Abstract
The default mode network (DMN) plays a crucial role in internal self-processing, rumination, and social functions. Disruptions to DMN connectivity have been linked with early adversity and the emergence of psychopathology in adolescence and early adulthood. Herein, we investigate how subclinical psychiatric symptoms can impact DMN functional connectivity during the pubertal transition. Resting-state fMRI data were collected annually from 190 typically-developing youth (9–15 years-old) at three timepoints and within-network DMN connectivity was computed. We used latent growth curve modeling to determine how self-reported depressive and posttraumatic stress symptoms predicted rates of change in DMN connectivity over the three-year period. In the baseline model without predictors, we found no systematic changes in DMN connectivity over time. However, significant modulation emerged after adding psychopathology predictors; greater depressive symptomatology was associated with significant decreases in connectivity over time, whereas posttraumatic stress symptoms were associated with significant increases in connectivity over time. Follow-up analyses revealed that these effects were driven by connectivity changes involving the dorsal medial prefrontal cortex subnetwork. In conclusion, these data suggest that subclinical depressive and posttraumatic symptoms alter the trajectory of DMN connectivity, which may indicate that this network is a nexus of clinical significance in mental health disorders.
Keywords: FMRI, Resting-state, Development, Trauma
1. Introduction
The default mode network (DMN) is one of three resting-state networks in the triple network model of cognitive dysfunction in psychopathology, which also includes the executive control and the salience networks (Menon, 2011). The DMN is a distributed network of brain regions that are reliably more active during rest than during task-related neuroimaging paradigms (Mak et al., 2017; Raichle, 2015; Raichle et al., 2001). The DMN is comprised of four core anatomical regions, including the medial prefrontal cortex, posterior cingulate cortex/precuneus (PCC), and left and right inferior parietal cortices (Garrity et al., 2007; Raichle et al., 2001; Supekar et al., 2010; Whitfield-Gabrieli and Ford, 2012). Additional brain regions, especially the medial temporal lobe, are also frequently included in the DMN (Buckner et al., 2008). These regions interact at rest to form three functional subdivisions of the DMN, including the medial temporal (MT) and dorsal medial prefrontal cortex (dmPFC) subsystems that converge on a separate core subsystem composed of the PCC and anterior medial prefrontal cortex (Andrews-Hanna et al., 2014, Yeo et al., 2011). Functional connectivity within and between these DMN regions has been associated with self-rumination, memory formation, and thinking about others (Bartova et al., 2015; Daselaar et al., 2004; Sambataro et al., 2010; Whitfield-Gabrieli and Ford, 2012). In addition, the ability to suppress the DMN during task performance has been associated with variability in multiple domains of cognitive performance, including working memory, general intelligence, and naturalistic viewing tendencies (Brandman et al., 2021; DeSerisy et al., 2021; Hearne et al., 2016; Sambataro et al., 2010).
Overall, there is limited work examining longitudinal connectivity changes of the DMN in healthy children and adolescents. The extant literature has unveiled that the structural and functional architecture of the DMN undergoes rapid changes during development (Fransson et al., 2007; Sanders et al., 2022; Supekar et al., 2010; Uddin et al., 2010). Resting state networks are already apparent in the infant brain and the DMN is present but weakly connected in young children, with increasing within network connectivity in older children and even greater within network connectivity in adults (de Bie et al., 2012; Fair et al., 2009). Though the DMN architecture of children and adolescents resembles that of adults by age 10 with established, but weak core connectivity, the whole brain’s overall connectivity profile continues to change from a regionally-dominant functional architecture to a more distributed network architecture during this developmental window, with increasing functional and structural segregation of brain networks (e.g., DMN, executive control network, salience network) that is thought to reflect specialization of each network (Baum et al., 2017; Fair et al., 2008; Fan et al., 2021; Sato et al., 2014; Sherman et al., 2014; Wang et al., 2022). Importantly, such changes are not uniform across the brain, as neuroimaging studies have identified significant structural and functional developmental changes to the DMN that varied in strength and direction in different subnetworks of the DMN (Fan et al., 2021, Supekar et al., 2010, Uddin et al., 2010).
Alterations in functional connectivity across the DMN have consistently been associated with neuropsychiatric disorders in adults, including autism, depression, posttraumatic stress disorder (PTSD), attention deficit hyperactivity disorder (ADHD), and schizophrenia (Doucet et al., 2020; Franzen et al., 2013; Garrity et al., 2007; Hyatt et al., 2022; Koch et al., 2012; Miller et al., 2017; Padmanabhan et al., 2017; Sha et al., 2019; Sheline et al., 2009; Wilson et al., 2013). In children and adolescents, early measures of allostatic load, including childhood trauma, maternal depression, and chronic early stress have been linked with disruptions to DMN functional connectivity and the emergence of psychopathology (Daniels et al., 2011; Gaffrey et al., 2012; Ho et al., 2015; Patriat et al., 2016; Zeev-Wolf et al., 2022, Zeev-Wolf et al., 2019). However, in developing youth this literature has not been fully consistent, especially in the areas of depression and PTSD, which may reflect the heterogeneity of the disorders themselves. These disorders are of particular importance as approximately 67% of children experience a traumatic event by age 16, and these events have been linked to future depression and posttraumatic symptoms (Chapman et al., 2004; Copeland et al., 2007; Finkelhor et al., 2013; Schalinski et al., 2016). In adolescents diagnosed with depression, there have been mixed findings of increased DMN connectivity in some samples and decreases in others, although these samples differed in medication, severity of depression, and methodological approaches (Cullen et al., 2009, Pannekoek et al., 2014, Sacchet et al., 2016). The literature regarding DMN connectivity in adolescents with PTSD also remains unclear, with disparate findings of both heightened and decreased DMN connectivity (Patriat et al., 2016, Viard et al., 2019, Wolf and Herringa, 2016). Such changes to the DMN architecture may be concomitant with the emergence of neuropsychiatric disorders, particularly mood and anxiety disorders in some youth, as approximately 75% of all diagnosable psychiatric disorders begin prior to age 24. Thus, there is a growing need to investigate how developmental changes in the DMN correspond to the initial emergence of psychopathology symptoms (Casey et al., 2000; Giedd et al., 1999; Kessler et al., 2005).
Despite the recent surge in literature focusing on how psychiatric symptomatology relates to aberrant functional connectivity in the DMN, the majority of these studies have employed a cross-sectional approach and few studies have examined how the DMN develops and relates to psychopathological traits in children and adolescents. Importantly, behavioral and neural changes can present prior to diagnosis when symptoms do not meet the clinical threshold (Carré et al., 2014; Fava, 1999; Kaiser et al., 2015). Thus, probing connectivity in healthy children and adolescents during the period of subclinical symptomatology using a longitudinal, within-subjects approach may provide a more detailed understanding of the emergence and potential progression of psychiatric disorders, as well as any concomitant alterations to DMN connectivity. To this end, we used latent growth curve modeling to examine whether subclinical depressive and posttraumatic stress symptoms were associated with DMN functional connectivity at the time of enrollment and/or the annual longitudinal changes that were observed in our sample of typically developing children without diagnoses of psychiatric disorders. Given the heterogeneity of findings in the literature and the focus on diagnosed youth or adults, and not typically developing youth with subclinical symptoms, we did not have specific directional hypotheses about the trajectory of DMN connectivity; however, we did hypothesize that subclinical depressive and posttraumatic stress symptoms would impact the longitudinal trajectory of DMN connectivity in typically developing youth.
2. Methods
2.1. Participants
We collected data from 202 typically developing youth (ages 9–15 years at enrollment) who were recruited from the local community as part of the Developmental Chronnecto-Genomics (Dev-CoG) study at the University of Nebraska Medical Center (UNMC) in Omaha, Nebraska and Mind Research Network in Albuquerque, New Mexico (MRN; Stephen et al., 2021). Of the sample, 100 participants were recruited at the UNMC site and 102 participants were recruited at MRN site; the same recruiting strategy was used at both sites. All participants were invited to return annually over a 3-year period for repeated neuroimaging and neuropsychological testing. Inclusion criteria included English as a primary language, age 9–15 years at enrollment, and assent/consent from the participant and parent. Exclusionary criteria included any medical illnesses that affected neurological function, history of neurological or psychiatric disorder, history of substance use, head trauma, and any metallic material contraindicated for MRI. After a complete description of the study, all parents provided written informed consent and children signed assent forms following the Institutional Review Board requirements for each study site.
2.2. Trauma measures
All participants completed a modified version of the Trauma History Profile (THP) assessing traumatic events (Hooper et al., 2011). The THP is a modified derivative of the UCLA PTSD Reaction Index for DSM IV and assesses a variety of trauma types and events (Steinberg et al., 2013). Participants endorsed whether they experienced 12 different types of trauma in their lifetime (No = 0, Yes = 1). Example items include seeing a family member being physically hurt, in a bad accident, or a painful medical treatment. A summed score of each participants’ trauma exposures was used.
A modified self-report Trauma Symptom Checklist for Children (TSCC) was also administered to all participants during their first visit (Briere, 1996). We removed two questions related to suicidality at the request of the IRB. This assessment is commonly used in children ages 8–16 years and loads onto six different clinically oriented subscales, with alpha coefficients of .81 for depression and .75 for posttraumatic stress (Nilsson et al., 2008). Participants indicated the frequency with which they experienced a variety of psychological symptoms on a four-point Likert scale from 0 to 3 (0 = never, 3 = almost all of the time). The depression subscale measures feelings of loneliness and sadness, and the posttraumatic subscale measures intrusive thoughts and memories/nightmares/fears about unpleasant past events. Given the developmental nature of this study, raw scores were used for analysis, with higher raw scores indicative of a greater number and/or severity of symptoms. The maximum range of raw scores for our two subscales of interest were 0–21 for depression and 0–30 for posttraumatic stress, where higher raw scores indicate a greater number of and/or severity of symptoms experienced.
2.3. Magnetic resonance image acquisition and processing
All participants underwent a five-minute eyes-open resting state fMRI (rs-fMRI) scan with a Siemens 3 T Skyra scanner at the UNMC site or with a Siemens 3 T TIM Trio at the MRN site (Stephen et al., 2021). At both sites, whole-brain blood oxygen level dependent (BOLD) data were acquired using a 32-channel head coil, with the following parameters: field of view (FOV) = 248 mm, resolution = 3 mm isotropic, flip angle = 44°, TE = 29 ms, and TR = 460 ms. Participants were instructed to rest with their eyes open and were monitored throughout the scan. A high-resolution T1-weighted anatomical scan was also acquired for co-registration using an MPRAGE sequence with the following parameters: field of view = 256 mm, resolution = 1 mm isotropic, flip angle = 8°, TE = 1.94 ms, TR = 2400 ms. The rs-fMRI data were preprocessed using the DPABI Toolbox (Yan et al., 2016). Preprocessing procedures included removal of the first 10 volumes, motion correction to the first volume with rigid-body alignment, co-registration of functional data to their anatomical T1-weighted images, linear detrending, regression of motion parameters and their derivatives (24-parameter model; Friston et al., 1996), scrubbing parameters (Power et al., 2012), white matter and cerebrospinal fluid time series using a component-based noise reduction method with 5 principal components (Behzadi et al., 2007), normalization of functional images to Montreal Neurological Institute (MNI) standardized space, spatial smoothing within the functional mask with a 6-mm full-width half-maximum Gaussian kernel, wavelet despiking (Patel et al., 2014), and bandpass filtering at 0.01 – 0.1 Hz (Cordes et al., 2001). Twelve participants with excess head motion for all available scans, defined as greater than 0.8 in mean framewise displacement (FD; Power et al., 2012) and/or greater than 2.5 mm of maximum motion, were excluded from further analyses. Of the remaining sample of 190 youth (93 UNMC, 97 MRN; 94 female), 137 returned and had complete data for year 2 (time between tests: UNMC: mean = 1.10 years, SD = 0.16; MRN: mean = 1.16 years, SD = 0.23) and 88 returned and had complete data for year 3 (time between tests: UNMC: 1.02 years, SD = 0.08; MRN: mean = 1.13 years, SD = 0.32). A detailed overview of recruitment, scans completed, and retention for this sample from the DevCoG study is available online (Stephen et al., 2021, Taylor et al., 2022).
2.4. Extraction of functional connectivity measures
We used the Yeo-7 network atlas, a previously established functional brain atlas based on 1000 resting state fMRI scans, to partition the functional connectome into 7 resting-state networks (RSNs): visual, somato-motor, dorsal attention, ventral attention, limbic, executive control, and default mode (Yeo et al., 2011). DMN functional connectivity, the variable of interest in this study, was determined in each participant by calculating the average Pearson’s correlation coefficient of each voxel’s BOLD signal time series with every other voxel within the DMN network. Thus, each participant had one correlation value representing DMN connectivity, for each of their available resting state scans across the 3-year collection period. The resulting values were further motion corrected by regressing out the mean framewise displacement; the residual values were used for subsequent analysis. Given the prior literature suggesting subnetwork-specific changes to DMN connectivity in psychopathology, we also used the Yeo-17 network atlas, which partitions the aforementioned Yeo-7 network atlas into more granular subnetworks; we used the three subnetworks (i.e., medial temporal [MT] subsystem, core subsystem, dorsal medial prefrontal cortex [dmPFC] subsystem) associated with the DMN to determine which subnetworks were most critical to our findings (Andrews-Hanna et al., 2014, Li et al., 2013, Tozzi et al., 2021, Yeo et al., 2011, Yokoyama et al., 2018). In this analysis, we did not compute between network functional connectivity.
2.5. Statistical analysis
All statistical analyses were completed using MPlus version 8.6 and the Statistical Package for Social Sciences (SPSS) version 25. We began this analysis by calculating descriptive statistics for all variables of interest. We sought to identify individual differences in the trajectories of DMN connectivity over three time points as a function of TSCC subclinical scores measured at the first time point. To that end, we used latent growth curve modeling (LGCM), which is well suited for estimating intraindividual change over time and dealing with partially missing data (Curran et al., 2010). For each of the four DMN delineations of interest (one model from the Yeo-7 atlas, and one model for each of the three Yeo-17 DMN parcellations), we used a 3-step process to examine the relationships between psychopathology symptom severity and the intercept and slope of change in DMN connectivity over time. Initially, we specified a baseline LGCM to examine changes in DMN connectivity over the three-year period without any control and predictor variables. The intercept was defined by DMN connectivity at each time point constrained to 1. The slope was constrained to 0, 1, and 2 for connectivity measures obtained during data collection years 1, 2, and 3, respectively. The second model added in covariates of age at time 1, sex, and data collection site as control variables. The latent intercept and slope variables were regressed on age, sex, and site to account for potential effects of systematic demographic differences, which may influence DMN connectivity measures. The final models added the predictor variables, including the depression and posttraumatic stress TSCC measures (raw scores) collected at time 1, which were regressed on age at time 1, sex, and site to account for potential maturational effects and demographic differences between sites and scanner differences. The latent intercept and slope variables were regressed on the TSCC measures and the aforementioned control variables. These final models allowed us to examine the extent to which subclinical symptoms in healthy youth were associated with baseline connectivity (i.e., at enrollment) and the rate of change in DMN connectivity over time. Models were assessed for goodness of fit using classical measures, including the Chi-square test, root mean square error of approximation (RMSEA, or a measure of “badness of fit”, with larger values indicating worse fit), and the comparative fit index, which indicates relative improvement in model fit from a baseline model (CFI; Hu and Bentler, 1999; Schermelleh-Engel et al., 2003). Furthermore, model fit comparisons were conducted by calculating the change in chi-squared values with each step in our model to test whether adding additional variables to the model significantly impaired the model fit, with a significant test suggesting that a more parsimonious model is favorable.
2.6. Missing data estimation
Of the total sample that was recruited, 190 children and adolescents had TSCC measures from time point 1 and resting state functional MRI data. However, not all children completed data collection procedures in years 2 and 3 (numbers for each year are detailed in the MRI Acquisition and Processing section). Thus, we completed LGCM estimation without and with missing data estimation, using full information maximum likelihood estimation (FIML; Enders, 2001; Enders and Bandalos, 2001). We drew similar conclusions from both models; thus, we used the model with FIML estimation to reduce the potential bias from missing data.
3. Results
3.1. Descriptive statistics
Sample demographics and descriptive statistics for measures of interest are presented in Table 1. A full correlation table of the variables of interest is available in Supplementary Table 1. The majority of participants (81%) reported experiencing at least 1 traumatic event (mean = 2.24 events, SD = 1.90), supporting our investigation of posttraumatic stress symptoms via the TSCC. Further, the majority of youth in the study reported at least some depressive and/or posttraumatic stress symptoms with a range of 0–11 for depressive and 0–22 for posttraumatic symptoms.
Table 1.
Sample Demographics and Descriptive Statistics.
|
Full Sample |
UNMC | MRN |
Comparison |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % | χ2 | p | |||
| Sex, male | 96 | 51 | 46 | 50 | 50 | 52 | 0.08 | 0.77 | |||
| Race | |||||||||||
| White | 161 | 85 | 78 | 84 | 83 | 86 | |||||
| Black / AA | 5 | 3 | 3 | 3 | 2 | 2 | |||||
| AI / AN | 6 | 3 | 0 | 0 | 6 | 6 | |||||
| MR | 10 | 9 | 11 | 13 | 6 | 6 | |||||
| U/NR | 8 | 3 | 6 | 7 | 2 | 2 | |||||
| Ethnicity (HL/NHL/U) | 21/161/8 | 11/85/4 | 8/84/1 | 8/91/1 | 37/60/0 | 38/62/0 | 22.57 | < .001 | |||
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | t-score | p | |
| Age Time 1 | 11.89 | 1.74 | 8.20–15.12 | 11.98 | 1.60 | 9.03–14.94 | 11.81 | 1.88 | 8.20–15.12 | 0.67 | .51 |
| Age Time 2 | 13.04 | 1.77 | 9.18–17.48 | 13.01 | 1.60 | 9.99–15.99 | 13.07 | 1.94 | 9.18–17.48 | -0.19 | .85 |
| Age Time 3 | 13.94 | 1.77 | 10.98–17.42 | 13.93 | 1.63 | 10.98–16.99 | 13.96 | 1.93 | 11.14–17.42 | -0.08 | .94 |
| DEP Time 1 | 3.23 | 2.74 | 0–11 | 2.84 | 2.70 | 0–11 | 3.70 | 2.73 | 0–11 | -2.16 | .03 |
| PTS Time 1 THP Time 1 |
6.10 2.25 |
4.66 1.90 |
0–22 0–10 |
5.36 2.32 |
4.04 2.00 |
0–17 0–10 |
6.79 2.18 |
5.09 1.81 |
0–22 0–7 |
-2.13 0.53 |
.04 .60 |
| DMN Time 1 | .48 | .10 | .24-.74 | .47 | .08 | .28-.63 | .48 | .11 | .24-.74 | -0.69 | .49 |
| DMN Time 2 | .45 | .11 | .12-.70 | .45 | .09 | .20-.68 | .46 | .12 | .12-.70 | -0.29 | .77 |
| DMN Time 3 | .41 | .13 | .12-.63 | .40 | .11 | .15-.59 | .4 | .15 | .12-.63 | -0.48 | .63 |
| MT Time 1 | .69 | .10 | .41-.97 | .69 | .10 | .49-.97 | .68 | .11 | .41-.96 | 0.73 | .47 |
| MT Time 2 | .64 | .13 | .25-.89 | .66 | .13 | .25-.89 | .63 | .13 | .27-.88 | 1.29 | .20 |
| MT Time 3 | .61 | .16 | .15-.95 | .61 | .15 | .15-.95 | .61 | .16 | .27-.84 | -0.02 | .98 |
| Core Time 1 | .61 | .11 | .28-.66 | .61 | .09 | .40-.81 | .62 | .12 | .28-.86 | -0.54 | .59 |
| Core Time 2 | .58 | .13 | .11-.92 | .58 | .12 | .28-.92 | .58 | .14 | .11-.92 | 0.25 | .80 |
| Core Time 3 | .53 | .17 | .17-.84 | .52 | .14 | .19-.77 | .54 | .18 | .17-.84 | -0.63 | .53 |
| dmPFC Time 1 | .53 | .10 | .29-.77 | .52 | .09 | .30-.67 | .53. | 10 | .29-.77 | -0.42 | .67 |
| dmPFC Time 2 | .50 | .12 | .16-.72 | .50 | .11 | .18-.72 | .51 | .12 | .16-.72 | -0.52 | .61 |
| dmPFC Time 3 | .45 | .15 | .12-.69 | .46 | .13. | 17-.65 | .45 | .16 | .12-.69 | 0.25 | .81 |
Note. Chi-squared tests compare count-based demographic variables between sites and independent-samples t-tests compare means between sites for continuous variables of interest. Race: AA = African American, AI/AN = American Indian/Alaska Native, MR = More than one Race, U = Unknown/Not Reported. Reported values for depressive (DEP) and posttraumatic stress (PTS) symptoms are based on Trauma Symptom Checklist for Children (TSCC) raw scores. The Trauma History Profile (THP) data reflects raw scores. Default mode network connectivity for each of the Yeo-7 or 17 atlas parcellations is specified by year (i.e., Time 1, 2, or 3). MT = Medial Temporal Subsystem, Core = Core Subsystem, dmPFC = dorsal medial prefrontal cortex subsystem.
3.2. Latent growth curve modeling results for default mode network connectivity
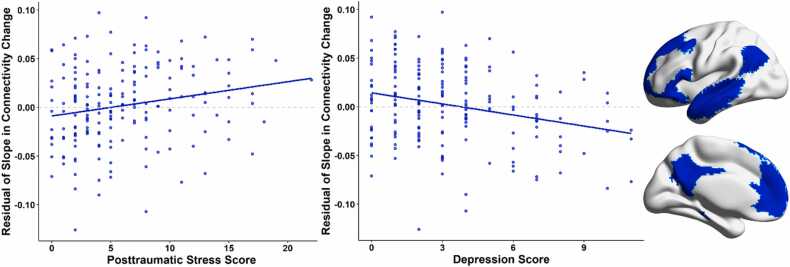
The baseline growth model using the Yeo-7 atlas to examine the trajectory of DMN connectivity without predictors or control variables had excellent model fit (χ2(1) = .001, p = 0.982; RMSEA = 0.000, 90% CI[0.00, 0.00]; CFI = 1.00). Using the motion-corrected residuals of DMN connectivity as the inputs for our model, the means of the latent intercept indicated that across the whole sample, average baseline DMN connectivity was 0.000 (p = 1.000) and the latent slope indicated that there was no systematic change in DMN connectivity over time (mean = 0.001, p = 0.896). In other words, the slope of change in DMN connectivity over time in our sample did not differ significantly from zero. In contrast, there was significant variability in the slope (variance = 0.004, p = 0.007) and intercept (mean = 0.004, p = 0.029). We then imposed control variables of age at time 1, sex, and site on the estimated latent intercept and slope variables. This model also had excellent fit (χ2(4) = 2.76, p = .60; RMSEA = 0.000, 90% CI[0.00, 0.09]; CFI = 1.00). No statistically significant effects of age, sex, or site were found on the latent intercept or slope variables. We then included the depressive and posttraumatic symptom scores from the TSCC in our LGCM. Our final model included age, sex, and site as control variables and raw scores of self-reported depressive and posttraumatic stress symptoms, and all were predictors of the intercept and slope of change in DMN connectivity. This model had excellent fit (χ2(6) = 3.30, p = .77; RMSEA = 0.000, 90% CI[0.00, 0.06]; CFI = 1.00). Age at time 1 was significantly associated with depressive (β = −.177, b = −0.278, p = 0.012) and posttraumatic symptoms (β = −.223, b = −0.595, p = 0.002), with older participants generally reporting fewer symptoms. Site was significantly associated with depressive (β = .148, b = 0.807, p = 0.038) and posttraumatic (β = .148, b = 1.334, p = 0.042) symptoms, such that participants at the MRN site tended to report more symptoms than youths at the UNMC site. Sex did not show any associations with the other variables. Depressive symptoms were associated with the latent slope variable, such that youth who self-reported more depressive symptoms tended to have decreased DMN connectivity over time (β = −.379, b = −0.009, p = 0.015). Posttraumatic stress symptoms showed the opposite pattern, where youth who self-reported more posttraumatic stress symptoms tended to have increased DMN connectivity over time (β = .338, b = 0.004, p = 0.029). Fig. 1 illustrates the effects of psychological symptoms on slope estimates in the overall DMN. Subclinical symptoms were not statistically significantly associated with the latent intercept variable.
Fig. 1.
Relationships Between Slope of Change in Connectivity within the Default Mode Network (DMN) and Psychological Symptoms. (Left) Scatterplots show the relationships between estimated residual slope of change in DMN connectivity and psychological symptoms. Slope values were adjusted by regressing out the effect of extraneous variables (i.e., sex, age, site) in the latent growth curve model. (Right) Default Mode Network from the Yeo-7 atlas.
3.3. LGCM results for the default mode network subsystems
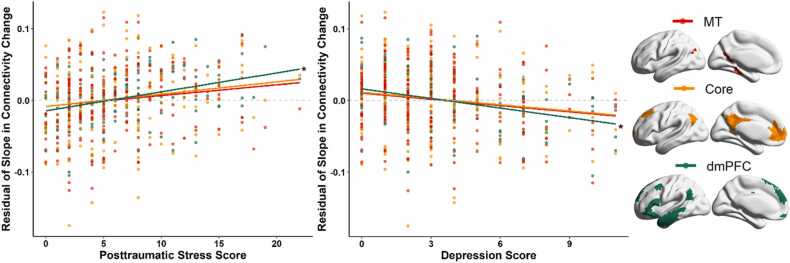
To further probe changes in DMN connectivity with greater specificity, we repeated this analysis connectivity measured within each of the three subsystems (i.e., medial temporal [MT] subsystem, core subsystem, dorsal medial prefrontal cortex [dmPFC] subsystem) of the DMN in the Yeo-17 parcellation. All three subnetworks without predictors or control variables had excellent model fit. The means for the intercepts and slopes were similar to the overall DMN model and the complete results for the baseline and control variables model are available in the Supplemental Material. Turning to the effects of interest, posttraumatic stress and depressive symptoms were significantly associated with connectivity metrics in the dmPFC subsystem of the DMN (see Fig. 2 for the full path model of the dmPFC subsystem). Youth who self-reported more depressive symptoms tended to have decreased DMN connectivity over time (β = −.524, b = −0.011, p = 0.003). Posttraumatic stress symptoms showed the opposite pattern, where youth who self-reported more posttraumatic stress symptoms tended to have increased DMN connectivity over time (β = .519, b = 0.006, p = 0.003). Fig. 3 illustrates the relationship between psychological symptoms and slope estimates for each of the three DMN subsystems. In contrast, the MT and Core subsystems did not show any longitudinal relationships with depressive and posttraumatic stress scores.
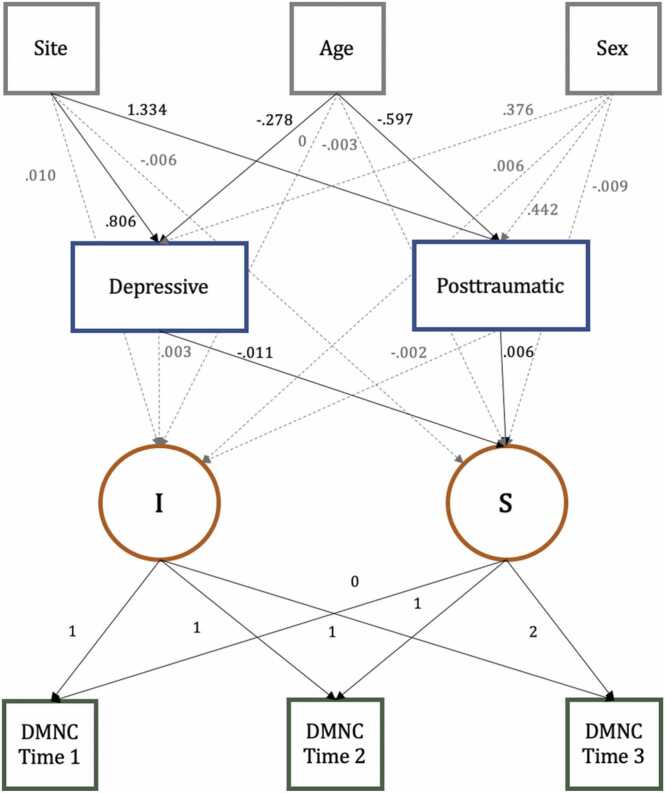
Fig. 2.
Latent Growth Curve Model Results for the Dorsal Medial Prefrontal Cortex Subsystem. Results of the final model, in which age at time 1, study site, sex, and psychological symptoms predict the latent intercept and slope of change in the dorsal medial prefrontal cortex (dmPFC) subsystem of the DMN. Solid black lines indicate statistically significant estimates at the p < .05 level, whereas dashed gray lines indicate nonsignificant relationships. Correlations among variables (not of interest) are not shown in the figure. All estimates are unstandardized. Depressive = depressive symptoms; Posttraumatic = posttraumatic stress symptoms; DMNC = Yeo-7 DMN connectivity. I = Intercept, S = Slope.
Fig. 3.
Relationships Between Slope of Change in Connectivity within the Default Mode Network Subsystems and Psychological Symptoms. (Left) Scatterplots show the relationships between estimated residual slope of change in dorsal medial prefrontal cortex (dmPFC) subsystem connectivity and psychological symptoms. Slope values were adjusted by regressing out the effect of extraneous variables in the latent growth curve model. * Indicates significant correlations. (Right) The Medial Temporal, Core, and dorsal medial prefrontal cortex subsystems of the Default Mode Network from the Yeo-17 atlas.
4. Discussion
The present study examined the longitudinal associations between resting state connectivity in the DMN and subclinical depressive and posttraumatic stress symptoms in a large cohort of typically developing youths. Our base models suggested that there were no significant changes in resting state connectivity within the DMN over time after correcting for motion. Our key model included subclinical depressive and posttraumatic stress symptoms as simultaneous predictors of DMN connectivity using the Yeo-7 atlas over the 3-year study period, controlling for age, sex, and data collection site. Using this model, we found that greater depressive symptoms related to decreasing connectivity over time and greater posttraumatic symptoms related to increasing connectivity over time. Interestingly, conducting the same LGCM analysis with three subdivisions of the DMN revealed that the results were driven by the dmPFC subsystem (Andrews-Hanna et al., 2014, Chen et al., 2020, Yeo et al., 2011). This report contributes to a small but growing body of work examining how neurodevelopmental trajectories vary among youth with subclinical levels of comorbid psychological symptoms.
Our most important findings were the association between subclinical depressive and posttraumatic symptoms in healthy youth and the trajectory of change in connectivity within the dmPFC subsystem, which includes the dmPFC, temporoparietal junction (TPJ), and lateral prefrontal areas including the lateral superior frontal cortex, ventrolateral PFC, and inferior frontal gyrus (Andrews-Hanna et al., 2014; Yeo et al., 2011). Several components of the dmPFC subsystem have previously been associated with playing a broad role in introspecting about mental states and self-referential processing (Andrews-Hanna, 2012, Andrews-Hanna et al., 2014, van Buuren et al., 2010, Wen et al., 2020). These processes occur when individuals reflect upon, evaluate, or appraise social information, interpersonal interactions, and during introspection about an individual’s own or others’ mental states (Lombardo et al., 2010, Rilling et al., 2004, Spreng and Grady, 2010, Van Overwalle, 2009). The dmPFC subsystem is also associated with emotional reappraisal and suppression, which has been found to be important in various forms of psychopathology (Buhle et al., 2014, Che et al., 2015, Modinos et al., 2010).
Of note, there is a dearth of literature examining the impact of subclinical symptoms on the developmental trajectories of resting state networks; thus, we focus primarily on highlighting our findings the context of existing clinical work. At least one study has shown that adolescents diagnosed with depression exhibit increased overall intra-network connectivity within the DMN (Sacchet et al., 2016). In addition, PCC connectivity in the core subsystem has been shown to be elevated in unmedicated adolescents with depression (Ho et al., 2015). However, another study found no alterations to DMN connectivity in treatment-naive adolescents with depression (Pannekoek et al., 2014). These discrepancies may be due to different methodological approaches (e.g., atlas-based vs ICA-based) and sample characteristics (e.g., age range, medication status, comorbidities; Cullen et al., 2009; Sacchet et al., 2016). In studies reporting positive findings in adolescents with depression, such DMN connectivity alterations have been found to correlate with symptom severity, suicidality, and emotion regulation (Rzepa and McCabe, 2018, Zhang et al., 2016). Specifically, decreased resting-state connectivity between the dmPFC and the precuneus correlates with depression severity (Rzepa and McCabe, 2018) and has been hypothesized to underlie less cognitive control, which may modulate vulnerability to increases in depression severity (Vilgis et al., 2018). Finally, it should be noted that the dmPFC subsystem contains crucial regions for attention reorienting and self-referential processing that may modulate the regulation and impact of depressive symptoms (Gusnard et al., 2001, Proskovec et al., 2018).
The impact of PTSD on DMN connectivity in adolescents also remains unclear. Pediatric PTSD has been characterized by heightened DMN connectivity in some studies, which may contribute to the re-experiencing symptoms of PTSD. This would be consistent with the role of the DMN in autobiographical memory (Patriat et al., 2016). In contrast, other studies have identified decreased DMN subsystem connectivity in adolescents with PTSD (Herringa et al., 2013, Viard et al., 2019). Discrepancies between studies may be explained by characteristics of the patient groups, focusing on patients with varying PTSD duration, severity, and trauma onset (Koch et al., 2016, Patriat et al., 2016, Viard et al., 2019, Wolf and Herringa, 2016). Components of the dmPFC subsystem have also been implicated in dysregulated emotional reappraisal in patients with PTSD (Liberzon and Sripada, 2007), and reduced DMN connectivity has been found to relate to avoidant behavior, hyperarousal, intrusive memories, and emotional and physical symptoms (Lanius et al., 2010, Patriat et al., 2016).
In both the depression and PTSD literature in children and adolescents, the majority of existing DMN functional connectivity studies are cross-sectional, which can obscure important developmental effects and within-subject variability. Generally, longitudinal studies probing the association of DMN connectivity to the emergence of psychopathology have been sparse, and none to our knowledge have focused on the relationship between subclinical psychological symptoms and DMN resting state functional connectivity. One of the few longitudinal studies of DMN connectivity in youth with depression identified a relationship between negative DMN connectivity and familial depression risk, suggesting that altered DMN connectivity may contribute to early-onset depression (Cai et al., 2021). Investigating the relationships between the altered trajectory of DMN connectivity in healthy youth with subclinical symptoms, and the likelihood and severity of future clinical diagnoses during adulthood will be critical in better understanding the significance of developmental connectivity alterations. The current work aims to partially remedy this gap by examining the relationships between subclinical symptoms in healthy youth and DMN connectivity alterations, which may ultimately contribute to overt clinical diagnoses during adulthood.
Finally, we did not find consistent longitudinal within-network changes in DMN connectivity at the group level. Prior work suggests that DMN connectivity is increased in adults relative to children and adolescents at the network level, though there is significant heterogeneity in the strength and directionality of such connectivity findings. Some of the most commonly reported increases in connectivity in adults compared to youth are between the medial prefrontal cortex and posterior cingulate nodes (Fair et al., 2008, Supekar et al., 2010), although this effect has not been universally observed between these specific nodes in longitudinal studies of children and adolescents (Pozzi et al., 2021, Sylvester et al., 2018). In addition, decreased interhemispheric connectivity among core DMN nodes and decreased regional homogeneity have been found in adults compared to youth, but again such developmental patterns are less clear in studies looking at changes from childhood to adolescence (Fair et al., 2008, Fan et al., 2021, Jolles et al., 2011, Lopez-Larson et al., 2011, Pozzi et al., 2021, Sylvester et al., 2018). The extant literature is also quite heterogeneous with respect to connectivity metrics (e.g., correlation vs. connectedness), analytic methods used (e.g., structural equation modeling vs. graph theory), architecture of neural data (e.g., atlas vs. independent components analysis), and study samples (e.g., age range; Fair et al., 2008; Fan et al., 2021; Mak et al., 2017; Sato et al., 2014; Supekar et al., 2010; Uddin et al., 2010). Given this broad range of methodological approaches, it is not surprising that this literature is not fully consistent and future larger-scale studies focusing on children and adolescents will be needed to fully clarify the developmental trajectory of DMN connectivity.
Although our study presents novel findings of an altered trajectory of DMN connectivity in youth with subclinical symptomatology, it is not without limitations. Our sample included only youths without clinical diagnoses and generalization of our findings to those with clinical levels of psychological symptoms should be approached with caution. Our findings do not necessarily suggest that the altered trajectory identified here will portend diagnosis. In addition, our work focuses on the Yeo atlas as it is one of the most reliable atlases currently available, though future work could investigate how connectivity findings may vary using graph theory- or ICA-based approaches (Doucet et al., 2019). Furthermore, we examined static connectivity in our study, but future investigations could expand on this work by exploring dynamic connectivity changes (e.g., the chronnectome) with respect to psychopathology (Calhoun et al., 2014, Wise et al., 2017). Future work could extend the current work examining resting state connectivity by evaluating the characteristics of DMN suppression during a task and their relation to individual outcomes (Bartova et al., 2015, Vilgis et al., 2018, Whitfield-Gabrieli and Ford, 2012). The current analyses will also be extended in future work to examine the within- and between-network connectivity of the DMN, salience network, and executive control network, as part of the triple network model of cognitive dysfunction, and probed for alterations in the developmental trajectory related to changes in subclinical anxiety levels.
To conclude, this study is the first to examine the extent to which subclinical symptomatology was associated with DMN connectivity changes in a longitudinal sample of typically developing youth. Our key findings were that subclinical depressive and posttraumatic symptoms modulated the trajectory of connectivity in the DMN, specifically the dmPFC subsystem. Components of the dmPFC subsystem have previously been identified as critical brain regions involved in self-referential processing, emotional reappraisal, and various additional psychological processes. Together, these novel results suggest partial overlap with neural findings documented in adult depression and PTSD, but also point to potential developmental abnormalities in pediatric psychiatric disorders.
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by grants R01-MH121101, P20-GM144641, P30-GM122734, P50-AA22534, F31-DA056296, R56-MH124925, and R01-MH118695 from the National Institutes of Health and grants 1539067 and 2112455 from the National Science Foundation. The funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation. We want to thank the participants for volunteering to participate in the study and our staff and local collaborators for contributing to the work.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2023.101216.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Data availability
The data used in this article will be made publicly available through the COINS framework at the completion of the study (https://coins.trendscenter.org/).
References
- Andrews-Hanna J.R. The brain’s default network and its adaptive role in internal mentation. Neurosci.: Review J. Bringing Neurobiol. Neurol. Psychiatry. 2012;18(3):251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartova L., Meyer B.M., Diers K., Rabl U., Scharinger C., Popovic A., Pail G., Kalcher K., Boubela R.N., Huemer J., Mandorfer D., Windischberger C., Sitte H.H., Kasper S., Praschak-Rieder N., Moser E., Brocke B., Pezawas L. Reduced default mode network suppression during a working memory task in remitted major depression. J. Psychiatr. Res. 2015;64:9–18. doi: 10.1016/j.jpsychires.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum G.L., Ciric R., Roalf D.R., Betzel R.F., Moore T.M., Shinohara R.T., Kahn A.E., Vandekar S.N., Rupert P.E., Quarmley M., Cook P.A., Elliott M.A., Ruparel K., Gur R.E., Gur R.C., Bassett D.S., Satterthwaite T.D. Modular Segregation of Structural Brain Networks Supports the Development of Executive Function in Youth. Current Biology: CB. 2017;27(11):1561–1572.e8. doi: 10.1016/j.cub.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bie, Boersma M., Boersma M., Adriaanse S., Veltman D.J., Wink A.M., Roosendaal S.D., Barkhof F., Stam C.J., Oostrom K.J., Delemarre-van de Waal, Sanz-Arigita E.J., Sanz-Arigita E.J. Resting-state networks in awake five- to eight-year old children. Human Brain Mapping. 2012;33(5):1189–1201. doi: 10.1002/hbm.21280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman T., Malach R., Simony E. The surprising role of the default mode network in naturalistic perception. Communications Biology. 2021;4(1):79. doi: 10.1038/s42003-020-01602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J. Psychological Assessment Resources; 1996. Trauma Symptom Checklist for Children (TSCC) [DOI] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H., Weber J., Ochsner K.N. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24(11):2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren M., Gladwin T.E., Zandbelt B.B., Kahn R.S., Vink M. Reduced functional coupling in the default-mode network during self-referential processing. Hum. Brain Mapp. 2010;31(8):1117–1127. doi: 10.1002/hbm.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Elsayed N.M., Barch D.M. Contributions from resting state functional connectivity and familial risk to early adolescent-onset MDD: Results from the Adolescent Brain Cognitive Development study. Journal of Affective Disorders. 2021;287:229–239. doi: 10.1016/j.jad.2021.03.031. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Miller R., Pearlson G., Adali T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84(2):262–274. doi: 10.1016/j.neuron.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré A., Gierski F., Lemogne C., Tran E., Raucher-Chéné D., Béra-Potelle C., Portefaix C., Kaladjian A., Pierot L., Besche-Richard C., Limosin F. Linear association between social anxiety symptoms and neural activations to angry faces: from subclinical to clinical levels. Social Cognitive and Affective Neuroscience. 2014;9(6):880–886. doi: 10.1093/scan/nst061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Giedd J.N., Thomas K.M. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chapman D.P., Whitfield C.L., Felitti V.J., Dube S.R., Edwards V.J., Anda R.F. Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders. 2004;82(2):217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Che X., Luo X., Tong D., Fitzgibbon B.M., Yang J. Habitual suppression relates to difficulty in regulating emotion with cognitive reappraisal. Biol. Psychol. 2015;112:20–26. doi: 10.1016/j.biopsycho.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Chen X., Chen N.-X., Shen Y.-Q., Li H.-X., Li L., Lu B., Zhu Z.-C., Fan Z., Yan C.-G. The subsystem mechanism of default mode network underlying rumination: a reproducible neuroimaging study. NeuroImage. 2020;221 doi: 10.1016/j.neuroimage.2020.117185. [DOI] [PubMed] [Google Scholar]
- Copeland W.E., Keeler G., Angold A., Costello E.J. Traumatic events and posttraumatic stress in childhood. Archives of General Psychiatry. 2007;64(5):577–584. doi: 10.1001/archpsyc.64.5.577. [DOI] [PubMed] [Google Scholar]
- Cordes D., Haughton V.M., Arfanakis K., Carew J.D., Turski P.A., Moritz C.H., Quigley M.A., Elizabeth Meyerand M. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am. J. Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Gee D.G., Klimes-Dougan B., Gabbay V., Hulvershorn L., Mueller B.A., Camchong J., Bell C.J., Houri A., Kumra S., Lim K.O., Castellanos F.X., Milham M.P. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci. Lett. 2009;460(3):227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran P.J., Obeidat K., Losardo D. Twelve frequently asked questions about growth curve modeling. J. Cogn. Dev.: Off. J. Cogn. Dev. Soc. 2010;11(2):121–136. doi: 10.1080/15248371003699969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels J.K., Frewen P., McKinnon M.C., Lanius R.A. Default mode alterations in posttraumatic stress disorder related to early-life trauma: a developmental perspective. Journal of Psychiatry & Neuroscience: JPN. 2011;36(1):56–59. doi: 10.1503/jpn.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar S.M., Prince S.E., Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. NeuroImage. 2004;23(3):921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- DeSerisy M., Ramphal B., Pagliaccio D., Raffanello E., Tau G., Marsh R., Posner J., Margolis A.E. Frontoparietal and default mode network connectivity varies with age and intelligence. Developmental Cognitive Neuroscience. 2021;48:100928. doi: 10.1016/j.dcn.2021.100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G.E., Lee W.H., Frangou S. Evaluation of the spatial variability in the major resting-state networks across human brain functional atlases. Hum. Brain Mapp. 2019;40(15):4577–4587. doi: 10.1002/hbm.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G.E., Janiri D., Howard R., O’Brien M., Andrews-Hanna J.R., Frangou S. Transdiagnostic and disease-specific abnormalities in the default-mode network hubs in psychiatric disorders: A meta-analysis of resting-state functional imaging studies. European Psychiatry: The Journal of the Association of European Psychiatrists. 2020;63(1):e57. doi: 10.1192/j.eurpsy.2020.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders C.K. The performance of the full information maximum likelihood estimator in multiple regression models with missing data. Educ. Psychol. Meas. 2001;61(5):713–740. [Google Scholar]
- Enders C.K., Bandalos D.L. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct. Equ. Model.: A Multidiscip. J. 2001;8(3):430–457. [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U.F., Church J.A., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology. 2009;5(5) doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U.F., Church J.A., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. The maturing architecture of the brain’s default network. Proc. Natl. Acad. Sci. USA. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F., Liao X., Lei T., Zhao T., Xia M., Men W., Wang Y., Hu M., Liu J., Qin S., Tan S., Gao J.-H., Dong Q., Tao S., He Y. Development of the default-mode network during childhood and adolescence: a longitudinal resting-state fMRI study. NeuroImage. 2021;226 doi: 10.1016/j.neuroimage.2020.117581. [DOI] [PubMed] [Google Scholar]
- Fava G.A. Subclinical symptoms in mood disorders: pathophysiological and therapeutic implications. Psychological Medicine. 1999;29(1):47–61. doi: 10.1017/s0033291798007429. [DOI] [PubMed] [Google Scholar]
- Finkelhor D., Turner H.A., Shattuck A., Hamby S.L. Violence, crime, and abuse exposure in a national sample of children and youth: an update. JAMA Pediatrics. 2013;167(7):614–621. doi: 10.1001/jamapediatrics.2013.42. [DOI] [PubMed] [Google Scholar]
- Fransson Peter, Beatrice Skiöld, Sandra Horsch, Anders Nordell, Mats Blennow, Hugo Lagercrantz, Ulrika Aden. Resting-State Networks in the Infant Brain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(39):15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen J.D., Heinrichs-Graham E., White M.L., Wetzel M.W., Knott N.L., Wilson T.W. Atypical coupling between posterior regions of the default mode network in attention-deficit/hyperactivity disorder: a pharmaco-magnetoencephalography study. Journal of Psychiatry & Neuroscience: JPN. 2013;38(5):333–340. doi: 10.1503/jpn.120054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med.: Off. J. Soc. Magn. Reson. Med. / Soc. Magn. Reson. Med. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gaffrey M.S., Luby J.L., Botteron K., Repovš G., Barch D.M. Default mode network connectivity in children with a history of preschool onset depression. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2012;53(9):964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity A.G., Pearlson G.D., McKiernan K., Lloyd D., Kiehl K.A., Calhoun V.D. Aberrant “Default Mode” Functional Connectivity in Schizophrenia. American Journal of Psychiatry. 2007;164(3):450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearne L.J., Mattingley J.B., Cocchi L. Functional brain networks related to individual differences in human intelligence at rest. Scientific Reports. 2016;6:32328. doi: 10.1038/srep32328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Birn R.M., Ruttle P.L., Burghy C.A., Stodola D.E., Davidson R.J., Essex M.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. USA. 2013;110(47):19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., Connolly C.G., Henje Blom E., LeWinn K.Z., Strigo I.A., Paulus M.P., Frank G., Max J.E., Wu J., Chan M., Tapert S.F., Simmons A.N., Yang T.T. Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biol. Psychiatry. 2015;78(9):635–646. doi: 10.1016/j.biopsych.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L.M., Stockton P., Krupnick J.L., Green B.L. Development, use, and psychometric properties of the trauma history questionnaire. J. Loss Trauma. 2011;16(3):258–283. [Google Scholar]
- Hu L., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Model.: A Multidiscip. J. 1999;6(1):1–55. [Google Scholar]
- Hyatt C.J., Wexler B.E., Pittman B., Nicholson A., Pearlson G.D., Corbera S., Bell M.D., Pelphrey K., Calhoun V.D., Assaf M. Atypical Dynamic Functional Network Connectivity State Engagement during Social-Emotional Processing in Schizophrenia and Autism. Cerebral Cortex. 2022;32(16):3406–3422. doi: 10.1093/cercor/bhab423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles D.D., van Buchem M.A., Crone E.A., Rombouts S.A.R.B. A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb. Cortex. 2011;21(2):385–391. doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Spielberg J.M., Warren S.L., Sutton B.P., Miller G.A., Heller W., Banich M.T. Distracted and down: neural mechanisms of affective interference in subclinical depression. Social Cognitive and Affective Neuroscience. 2015;10(5):654–663. doi: 10.1093/scan/nsu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Koch S.B.J., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress Anxiety. 2016;33(7):592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Koch W., Teipel S., Mueller S., Benninghoff J., Wagner M., Bokde A.L.W., Hampel H., Coates U., Reiser M., Meindl T. Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer’s disease. Neurobiology of Aging. 2012;33(3):466–478. doi: 10.1016/j.neurobiolaging.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R.L., Coupland N.J., Hegadoren K.M., Rowe B., Théberge J., Neufeld R.W.J., Williamson P.C., Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr. Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Li B., Liu L., Friston K.J., Shen H., Wang L., Zeng L.-L., Hu D. A treatment-resistant default mode subnetwork in major depression. Biol. Psychiatry. 2013;74(1):48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Liberzon, I., & Sripada, C.S. (2007). The functional neuroanatomy of PTSD: a critical review. In E. R. De Kloet, M. S. Oitzl, & E. Vermetten (Eds.), Progress in Brain Research (Vol. 167, pp. 151–169). Elsevier. [DOI] [PubMed]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., Wheelwright S.J., Sadek S.A., Suckling J., MRC AIMS Consortium, Baron-Cohen S. Shared neural circuits for mentalizing about the self and others. J. Cogn. Neurosci. 2010;22(7):1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson M.P., Anderson J.S., Ferguson M.A., Yurgelun-Todd D. Local brain connectivity and associations with gender and age. Dev. Cogn. Neurosci. 2011;1(2):187–197. doi: 10.1016/j.dcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak L.E., Minuzzi L., MacQueen G., Hall G., Kennedy S.H., Milev R. The default mode network in healthy individuals: a systematic review and meta-analysis. Brain Connect. 2017;7(1):25–33. doi: 10.1089/brain.2016.0438. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Miller D.R., Hayes S.M., Hayes J.P., Spielberg J.M., Lafleche G., Verfaellie M. Default Mode Network Subsystems are Differentially Disrupted in Posttraumatic Stress Disorder. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging. 2017;2(4):363–371. doi: 10.1016/j.bpsc.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G., Ormel J., Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Soc. Cogn. Affect. Neurosci. 2010;5(4):369–377. doi: 10.1093/scan/nsq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson D., Wadsby M., Svedin C.G. The psychometric properties of the Trauma Symptom Checklist For Children (TSCC) in a sample of Swedish children. Child Abus. Negl. 2008;32(6):627–636. doi: 10.1016/j.chiabu.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A., Lynch C.J., Schaer M., Menon V. The Default Mode Network in Autism. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging. 2017;2(6):476–486. doi: 10.1016/j.bpsc.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek J.N., van der Werff S.J.A., Meens P.H.F., van den Bulk B.G., Jolles D.D., Veer I.M., van Lang N.D.J., Rombouts S.A.R.B., van der Wee N.J.A., Vermeiren R.R.J.M. Aberrant resting-state functional connectivity in limbic and salience networks in treatment--naïve clinically depressed adolescents. J. Child Psychol. Psychiatry, Allied Discip. 2014;55(12):1317–1327. doi: 10.1111/jcpp.12266. [DOI] [PubMed] [Google Scholar]
- Patel A.X., Kundu P., Rubinov M., Jones P.S., Vértes P.E., Ersche K.D., Suckling J., Bullmore E.T. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. NeuroImage. 2014;95:287–304. doi: 10.1016/j.neuroimage.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriat R., Birn R.M., Keding T.J., Herringa R.J. Default-mode network abnormalities in pediatric posttraumatic stress disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(4):319–327. doi: 10.1016/j.jaac.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi E., Vijayakumar N., Byrne M.L., Bray K.O., Seal M., Richmond S., Zalesky A., Whittle S.L. Maternal parenting behavior and functional connectivity development in children: a longitudinal fMRI study. Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec A.L., Heinrichs-Graham E., Wiesman A.I., McDermott T.J., Wilson T.W. Oscillatory dynamics in the dorsal and ventral attention networks during the reorienting of attention. Hum. Brain Mapp. 2018;39(5):2177–2190. doi: 10.1002/hbm.23997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The brain’s default mode network. Annual Review of Neuroscience. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A. A Default Mode of Brain Function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., Sanfey A.G., Aronson J.A., Nystrom L.E., Cohen J.D. The neural correlates of theory of mind within interpersonal interactions. NeuroImage. 2004;22(4):1694–1703. doi: 10.1016/j.neuroimage.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Rzepa E., McCabe C. Anhedonia and depression severity dissociated by dmPFC resting-state functional connectivity in adolescents. J. Psychopharmacol. 2018;32(10):1067–1074. doi: 10.1177/0269881118799935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchet M.D., Ho T.C., Connolly C.G., Tymofiyeva O., Lewinn K.Z., Han L.K., Blom E.H., Tapert S.F., Max J.E., Frank G.K., Paulus M.P., Simmons A.N., Gotlib I.H., Yang T.T. Large-scale hypoconnectivity between resting-state functional networks in unmedicated adolescent major depressive disorder. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2016;41(12):2951–2960. doi: 10.1038/npp.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F., Murty V.P., Callicott J.H., Tan H.-Y., Das S., Weinberger D.R., Mattay V.S. Age-related alterations in default mode network: impact on working memory performance. Neurobiology of Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders A.F.P., Baum G.L., Harms M.P., Kandala S., Bookheimer S.Y., Dapretto M., Somerville L.H., Thomas K.M., Van Essen D.C., Yacoub E., Barch D.M. Developmental trajectories of cortical thickness by functional brain network: The roles of pubertal timing and socioeconomic status. Developmental Cognitive Neuroscience. 2022;57:101145. doi: 10.1016/j.dcn.2022.101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J.R., Salum G.A., Gadelha A., Picon F.A., Pan P.M., Vieira G., Zugman A., Hoexter M.Q., Anés M., Moura L.M., Gomes Del’Aquilla M.A., Amaro E., Jr, McGuire P., Crossley N., Lacerda A., Rohde L.A., Miguel E.C., Bressan R.A., Jackowski A.P. Age effects on the default mode and control networks in typically developing children. J. Psychiatr. Res. 2014;58:89–95. doi: 10.1016/j.jpsychires.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Schalinski I., Teicher M.H., Nischk D., Hinderer E., Müller O., Rockstroh B. Type and timing of adverse childhood experiences differentially affect severity of PTSD, dissociative and depressive symptoms in adult inpatients. BMC Psychiatry. 2016;16:295. doi: 10.1186/s12888-016-1004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermelleh-Engel K., Moosbrugger H., Müller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. methods of psychological research. 2003;8(2):23–74. [Google Scholar]
- Sha Z., Wager T.D., Mechelli A., He Y. Common Dysfunction of Large-Scale Neurocognitive Networks Across Psychiatric Disorders. Biological Psychiatry. 2019;85(5):379–388. doi: 10.1016/j.biopsych.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z., Mintun M.A., Wang S., Coalson R.S., Raichle M.E. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L.E., Rudie J.D., Pfeifer J.H., Masten C.L., McNealy K., Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Developmental Cognitive Neuroscience. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Grady C.L. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 2010;22(6):1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Steinberg A.M., Brymer M.J., Kim S., Briggs E.C., Ippen C.G., Ostrowski S.A., Gully K.J., Pynoos R.S. Psychometric properties of the UCLA PTSD reaction index: part I. J. Trauma. Stress. 2013;26(1):1–9. doi: 10.1002/jts.21780. [DOI] [PubMed] [Google Scholar]
- Stephen J.M., Solis I., Janowich J., Stern M., Frenzel M.R., Eastman J.A., Mills M.S., Embury C.M., Coolidge N.M., Heinrichs-Graham E., Mayer A., Liu J., Wang Y.P., Wilson T.W., Calhoun V.D. The developmental chronnecto-genomics (Dev-CoG) study: a multimodal study on the developing brain. NeuroImage. 2021;225 doi: 10.1016/j.neuroimage.2020.117438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Prater K., Amin H., Greicius M.D., Menon V. Development of functional and structural connectivity within the default mode network in young children. NeuroImage. 2010;52(1):290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C.M., Whalen D.J., Belden A.C., Sanchez S.L., Luby J.L., Barch D.M. Shyness and trajectories of functional network connectivity over early adolescence. Child Dev. 2018;89(3):734–745. doi: 10.1111/cdev.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B.K., Frenzel M.R., Eastman J.A., Embury C.M., Agcaoglu O., Wang Y.-P., Stephen J.M., Calhoun V.D., Wilson T.W. Individual differences in amygdala volumes predict changes in functional connectivity between subcortical and cognitive control networks throughout adolescence. NeuroImage. 2022;247 doi: 10.1016/j.neuroimage.2021.118852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi L., Zhang X., Chesnut M., Holt-Gosselin B., Ramirez C.A., Williams L.M. Reduced functional connectivity of default mode network subsystems in depression: Meta-analytic evidence and relationship with trait rumination. NeuroImage. Clin. 2021;30 doi: 10.1016/j.nicl.2021.102570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front. Syst. Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum. Brain Mapp. 2009;30(3):829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A., Mutlu J., Chanraud S., Guenolé F., Egler P.-J., Gérardin P., Baleyte J.-M., Dayan J., Eustache F., Guillery-Girard B. Altered default mode network connectivity in adolescents with post-traumatic stress disorder. NeuroImage. Clin. 2019;22 doi: 10.1016/j.nicl.2019.101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgis V., Gelardi K.L., Helm J.L., Forbes E.E., Hipwell A.E., Keenan K., Guyer A.E. Dorsomedial prefrontal activity to sadness predicts later emotion suppression and depression severity in adolescent girls. Child Dev. 2018;89(3):758–772. doi: 10.1111/cdev.13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Ren T., Zhang X., Dou W., Jia X., Li B.-M. The longitudinal development of large-scale functional brain networks for arithmetic ability from childhood to adolescence. The European Journal of Neuroscience. 2022;55(7):1825–1839. doi: 10.1111/ejn.15651. [DOI] [PubMed] [Google Scholar]
- Wen T., Mitchell D.J., Duncan J. The functional convergence and heterogeneity of social, episodic, and self-referential thought in the default mode network. Cereb. Cortex. 2020;30(11):5915–5929. doi: 10.1093/cercor/bhaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Wilson T.W., Franzen J.D., Heinrichs-Graham E., White M.L., Knott N.L., Wetzel M.W. Broadband neurophysiological abnormalities in the medial prefrontal region of the default-mode network in adults with ADHD. Human Brain Mapping. 2013;34(3):566–574. doi: 10.1002/hbm.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T., Marwood L., Perkins A.M., Herane-Vives A., Joules R., Lythgoe D.J., Luh W.-M., Williams S.C.R., Young A.H., Cleare A.J., Arnone D. Instability of default mode network connectivity in major depression: a two-sample confirmation study. Transl. Psychiatry. 2017;7(4) doi: 10.1038/tp.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R.C., Herringa R.J. Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2016;41(3):822–831. doi: 10.1038/npp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.-G., Wang X.-D., Zuo X.-N., Zang Y.-F. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14(3):339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R., Fischl B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Okamoto Y., Takagaki K., Okada G., Takamura M., Mori A., Shiota S., Ichikawa N., Jinnin R., Yamawaki S. Effects of behavioral activation on default mode network connectivity in subthreshold depression: a preliminary resting-state fMRI study. J. Affect. Disord. 2018;227:156–163. doi: 10.1016/j.jad.2017.10.021. [DOI] [PubMed] [Google Scholar]
- Zeev-Wolf M, Levy J., Goldstein A., Zagoory-Sharon O., Feldman R. Chronic Early Stress Impairs Default Mode Network Connectivity in Preadolescents and Their Mothers. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging. 2019;4(1):72–80. doi: 10.1016/j.bpsc.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Zeev-Wolf M., Dor-Ziderman Y., Pratt M., Goldstein A., Feldman R. Investigating default mode network connectivity disruption in children of mothers with depression. The British Journal of Psychiatry: The Journal of Mental Science. 2022:1–10. doi: 10.1192/bjp.2021.164. [DOI] [PubMed] [Google Scholar]
- Zhang S., Chen J.-M., Kuang L., Cao J., Zhang H., Ai M., Wang W., Zhang S.-D., Wang S.-Y., Liu S.-J., Fang W.-D. Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry. 2016;16(1):337. doi: 10.1186/s12888-016-1047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Data Availability Statement
The data used in this article will be made publicly available through the COINS framework at the completion of the study (https://coins.trendscenter.org/).