Abstract
Only few studies reported in literature that has elucidated in detail the implications of molecular typing in metastatic and recurrent breast cancer. In this prospective study, we have analysed in depth the pattern of expression, discordances of molecular markers in various metastatic sites, and recurrent cases and their response to chemotherapy/targeted agents and the prognostic outcome. The primary aim of the study was to determine ER, PR, HER2/NEU, and Ki-67 from recurrent and metastatic carcinoma breast to study the expression pattern and discordance and also to study the degree of discordance in relation to the site of metastasis and pattern of metastasis (synchronous vs metachronous) and discordance pattern with the response to chemotherapy and median overall survival rates in months in available subset of patients. Prospective open-label study done at the Government Rajaji Hospital, Madurai Medical College, and Government Royapettah Hospital, Kilpauk Medical College, India, from November 2014 to August 2021. All breast carcinoma patients with recurrence or oligo metastasis (defined as one organ with less than 5 metastases in our study) with known receptor status were eligible and 110 patients were enrolled in the study. ER (ER + to ER −) discordance was seen in 19 (26.38%) cases. PR (PR + to PR -Ve) discordance was seen in 14 (19.17%) cases. HER2/NEU (HER2/NEU + Ve to -Ve) discordance was seen in 3 (16.6%) cases. Ki-67 discordance was seen in 54 (49.09%) cases. High Ki-67 as a proliferative marker has increased response to chemotherapy but earlier relapse and disease progression especially in Luminal B type. In further subset analysis, ER, PR, and HER2/NEU discordance is higher for lung metastasis (ER, PR 61.1%, p value .001, HER2/NEU 5.5%), followed by liver metastasis (ER, PR 50%, p value .0023, 1 case turning ER -ve to + ve, HER2/NEU 1 (10%)). In lung, discordance is more for metachronous metastasis. In liver, discordance is 100% for synchronous metastasis. Synchronous metastasis with discordance in ER and PR is associated with rapid disease progression. High Ki-67 subset of Luminal B–like tumors progressed rapidly than triple negative and HER2/NEU positive subset. Complete clinical response rate in contralateral axillary node metastasis group was 87.8%, followed by local only recurrence with high Ki-67 where chemotherapy had response rate of 81% and 2 years DFS of 93.12% after excision. Certain subsets like contralateral axillary nodes and supraclavicular nodes which present as oligo metastatic disease with discordance and high Ki-67 respond well to chemotherapeutics and targeted agents improving the OS in this subset of patients. Molecular markers and their expression and discordance pattern determine the therapeutic outcome and prognosis of the disease. Early identification and targeting the discordance would go a long way in improving the outcome and DFS and OS of breast cancer patients.
Keywords: ER, PR, HER2/NEU, Ki-67, Breast cancer, Discordance
Introduction
There is a paradigm shift in the treatment of breast cancer with molecular markers playing a major prognostic role. Biomarkers, including estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2), and proliferation marker Ki-67, have been used for several years to predict the prognosis of breast cancer and to guide its therapy. DNA microarray profiling studies of breast tumors have identified distinct subtypes of breast carcinomas that are associated with different clinical outcomes [1]. Cheang and colleagues described an immunopanel of ER, PR, HER2, and Ki-67 that can segregate the luminal A and B subtypes [2]. Luminal B breast cancers with Ki-67 levels of at least 14% had a worse prognosis for both breast cancer recurrence and survival compared with Luminal A tumors with Ki-67 levels of less than 14% [2].
HER2 is a member of the Erb family that plays an important role in promoting oncogenic transformation and tumor growth [3]. The tumors of approximately 25–30% of the patients with breast cancer over express HER2 protein, and this overexpression is correlated with a poor clinical outcome [4]. The HER2 receptor has become important as a target for antibody-based therapy with trastuzumab. In addition to the treatment of the metastatic disease, adjuvant treatment of primary HER2-positive breast cancers with trastuzumab has been shown to markedly improve the outcome of the patients [5]. In patients with metastatic disease, selection for therapy with trastuzumab has traditionally been based on the HER2 status of the primary tumor.
Ki-67 is a nuclear non-histone protein and an antigen associated with cell proliferation. It was identified after immunization of mice with Hodgkin’s lymphoma [6]. The murine monoclonal antibody Ki-67 reacts with a human nuclear antigen that is expressed in G1, S, G2, and mitosis, but not in G0 [7]. Numerous studies have shown that Ki-67 is of prognostic value in many types of malignant tumors. In breast cancer, a strong correlation has been found between the percentage of cells positive for Ki-67 and nuclear grade, age, and mitotic rate [8]. Only a few studies are reported in the literature that have elucidated in detail the implications of molecular typing in metastatic and recurrent breast cancer and almost nil or just a few case reports on its implications in recurrent breast cancers. There are only a few studies on Ki-67 discordance and its prognostic outcome being analysed in detail. In this prospective study, we have analysed in depth the pattern of expression, discordances of molecular markers in various metastatic sites, and recurrent cases and their response to chemotherapy/targeted agents and the prognostic outcome.
Materials and Methods
This study was a prospective observational study at a tertiary referral academic medical centre, viz. Government Royapettah Hospital, Kilpauk Medical College, and Government Rajaji Hospital, Madurai Medical College, India, from November 2014 to August 2021. This study included all breast carcinoma patients with recurrence in the primary and metastasis (synchronous or metachronous) in which previous receptor status is available. We included only oligometastatic disease (defined as one organ with less than 5 metastases in our study). Cases in which records/slides/blocks/previous receptor status were not available, poor PS (performance status) patients, and those with brain metastasis were excluded. The study protocol was approved by the institute’s ethical committee and informed consent was signed by all the patients.
The primary aim of the study was to determine ER, PR, HER2/NEU, and Ki-67 from recurrent and metastatic carcinoma breast to study the expression pattern and discordance rates.
The main objectives were to study the degree of discordance in relation to the site of metastasis and pattern of metastasis (synchronous vs metachronous) and disease outcome and also to determine the correlation of expression and discordance pattern with the response to chemotherapy and median overall survival rates in months in available subset of patients.
One hundred ten patients were enrolled in the study and 110 patients were analysed. Out of 110 patients, 41 (37.3%) patients were premenopausal patients and 69 (62.7%) patients were postmenopausal patients. Core needle biopsy specimen was obtained from all patients from primary and metastatic sites. Core needle biopsy specimen obtained was immediately fixed in formalin within 10 min, in order to prevent delay and false negative results. Tissue biopsy specimens were sectioned using thin slices, for receptor assay such as ER, PR, HER2, and Ki-67. Ki-67 cutoff value was taken as 14%. High Ki-67 significance was considered when value more than 30%. Confirmation of receptor status from primary and metastatic site was done by following standard protocol for hormone receptor assay with thin slices of specimen immediately fixed in formalin within a period of 15 min and transported under proper conditions to the same standard reference laboratory with quality control and same IHC methodology was followed in both the times to avoid bias in the report. The chemotherapy regimen taken by the patient was also documented. After verifying the eligibility and PET/CT reports, patients were classified into three groups as group A (synchronous metastasis), group B (locally recurrent disease), and group C (metachronous metastasis).
The data collected was then recorded in a study proforma, entered into an Excel worksheet, and analysed using statistical software, namely SPSS 23.0 and Microsoft Office tools were used to generate graphs and tables. Descriptive and other statistical analyses were carried out in the present study. Results on categorical measurements are presented in number and its percentage (%). The chi-square test and the Fisher exact test were used to study the significance of parameters on a categorical scale between two or more groups. The probability of committing a type I error was bilaterally set at a value of 0.05 and committing a type II error at a b value of 0.20.
Results
In this study, the total number of ER and PR + Ve cases was 72, out of which 19 were premenopausal and 53 were postmenopausal. The total number of HER2/NEU + ve cases was 18, out of which 5 were premenopausal and 13 were postmenopausal. When Luminal classification was applied, 22 belonged to Luminal A (ER + , PR + , HER2 − , Ki-67 < 14) and 37 belonged to Luminal B (ER + , PR + , HER2 + / − , Ki-67 > 1 4) out of which HER2/NEU positive were 17 and HER2/NEU negative were 20. Nineteen patients were triple negative with 11 patients confirmed basal type by expression of basal cytokeratin markers.
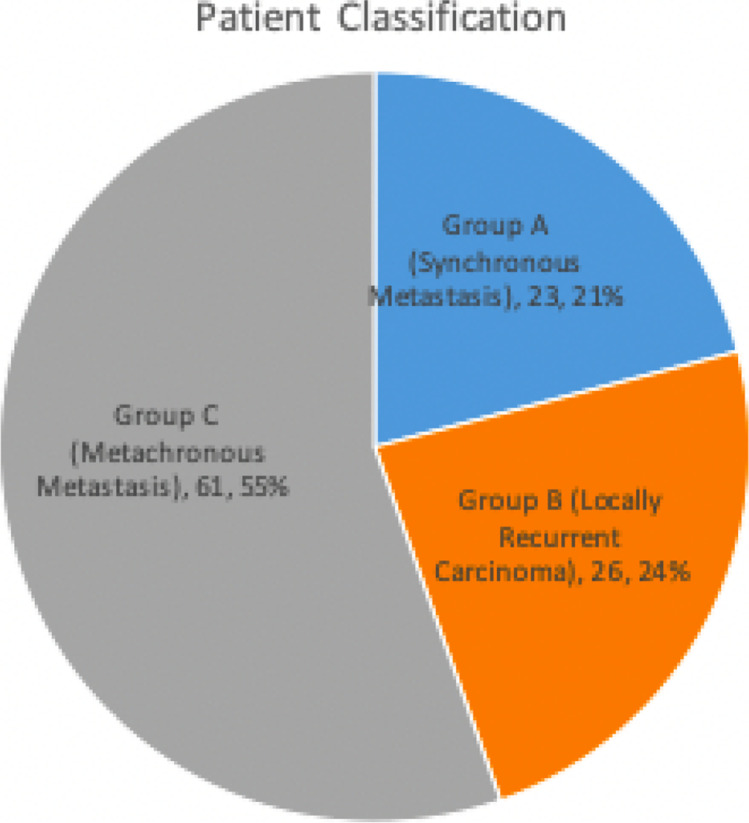
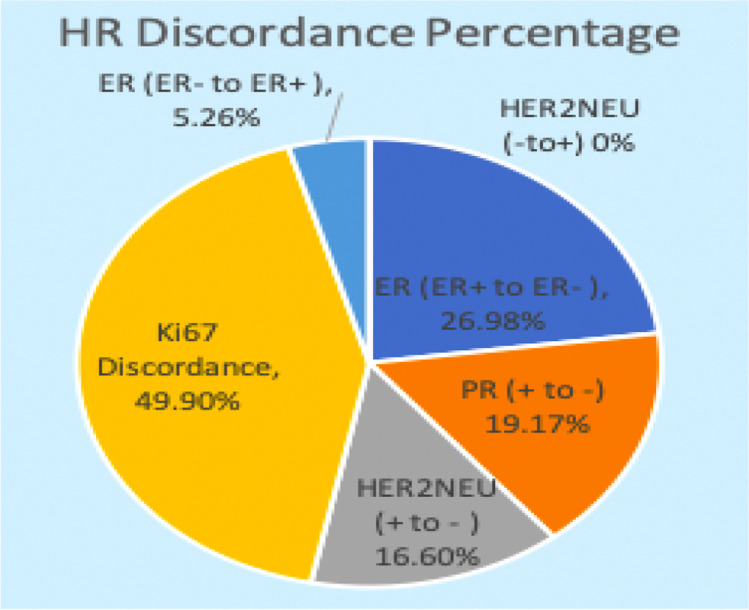
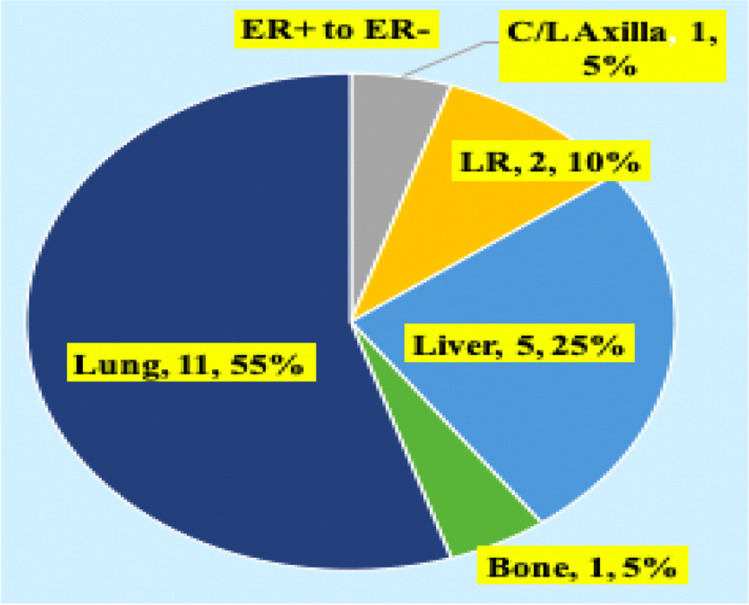
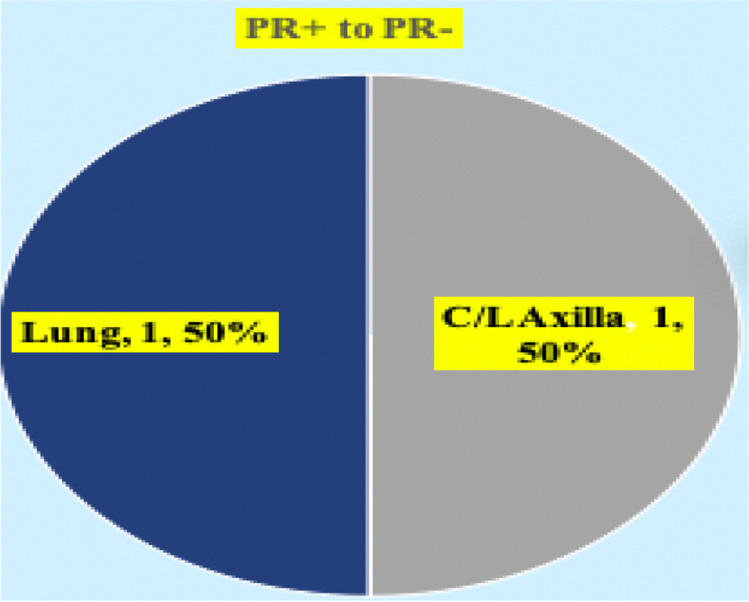
Patients were classified into three groups (Fig. 1). Out of the 110 cases, prospectively analyzed so far, the discordance pattern and expression of molecular markers are mentioned (Fig. 2). ER (ER + to ER −) discordance was seen in 19 (26.98%) cases (Fig. 3) and ER − to ER + was observed in two patients. PR (PR + to PR -Ve) discordance was seen in 14 (19.17%) cases (Fig. 4). Twelve patients in this PR discordance were from those 19 patients with ER discordance. All ER-positive patients in our study were on adjuvant hormone therapy and discordances and recurrences developed while on adjuvant therapy. HER2/NEU (HER2/NEU + Ve to -Ve) discordance was seen in 3 (16.6%) cases (Fig. 5).
Fig. 1.
Patient classification: Group A (synchronous metastasis) — 23 cases (bone, 7; liver, 1; lung, 3; contralateral supraclavicular node, 3; opposite axillary metastasis, 9). Group B (locally recurrent carcinoma) — 26 cases. Group C (metachronous metastasis) — 61 cases (bone, 20; liver, 9; lung, 15; contralateral supraclavicular node, 12; opposite axillary metastasis, 4; subcutaneous metastasis, 1)
Fig. 2.
Hormonal receptor (HR) discordance percentage. ER, estrogen receptor; PR, progesterone receptor
Fig. 3.
Subset analysis of estrogen receptor (ER) discordance. C/L, contralateral; LR, locally recurrent
Fig. 4.
Subset analysis of progesterone receptor (PR) discordance. C/L, contralateral
Fig. 5.
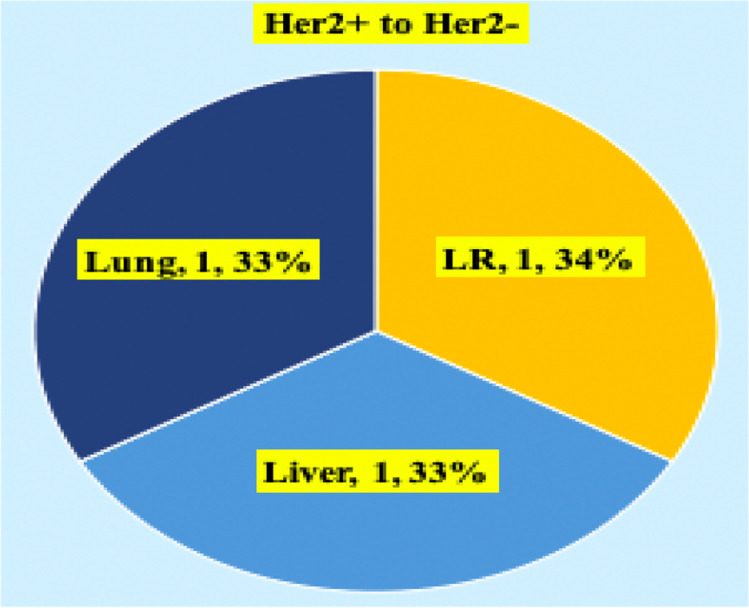
Subset analysis of HER2 receptor discordance. LR, locally recurrent
Metastatic HER2/NEU discordance cases were metachronous lesions. So far only two cases of ER negative in primary became positive in metastasis in the liver and bone metastasis was observed. No case of negative to positive in HER2/NEU receptor status was documented. Ki-67 discordance was seen in 54 (49.9%) cases (Fig. 6). All these patients had Ki-67 in primary < 14% and increased at least beyond 30% in the metastatic site. Of 54 cases, three cases had decreased Ki-67 in the metastatic site against primary (bone 2, lung 1). Ki-67 cutoff was taken as 14%. A value more than 30% is taken as very high. In further subset analysis, ER, PR, and HER2/NEU discordance is higher for lung metastasis (ER, PR 61.1%, p value 0.001, HER2/NEU 5.5%), followed by liver metastasis (ER, PR 50%, p value 0.0023, 1 case turning ER -ve to + ve, HER2/NEU 1 (10%)). In lung, discordance is more for metachronous metastasis. In liver, discordance is 100% for synchronous metastasis.
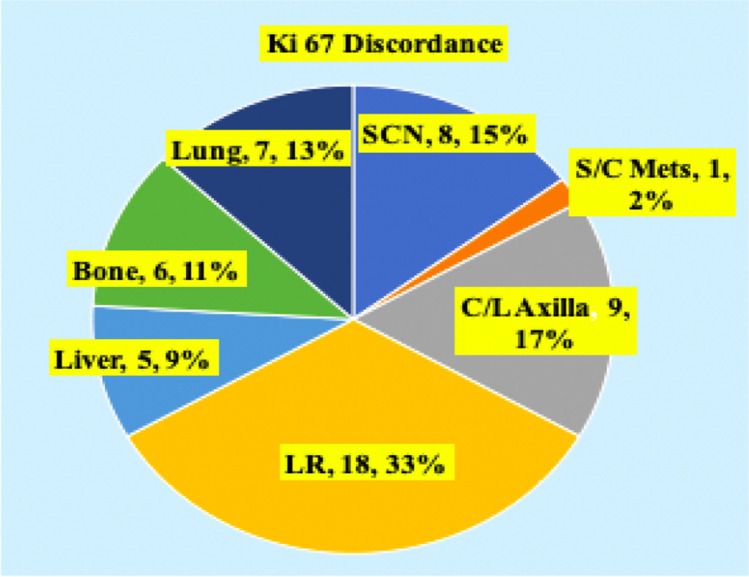
Fig. 6.
Subset analysis of Ki-67 discordance. SCN, supracalvicular lymph nodes; S/C, subcutaneous; C/L, contralateral; LR, locally recurrent
High Ki-67 as a proliferative marker has increased response to chemotherapy but earlier relapse and disease progression especially in Luminal B type. Ki-67 discordance was highest for contralateral axillary nodal metastasis, with overall discordance 17% (p value < 0.001) more for synchronous metastasis. This is followed by local recurrence (33%), then for supraclavicular nodal metastasis (15%). Discordance to Ki-67 was lower for liver metastasis (9%) (p value 0.06) and is only 10% for metachronous metastasis.
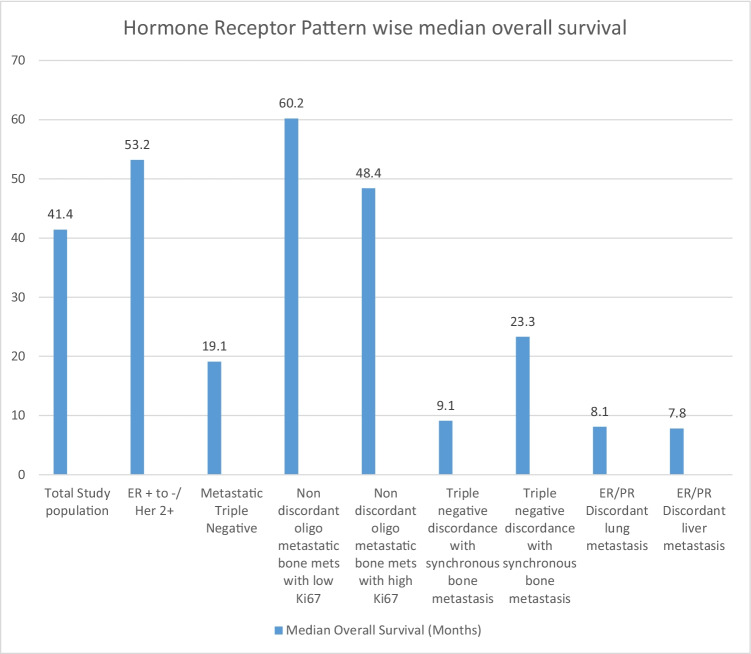
The median overall survival for different categories was documented (Fig. 7).
Fig. 7.
Hormone receptor pattern wise median overall survival in months. ER, estrogen receptor; PR, progesterone receptor
Synchronous metastasis with discordance in ER and PR is associated with rapid disease progression of disease. Synchronous metastasis with discordant Ki-67 is associated with rapid disease progression than triple negative disease. Contralateral axillary metastasis with high Ki-67 and no receptor discordance had complete response with chemotherapy and good prognosis. Complete clinical response rate in contralateral axillary metastasis group was 87.8%, followed by local only recurrence with high Ki-67 where chemotherapy had response rate of 81%. Non discordant bone-only metastasis also have high rate of chemotherapy response and complete clinical response rate of 63.2% in our series. Increased Ki-67 was more often associated with synchronous metastasis and node-only metastasis where early systemic chemotherapy incorporating both taxanes and anthracycline produced clinical response of about 80% which was statistically significant (p-0.001). No mortality was reported in the subset with contralateral axillary lymph nodes.
Discussion
Biomarkers like ER, PR, HER2/NEU, and Ki-67 are routinely performed and very important for choosing the most optimal adjuvant therapy for breast carcinoma. In cases of primary breast cancer, tamoxifen will be given in ER, PR-positive and trastuzumab in HER2-positive cases. Tamoxifen reduces the risk of breast cancer recurrence by 50%, after 5 years of treatment with early breast cancer [9]. As most targeted treatment decisions are made on the basis of biomarkers for hormone receptors and HER2/NEU, a change in biomarkers will have great impact on treatment recommendations. Many studies have previously shown a substantial discordance rate between primary tumor and metastatic disease, with studies reporting hormone receptor discordance rates between 30 and 40% and HER2/NEU discordance rates between 10 and 30% [10–12].
Primary and metastatic discordance could involve tumor sampling as well as intrinsic tumor heterogeneity as etiology. Host environmental changes and treatment consequences could cause heterogeneous tumor clones with different hormone receptor and HER2/NEU biomarkers [13]. In particular, tumors may develop receptor downregulation or resistance from patients who previously received adjuvant hormone or HER2/NEU-targeted treatment. Discordance between primary and metastatic breast cancer has been suggested for many years; however, most studies were retrospective, with only a few prospective studies now reporting biomarker discordance rates [10, 11].
In our multi-institutional prospective study of 110 patients of breast carcinoma with recurrent and/(or) metastatic disease, ER discordance was seen in 26.38% and PR discordance seen in 19.17% cases. Twelve cases had both ER and PR discordance. HER2/NEU discordance was seen in 16.6% cases. A single-institution analysis by M.V. Dieci et al. [14] evaluated the rates of changes in single-receptor expression, with results of 13.4%, 39%, and 11.8% for ER, PR, and HER2, respectively [14]. In a study by Aitken SJ et al, patients with hormone receptor–positive primary breast cancer treated with endocrine therapy where result was suboptimal because they were hormone receptor–negative metastatic disease [15]. It shows the importance of receptor studies and discordance in primary and metastatic cancer. Herceptin (trastuzumab) is administered in patients whom tumor in the breast overexpresses HER2/NEU. However, if the HER2/NEU status at the metastatic site is different than what it is at the primary site, then therapy would have to be revised. Biopsy of the metastatic site and HER2/NEU status are essential [16]. In our study, HER2/NEU discordance was seen in 16.6% cases where no cases turned out to be positive in the metastatic site which was negative in primary.
Ki-67 was positive in 49.9% cases. All these patients had Ki-67 in primary > 14% and increased at least beyond 30% in the metastatic site. Our results strongly highlight the prognostic effect of discordance between matched primary breast cancer and recurrence.
Within the discordant group, a loss of a receptor expression rather than gain resulted as the main determinant of poor prognosis. High Ki-67 as a proliferative marker has increased response to chemotherapy but earlier relapse and disease progression especially in Luminal B type.
Ki-67 discordance was highest for contralateral axillary nodal metastasis, with overall discordance 71% followed by local recurrence 69.23%, then for contralateral supraclavicular nodal metastasis 53%. Discordance to Ki-67 was lower for bone metastasis 14%. In a study by Park et al., discordance was observed in 28.4% cases [17]. In another study done by Tawfiq et al., it was observed that Ki-67 expression showed 81.3% concordance in primary breast cancer and metastatic cancer [18]. Synchronous metastases with discordance in ER and PR are associated with rapid disease progression and decreased OS. Synchronous metastasis with discordant Ki-67 is associated with rapid disease progression than triple negative disease.
Contralateral axillary metastasis with high Ki-67 and no receptor discordance had complete response with chemotherapy and good prognosis. In a study done by Tawfiq et al., a small subgroup of 6.7% cases were noted to have a nodal Ki-67 of ≥ 10% as compared with < 10% in primary breast cancer and these patients were reported to have worse survival [18].
Non discordant bone-only metastasis also have high rate of chemo response and complete clinical response rate of 63.2% in our series. Increased Ki-67 was more often associated with synchronous metastasis and node-only metastasis where early systemic chemotherapy incorporating both taxol and anthracycline produced clinical response of about 80%. This proves that high Ki-67 subset of Luminal B–like tumors progressed rapidly than triple negative and HER2/NEU-positive subset.
In conclusion, receptor discordance in metastatic breast cancer is a known entity. However, discordance in synchronous metastasis and its implication on therapeutic and prognostic outcome is a new area for research. Non discordant oligometastatic bone disease have high rate of chemo response. Molecular markers and their expression and discordance pattern determine the therapeutic outcome and prognosis of the disease. Early identification and targeting the discordance would go a long way in improving the outcome and OS of breast cancer patients.
Declarations
Ethics Approval
This is an observational study and hence exempted from ethical approval by institutional ethical committee.
Informed Consent
Informed consent was obtained from all individuals included in this study for participation in study and publication.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maheswaran Satishkumar and Muthuvel Ramesh contributed equally to this work.
Contributor Information
Maheswaran Satishkumar, Email: drmsatishkumar@gmail.com.
Muthuvel Ramesh, Email: oncomramesh@gmail.com.
Jeevan G. Sanjive, Email: jeevansanjeev@gmail.com
References
- 1.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheang MC, Chia SK, Voduc D, et al. Ki-67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Ross JS, Fletcher JA. HER-2/neu (c-erb-B2) gene and protein in breast cancer. Am J Clin Pathol. 1999;112(1 Suppl 1):53–67. [PubMed] [Google Scholar]
- 5.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27(34):5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes J, Li L, Schlueter C, et al. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;138(4):867–873. [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin AA, Ro J, Ro JY, et al. Ki-67 immunostaining in node-negative stage I/II breast carcinoma Significant correlation with prognosis. Cancer. 1991;68(3):549–557. doi: 10.1002/1097-0142(19910801)68:3<549::AID-CNCR2820680318>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 8.Keshgegian AA, Cnaan A. Proliferation markers in breast carcinoma Mitotic figure count, S-phase fraction, proliferating cell nuclear antigen, Ki-67 and MIB-1. Am J Clin Pathol. 1995;104(1):42–9. doi: 10.1093/ajcp/104.1.42. [DOI] [PubMed] [Google Scholar]
- 9.Santinelli A, Pisa E, Stramazzotti D, Fabris G. HER-2 status discrepancy between primary breast cancer and metastatic sites Impact on target therapy. Int J Cancer. 2008;122:999–1004. doi: 10.1002/ijc.23051. [DOI] [PubMed] [Google Scholar]
- 10.Kulka J, Székely B, Lukács LV, et al. Comparison of predictive immunohistochemical marker expression of primary breast cancer and paired distant metastasis using surgical material: a practice-based study. J Histochem Cytochem. 2016;64(4):256–267. doi: 10.1369/0022155416639013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li MH, Hou CL, Wang C, Sun AJ. HER-2, ER, PR status concordance in primary breast cancer and corresponding metastatic lesion in lymph node in Chinese women. Pathol Res Pract. 2016;212(4):252–257. doi: 10.1016/j.prp.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Domanski AM, Monsef N, Domanski HA, Grabau D, Fernö M. Comparison of the oestrogen and progesterone receptor status in primary breast carcinomas as evaluated by immunohistochemistry and immunocytochemistry: a consecutive series of 267 patients. Cytopathology. 2013;24(1):21–25. doi: 10.1111/j.1365-2303.2012.00997.x. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson E, Appelgren J, Solterbeck A, Bergenheim M, Alvariza V, Bergh J. Breast cancer during follow-up and progression – a population based cohort on new cancers and changed biology. Eur J Cancer. 2014;50(17):2916–2924. doi: 10.1016/j.ejca.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Dieci MV, Barbieri E, Piacentini F, et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann Oncol. 2013;24:101–108. doi: 10.1093/annonc/mds248. [DOI] [PubMed] [Google Scholar]
- 15.Aitken SJ, Thomas JS, Langdon SP, Harrison DJ, Faratian D. Quantitative analysis of changes in ER, PR and HER2 expression in primary breast cancer and paired nodal metastases. Ann Oncol. 2010;21:1254–1261. doi: 10.1093/annonc/mdp427. [DOI] [PubMed] [Google Scholar]
- 16.Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2 overexpressing primary breast tumors. J Clin Oncol. 2012;30:593–599. doi: 10.1200/JCO.2010.33.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park D, Karesen R, Noren T, Sauer T. Ki-67 expression in primary breast carcinomas and their axillary lymph node metastases: clinical implications. Virchows Arch. 2007;451:11–18. doi: 10.1007/s00428-007-0435-2. [DOI] [PubMed] [Google Scholar]
- 18.Tawfik K, Kimler BF, Davis MK, Fan F, Tawfik O. Ki-67 expression in axillary lymph node metastases in breast cancer is prognostically significant. Hum Pathol. 2013;44:39–46. doi: 10.1016/j.humpath.2012.05.007. [DOI] [PubMed] [Google Scholar]