Introduction
Ovarian mature cystic teratoma (OMCT) arise from defective germ cells with failed meiosis I/II and form the commonest ovarian germ cell neoplasm in women < 40 years [1]. Somatic malignant transformation (MT) in OMCT, hereafter referred to as transformed OMCT (tOMCT), occurs only in 1–3% of cases. Here, we present our 4-year experience in this rare entity.
Case 1
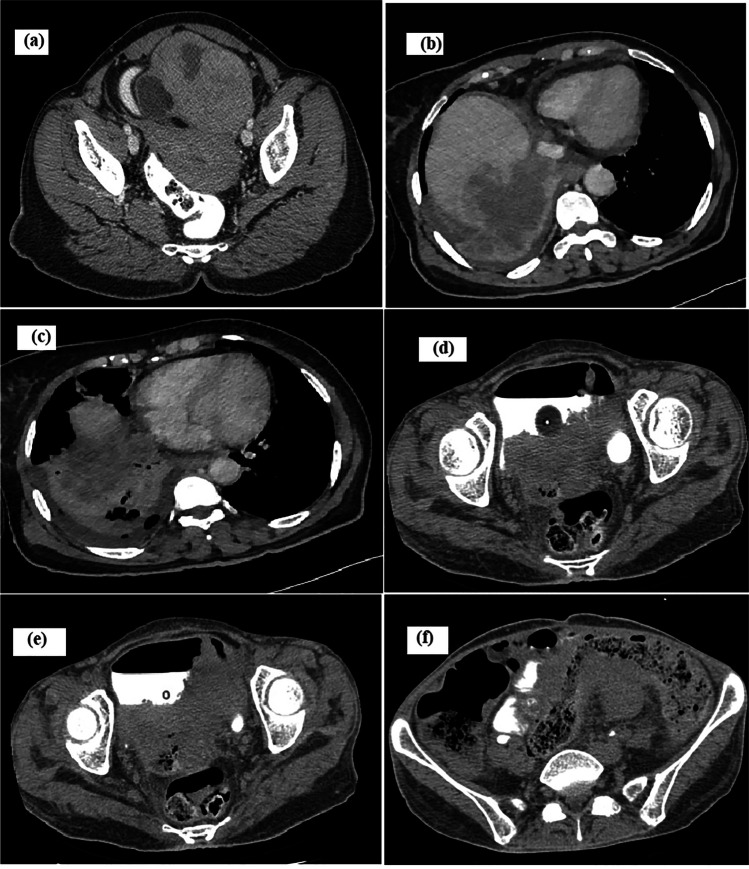
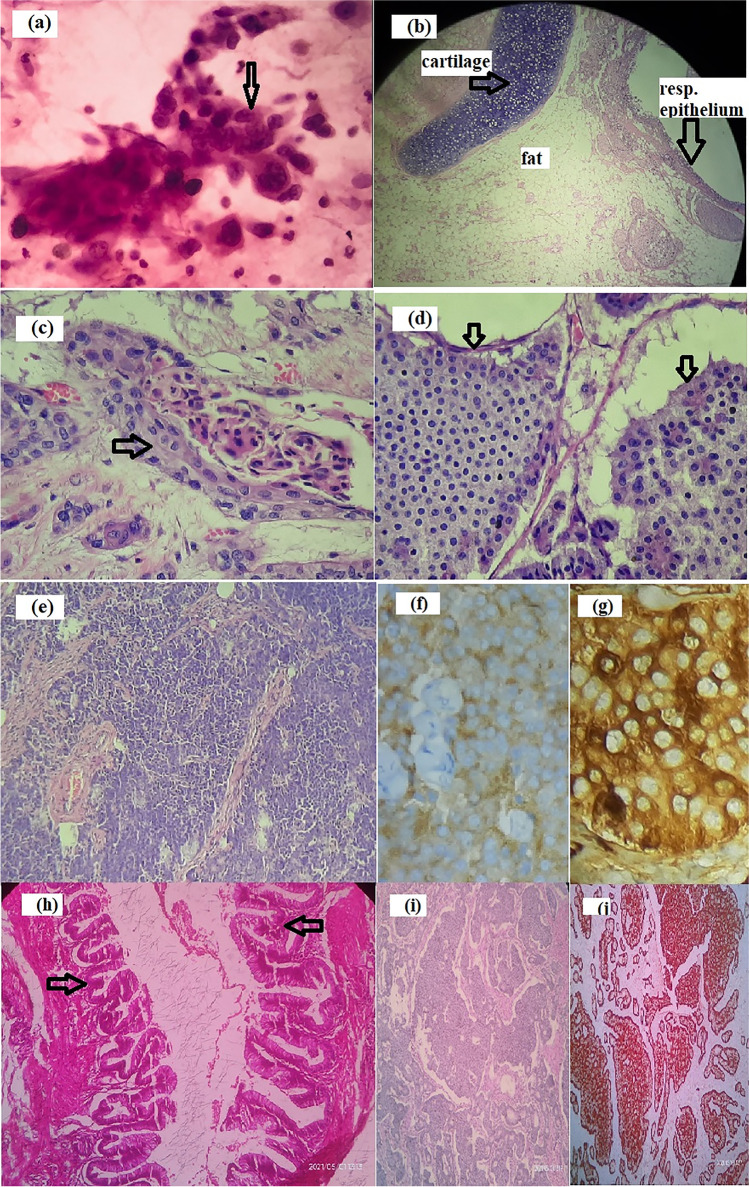
A 64-year-old lady, P3L3, presented with abdominal pain and early satiety of 5 months duration. She denied having bleeding per vaginum, dyspnea, weight loss, or other systemic symptoms. She reported no addictions/drug allergies and was on regular medications for hypothyroidism and hypertension. At presentation, she was of Eastern Cooperative Oncology Group Performance Status (ECOG PS) 1. A firm mass of size 20 × 15 cm was filling the infra-umbilical quadrants and a pouch of Douglas (POD) deposit was appreciated. Contrast-enhanced computed tomography (CECT) of the abdomen/pelvis showed heterogeneous enhancing 15.5 × 13.7 × 9.5 cm left adnexal mass with solid areas, irregular fluid/fat densities and macrocalcifications probably malignant teratoma, along with POD mass and multiple enlarged para-aortic nodes (Fig. 1a). Her CA-125 was 250 U/ml and CEA was 7.6 ng/ml. Fine needle aspiration cytology from POD suggested adenocarcinoma (Fig. 2a). The multidisciplinary tumour board decided for neoadjuvant chemotherapy with a provisional diagnosis of advanced carcinoma ovary. Post 6 cycles of taxane/carboplatin neoadjuvant chemotherapy, total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO), omentectomy, para-aortic lymph node debulking, resection of the involved ileum and bowel anastomosis was done. Grossly, the left adenexal mass measured 20 × 16 × 4 cm, which on microscopy showed moderately differentiated SCC and well-differentiated neuroendocrine tumour arising from a left OMCT (Fig. 2b–d). The SCC component infiltrated the peri-intestinal fat and muscularis propria. Lymphovascular emboli were seen. Three out of five para-aortic lymph nodes showed metastasis from neuroendocrine tumour, which were immunoreactive for synaptophysin and chromogranin (Fig. 2e–g). The MIB was 5–8%.
Fig. 1.
Radiology images. a Contrast-enhanced computed tomography (CECT) of the abdomen/pelvis showed heterogeneous enhancing left adnexal mass with solid areas, fluid/fat densities, and macrocalcifications. b CECT of the thorax/abdomen showing rupture of right liver lobe lesion into the right pleural cavity. c CECT of the thorax/abdomen showing necrotic bilobar liver lesions. d–f CT of the abdomen/pelvis showing a large irregular cavitating pelvic mass containing air pockets with vaginal infiltration (d), bladder infiltration (e), and rectosigmoid infiltration (f)
Fig. 2.
Histopathology images. a Cytology smear showing atypical cells in clusters suggestive of adenocarcinoma. b Ovarian mature teratoma showing cartilage, fat, and respiratory epithelium (H&E). c Ovarian mature teratoma with squamous cell carcinoma component (H&E, × 40). d Teratoma with neuroendocrine tumour (H&E, × 40). e Lymph node metastases with neuroendocrine tumour (H&E, × 40). f Immunohistochemistry (IHC) showing synaptophysin positivity in neuroendocrine component (IHC, × 40). g IHC showing chromogranin positivity in neuroendocrine component (IHC, × 40). h Borderline mucinous tumour with atypically proliferating mucinous cells in papillary pattern (H&E, × 40). i Neoplastic cells in tubular and rosettoid pattern suggestive of carcinoid (H&E, × 40). j IHC showing chromogranin positivity in carcinoid areas
Within 2 months, she progressed with abdominal symptoms. CECT of the thorax/abdomen showed necrotic bilobar liver lesions with rupture of right lobe lesion into the right pleural cavity, multiple nodular lung lesions, periportal/pericardiophrenic nodes, and multiple deposits in gastrohepatic ligament/mesentery (Fig. 1b, c). Liver biopsy showed metastasis from SCC. The patient opted for best supportive care and succumbed to death after 1 month.
Case 2
A 47-year-old postmenopausal lady, P3L3, with no comorbidities, presented to us with abdominal pain/distension of 6 months duration. Magnetic resonance imaging (MRI) of the abdomen revealed a left ovarian encapsulated, thin-walled lesion measuring 18 × 17 × 11 cm with enhancing irregular internal septae and loculations. Her ECOG PS was 2 and she underwent TAH BSO, appendicectomy, and infracolic omentectomy. The 15 × 15 × 10 cm left ovarian mass was patholocally suggestive of OMCT with MT into SCC with vascular invasion. The omentum was involved with a metastatic SCC deposit. She defaulted after receiving 2 cycles of ifosfamide/cisplatin and unfortunately, presented after 4 months in a moribund state with hypotension. On clinical examination, she had rectovaginal fistula. CT of the abdomen/pelvis showed a large irregular cavitating pelvic mass of size 12 × 12 × 9 cm, with air pockets inside. The mass was in continuity with vaginal lumen. It infiltrated rectosigmoid superiorly and bladder inferiorly with fistula formation and intravesical extension (Fig. 1d–f). The small bowel loops also had fistulous communication to the mass at multiple sites. The patient was managed conservatively and she expired after 2 weeks.
Case 3
A 51-year-old postmenopausal lady, P2L2, sought medical attention for dull aching pain in the right loin of 1-month duration. A mass was palpable in the right iliac fossa/hypogastrium of 12-week size. MRI of the abdomen/pelvis revealed a large thick-walled complex right adnexal lesion of 13.8 × 11.2 × 9.8 cm. Her CA-125 was 31.3 IU/ml. She underwent TAH BSO omentectomy and peritoneal fluid cytology and got referred to us. Intraoperatively, the cyst was ruptured. Histopathological review showed right OMCT with moderately differentiated SCC. After 6 cycles of platinum-based chemotherapy (TIP), she is clinically well and CT of the abdomen/pelvis after 3 years shows no evidence of recurrence.
Case 4
A 57-year-old lady, diabetic and hypertensive on regular medicine, was evaluated for lower abdominal pain of 3 months duration. Her CT of the abdomen/pelvis showed right adnexal mass measuring 17.5 × 11.6 × 25 cm (with features of torsion) and a left adnexal mass measuring 11.4 × 10.4 × 10.8 cm. Histopathology of TAH BSO specimen showed left OMCT with pseudomyxoma ovarii, low-grade mucinous tumour, and insular carcinoid (Fig. 2h–j). Chromogranin was immunoreactive and MIB1 labelling index was 1%. Other ovary showed gangrenous cyst with features of torsion. She is under follow-up and is recurrence free at 6 months of follow-up.
Discussion
Pure OMCT is the most common ovarian germ cell neoplasm. Transformation in OMCT is mostly in germ lineage. A dreaded somatic MT can happen in 1–3% of cases; thereby, the benign disease gets transformed into a much lethal disease termed tOMCT [1].
As per the Indian study by Rathore et al., the MT risk was 3.5% (8/230), whereas other studies find these percentages as 0.6%, 5%, and 6.67% [1, 2]. It is mostly seen in postmenopausal women [3]. The mean age of patients with tOMCT as per Rathore et al. was 44.2 ± 8.94 years and in a much larger study by Hackethal et al., the median age was 55 years [1, 4]. In our series also, all 4 patients were postmenopausal and the median age was 54 years, in accordance with the above studies.
Prolonged carcinogen exposure in the pelvic cavity and human papilloma virus infection are proposed as causative factors for MT in an existing unintervened OMCT [5]. The most common somatic MT is to SCC (80%), followed by adenocarcinoma, adeno-squamous carcinoma, transitional cell carcinoma, malignant melanoma, neuroendocrine carcinomas, and very rarely carcinoids [6]. In our series, the MTs observed in patient 1 were SCC, adenocarcinoma, and well-differentiated neuroendocrine tumour, whereas in patients 2 and 3, it was SCC and in patient 4, it was low-grade mucinous tumour and insular carcinoid.
Lack of unique symptoms in OMCT patients harbouring MT makes its preoperative diagnosis impossible. Main symptoms are abdominal fullness/pain, pressure-related symptoms, and rarely, acute abdominal pain due to torsion. In our study, all 4 patients presented with abdominal pain and/or distension among which one had torsion.
The risk of MT is directly proportional to patient’s age and tumour size. In our series, all patients had a tumour volume of 1000 cm3 and above. Advanced age along with elevated SCC antigen ((SCCAg, > 2.5 ng/ml),CA125,CA19-9,CEA, and macrophage colony-stimulating factor) might hint MT in OMCT [4]. Although the diagnostic role of SCCAg is debated, high SCCAg was correlated with adverse prognosis rather than disease stage in a study [4]. Radiological characteristics in benign and tOMCT are almost similar, but areas of haemorrhage/necrosis, bigger tumour, adhesions, solid/firm, friable, myxomatous, or variegated areas should arouse suspicion of MT in OMCT [4].
Poor prognostic factors of the disease are stage (most important), presence of additional invasive tumour subtypes, ascites, capsule invasion, vascular invasion, rupture, and adhesion [3]. The poor prognostic findings in our series were local infiltration (patient 1), lymphovascular invasion (patients 1 and 2), node positivity/higher stage (patients 1 and 2), and tumour rupture (patient 3).
The surgical management with TAH BSO, omentectomy, and/or retroperitoneal lymphadenectomy is done for complete surgical staging and optimal cytoreduction. Frozen-section is strongly considered as the reported yield in detecting MT is 100% as per Black et al. [7]. Intraoperative tumour spillage is associated with tumour recurrence [8]. The 2-year survival and 5-year survival in those with stage I are 95% and in those with stage II and above, it is 0–30% and 0%, respectively [3].
The optimal adjuvant therapy for tOMCT is still unclear. Patients who received adjuvant chemotherapy survived better than those who did not [3, 9]. Platinum/taxane combination chemotherapy is the most common adjuvant chemotherapeutic agent received by such patients [10]. From a larger review on 119 patients with tOMCT, other regimens used were anthracyclines, antimetabolites, vinca alkaloids, and alkylating drugs [4]. Despite optimal surgery and adjuvant chemotherapy, this disease carries high mortality.
Our first patient was provisionally diagnosed as advanced adenocarcinoma ovary and received neoadjuvant chemotherapy with taxane/carboplatin regimen. Post neoadjuvant chemotherapy, she underwent interval cytoreduction and histopathology showed OMCT with SCC and well-differentiated neuroendocrine tumour. It is possible that the chemotherapy would have acted upon the adenocarcinoma component, but within a short span of time, she developed extensive supradiaphragmatic visceral/nodal metastasis proving the aggressive nature of the disease. Patient 2 had vascular invasion and omental involvement in the surgically excised specimen. She defaulted after 2 cycles of chemotherapy and presented with extensive inter-organ abdominal fistulous communications, hinting towards the adhesive and local infiltrative nature of the disease. The third patient in our series had an early disease and is disease free for 3 years after adjuvant chemotherapy in spite of intraoperative tumour rupture. The fourth patient, in whom MT to focal pseudomyxoma ovarii, low-grade mucinous tumour, and insular carcinoid was seen, is disease free at short-term follow-up (Table 1).
Table 1.
Patient characteristics, treatment and outcome of the 4 patients with ovarian mature cystic teratoma harbouring malignant transformation
| Details | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age (years) | 64 | 47 | 51 | 57 |
| Symptom/duration | Abdominal pain, 5 months | Abdominal pain and distension, 6 months | Abdominal pain, 1 month | Abdominal pain, 3 months |
| Imaging features | Ovarian teratoma | Ovarian mass with enhancing septae | Thick-walled ovarian lesion | Complex ovarian lesion |
|
S. CA-125 (0–35 U/ml) |
250 U/ml | Not done | 31.3 U/ml | Not done |
| Preop diagnosis | Adenocarcinoma ovary | Abdominopelvic mass | Abdominopelvic mass | Abdominopelvic mass |
| Surgery | TAH, BSO, omentectomy, para-aortic lymph node debulking, resection of the involved ileum and bowel anastomosis | TAH BSO, appendicectomy, and infracolic omentectomy | TAH BSO, omentectomy | TAH BSO |
| Histopathology | OMCT with adenocarcinoma, SCC, well-differentiated neuroendocrine tumour | OMCT with SCC | OMCT with SCC | OMCT with low-grade mucinous tumour, insular carcinoid |
| Poor risk factors | Local infiltration, LVI, node positivity | Vascular invasion, higher stage | Tumour rupture | Nil |
| Adjuvant treatment | Taxane/platinum 6 cycles | Ifosfamide/platinum. Defaulted after 2 cycles | TIP | Nil |
| Outcome | Dead | Dead | No evidence of disease at 3 years | No recurrence at 6 months follow-up |
Abbreviations: MT malignant transformation, TAH total abdominal hysterectomy, BSO bilateral salpingo-oophorectomy, OMCT ovarian mature cystic teratoma, SCC squamous cell carcinoma, LVI lymphovascular invasion, TIP paclitaxel, ifosfamide, cisplatin
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Previous Publications/Presentations
Nil.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Concluding Points
• MT arising from OMCT is a rare, aggressive malignancy with dismal outcome.
• Early detection and early FIGO stage carries better prognosis.
• Less diagnosed preoperatively than pathological. High index of suspicion and thorough histological examination is crucial in diagnosis.
• Optimal cytoreduction is the surgical dictum.
• Combined platinum/taxane chemotherapy seems to improve survival in advanced-stage disease.
References
- 1.Rathore R, Sharma S, Agarwal S. Malignant transformation in mature cystic teratoma of the ovary: a retrospective study of eight cases and review of literature. Prz Menopauzalny. 2018;17(2):63–68. doi: 10.5114/pm.2018.77304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim MJ, Kim NY, Lee D-Y, Yoon B-K, Choi D. Clinical characteristics of ovarian teratoma: age-focused retrospective analysis of 580 cases. Am J Obstet Gynecol. 2011;205(1):32.e1–4. doi: 10.1016/j.ajog.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 3.Tehranian A, Ghahghaei-Nezamabadi A, Seifollahi A, Kasraei S, Dehghani-Nejad H, Maleki-Hajiagha A. Ovarian mature cystic teratoma with malignant transformation: two case reports. J Med Case Reports. 2021;15(1):23. doi: 10.1186/s13256-020-02594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hackethal A, Brueggmann D, Bohlmann MK, Franke FE, Tinneberg H-R, Münstedt K. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol. 2008;9(12):1173–1180. doi: 10.1016/S1470-2045(08)70306-1. [DOI] [PubMed] [Google Scholar]
- 5.Chiang AJ, Chen MY, Weng CS, Lin H, Lu CH, Wang PH, et al. Malignant transformation of ovarian mature cystic teratoma into squamous cell carcinoma: a Taiwanese Gynecologic Oncology Group (TGOG) study. J Gynecol Oncol. 2017;28(5):e69. doi: 10.3802/jgo.2017.28.e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das P, Sable M, Nath D, Chumbar S, Das C, Priyadarshini P, et al. Pelvic mature cystic teratoma with neuroendocrine carcinoma: report of a rare association and review of literature. Indian J Pathol Microbiol. 2014;57(1):113–115. doi: 10.4103/0377-4929.130916. [DOI] [PubMed] [Google Scholar]
- 7.Black JD, Roque DM, Pasternak MC, Buza N, Rutherford TJ, Schwartz PE, et al. A series of malignant ovarian cancers arising from within a mature cystic teratoma: a single institution experience. Int J Gynecol Cancer. 2015;25(5):792–797. doi: 10.1097/IGC.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 8.Wen K-C, Hu W-M, Twu N-F, Chen P, Wang P-H. Poor prognosis of intraoperative rupture of mature cystic teratoma with malignant transformation. Taiwan J Obstet Gynecol. 2006;45(3):253–256. doi: 10.1016/S1028-4559(09)60236-9. [DOI] [PubMed] [Google Scholar]
- 9.Park J-Y, Kim D-Y, Kim J-H, Kim Y-M, Kim Y-T, Nam J-H. Malignant transformation of mature cystic teratoma of the ovary: experience at a single institution. Eur J Obstet Gynecol Reprod Biol. 2008;141(2):173–178. doi: 10.1016/j.ejogrb.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Sakuma M, Otsuki T, Yoshinaga K, Utsunomiya H, Nagase S, Takano T, et al. Malignant transformation arising from mature cystic teratoma of the ovary: a retrospective study of 20 cases. Int J Gynecol Cancer. 2010;20(5):766–771. doi: 10.1111/igc.0b013e3181daaf1d. [DOI] [PubMed] [Google Scholar]