Abstract
The mitogen-activated protein kinases (MAPKs) are signaling molecules that become enzymatically activated through phosphorylation by diverse stimuli. Hematopoietic cytokines, growth factors, and stimulated lymphocyte antigen receptors may activate specific MAPKs by altering the balance of MAPK-activating protein kinases and the protein phosphatases that target their activation sites. Hematopoietic protein tyrosine phosphatase (HePTP) is a hematopoiesis-specific cytoplasmic protein tyrosine phosphatase whose expression is induced by mitogenic stimuli. To investigate the role of HePTP in hematopoietic development, we constructed mice deficient in this phosphatase using the technique of homologous recombination. Primary lymphocytes from HePTP−/− mice show enhanced activation of extracellular stimulus-regulated kinase (ERK) after both phorbol myristate acetate (PMA) and anti-CD3-mediated T-cell receptor (TCR) stimulation, suggesting a true physiological relationship between these two molecules. Activation of MEK, the physiological activator of ERK, by anti-CD3 or PMA is not affected by HePTP deletion. The distribution of hematopoietic lineages in bone marrow and peripheral blood samples and the in vitro proliferative capacity of bone marrow progenitors from HePTP deletion mice do not deviate from those of matched littermate controls. Similarly, lymphocyte activation and development are indistinguishable in HePTP−/− mice and controls. We conclude that HePTP is a physiological regulator of ERK on the basis of these studies and hypothesize that its deletion is well compensated for in the developing mouse through reduction of ERK targets or enhancement of physiologically opposed signaling pathways.
Extracellular stimuli modify cells by altering traffic through intracellular networks of protein kinases and related molecules. The mitogen-activated protein kinase (MAPK) family plays a central role in signaling pathways stimulated by extracellular stimuli such as growth factors, cytokines, and physical stress and is conserved over a huge evolutionary distance (8). In higher organisms, this broad kinase family includes the extracellular stimulus-regulated kinases (ERKs) and two stress-stimulated kinase groups, the stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) and the p38HOG kinases (2, 11, 19, 33).
In response to specific stimulation, the MAPKs are activated by phosphorylation on conserved threonine and tyrosine amino acids which are found within an activation motif (27, 28, 31). This critical regulation site is located in a loop linking kinase domains VII and VIII and varies in length between individual MAPKs (13, 16). Both the length of this loop and the identity of adjacent amino acids determine the specificity of MAPK kinases, which are the principal determinants of MAPK activity (16, 43).
While extracellular stimuli may induce activating phosphorylation of MAPKs by activating MAPK kinases, the MAPK-specific dual specificity protein phosphatases are potential negative regulators of mitogenic cascades by targeting these same sites (24, 25, 35). These nuclear MAPK phosphatases are typified by MAPK phosphatase 1 (MKP-1), which targets both phosphothreonine and phosphotyrosine on most activated MAPKs. Its overexpression can counter the transforming action of Ras activation in fibroblasts (35), presumably by dephosphorylating activated ERK, antagonizing the activity of MAPK kinases directly. The dual-specificity phosphatases MKP-3 and MKP-4 also inactivate the ERKs, while the phosphatase M3/6 may have specificity for the SAPK/JNK family (24, 25). Similarly, the neuronal ERK tyrosine-specific phosphatases phosphoprotein phosphatase (PTP-SL) and striatum-enriched phosphatase-SL (STEP) associate with ERK through a specific docking site, termed the kinase interaction motif (KIM), resulting in specific ERK inactivation through tyrosine dephosphorylation (29).
Important signaling events may reflect the balance between MAPK kinases and MAPK phosphatases. MAPK phosphatases may be controlled as part of a coordinated cellular response to extracellular signals. For example, stress stimuli, acting through the JNK (SAPK) family of MAPKs, induce the expression of MKP-1, which targets sites of activating phosphorylation on ERK (4). Such cross-regulation may act to prevent the activation of physiologically conflicting pathways after multiple stimuli. Alternatively, MAPK phosphatases may be activated in parallel to their targets, causing the activation of these powerful signaling molecules to be self-limited. For instance, the MAPK phosphatase MKP-3 is activated after phosphorylation of ERK-2 by binding of ERK-2 to the noncatalytic amino terminus of MKP-3 (6).
We have described a hematopoiesis-specific protein tyrosine phosphatase (HePTP) that is induced in primary lymphocytes by extracellular stimuli such as the mitogens concanavalin A, phytohemagglutinin, and interleukin-2 (IL-2) and by gene transfer of activated Lck or c-Raf (1, 44). Overexpression of HePTP reduces T-cell receptor (TCR)-induced activation of ERK2 and blocks TCR-induced transcriptional activation of an interleukin-2 promoter-derived ERK-responsive element (32). Overexpression of HePTP also reduces ERK activation after phorbol myristate acetate (PMA) or TCR stimulation of cultured Jurkat cells and interferes with PMA- and growth factor-induced MAPK activation in myeloid cells. Consistent with its role as an ERK phosphatase, HePTP contains a KIM, as observed in PTP-SL and STEP (29). Collectively these data suggest that HePTP may have a unique role in hematopoietic mitogenic signaling cascades, possibly through direct ERK dephosphorylation.
While existing data suggest that HePTP may be an ERK tyrosine phosphatase, studies based on gene transfer-induced overexpression and in vitro enzymatic reactions often do not identify true physiological activities. For example, MEKK1, a mammalian kinase related to the Saccharomyces cerevisiae pheromone signaling kinase Ste11, was initially identified through cell transfection experiments as an activator of the ERK kinase MEK (from which it derives its name) (20). Subsequent studies demonstrated that MEKK-1 is unlikely to be a physiologic activator of MEK-1, having much higher specificity for SEK-1, the upstream activator of the SAPK/JNK family (42). To further characterize the role of HePTP in hematopoietic cell signaling, we generated mice deficient in this enzyme using the technique of homologous recombination. We report here that HePTP−/− mice are fertile and healthy in appearance, with normal solid organ and bone marrow morphology. Despite increased ERK activation observed after PMA or TCR stimulation of HePTP−/− lymphocytes, proliferative response and cytokine secretion are normal. This work demonstrates that HePTP is a physiologically relevant ERK hematopoietic phosphatase, though its absence appears to be well compensated for in genetically deficient mice.
MATERIALS AND METHODS
Generation of mice genetically deficient in HePTP.
A plasmid used to introduce a targeted HePTP mutation by homologous recombination was constructed by inserting the pMC1Neo gene cassette between the PstI site in exon 2 and the BglII site found in the second intron of the HePTP gene. This plasmid was transfected into the E14K embryonic stem (ES) cell line (kindly provided by K. Rajewsky, Cologne, Germany) by electroporation. G418-resistant ES clones were screened by PCR using primers producing products distinguishing wild-type and mutant forms. Two cell lines with proper homologous recombination were obtained and verified by Southern hybridization analysis. Each cell line was injected into blastocysts of C57BL/6 mice (Jackson Laboratory), producing chimeras. These chimeric mice, derived from two different ES clones, were mated with C57BL/6 mice. Tail DNA from offspring with agouti coat color was analyzed by PCR or Southern hybridization to confirm the germ line integration of the mutated HePTP gene. Heterozygous mice were mated to generate +/+ and −/− littermates to be used for experimentation. Mice were mated and maintained in the animal facility of the Ontario Cancer Institute. Animals were housed in a semibarrier set which includes sterile static microisolators, acidified (pH 2.8) drinking water by bottle, and sterile standard laboratory rodent chow. Animal cages were serviced in HEPA-filtered room air.
Products of HePTP matings were characterized at the HePTP locus by PCR. Mice were weaned and ear-marked at day 21. At week 4, 2 mm of tail was cut using a surgical blade, and mice were bled using a heparinized capillary tube (Microvette CB 300; Sarsted). Total DNA was extracted from blood (40 μl) and the tail tip using a DNeasy tissue kit (Qiagen). Extracted DNA (0.1 μg) was used as a template in 50 μl of a final reaction mixture which contained deoxynucleoside triphosphates (100 μM each) (Gibco-BRL), HePTP E2-S2 primer (CAAGAAGCATGTGCGCCTGC) (10 μM), HePTP E3-AS primer (TGCTGTAGCGACCAGCGTGT) (10 μM), MgCl2 (1.5 mM) (Gibco-BRL), and Taq polymerase enzyme (0.25-μl stock) (Gibco-BRL). HePTP−/− mice were identified by a 1.8-kb amplification product, since amplification of template genomic DNA from HePTP+/+ mice produces a 0.7-kb band.
Lymphocyte proliferation assays.
Spleen, thymus, and lymph nodes were surgically removed and meshed to obtain a single-cell suspension. Cells were washed, counted, and cultured in Iscove modified Dulbecco's medium (IMDM) containing fetal calf serum (FCS) (10%). Spleen cells (105/100 μl) were placed in 96-well plates in the presence of anti-CD3Ε with anti-CD28 (both from PharMingen) (12). Proliferation studies were performed using [3H]dT (1 μCi/well) (NEN) added for 16 h after 24 and 48 h of stimulation. Cells were harvested (Packard Filtermate cell harvester), and the incorporated thymidine was measured on a Top Count NXT (Packard). Cells harvested at different intervals were stained with biotinylated anti-CD69 and anti-CD25 and analyzed using a FACScan (Becton Dickinson).
Hematopoietic colony assay.
Marrow cells were flushed from the femora of 8- to 12-week-old HePTP+/+ and HePTP−/− littermate mice. Cells were resuspended in IMDM, counted, and plated at various concentrations in methylcellulose (1 ml) containing erythropoietin (1 U) (EPO; Kirin Brewery), fetal bovine serum (4%; ICN/Flow), bovine serum albumin (0.5%; Sigma), human transferrin (0.1 mg/ml; Boehringer), recombinant human IL-11 (rhIL-11) (0.01 μg; Genetics Institute), rhIL-1α (0.001 μg; Biogen), cystine (0.02 mg; Sigma), insulin (0.01 mg; Sigma), lipids mix (1.6 μl; Sigma), IL-3-containing conditioned medium (15 U), CHO cell line-conditioned medium containing the c-kit ligand (3%), and 5637 cell line-conditioned medium (10%) (39). Cultures were incubated for 3 to 10 days at 37°C in a humidified atmosphere containing CO2 (5%). Myeloid, erythroid, and megakaryocytic colonies were identified morphologically using May-Grunwald-Giemsa staining.
Immunostaining and flow cytometry.
Single-cell suspensions (106 cells) from thymus, spleen, or axillary/cervical lymph nodes were resuspended in phosphate-buffered saline (PBS) and incubated for 30 min on ice with phycoerythrin (PE), fluorescein isothiocyanate (FITC), or biotin-conjugated anti-CD4, anti-CD8, anti-TCRαβ, anti-CD3, anti-CD25/IL-2Rα, anti-CD28, anti-CD69, anti-CD5, anti-immunoglobulin M (IgM), anti-CD11B, anti-CD45, or anti-B220. Biotinylated antibodies were visualized using streptavidin-RED670 (Life Sciences). Samples were analyzed using a FACScan (Becton Dickinson).
Detection of MAPK and MEK phosphorylation in primary mouse lymphoid tissues.
Splenocytes, thymocytes, or lymph node cells were washed and incubated in IMDM containing FCS (0.5%) with either anti-CD3Ε antibody (10 μg/ml) (PharMingen) or PMA (10 nM) (Sigma) to induce ERK pathway activation. Sorbitol (400 mM) was used to induce p38HOG and SAPK activation. Cells (2 × 107) were placed in lysis buffer (200 μl) consisting of Nonidet P-40 (0.1%), Tris (pH 7.5) (50 mM), NaCl (150 mM), sodium orthovanadate (5 mM), dibasic sodium pyrophosphate (50 mM), and the protease inhibitors leupeptin (1 μg/ml), aprotinin (1 μg/ml), and phenylmethylsulfonyl fluoride (1 mM) (all from Sigma). Protein concentration was measured in each lysate using Bio-Rad reagent. Equal amounts of proteins were analyzed by polyacrylamide gel electrophoresis (PAGE) followed by Western analysis using antibodies specific for the enzymatically activated phospho-specific forms of SAPK, p38HOG, MEK, and ERK1 and -2 (all from New England Biolabs). Western blots to detect total MAPK and MEK were performed to ensure equal loading in each lane.
Capture ELISA.
Lymph node cells from HePTP+/+ and HePTP−/− mice were cultured in IMDM containing FCS (10%) in the presence of anti-CD3Ε (1 μg/ml) plus anti-CD28 (1 μg/ml) (both from PharMingen). After 24 and 48 h of stimulation, supernatants (100 μl/well) were collected and kept at −70°C for subsequent IL-2 determination. IL-2 levels were measured with the mouse IL-2 Duo Set enzyme-linked immunosorbent assay (ELISA) development system (R&D) using a Bio-Rad plate reader.
Th1 and Th2 differentiation.
Spleen cells from HePTP+/+ and HePTP−/− mice were passed through a stainless steel screen to make single-cell suspensions. The cells were washed in α minimal essential medium (αMEM) (Gibco) supplemented with glutamine (2 mM), sodium pyruvate (1 mM), HEPES (pH 7.3) (15 mM), mercaptoethanol (50 μM), and FCS (10%). Splenocytes (4 × 106 cells) were cultured for 48 h in 24-well flat-bottomed plates as described (15), and T cells were activated by incubation with a 1:40 dilution of anti-CD3Ε monoclonal antibody culture supernatant. Cultures were harvested, and activated T cells were recovered by centrifugation with Lympholyte and reactivated by incubation for an additional 16 h in 96-well plates coated with anti-CD3Ε antibody. During the last 3 h of incubation, cells were exposed to brefeldin A (10 μg/ml; Sigma). Cells were subsequently washed in PBS, fixed with paraformaldehyde (4%), and permeabilized with saponin (0.1%). T-cell subsets were identified by flow cytometry using appropriate concentrations of monoclonal antibodies directed against CD4, CD8, IL-4, IL-10, tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) as described (14).
RESULTS
Generation of HePTP deletion mice.
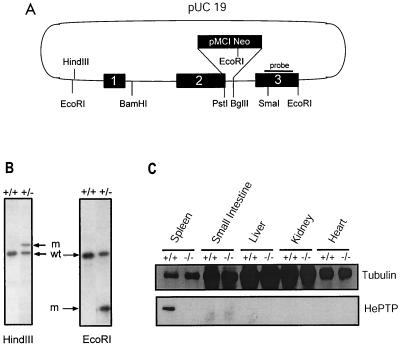
To further characterize the physiological role of HePTP, we generated deletion mice through the process of homologous recombination. Exon 2 of the HePTP gene was disrupted in E14K embryonic stem cells using a replacement construct containing the pMC1Neo gene expression cassette (Fig. 1A) (38). The neo gene was inserted between the PstI site in exon 2 and the BglII site found in the second intron of the HePTP gene. Two recombinant ES cell clones were identified by PCR and confirmed on Southern blotting of DNA (Fig. 1B). Each clone was injected into blastocysts of C57BL/6 mice, producing chimeras which were mated with C57BL/6 mice. Tail DNA from offspring with agouti coat color were analyzed by PCR or Southern hybridization to confirm the germ line integration of the mutated HePTP gene. Expression of HePTP was tested by Western analysis of protein extracts from mouse tissues. As shown in Fig. 1C, expression was totally absent in HePTP−/− mice, which are fertile and appear healthy. The distribution of genotypes +/+, −/−, and +/− followed Mendelian inheritance (data not shown).
FIG. 1.
Generation of mice deficient in HePTP through homologous recombination. (A) A 2.6-kb EcoRI-EcoRI genomic fragment encompassing the first three exons of HePTP was cloned into pUC19. An antisense neo gene cassette replaced sequence between the PstI site in exon 2 and the BglII site found in the second intron. This construct was transfected into the E14K ES cell line by electroporation. G418-resistant ES clones were screened by PCR using primers at positions indicated by arrows. The solid bar indicates the probe used for Southern confirmation of homologous recombination. (B) Southern analysis of DNA from PCR-positive ES cells, demonstrating successful single-site homologous integration. wt, wild-type ES; m, mutant ES. The mutant HindIII-cut fragment is increased in size by the inserted neomycin gene, while the mutant EcoRI fragment is reduced in size due to the introduction of a new EcoRI site within the neomycin gene. (C) Northern blot analysis of tissues from HePTP wild-type and −/− mice for HePTP mRNA expression. The wild-type form of the mRNA (arrow) is expressed in thymus and spleen cells obtained from wild-type animals but not from −/− mice. In the latter, a higher-molecular-weight form is expressed as a consequence of the neo cassette insertion. A human β-actin probe (Clontech) was used as a loading control. (D) Western blot analysis of tissues from HePTP+/+ and HePTP−/− mice for HePTP expression. While HePTP+/+ mice express this phosphatase in spleen, HePTP−/− mice show no expression in any organ, confirming gene targeting. Equal amounts of total protein (20 μg) were loaded in each lane. Antitubulin immunoblotting demonstrates organ-specific equivalence.
ERK stimulation is enhanced in HePTP −/− primary lymphoid cells.
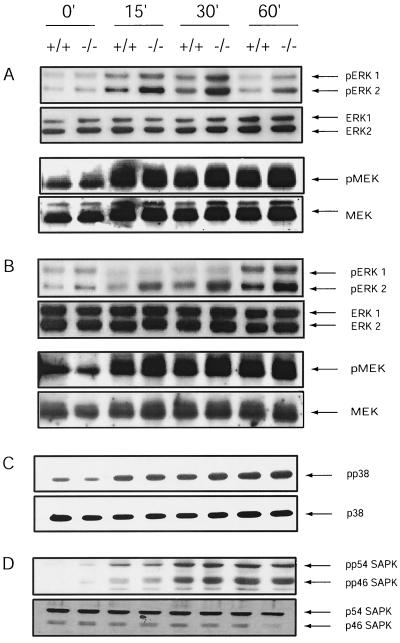
To address the intersection of HePTP and the ERK signaling cascades, we evaluated PMA-induced ERK and MEK activation in mouse lymphocytes lacking HePTP. Isolated spleen cells were incubated ex vivo with PMA at various concentrations. Western blot analysis using antibodies specific for the phosphorylated forms of ERK showed enhanced phosphorylation in HePTP−/− cells in comparison to HePTP+/+ cells, while no change in MEK activation was observed (Fig. 2A). Since HePTP may inhibit TCR-induced ERK activation (32), ERK and MEK activity after antibody-induced TCR stimulation of HePTP−/− lymphocytes was also evaluated. Lymphocytes incubated with anti-CD3Ε for 0 to 60 min were immediately lysed in buffer containing protease and phosphatase inhibitors. Despite equivalent total ERK expression, TCR-stimulated HePTP−/− lymphocytes demonstrated enhanced TCR-mediated ERK phosphorylation. MEK expression and activation were unchanged (Fig. 2B). When splenic lymphocytes were treated with sorbitol to induce p38HOG and SAPK activation, no difference was noted between HePTP−/− and HePTP+/+ mice, suggesting that ERK but not the stress-induced kinase cascades are altered in HePTP−/− mice (Fig. 2C and D). These data suggest that HePTP is a physiologic downmodulator of TCR-stimulated ERK activity and does not seem to have an inhibitory effect on the other MAPKs or on proximal signaling pathways.
FIG. 2.
PMA- and TCR-induced ERK activation is increased in spleen cells from HePTP−/− mice. (A) Spleen cells (2 × 107) were unstimulated or stimulated with 10 nM PMA for 15, 30, and 60 min. Activated, phosphorylated ERK, total ERK, activated phosphorylated MEK, and total MEK were detected by immunoblotting. While HePTP+/+ and HePTP−/− splenocytes have similar amounts of total ERK and MEK, HePTP−/− cells demonstrate increased activation of ERK but not MEK after PMA stimulation. (B) TCR stimulation-induced activation of ERK and MEK was evaluated in total splenocytes from wild-type and HePTP deletion mice. Cells were incubated for 0, 15, 30, or 60 min with anti-CD3Ε (10 μg/ml), lysed, and evaluated by immunoblotting for ERK1-2, MEK, phospho-ERK, and phospho-MEK. HePTP−/− lymphocytes demonstrate increased activation of ERK after TCR stimulation compared with that of wild-type controls but no difference in MEK activation. (C and D) p38HOG activation (C) and SAPK activation (D) in HePTP+/+ and HePTP−/− splenocytes. Cells (2 × 107) were treated with sorbitol (400 mM) for the indicated times. Lysates were analyzed by Western blotting for total p38HOG or SAPK and for the phosphorylated activated forms as indicated. Cells from wild-type HePTP and HePTP−/− mice show similar activation profiles for p38HOG and SAPK.
Myelopoiesis and lymphopoiesis are normal in HePTP−/− mice.
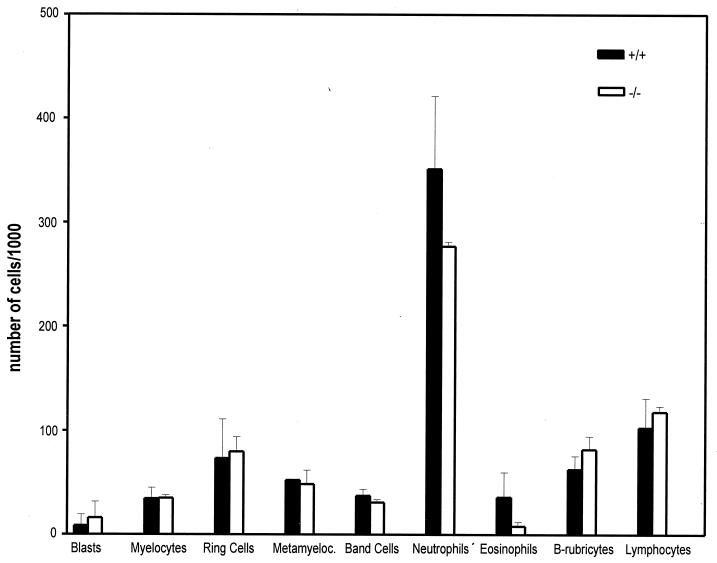
Since ERK stimulation is an important component of hematopoietic clonal expansion and development, we performed a quantitative analysis of bone marrow cells in HePTP+/+ and HePTP−/− mice. Bone marrow samples were stained with May-Grunwald-Giemsa stain, and differential cell counts were performed. Figure 3 shows that no significant difference was found in the distribution of cell lineages between wild-type and HePTP-deleted mice.
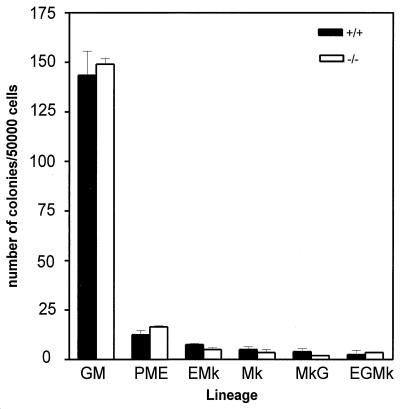
FIG. 3.
In vitro development of bone marrow progenitor cells is similar in HePTP−/− mice (open bars) to that in HePTP+/+ (solid bars) mice. Bone marrow progenitor cells were harvested from mouse femora and plated in methylcellulose suspension for 10 days in the presence of standard growth factors and cytokines, as described in Materials and Methods. Granulomonocytic (GM), pure mature erythroid (PME), erythromegakaryocytic (EMk), megakaryocytic (Mk), granulomegakaryocytic (MkG), and erythogranulomegakaryocytic (EGMk) colonies were identified morphologically by inverted light microscopy. Colony counts from duplicate experiments are shown with 95% confidence limits.
To study hematopoiesis in vitro, bone marrow progenitor cell development was evaluated by methylcellulose culture. At days 3 and 7, pure erythroid, granulocytic, megakaryocytic, and mixed colonies were identified morphologically and counted. No significant difference was found between HePTP−/− and HePTP+/+ mice (Fig. 4).
FIG. 4.
Mature bone marrow cytology is not different between HePTP−/− and HePTP+/+ mice. Bone marrow samples were obtained from the femora of two pairs of age matched HePTP−/− and HePTP+/+ mice and stained with May-Grünwald-Giemsa stain. One thousand cell differential counts were performed for each sample. Data are mean percentages ± standard deviations (SD).
Spleen, thymus, and lymph nodes from HePTP−/− and HePTP+/+ mice were stained with hematoxylin-eosin (HE) and studied by light microscopy. Fresh single-cell suspensions from these organs were also isolated, and different lymphoid subpopulations were determined by flow cytometry. No difference in organ architecture between HePTP−/− and HePTP+/+ mice was observed. Levels of surface expression of all lymphoid markers were similar in lymphoid organs from both mice, suggesting the preservation of lymphoid subsets (Table 1).
TABLE 1.
Surface expression of lineage and activation markers on lymphoid cellsa
| Cells | Marker | +/+ Mice
|
−/− Mice
|
||
|---|---|---|---|---|---|
| Expression (mean % of cells) | SD | Expression (mean % of cells) | SD | ||
| Lymph node | CD4 | 55.67 | 5.11 | 44.84 | 6.67 |
| CD8 | 33.40 | 1.72 | 42.81 | 6.38 | |
| TCRαβ | 86.14 | 7.92 | 82.75 | 1.22 | |
| CD11 | 0.76 | 0.13 | 2.35 | 0.50 | |
| CD25 | 8.34 | 5.41 | 17.02 | 5.74 | |
| CD28 | 1.17 | 0.44 | 3.23 | 1.70 | |
| CD45 | 98.25 | 0.53 | 97.71 | 2.04 | |
| Spleen | B220 | 16.52 | 2.79 | 12.95 | 3.37 |
| IgM | 11.72 | 1.25 | 10.11 | 0.02 | |
| IgD | 16.04 | 1.50 | 11.81 | 2.05 | |
| CD4 | 43.43 | 6.15 | 43.97 | 1.24 | |
| CD8 | 9.39 | 0.48 | 17.19 | 8.39 | |
| Thymus | CD4 | 85.03 | 2.49 | 86.025 | 3.44 |
| CD8 | 57.91 | 4.65 | 71.38 | 0.32 | |
| CD4 + CD8 | 51.67 | 6.25 | 64.35 | 3.78 | |
| TCRαβ | 37.82 | 6.38 | 23.92 | 1.5 | |
| CD3 | 12.93 | 3.07 | 11.35 | 3.55 | |
| CD5 | 24.21 | 8.08 | 25.63 | 2.07 | |
| PMA-stimulated spleen | CD69 | ||||
| 0 h | 1.63 | 0.21 | 2.55 | 1.41 | |
| 12 h | 75.72 | 5.27 | 77.09 | 10.22 | |
Unstimulated spleen, thymus, and lymph node cells from two pairs of HePTP−/− and HePTP+/+ mice were stained with the indicated monoclonal antibodies conjugated directly to PE, FITC, or biotin. Surface expression was analyzed by flow cytometry.
Mitogen-induced expression of activation-induced surface marker and proliferation are not affected by HePTP deletion.
Since HePTP is expressed in differentiated blood cells, we examined proliferation and activation of mature lymphocytes in cells lacking this phosphatase. Expression of the ERK-dependent activation marker CD69 after PMA or anti-CD3 stimulation of splenic lymphocytes was evaluated (36, 37). The levels of expression of CD69 were comparable in HePTP+/+ and HePTP−/− mice (Table 1) (5, 37).
Proliferation of spleen cells after TCR and CD28 stimulation was evaluated by pulse-labeling with tritiated thymidine. Isolated cells were stimulated for 24 and 48 h and incubated for 16 h with [3H]dT to detect induced entry into S phase. No significant difference was found between stimulated HePTP+/+ and HePTP−/− cells (Fig. 5). As an additional marker of T-cell activation, IL-2 production was measured using capture ELISA after TCR and CD28 stimulation of primary lymphocytes. No significant difference was found between HePTP−/− and HePTP+/+ cells in the level of IL-2 produced (Fig. 6).
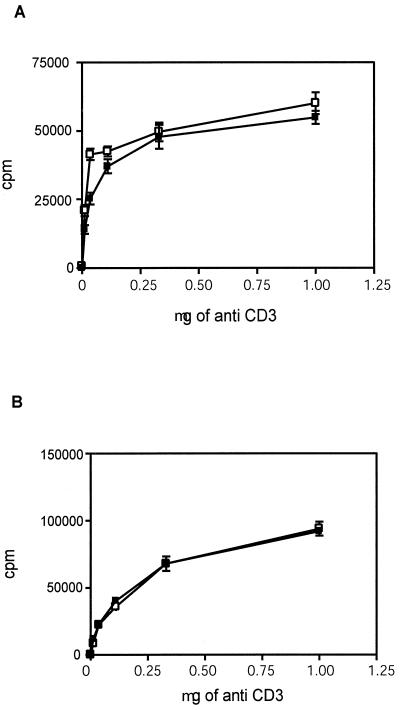
FIG. 5.
Levels of activation-induced proliferation of spleen cells are similar in HePTP−/− (solid squares) and HePTP+/+ (open squares) mice. Spleen cell suspensions (105) were incubated in 96-well dishes with anti-CD3 at the concentrations shown and with anti-CD28 (1 μg/ml) for 24 h (A) or 48 h (B). Proliferation was assessed by the incorporation of [3H]dT and expressed as counts per minute (cpm). No significant difference was observed between HePTP+/+ and HePTP−/− splenocytes. Mean [3H]dT uptake ± SD is shown.
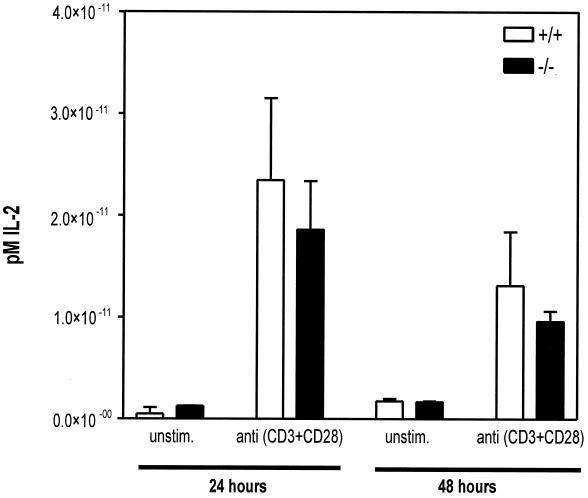
FIG. 6.
IL-2 production by stimulated spleen cells is the same in HePTP−/− and HePTP+/+ mice. Freshly harvested spleen cells from HePTP+/+ and HePTP−/− mice were incubated for 24 or 48 h in the presence of anti-CD3 plus anti-CD28 (1 μg/ml each). Supernatants were collected, and IL-2 levels were determined by capture ELISA. No significant difference was detected between HePTP−/− and HePTP+/+ mice. Mean production of IL-2 ± SD is shown.
The differentiation of T helper 1 and T helper 2 cells from naive T cells requires the presence of exogenous cytokines during primary antigenic stimulation. While IL-12 promotes differentiation into Th1 cells through a p38HOG-dependent pathway, IL-4 supports Th2 differentiation by cross talk between the TCR-mediated activation of the RAS/ERK pathway and the IL-4 receptor (IL-4R)-mediated STAT6 pathway (30). We therefore tested Th2 differentiation in HePTP−/− mice. Spleen-derived naive T lymphocytes were stimulated with anti-CD3Ε antibody. The production of Th2 cells was assessed by intracellular cytokine staining with anti-IL-4 and anti-IL-10 antibodies. Differentiation into Th1 cells was identified by IFN-α and IFN-γ expression using flow cytometry analysis. Comparison of HePTP+/+ and HePTP−/− mouse Th1 subpopulations did not reveal significant differences (data not shown).
DISCUSSION
Phosphorylation of signaling protein kinases can stimulate their enzymatic activity, as is observed after MAPK phosphorylation (8), or can be inhibitory, as is seen after phosphorylation of Src family kinases, such as Lck (3). Protein phosphatases are hypothesized to be important counterbalances to the activities of regulatory kinases. Through gene targeting and other techniques, specific physiological roles for some phosphatases are becoming understood. For instance, the tyrosine phosphatase CD45 functions in signaling from the lymphocyte antigen receptors by specifically removing phosphate from a carboxy-terminal inhibitory tyrosine residue from Lck (18, 26). The very restricted pattern of CD45 expression to hematopoietic cells is consistent with such a central role in cell signaling. For few tyrosine phosphatases has such a discrete molecular activity been defined.
The MAPK phosphatase/PAC1 family of dual-specificity phosphatases target conserved phosphoserine and phosphothreonine residues found within the activation motif of the MAPKs (17, 21–25, 34, 40–47, 49). MKP-1, the best-characterized member of this growing family, dephosphorylates ERK2, SAPK, and p38HOG in transient-transfection studies (7). Its expression is induced in U937 cells by PMA, coincident with the eventual inactivation of ERK and consistent with its presumed role as a MAPK repressor (10). Overexpression of MKP-1 prevents ERK activation and prevents Ras-induced stimulation of DNA synthesis. Interestingly, mice deficient in this gene, unlike HePTP−/− mice, still activate ERK normally, suggesting functional redundancy within this gene family (7, 9, 35). The physiologic role of MKP-1 and others in this family of nuclear dual specificity phosphatases may be both to reset MAPK signaling pathways and to regulate the relative traffic through each of them. For example, SAPK activation induces MKP-1 expression, inhibiting ERK activation, thereby limiting cell division during times of cell stress (4).
HePTP is expressed only in the cytoplasm of cells of hematopoietic origin, such as mature erythrocytes, macrophages, neutrophils, and megakaryocytes, and also macrophagic progentor cells. Like MKP-1, its expression is induced by mitogens, although the temporal profiles differ. While MKP-1 expression is maximal 4 h after PMA stimulation of U937 cells, significant expression of HePTP is not observed in primary lymphocytes until 24 h after lipopolysaccharide or concanavalin A stimulation (10, 44).
To study the physiological role of HePTP in vivo, we generated and evaluated mice in which HePTP was disrupted by homologous recombination. We demonstrated that ERK activation in HePTP−/− mice is enhanced in response to TCR stimulation and mitogens such as PMA. MEK activation after mitogen stimulation is not HePTP dependent, suggesting that ERK is a physiological target for this phosphatase but proximal signaling elements are unaffected.
Since HePTP is expressed widely in mature hematopoietic cells, we examined the development and function of bone marrow, peripheral blood, and lymphoid tissues in HePTP−/− mice. We show that HePTP−/− mice have normal hematopoiesis, as indicated by bone marrow cultures. Lymphocyte subset numbers and function are normal in HePTP−/− mice, as shown by anti-CD3-induced proliferation and IL-2 production.
Marked physiological derangement of HePTP−/− mice induced by ERK activation may be attenuated through compensatory biochemical pathways which become induced during development. The consequences of physiologically inappropriate ERK activation may be modified through the downregulation of ERK targets or the induction of counterbalancing signaling pathways in HePTP−/− mice. Opposite mechanisms may be operative in mice deficient in p44ERK1, which appear normal, having only subtle changes in thymocyte maturation (22). In these mice, p44ERK2 activation and other alterations may compensate for the deficiency of this major effector of mitogenic signaling.
While there are many examples of nuclear threonine/tyrosine phosphatases, few cytoplasmic MAPK-targeted phosphatases have been recognized. The neuron-specific tyrosine phosphatases PTP-SL and STEP bind to ERK through a 14-residue KIM, consisting of the consensus motif LQERRGSNVXLXLD (29). These two tyrosine phosphatases extinguish ERK enzymatic activity and are themselves phosphorylated by ERK. HePTP contains the sequence LQERRGSNVALMLD (residues 16 to 30), which is consistent with its ability to associate with ERK (44). These observations suggest that HePTP, PTP-SL, and STEP have similar tissue-specific functions as cytoplasmic regulators of ERK-dependent signaling cascades.
Hematopoietic cells are exposed to a spectrum of antigenic or cytokine stimuli which may potentially induce all three families of MAPKs. ERK stimulation, induced by both growth factors and mitogenic cytokines, often propels cells into division or differentiation, while SAPK and p38HOG activation may be associated with cell growth delay, differentiation, or apoptosis. Additional intracellular mechanisms of MAPK control are needed to integrate potentially conflicting signals and allow a coordinated cell outcome. MAPK phosphatases, such as the MKPs, PAC1, and HePTP, provide an additional layer of regulation over MAPK-induced physiological responses to extracellular stimuli. For instance, prolonged stimulation of hematopoietic cells by inflammatory cytokines, a circumstance in which HePTP is induced, may trigger relative stimulation of the SAPK pathway, since HePTP preferentially inactivates ERK and p38HOG. Unopposed SAPK or p38HOG activation in this circumstance may lead to growth arrest or apoptosis, thus limiting the inflammatory response.
Although HePTP is among the first examples of an inducible tyrosine phosphatase that acts as a mammalian MAPK inhibitor, several have been identified in S. cerevisiae, suggesting phylogenetic conservation of tyrosine phosphatase-induced control of MAPK activation. The Schizosaccharomyces pombe MAPK StyI, like mammalian MAPKs, becomes activated through dual phosphorylation, initiating mitotic fission. StyI is regulated by the MAPK kinase Wis1, which is sensitive to changes in external osmolarity. Under conditions of hyperosmolality, Wis1 induces the transcription of pyp2, a tyrosine phosphatase functionally redundant to pyp1, which specifically targets StyI, resulting in its inactivation (21). Similarly, in budding yeast, the tyrosine phosphatases PTP2 and PTP3 specifically target the Hog1 MAPK, thereby inactivating the response to osmotic stress. Similar to pyp2 in fission yeast, PTP2 and PTP3 are induced by osmotic stress, suggesting that they function in a negative feedback loop (41). We have shown that HePTP is induced by mitotic stimuli such as PMA and concanavalin A in murine lymphocytes (44). The regulation of mitotic signaling by HePTP is in this way similar to the regulation of the yeast hyperosmolar response by PTP2 and PTP3, suggesting conservation of a general signaling mechanism.
ACKNOWLEDGMENTS
This work was supported by grants from the National Cancer Institute of Canada to B. Zanke. We thank T. W. Mak for assistance with homologous recombination experiments, Joseph Penninger for his guidance in experiments evaluating T-cell proliferation and TCR-induced ERK activation, Norman Iscove and Deborah Hyam for assistance with experiments involving the hybridization of lineage blots and culture of mouse bone marrow, Kaliannan Raju for assistance with T-helper 1 and 2 differentiation experiments, and Christine Quarrinton and her team at the OCI Animal Resource Centre for assistance with animal care.
REFERENCES
- 1.Adachi M, Torigoe T, Sekiya M, Minami Y, Taniguchi T, Hinoda Y, Yachi A, Reed J C, Imai K. IL-2-induced gene expression of protein-tyrosine phosphatase LC-PTP requires acidic and serine-rich regions within IL-2 receptor beta chain. FEBS Lett. 1995;372:113–118. doi: 10.1016/0014-5793(95)00952-6. [DOI] [PubMed] [Google Scholar]
- 2.Ahn N G, Seger R, Bratlien R L, Krebs E G. Growth factor-stimulated phosphorylation cascades: activation of growth factor-stimulated MAPK. CIBA Found Symp. 1992;164:113–126. doi: 10.1002/9780470514207.ch8. [DOI] [PubMed] [Google Scholar]
- 3.Bergman M, Mustelin T, Oetken C, Partanen T, Flint N A, Amrein K E, Autero M, Burn P, Alitalo K. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J. 1992;11:2919–2924. doi: 10.1002/j.1460-2075.1992.tb05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokemeyer D, Sorokin A, Yan M, Ahn N G, Templeton D J, Dunn M J. Induction of mitogen-activated protein kinase phosphatase 1 by the stress-activated protein kinase signaling pathway but not by extracellular signal-regulated kinase in fibroblasts. J Biol Chem. 1996;271:639–642. doi: 10.1074/jbc.271.2.639. [DOI] [PubMed] [Google Scholar]
- 5.Borrego F, Pena J, Solana R. Regulation of CD69 expression on human natural killer cells: differential involvement of protein kinase C and protein tyrosine kinases. Eur J Immunol. 1993;23:1039–1043. doi: 10.1002/eji.1830230509. [DOI] [PubMed] [Google Scholar]
- 6.Camps M, Nichols A, Gillieron C, Antonsson B, Muda M, Chabert C, Boschert U, Arkinstall S. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science. 1998;280:1262–1265. doi: 10.1126/science.280.5367.1262. [DOI] [PubMed] [Google Scholar]
- 7.Chu Y, Solski P A, Khosravi-Far R, Der C J, Kelly K. The mitogen-activated protein kinase phosphatases PAC1: MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem. 1996;271:6497–6501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 8.Davis R J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 9.Dorfman K, Carrasco D, Gruda M, Ryan C, Lira S A, Bravo R. Disruption of the ERP/MKP-1 gene does not affect mouse development: normal MAPK activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 1996;13:925–931. [PubMed] [Google Scholar]
- 10.Franklin C C, Kraft A S. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 11.Galcheva-Gargova Z, Derijard B, Wu I H, Davis R J. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- 12.Greulich H, Erikson R L. An analysis of Mek1 signaling in cell proliferation and transformation. J Biol Chem. 1998;273:13280–13288. doi: 10.1074/jbc.273.21.13280. [DOI] [PubMed] [Google Scholar]
- 13.Hanks S K, Quinn A M, Hunter T. The protein kinase family: Conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 14.Heiskanen M A, Bittner M L, Chen Y, Khan J, Adler K E, Trent J M, Meltzer P S. Detection of gene amplification by genomic hybridization to cDNA microarrays. Cancer Res. 2000;60:799–802. [PubMed] [Google Scholar]
- 15.Hughes T R, Roberts C J, Dai H, Jones A R, Meyer M R, Slade D, Burchard J, Dow S, Ward T R, Kidd M J, Friend S H, Marton M J. Widespread aneuploidy revealed by DNA microarray expression profiling. Nat Genet. 2000;25:333–337. doi: 10.1038/77116. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Li Z, Schwarz E M, Lin A, Guan K, Ulevitch R J, Han J. Structure-function studies of p38 mitogen-activated protein kinase. Loop 12 influences substrate specificity and autophosphorylation, but not upstream kinase selection. J Biol Chem. 1997;272:11096–11102. doi: 10.1074/jbc.272.17.11096. [DOI] [PubMed] [Google Scholar]
- 17.Keyse S M. An emerging family of dual specificity MAPK phosphatases. Biochim Biophys Acta. 1995;1265:152–160. doi: 10.1016/0167-4889(94)00211-v. [DOI] [PubMed] [Google Scholar]
- 18.Kishihara K, Penninger J, Wallace V A, Kundig T M, Kawai K, Wakeham A, Timms E, Pfeffer K, Ohashi P S, Thomas M L, Furlonger C, Paige C J, Mak T W. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 19.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 20.Lange-Carter C A, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. A divergence in the MAPK regulatory network defined by MEK kinase and Raf. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 21.Millar J B A, Buck V, Wilkinson M G. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAPK controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- 22.Morgan D O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 23.Muda M, Boschert R, Dickinson R, Martinou J C, Martinou I, Camps M, Schlegel W, Arkinstall S. MKP-3: a novel cytosolic protein tyrosine phosphatase that exemplifies a new class of mitogen activated protein kinase phosphatase. J Biol Chem. 1996;271:4319–4326. doi: 10.1074/jbc.271.8.4319. [DOI] [PubMed] [Google Scholar]
- 24.Muda M, Boschert U, Smith A, Antonsson B, Gillieron C, Chabert C, Camps M, Martinou I, Ashworth A, Arkinstall S. Molecular cloning and functional characterization of a novel mitogen-activated protein kinase phosphatase, MKP-4. J Biol Chem. 1997;272:5141–5151. doi: 10.1074/jbc.272.8.5141. [DOI] [PubMed] [Google Scholar]
- 25.Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem. 1996;271:27205–27208. doi: 10.1074/jbc.271.44.27205. [DOI] [PubMed] [Google Scholar]
- 26.Mustelin T, Coggeshall K M, Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci USA. 1989;86:6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payne D M, Rossomando A J, Martino P, Erickson A K, Her J H, Shabanowitz J, Hunt D F, Weber M J, Sturgill T W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAPK) EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posada J, Cooper J A. Requirements for phosphorylation of MAPK during meiosis in Xenopus oocytes. Science. 1992;255:212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- 29.Pulido R, Zuniga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J. 1998;17:7337–7350. doi: 10.1093/emboj/17.24.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts C J, Nelson B, Marton M J, Stoughton R, Meyer M R, Bennett H A, He Y D, Dai H, Walker W L, Hughes T R, Tyers M, Boone C, Friend S H. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science. 2000;287:873–880. doi: 10.1126/science.287.5454.873. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 32.Saxena M, Williams S, Gilman J, Mustelin T. Negative regulation of T cell antigen receptor signal transduction by hematopoietic tyrosine phosphatase (HePTP) J Biol Chem. 1998;273:15340–15344. doi: 10.1074/jbc.273.25.15340. [DOI] [PubMed] [Google Scholar]
- 33.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 34.Sun H, Charles C H, Lau L F, Tonks N K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAPK in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 35.Sun H, Tonks N K, Bar-Sagi D. Inhibition of Ras-induced DNA synthesis by expression of the phosphatase MKP-1. Science. 1994;266:285–288. doi: 10.1126/science.7939666. [DOI] [PubMed] [Google Scholar]
- 36.Sutherland C L, Heath A W, Pelech S L, Young P R, Gold M R. Differential activation of the ERK, JNK, and p38 mitogen-activated protein kinases by CD40 and the B cell antigen receptor. J Immunol. 1996;157:3381–3390. [PubMed] [Google Scholar]
- 37.Terada K, Kaziro Y, Satoh T. Ras-dependent activation of c-Jun N-terminal kinase/stress-activated protein kinase in response to interleukin-3 stimulation in hematopoietic BaF3 cells. J Biol Chem. 1997;272:4544–4548. doi: 10.1074/jbc.272.7.4544. [DOI] [PubMed] [Google Scholar]
- 38.Thomas K R, Capecchi M R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 39.Trevisan M, Iscove N N. Phenotypic analysis of murine long-term hemopoietic reconstituting cells quantitated competitively in vivo and comparison with more advanced colony-forming progeny. J Exp Med. 1995;181:93–103. doi: 10.1084/jem.181.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward Y, Gupta S, Jensen P, Wartmann M, Davis R J, Kelly K. Control of MAPK activation by the mitogen-induced threonine/ tyrosine phosphatase PAC1. Nature. 1994;367:651–654. doi: 10.1038/367651a0. [DOI] [PubMed] [Google Scholar]
- 41.Wurgler-Murphy S M, Maeda T, Witten E A, Saito H. Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol Cell Biol. 1997;17:1289–1297. doi: 10.1128/mcb.17.3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 43.Zanke B W, Rubie E A, Boudreau K, McGinnis M, Yan M, Templeton D J, Woodgett J R. Insulation of mammalian MAPK pathways through formation of specific kinase:activator complexes. J Biol Chem. 1996;271:29876–29881. doi: 10.1074/jbc.271.47.29876. [DOI] [PubMed] [Google Scholar]
- 44.Zanke B W, Suzuki H, Kishihara K, Mizzen L, Minden M, Pawson A, Mak T W. Cloning and expression of an inducible lymphoid-specific, protein tyrosine phosphatase (HePTPase) Eur J Immunol. 1992;22:235–239. doi: 10.1002/eji.1830220134. [DOI] [PubMed] [Google Scholar]