Abstract
Background
Although differentiated thyroid carcinoma (DTC) is the most frequent endocrine pediatric cancer, it is rare in childhood and adolescence. While tumor persistence and recurrence are not uncommon, mortality remains extremely low. Complications of treatment are however reported in up to 48% of the survivors. Due to the rarity of the disease, current treatment guidelines are predominantly based on the results of small observational retrospective studies and extrapolations from results in adult patients. In order to develop more personalized treatment and follow-up strategies (aiming to reduce complication rates), there is an unmet need for uniform international prospective data collection and clinical trials.
Methods and analysis
The European pediatric thyroid carcinoma registry aims to collect clinical data for all patients ≤18 years of age with a confirmed diagnosis of DTC who have been diagnosed, assessed, or treated at a participating site. This registry will be a component of the wider European Registries for Rare Endocrine Conditions project which has close links to Endo-ERN, the European Reference Network for Rare Endocrine Conditions. A multidisciplinary expert working group was formed to develop a minimal dataset comprising information regarding demographic data, diagnosis, treatment, and outcome. We constructed an umbrella-type registry, with a detailed basic dataset. In the future, this may provide the opportunity for research teams to integrate clinical research questions.
Ethics and dissemination
Written informed consent will be obtained from all participants and/or their parents/guardians. Summaries and descriptive analyses of the registry will be disseminated via conference presentations and peer-reviewed publications.
Keywords: registry, DTC, thyroid carcinoma, childhood
Background
Pediatric differentiated thyroid carcinoma (DTC) is a rare disease, although it is the most frequent endocrine malignancy in children, representing 2–4% of all pediatric malignancies. According to the Surveillance, Epidemiology, and End Results Program, the annual percent change in pediatric thyroid cancer incidence increased from 1.1% per year (1973–2006) to 9.6% per year (2006–2013) (1). The increasing incidence is likely the result of better detection of small and early-stage papillary tumors due to improvement of diagnostic tools and changes in environmental risk factors resulting in a real increase in pediatric thyroid carcinoma (ped-DTC) (2). DTC is the most commonly found (80–90% papillary; ~10% follicular) followed by medullary thyroid carcinoma (3–5%) and rarely anaplastic and poorly DTC (3). Generally, in comparison with adults, pediatric DTC patients present more frequently with ‘advanced disease’ at diagnosis, harboring larger tumor sizes, more frequent lymph node involvement, distant metastases, and multifocal disease. Nevertheless, the prognosis of children with DTC is excellent, and although tumor recurrence/persistence is not uncommon, mortality remains extremely low (4, 5, 6, 7, 8, 9, 10, 11).
The standard of care for pediatric DTC is surgery followed by 131I treatment. Surgery involves total thyroidectomy in almost all patients, although for selected patients there may be an indication for hemithyroidectomy (for instance in those children with microcarcinoma <1 cm limited to the thyroid gland and no signs of cervical lymphadenopathy) (12, 13). In the pediatric American Thyroid Association (ATA) recommendation, prophylactic central neck dissection is recommended for children with malignant cytology and clinical evidence of gross extrathyroidal invasion and/or locoregional metastasis on preoperative staging or intraoperative findings (12). In the upcoming European recommendation, central neck dissection has not been recommended routinely, only to be performed in advanced pediatric thyroid cancer. Routine prophylactic lateral neck dissection is not recommended. However, lateral neck dissection should be performed in patients with cytologic evidence of metastases to the lateral neck (12).
The consequence of total thyroidectomy includes the lifelong need for thyroid hormone supplementation and harbors the risk of transient or permanent hypoparathyroidism and recurrent laryngeal nerve damage (14). Complication rates vary widely in the literature depending on the type of surgery performed (hemithyroidectomy versus total thyroidectomy, with or without lymph node dissection), definitions of surgical complications, and experience of the surgeon, but they are sensibly more frequent than the complications observed in adult patients (4, 14, 15).
Following total thyroidectomy, 131I therapy is recommended in almost all pediatric DTC patients with the exception of patients with microcarcinoma (tumor <1 cm, limited to the thyroid gland) aiming to destroy any (iodine-avid) thyroid cancer cells, that is unknown microscopic, locoregional, and/or distant metastatic disease (12, 13). The activity of 131I administered depends on the extent of surgery, tumor size, presence of metastases, body weight, and pubertal stage. Adverse effects associated with 131I therapy depend on the cumulative activity administered and include salivary gland dysfunction and lacrimal gland dysfunction, and concerns exist upon an increased risk for secondary primary malignancies (16, 17, 18). With current treatment strategies, the overall survival in pediatric patients with DTC is excellent. This excellent survival raises the question whether treatment may be given less ‘aggressively’ aiming to reduce the number and severity of current adverse effects. It seems like the ‘one-size-fits-all’ approach to DTC should be revised and transitioned into more personalized treatment strategies. We hypothesize that modification of current treatment protocols will not affect disease-specific morbidity and mortality, yet may reduce treatment-induced adverse outcome.
As a consequence of the rarity of the disease during childhood and adolescence, current treatment guidelines are predominantly based on the results of small retrospective observational studies. Results from retrospective observational studies should however be interpreted with caution due to their inherent limitations and potential bias. Given the important differences in behavior of DTC in children compared to adults, evidence from large-sized studies performed in adults cannot and therefore in an ideal world should not directly be extrapolated to children. In order to improve treatment and outcome of pediatric DTC patients, there is an unmet need for uniform prospective data collection of larger cohorts and randomized controlled clinical trials.
Prospective collection of international collaborative robust individual patient data may increase our knowledge of clinical behavior of pediatric DTC, identify risk factors for recurrence, and assess late effects of treatment. Collectively, this will not only improve clinical care and outcomes for pediatric DTC patients in the future but will also generate novel hypothesis and will be the framework for European collaborative studies.
Which initiatives preceded the current proposal?
2016: At the European Thyroid Association-Cancer Research Network (ETA-CRN) meeting in Copenhagen, an initiative to collaborate on ped-DTC within a European network was started (initiative: T.P. Links).
2017: Dekker et al. conducted a survey on the care for pediatric DTC in different European countries and concluded that national registries for pediatric DTC are limited, and treatment is very scattered with highly variable numbers of children per center (19).
2020: The ETA-CRN network formed a working group to develop the first European recommendation for diagnosis and treatment of pediatric thyroid nodules and DTC (chair: H.M. van Santen).
2021: On December 15, an ENDO-ERN/ETA-CRN webinar was organized on the ‘ETA recommendations for pediatric thyroid nodules and DTC’.
2022: Initiative to perform a pooled analysis from different European cohorts, to provide a comprehensive assessment of the association between risk factors and DTC outcome, that isrecurrence and persistent disease (coordinator: S.C. Clement).
Rationale for the pediatric European Registry
As pediatric DTC is a rare disease, no single study site is in the position to obtain clinical data in sufficient numbers to conduct conclusive studies. The European ped-DTC registry is a cooperative effort that will provide large enough clinical datasets to answer questions conclusively by conducting well-powered studies. The registry will offer detailed information about each patient regarding his/her demographic details and clinical information and will serve in the future as the umbrella for linked studies. Importantly, a European pediatric registry for DTC can provide the basis for a collaborative European thyroid cancer registry across all ages and all disease states.
Methods
The European Registries for Rare Endocrine Conditions (EuRRECa) detailed patient registry is a prospective, multicenter, European registry collecting validated, standardized, and well-characterized patient data. While the registry has primary focus on descriptive outcomes, it is anticipated that in the future collection of specific data for linked studies will add significant further value to the registry (umbrella-type registry) (20). Registry subjects will be enrolled at participating sites. Currently, at least 25 institutions have given their commitment to participate at the implementation of the registry. As such, we anticipate an inclusion rate of approximately 100 patients per year in the first 2–3 years, based on historical experience at the member institutions.
Objectives of the registry
The objectives of the registry were to collect prospective data on demographics, tumor characteristics, given treatment, and outcome of pediatric DTC patients, aiming
-
To increase knowledge on prevalence of pediatric DTC, its treatment across Europe, and its outcome. With the registry, we will:
Evaluate the incidence and outcome (i.e. recurrence/persistent disease, death of disease, and adverse effects of given treatment) of pediatric DTC in Europe.
Identify the impact (risk factor analysis) of several patient- and tumor-related characteristics on outcome of pediatric DTC, that is recurrence rate/persistent disease and death of disease.
-
To perform collaborative international studies. With this registry, we will:
Create a European pediatric DTC cohort that will provide researchers and clinicians access to accurate, validated, standardized, and well-characterized patient data.
Facilitate enrollment into linked studies/clinical trials.
Organization and management of the registry
The ped-DTC registry will be established and managed by the EuRRECa (https://eurreca.net/) which was launched in February 2018 and funded by the EU Health Programme with additional support from the European Society for Pediatric Endocrinology and the European Society of Endocrinology. The EuRRECa project was initially developed to support the European Reference Network for Rare Endocrine Conditions (Endo-ERN) but is open to use by the wider endocrine community. Endo-ERN is the largest ERN with 111 reference centers from 28 member states that are estimated to care for over 60,000 patients (https://endo-ern.eu/about/reference-centre). Endo-ERN includes 36 groups of conditions with orphacodes that are organized into eight ‘main thematic groups’. It aims to maximize the opportunity for patients, healthcare professionals, and researchers to participate and use high‐quality, patient-centered registries for rare endocrine conditions that are covered within Endo‐ERN. The EuRRECa project offers two platforms for patients’ registration. The electronic reporting system (e-REC) provides a better understanding of the occurrence of the conditions covered by Endo-ERN. To date, 31 participating centers have reported over 1850 cases of non-metastatic thyroid carcinoma; of these, 64 cases were pediatric patients.
Participation is open to all members of Endo-ERN and to all other professionals providing endocrine care. A comprehensive description of the methods of EuRRECa has been previously published (21). Ultimately, in close collaboration with other European partners such as the European Reference Network on adult cancers (solid tumors) (ERN EURACAN), the registry can be extended into a collaborative European thyroid cancer registry across all ages and all disease states.
Management team
The management team comprises clinical specialists with expertise and interest in ped-DTC and representatives of the EuRRECa project management group (Supplementary Appendix A, see section on supplementary materials given at the end of this article). The management team will meet monthly (electronically) and will oversee the day-to-day running of the registry, under the direction of the expert working group.
Expert working group
The ped-DTC expert working group comprises representation of experts in pediatric thyroid nodule/cancer management and its follow-up including all necessary medical specialists (pediatric/adult endocrinology, radiology, thyroid surgery, clinical genetics, pathology, and nuclear medicine) and geographic regions (Supplementary Appendix B). It meets every 3 months (electronically) and has an important advising role in setting up the registry, monitoring data collection, producing of annual data reports and publications, and reviewing study proposals of linked studies in the future.
Primary physician/site investigator
Each participating site will be appointed a primary physician/site investigator who is responsible for the organization and management of the registry activities at the local site. Main site activities include obtaining local ethics committee approval, identification of eligible patients for recruitment and data collection, and data quality control. For local ethics committee approval, site investigators will be applied with easy adoptable forms.
Registry population
All patients with a confirmed diagnosis of thyroid carcinoma <18 years of age who have been diagnosed, assessed, or treated at a participating site are eligible to be entered into the registry. The ped-DTC registry will collect retrospective data from January 2020 to December 2022 and prospective data from January 2023 onwards.
Inclusion criteria:
The subject and parents/guardians are able and willing to sign the informed consent document.
Age <18 at the time of diagnosis.
Histological confirmation of well-differentiated thyroid carcinoma.
Exclusion criteria:
Medullary thyroid carcinoma and anaplastic thyroid carcinoma.
Participant recruitment
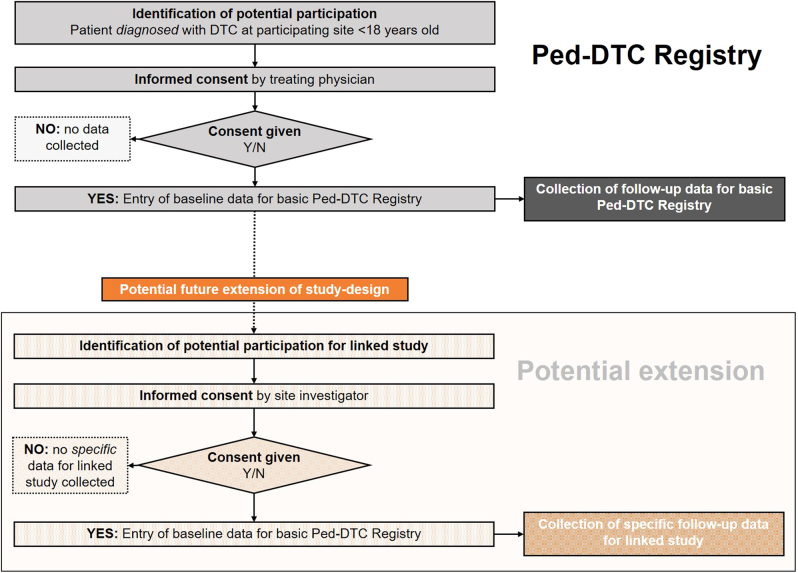
Each study site has to be approved by the respective ethics committee. The treating physician/site investigator will recruit potentially eligible subjects and inquire as to their willingness to participate in this study (Fig. 1).
Figure 1.
Patient recruitment framework of the ped-DTC registry.
Dedicated (ERN affiliated) clinicians can enter patient data into the registry after obtaining written informed consent from patients and/or parents/guardians. Patients will be treated according to their physician’s standard practice and discretion. There are no additional treatments or exams that are required to take place within the basic ped-TC registry. No alterations to a patient’s clinic schedule are expected as a result of participation in the basic registry. Primary site physicians/investigators will abstract specified data from the participant’s medical record and enter these data into the web-based registry system. Measurement points include at the time of enrollment (T0), 6 months (T1) and 12 months after diagnosis (T2), as well as further consultation at year 2 (T3), year 5 (T4), and year 10 (T5). A summary of the study design is outlined in Table 1.
Table 1.
Registry activities.
| Registry period | |||||||
|---|---|---|---|---|---|---|---|
| Enrollment and entry of baseline data | Follow-up | Early termination | |||||
| Time point | T0 | T1 | T2 | T3 | T4 | T5 | |
| Eligibility screening | X | ||||||
| Informed consent | X | ||||||
| Demographic data | X | ||||||
| Preoperative results | X | ||||||
| Surgical treatment | X | ||||||
| Postoperative results | X | ||||||
| 131I therapy | X | X | X | ||||
| Outcome | X | X | X | X | X | X | |
| Reason for early termination | X | ||||||
Measurement points include at the time of enrollment (T0), 6 months (T1) and 12 months after diagnosis (T2), as well as further consultation at year 2 (T3), year 5 (T4), and year 10 (T5).
The data collected in the basic ped-DTC registry will be used to improve clinical care as well as research with data access governed by a DAC (21). The results of any (linked) studies performed will be disseminated widely. Patients will be able to access the registry to view their own record, set preferences for data sharing, and complete patient-reported outcomes; patients are required to provide their email address to their clinician for online access, and this is captured on the registry consent form. Patient-reported outcomes are increasingly being used in registries to understand patient experiences and preferences.
Data collection
For each patient, the registry collects a basic set of data. In the future, approved linked studies requiring specific data collection can be added to the database. Patient demographic and clinical data are submitted by sites via direct data entry using a secured web-based database. The primary physician/site investigator is responsible for entering patient data directly into the database. Sites will be trained to use the database and will be provided with a data entry manual to assist with good-quality data collection. Data to be collected in the basic ped-DTC registry include the following domains: (i) demographics; (ii) preoperative results; (iii) surgical treatment; (iv) postoperative results; (v) 131I therapy; and (vi) outcome. Data items collected by the basic ped-DTC registry are outlined in Table 2. A detailed case report form (CRF) will be developed by the expert working group.
Table 2.
Data items collected by basic pediatric-DTC registry.
| 1. Patient details | 2. Preoperative | 3. Surgical treatment | 4. Postoperative | 5. (131I) therapy | 6. Outcome |
|---|---|---|---|---|---|
| Patient ID | Presence of comorbidities | Surgeon (pediatric/ adult/ endocrine/ combination) | TNM staging | Indication | Serum Tg antibodies |
| Sex | Family history of DTC | Procedure type | Histology (PA) | Number of 131I treatments | Maximum stimulated Tg |
| Date of enrollment | Typical manifestations of familial syndromes | Indication for procedure | Genetic testing results | Cumulative activity of 131I | Death of disease |
| Country/hospital | Previous exposure to radiation | Lymph node dissection (levels) | Transient hypoparathyroidism | Distant metastases | Death of any cause |
| Age at diagnosis | Previous malignancy | Lymph node dissection intent | Transient recurrent nerve injury | Distant metastasis sites | Date of death |
| Date of diagnosis | Previous thyroid surgery | Other adverse events/surgical complications | Other adjuvant therapy (i.e. radiotherapy or TKI) | Date of last known alive | |
| Clinical symptoms | Recurrence | ||||
| Dominant finding at physical exam | Persistent disease | ||||
| Thyroid function at diagnosis | Remission | ||||
| Preoperative imaging results | Persistent hypoparathyroidism | ||||
| Results of fine needle aspiration (Bethesda classification) | Persistent recurrent nerve injury | ||||
| Clinical voice abnormality | Dry mouth/hyposalivation | ||||
| Preoperative laryngeal exam | Second primary malignancies | ||||
| Weight SDS | Infertility | ||||
| Height SDS | Other adverse events | ||||
| Calculated BMI SDS |
BMI, body mass index; DTC, differentiated thyroid carcinoma; PA, pathology; SDS, standard deviation scores; Tg, thyroglobulin; TKI, tyrosine kinase inhibitor; TNM, tumor, node, metastases.
Data access, data quality, and data governance
The EuRRECa project aims to promote good standards of practice by adherence to the highest standards of data security, and the data collected are subject to stringent governance (21). The project complies with the UK Data Protection Act (2018) and General Data Protection Regulation (GDPR 2016/679). All participating centers obtain their own local institutional approvals for using the basic patient registry. The analysis of registry data will lead to aggregated reports summarizing the epidemiology of pediatric DTC, treatment, and outcomes. These reports will include a public annual data report. The data access process governed by a Data Access Committee (DAC) have been previously described (21, 22). In short, stakeholders request data by completing a Data Request Form and a Data Sharing Agreement. The request is reviewed by the DAC who provide feedback to the applicant, who may be asked to revise the request. Once approved, data are released to the applicant.
Discussion
To our knowledge, this is the first prospective international registry on pediatric DTC. The ped-DTC registry will facilitate the efficient capture of prospective longitudinal data on the clinical behavior of ped-DTC, which is currently lacking, with the aim to develop more personalized treatment and follow-up strategies. The ped-DTC registry will provide real-world data and identify areas of excellence as well as areas of concern in the management of DTC during childhood and adolescence. Furthermore, the registry will facilitate the identification and recruitment of eligible participants for potential future linked studies.
The development of a patient registry is a complex process which can globally be divided into three major stages: i) preparatory phase, ii) implementation, and iii) output. The ped-DTC registry is in progress, and we are currently in the preparatory phase; the next step will be the development of a detailed CRF. To be fully operational and responding to its aims, the registry will have to manage several challenges. The main challenge will be to attract participating hospitals throughout the whole of Europe to decrease the risk of biased selections. By taking part in the ped-DTC registry, participating hospitals will get the opportunity to compare their patient outcomes with those from other hospitals. In addition, these hospitals will benefit from participating in multicenter clinical studies that could improve their clinical care for patients with pediatric DTC. Furthermore, after approval, each hospital could become the principal investigator of a linked study in the future. On the other hand, to commence patient recruitment, the registry needs to seek ethics approval at each participating site. EuRRECa will facilitate this process which will make it more manageable. Nevertheless, the process of obtaining site approval remains time consuming and labor intensive. The second challenge will be to sustainably maintain the mobilization of all primary physicians/site investigators. Especially the large number of data items requested as well as the 10-year follow-up may result in missing data and dropouts. To prevent this, the registry will be an easy-to-use electronic reporting system which allows continuous reporting, and physicians will receive a reminder as to when to enter follow-up data for a specific patient.
Conclusion
Patient registries are powerful tools used to monitor disease and facilitate research and are predominantly important for rare diseases, where data collection is challenging due to their low prevalence. Although in essence a clinic-based patient registry, the umbrella-type registry design allows us to perform linked studies in the future. Data collected by ped-DTC registry may ultimately result in improvement of patient care.
Supplementary Material
Declaration of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
SFA and ALP are supported by the European Union’s Health Programme (2014–2020) on the EuRRECa project ‘777215/EuRRECa’ and the EuRR-Bone project ‘946831/EuRR-Bone’. The EuRRECa project is also grateful to the European Society of Endocrinology and the European Society for Paediatric Endocrinology for funding support.
Ethical Statement
All participating centers obtain their own local institutional approvals for using the basic patient registry.
Acknowledgements
The authors would like to thank Jillian Bryce for project management support to the EuRRECa project.
References
- 1.Qian ZJ, Jin MC, Meister KD, Megwalu UC. Pediatric thyroid cancer incidence and mortality trends in the United States, 1973–2013. JAMA Otolaryngology– Head and Neck Surgery 2019145 617–623. ( 10.1001/jamaoto.2019.0898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nature Reviews. Endocrinology 201612646–653. ( 10.1038/nrendo.2016.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulson VA, Rudzinski ER, Hawkins DS. Thyroid cancer in the pediatric population. Genes 201910 723. ( 10.3390/genes10090723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein Hesselink MS, Nies M, Bocca G, Brouwers AH, Burgerhof JGM, Dam van EWCM, Havekes B, Heuvel-Eibrink van den MM, Corssmit EPM, Kremer LCMet al. Pediatric differentiated thyroid carcinoma in the Netherlands: a nationwide follow-up study. Journal of Clinical Endocrinology and Metabolism 20161012031–2039. ( 10.1210/jc.2015-3290) [DOI] [PubMed] [Google Scholar]
- 5.Redlich A, Luster M, Lorenz K, Lessel L, Rohrer TR, Schmid KW, Frühwald MC, Vorwerk P, Kuhlen M. Age, American Thyroid Association Risk Group, and Response to Therapy Are Prognostic Factors in Children With Differentiated Thyroid Cancer. Journal of Clinical Endocrinology and Metabolism 2022107e165–e177. ( 10.1210/clinem/dgab622) [DOI] [PubMed] [Google Scholar]
- 6.Jong de MC, Gaze MN, Szychot E, Rozalén García V, Brain C, Dattani M, Spoudeas H, Hindmarsh P, Abdel-Aziz TE, Bomanji Jet al. Treating papillary and follicular thyroid cancer in children and young people: single UK-center experience between 2003 and 2018. Journal of Pediatric Surgery 202156534–539. ( 10.1016/j.jpedsurg.2020.07.034) [DOI] [PubMed] [Google Scholar]
- 7.Cistaro A, Quartuccio N, Garganese MC, Villani MF, Altini C, Pizzoferro M, Piccardo A, Cabria M, Massollo M, Maghnie Met al. Prognostic factors in children and adolescents with differentiated thyroid carcinoma treated with total thyroidectomy and RAI: a real-life multicentric study. European Journal of Nuclear Medicine and Molecular Imaging 2022491374–1385. ( 10.1007/s00259-021-05586-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo M, Malandrino P, Moleti M, Vermiglio F, D’Angelo A, la Rosa G, Sapuppo G, Calaciura F, Regalbuto C, Belfiore Aet al. Differentiated thyroid cancer in children: heterogeneity of predictive risk factors. Pediatric Blood and Cancer 201865 e27226. ( 10.1002/pbc.27226) [DOI] [PubMed] [Google Scholar]
- 9.Sapuppo G, Hartl D, Fresneau B, Hadoux J, Breuskin I, Baudin E, Rigaud C, Guerlain J, Ghuzlan al A, Leboulleux Set al. Differentiated thyroid cancer in children and adolescents: long term outcome and risk factors for persistent disease. Cancers 202113 3732. ( 10.3390/cancers13153732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negre Busó M, García Burillo A, Simó Perdigó M, Galofré Mora P, Boronat De Ferrater M, Cuberas Borrós G, Sábado Álvarez C, Castell Conesa J. Long-term follow-up of differentiated thyroid carcinoma in children and adolescents. Journal of Pediatric Endocrinology and Metabolism 2020331431–1441. ( 10.1515/jpem-2020-0194) [DOI] [PubMed] [Google Scholar]
- 11.Karapanou O, Tzanela M, Rondogianni P, Dacou-Voutetakis C, Chiotis D, Vlassopoulou B, Vassiliadi D, Kanaka-Gantenbein C, Tsagarakis S. Long-term outcome of differentiated thyroid cancer in children and young adults: risk stratification by ATA criteria and assessment of pre-ablation stimulated thyroglobulin as predictors of disease persistence. Endocrine 202070566–574. ( 10.1007/s12020-020-02378-2) [DOI] [PubMed] [Google Scholar]
- 12.Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster Met al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 201525716–759. ( 10.1089/thy.2014.0460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebbink CA, Dekker BL, Bocca G, Braat AJAT, Derikx JPM, Dierselhuis MP, Keizer de B, Kruijff S, Kwast ABG, Nederveen van FHet al. New national recommendations for the treatment of pediatric differentiated thyroid carcinoma in the Netherlands. European Journal of Endocrinology 2020183 11. ( 10.1530/EJE-20-0191) [DOI] [PubMed] [Google Scholar]
- 14.Hanba C, Svider PF, Siegel B, Sheyn A, Shkoukani M, Lin HS, Raza SN. Pediatric thyroidectomy. Otolaryngology–Head and Neck Surgery 2017156360–367. ( 10.1177/0194599816677527) [DOI] [PubMed] [Google Scholar]
- 15.Breuer C, Tuggle C, Solomon D, Sosa JA. Pediatric thyroid disease: when is surgery necessary, and who should be operating on our children? Journal of Clinical Research in Pediatric Endocrinology 20135(Supplement 1) 79–85. ( 10.4274/jcrpe.817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clement SC, Peeters RP, Ronckers CM, Links TP, Heuvel-Eibrink van den MM, Nieveen van Dijkum EJM, Rijn van RR, Pal van der HJH, Neggers SJ, Kremer LCMet al. Intermediate and long-term adverse effects of radioiodine therapy for differentiated thyroid carcinoma – A systematic review. Cancer Treatment Reviews 201541925–934. ( 10.1016/j.ctrv.2015.09.001) [DOI] [PubMed] [Google Scholar]
- 17.Albano D, Bertagna F, Panarotto MB, Giubbini R. Early and late adverse effects of radioiodine for pediatric differentiated thyroid cancer. Pediatric Blood and Cancer 201764 e26595. ( 10.1002/pbc.26595) [DOI] [PubMed] [Google Scholar]
- 18.Reinecke MJ, Ahlers G, Burchert A, Eilsberger F, Flux GD, Marlowe RJ, Mueller HH, Reiners C, Rohde F, Santen van HMet al. Second primary malignancies induced by radioactive iodine treatment of differentiated thyroid carcinoma — a critical review and evaluation of the existing evidence. European Journal of Nuclear Medicine and Molecular Imaging 2022493247–3256. ( 10.1007/s00259-022-05762-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dekker BL, Newbold KL, Führer D, Waguespack SG, Handkiewicz-Junak D, Links TP. & European Initiative on Collaboration on Paediatric Thyroid Cancer. Survey on paediatric differentiated thyroid cancer care in Europe. Hormone Research in Paediatrics 20188958–62. ( 10.1159/000484170) [DOI] [PubMed] [Google Scholar]
- 20.Nishii K, Inoue M, Obata H, Ueda Y, Kozuki T, Yamasaki M, Moritaka T, Awaya Y, Sugimoto K, Gemba Ket al. Novel prospective umbrella‐type lung cancer registry study for clarifying clinical practice patterns: CS‐Lung‐003 study protocol. Thoracic Cancer 202112725–731. ( 10.1111/1759-7714.13789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali SR, Bryce J, Smythe C, Hytiris M, Priego AL, Appelman-Dijkstra NM, Ahmed SF. Supporting international networks through platforms for standardised data collection—the European Registries for Rare Endocrine Conditions (EuRRECa) model. Endocrine 202171555–560. ( 10.1007/s12020-021-02617-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali SR, Bryce J, Tan LE, Hiort O, Pereira AM, Akker van den ELT, Appelman-Dijkstra NM, Bertherat J, Cools M, Dekkers OMet al. The EuRRECa project as a model for data access and governance policies for rare disease registries that collect clinical outcomes. International Journal of Environmental Research and Public Health 202017 8743. ( 10.3390/ijerph17238743) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a