Abstract
Mental disorders such as anxiety and depression induced by chronic pain are common in clinical practice, and there are significant sex differences in their epidemiology. However, the circuit mechanism of this difference has not been fully studied, as preclinical studies have traditionally excluded female rodents. Recently, this oversight has begun to be resolved and studies including male and female rodents are revealing sex differences in the neurobiological processes behind mental disorder features. This paper reviews the structural functions involved in the injury perception circuit and advanced emotional cortex circuit. In addition, we also summarize the latest breakthroughs and insights into sex differences in neuromodulation through endogenous dopamine, 5-hydroxytryptamine, GABAergic inhibition, norepinephrine, and peptide pathways like oxytocin, as well as their receptors. By comparing sex differences, we hope to identify new therapeutic targets to offer safer and more effective treatments.
Keywords: sex differences, pain, anxiety, depression, neural circuit
1. Introduction
Chronic pain has a high comorbidity rate with anxiety and depression, and the incidence is as high as 50% (Vos et al., 2020). Epidemiology suggests that there are sex differences in pain-induced mental disorders, and sex factors also affect the efficacy of clinical anti-anxiety and antidepressant drugs (Sramek et al., 2016; LeGates et al., 2019). Most of the experimental studies in neuroscience are carried out in male animals. The excessive dependence on male animals and cells in preclinical studies may mask the key sex differences that may guide clinical research (Madla et al., 2021). Therefore, this paper reviews the research on sex differences in the field of pain and related mental behaviors in recent years, analyzes the sex differences of dopamine, serotonin, GABA, oxytocin, and norepinephrine pathways involved in pain and related emotional behaviors, and combs out the brain circuit mechanism that may be involved in the regulation of sex differences in pain-induced mental disorders. In the era of individualized medical care, emphasis on sex medicine is essential to promote personalized care for patients.
2. Sex differences in neural circuits
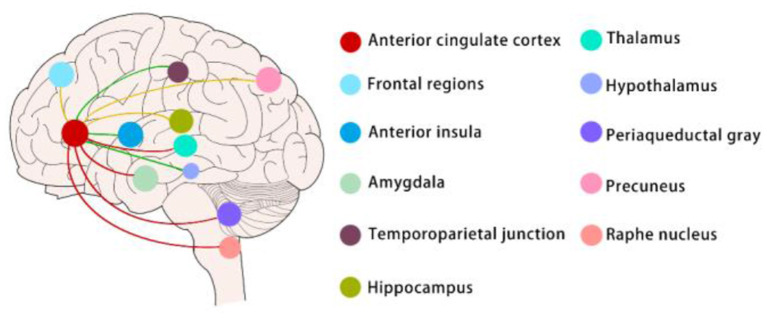
Many brain regions, such as the anterior cingulate cortex, thalamus, amygdala, medial prefrontal cortex, and periaqueductal gray, are involved in the regulation of both chronic pain and emotions (Bushnell et al., 2013). However, a growing number of researchers believe that no neuron is an isolated island and that the connections between brain regions are more critical than brain subdivisions (Han and Domaille, 2022). Identifying specific or shared circuits that regulate pain and emotions is the key to unraveling the complex manifestations of pain and emotions. In clinical experiments, graph theory, modular analysis, and machine learning were used to analyze the default mode, central, visual, and sensorimotor modules in patients with chronic pain (Figure 1). In preclinical experiments, there were also more nuanced circuit manipulation experiments which were now being carried out extensively in animal experiments (Figure 2). Interestingly, both the clinical and preclinical results showed that sex difference was a non-negligible factor in the study of pain and emotions (Mogil, 2020). Brain regions and connections related to pain and emotions such as anterior cingulate cortex, amygdala, locus coeruleus, ventral tegmental area, and periaqueductal gray were all sexually differentiated.
Figure 1.
Sex differences in neural circuits (mainly in clinical experiments).
Figure 2.
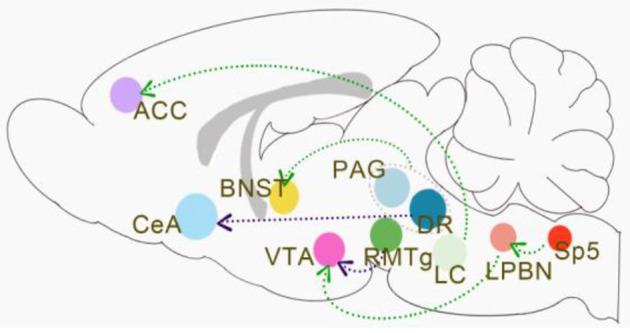
Sex differences in neural circuits (mainly in preclinical experiments).
Sex-difference neural circuits mentioned in this article are mainly about the functional connectivity (FC) of ACC. The red lines indicate that women exhibit greater FC between the anterior cingulate cortex and the thalamus, amygdala, periaqueductal gray, and raphe nucleus than men when chronic pain and pain emotions exist; The green lines indicate that men exhibit greater FC between the anterior cingulate cortex and temporoparietal junction, anterior insula, and hypothalamus than women when chronic pain and pain emotions exist. The yellow lines indicate that only women with chronic pain had greater ACC FC to the precuneus and lower FC to the hippocampus and frontal regions, men with chronic pain have no change in these connectivities compared to healthy men.
The green lines indicate that activation of the pathways induces pain-related depression in males but not females. Activation of the LC → ACC pathway leads to pain-induced depression in males, but it remains unknown whether the LC → ACC pathway conducts the same function in females. Increased VTA DA neuronal activity was associated with chronic neuropathic pain-induced depression-like behaviors. The activation of the Sp5C-LPBNGlu-VTADA pathway was directly involved in the modulation of pain-related depression in males, but this circuit was not manipulated in females. Activation of the terminals in vlPAG/DRDA+ neurons or vlPAG/DRDA+-BNST can reduce nociceptive sensitivity in naïve male mice and inflammatory pain states in males, whereas in female mice resulted in increased locomotion in the presence of significant stimuli. The purple lines indicate that activation of the pathways induces pain-related depression both in males and females. The inhibition of DRN serotonergic neurons → CeA somatostatin neurons pathway produces depression-like behaviors both in male and female mice models with chronic pain. There are also no sex differences in the regulation of the RMTgGABA-VTADA circuit for chronic pain-induced pleasure deficits.
2.1. Anterior cingulate cortex (ACC)
Recent studies indicate that the anterior cingulate cortex (ACC) plays a critical role in chronic pain and pain-related emotional responses (Li et al., 2021). As an important part of the limbic system, ACC involves in pain and pain-related emotions via connectivity with other brain regions such as the descending pain antinociceptive system and so on (Chen et al., 2021). In a cross-sectional resting-state functional connectivity (RSFC) study among older adults, researchers found that the strongest evidence for sex differences emerged in the associations of thermal pain with RSFC between the ACC and amygdala and between the ACC and PAG in older females relative to older males (Figure 1) (Monroe et al., 2018). Researchers also investigated whether women have stronger functional connectivity (FC) and greater structural connectivity (SC) compared to men between the subgenual ACC (sgACC) and the descending antinociceptive system. They revealed that brain circuitry in women may provide for greater engagement of the descending modulation system mediating pain habituation but not in men. Between the sgACC and the periaqueductal gray (PAG), raphe nucleus, medial thalamus, and anterior midcingulate cortex (aMCC) women exhibited greater FC than men (Figure 1) (Wang et al., 2014). There were also findings indicating that abnormal sgACC circuitry is unique to women but not men with ankylosing spondylitis-related chronic pain. Compared to men, women had greater sgACC FC to the default mode and sensorimotor networks (Figure 1) (Osborne et al., 2021). Besides the connectivity, the anatomy of the ACC of rats was also sexually dimorphic with males having greater dendritic spine density as well as arborization. And this reduction was more pronounced for males with increasing age (Markham and Juraska, 2002). Synaptic plasticity is a key cellular mechanism for pain perception and emotional regulation. In preclinical studies, excitatory transmission and plasticity in the anterior cingulate cortex are critical in chronic pain-related emotions. Researchers used a 64-channel multielectrode (MED64) system to record synaptic plasticity in the ACC and found that long-term depression (LTD) was greater in ACC in male mice than females while long-term potentiation (LTP) did not show a sex-related difference (Liu et al., 2020). Besides, some studies reported that Sex differences in GABAergic gene expression occur in the ACC in schizophrenia (Bristow et al., 2015). It remains unknown whether Sex-difference GABAergic gene expression involves in pain and pain-related emotion.
2.2. Amygdala
It has been suggested that the amygdala receives inputs from the parabrachial nucleus and mediates differentiated pain and affective responses in males and females (Sun et al., 2020). Calcitonin gene-related peptide 1 (CGRP1) receptors in the central nucleus of the amygdala (CeA) are involved in neuropathic pain-related amygdala activity and contribute to nociception in both sexes (Presto and Neugebauer, 2022). However, they elicit emotional-affective pain responses such as ultrasound onset and anxiety-like behaviors mainly in females (Neugebauer et al., 2020). The dorsal raphe nucleus (DRN) is the main brain region for the synthesis and release of serotonin, and its involvement in pain-affective reactions has been discussed previously. It has been suggested that the inhibition of DRN serotonergic neurons → CeA somatostatin neurons pathway produces depression-like behaviors in male mice models with chronic pain. Activation of this pathway using pharmacological or optogenetic approaches reduced depression-like behavior in these mice (Figure 2) (Zhou et al., 2019). In this study, the researchers also included MRI data of the patient's FC to corroborate this finding. However, these data were not sex-differentiated, which seems to indicate that this circuit is not sexually dimorphic.
2.3. Locus coeruleus (LC)
The locus coeruleus (LC) acts as a nucleus that regulates pain and emotion (Hirschberg et al., 2017). Resilience to chronic stress is mediated by the noradrenergic regulation of dopamine neurons (Llorca-Torralba et al., 2016). The circuits in which it is involved have also received considerable attention. In studies performed on male rats only, it was believed that bilateral chemogenetic inhibition of the LC → ACC pathway relieves pain-induced depression (Figure 2) (Llorca-Torralba et al., 2022), while activation of the noradrenergic LC → spinal cord pathway relieves pain. However, the noradrenergic system is considered to be sexually dimorphic in much of the literature. Studies have shown differences in the structure and function of LC between male and female rodents in many ways (Bangasser et al., 2015). The dendrites of the neurons in the LC of female rodents were more complex than those of the males (Bangasser et al., 2011), and the female LC dendrites further extended to the areas around the LC, as well as the afferent limbic systems involved in the stress response, such as the central nucleus of the amygdala and the bed nucleus of the stria terminalis (Van Bockstaele et al., 2001). And due to the increased synaptic density in females relative to males, female LC dendrites may receive more synaptic input (Bangasser et al., 2011). This means that anatomically the noradrenergic neurons of the LC may then form different circuits from other brain regions. Therefore, it remains unknown whether inhibition of the LC → ACC pathway relieves pain-induced depression is applicable to female rats.
2.4. Ventral tegmental area (VTA)
In addition to the involvement of the LC noradrenergic system in pain and depression co-morbidity, dopaminergic neuronal projections from the ventral tegmental area (VTA) to the prefrontal cortex (PFC), amygdala and nucleus ambiguus (NAc) play a key role in the perception and regulation of chronic pain symptoms (Hipólito et al., 2015). Rostromedial tegmental nucleus (RMTg) GABA hyperinhibition of the VTA dopamine (DA) neurons mediates pain-induced pleasure deprivation (Figure 2). In their experiments, the researchers concluded that no differences were found in the behaviors of males and females in the disease state or the alterations in their behaviors after the manipulation of the circuits (Markovic et al., 2021). This suggests that there are no sex differences in the regulation of the RMTg GABA-VTA DA circuit for chronic pain-induced pleasure deficits (Figure 2) (Lowes et al., 2021). In another study, it was suggested that chronic neuropathic pain-induced depression-like behaviors were associated with increased VTA DA neuronal activity and that upstream spinal trigeminal sub-nucleus caudalis to the lateral parabrachial nucleus (Sp5C-LPBN) glutamatergic neuronal projections were directly involved in the modulation of VTA DA neurons (Figure 2) (Zhang et al., 2021). However, this circuit was not manipulated in female rats. Some studies support that the projections, gene expression levels, and electrical activity of VTA DA neurons do not differ significantly in males and females, which may explain the absence of functional differences in VTA DA-involved circuits in the co-morbidity of chronic pain and depression (Chung et al., 2017).
2.5. Periaqueductal gray (PAG)
In contrast, the DA neuron to the bed nucleus of the stria terminalis (BNST) projection circuit in the ventral lateral aqueduct periaqueductal gray/dorsal fissure (vlPAG/DR) has male and female differences in pain-related behaviors (Figure 2) (Yu et al., 2021). It has been found that activation of the terminals in vlPAG/DRDA+ neurons or vlPAG/DRDA+-BNST can reduce nociceptive sensitivity in naïve male mice and inflammatory pain states in males, whereas activation of this pathway in female mice resulted in increased locomotion in the presence of significant stimuli (Yu et al., 2021). There is still insufficient fundamental research to answer the question of whether DA in PAG/DR is indeed sex-differentiated in projections and functions. Early studies suggested that the projection from the PAG to the rostral ventromedial medulla (RVM) is sexually dimorphic and that systemic administration of morphine significantly inhibited pain-induced PAG activation in male rather than female rats. It has also been suggested that microglia (Doyle et al., 2017) in the PAG, opioid receptor signaling (Loyd and Murphy, 2006), morphine metabolites (Doyle and Murphy, 2018), and endocannabinoids (Llorente-Berzal et al., 2022) are all sexually differentiated in their involvement in pain regulation. The PAG is a key structure in a number of regulatory pathways of nociception, emitting neuro fibers projections to the amygdala, hypothalamus, frontal cortex, hippocampus, and BNST to regulate the generation of pain and pain-related behaviors. We thus hypothesize, with little knowledge, that the brain circuits involving PAG are more likely to exhibit sexual dimorphism in their involvement in pain and related emotions.
In conclusion, whether different brain circuits are sexually dimorphic in their involvement in pain and related emotions may be related to differences in their own anatomies, molecular levels, and electrophysiological activities per se, and cannot be generalized in a simple way.
3. Sex differences in neurotransmitters and neuromodulators
The past and current literature have indicated that neurotransmitters and neuromodulators systems such as norepinephrine (Joshi and Chandler, 2020), dopamine (Hasbi et al., 2020), serotonin (Zhang et al., 2017), GABA (Cerne et al., 2022), and oxytocin (Tamborski et al., 2016; Aulino and Caldwell, 2020) seemed to be strongly involved in pain-related sexually dimorphic mental disorders (Dazzi and Scicchitano, 2014). Differences in concentrations (Busch et al., 1997), receptors (Hasbi et al., 2020), and transporters (Zachry et al., 2021) of these neurotransmitters and neuromodulators sexual differences may be potential targets for explaining sex differences in pain-related mental disorders.
3.1. Norepinephrine
Norepinephrine (NE) mediates the pathogenesis of pain and anxiety co-morbidity (Phillips et al., 2018). Serotonin-noradrenaline reuptake inhibitors (SNRI) antidepressants are widely used in anxiety disorders and they block the reuptake of NE and 5-HT, making them psychotropic drugs for the treatment of neuropathic pain in clinical settings as well (Fava et al., 2018). Norepinephrinergic neurons are mainly found in the LC and widely project to the cerebral cortex, hippocampus, hypothalamus, cerebellum, brainstem nuclei, and spinal cord (Mason, 1979). Previous studies have suggested that sex differences in the locus coeruleus noradrenergic system (Bangasser et al., 2015; Mulvey et al., 2018; Joshi and Chandler, 2020) may be one of the reasons why pain and depression occur frequently in females.
It has been reported that the LC of adult female rats is larger than that of male rats (Pinos et al., 2001) and contains more NE-ergic neurons (Guillamón et al., 1988), which corresponds to the phenomenon in humans (Busch et al., 1997). This may have increased the capacity for NE generation and release in females. In addition to differences in the number of neurons, there are also sex differences in the LC dendritic morphology (Bangasser et al., 2011). Morphological analysis of individual LC neurons by the researchers revealed that female LC dendrites are longer and more complex than those of males, which may increase synaptic afferent contacts in the peri-LC region (Mulvey et al., 2018). For example, increased nociception introduced by PAG (Bangasser et al., 2011) may be one of the neurobiological mechanisms underlying the sex differences in pain.
Apart from the differences in the number and morphology of NE-ergic neurons in LC, it has been reported that estradiol treatment increases NE levels in the ventral hippocampus, cortex, and hypothalamus of ovariectomized female rats (Bangasser et al., 2011). In addition, estrogen can increase NE synthesis and decrease NE degradation, while ovarian hormones increase NE levels in LC target regions through presynaptic modulation of NE release (Vathy and Etgen, 1988). This can occur through estrogenic regulation of the NE biosynthetic enzyme tyrosine hydroxylase (TH) (Serova et al., 2002; Dalla et al., 2010). The widespread projection system of the LC can release NE into the forebrain and regulate emotional behaviors by targeting forebrain regions. LC activation in animal models of chronic pain exhibited an anxiolytic-depressive phenotype (Landau et al., 2015) but these experiments did not involve females (Alba-Delgado et al., 2013).
However, in reports on other excitatory mediators associated with LC and neuropathic pain, the main excitatory neurotransmitter of the associated stress response, corticotropin-releasing factor (CRF), was enhanced in LC with sex differences. The expression of the CRF1 receptor was increased in the LC of male mice (Bangasser et al., 2013) with chronic pain and anxiogenic phenotypes, whereas this receptor was weakly expressed in anxiety-resilient female mice with pain. Interestingly, increased sensitivity of LC to CRF signaling in females leads to enhanced LC responses of females to non-pain stresses.
In conclusion, sex differences in the locus coeruleus norepinephrine system in the phenotype of pain and related anxiety and depression is an area of research interest.
3.2. Dopamine
Dopaminergic neurons in the brain are widely distributed in the substantia nigra pars compacta (Poulin et al., 2018), ventral tegmental area (Markovic et al., 2021), hypothalamus (Kim et al., 2019) and periventricular area, periaqueductal gray, dorsal raphe nucleus (Yu et al., 2021), and the olfactory bulb (Pignatelli and Belluzzi, 2017). Regions such as the prefrontal cortex (Bhattacherjee et al., 2019), striatum (Dentresangle et al., 2001), nucleus ambiguus, amygdala (Janak and Tye, 2015), thalamus, hippocampus, periaqueductal gray (Yu et al., 2021), and dorsal horn of the spinal cord are all innervated by dopaminergic neurons and are involved in the transduction of pain and related behaviors (Mercer Lindsay et al., 2021; Yang H. et al., 2021). The nigrostriatal dopaminergic system affects pain transduction and perception through the ascending and descending pathways (Dieb et al., 2016). The mesolimbic dopaminergic system regulates pain perception mainly through the reward or motivational pathways in the ventral tegmental area innervating the vomeronasal nucleus, amygdala, thalamus, and hippocampus (Serafini et al., 2020). It also influences learning and memory as well as sensory evaluation of pain through projections to the prefrontal cortex (Huang et al., 2020).
In recent years, it has become a consensus that there are sex differences in the incidence of dopamine-related neuropsychiatric disorders and sensitivity to dopamine-enhancing drugs such as stimulants, and previous studies have shown that dopaminergic circuits often act through dopamine receptors D1 and D2 (Fasano et al., 2013; Stalter et al., 2020; Allichon et al., 2021). It has been shown in the chronic constriction injury (CCI) pain model of the sciatic nerve in mice that dopaminergic projections from the VTA to the nucleus accumbens (NAc) are involved in pain modulation (Ding et al., 2021). In addition, optogenetic activation of dopaminergic neurons in the VTA and their nerve endings in the NAc significantly increases the nociceptive threshold in CCI rats, and the analgesic effect is exerted mainly through D2 (Gao et al., 2020). D1 and D2 may be involved in pain and analgesia in different ways. In the PAG, an important component of the nociceptive descending regulatory system, the analgesic efficacy of opioid receptor agonists on thermal pain stimuli in mice is significantly reduced following damage to dopaminergic nerve endings. Microinjection of D1 antagonists attenuated the analgesic effect of opioids in the hot plate tests, whereas D2 antagonists showed no such effect (Tobaldini et al., 2018). In another study, pharmacological experiments revealed that both D1 and D2 antagonists significantly antagonized the analgesic effects of opioids. Injections of D2 agonists into PAG increased the nociceptive threshold in mice, and the analgesic effects of D2 agonists were blocked by D2 antagonists and γ-aminobutyric acid receptor agonists or opioid receptor antagonists. This study also demonstrated that the combination of D1 and D2 agonists had a greater anti-injurious effect than any one of the receptor agonists alone (Wang et al., 2021).
More interestingly, a number of studies have found that D1 and D2 are involved in sex-differentiated regulation of pain and related behaviors such as anxiety and depression (Hasbi et al., 2020). For example, in the caudate nucleus of non-human primates and the rat striatum, females express a higher density of D1–D2 heteromeric complexes and more D1–D2-expressing neurons compared to males (Hasbi et al., 2020). Signaling pathway analysis showed that sex differences in D1–D2 heteromer expression resulted in differences in the basal and heteromer-stimulated activity of two important signaling pathways, BDNF/TrkB and Akt/GSK3/β-linked protein. The dopamine D1–D2 heteromeric complex is involved in depressive and anxiety-like behaviors, and higher D1–D2 heteromer expression in females may significantly increase the propensity for depressive- and anxiety-like behaviors (Hasbi et al., 2020). Furthermore, in the field of non-pain-related emotions, it has been demonstrated that sex differences in D1 receptor-regulated molecular pathways lead to sex differences in social withdrawal behaviors (Campi et al., 2014). Although social defeat increased dopamine levels in male and female NAc, social withdrawal was induced only in female California mice, but not in male ones. Pharmacological experiments showed that D1 receptor activation was sufficient to induce social withdrawal in females, but not in males. D1 antagonists increased social approach behavior in females exposed to social defeat but did not affect naïve females (Campi et al., 2014). Apart from the fact that D1 and D2 are differentially expressed between males and females, they may be a potential target for revealing the differences in pain-related emotions between the two sexes. Sex differences in neurological dopamine sensitivity as well as in the balanced dopamine release among the circuits could be another area of concern.
In clinical and preclinical studies, using pharmacological methods combined with PET-CT imaging, researchers have suggested that the increased sensitivity of the striatal dopamine reward system in females compared to males may underlie the sex differences in substance use disorders and attention-deficit/hyperactivity disorder (ADHD) (Manza et al., 2022). However, some researchers pointed out that there is little sex difference in the encoding of VTA neurons as well as dopamine release and vesicle depletion in the NAc during the learning process of cue-action-reward instrumental tasks, and that dopamine-related sex differences may be mediated by secondary mechanisms that flexibly affect dopamine cell and circuit functions (Rivera-Garcia et al., 2020). Much of the research on the dopamine system has focused on addiction-related disorders, and it has been suggested that sex differences between different dopamine projections underlie sex differences in addiction (Becker, 2016). In rodents, ovariectomized female rats exhibit smaller initial dopamine increases after cocaine treatment than castrated male rats. Estradiol treatment of ovariectomized female rats enhanced stimulated dopamine release in the dorsolateral striatum but not in the vomeronasal nucleus, resulting in sex differences in the balance between these two dopaminergic projections (Becker, 2016). Moreover, it is not clear whether sex differences regarding the balance of the dopaminergic nervous systems are involved in the generation of sex differences in pain and pain-related emotions. It may be an important potential target.
While many of the previous studies have focused on sex differences in the anatomical structure of dopamine neurons and sex differences related to dopamine levels, it has been suggested that how sex differences in microcircuit regulatory mechanisms mediate sex-differentiated dopamine dynamics is worthy of equal attention (Zachry et al., 2021). Studies suggested that there are local regulatory mechanisms in the ventral tegmental area of the midbrain limbic dopamine system to the striatal circuits that are independent of somatic activity and that these processes can occur through both homogeneous synaptic mechanisms (e.g., presynaptic dopamine self-receptors and dopamine transporter proteins) and heterosynaptic mechanisms (e.g., retrograde signaling of postsynaptic cholinergic and GABAergic systems, etc.) (Zachry et al., 2021), so that the dopamine released by the striatal axonal terminals can be independently and rapidly regulated. In addition, these regulations are potential targets of sex differences in ovarian hormone-dependent and non-dependent dopamine regulation (Zachry et al., 2021). These mechanisms have been shown to be key mediators of multiple psychiatric disorders and involved in the expression of sex-specific behaviors.
3.3. Serotonin
As a monoamine neurotransmitter, serotonin plays a vital role in regulating emotions (Kraus et al., 2017), learning (Grossman et al., 2022), memory (Wu et al., 2021), sleep (Monti, 2011), and appetite (Blundell, 1984), and is closely related to pain and neuropsychiatric disorders such as major depression and anxiety (Zhou et al., 2022). In recent decades, selective serotonin reuptake inhibitor (SSRI) drugs have been the most commonly prescribed medications for depression. Clinical and preclinical studies suggest that amitriptyline, a tricyclic antidepressant used to treat mood disorders, neuropathic pain, and migraine, can increase serotonin levels and restore behavioral responses associated with pain and depression (Zhang et al., 2017). Long-term administration of fluoxetine, an SSRI, was found to prevent anxiety and depression caused by sciatic nerve injury without affecting mechanical allodynia (Barthas et al., 2017). Researchers had found, but controversially, that SSRIs were more effective for females compared to tricyclic antidepressants (Kornstein et al., 2000). Sex differences regarding the serotonergic system had been much reported. In particular, 5-Hydroxy indoleacetic acid (5-HIAA) was reported to be increased in the cerebrospinal fluid of women suffering from depression (Rubinow et al., 1998). Studies have shown increases in 5-HT activity, 5-HT synthesis, and 5-HT metabolites in the brains of female rats compared to male ones (Carlsson and Carlsson, 1988; Haleem et al., 1990). In a clinical study comparing SSRIs with tricyclics, researchers found that menopause significantly affected treatment outcomes and pointed out that this sex-specific difference may be related to the hormonal milieu (Yonkers and Simoni, 2018). Studies conducted with multiple models of depression such as the Chronic Mild Stress Model, the Learned Helplessness Model, the Flinders Sensitive Line rats, and the Lipopolysaccharide-Induced Sickness Behavior in mice have shown that serotonergic neurochemical responses are affected differently in males and females, resulting in sex-dependent behavioral effects (Serova et al., 2002; Dalla et al., 2010).
In previous studies, researchers have noted that sex steroids such as testosterone, progesterone, estrogen, and HPA axis, all have effects on the serotonin pathway (Songtachalert et al., 2018). Activated immune inflammation induces the indoleamine-2,3-dioxygenase (IDO) and tryptophan catabolite (TRYCAT) pathways, thereby enhancing tryptophan degradation and increasing the generation of TRYCATs, including kynurenine and quinolinic acid, exerting an overall anxiogenic effect. The effect of immune activation on IDO is greater in females than in males, therefore, females are more likely to exhibit elevated anxiogenic TRYCAT levels following immune challenge. Moreover, aberrations in the IDO-activated TRYCAT pathway are observed in pregnant females and parturients and are associated with increased levels of postpartum anxiety (Songtachalert et al., 2018).
In addition to the metabolic pathways of serotonin, studies have also found that there are sex differences in serotonin receptors (Zhang et al., 1999; Snoeren et al., 2014; Yamada et al., 2015). There were also differences in 5-HT1A receptor responses between males and females in the repeated stress restraint model in rats. Only males exhibited elevated 5-HT1A receptor G protein coupling responses after repetitive restraint, whereas only females showed increased 5-HT1A receptor responses in the hippocampus following single or repeated exposure (Philippe et al., 2022). In studies exploring sex-related differences in genetics, stress, and the nervous system, female 5-HT1B receptor knockout mice showed significantly lower immobility time and significantly higher baseline hippocampal 5-HT levels than male 5-HT1B receptor knockout mice or male and female wild-type mice in tail suspension and forced swimming tests (Jones and Lucki, 2005). This suggests that female 5-HT1B receptor knockout mice exhibit sex-related disinhibition of 5-HT release, which maintains higher baseline levels of hippocampal 5-HT and behavioral vulnerability to 5-HT depletion (Jones and Lucki, 2005). Moreover, the serotonin transporter protein is also worthy of investigation as a target for many anxiolytic and antidepressant drugs. Using 5-HT transporter (5-HTT) gene-deficient mice as an anxiety animal model, researchers examined cerebral blood flow during resting and amygdala hyperresponsiveness periods using resting-state functional magnetic resonance imaging (rs-fMRI) (Kolter et al., 2021). The results indicated that amygdala reactivity in 5-HTT-deficient mice is regulated by the 5-HTT genotype in males. Whereas, in females it is regulated by the estrous cycle and the predominant influence of gonadotropins may mask genotypic effects (Kolter et al., 2021).
In conclusion, the role of the serotonin system in the sex-differentiated modulation of pain, anxiety, and depression is a matter worthy of investigation.
3.4. Gamma-aminobutyric acid (GABA)
GABA, as one of the important inhibitory neurotransmitters, regulates the encoding of pain and anxiety-depressive mood (Cerne et al., 2022). It has been reported that the anxiolytic effect of GABA depends mainly on its binding to the GABAA receptors (GABAAR). The benzodiazepine anxiolytic GABAAR modulators have been in clinical use for decades (Sollozo-Dupont et al., 2015). GABAAR functions through its subunit composition, the activation of which allows GABA to exert trophic effects in immature neurons.
Recent studies found that GABA-mediated responses were sexually dimorphic even in the absence of gonadal hormone and that there were sex differences in the expression of GABAAR subtypes (Mir et al., 2020). Researchers assessed sex differences in GABAAR function of hypothalamic neurons before brain masculinization by gonadal hormones by culturing 16-day rat embryonic ventral medial hypothalamus neurons in vitro, combined with calcium imaging and electrophysiological recordings (Mir et al., 2020). Optogenetic-specific activation of the dmPFC/vlPAG neural pathway had been reported to produce analgesic and anxiolytic effects in chronic pain-anxiety mice. dmPFC-specific activation of inhibitory neurons in dmPFC was reported to induce nociception and anxiety under normal conditions and chronic pain, and the GABAAR or mGluR1 antagonists can produce analgesic and anxiolytic effects (Yin et al., 2020). However, this study focused only on male mice and it is unknown whether female ones have the same phenotype.
In studies of disorders associated with altered mPFC functions such as schizophrenia, ADHD (Aoki et al., 2013), post-traumatic stress disorder (Lou et al., 2020), depression (Yang L. et al., 2021), and drug addiction (Jasinska et al., 2015), etc., researchers suggested that sex-differentiated manifestations can be partially explained by sex differences in G protein gated inwardly-rectifying K+ (GIRK)-dependent signaling in mPFC pyramidal neurons. Neuronal GIRK channels are formed by homo- or heteromeric assembly of GIRK1/GIRK2/GIRK3 subunits. They play a key role in regulating excitability throughout the brain and are associated with a variety of neurological disorders as well as sex differences in cellular functions. Sex differences in GABABR-GIRK signaling are attributed to a phosphorylation-dependent transport mechanism (Marron Fernandez De Velasco et al., 2015). There are sex differences in the GABABR-GIRK signaling pathway in these neurons. GABABR-dependent GIRK currents in the anterior limbic region of the mPFC were greater in adolescent male mice than in females, but this sex difference was not observed in pyramidal neurons in layer 5/6 of the adjacent limbic cortex.
Previous studies have revealed sex differences in the expression levels of the GABA signaling components, namely, glutamic acid decarboxylase (GAD), GABA receptor subunit, and GABA transporter (GAT) (Pandya et al., 2019). Analysis of sex-specific changes in the expression of GAD, GABAA/BR subunit, and GAT in the human primary sensory and motor cortex revealed sex-dependent differences in the expression of the GABAAR subunit in the superior cerebral gyrus (STG). There is a significant sex-dependent difference in the expression of the α1 subunit of STG: males present significantly higher levels of expression compared to women across all stages of life in STG. Older females had significantly lower α2, α5, and β3 subunit expression in the STG compared to older males. These changes found in the STG may significantly affect GABAergic neurotransmission and lead to sex-specific disease susceptibility and progression (Pandya et al., 2019). There is still a lack of evidence as to whether these baseline differences are involved in the sex-differentiated manifestations of pain and related emotions and behaviors.
In studies on male and female smokers in terms of nicotine dependence, cigarette cravings, and mood or pain sensitivity, researchers used single-photon emission computed tomography (SPECT) to image subjects (Cosgrove et al., 2011). The results showed that females (both female smokers and female non-smokers) had higher GABAA-benzodiazepine receptor (GABAA-BZR) availability than all males. GABAA-BZR availability was negatively correlated with craving and pain sensitivity in female smokers, but not in male smokers. This suggests a sex-specific modulation of GABAA-BZR availability and demonstrates the potential of GABAA-BZRs to mediate smoking cravings and pain symptoms in female and male smokers (Cosgrove et al., 2011).
It is estimated that GABAergic neurons account for more than half of the hypothalamic neuronal population (Searles et al., 2000), and they may explain some of the structural and functional sex differences observed in the mammalian brain. Studies have reported sex differences in GABA turnover rates in discrete hypothalamic structures in adult rats and determined that these differences may be related to differences in GAD65 and/or GAD67 mRNA levels (Sagrillo and Selmanoff, 1997). There is evidence that GAD65 mRNA levels are significantly higher in female rats in the dorsomedial nucleus (DMN), while GAD67 mRNA levels are higher in male rats in the medial amygdala. These data reveal significant sex differences in GABA turnover and GAD mRNA levels in hypothalamic GABAergic neurons of specific populations (Bowman et al., 2013).
Whether the differential manifestations of these GABAergic neurons participate in the sex differences in pain and pain-related affective behaviors remains to be further studied.
3.5. Oxytocin
Oxytocin (Oxt) is a nine-amino-acid peptide hormone that is synthesized and released in the brain primarily by neurons in the paraventricular (PVN) and supraoptic nuclei (SON) of the hypothalamus (Rossoni et al., 2008). Oxt is thought to be associated with pain, and clinically, plasma oxytocin levels are reduced in women with fibromyalgia syndrome (Anderberg and Uvnas-Moberg, 2000). Intranasal administration of Oxt leads to changes in the activity of the bilateral thalamus, left caudate nucleus, and right amygdala, and ameliorates pain in patients with chronic low back pain (Schneider et al., 2020). Epidural oxytocin induces analgesia in patients with severe chronic pain and also improves patients' moods and quality of life.
Oxt has anti-injurious and antinociceptive hormonal effects on neuropathic pain (Xin et al., 2017) induced by nerve injury, which is mediated by its receptor (OTR) and likely occurs due to co-localization of these neurons within OTR-binding sites, such as the spinal dorsal horn (Veronneau-Longueville et al., 1999; Wrobel et al., 2011). Interestingly, in the pain model in rats, Oxt content in the PVN was found to be significantly reduced, but Oxt content in the spinal cord remained unchanged. The researchers also observed that intracerebroventricular injection of Oxt increased the mechanical hypersensitivity response threshold in a dose-dependent manner, whereas intrathecal injection of Oxt did not induce any analgesia. These results indirectly suggest that Oxt in the brain may have an analgesic effect independent of the spinal cord (Zhang et al., 2015). Furthermore, Oxt also plays a crucial role in social behavior, stress, and depression, as verified in animal experiments (Neumann, 2008; Massey et al., 2016). More than one study has suggested that activation of OTR in the VTA is critical for the expression of reward-like properties of social interactions (Song et al., 2016; Borland et al., 2018). However, there are clear sex differences in the embryonic development of Oxt /OTR, which may indicate sex differences in their involvement in pain/behavior (Tamborski et al., 2016; Aulino and Caldwell, 2020). In clinical studies, the expression of Oxt at rs4813625, a single nucleotide polymorphism linked to Oxt was found to correlate more with nociception, anxiety, and wellbeing in females, while no such correlation was found in males (Love et al., 2012).
Another study showed a correlation between depression severity and methylation of the Oxt promoter region. There was a significant negative correlation between critical life events and the mean methylation status as well as the methylation status of single CpG sites in the Oxt promoter region. Whereas, there was no association between depression severity and Oxt methylation. However, there were significant sex differences in the methylation status of Oxt, with females having higher methylation rates than males, suggesting that in patients with depressive disorders, Oxt activation is lower in female patients compared to male ones (Sanwald et al., 2020). Interestingly, in an animal experiment, 3-nitropropionic acid (3-NP)-induced Huntington's disease model was found to have both anxiety and depressive behaviors. 3-NP also reduced the levels of OTR and mGluR2 in the striatum and increased mGluR5. Oxt pretreatment was performed to ameliorate anxiety and depression and to reverse the abnormal expression of OTR, mGluR2, and mGluR5 under the disease state. These behavioral and molecular alterations act similarly between male and female animals (Khodagholi et al., 2022). Meanwhile, some studies support that Oxt may interact with mGluR2 and influence addictive behavior in rats and that this receptor interaction is similar between females and males (Bernheim et al., 2017). However, some researchers have suggested that sex differences in Oxt -regulated emotions tend to occur in the presence of negative stress, for example, one study found that male rats exposed to the stress of social defeat exhibited reduced social avoidance after receiving Intracerebroventricular (ICV) infusions of Oxt (Lukas et al., 2011). Whereas, ICV infusion of Oxt did not reduce social avoidance in stressed female rats (Lukas and Neumann, 2014). The same dose of intranasal Oxt reversed social avoidance in male mice exposed to social defeat, a phenomenon not observed in female mice (Steinman et al., 2016). Nevertheless, sex differences of Oxt in pain affective reactions remain worthy of further study.
4. Conclusion
In the study of pain-induced emotional disorders, most animal experiments only use male mice. It is generally considered that many neurological and behavioral functions are affected by estrogen, including emotion, cognitive function, and pain (McEwen and Milner, 2017). Sex hormones, particularly estradiol and progesterone, play an important role in pain perception and mood swings (Vincent and Tracey, 2010). It has been reported that there is a strong link between mood swings and sex hormones, particularly endogenous hormones (Hernandez-Hernandez et al., 2019; Frokjaer, 2020). Menstrual (or estrous) cycles in females altered pain perception (Kaur et al., 2018), depression (Kaur et al., 2018; Zhao et al., 2021), and even neuronal activity in certain brain regions (D'Souza and Sadananda, 2017). Researchers often do not use female mice for research based on these complexities. However, there is also a contrary view. Studies have shown that there is no significant difference in social behavior, depression-like, anxiety-like behavior, and pain threshold in female mice with pain during different estrus periods (Zhao et al., 2021). In any case, it has been reported in the literature that women have higher rates of pain, depression, and anxiety, and the extensive use of male animal experiments has slowed the process of developing drugs that are more suitable for women, such as analgesia, antidepressants, and antianxiety. There is a huge contradiction in this. We cannot ignore these differences. In the future, research needs to include females to further clarify the mechanism of pain-induced emotional disorders and the targets of sex-differentiated regulation of related neurotransmitters and modulation, which is believed to provide better background support for individual precision medicine.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding Statement
This work was supported by Natural Science Foundation of Shandong Province (No. ZR2021QH103), National Natural Science Foundation of China (No. 82174499), Innovative Research Team of High-level Local Universities in Shanghai, Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-C-202008), and Shanghai Key Laboratory for Acupuncture Mechanism and Ac-upoint Function (No. 21DZ2271800).
Abbreviations
CGRP1, calcitonin gene-related peptide 1; CeA, central nucleus of the amygdala; DRN, dorsal raphe nucleus; LC, locus coeruleus; ACC, anterior cingulate cortex; sgACC, subgenual ACC; FC, functional connectivity; LTD, long-term depression; LTP, long-term potentiation; VTA, ventral tegmental area; PFC, prefrontal cortex; NAc, nucleus ambiguus; RMTg, rostromedial tegmental nucleus; DA, dopamine; Sp5C-LPBN, spinal trigeminal subnucleus caudalis to the lateral parabrachial nucleus; BNST, bed nucleus of the stria terminalis; vlPAG/DR, ventral lateral aqueduct periaqueductal gray/dorsal fissure; RVM, rostral ventromedial medulla; NE, norepinephrine; SNRI, serotonin-noradrenaline reuptake inhibitors; TH, tyrosine hydroxylase; CRF, corticotropin-releasing factor; D1 and D2, dopamine receptor 1 and 2; CCI, chronic constriction injury; NAc, nucleus accumbens; ADHD, attention-deficit/hyperactivity disorder; SSRI, selective serotonin reuptake inhibitor; 5-HIAA, 5-Hydroxy indoleacetic acid; IDO, indoleamine-2,3-dioxygenase; TRYCAT, tryptophan catabolite pathways; 5-HTT, 5-HT transporter; rs-fMRI, resting-state functional magnetic resonance imaging; GABA, gamma-aminobutyric acid; GABAA/BR, GABAA/B receptors; GIRK, G proteingated inwardly-rectifying K+; GAD, glutamic acid decarboxylase; GAT, GABA transporter; STG, superior cerebral gyrus; SPECT, single-photon emission computed tomography; GABAA-BZR, GABAA-benzodiazepine receptor; DMN, dorsomedial nucleus; Oxt, Oxytocin; PVN, paraventricular; SON, supraoptic nuclei; ICV, intracerebroventricular.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Alba-Delgado C., Llorca-Torralba M., Horrillo I., Ortega J. E., Mico J. A., Sánchez-Blázquez P., et al. (2013). Chronic pain leads to concomitant noradrenergic impairment and mood disorders. Biol. Psychiatry 73, 54–62. 10.1016/j.biopsych.2012.06.033 [DOI] [PubMed] [Google Scholar]
- Allichon M. C., Ortiz V., Pousinha P., Andrianarivelo A., Petitbon A., Heck N., et al. (2021). Cell-type-specific adaptions in striatal medium-sized spiny neurons and their roles in behavioral responses to drugs of abuse. Front. Synapt. Neurosci. 65, 13799274. 10.3389/fnsyn.2021.799274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderberg U. M., Uvnas-Moberg K. (2000). Plasma oxytocin levels in female fibromyalgia syndrome patients. Z. Rheumatol. 59, 373–379. 10.1007/s003930070045 [DOI] [PubMed] [Google Scholar]
- Aoki Y., Inokuchi R., Suwa H., Aoki A. (2013). Age-related change of neurochemical abnormality in attention-deficit hyperactivity disorder: a meta-analysis. Neurosci. Biobehav. Rev. 37, 1692–1701. 10.1016/j.neubiorev.2013.04.019 [DOI] [PubMed] [Google Scholar]
- Aulino E. A., Caldwell H. K. (2020). Subtle sex differences in vasopressin mRNA expression in the embryonic mouse brain. J. Neuroendocrinol. 32, e12835. 10.1111/jne.12835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D. A., Reyes B. A., Piel D., Garachh V., Zhang X. Y., Plona Z. M., et al. (2013). Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol. Psychiatry 18, 166–173. 10.1038/mp.2012.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D. A., Wiersielis K. R., Khantsis S. (2015). Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 1641, 177–188. 10.1016/j.brainres.2015.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D. A., Zhang X., Garachh V., Hanhauser E., Valentino R. J. (2011). Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol. Behav. 103, 342–351. 10.1016/j.physbeh.2011.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthas F., Humo M., Gilsbach R., Waltisperger E., Karatas M., Leman S., et al. (2017). Cingulate overexpression of mitogen-activated protein kinase phosphatase-1 as a key factor for depression. Biol. Psychiatry 82, 370–379. 10.1016/j.biopsych.2017.01.019 [DOI] [PubMed] [Google Scholar]
- Becker J. B. (2016). Sex differences in addiction. Dialog. Clin. Neurosci. 18, 395–402. 10.31887/DCNS.2016.18.4/jbecker [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim A., Leong K., Berini C., Reichel C. M. (2017). Antagonism of mGlu2/3 receptors in the nucleus accumbens prevents oxytocin from reducing cued methamphetamine seeking in male and female rats. Pharmacol. Biochem. Behav. 161, 13–21. 10.1016/j.pbb.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacherjee A., Djekidel M. N., Chen R., Chen W., Tuesta L. M., Zhang Y. (2019). Cell type-specific transcriptional programs in mouse prefrontal cortex during adolescence and addiction. Nat. Commun. 10, 4169–4118. 10.1038/s41467-019-12054-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J. E. (1984). Serotonin and appetite. Neuropharmacology 23, 1537–1551. [DOI] [PubMed] [Google Scholar]
- Borland J. M., Grantham K. N., Aiani L. M., Frantz K. J., Albers H. E. (2018). Role of oxytocin in the ventral tegmental area in social reinforcement. Psychoneuroendocrinology 95, 128–137. 10.1016/j.psyneuen.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. R., Kumar N. N., Hassan S. F., McMullan S., Goodchild A. K. (2013). Brain sources of inhibitory input to the rat rostral ventrolateral medulla. J. Compar. Neurol. 521, 213–232. 10.1002/cne.23175 [DOI] [PubMed] [Google Scholar]
- Bristow G. C., Bostrom J. A., Haroutunian V., Sodhi M. S. (2015). Sex differences in GABAergic gene expression occur in the anterior cingulate cortex in schizophrenia. Schizoph. Res. 167, 57–63. 10.1016/j.schres.2015.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch C., Bohl J., Ohm T. G. (1997). Spatial, temporal and numeric analysis of Alzheimer changes in the nucleus coeruleus. Neurobiol. Aging 18, 401–406. [DOI] [PubMed] [Google Scholar]
- Bushnell M. C., Ceko M., Low L. A. (2013). Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 14, 502–511. 10.1038/nrn3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi K. L., Greenberg G. D., Kapoor A., Ziegler T. E., Trainor B. C. (2014). Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology 77, 208–216. 10.1016/j.neuropharm.2013.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson M., Carlsson A. (1988). A regional study of sex differences in rat brain serotonin. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 12, 53. [DOI] [PubMed] [Google Scholar]
- Cerne R., Lippa A., Poe M. M., Smith J. L., Jin X., Ping X., et al. (2022). GABAkines: advances in the discovery, development, and commercialization of positive allosteric modulators of GABAA receptors. Pharmacol. Therapeut. 234, 108035. 10.1016/j.pharmthera.2021.108035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Li X., Zhuo M. (2021). NMDA receptors and synaptic plasticity in the anterior cingulate cortex. Neuropharmacology 197, 108749. 10.1016/j.neuropharm.2021.108749 [DOI] [PubMed] [Google Scholar]
- Chung A. S., Miller S. M., Sun Y., Xu X., Zweifel L. S. (2017). Sexual congruency in the connectome and translatome of VTA dopamine neurons. Sci. Rep. 7, 11120. 10.1038/s41598-017-11478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove K. P., Esterlis I., Mason G. F., Bois F., O'Malley S. S., Krystal J. H. (2011). Neuroimaging insights into the role of cortical GABA systems and the influence of nicotine on the recovery from alcohol dependence. Neuropharmacology 60, 1318–1325. 10.1016/j.neuropharm.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C., Pitychoutis P. M., Kokras N., Papadopoulou-Daifoti Z. (2010). Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 106, 226–233. 10.1111/j.1742-7843.2009.00516.x [DOI] [PubMed] [Google Scholar]
- Dazzi F., Scicchitano C. (2014). Neurotransmitters: gender's differences. Rivista di Psichiatria 49, 237. 10.1708/1668.18264 [DOI] [PubMed] [Google Scholar]
- Dentresangle C., Le Cavorsin M., Savasta M., Leviel V. (2001). Increased extracellular DA and normal evoked DA release in the rat striatum after a partial lesion of the substantia nigra. Brain Res. 893, 178–185. 10.1016/S0006-8993(00)03311-4 [DOI] [PubMed] [Google Scholar]
- Dieb W., Ouachikh O., Alves S., Boucher Y., Durif F., Hafidi A. (2016). Nigrostriatal dopaminergic depletion increases static orofacial allodynia. J. Headache Pain 17, 11. 10.1186/s10194-016-0607-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Gao X., Wang Z., Jiang X., Lu S., Xu J., et al. (2021). Preoperative chronic and acute pain affects postoperative cognitive function mediated by neurotransmitters. J. Mol. Neurosci. 71, 515–526. 10.1007/s12031-020-01673-x [DOI] [PubMed] [Google Scholar]
- Doyle H. H., Eidson L. N., Sinkiewicz D. M., Murphy A. Z. (2017). Sex Differences in microglia activity within the periaqueductal gray of the rat: a potential mechanism driving the dimorphic effects of morphine. J. Neurosci. 37, 3202–3214. 10.1523/JNEUROSCI.2906-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle H. H., Murphy A. Z. (2018). Sex-dependent influences of morphine and its metabolites on pain sensitivity in the rat. Physiol. Behav. 187, 32–41. 10.1016/j.physbeh.2017.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza D., Sadananda M. (2017). Estrous cycle phase-dependent changes in anxiety- and depression-like profiles in the late adolescent Wistar-Kyoto rat. Ann. Neurosci. 24, 136–145. 10.1159/000477151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano C., Bourque M., Lapointe G., Leo D., Thibault D., Haber M., et al. (2013). Dopamine facilitates dendritic spine formation by cultured striatal medium spiny neurons through both D1 and D2 dopamine receptors. Neuropharmacology 67, 432–443. 10.1016/j.neuropharm.2012.11.030 [DOI] [PubMed] [Google Scholar]
- Fava G. A., Benasi G., Lucente M., Offidani E., Cosci F., Guidi J. (2018). Withdrawal symptoms after serotonin-noradrenaline reuptake inhibitor discontinuation: systematic review. Psychother. Psychosom. 87, 195–203. 10.1159/000491524 [DOI] [PubMed] [Google Scholar]
- Frokjaer V. G. (2020). Pharmacological sex hormone manipulation as a risk model for depression. J. Neurosci. Res. 98, 1283–1292. 10.1002/jnr.24632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Shen L., Wen H., Zhao Y., Chen P., Ruan H. (2020). The projections from the anterior cingulate cortex to the nucleus accumbens and ventral tegmental area contribute to neuropathic pain-evoked aversion in rats. Neurobiol. Dis. 140, 104862. 10.1016/j.nbd.2020.104862 [DOI] [PubMed] [Google Scholar]
- Grossman C. D., Bari B. A., Cohen J. Y. (2022). Serotonin neurons modulate learning rate through uncertainty. Curr. Biol. 32, 586–599.e7. 10.1016/j.cub.2021.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamón A., de Blas M. R., Segovia S. (1988). Effects of sex steroids on the development of the locus coeruleus in the rat. Dev. Brain Res. 40, 306–310. [DOI] [PubMed] [Google Scholar]
- Haleem D. J., Kennett G. A., Curzon G. (1990). Hippocampal 5-hydroxytryptamine synthesis is greater in female rats than in males and more decreased by the 5-HT1A agonist 8-OH-DPAT. J. Neural Trans. 79, 93–101. [DOI] [PubMed] [Google Scholar]
- Han G. S., Domaille D. W. (2022). Connecting the dynamics and reactivity of arylboronic acids to emergent and stimuli-responsive material properties. J. Mater. Chem. B Mater. Biol. Med. 1, 6263–6278. 10.1039/D2TB00968D [DOI] [PubMed] [Google Scholar]
- Hasbi A., Nguyen T., Rahal H., Manduca J. D., Miksys S., Tyndale R. F., et al. (2020). Sex difference in dopamine D1–D2 receptor complex expression and signaling affects depression- and anxiety-like behaviors. Biol. Sex Differ. 11, 8. 10.1186/s13293-020-00285-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Hernandez O. T., Martinez-Mota L., Herrera-Perez J. J., Jimenez-Rubio G. (2019). Role of estradiol in the expression of genes involved in serotonin neurotransmission: implications for female depression. Curr. Neuropharmacol. 17, 459–471. 10.2174/1570159X16666180628165107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipólito L., Wilson-Poe A., Campos-Jurado Y., Zhong E., Gonzalez-Romero J., Virag L., et al. (2015). Inflammatory pain promotes increased opioid self-administration: role of dysregulated ventral tegmental area μ opioid receptors. J. Neurosci. 35, 12217–12231. 10.1523/JNEUROSCI.1053-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg S., Li Y., Randall A., Kremer E. J., Pickering A. E. (2017). Functional dichotomy in spinal- vs. prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats. eLife 6, e29808. 10.7554/eLife.29808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Zhang Z., Gambeta E., Xu S. C., Thomas C., Godfrey N., et al. (2020). Dopamine inputs from the ventral tegmental area into the medial prefrontal cortex modulate neuropathic pain-associated behaviors in mice. Cell Rep. 31, 107812. 10.1016/j.celrep.2020.107812 [DOI] [PubMed] [Google Scholar]
- Janak P. H., Tye K. M. (2015). From circuits to behaviour in the amygdala. Nature 517, 284–292. 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska A. J., Chen B. T., Bonci A., Stein E. A. (2015). Dorsal medial prefrontal cortex (MPFC) circuitry in rodent models of cocaine use: implications for drug addiction therapies. Addict. Biol. 20, 215–226. 10.1111/adb.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. D., Lucki I. (2005). Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology 30, 1039–1047. 10.1038/sj.npp.1300664 [DOI] [PubMed] [Google Scholar]
- Joshi N., Chandler D. (2020). “Chapter 10: sex and the noradrenergic system,” in Handbook of Clinical Neurology, eds R. Lanzenberger, G. S. Kranz, and I. Savic (Amsterdam: Elsevier; ), 167–176. 10.1016/B978-0-444-64123-6.00012-6 [DOI] [PubMed] [Google Scholar]
- Kaur S., Benton W. L., Tongkhuya S. A., Lopez C. M. C., Uphouse L., Averitt D. L. (2018). Sex differences and estrous cycle effects of peripheral serotonin-evoked rodent pain behaviors. Neuroscience 38, 487–100. 10.1016/j.neuroscience.2018.05.017 [DOI] [PubMed] [Google Scholar]
- Khodagholi F., Maleki A., Motamedi F., Mousavi M. A., Rafiei S., Moslemi M. (2022). Oxytocin prevents the development of 3-NP-induced anxiety and depression in male and female rats: possible interaction of OXTR and mGluR2. Cell Mol. Neurobiol. 42, 1105–1123. 10.1007/s10571-020-01003-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Yao Z., Graybuck L. T., Kim T. K., Nguyen T. N., Smith K. A., et al. (2019). Multimodal analysis of cell types in a hypothalamic node controlling social behavior. Cell 179, 713–728.e17. 10.1016/j.cell.2019.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter J. F., Hildenbrand M. F., Popp S., Nauroth S., Bankmann J., Rother L., et al. (2021). Serotonin transporter genotype modulates resting state and predator stress-induced amygdala perfusion in mice in a sex-dependent manner. PLoS ONE 16, e0247311. 10.1371/journal.pone.0247311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein S. G., Schatzberg A. F., Thase M. E., Yonkers K. A., McCullough J. P., Keitner G. I., et al. (2000). Gender differences in treatment response to sertraline vs. imipramine in chronic depression. Am. J. Psychiatry 157, 1445–1452. 10.1176/appi.ajp.157.9.1445 [DOI] [PubMed] [Google Scholar]
- Kraus C., Castren E., Kasper S., Lanzenberger R. (2017). Serotonin and neuroplasticity: links between molecular, functional and structural pathophysiology in depression. Neurosci. Biobehav. Rev. 77, 317–326. 10.1016/j.neubiorev.2017.03.007 [DOI] [PubMed] [Google Scholar]
- Landau A. M., Phan J., Iversen P., Lillethorup T. P., Simonsen M., Wegener G., et al. (2015). Decreased in vivo α2 adrenoceptor binding in the flinders sensitive line rat model of depression. Neuropharmacology 91, 97–102. 10.1016/j.neuropharm.2014.12.025 [DOI] [PubMed] [Google Scholar]
- LeGates T. A., Kvarta M. D., Thompson S. M. (2019). Sex differences in antidepressant efficacy. Neuropsychopharmacology 44, 140–154. 10.1038/s41386-018-0156-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Matsuura T., Xue M., Chen Q., Liu R., Lu J., et al. (2021). Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep. 36, 109411–109411. 10.1016/j.celrep.2021.109411 [DOI] [PubMed] [Google Scholar]
- Liu R., Xue M., Li X., Zhuo M. (2020). Sex difference in synaptic plasticity in the anterior cingulate cortex of adult mice. Molecul. Brain 13, 41–41. 10.1186/s13041-020-00583-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca-Torralba M., Borges G., Neto F., Mico J. A., Berrocoso E. (2016). Noradrenergic locus coeruleus pathways in pain modulation. Neuroscience 338, 93–113. 10.1016/j.neuroscience.2016.05.057 [DOI] [PubMed] [Google Scholar]
- Llorca-Torralba M., Camarena-Delgado C., Suárez-Pereira I., Bravo L., Mariscal P., Garcia-Partida J. A., et al. (2022). Pain and depression comorbidity causes asymmetric plasticity in the locus coeruleus neurons. Brain 145, 154–167. 10.1093/brain/awab239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Berzal A., McGowan F., Gaspar J. C., Rea K., Roche M., Finn D. P. (2022). Sexually dimorphic expression of fear-conditioned analgesia in rats and associated alterations in the endocannabinoid system in the periaqueductal grey. Neuroscience 480, 117–130. 10.1016/j.neuroscience.2021.11.005 [DOI] [PubMed] [Google Scholar]
- Lou T., Ma J., Wang Z., Terakoshi Y., Lee C. Y., Asher G., et al. (2020). Hyper-activation of mPFC underlies specific traumatic stress-induced sleep-wake EEG disturbances. Front. Neurosci. 14, 883. 10.3389/fnins.2020.00883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love T. M., Enoch M., Hodgkinson C. A., Peciña M., Mickey B., Koeppe R. A., et al. (2012). Oxytocin gene polymorphisms influence human dopaminergic function in a sex-dependent manner. Biol. Psychiatry 72, 198–206. 10.1016/j.biopsych.2012.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes D. C., Chamberlin L. A., Kretsge L. N., Holt E. S., Abbas A. I., Park A. J., et al. (2021). Ventral tegmental area GABA neurons mediate stress-induced blunted reward-seeking in mice. Nat. Commun. 12, 3539. 10.1038/s41467-021-23906-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd D. R., Murphy A. Z. (2006). Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J. Comp. Neurol. 496, 723–738. 10.1002/cne.20962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M., Neumann I. D. (2014). Social preference and maternal defeat-induced social avoidance in virgin female rats: sex differences in involvement of brain oxytocin and vasopressin. J. Neurosci. Methods 234, 101–107. 10.1016/j.jneumeth.2014.03.013 [DOI] [PubMed] [Google Scholar]
- Lukas M., Toth I., Reber S. O., Slattery D. A., Veenema A. H., Neumann I. D. (2011). The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 36, 2159–2168. 10.1038/npp.2011.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madla C. M., Gavins F. K. H., Merchant H. A., Orlu M., Murdan S., Basit A. W. (2021). Let's talk about sex: differences in drug therapy in males and females. Adv. Drug Deliv. Rev. 175, 113804. 10.1016/j.addr.2021.05.014 [DOI] [PubMed] [Google Scholar]
- Manza P., Shokri-Kojori E., Wiers C. E., Kroll D., Feldman D., McPherson K., et al. (2022). Sex differences in methylphenidate-induced dopamine increases in ventral striatum. Mol. Psychiatry 27, 939–946. 10.1038/s41380-021-01294-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J. A., Juraska J. M. (2002). Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol. Aging 23, 579–588. 10.1016/S0197-4580(02)00004-0 [DOI] [PubMed] [Google Scholar]
- Markovic T., Pedersen C. E., Massaly N., Vachez Y. M., Ruyle B., Murphy C. A., et al. (2021). Pain induces adaptations in ventral tegmental area dopamine neurons to drive anhedonia-like behavior. Nat. Neurosci. 24, 1601–1613. 10.1038/s41593-021-00924-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron Fernandez De Velasco E., Hearing M., Xia Z., Victoria N. C., Luján R., Wickman K. (2015). Sex differences in GABA(B)R-GIRK signaling in layer 5/6 pyramidal neurons of the mouse prelimbic cortex. Neuropharmacology 95, 353–360. 10.1016/j.neuropharm.2015.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason S. T. (1979). Noradrenaline and behaviour. Trends Neurosci. 2, 82–84. [Google Scholar]
- Massey S. H., Backes K. A., Schuette S. A. (2016). Plasma oxytocin concentration and depressive symptoms: a review of current evidence and directions for future research. Depress Anxiety 33, 316–322. 10.1002/da.22467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S., Milner T. A. (2017). Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 95, 24–39. 10.1002/jnr.23809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer Lindsay N., Chen C., Gilam G., Mackey S., Scherrer G. (2021). Brain circuits for pain and its treatment. Sci. Transl. Med. 13, eabj7360. 10.1126/scitranslmed.abj7360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir F. R., Wilson C., Cabrera Zapata L. E., Aguayo L. G., Cambiasso M. J. (2020). Gonadal hormone-independent sex differences in GABAA receptor activation in rat embryonic hypothalamic neurons. Br. J. Pharmacol. 177, 3075–3090. 10.1111/bph.15037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil J. S. (2020). Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat. Rev. Neurosci. 21, 353–365. 10.1038/s41583-020-0310-6 [DOI] [PubMed] [Google Scholar]
- Monroe T. B., Fillingim R. B., Bruehl S. P., Rogers B. P., Dietrich M. S., Gore J. C., et al. (2018). Sex differences in brain regions modulating pain among older adults: a cross-sectional resting state functional connectivity study. Pain Med. 19, 1737–1747. 10.1093/pm/pnx084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti J. M. (2011). Serotonin control of sleep-wake behavior. Sleep Med. Rev. 15, 269–281. 10.1016/j.smrv.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Mulvey B., Bhatti D. L., Gyawali S., Lake A. M., Kriaucionis S., Ford C. P., et al. (2018). Molecular and functional sex differences of noradrenergic neurons in the mouse locus coeruleus. Cell Rep. 23, 2225–2235. 10.1016/j.celrep.2018.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V., Mazzitelli M., Cragg B., Ji G., Navratilova E., Porreca F. (2020). Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology 170, 108052. 10.1016/j.neuropharm.2020.108052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I. D. (2008). Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J. Neuroendocrinol. 20, 858–865. 10.1111/j.1365-2826.2008.01726.x [DOI] [PubMed] [Google Scholar]
- Osborne N. R., Cheng J. C., Rogachov A., Kim J. A., Hemington K. S., Bosma R. L., et al. (2021). Abnormal subgenual anterior cingulate circuitry is unique to women but not men with chronic pain. Pain 162, 97. 10.1097/j.pain.0000000000002016 [DOI] [PubMed] [Google Scholar]
- Pandya M., Palpagama T. H., Turner C., Waldvogel H. J., Faull R. L., Kwakowsky A. (2019). Sex- and age-related changes in GABA signaling components in the human cortex. Biol. Sex Differ. 10, 5. 10.1186/s13293-018-0214-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe T. J., Bao L., Koblanski M. E., Viau V. (2022). Sex differences in serotonin 5-HT 1A receptor responses to repeated restraint stress in adult male and female rats. Int. J. Neuropsychopharmacol. 25, 863–876. 10.1093/ijnp/pyac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. L., Burr R. L., Dunner D. L. (2018). rTMS effects in patients with co-morbid somatic pain and depressive mood disorders. J. Affect. Disord. 241, 411–416. 10.1016/j.jad.2018.08.065 [DOI] [PubMed] [Google Scholar]
- Pignatelli A., Belluzzi O. (2017). Dopaminergic neurones in the main olfactory bulb: an overview from an electrophysiological perspective. Front. Neuroanat. 11, 7. 10.3389/fnana.2017.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinos H., Collado P., Rodriguez-Zafra M., Rodriguez C., Segovia S., Guillamón A. (2001). The development of sex differences in the locus coeruleus of the rat. Brain Res. Bull. 56, 73–78. 10.1016/S0361-9230(01)00540-8 [DOI] [PubMed] [Google Scholar]
- Poulin J., Caronia G., Hofer C., Cui Q., Helm B., Ramakrishnan C., et al. (2018). Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nature neuroscience 21, 1260–1271. 10.1038/s41593-018-0203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presto P., Neugebauer V. (2022). Sex differences in CGRP regulation and function in the amygdala in a rat model of neuropathic pain. Front. Mol. Neurosci. 15, 928587. 10.3389/fnmol.2022.928587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Garcia M. T., McCane A. M., Chowdhury T. G., Wallin-Miller K. G., Moghaddam B. (2020). Sex and strain differences in dynamic and static properties of the mesolimbic dopamine system. Neuropsychopharmacology 45, 2079–2086. 10.1038/s41386-020-0765-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossoni E., Feng J., Tirozzi B., Brown D., Leng G., Moos F. (2008). Emergent synchronous bursting of oxytocin neuronal network. PLoS Comput. Biol. 4, e1000123. 10.1371/journal.pcbi.1000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow D. R., Schmidt P. J., Roca C. A. (1998). Estrogen-serotonin interactions: implications for affective regulation. Biol. Psychiatry 44, 839–850. [DOI] [PubMed] [Google Scholar]
- Sagrillo C. A., Selmanoff M. (1997). Castration decreases single cell levels of mRNA encoding glutamic acid decarboxylase in the diagonal band of broca and the sexually dimorphic nucleus of the preoptic area. J. Neuroendocrinol. 9, 699–706. [DOI] [PubMed] [Google Scholar]
- Sanwald S., Gahr M., Widenhorn-Muller K., Schonfeldt-Lecuona C., Richter K., Connemann B. J., et al. (2020). Relation of promoter methylation of the oxytocin gene to stressful life events and depression severity. J. Mol. Neurosci. 70, 201–211. 10.1007/s12031-019-01446-1 [DOI] [PubMed] [Google Scholar]
- Schneider I., Schmitgen M. M., Boll S., Roth C., Nees F., Usai K., et al. (2020). Oxytocin modulates intrinsic neural activity in patients with chronic low back pain. Eur. J. Pain 24, 945–955. 10.1002/ejp.1543 [DOI] [PubMed] [Google Scholar]
- Searles R. V., Yoo M. J., He J. R., Shen W. B., Selmanoff M. (2000). Sex differences in GABA turnover and glutamic acid decarboxylase (GAD(65) and GAD(67)) mRNA in the rat hypothalamus. Brain Res. 878, 11–19. 10.1016/S0006-8993(00)02648-2 [DOI] [PubMed] [Google Scholar]
- Serafini R. A., Pryce K. D., Zachariou V. (2020). The mesolimbic dopamine system in chronic pain and associated affective comorbidities. Biol. Psychiatry 87, 64–73. 10.1016/j.biopsych.2019.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serova L., Rivkin M., Nakashima A., Sabban E. L. (2002). Estradiol stimulates gene expression of norepinephrine biosynthetic enzymes in rat locus coeruleus. Neuroendocrinology 75, 193–200. 10.1159/000048237 [DOI] [PubMed] [Google Scholar]
- Snoeren E. M. S., Veening J. G., Olivier B., Oosting R. S. (2014). Serotonin 1A receptors and sexual behavior in female rats: a review. Pharmacol. Biochem. Behav. 121, 43–52. 10.1016/j.pbb.2013.11.017 [DOI] [PubMed] [Google Scholar]
- Sollozo-Dupont I., Estrada-Camarena E., Carro-Juarez M., Lopez-Rubalcava C. (2015). GABAA/benzodiazepine receptor complex mediates the anxiolytic-like effect of Montanoa tomentosa. J. Ethnopharmacol. 162, 278–286. 10.1016/j.jep.2014.12.070 [DOI] [PubMed] [Google Scholar]
- Song Z., Borland J. M., Larkin T. E. O, Malley M., Albers H. E. (2016). Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology 74, 164–172. 10.1016/j.psyneuen.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songtachalert T., Roomruangwong C., Carvalho A. F., Bourin M., Maes M. (2018). Anxiety disorders: sex differences in serotonin and tryptophan metabolism. Curr. Top. Med. Chem. 18, 1704–1715. 10.2174/1568026618666181115093136 [DOI] [PubMed] [Google Scholar]
- Sramek J. J., Murphy M. F., Cutler N. R. (2016). Sex differences in the psychopharmacological treatment of depression. Dialog. Clin. Neurosci. 18, 447–457. 10.31887/DCNS.2016.18.4/ncutler [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalter M., Westendorff S., Nieder A. (2020). Dopamine gates visual signals in monkey prefrontal cortex neurons. Cell Rep. 30, 164–172.e4. 10.1016/j.celrep.2019.11.082 [DOI] [PubMed] [Google Scholar]
- Steinman M. Q., Duque-Wilckens N., Greenberg G. D., Hao R., Campi K. L., Laredo S. A., et al. (2016). Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol. Psychiatry 80, 406–414. 10.1016/j.biopsych.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Liu R., Guo F., Wen M., Ma X., Li K., et al. (2020). Parabrachial nucleus circuit governs neuropathic pain-like behavior. Nat. Commun. 11, 5974–5974. 10.1038/s41467-020-19767-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborski S., Mintz E. M., Caldwell H. K. (2016). Sex differences in the embryonic development of the central oxytocin system in mice. J. Neuroendocrinol. 28, 4. 10.1111/jne.12364 [DOI] [PubMed] [Google Scholar]
- Tobaldini G., Reis R. A., Sardi N. F., Lazzarim M. K., Tomim D. H., Lima M. M. S., et al. (2018). Dopaminergic mechanisms in periaqueductal gray-mediated antinociception. Behav. Pharmacol. 29, 225–233. 10.1097/FBP.0000000000000346 [DOI] [PubMed] [Google Scholar]
- Van Bockstaele E. J., Bajic D., Proudfit H., Valentino R. J. (2001). Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol. Behav. 73, 273–283. 10.1016/S0031-9384(01)00448-6 [DOI] [PubMed] [Google Scholar]
- Vathy I., Etgen A. M. (1988). Ovarian steroids and hypothalamic norepinephrine release: studies using in vivo brain microdialysis. Life Sci. 43, 1493–1499. [DOI] [PubMed] [Google Scholar]
- Veronneau-Longueville F., Rampin O., Freund-Mercier M. J., Tang Y., Calas A., Marson L., et al. (1999). Oxytocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neuroscience 93, 1437–1447. [DOI] [PubMed] [Google Scholar]
- Vincent K., Tracey I. (2010). Sex hormones and pain: the evidence from functional imaging. Curr. Pain Headache Rep. 14, 396–403. 10.1007/s11916-010-0139-1 [DOI] [PubMed] [Google Scholar]
- Vos T., Lim S. S., Abbafati C., Abbas K. M., Abbasi M., Abbasifard M., et al. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 396, 1204–1222. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Erpelding N., Davis K. D. (2014). Sex differences in connectivity of the subgenual anterior cingulate cortex. Pain 155, 755–763. 10.1016/j.pain.2014.01.005 [DOI] [PubMed] [Google Scholar]
- Wang X., Mokhtari T., Zeng Y., Yue L., Hu L., Xue-Qiang W., et al. (2021). The distinct functions of dopaminergic receptors on pain modulation: a narrative review. J. Neural Transpl. Plast. 202, 16682275. 10.1155/2021/6682275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel L., Schorscher-Petcu A., Dupre A., Yoshida M., Nishimori K., Tribollet E. (2011). Distribution and identity of neurons expressing the oxytocin receptor in the mouse spinal cord. Neurosci. Lett. 495, 49–54. 10.1016/j.neulet.2011.03.033 [DOI] [PubMed] [Google Scholar]
- Wu X., Morishita W., Beier K. T., Heifets B. D., Malenka R. C. (2021). 5-HT modulation of a medial septal circuit tunes social memory stability. Nature 599, 96–101. 10.1038/s41586-021-03956-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Q., Bai B., Liu W. (2017). The analgesic effects of oxytocin in the peripheral and central nervous system. Neurochem. Int. 10, 357–364. 10.1016/j.neuint.2016.12.021 [DOI] [PubMed] [Google Scholar]
- Yamada C., Sadakane C., Nahata M., Saegusa Y., Nakagawa K., Okubo N., et al. (2015). Serotonin 2C receptor contributes to gender differences in stress-induced hypophagia in aged mice. Psychoneuroendocrinology 55, 81–93. 10.1016/j.psyneuen.2015.02.006 [DOI] [PubMed] [Google Scholar]
- Yang H., de Jong J. W., Cerniauskas I., Peck J. R., Lim B. K., Gong H., et al. (2021). Pain modulates dopamine neurons via a spinal-parabrachial-mesencephalic circuit. Nat. Neurosci. 24, 1402–1413. 10.1038/s41593-021-00903-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Liu C., Li W., Ma Y., Huo S., Ozathaley A., et al. (2021). Depression-like behavior associated with E/I imbalance of mPFC and amygdala without TRPC channels in mice of knockout IL-10 from microglia. Brain Behav. Immun. 97, 68–78. 10.1016/j.bbi.2021.06.015 [DOI] [PubMed] [Google Scholar]
- Yin J. B., Liang S. H., Li F., Zhao W. J., Bai Y., Sun Y., et al. (2020). dmPFC-vlPAG projection neurons contribute to pain threshold maintenance and antianxiety behaviors. J. Clin. Invest. 130, 6555–6570. 10.1172/JCI127607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers K. A., Simoni M. K. (2018). Premenstrual disorders. Am. J. Obstet. Gynecol. 218, 68–74. 10.1016/j.ajog.2017.05.045 [DOI] [PubMed] [Google Scholar]
- Yu W., Pati D., Pina M. M., Schmidt K. T., Boyt K. M., Hunker A. C., et al. (2021). Periaqueductal gray/dorsal raphe dopamine neurons contribute to sex differences in pain-related behaviors. Neuron 109, 1365–1380.e5. 10.1016/j.neuron.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachry J. E., Nolan S. O., Brady L. J., Kelly S. J., Siciliano C. A., Calipari E. S. (2021). Sex differences in dopamine release regulation in the striatum. Neuropsychopharmacology 46, 491–499. 10.1038/s41386-020-00915-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ma W., Barker J. L., Rubinow D. R. (1999). Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience 94, 251–259. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang J., Niu C., Zhang Y., Zhu T., Huang D., et al. (2021). Activation of parabrachial nucleus: ventral tegmental area pathway underlies the comorbid depression in chronic neuropathic pain in mice. Cell Rep. 37, 109936. 10.1016/j.celrep.2021.109936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Liu Y., Zhao M., Tang W., Wang X., Dong Z., et al. (2017). Depression and anxiety behaviour in a rat model of chronic migraine. J. Headache Pain 18, 27–27. 10.1186/s10194-017-0736-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yang Y., Dai R., Wu H., Li C., Guo Q. (2015). Oxytocin in the paraventricular nucleus attenuates incision-induced mechanical allodynia. Exp. Ther. Med. 9, 1351–1356. 10.3892/etm.2015.2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Li Q., Ma Y., Wang Z., Fan B., Zhai X., et al. (2021). Behaviors related to psychiatric disorders and pain perception in C57BL/6J mice during different phases of estrous cycle. Front. Neurosci. 156, 50793. 10.3389/fnins.2021.650793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Jin Y., Meng Q., Zhu X., Bai T., Tian Y., et al. (2019). A neural circuit for comorbid depressive symptoms in chronic pain. Nat. Neurosci. 22, 1649–1658. 10.1038/s41593-019-0468-2 [DOI] [PubMed] [Google Scholar]
- Zhou Y. S., Meng F. C., Cui Y., Xiong Y. L., Li X. Y., Meng F. B., et al. (2022). Regular aerobic exercise attenuates pain and anxiety in mice by restoring serotonin-modulated synaptic plasticity in the anterior cingulate cortex. Med. Sci. Sports Exerc. 54, 566–581. 10.1249/MSS.0000000000002841 [DOI] [PMC free article] [PubMed] [Google Scholar]