Abstract
Purpose
This study aims to investigate the associations of the systemic immune-inflammation index (SII) with bone mineral density (BMD) and osteoporosis in adult females from a nationally representative sample.
Methods
A cross-sectional study was performed among 4092 females aged ≥20 years from the National Health and Nutrition Examination Survey 2007–2010. Linear and logistic regressions were applied to explore the relationships of SII with BMD and the risk of osteoporosis, respectively.
Results
Linear regression analyses found that a doubling of SII levels was significantly correlated with a 1.39% (95% CI: 0.57%, 2.20%) decrease in total femur BMD, a 1.16% (95% CI: 0.31%, 2.00%) decrease in femur neck BMD, a 1.73% (95% CI: 0.78%, 2.66%) decrease in trochanter BMD, and a 1.35% (95% CI: 0.50%, 2.20%) decrease in intertrochanteric BMD among postmenopausal women, after adjusting for covariates. Logistic regression analyses showed that compared with postmenopausal women in the lowest SII quartile, those in the highest quartile had higher risks of osteoporosis in the total femur (odds ratio (OR) = 1.70, 95% CI: 1.04, 2.76), trochanter (OR = 1.86, 95% CI: 1.07, 3.38), intertrochanter (OR = 2.01, 95% CI: 1.05, 4.04) as well as overall osteoporosis (OR = 1.57, 95% CI: 1.04, 2.37). In contrast, there was no significant association between SII and BMD in premenopausal women.
Conclusions
SII levels were negatively associated with BMD levels in postmenopausal women but not in premenopausal women. Elevated SII levels could be a potential risk factor for osteoporosis in postmenopausal women.
Keywords: bone mass density, osteoporosis, systemic immune-inflammation index, menopause, NHANES
Introduction
Osteoporosis is a metabolic bone disease characterized by decreased bone mineral density (BMD) and perturbation of bone quality. World Health Organization (WHO) guidelines use dual-energy X-ray absorptiometry (DXA) scans to measure BMD and define osteoporosis as being ≤2.5 standard deviations (s.d.) of the mean BMD in young healthy females (1). Osteoporosis can cause increased bone fragility and risks of osteoporotic fractures (2). It was estimated that the annual case and healthcare burden of osteoporosis-related fractures were expected to reach approximately three million cases and $25.3 billion in the United States in 2025, respectively (3). The increasing incidence of osteoporosis and the resulting number of fractures places a heavy burden on public health services, especially among women. Osteoporosis and low bone mass are more common in women than in men (4, 5), and one-third of women aged >50 years worldwide have been reported to suffer from osteoporotic fractures (6). This situation prompts a global effort to find more effective preventions for osteoporosis in women. Early detection of osteoporosis and intervention with protective measures are important healthcare strategies. Therefore, it is also very important to explore more indicators that can reflect osteoporosis.
Osteoporosis is generally accompanied by low-grade inflammation development (7). Previous in vitro experiments showed that the inflammatory response could cause differentiation or apoptosis of bone mesenchymal stem cells and osteoblasts through oxidative stress, which is related to the pathogenesis of osteoporosis (8, 9). The systemic immune-inflammation index (SII) is a new inflammatory index based on peripheral blood neutrophils, lymphocytes, and platelet counts. Previous studies have confirmed that SII can objectively reflect inflammatory status in tumor patients (10, 11). It was suggested that SII could be used as a marker to predict the prognosis of solid tumors (12), but still few studies have explored the relationships of SII with BMD and osteoporosis. Two previous clinical studies proposed that SII could be a risk predictor for osteoporosis and fracture in women (13, 14). According to these studies, SII may be correlated with BMD and osteoporosis. However, these previous results came from limited sample size or hospitalized patients, so they may not represent the characteristics of the general population.
Therefore, the present study aims to investigate whether SII is associated with BMD and osteoporosis in female adults using data from a nationally representative population.
Methods and materials
Study population
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional survey designed to collect information on the health status of the U.S. household population. Public-use NHANES data are released in a 2-year cycle by the National Center for Health Statistics (NCHS). All participants received a set of comprehensive questionnaires, physical examinations, and laboratory tests. The datasets from NHANES were widely applied in health-related studies (15, 16, 17). Each participant provided informed consent, and all study protocols were approved by the NCHS’s Institutional Review Board. No further ethical approval is needed. Details regarding the survey design, methods, and datasets have been published and are publicly available on the NHANES website.
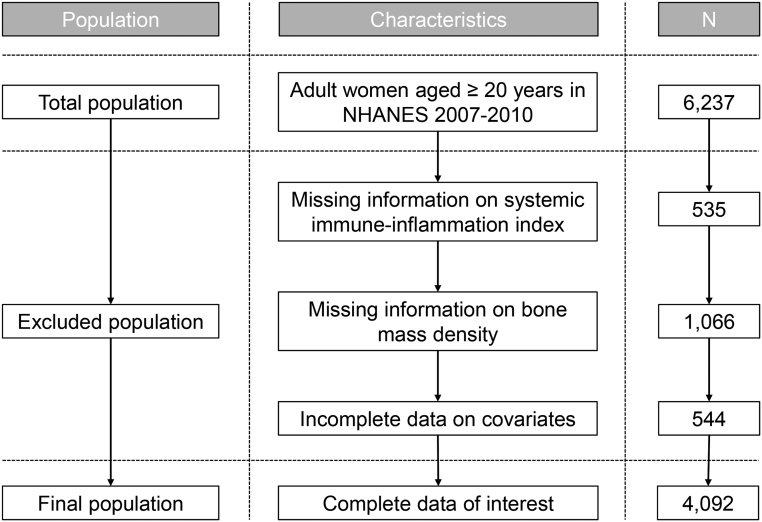
Given that there was no DXA femur bone data in the NHANES 2011–2012 cycle, survey data used for analysis were extracted from the 2007–2008 and 2009–2010 cycles. The flow chart is presented in Fig. 1. Initially, 6237 women aged ≥20 years were selected from the continuous NHANES (2007–2010) datasets. Women without information regarding SII (n = 535) and BMD (n = 1066) were excluded. We then excluded 544 participants who had incomplete data on the covariates of interest and who suffered from lymphoma, infections, or autoimmune disease. Finally, a total of 4092 adult women were included in this study. The menopausal status was defined based on the absence of regular periods in the past 12 months or age over 55 years described previously (18). The final analytic sample contained 1948 premenopausal women and 2144 postmenopausal women.
Figure 1.
Flowchart of the study participant selection process.
Bone mineral density measurements and definition of osteoporosis
BMD testing was measured by DXA. The BMD values (gm/cm2) were obtained in four femoral regions, including the femur neck, trochanter, total femur, and intertrochanter. The details about methods and quality control have been shown in the DXA procedures online manual. The exclusion criteria for participants in the DXA examination were also described in previous studies (6, 19).
BMD levels that surpassed 2.5 s.d. below the mean of young adults aged 20–29 years were defined as osteoporosis based on WHO guidelines. The young reference group used in our analyses was described in previous papers (6, 19). Osteoporosis was evaluated in the femur neck, total femur, intertrochanter, and trochanter, whose thresholds were 0.56 g/cm2, 0.67 g/cm2, 0.79 g/cm2, and 0.46 g/cm2, respectively (19). The BMD value below the threshold in any femoral region was considered overall osteoporosis.
Measurement of peripheral blood cell counts
Blood specimens were measured with a Coulter HMX Hematology Analyser in the Mobile Examination Centers (MECs). Detailed information regarding the quality assurance and quality control procedures is described in the NHANES Laboratory Procedure Manual (https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/CBC_F_met_HE.pdf). Data on platelet counts, neutrophils, and lymphocytes were collected. The SII level was calculated using the formula (platelet count × neutrophil count/lymphocyte count) (10, 13).
Covariates
Covariates were identified by literature reviews (19, 20). Information about covariates was also collected through the questionnaires and health examination in the mobile centers, including age, race, education levels, annual family income, body mass index (BMI), physical activity, serum cotinine, marital status, calcium intake, smoking status, drinking status, and use of prednisone or cortisone. Study participants who had at least 12 alcoholic drinks/year were considered as drinkers, and those who smoked at least 100 cigarettes in life were considered as smokers following previous studies (21, 22). The calcium intake was classified into three groups based on tertiles of estimated calcium intake during the 24 hours before the interview (low, <621 mg/day; middle, 621–1026 mg/day; high, >1026 mg/day). The use of prednisone/cortisone was defined as having ever taken any prednisone or cortisone nearly every day for a month or longer. The different levels of physical activity were defined following the Physical Activity Guidelines for Americans (23).
Statistical analysis
All statistical analyses were conducted using R version 4.0.5 (The R Foundation for Statistical Computing, Vienna, Austria). Weighted descriptive information (Full Sample 2 Year MEC Exam Weight, WTMEC2YR) stratified by menopausal status was performed on all variables using the ‘survey’ package (16, 24). The significance of the differences in variables of basic characteristics between premenopausal and postmenopausal women was evaluated. A separate analysis stratified by menopausal status was conducted, given that menopausal status is an important factor of BMD in women. The significance of the differences in BMD levels at different femoral regions across quartiles of SII was evaluated by the Kruskal–Wallis test.
The associations of SII levels as continuous variables with BMD in females were first analyzed by multivariable linear regression models. For regression analysis, both SII and BMD were Ln-transformed to approximate a normal distribution. The SII levels were also set as categorical variables and analyzed using the first SII quartile group as the reference group. The results were expressed as estimated percent changes with 95% confidence intervals (CIs) for a doubling of SII levels in BMD levels according to our previous studies (15, 24). Adjusted odds ratios (ORs) for osteoporosis were evaluated by logistic regression models. All models were performed adjusting for the covariates mentioned earlier, including age, race, education levels, serum cotinine, annual family income, BMI, physical activity, marital status, calcium intake, smoking status, drinking status, and use of prednisone or cortisone. Furthermore, the receiver operating characteristic (ROC) curves were applied to evaluate SII as a diagnostic marker for osteoporosis. The optimal cut-off values of SII in any femoral regions of interest were selected when Youden’s index reached the maximum value. A two-sided P-value < 0.05 was considered statistically significant.
Results
Characteristics of participants
The study population included 1948 premenopausal women (mean age = 35.31 ± 9.48 years) and 2144 postmenopausal women (mean age = 62.55 ± 12.26 years). The basic characteristics of the study participants stratified by menopausal status are presented in Table 1. Compared with premenopausal women, postmenopausal women were more likely to be non-Hispanic White, have a lower level of education, be poorer, have a higher BMI level, be sedentary, be widowed/divorced/separated, be smokers, be non-drinkers, have less in calcium intake, and use prednisone or cortisone. No differences in serum cotinine levels were found.
Table 1.
Basic characteristics of study population stratified by menopausal status.
| Characteristicsa | Overall | Premenopause | Postmenopause | P valuesb |
|---|---|---|---|---|
| n | 4092 | 1948 | 2144 | |
| Age, years | 49.58 ± 17.51 | 35.31 ± 9.48 | 62.55 ± 12.26 | <0.001 |
| BMI, kg/m2 | 28.54 ± 6.29 | 28.08 ± 6.46 | 28.95 ± 6.10 | <0.001 |
| Serum cotinine, ng/mL | 48.90 ± 115.06 | 51.68 ± 111.55 | 46.35 ± 118.15 | 0.141 |
| Race, n (%) | <0.001 | |||
| Mexican American | 724 (17.69%) | 385 (19.76%) | 339 (15.81%) | |
| Non-Hispanic White | 2052 (50.15%) | 897 (46.05%) | 1155 (53.87%) | |
| Non-Hispanic Black | 703 (17.18%) | 335 (17.20%) | 368 (17.16%) | |
| Others | 613 (14.98%) | 331 (16.99%) | 282 (13.15%) | |
| Education, n (%) | <0.001 | |||
| <High school | 1082 (26.44%) | 439 (22.54%) | 643 (29.99%) | |
| High school | 922 (22.53%) | 391 (20.07%) | 531 (24.77%) | |
| >High school | 2088 (51.03%) | 1118 (57.39%) | 970 (45.24%) | |
| Annual family income, n (%) | <0.001 | |||
| <$20,000 | 950 (23.22%) | 380 (19.51%) | 570 (26.59%) | |
| $20,000–44,999 | 1404 (34.31%) | 644 (33.06%) | 760 (35.45%) | |
| ≥$45,000 | 1738 (42.47%) | 924 (47.43%) | 814 (37.97%) | |
| Physical activity, n (%) | <0.001 | |||
| Sedentary | 1249 (30.52%) | 434 (22.28%) | 815 (38.01%) | |
| Insufficient | 615 (15.03%) | 267 (13.71%) | 348 (16.23%) | |
| Moderate | 490 (11.97%) | 250 (12.83%) | 240 (11.19%) | |
| High | 1738 (42.47%) | 997 (51.18%) | 741 (34.56%) | |
| Marital status, n (%) | <0.001 | |||
| Married/cohabiting | 2261 (55.25%) | 1137 (58.37%) | 1124 (52.43%) | |
| Widowed/divorced/separated | 1177 (28.76%) | 304 (15.61%) | 873 (40.72%) | |
| Never married | 654 (15.98%) | 507 (26.03%) | 147 (6.86%) | |
| Smoker, n (%) | 0.002 | |||
| No | 2473 (60.43%) | 1225 (62.89%) | 1248 (58.21%) | |
| Yes | 1619 (39.57%) | 723 (37.11%) | 896 (41.79%) | |
| Drinker, n (%) | <0.001 | |||
| No | 2573 (62.88%) | 557 (28.59%) | 962 (44.87%) | |
| Yes | 1519 (37.12%) | 1391 (71.41%) | 1182 (55.13%) | |
| Calcium intake, n (%) | <0.001 | |||
| Low | 1531 (37.41%) | 680 (34.91%) | 851 (39.69%) | |
| Middle | 1462 (35.73%) | 656 (33.68%) | 806 (37.59%) | |
| High | 1099 (26.86%) | 612 (31.42%) | 487 (22.71%) | |
| Use of prednisone or cortisone, n (%) | <0.001 | |||
| No | 3853 (94.16%) | 1866 (95.79%) | 1987 (92.68%) | |
| Yes | 239 (5.84%) | 82 (4.21%) | 157 (7.32%) |
aMean ± S.D. for continuous variables and percentages for categorical variables were weighted.
bP values for differences between the premenopause and postmenopause groups were calculated by Mann–Whitney U test and chi-square test, respectively.
SII levels were then categorized into quartiles among pre- and postmenopausal women (cutoff points were 376.20, 514.90, and 705.50 for premenopausal women, and 337.80, 463.00, and 644.00 for postmenopausal women). The distributions of BMD levels in different bone sites according to the SII quartile groups are depicted in Table 2. There was a decreasing tendency for BMD in each bone site, as the SII increased in postmenopausal women. However, no significant trend was observed in premenopausal women.
Table 2.
The distributions of bone mass density stratified by quartiles of systemic immune-inflammation index.
| Characteristicsa | Overall | Systemic immune-inflammation indexb | P valuesc | |||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| Premenopause | ||||||
| Total femur BMD, g/cm2 | 0.972 ± 0.129 | 0.971 ± 0.132 | 0.965 ± 0.122 | 0.974 ± 0.132 | 0.979 ± 0.129 | 0.394 |
| Femur neck BMD, g/cm2 | 0.873 ± 0.130 | 0.876 ± 0.135 | 0.867 ± 0.122 | 0.875 ± 0.132 | 0.872 ± 0.130 | 0.732 |
| Trochanter BMD, g/cm2 | 0.727 ± 0.110 | 0.728 ± 0.111 | 0.723 ± 0.105 | 0.725 ± 0.112 | 0.732 ± 0.109 | 0.610 |
| Intertrochanter BMD, g/cm2 | 1.145 ± 0.151 | 1.140 ± 0.156 | 1.137 ± 0.145 | 1.148 ± 0.153 | 1.156 ± 0.151 | 0.208 |
| Postmenopause | ||||||
| Total femur BMD, g/cm2 | 0.857 ± 0.146 | 0.867 ± 0.142 | 0.863 ± 0.149 | 0.854 ± 0.138 | 0.845 ± 0.154 | 0.056 |
| Femur neck BMD, g/cm2 | 0.739 ± 0.137 | 0.752 ± 0.138 | 0.740 ± 0.135 | 0.738 ± 0.135 | 0.727 ± 0.139 | 0.028 |
| Trochanter BMD, g/cm2 | 0.643 ± 0.121 | 0.651 ± 0.120 | 0.648 ± 0.122 | 0.640 ± 0.117 | 0.633 ± 0.126 | 0.067 |
| Intertrochanter BMD, g/cm2 | 1.020 ± 0.176 | 1.031 ± 0.169 | 1.029 ± 0.181 | 1.015 ± 0.166 | 1.005 ± 0.187 | 0.049 |
aBMD levels are expressed as the mean ± s.d.
bThe ranges of SII quartiles 1–4 were <376.20, 376.20–514.90, 515.00–705.50, and >705.50 in premenopausal women and <337.80, 337.80–463.00, 463.10–644.00, and >644.00 in postmenopausal women, respectively.
cP values were derived from Kruskal–Wallis tests among different SII quartile groups.
Associations between SII and BMD
Table 3 presents the estimated association between BMD levels at each site and SII levels using linear regression analyses. After adjusting covariates, a doubling of SII levels was significantly correlated with a 1.39% (95% CI: 0.57%, 2.20%) decrease in total femur BMD, a 1.16% (95% CI: 0.31%, 2.00%) decrease in femur neck BMD, a 1.73% (95% CI: 0.78%, 2.66%) decrease in trochanter BMD, and a 1.35% (95% CI: 0.50%, 2.20%) decrease in intertrochanteric BMD in postmenopausal women. When SII levels were modeled as categorical variables, postmenopausal women whose SII levels in the fourth quartile had 1.85% (95% CI: 0.63%, 3.08%) lower total femur BMD, 1.58% (95% CI: 0.30%, 2.84%) lower femur neck BMD, 2.19% (95% CI: 0.77%, 3.59%) lower trochanter BMD, and 1.97% (95% CI: 0.59%, 3.13%) lower intertrochanteric BMD than those in the first quartile. Moreover, P-values for the trend in the models of the total femur, trochanter, and intertrochanter were significant in postmenopausal women. However, no significant associations of SII with BMD in any bone sites in premenopausal women were observed.
Table 3.
Percent change (95% CI) in bone mass density by the systemic immune-inflammation index in pre- and postmenopausal women.
| Variables | n | Total femur | Femur neck | Trochanter | Intertrochanter |
|---|---|---|---|---|---|
| Premenopause | |||||
| Per doubling of SII | 1948 | −0.06 (−0.81, 0.69) | −0.39 (−1.21, 0.44) | −0.40 (−1.28, 0.49) | 0.18 (−0.58, 0.94) |
| SII quartiles | |||||
| Quartile 1 (<376.20) | 487 | Reference | Reference | Reference | Reference |
| Quartile 2 (376.20–514.90) | 487 | 0.08 (−0.06, 1.13) | 0.08 (−1.07, 1.25) | 0.02 (−1.20, 1.27) | 0.18 (−0.87, 1.24) |
| Quartile 3 (515.00–705.50) | 487 | −0.06 (−1.11, 0.99) | −0.21 (−1.36, 0.95) | −0.50 (−1.72, 0.74) | 0.14 (−0.91, 1.20) |
| Quartile 4 (>705.50) | 487 | 0.38 (−0.68, 1.45) | −0.16 (−1.32, 1.10) | 0.12 (−1.12, 1.38) | 0.71 (−0.36, 1.79) |
| P for trend | 0.558 | 0.677 | 0.936 | 0.228 | |
| Postmenopause | |||||
| Per doubling of SII | 2144 | −1.39 (−2.20, −0.57)c | −1.16 (−2.00, −0.31)b | −1.73 (−2.66, −0.78)c | −1.35 (−2.20, −0.50)b |
| SII quartiles | |||||
| Quartile 1 (<337.80) | 536 | Reference | Reference | Reference | Reference |
| Quartile 2 (337.80–463.00) | 537 | −0.48 (−1.70, 0.75) | −0.79 (−2.05, 0.47) | −0.43 (−1.84, 0.99) | −0.38 (−1.64, 0.90) |
| Quartile 3 (463.10–644.00) | 536 | −0.13 (−1.35, 1.11) | 0.16 (−1.11, 1.45) | −0.39 (−1.80, 1.04) | −0.18 (−1.46, 1.11) |
| Quartile 4 (>644.00) | 535 | −1.86 (−3.08, −0.63)b | −1.58 (−2.84, −0.30)a | −2.19 (−3.59, −0.77)b | −1.97 (−3.13, −0.59)b |
| P for trend | 0.008 | 0.068 | 0.005 | 0.009 |
All models were adjusted for age, race, education levels, annual family income, BMI, serum cotinine, physical activity, marital status, calcium intake, smoking status, drinking status, and use of prednisone or cortisone. aP < 0.05, bP < 0.01, cP < 0.001.
Associations between SII and osteoporosis
Given the very limited number of premenopausal women who had osteoporosis in this study, associations between SII and osteoporosis were analyzed only in postmenopausal women since the number of postmenopausal women with osteoporosis in this dataset was enough to conduct analysis. Table 4 shows the fully adjusted ORs of SII for osteoporosis among the study participants. A doubling of SII was associated with 1.48 (95% CI: 1.08, 2.04) OR for total femur osteoporosis, 1.72 (95% CI: 1.16, 2.54) OR for trochanter osteoporosis, 1.56 (95% CI: 1.14, 2.13) OR for intertrochanteric osteoporosis, and 1.43 (95% CI: 1.09, 1.89) OR for overall osteoporosis. After SII was converted into a categorical variable, postmenopausal women at the highest SII quartile had a significantly increased risk of osteoporosis in the total femur (OR = 1.70, 95% CI: 1.04, 2.76), trochanter (OR = 1.81, 95% CI: 1.07, 3.38), intertrochanter (OR = 2.01, 95% CI: 1.05, 4.04), and overall osteoporosis (OR = 1.57, 95% CI: 1.04, 2.37) than those at the lowest quartile of SII. However, no significant associations were observed for SII with femur neck osteoporosis in any models.
Table 4.
Association of the systemic immune-inflammation index with osteoporosis in postmenopausal women.
| Variables | n | OR (95% CI) of osteoporosis | ||||
|---|---|---|---|---|---|---|
| Total femur | Femur neck | Trochanter | Intertrochanter | Overall | ||
| Per doubling of SII | 2144 | 1.48 (1.08, 2.04)a | 1.32 (0.95, 1.85) | 1.72 (1.16, 2.54)b | 1.56 (1.14, 2.13)b | 1.43 (1.09, 1.89)a |
| SII quartiles | ||||||
| Quartile 1 (<337.80) | 536 | Reference | Reference | Reference | Reference | Reference |
| Quartile 2 (337.80–463.00) | 537 | 1.34 (0.81, 2.22) | 1.02 (0.60, 1.40) | 1.25 (0.65, 2.23) | 0.89 (0.59, 1.33) | 1.01 (0.65, 1.56) |
| Quartile 3 (463.10–644.00) | 536 | 1.02 (0.61, 1.70) | 1.00 (0.59, 1.68) | 1.31 (0.69, 2.51) | 1.53 (0.92, 2.53) | 0.97 (0.64, 150) |
| Quartile 4 (>644.00) | 535 | 1.70 (1.04, 2.76)a | 1.27 (0.76, 2.22) | 1.81(1.07, 3.38)a | 2.01 (1.05, 4.04)a | 1.57 (1.04, 2.37) a |
| P for trend | 0.048 | 0.362 | 0.039 | 0.013 | 0.034 | |
All models were adjusted for age, race, education levels, annual family income, BMI, serum cotinine, physical activity, marital status, calcium intake, smoking status, drinking status, and use of prednisone or cortisone. aP < 0.05, bP < 0.01.
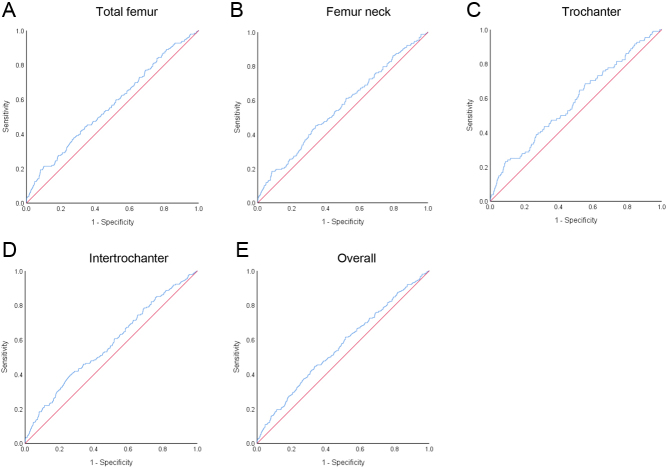
ROC curves were used to further evaluate the diagnostic value of SII for osteoporosis in the femur neck, total femur, intertrochanter, and trochanter, and each ROC curve is displayed in Fig. 2. The areas under the ROC curve (AUC) of SII for osteoporosis were 0.57 (95% CI:0.52, 0.61; P = 0.022) in the total femur, 0.56 (95% CI:0.51, 0.61; P = 0.009) in the femur neck, 0.58 (95% CI:0.52, 0.64; P = 0.005) in the trochanter, and 0.58 (95% CI:0.53, 0.62; P < 0.001) in the intertrochanter as well as 0.56 (95% CI: 0.53, 0.60; P = 0.001) for overall. The best cutoff values of SII for the diagnosis of osteoporosis were 865.5 in the total femur, 567.5 in the femur neck, 888.9 in the trochanter, and 623.4 in the intertrochanter as well as 567.6 for overall.
Figure 2.
Efficacy of SII in discriminating osteoporosis in postmenopausal women. Receiver operating characteristic (ROC) curves of SII in discriminating osteoporosis regarding the total femur (A), femur neck (B), trochanter (C), and intertrochanter (D) as well as overall osteoporosis (E) are presented.
Discussion
Our study examined the relationships of SII with BMD and osteoporosis in pre- and postmenopausal women using nationally representative data. We observed that SII was negatively correlated with BMD levels at the femur neck, total femur, trochanter, and intertrochanteric sites in postmenopausal women, even after adjusting for covariates. Similarly, compared with individuals in the lowest quartile of SII, postmenopausal women in the highest quartile have higher risks of osteoporosis at the total femur, trochanter, and intertrochanteric sites. In contrast, no significant associations between SII and BMD were observed in premenopausal women. To our knowledge, this is the first report using the data of NHANES to study the relationships of SII with BMD and osteoporosis in both postmenopausal women and premenopausal women, highlighting the importance of the role of immuno-inflammatory responses in the risk of osteoporosis.
It is well known that normal bone formation and function in the body depend on a homeostatic process between osteogenesis and bone resorption (25). Activation of the inflammatory microenvironment and a compromised immune system significantly affect the microarchitecture of bone (26). Multiple immune cells can reside in the bone marrow cavity (27). Dysfunctional lymphocytes can initiate cascades of inflammatory cytokines and chemokines and trigger the aggregation of inflammatory cells (28, 29). The homeostasis of bone function is thereby disrupted, which generally favors osteoclast-induced bone resorption. Therefore, the immune-inflammation imbalance may lead to osteopenia and weaken bone strength and density, ultimately leading to osteoporosis.
SII is a promising inflammatory indicator that can comprehensively reflect the immune and inflammatory state of the body. Considering that platelets, neutrophils, and lymphocytes are part of routine blood examination and that routine blood examination is simple, economical, and rich in useful parameters, the application of SII is worthy of further study. A few studies about SII and osteoporosis in women were conducted in a clinical setting. A prospective cohort study of 238 postmenopausal women from two hospitals reported that postmenopausal osteoporosis patients had higher SII than the controls (13). Another study enrolled 413 postmenopausal women and showed a negative association between SII and the mean BMD of the femoral neck (14). However, researches on a large population are still very needed. In our study, we explored the association in representative adult populations to ensure more valid and representative results. Consistent with a previous study (14), our study also showed the relationship between SII and femoral neck BMD among postmenopausal women. Furthermore, our study conducted a more comprehensive analysis and found that SII was also correlated with decreased BMD at the total femur, trochanter, and intertrochanteric sites.
In this study, risks for osteoporosis were next evaluated by logistic regression models. Our results found that SII was associated with increased risks of osteoporosis in postmenopausal women. Considering osteoporosis as a risk factor for fracture, it is possible that SII could be a risk factor for fracture. Our results showed that the reduction of BMD could reach 2% with a doubling of SII change. A previous study using a meta-regression of published trials found a 2% improvement in femoral neck BMD was associated with a 28% reduction in vertebral fracture, a 15% reduction in hip fracture, and a 11% reduction in nonvertebral fracture (30). Thus, these about 2% reductions of BMD with a doubling of SII could be large enough to affect fracture risks. However, since our data on fracture was still lacking, more data on fracture are still needed to support the association between SII and fracture. The predictive/diagnostic capabilities of SII were also assessed using ROC curves in this study. Although ROC curves showed that SII had significant differences in discriminating osteoporosis in postmenopausal women, it seemed that there is relatively low discriminatory power for SII in osteoporosis diagnosis (all AUCs less than 0.7). The results of ROC curves suggested that the efficacy of the SII indicator alone in discriminating subjects with osteoporosis was not high enough. Thus, more studies are needed to explore SII as an early marker of osteoporosis in postmenopausal women.
The association between SII and testosterone may differ by menopausal status since we observed significant associations in postmenopausal women but no association in premenopausal women. However, the possible mechanism is unclear, since the present study only provided a correlation in the populations. Combined with the previous studies, there were several possibilities. It is well known that estrogen is strongly related to osteoporosis, and estrogen deficiency can lead to increased apoptosis on osteoblast, bone loss, and osteoporosis (7, 31). We speculated that it might be related to changes in sex hormone levels after menopause. Numerous studies have demonstrated the ability of sex hormones to modulate various aspects of the immune system by acting on estrogen receptors expressed in various inflammatory and immune cells (7). Several studies showed that estrogen deficiency could lead to the activation of multiple immune cells to produce multiple pro-inflammatory factors that induce bone remodeling dysfunction and decreased bone density in postmenopausal women (7, 31, 32). The inflammatory marker was likely able to be strongly influenced by estrogen levels. However, the data in this study showed that mean SII was higher in the premenopausal group than in the postmenopausal group. Due to the large differences in estrogen levels and BMD, the relationships between post- and premenopausal women cannot be compared. Thus, the inflammatory response might not be directly responsible for lowering BMD but rather exacerbate osteoporosis in the presence of estrogen deficiency. Since the SII index is only a cruder indication of the level of inflammation in the human body, more specific inflammatory indexes are still needed for more detailed and in-depth exploration. These may explain why our study found a significant correlation in postmenopausal women but not in premenopausal women.
The strengths of our study included a nationally representative population and statistical models with adjustments for a variety of important confounders. We believe our findings are likely applicable to the general population. In addition, the SII levels were treated as both continuous variables and categorical variables to reduce the contingency in the statistical analysis process and enhance the robustness of our results. Some limitations should also be considered. First, it was difficult to identify the temporal relationship of SII with osteoporosis because of the cross-sectional design. Prospective studies are still needed to clarify the causal relationship. Second, many genetic and non-genetic factors were linked to the pathogenesis of osteoporosis, but our data analysis was primarily adjusted for some demographic and lifestyle variables. Third, some common inflammatory biomarkers, such as hs-CRP, IL-6, and TNF-alpha, may also be associated with osteoporosis, but they are not available in the NHANES dataset. Finally, this study excluded all patients with lymphoma, infections, or autoimmune disease from the analysis, which might influence both SII and BMD. This also limited our application in clinical patients. Besides, we need to acknowledge that there are indeed many confounding factors in real-world patient populations but not included in the present analysis due to the availability of relevant data in the NHANES dataset, which also limited our research. It requires us to carry out more detailed research in the future among real-world patient populations with different diseases.
Conclusions
In summary, this study revealed significant positive correlations between SII levels and decreased BMD in postmenopausal women but not in premenopausal women. Elevated SII levels showed higher risks for osteoporosis in postmenopausal women. Our study provides new evidence for the role of immuno-inflammatory responses in bone loss in women. Further longitudinal population studies and experimental studies are still needed to verify our findings.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study was supported by the Scientific Research Fund of Zhejiang Provincial Education Department (Y202249200), the Qiuzhen Excellent Young Talent Program of Hangzhou Medical College (00004F1RCYJ2203), and the Scientific Research Project of Wenzhou Science and Technology Bureau (2021Y0395). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contribution statement
JZ contributed to the conceptualization, methodology, analysis, and drafting of the article; JJ, YQ, and YZ contributed to the acquisition of data, validation, and reviewing of the article; YW contributed to the conceptualization, methodology, and reviewing of the article; HX contributed to conceptualization, methodology, project administration, drafting, and reviewing of the article. All authors read and approved the version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgements
The authors express our gratitude to the National Center for Health Statistics of the CDC who was responsible for the planning and execution of the NHANES.
References
- 1.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R. & National Osteoporosis Foundation. Clinician’ s guide to prevention and treatment of osteoporosis. Osteoporosis International 2014252359–2381. ( 10.1007/s00198-014-2794-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 20113771276–1287. ( 10.1016/S0140-6736(1062349-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu QY, Li X, Miao ZN, Ye JX, Wang B, Zhang F, Xu RS, Jiang DL, Zhao MD, Yuan FL. Long non-coding RNAs: a new regulatory code for osteoporosis. Frontiers in Endocrinology 20189 587. ( 10.3389/fendo.2018.00587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan AW, Zadran N, Khan A, Ishaq M, Kumar J, Ibrar A, Tahir A. Vitamin D levels and bone mineral density in premenopausal women compared to postmenopausal women: a multi-centre study from Pakistan. Cureus 202012 e11439. ( 10.7759/cureus.11439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporosis International 200213105–112. ( 10.1007/s001980200001) [DOI] [PubMed] [Google Scholar]
- 6.Guo J, Huang Y, Bian S, Zhao C, Jin Y, Yu D, Wu X, Zhang D, Cao W, Jing F.et al. Associations of urinary polycyclic aromatic hydrocarbons with bone mass density and osteoporosis in U.S. adults, NHANES 2005–2010. Environmental Pollution 2018240209–218. ( 10.1016/j.envpol.2018.04.108) [DOI] [PubMed] [Google Scholar]
- 7.Livshits G, Kalinkovich A. Targeting chronic inflammation as a potential adjuvant therapy for osteoporosis. Life Sciences 2022306 120847. ( 10.1016/j.lfs.2022.120847) [DOI] [PubMed] [Google Scholar]
- 8.Al-Hakami A, Alqhatani SQ, Shaik S, Jalfan SM, Dhammam MSA, Asiri W, Alkahtani AM, Devaraj A, Chandramoorthy HC. Cytokine physiognomies of MSCs from varied sources confirm the regenerative commitment post-coculture with activated neutrophils. Journal of Cellular Physiology 20202358691–8701. ( 10.1002/jcp.29713) [DOI] [PubMed] [Google Scholar]
- 9.Singh P, Hu P, Hoggatt J, Moh A, Pelus LM. Expansion of bone marrow neutrophils following G-CSF administration in mice results in osteolineage cell apoptosis and mobilization of hematopoietic stem and progenitor cells. Leukemia 2012262375–2383. ( 10.1038/leu.2012.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J.et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clinical Cancer Research 2014206212–6222. ( 10.1158/1078-0432.CCR-14-0442) [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Luo J, Wen J, Jiang M. The relationship between systemic Immune inflammatory index and prognosis of patients with non-small cell lung cancer: a meta-analysis and systematic review. Frontiers in Surgery 20229898304. ( 10.3389/fsurg.2022.898304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Wang C, Wang J, Huang X, Cheng Y. A novel systemic immune-inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma. Journal of Cancer Research and Clinical Oncology 20171432077–2086. ( 10.1007/s00432-017-2451-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang H, Zhang H, Wang Z, Zhou Z, Li Y, Lu L. Systemic immune-inflammation index acts as a novel diagnostic biomarker for postmenopausal osteoporosis and could predict the risk of osteoporotic fracture. Journal of Clinical Laboratory Analysis 202034e23016. ( 10.1002/jcla.23016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du YN, Chen YJ, Zhang HY, Wang X, Zhang ZF. Inverse association between systemic immune-inflammation index and bone mineral density in postmenopausal women. Gynecological Endocrinology 202137650–654. ( 10.1080/09513590.2021.1885642) [DOI] [PubMed] [Google Scholar]
- 15.Xu H, Mao Y, Hu Y, Xu B. Association between exposure to polyfluoroalkyl chemicals and increased fractional exhaled nitric oxide in adults. Environmental Research 2021198 110450. ( 10.1016/j.envres.2020.110450) [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Mao Y, Xu B. Association between pyrethroid pesticide exposure and hearing loss in adolescents. Environmental Research 2020187 109640. ( 10.1016/j.envres.2020.109640) [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Mao Y, Xu B, Hu Y. Low-level environmental lead and cadmium exposures and dyslipidemia in adults: findings from the NHANES 2005–2016. Journal of Trace Elements in Medicine and Biology 202163 126651. ( 10.1016/j.jtemb.2020.126651) [DOI] [PubMed] [Google Scholar]
- 18.Long SE, Kahn LG, Trasande L, Jacobson MH. Urinary phthalate metabolites and alternatives and serum sex steroid hormones among pre- and postmenopausal women from NHANES, 2013–16. Science of the Total Environment 2021769 144560. ( 10.1016/j.scitotenv.2020.144560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai S, Fan J, Zhu L, Ye J, Rao X, Fan C, Zhong Y, Li Y. Bone mineral density and osteoporosis in relation to all-cause and cause-specific mortality in NHANES: a population-based cohort study. Bone 2020141 115597. ( 10.1016/j.bone.2020.115597) [DOI] [PubMed] [Google Scholar]
- 20.Fan Y, Ni S, Zhang H. Associations of copper intake with bone mineral density and osteoporosis in adults: data from the national health and nutrition examination survey. Biological Trace Element Research 20222002062–2068. ( 10.1007/s12011-021-02845-5) [DOI] [PubMed] [Google Scholar]
- 21.Liao S, Zhang J, Shi S, Gong D, Lu X, Cheang I, Zhang H, Li X. Association of aldehyde exposure with cardiovascular disease. Ecotoxicology and Environmental Safety 2020206 111385. ( 10.1016/j.ecoenv.2020.111385) [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Sun J, Tang C, Gu L, Du C, Wang H, Ma Y, Wang L. Association between urinary thallium exposure and cardiovascular disease in U.S. adult population. Chemosphere 2022294 133669. ( 10.1016/j.chemosphere.2022.133669) [DOI] [PubMed] [Google Scholar]
- 23.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA 20183202020–2028. ( 10.1001/jama.2018.14854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Bo Y. Associations between pyrethroid exposure and serum sex steroid hormones in adults: findings from a nationally representative sample. Chemosphere 2022300 134591. ( 10.1016/j.chemosphere.2022.134591) [DOI] [PubMed] [Google Scholar]
- 25.Dirckx N, Hul Van M, Maes C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Research Part C 201399170–191. ( 10.1002/bdrc.21047) [DOI] [PubMed] [Google Scholar]
- 26.Lin Z, Shen D, Zhou W, Zheng Y, Kong T, Liu X, Wu S, Chu PK, Zhao Y, Wu J.et al. Regulation of extracellular bioactive cations in bone tissue microenvironment induces favorable osteoimmune conditions to accelerate in situ bone regeneration. Bioactive Materials 202162315–2330. ( 10.1016/j.bioactmat.2021.01.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caputa G, Castoldi A, Pearce EJ. Metabolic adaptations of tissue-resident immune cells. Nature Immunology 201920793–801. ( 10.1038/s41590-019-0407-0) [DOI] [PubMed] [Google Scholar]
- 28.Weitzmann MN.T-cells and B-cells in osteoporosis. Current Opinion in Endocrinology, Diabetes, and Obesity 201421461–467. ( 10.1097/MED.0000000000000103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baht GS, Vi L, Alman BA. The role of the immune cells in fracture healing. Current Osteoporosis Reports 201816138–145. ( 10.1007/s11914-018-0423-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouxsein ML, Eastell R, Lui LY, Wu LA, Papp de AE, Grauer A, Marin F, Cauley JA, Bauer DC, Black DM.et al. Change in bone density and reduction in fracture risk: a meta-regression of published trials. Journal of Bone and Mineral Research 201934632–642. ( 10.1002/jbmr.3641) [DOI] [PubMed] [Google Scholar]
- 31.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. Journal of Clinical Investigation 20061161186–1194. ( 10.1172/JCI28550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu D, Cline-Smith A, Shashkova E, Perla A, Katyal A, Aurora R. T-cell mediated inflammation in postmenopausal osteoporosis. Frontiers in Immunology 202112 687551. ( 10.3389/fimmu.2021.687551) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a