1001/jamanetworkopen.2020.13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aygencel G, Turkoglu M. Characteristics, outcomes and costs of prolonged stay ICU patients. Turk J Med Surg Intensive Care. 2011;2:53–58. doi: 10.5152/dcbybd.2011.12. [DOI] [Google Scholar]
- 11.Low PA, Gilden JL, Freeman R, et al. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA. 1997;277(13):1046–1051. doi: 10.1001/jama.1997.03540370036033. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed Ali AT, Abd El-Aziz MA, Mohamed Abdelhafez A, et al. Effect of oral vasopressors used for liberation from intravenous vasopressors in intensive care unit patients recovering from spinal shock: a randomized controlled trial. Crit Care Res Pract. 2022;2022:6448504. doi: 10.1155/2022/6448504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santer P, Anstey MH, Patrocinio MD, et al. Effect of midodrine versus placebo on time to vasopressor discontinuation in patients with persistent hypotension in the intensive care unit (MIDAS): an international randomised clinical trial. Intensive Care Med. 2020;46(10):1884–1893. doi: 10.1007/s00134-020-06216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa-Pinto R, Yong ZT, Yanase F, et al. A pilot, feasibility, randomised controlled trial of midodrine as adjunctive vasopressor for low-dose vasopressor-dependent hypotension in intensive care patients: the MAVERIC study. J Crit Care. 2022;67:166–171. doi: 10.1016/j.jcrc.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Luka B, Kolli R, et al. Use of oral midodrine in weaning-off intravenous vasopressors in patients with septic shock. 2010.
- 19.Hailu K, Allen B, Rottman-Pietrzak K, et al. 15010: impact of adjuvant use of midodrine in septic patients receiving vasopressors in the ICU setting. Crit Care Med. 2020;48(1):771–771. doi: 10.1097/01.ccm.0000648268.34861.39. [DOI] [Google Scholar]
- 20.Fiorenza M, Barkes J, Naik S. 924: adjunctive oral midodrine use in critically ill patients. Crit Care Med. 2020;48:441. doi: 10.1097/01.ccm.0000633232.77165.17. [DOI] [Google Scholar]
- 21.Roach E, Adie S, Gowan M, et al. 200: impact of oral midodrine on duration of intravenous vasopressor therapy. Crit Care Med. 2018;46(1):82. doi: 10.1097/01.ccm.0000528219.66628.6d. [DOI] [Google Scholar]
- 22.Nadhim A, Tun Z, Chua LC, et al. The impact of midodrine on duration of vasopressor infusion in patents with septic shock in a community hospital setting. Chest. 2019;156(4):A1584. doi: 10.1016/j.chest.2019.08.1396. [DOI] [Google Scholar]
- 23.Sharma S, Lardizabal JA, Bhambi B. Oral midodrine is effective for the treatment of hypotension associated with carotid artery stenting. J Cardiovasc Pharmacol Ther. 2008;13(2):94–97. doi: 10.1177/1074248408317709. [DOI] [PubMed] [Google Scholar]
- 24.Whitson MR, Mo E, Nabi T, et al. Feasibility, utility, and safety of midodrine during recovery phase from septic shock. Chest. 2016;149(6):1380–1383. doi: 10.1016/j.chest.2016.02.657. [DOI] [PubMed] [Google Scholar]
- 25.Poveromo LB, Michalets EL, Sutherland SE. Midodrine for the weaning of vasopressor infusions. J Clin Pharm Ther. 2016;41(3):260–265. doi: 10.1111/jcpt.12375. [DOI] [PubMed] [Google Scholar]
- 26.Al-Abdouh A, Haddadin S, Matta A, et al. Impact of adjuvant use of midodrine to intravenous vasopressors: a systematic review and meta-analysis. Crit Care Res Pract. 2021;2021:5588483. doi: 10.1155/2021/5588483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Sainz JA, Vazquez-Prado J, del Carmen ML. Alpha 1-adrenoceptors: function and phosphorylation. Eur J Pharmacol. 2000;389(1):1–12. doi: 10.1016/S0014-2999(99)00896-1. [DOI] [PubMed] [Google Scholar]
- 28.Wright RA, Kaufmann HC, Perera R, et al. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology. 1998;51(1):120–124. doi: 10.1212/WNL.51.1.120. [DOI] [PubMed] [Google Scholar]
- 29.Zachariah PK, Bloedow DC, Moyer TP, et al. Pharmacodynamics of midodrine, an antihypotensive agent. Clin Pharmacol Ther. 1986;39(5):586–591. doi: 10.1038/clpt.1986.101. [DOI] [PubMed] [Google Scholar]
- 30.Grobecker H, Kees F, Linden M, et al. The bioavailability of midodrin and alpha-2,5-dimethoxyphenyl-beta-aminoethanol hydrochloride. Arzneimittelforschung. 1987;37(4):447–450. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.