Abstract
A 14-year-old boy with movement disorder and epilepsy developed status dystonicus leading to rhabdomyolysis and subsequent acute kidney injury requiring continuous renal replacement therapy (CRRT). He was given multiple intravenous sedatives and analgesics to control his dystonia and dyskinesia. 8 days after admission, his condition had improved and a trial termination of CRRT was carried out. The sedatives and analgesics were switched to oral diazepam, morphine, clonidine, and chloral hydrate. However, his renal function did not recover fully. There was rising trend of serum creatinine level with evolving hyperphosphatemia and metabolic acidosis. He also gradually developed hypoventilation, hypercapnia and pinpoint pupils after weaning CRRT. The clinical impression was over-sedation resulting in hypoventilation and respiratory failure, contributed by the deteriorating renal function. Non-invasive ventilatory support was then started and CRRT was resumed. His condition improved over the next 24 hours. Dexmedetomidine infusion was used during CRRT and he slowly required stepping up of sedatives again. A separate set of dosage for all his oral sedative agents was prepared for his subsequent CRRT weaning challenge and no more over-sedative episode was then encountered. Our case illustrated that patients at recovery phase of AKI are susceptible to medication overdose, especially during the period of CRRT weaning. Sedatives and analgesics including morphine and benzodiazepines should be used with caution during this period and alternatives may need to be considered. Advanced planning of medication dosage adjustment is advised to reduce the risk of medication overdose.
Keywords: critical care, medication safety, nephrology, pediatrics, pharmacokinetics, adverse drug reactions
Introduction
Critically ill patients with acute kidney injury (AKI) pose a great challenge to optimal drug dosing as drug absorption, distribution, metabolism and elimination are altered by the pathophysiologic changes. 1 Continuous renal replacement therapy (CRRT) has become a popular modality of renal replacement therapy for critically ill patients and weaning from CRRT is an integral part of AKI management as it determines the subsequent need of long-term renal replacement therapy. 2 However, this is also a period that is susceptible to medication overdose as drug clearance may exhibit huge variation when CRRT is stopped but the renal function is not yet fully recovered. A previous study also revealed that adverse drug events occurred both during AKI and at recovery phase of AKI. 3 We here reported a case of over-sedation occurring at the recovery phase of AKI during CRRT weaning.
Case Presentation
A 14-year-boy was admitted due to breakthrough seizure and status dystonicus after acquiring coronavirus disease 2019 (COVID-19) infection. He has GNAO1-mutation complicating movement disorder, epilepsy and severe intellectual disability requiring long term use of baclofen, tetrabenazine, carbamazepine and clobazam. He was found to have rhabdomyolysis after admission with a serum creatine kinase (CK) level of 217 030 IU/L, there was also AKI with a serum creatinine level of 79 μmol/L and estimated glomerular filtration rate [eGFR] of 65.6 mL/min/1.73 m2 (baseline creatinine level 31 μmol/L). He was started on alkaline diuresis and then transferred to the Pediatric Intensive Care Unit (PICU).
He soon developed respiratory failure and hypotension requiring intubation for mechanical ventilation and the use of inotropes. His CK level also rose sharply reaching a peak level of 449 100 IU/L, therefore CRRT was initiated. He required multiple medications for sedation and control of his underlying dystonia / dyskinesia which included intravenous infusion of midazolam (starting dose of 0.2 mg/kg/h), morphine (starting dose of 20 µg/kg/hour) and ketamine (starting dose of 5 µg/kg/minute), as well as oral clonidine (starting dose of 0.58 µg/kg/dose Q8H) and chloral hydrate (starting dose of 23 mg/kg/dose Q6H) in addition to his usual medications. Subsequently his condition was stabilized and he managed to wean off invasive ventilatory support. There was no recurrence of abnormal movement, and his sedation was then switched to oral diazepam, morphine, clonidine and chloral hydrate.
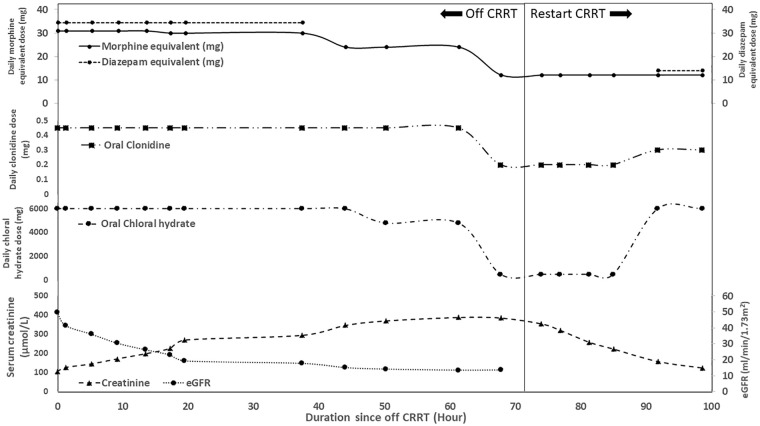
Eight days after admission his CRRT dose was much reduced and the CK level was 15 069 IU/L. Therefore, termination of CRRT was attempted and furosemide infusion at 1.2 mg/kg/hour was commenced for prevention of fluid overload. The renal function deteriorated slowly as evidenced by rising trend of serum creatinine level with evolving hyperphosphatemia and metabolic acidosis despite a satisfactory urine output. He also gradually developed hypoventilation with a rising trend of pCO2 up to 7.4 kPa despite reduction of doses of his sedatives and analgesics. 60 hours after stopping CRRT, his serum urea and creatinine level rose to 30 mmol/L and 389 μmol/L (eGFR 13.3 mL/min/1.73 m2) respectively. Physical examination revealed a respiratory rate of 8 breaths/minute only, and the pupil sizes were 2 mm bilaterally. There was no focal neurological deficit. The state behavioral scale (SBS) was −2 (responsive to noxious stimuli) indicating a deep sedation state. The clinical impression was over-sedation resulting in hypoventilation and respiratory failure, contributed by the deteriorating renal function.
CRRT support was restarted immediately and non-invasive ventilatory support was given. After discussion with the clinical pharmacist, his oral sedatives and analgesics were reduced (Figure 1) and short-term dexmedetomidine infusion (starting dose of 0.3 µg/kg/hour) was used during CRRT. Over the next 24 hours, his breathing effort improved and he managed to wean off the non-invasive ventilatory support. Physical examination showed bilateral pupil sizes of 3 mm and a respiratory rate of 16 breaths/minute. He became more alert and his SBS rose to 2 with development of tachycardia. The dosages of his sedative agents were then stepped up and a separate set of dosage for all his oral sedative agents was prepared when his CRRT was stopped (oral morphine 3 mg Q6H, clonidine 0.05 mg Q6H and chloral hydrate 500 mg daily). No recurrence of over-sedation was noted upon subsequent challenge of CRRT termination, and his sedatives and analgesics were weaned off slowly over a period of few weeks.
Figure 1.
The change of doses of the sedatives and analgesics during CRRT weaning.
Note. The combination of sedatives and analgesics at the time when serum creatinine level rose to peak included oral morphine 3 mg Q6H, clonidine 0.05 mg Q6H and chloral hydrate 500 mg daily. CRRT = continuous renal replacement therapy; eGFR = estimated glomerular filtration rate.
Discussion
Patients with AKI are at risk of medication overdose, and the impact of drugs with potentially serious side effects such as sedatives and analgesics can be huge as illustrated by the occurrence of over-sedation in our patient during CRRT weaning. Sedatives and analgesics are essential in pediatric practice and multiple medications, particularly opioids, benzodiazepines and α-agonists, are usually required for successful delivery of invasive treatment including mechanical ventilation and CRRT. 4 In fact, concomitant administration of benzodiazepines and opioids in patients with renal impairment would increase the risk of respiratory depression. 5 The unstable and deteriorating renal function, co-administration of opioids and benzodiazepines in addition to his usual CNS suppressant and anti-epileptics certainly increased our patient’s risk of developing respiratory compromise when CRRT was stopped. And it is equally important not to abruptly terminate benzodiazepines and opioids for risk of acute withdrawal. Unlike many of the antibiotics or anti-epileptics, drug level monitoring is not available for most analgesics and sedatives. Hence monitoring of its clinical effect is an essential part for early detection of potential adverse events. In addition to SBS, COMFORT behavior scale (COMFORT-B scale) and Richmond Agitation Sedation Scale (RASS) are validated scores that can be applied to critically ill children. 4 Moreover, physiological parameters such as respiratory rate, oxygen saturation, heart rate and end-tidal carbon dioxide or partial pressure of carbon dioxide are useful indicators of sedation level.
Clinicians and pharmacists may often need to make clinical judgment of drug dosage adjustment as specific recommendations on safe prescription for children recovering AKI or weaning CRRT are scarce. There are 2 main factors that merit consideration before making a prescription adjustment. Firstly, the degree of kidney function recovery based on factors such as the underlying cause of AKI, hemodynamic parameters, trend of renal function, CRRT dose before termination and urine output in response to diuretics challenge. All these parameters will provide important clues for weaning success.2,6 Although advanced technique such as transdermal measurement of fluorescent molecules may provide a novel method for timely estimation of GFR, it is not readily available in most units. 7 The bedside eGFR estimating equation remains one of the most convenient methods to estimate the GFR, 8 and regular renal function monitoring may be required. Secondly, the pharmacological properties of individual medications including the degree of protein binding, the volume of distribution (Vd), the lipophilicity and the molecular weight should also be considered 1 (Table 1). As the Vd of medications may increase among patients in critical illness, 1 this may cause a longer medication half-life leading to more sustained clinical effects. Together with the termination of CRRT, this may explain the over-sedation in our patient. Opioids and benzodiazepines, morphine and midazolam in particular, are one of the most commonly used analgesics and sedative agents in the PICUs locally. Opioid analgesics are frequently used for pain control among critically ill patients and those with renal impairment.4,9 Dose adjustment for renal impairment is required for most opioid analgesics and a few types of opioids such as morphine, oxycodone and codeine are not recommended in those with very low eGFR. 9 In fact, avoiding morphine and switching to other opioids such as fentanyl may be a safer alternative in this circumstance. Benzodiazepines should also be used with cautious in those with renal impairment as the side effect profiles may be enhanced, particularly in conjunction with opioids.10,11 Given the risk of aggravating hypotension and involuntary movement, propofol was not used at that juncture. It should also be noted that most pharmacokinetic information for renal impairment is based on studies conducted on patients with chronic kidney disease. Clinicians and pharmacists may need to extrapolate those results when making decision for patients with AKI. Therefore, a “start-low” and “slow-and-small dose titration” approach may be rational for patients recovering AKI, and dose reduction or even medication termination should be exercised when CRRT is stopped.9,10 Frequent monitoring of the renal function and evaluation of medication side effects are needed to prevent under- or overdosing.
Table 1.
Pharmacological Properties of the Sedatives and Analgesics Used During CRRT Weaning.
| Drug | Molecular weight (Dalton) | Protein binding (%) | Volume of distribution (L/kg) | Half-life (hour) | Urine elimination | Bioavailability | Dose adjustment for eGFR < 15 mL/min/1.73 m2 |
|---|---|---|---|---|---|---|---|
| Chloral hydrate | 165.4 | 35-40 | 0.6 | Children: 10 Adult 8-12 |
Mostly urine (as metabolites) | >95% | Avoid in severe renal impairment |
| Clonidine | 230 | 20 | Children: 1 Adults: 2-3 |
Children: 6 Adults: 12-16 Renal impairment: ≤41 |
40% (as unchanged drug) | 70-80% | Lower dose is recommended |
| Diazepam | 285 | 96 | 1 | Children: 15-21 Adults: 44-48 |
100% (mostly as glucuronide conjugates) | >90% | No reference |
| Midazolam | 326 | 97 | Children: 1-2 Adults: 1-3 |
Children: 2.9-4.5 Adults: 3 |
90% (mostly as glucuronide conjugates) | — | 50% dose reduction if <10 mL/min/1.73 m2 |
| Morphine | 285 | 20-35 | 1 | Children: 2-9 Adults: 2 |
~90% (10% as unchanged drug) |
17-33% | 25-50% of usual dose and Increase dosing interval Avoid in those with <15 mL/min/1.73 m2 due to delayed clearance of active metabolite morphine-6-glucuronide |
Note. eGFR = estimated glomerular filtration rate.
The impact of CRRT on the degree of drug removal should also be considered when prescribing for patients weaning CRRT. However, the information on optimal medication dosing during CRRT is very limited. It is estimated that drug dosing studies were available for only <20% of commonly used medications for patients receiving CRRT. 12 Medications with lower degree of protein binding, smaller Vd and smaller molecular weight would be more readily removed by CRRT. 1 To further complicate the problem, various modalities of CRRT or the increasingly employed hemoadsorption column may all have different effects on the clearance of medications. 1 For clearance of small-sized molecules (<500-1000 Daltons), solute clearance occurs with simple diffusion (modality: continuous veno-venous hemodialysis [CVVHD]) and is affected by both the fraction of drug in the dialysate fluid (saturation coefficient [SA]) and the dialysate flow rate. For clearance of middle-sized molecules (1000-15 000 Daltons), convection is the main mechanism of solute clearance (modality: continuous veno-venous hemofiltration [CVVH]). The ultrafiltration rate is the main determinant of drug clearance, which is dependent on the sieving coefficient (Sc) of the drug and is dependent on the molecular weight of the drug and the membrane pore size. In continuous veno-venous hemodiafiltration (CVVHDF), the effect of convection and diffusion are combined. 1 Hemoadsorption column may cause significant adsorption of various molecules including medications, 13 however in-vivo information on their impact on drug levels may be limited, and drug monitoring should be performed whenever available.
Given the dynamic change of GFR at the time of AKI recovery with various factors mentioned above, the input from a clinical pharmacist is indispensable. It has been demonstrated that pharmacist interventions in critical care settings may ensure proper medication dosing14,15 and even reduce the occurrence of medication error. 16 A well-defined critical care pharmacy service model is important for delivery of high-quality critical care services. 17
Conclusion
Patients at recovery phase of AKI are susceptible to adverse effects of medications, especially during the period of CRRT weaning. Advanced planning on medication dosage adjustment is advised to minimize the development of side effects. Both patient and pharmacokinetic factors should be considered when adjusting medication dosage. Cautious use of medications, appropriate dosage adjustment and frequent monitoring of renal function, serum drug level and manifestation of medication side effects are indispensable to prevent medication overdose at this vulnerable period.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Informed consent has been obtained from the parents.
ORCID iD: Wun Fung Hui  https://orcid.org/0000-0001-5941-1478
https://orcid.org/0000-0001-5941-1478
References
- 1. Bouajram RH, Awdishu L. A clinician’s guide to dosing analgesics, anticonvulsants, and psychotropic medications in continuous renal replacement therapy. Kidney Int Rep. 2021;6(8):2033-2048. doi: 10.1016/j.ekir.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gautam SC, Srialluri N, Jaar BG. Strategies for continuous renal replacement therapy de-escalation. Kidney360. 2021;2(7):1166-1169. doi: 10.34067/KID.0000912021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cox ZL, McCoy AB, Matheny ME, et al. Adverse drug events during AKI and its recovery. Clin J Am Soc Nephrol. 2013;8(7):1070-1078. doi: 10.2215/CJN.11921112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egbuta C, Mason KP. Current state of analgesia and sedation in the Pediatric Intensive Care Unit. J Clin Med. 2021;10(9):1847. doi: 10.3390/jcm10091847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta K, Prasad A, Nagappa M, Wong J, Abrahamyan L, Chung FF. Risk factors for opioid-induced respiratory depression and failure to rescue: a review. Curr Opin Anaesthesiol. 2018;31(1):110-119. [DOI] [PubMed] [Google Scholar]
- 6. Raurich JM, Llompart-Pou JA, Novo MA, Talavera C, Ferreruela M, Ayestarán I. Successful weaning from continuous renal replacement therapy. Associated risk factors. J Crit Care. 2018;45:144-148. doi: 10.1016/j.jcrc.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 7. Solomon R, Goldstein S. Real-time measurement of glomerular filtration rate. Curr Opin Crit Care. 2017;23(6):470-474. doi: 10.1097/MCC.0000000000000456 [DOI] [PubMed] [Google Scholar]
- 8. Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629-637. doi: 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gelot S, Nakhla E. Opioid dosing in renal and hepatic impairment. US Pharm. 2014;39(8):34-38. [Google Scholar]
- 10. Wilcock A, Charlesworth S, Twycross R, et al. Prescribing non-opioid drugs in end-stage kidney disease. J Pain Symptom Manag. 2017;54(5):776-787. doi: 10.1016/j.jpainsymman.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: Drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12(6):235. doi: 10.1186/cc7093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mueller BA, Smoyer WE. Challenges in developing evidence-based drug dosing guidelines for adults and children receiving renal replacement therapy. Clin Pharmacol Ther. 2009;86(5):479-482. doi: 10.1038/clpt.2009.150 [DOI] [PubMed] [Google Scholar]
- 13. Schneider AG, André P, Scheier J, et al. Pharmacokinetics of anti-infective agents during CytoSorb hemoadsorption. Sci Rep. 2021;11(1):10493. doi: 10.1038/s41598-021-89965-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tawhari MM, Tawhari MA, Noshily MA, Mathkur MH, Abutaleb MH. Hospital pharmacists interventions to drug-related problems at tertiary critical care pediatric settings in Jazan, Saudi Arabia. Hosp Pharm. 2022;57(1):146-153. doi: 10.1177/0018578721990889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chiang LH, Huang YL, Tsai TC. Clinical pharmacy interventions in intensive care unit patients. J Clin Pharm Ther. 2021;46(1):128-133. doi: 10.1111/jcpt.13265 [DOI] [PubMed] [Google Scholar]
- 16. Naseralallah LM, Hussain TA, Jaam M, Pawluk SA. Impact of pharmacist interventions on medication errors in hospitalized pediatric patients: a systematic review and meta-analysis. Int J Clin Pharm. 2020;42(4):979-994. [DOI] [PubMed] [Google Scholar]
- 17. Lat I, Paciullo C, Daley MJ, et al. Position Paper on Critical Care Pharmacy Services: 2020 update. Crit Care Med. 2020;48(9):e813-e834. doi: 10.1097/CCM.0000000000004437 [DOI] [PubMed] [Google Scholar]