Abstract
Background
The brain-derived neurotrophic factor (BDNF) may promote development of pulmonary hypertension and right ventricular (RV) failure. However, BDNF plasma levels were decreased in patients with left ventricular (LV) failure. Therefore, we investigated BDNF plasma levels in pulmonary hypertension patients and the role of BDNF in mouse models of pulmonary hypertension and isolated RV failure.
Methods
BDNF plasma levels were correlated to pulmonary hypertension in two patient cohorts, including either post- and pre-capillary pulmonary hypertension patients (first cohort) or only pre-capillary pulmonary hypertension patients (second cohort). In the second cohort, RV dimensions and load-independent function were determined by imaging and pressure–volume catheter measurements, respectively. For induction of isolated RV pressure overload, heterozygous Bdnf knockout (Bdnf+/−) mice were subjected to pulmonary arterial banding (PAB). For induction of pulmonary hypertension, mice with inducible knockout of BDNF in smooth muscle cells (Bdnf/Smmhc knockout) were exposed to chronic hypoxia.
Results
Plasma BDNF levels were decreased in patients with pulmonary hypertension. Following adjustment for covariables, BDNF levels negatively correlated in both cohorts with central venous pressure. In the second cohort, BDNF levels additionally negatively correlated with RV dilatation. In animal models, BDNF downregulation attenuated RV dilatation in Bdnf+/−mice after PAB or hypoxic Bdnf/Smmhc knockout mice, although they developed pulmonary hypertension to a similar extent.
Conclusions
Similar to LV failure, circulating levels of BDNF were decreased in pulmonary hypertension patients, and low BDNF levels were associated with right heart congestion. Decreased BDNF levels did not worsen RV dilatation in animal models, and thus, may be the consequence, but not the cause of RV dilatation.
Short abstract
Plasma BDNF levels are decreased in IPAH patients, and correlate with central venous pressure and RV dilatation but not heart function. Low BDNF levels in animal models attenuate RV dilatation. https://bit.ly/3e2dwTK
Introduction
Pulmonary hypertension is a life-threatening disease of the pulmonary circulation characterised by an increased pulmonary arterial pressure (PAP), which leads to increased right ventricular (RV) afterload and, ultimately, RV failure. Based on aetiology, pathological findings and haemodynamic features, different forms of pulmonary hypertension can be distinguished according to the World Symposium on Pulmonary Hypertension [1]. Pre-capillary forms of pulmonary hypertension are defined by an increased pulmonary vascular resistance (PVR) caused by pulmonary vascular remodelling, while post-capillary forms are caused by left heart disease leading to an increase in pulmonary venous pressure and subsequently PAP without increased PVR [1]. Different triggers, such as chronic hypoxic exposure, can lead to contraction, proliferation and migration of pulmonary arterial smooth muscle cells (PASMCs), which are characteristics of pre-capillary forms of pulmonary hypertension [2]. Despite therapeutic improvement over recent decades, there is currently no cure for pulmonary vascular remodelling or subsequent RV failure [3].
It has been described that plasma levels of the brain-derived neurotrophic factor (BDNF) were decreased in patients with heart failure and associated with heart failure severity [4] as well as with adverse outcomes [5]. In contrast, expression of BDNF and one of its receptors, the tyrosine kinase receptor B (TrkB), was increased in arteries of patients with idiopathic pulmonary arterial hypertension (IPAH) and mice with chronic hypoxia-induced pulmonary hypertension [6]. Hypoxic exposure of human PASMCs and endothelial cells also increased BDNF protein expression as well as secretion in vitro [7, 8]. As BDNF treatment resulted in an augmented proliferation of human PASMCs, it was suggested that BDNF promotes development of pulmonary hypertension [6]. Along these lines, it is well known that tyrosine kinase receptors induce major intracellular signalling cascades resulting in proliferation, migration and resistance to apoptosis of PASMCs [9]. In particular, BDNF can bind to the high-affinity TrkB receptor and the low-affinity p75 neurotrophin receptor, which leads to activation of several proliferative pathways [10, 11]. Previously, BDNF as a member of the neurotrophin family was mainly studied in the nervous system. However, BDNF is released by several other organs including muscles, the liver and adipose tissue, as well as circulating blood cells such as activated immune cells, and platelets, which can store BDNF [12]. Moreover, BDNF can be released by cardiomyocytes and enhanced the cardiac contractile force by increasing calcium transients in cardiomyocytes [13]. Thus, BDNF may not only play a role in pulmonary vascular remodelling, but also RV adaptation to increased afterload. In this regard, opposing results were published showing both worse and better outcome after myocardial infarction in genetic mouse models of decreased BDNF expression [14, 15]. However, the role of BDNF in the development of pulmonary hypertension and right heart hypertrophy and failure is currently unknown.
Against this background, we aimed to determine whether BDNF could serve as a potential biomarker and/or treatment target for pulmonary hypertension and RV failure. Therefore, we conducted a study to 1) asses the relationship between BDNF plasma levels and the severity of pulmonary hypertension and RV function in patients with different forms of pulmonary hypertension; 2) investigate the development of RV remodelling and function in BDNF haploinsufficient mice after pulmonary arterial banding (PAB); and 3) determine the effect of smooth muscle cell-specific BDNF deletion in mice on the development of hypoxia-induced pulmonary hypertension.
Materials and methods
A more detailed description of the methods is in the supplementary material.
Clinical study
Two cohorts were included in the study. The first cohort included pulmonary hypertension and control patients from the Giessen Pulmonary Hypertension Registry. Pulmonary hypertension patients were grouped into pre-capillary pulmonary hypertension (only patients with IPAH were included to avoid confounding factors of associated forms of pulmonary hypertension), isolated post-capillary pulmonary hypertension and combined pre- and post-capillary pulmonary hypertension (cpcPH) according to the current guidelines [1]. The second cohort included IPAH patients from the Right Heart 1 trial (clinicaltrials.gov identifier NCT03403868) with available conductance catheter and/or magnetic resonance imaging (MRI) measurements. Patient informed consent was obtained before the study, as approved by the local authorities (ethics proposal number 100/2013) and in accordance with the ethical principles for medical research involving human subjects as set out in the Declaration of Helsinki.
Clinical investigations
Clinical investigations such as right heart catheterisation, cardiopulmonary exercise testing (CPET), and echocardiography were performed in the course of the Right Heart 1 study, as described previously [16, 17]. MRI was performed using a 1.5 T scanner system (Avanto; Siemens Healthineers). For the analysis of online pressure–volume loops, conductance catheterisation was performed by using a 4F pressure–volume catheter (CA-Nr 41063; CD Leycom) positioned in the RV apex. End-systolic elastance was calculated as maximal pressure − end-systolic pressure/stroke volume and arterial elastance as end-systolic pressure/stroke volume from single beat analysis, as described previously [18, 19].
BDNF ELISA
BDNF level in plasma was measured using BDNF ELISA (R&D Systems).
Animal studies
All animal experiments were approved by the local authorities of Regierungspräsidium Giessen (GI 20/10, number 92/2010 and 24/2015 for hypoxic experiments; GI 20/10 number 63/2012 for PAB; GI 20/10 number 115/2014 for cell isolation) and in compliance with the guidelines from directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes and to the Declaration of Helsinki. All mice used in this study were adult males with a weight of 20–30 g. For the hypoxic experiments, Smmhc-CreERT2 mice (Tg(Myh11-cre/ERT2)) mice were obtained from Stefan Offermanns (Max Planck Institute for Heart and Lung Research, Bad Nauheim, Germany) and crossbred with BDNF-floxed mice (Bdnftm3Jae/J) from the Jackson Laboratory to obtain mice expressing tamoxifen-inducible Cre-recombinase under the promoter of smooth muscle myosin heavy chain (Myh11, also known as Smmhc) and Bdnf flanked by loxP sites (Bdnf/Smmhc). Induction of the smooth muscle cell (SMC)-type specific knockout was achieved by tamoxifen feeding 10 days prior to normoxic or hypoxic exposure. In total, three different groups were evaluated: the Bdnf/Smmhc control group without induction of the BDNF knockout by feeding with tamoxifen-free food; the Bdnf/Smmhc knockout group with induction of Bdnf knockout in SMCs by feeding with tamoxifen-containing food; and to evaluate the effect of tamoxifen feeding, we additionally analysed Cre-negative, Bdnf-floxed mice that were fed with tamoxifen without induction of BDNF knockout (Smmhc control group).
For the PAB experiments, mice heterozygous for the Bdnftm1Jae mutation (B6.129S4-Bdnftm1Jae/J, Bdnf+/−), which show approximately half normal levels of Bdnf mRNA, originating from the Jackson Laboratory and bred in our local animal facility, were used. For the control, we used littermates without mutation (WT) bred in our local animal facility. For cell isolation, WT (C57BL/6J) mice were obtained from the Jackson Laboratory. Numbers given for in vivo measurements may vary for different parameters due to technical issues (e.g. displacement of catheter).
Hypoxic exposure, pulmonary artery banding, in vivo studies
For chronic hypoxic exposure, Bdnf/Smmhc knockout (+/− tamoxifen) and Smmhc control (+ tamoxifen) mice were kept in a ventilated chamber under normobaric hypoxic (10% oxygen) conditions for 4 weeks. PAB was performed as described previously [20]. Echocardiographic images were obtained with a high-resolution imaging system (Vevo 2100; VisualSonics), as described previously [21, 22]. In vivo haemodynamics were performed as described previously [21, 22].
Histology
To evaluate the extent of pulmonary vascular remodelling, double immunohistochemical staining was performed using a monoclonal anti-α-smooth muscle actin antibody (#A2547; Sigma-Aldrich) to detect the vascular smooth muscle layer and an anti-von-Willebrand factor antibody (#GA527; Dako) to detect the endothelium. To evaluate cardiac fibrosis, Picro-Sirius Red staining (Picro-Sirius Red Stain Kit, #ab150681; Abcam) was performed.
Isolation of PASMCs
Isolation of mouse PASMCs from pre-capillary pulmonary arterial vessels was performed, as described previously [23].
Proliferation assay
Cell proliferation of PASMCs was assessed using 5-bromo-2′-desoxyuridine incorporation (Roche).
Quantitative real-time PCR
Real-time PCR was carried out in a CFX Connect Real-Time PCR Detection System (Bio-Rad) using SYBR-Green (Bio-Rad).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.05 (GraphPad Software). All data in the figures are presented as mean±sem. A p-value ≤0.05 was considered to be significant. For the evaluation of the clinical study, a linear regression analysis, multiple regression analysis or a one-factorial ANOVA was performed. p-values are provided for the linear regression analysis: p#-values for multiple regression analysis with adjustment for body mass index (BMI), age, gender, thrombocyte counts, haemoglobin levels, thyroid-stimulating hormone and C-reactive protein; and p¶-values for multiple regression analysis with adjustment for BMI, age and gender. The results of the animal experiments were analysed using two-way ANOVA with a post hoc multiple comparison test according to Tukey.
Results
BDNF plasma levels were decreased in pulmonary hypertension patients and correlated with RV and left ventricular function
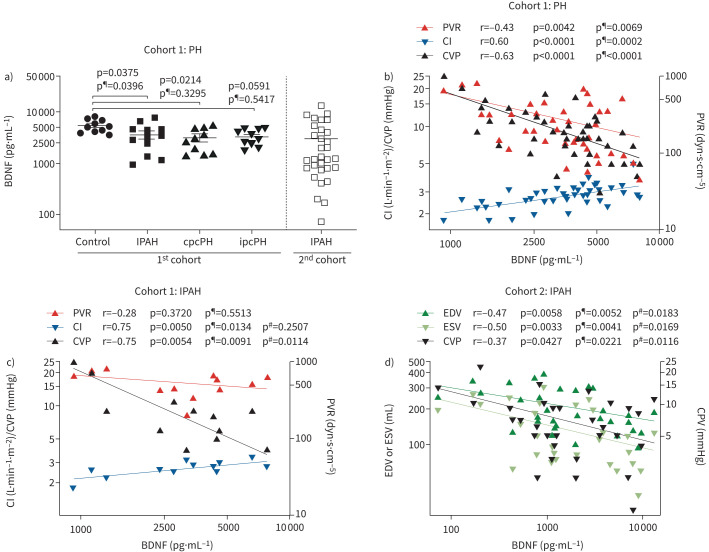
To assess whether BDNF could serve as a potential biomarker of specific pulmonary hypertension forms, BDNF concentration was first measured in plasma of control patients and patients with pre-, post- or combined pre- and post-capillary pulmonary hypertension (patient characteristics of cohort 1 are shown in table 1 and supplementary table S1). BDNF plasma levels were significantly decreased in pulmonary hypertension patients with IPAH and cpcPH compared to control patients, and only in IPAH patients after adjustment for BMI, age and gender (figure 1a). These data indicated that these covariables explain some of the difference in BDNF levels between patients with a post-capillary component and control patients (figure 1a). BDNF plasma levels negatively correlated with PVR and central venous pressure (CVP), and positively with cardiac index in all pulmonary hypertension patients and correlation was preserved after adjustment for BMI, age and gender (figure 1b). Analysis of IPAH patients of the first cohort further showed correlation of BDNF plasma levels with CVP (p¶=0.0091) and cardiac index (p¶=0.0134), but not PVR (p¶=0.5513) after adjustment for these covariables (figure 1c).
TABLE 1.
Pulmonary hypertension patient characteristics (first cohort)
| Control | IPAH | p-value | cpcPH | p-value | ipcPH | p-value | |
| Patients | 10 | 12 | 10 | 10 | |||
| Male/female | 4–6 | 5–7¶ | 3–7¶ | 3–7¶ | |||
| Age (years) | 43 (39–67) | 49 (42–57) | 0.9440 | 78 (71–80) | <0.0001 | 71 (60–78) | 0.0009 |
| BMI (kg·m−2) | 27 (25–29) | 27 (22–30) | 0.8710 | 26 (24–30) | 0.9672 | 31 (27–39) | 0.1906 |
| mPAP (mmHg) | 18 (16–20) | 43 (37–54) | <0.0001 | 39 (32–45) | <0.0001 | 30 (24–34) | 0.0037 |
| PAWP (mmHg) | 10 (9–11) | 11 (8–13) | 0.9726 | 21 (19–23) | <0.0001 | 20 (17–22) | <0.0001 |
| PVR (dyn·s·cm−5) | 95 (73–135) | 558 (437–667) | <0.0001 | 319 (265–349) | <0.0001 | 145 (99–167) | 0.8643 |
| Cardiac index (L·min−1·m−2) | 3.1 (2.9–3.4) | 2.7 (2.5–3.0) | 0.0279 | 2.4 (1.9–2.6) | 0.0014 | 2.7 (2.4–3.0) | 0.0811 |
| CVP (mmHg) | 7 (5–8) | 9 (5–11) | 0.4019 | 11 (9–13) | 0.2244 | 10 (8–15) | 0.1041 |
| BNP (pg·mL−1)# | 17 (6–41) | 86 (33–257) | 0.1783 | 309 (209–541) | 0.0002 | 99 (77–165) | 0.1640 |
| BDNF (pg·mL−1) | 5074 (4031–6901) | 3362 (1618–4613) | 0.0375 0.0396+ | 2838 (1528–4999) | 0.0214 0.3295+ | 3220 (2200–4501) | 0.0591 0.5417+ |
Data are presented as n or median (interquartile range), unless otherwise stated. IPAH: idiopathic pulmonary hypertension; cpcPH: combined pre- and post-capillary pulmonary hypertension; ipcPH: isolated post-capillary pulmonary hypertension; BMI: body mass index; mPAP: mean pulmonary arterial pressure, PAWP: pulmonary arterial wedge pressure; PVR: pulmonary vascular resistance; CVP: central venous pressure; BNP: brain natriuretic peptide; BDNF: brain-derived neurotrophic factor. Statistical analysis was performed using one-way-ANOVA with Dunnett's post hoc multiple comparison test. p-values are provided in comparison to the control group. #: n=9 for control and ipcPH; ¶: no statistically significant difference compared to control; +: p-value adjusted for BMI, age and gender.
FIGURE 1.
Brain-derived neurotrophic factor (BDNF) plasma concentration in control and pulmonary hypertension patients. a) BDNF plasma concentration determined in peripheral blood samples from control patients and patients with idiopathic pulmonary arterial hypertension (IPAH), combined pre-and post-capillary pulmonary hypertension (cpcPH) and isolated post-capillary pulmonary hypertension (ipcPH). b) Correlation of BDNF plasma concentration to pulmonary vascular resistance (PVR), cardiac index (CI) or central venous pressure (CVP) in all pulmonary hypertension patients. c) Correlation of BDNF plasma concentration to PVR, CI or CVP in IPAH patients. d) Correlation of BDNF plasma concentration to end-diastolic volume (EDV), end-systolic volume (ESV) or CVP. a) Disease groups were compared to the control group with Dunnett's post hoc multiple comparison test using appropriate transformations (log for concentrations). b, c, d) Statistical analysis was performed by linear regression analysis for calculation of Pearson's r and p-value. #: multiple regression analysis was performed to adjust for body mass index (BMI), age, gender, thrombocyte counts, haemoglobin levels, thyroid-stimulating hormone and C-reactive protein; ¶: multiple regression analysis was performed to adjust for BMI, age and gender only.
Next, we investigated the relationship between BDNF plasma levels and specifically RV remodelling and function. For this purpose, we collected blood from a second cohort of IPAH patients (patient characteristics of cohort 2 are presented in table 2 and supplementary table S1) and compared BDNF plasma levels (displayed in figure 1a) to parameters obtained using CPET and echocardiography during routine clinical presentation or, if available, to MRI and conductance catheter measurements from the Right Heart 1 trial (clinicaltrials.gov identifier NCT03403868).
TABLE 2.
Correlation of brain-derived neurotrophic factor (BDNF) plasma levels to structural and functional heart parameters and clinical parameters (second cohort)
| Patients (n) | Median (interquartile range) | Pearson r | p-value | p-value adjusted for BMI, age and gender | |
| Pulmonary vascular parameters | 0 . 0778 | 0 . 1226 | |||
| PASP (mmHg), echo | 23 | 66 (46–90) | −0.52 | 0.0110 | 0.0245 |
| PCWP (mmHg), RHC | 31 | 9 (7–10) | −0.01 | 0.9725 | 0.4350 |
| PVR (dyn·s·cm−5), RHC | 31 | 529 (335–776) | −0.19 | 0.3189 | 0.6167 |
| RV dimensions/structure | <0 . 0001 | <0 . 0001 | |||
| EDV (mL), MRI | 31 | 179 (145–291) | −0.48 | 0.0058 | 0.0052 |
| ESV (mL), MRI | 31 | 116 (73–196) | −0.51 | 0.0033 | 0.0041 |
| Myocardial mass diastole (g), MRI | 31 | 60 (47–97) | −0.34 | 0.0313 | 0.0134 |
| Myocardial mass systole (g), MRI | 31 | 59 (44–87) | −0.35 | 0.0527 | 0.0083 |
| T1 uRVIP (ms), MRI | 28 | 1053 (1011–1096) | −0.42 | 0.0270 | 0.1328 |
| T2 uRVIP (ms), MRI | 31 | 63 (59–70) | 0.20 | 0.767 | 0.5558 |
| RV function | 0 . 0297 | 0 . 0525 | |||
| TAPSE (mm), echo | 27 | 20 (18–26) | 0.34 | 0.0817 | 0.3945 |
| EF (%), MRI | 31 | 39 (27–51) | 0.45 | 0.0105 | 0.0135 |
| SV (mL), MRI | 31 | 68 (60–90) | −0.11 | 0.5451 | 0.4010 |
| Ees, PV | 28 | 49 (38–73) | −0.16 | 0.4262 | 0.2164 |
| Coupling parameters | 0.0032 | 0.0139 | |||
| Ees/Ea, PV | 28 | 0.84 (0.43–1.13) | −0.10 | 0 . 6009 | 0 . 3071 |
| TAPSE/sPAP, echo | 23 | 0.42 (31–97) | 0.56 | 0.0068 | 0.0408 |
| SV/ESV, MRI | 31 | 0.65 (0.37–1.02) | 0.44 | 0.0131 | 0.0272 |
| Global heart function | 0 . 2427 | 0 . 7997 | |||
| Cardiac index (L·min−1·m−2), RHC | 31 | 2.6 (2.1–3.2) | <0.01 | 0.9865 | 0.7428 |
| Cardiac index (L·min−1·m−2), MRI | 31 | 2.5 (2.1–2.9) | −0.19 | 0.3144 | 0.7980 |
| BNP (pg·mL−1) | 28 | 91 (30–221) | 0.36 | 0.0609 | 0.3631 |
| Left heart parameters | 0 . 0008 | 0 . 0008 | |||
| EDV (mL), MRI | 30 | 97 (82–113) | −0.24 | 0.1944 | 0.4338 |
| ESV (mL), MRI | 30 | 35 (25–45) | −0.37 | 0.0430 | 0.0154 |
| SV (mL), MRI | 30 | 63 (46–70) | −0.02 | 0.9253 | 0.6759 |
| Myocardial mass diastole (g), MRI | 30 | 104 (87–117) | −0.20 | 0.2895 | 0.1600 |
| Myocardial mass systole (g), MRI | 30 | 87 (80–101) | −0.23 | 0.2179 | 0.0559 |
| EF (%), MRI | 30 | 65 (56–75) | 0.40 | 0.0285 | 0.0240 |
| T1 LV global (ms), MRI | 29 | 1044 (1010–1088) | −0.37 | 0.0470 | 0.2124 |
| T2 LV global (ms), MRI | 29 | 58 (55–60) | −0.53 | 0.0033 | 0.0059 |
| Exertional parameters | 0 . 1055 | 0 . 4779 | |||
| 6MWD (m) | 29 | 406 (272–480) | 0.32 | 0.0917 | 0.3725 |
| V′O2max (mL·min−1·kg−1) | 20 | 13.3 (10.5–16.2) | 0.02 | 0.9459 | 0.5498 |
| Maximum workload (W) | 20 | 63 (38–90) | 0.42 | 0.0608 | 0.3076 |
| General characteristics | |||||
| Age (years) | 31 | 55 (46–60) | −0.14 | 0.4529 | |
| BMI (kg·m−2) | 31 | 25 (22–30) | 0.31 | 0.0888 | |
| BDNF | 31 | 1262 (791–4619) |
Linear regression analysis with log-values was performed to calculate the p-value and Pearson's r for correlation of BDNF and the respective parameter. Combined p-values (bold) were calculated according to Fisher's method. Multiple regression analysis was used to adjust for body mass index (BMI), age and gender. PASP: pulmonary arterial systolic pressure; echo: echocardiography; PCWP: pulmonary capillary wedge pressure; RHC: right heart catheterisation; PVR: pulmonary vascular resistance; RV: right ventricle; EDV: end-diastolic volume; MRI: magnetic resonance imaging; ESV: end-systolic volume; T1/T2: MRI relaxation times; uRVIP: upper RV insertion point; TAPSE: tricuspid annular plane systolic excursion; EF: ejection fraction; SV: systolic volume; Ees: end-systolic elastance; PV: pressure–volume loop catheter; Ea: arterial elastance; sPAP: systolic pulmonary artery pressure; BNP: brain natriuretic peptide; LV: left ventricle; 6MWD: 6-min walk distance; V′O2max: maximal oxygen uptake.
In line with the results of the first patient cohort, we found that BDNF plasma levels correlated with CVP, and most importantly with parameters of RV dimension, in particular with the end-systolic and end-diastolic volume of the RV (RV-ESV and RV-EDV; figure 1d, table 2). However, the correlation with pulmonary vascular and exertional parameters was not statistically significant. Moreover, in contrast to the first cohort, the correlation of BDNF levels to heart function was not statistically significant, although RV coupling parameters correlated with BDNF levels. Thus, we compared both cohorts (supplementary table S1), but did not detect statistically significant differences between haemodynamic, biometric or blood markers that could explain the discrepant results with regard to correlation of BDNF with cardiac index. However, there was a tendency for decreased thrombocyte counts, increased haemoglobin levels, decreased thyroid-stimulating hormone and increased C-reactive protein in the second cohort compared to the first cohort, which may have affected BDNF plasma levels. Therefore, we adjusted p-values of correlation for these covariables and found that RV-EDV, RV-ESV and CVP but not cardiac index significantly correlated with BDNF levels after adjustment (first cohort: CVP p#=0.0114, cardiac index p#=0.2507; second cohort: CVP p#=0.0116, cardiac index p#=0.9304, RV-EDV p#=0.0183; RV-ESV p#=0.0169). These results indicate that these covariables contributed to correlation of BDNF and cardiac index in IPAH patients, and only markers of right heart congestion were independently correlated to BDNF levels.
In summary, the human study showed low BDNF plasma levels in IPAH patients, which correlate with markers of right heart congestion.
Bdnf heterozygous mice developed less RV dilatation after pulmonary arterial banding
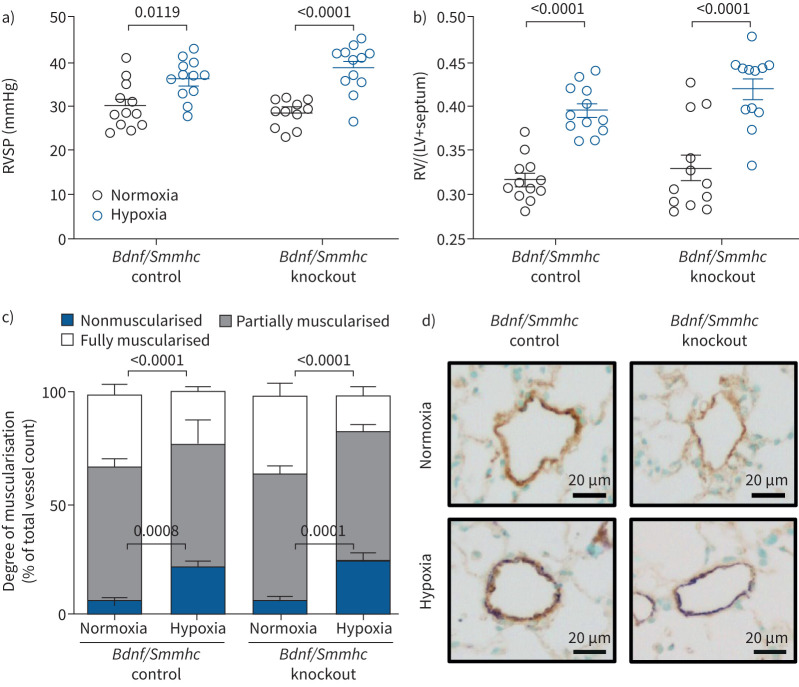
To investigate the impact of low BDNF levels on RV adaptation to increased afterload, we subjected heterozygous Bdnf knockout (Bdnf+/−) mice (because homozygous Bdnf knockout mice are not viable) to PAB. 3 weeks after PAB, right ventricular systolic pressure (RVSP) elevation and the increase of the heart ratio were comparable in WT and Bdnf+/− mice (figure 2a and b). However, right ventricular internal diameter was increased to a lesser extent in banded Bdnf+/−mice compared to banded WT mice (figure 2c), whereas right ventricular wall thickness (RVWT) was increased to a similar level (figure 2d). Tricuspid annular plane systolic excursion (TAPSE) and cardiac output were similarly decreased after PAB in both genotypes (figure 2e and f). In addition, collagen content in the RV tissue was not significantly different between PAB WT and PAB Bdnf+/−mice (figure 2g and h). PAB-induced RV hypertrophy was associated with enhanced Bdnf and TrkB mRNA expression in the RV tissue (supplementary figure S2a and b). These data indicate that low BDNF levels do not promote RV dysfunction, but may have a beneficial effect on RV remodelling.
FIGURE 2.
Degree of right ventricular (RV) remodelling and dysfunction in brain-derived neurotrophic factor (Bdnf+/−) mice after pulmonary arterial banding (PAB). a) Right ventricular systolic pressure (RVSP); b) heart ratio: weight of RV as ratio of the weight of the RV to the weight of the left ventricle (LV)+septum; c) right ventricular internal diameter (RVID); d) right ventricular wall thickness (RVWT); e) tricuspid annular plane systolic excursion (TAPSE); f) cardiac output; g) percentage of collagen in the RV determined by Picro-Sirius-Red staining; h) representative pictures of Picro-Sirius-Red staining of the RV. Collagen fibres are stained red, cardiomyocytes are stained yellow. Data are presented as mean±sem. Statistical analysis was performed using two-way ANOVA with Tukey's post hoc test (log for collagen content). WT: wild-type.
SMC-specific Bdnf knockout showed attenuated RV dilatation after chronic hypoxic exposure without effect on pulmonary hypertension
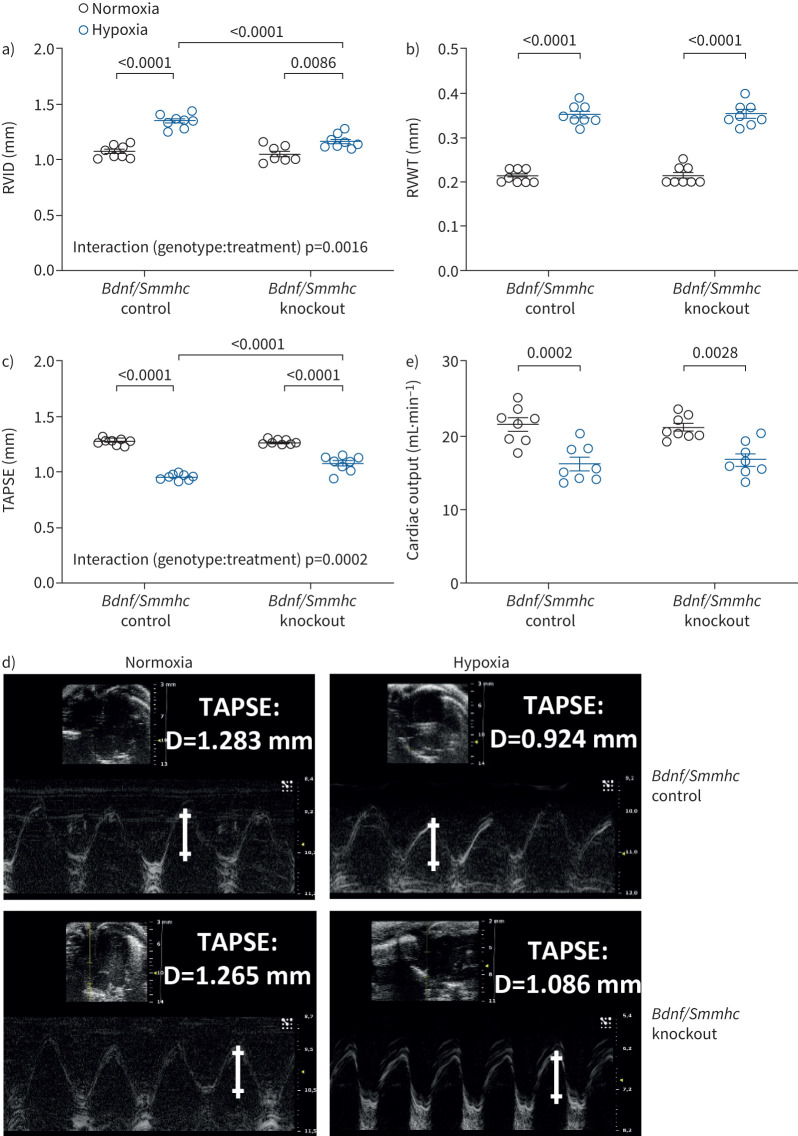
As a second mouse model to investigate the effect of BDNF on RV remodelling in pulmonary hypertension, we used chronic hypoxia-induced pulmonary hypertension in mice with SMC-specific knockout of Bdnf (Bdnf/Smmhc knockout induced by tamoxifen feeding). This model was chosen to investigate the effect of BDNF knockdown on the RV and pulmonary vasculature, as human PASMCs showed higher proliferation after exogenous BDNF stimulation, suggesting that BDNF promotes development of pulmonary hypertension [6]. In these mice, we did not detect any statistical significant difference in development of pulmonary hypertension after 4 weeks of hypoxic exposure (10% oxygen), determined by RVSP (figure 3a), the Fulton index (weight of the RV/(+septum)) as measure for RV hypertrophy; figure 3b) or pulmonary vascular remodelling (figure 3c and d) compared to mice without knockout (Bdnf/Smmhc control, without tamoxifen feeding). However, in line with the findings in the PAB model, RV dilatation was attenuated in Bdnf/Smmhc knockout mice compared to Bdnf/Smmhc control mice (figure 4a), while RVWT was not statistically significant different between the genotypes (figure 4b). Interestingly, TAPSE, a parameter for systolic RV function, was less decreased in hypoxic Bdnf/Smmhc knockout mice compared to Bdnf/Smmhc control mice (figure 4c and d). In contrast, hypoxic exposure decreased cardiac output to a similar extent in both genotypes (figure 4e). Moreover, we did not detect any statistically significant differences in systemic arterial pressure between these groups (supplementary figure S3a). To determine whether tamoxifen feeding may affect the development of pulmonary hypertension, we compared the Bdnf/Smmhc control group with the Smmhc control group after tamoxifen feeding. No differences in heart ratio, RVSP or vascular remodelling after normoxic or hypoxic exposure were detected between these groups (supplementary figure S3b–d) Interestingly, tamoxifen feeding even enhanced hypoxia-induced RV dilatation (supplementary figure S3e) without affecting RVWT (supplementary figure S3f), TAPSE (supplementary figure S3g) or cardiac output (supplementary figure S3h) suggesting that attenuation of RV dilatation and decrease in TAPSE in Bdnf/Smmhc knockout was not due to tamoxifen feeding.
FIGURE 3.
Degree of hypoxia-induced pulmonary hypertension in brain-derived neurotrophic factor (Bdnf) smooth muscle cell (SMC)-specific (smooth muscle myosin heavy chain (Smmhc)) knockout mice after 4 weeks of hypoxic exposure. a) Right ventricular systolic pressure (RVSP) quantified in vivo. b) Heart ratio: weight of right ventricle (RV) as ratio of the weight of the RV to the weight of the left ventricle (LV)+septum. c) Pulmonary vascular remodelling of small (20–70 μm) pulmonary vessels determined as the amount of fully, partially and nonmuscularised vessels in percentage total vessel count. Pulmonary vessels were categorised as nonmuscularised vessels (percentage of α-actin positive cells <5% in relation to the vessel circumference), partially muscularised vessels (percentage of α-actin positive cells between 5% and 75% in relation to the vessel circumference) and fully muscularised vessels (percentage of α-actin positive cells >75% in relation to the vessel circumference). n=12 mice each group. d) Representative images of pulmonary vessels. Purple: staining for α-smooth muscle actin. Brown: staining for von Willebrand factor. Data are presented as mean±sem. Statistical analysis was performed using two-way ANOVA with Tukey's post hoc test.
FIGURE 4.
Degree of hypoxia-induced right ventricular remodelling and dysfunction in brain-derived neurotrophic factor (Bdnf) smooth muscle cell (SMC)-specific (smooth muscle myosin heavy chain (Smmhc)) knockout mice after 4 weeks of hypoxic exposure. a) Right ventricular internal diameter (RVID); b) right ventricular wall thickness (RVWT); c) tricuspid annular plane systolic excursion (TAPSE); d) representative echocardiographic images of TAPSE; e) cardiac output. Data are presented as mean±sem. Statistical analysis was performed using two-way ANOVA with Tukey's post hoc test. D: distance.
BDNF protein levels were increased in lung homogenate after 4 weeks of hypoxic exposure independent of BDNF knockout
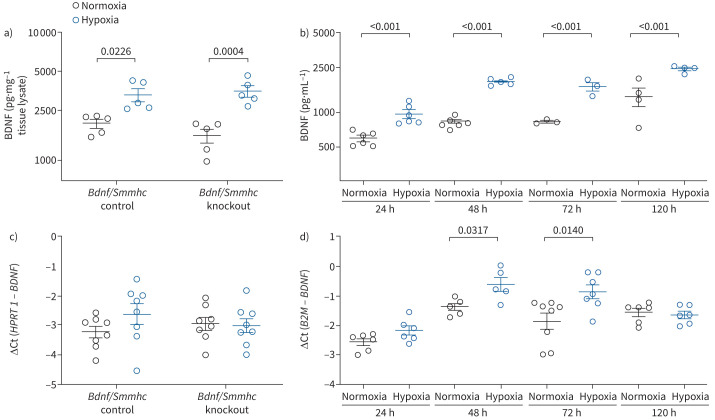
In contrast to the decreased circulating levels of BDNF in pulmonary hypertension patients, BDNF protein levels were increased in lung homogenate of chronic hypoxic mice (figure 5a) and isolated PASMCs after hypoxic exposure (figure 5b), with no detectable difference between Bdnf/Smmhc knockout and control lung homogenate (figure 5a). Increased mRNA levels were not detectable in lung homogenate, but were detected in isolated PASMCs after hypoxic exposure (figure 5c, d). Successful knockout of BDNF was proven in aortic tissue (supplementary figure S4a). Interestingly, TrkB mRNA expression was higher in Bdnf/Smmhc knockout compared to control in aortic tissue (supplementary figure S4b), while we could not detect a difference in lung homogenate (supplementary figure S4c). TrkB mRNA was also upregulated in hypoxia in lung homogenate and isolated PASMCs (supplementary figure S4c and d).
FIGURE 5.
Expression of brain-derived neurotrophic factor (BDNF) protein and mRNA in lung homogenate and pulmonary arterial smooth muscle cells (PASMCs). BDNF protein or mRNA levels in a, c) lung homogenate of mice after 4 weeks of normoxic or hypoxic exposure and b, d) isolated PASMCs after different time periods of in vitro hypoxic exposure. Data are presented as mean±sem. a, c) Statistical analysis was performed using two-way ANOVA with Tukey's post hoc test (log for concentrations); b, d) statistical analysis was performed using Mann–Whitney's U-test (log for concentrations).
Discussion
By using advanced methods for in-depth characterisation of RV remodelling in pulmonary hypertension patients, we showed for the first time that BDNF plasma levels were decreased in IPAH patients, and low BDNF levels were associated with signs of right heart congestion. In two animal models of pulmonary hypertension, low BDNF levels did not promote pressure overload-induced RV remodelling, suggesting that low BDNF levels are not causatively related to maladaptive RV remodelling as seen in pulmonary hypertension patients. Increased BDNF expression in the hypoxic lung and RV of the PAB animals suggests that neither the heart nor the lung accounted for low BDNF plasma levels in pulmonary hypertension patients. Moreover, SMC-specific knockdown of BDNF did not affect development of chronic hypoxia-induced pulmonary hypertension.
The low BDNF levels in pulmonary hypertension patients of our first patient cohort and correlation to global heart function are in line with previous investigations on the left heart, which demonstrated an association between low BDNF plasma levels and left heart failure [4, 5, 24]. Moreover, low BDNF levels have been shown to be associated with heart failure severity [4], death and adverse outcomes [25]. Interestingly, a recent study using data from a large population revealed that low BDNF levels were related to adverse early and subclinical left ventricular (LV) remodelling and higher levels of N-terminal pro-brain natriuretic peptide [24]. Accordingly, we found decreased BDNF levels in patients with a post-capillary component of pulmonary hypertension. However, our study cohort did not allow us to discern the effects of pulmonary hypertension in these patients from effects of the relevant covariables (age, BMI, gender [26]) with sufficient statistical confidence. Future larger studies are necessary to determine the relevance of a pre- versus post-capillary component on BDNF levels. However, BDNF levels in IPAH patients were also significantly decreased after adjustment for covariables. Thus, we speculated that BDNF plasma levels in IPAH patients are associated with RV dysfunction. Therefore, we investigated a second cohort of PAH patients that had undergone a detailed analysis of heart function using advanced methods such as PV-loop analysis and MRI. Indeed, we found that BDNF plasma levels correlated with RV dimensions/structure and CVP, but not cardiac index, after adjustment for several covariables. These data indicate that low BDNF levels are more related to venous congestions (backward failure) than to forward heart failure. Interestingly, BDNF also correlated with LV parameters which, however, are affected in IPAH by RV dysfunction due to RV–LV interdependence [27], so that an independent effect of LV function could not be analysed in this study.
To investigate the direct effects of low BDNF levels on RV remodelling we used two mouse models of increased RV afterload. First, we investigated RV remodelling in mice heterozygous for the Bdnf tm1Jae mutation (which show approximately half normal levels of Bdnf mRNA; homozygous mice mostly die within the second post-natal week) using the PAB model to induce isolated RV afterload. Bdnf+/− mice showed less RV dilatation after PAB compared to WT mice; however, this increase was rather small and not accompanied by better RV function, so that the physiological relevance remains unclear. As the second model we used hypoxia-induced pulmonary hypertension in SMC-specific Bdnf knockout mice. Bdnf/Smmhc knockout mice displayed attenuated RV dilatation and improved TAPSE compared to Bdnf/Smmhc control mice, despite a comparable severity of hypoxia-induced pulmonary hypertension. These findings suggest that diminished BDNF expression even only in SMCs exerts beneficial effects on RV adaptation to increased RV afterload. Along these lines, in a model of myocardial infarction Bdnf+/− mice showed reduced adverse cardiac remodelling and improved survival [15]. In contrast, conditional global BDNF deletion in mice was associated with a worsening of heart function following myocardial infarction [14]. Further studies should investigate the reason for these discrepancies, pathomechanisms underlying the effects of BDNF on RV remodelling and function, and its effect on TrkB signalling.
We further investigated whether BDNF affects pulmonary vascular remodelling in the Bdnf/Smmhc hypoxic mouse model. Previously, we demonstrated proliferative effects of exogenous BDNF on human PASMCs [6]. However, we did not find any effect of Bdnf deletion on the development of pulmonary hypertension. BDNF release by cell types other than PASMCs during chronic hypoxia may account for these findings. Indeed, we observed increased BDNF levels in lung homogenates after 4 weeks of hypoxic exposure, and this increase was independent of BDNF deletion. Thus, in vivo non-SMC cell types, particularly endothelial cells, may blunt the effect of SMC-specific BDNF downregulation. In this regard, human pulmonary arterial endothelial cells have been shown to secrete BDNF in response to hypoxia [7]. Moreover, we examined the expression of BDNF and TrkB in aortas, which served as an indicator tissue for their regulation in SMCs. Importantly, upregulation of the BDNF-receptor TrkB mRNA expression, probably as a compensatory mechanism, in aortas of Bdnf/Smmhc knockout mice suggested unimpaired TrkB signalling in Bdnf/Smmhc knockout mice.
In contrast to circulating levels in pulmonary hypertension patients, Bdnf and TrkB mRNA was upregulated in the RV after PAB, as well as in the lung homogenate of hypoxic mice. Previous studies have also shown that endothelial cells of the coronary artery and cardiomyocytes in the adult heart express Bdnf [13, 28]. Along this line, an upregulation of BDNF protein was also found in left heart failure [29]. Moreover, BDNF plasma levels were increased after 3 days of a high-altitude sojourn [7] and human PASMCs released BDNF during hypoxia in vitro [8]. Accordingly, previous studies demonstrated increased Bdnf and TrkB mRNA expression in pulmonary vessels of patients with IPAH and mice with chronic hypoxia-induced pulmonary hypertension; however, plasma levels were not reported [6]. Currently the reason of the decreased circulating BDNF levels in IPAH, which may not reflect local BDNF levels in the lung or heart, remains unknown. BDNF plasma levels may be affected by systemic factors associated with IPAH and could be decreased due to less exercise of the patients [30], high glucose levels [12] or potentially psychological conditions, such as depression and cognitive impairment [31, 32]. In this regard, a brain–heart loop has been described for the left heart [14]. Other factors have been frequently described to affect plasma BDNF levels, such as thyroid hormones [33, 34] and BDNF released from thrombocytes [35]. Moreover, plasma BDNF levels correlated with C-reactive protein [36] and haemoglobin levels [37] in human studies. Therefore, for these factors we adjusted in our multivariate analysis. Furthermore, other non-neuronal cells, including vascular endothelial cells, and immune cells could release BDNF into plasma [38]. In summary, decreased BDNF levels were detected in IPAH patients and low BDNF levels were associated with RV dilatation and CVP. Animal experiments do not indicate induction of RV remodelling and dysfunction by low BDNF levels, but rather mild protective effects on RV dilatation. These data suggest that low BDNF levels detected in IPAH patients do not cause RV dysfunction, but are rather a consequence of right heart congestion.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00230-2022.SUPPLEMENT (511.3KB, pdf)
Acknowledgements
The authors gratefully thank I. Breitenborn-Mueller, C. Homberger, Andrea Mohr and N. Schupp (Universities of Giessen and Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Excellence Cluster Cardio-Pulmonary Institute (CPI), Justus-Liebig University, Giessen, Germany) for technical assistance. We thank S. Offermanns (Max Planck Institute for Heart and Lung Research, Bad Nauheim, Germany) for kindly providing the mouse line STOCK Tg (Myh11-cre/ERT2).
Provenance: Submitted article, peer reviewed.
Author contributions: K. Schäfer, K. Tello and O. Pak performed the majority of the experiments, and assisted with interpretation of results and manuscript preparation. M. Richter, H. Gall, M. Hecker and H.A. Ghofrani performed the human studies. M. Gierhardt, B. Kojonazarov, S. Kraut and D. Zahner performed animal experiments. C. Veith and K. Lo performed in vitro experiments. J. Wilhelm performed statistical analysis. R.T. Schermuly, F. Grimminger, G. Kwapiszewska, L. Fink and W. Seeger assisted with experimental studies and performed interpretation of results. N. Weissmann and R. Gerstberger performed interpretation of results and manuscript preparation. A. Sydykov and N. Sommer designed experimental studies, performed analysis and interpretation of results, and prepared the manuscript.
Conflict of interest: No disclosures.
Support statement: This work was supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) project number 268555672 – SFB 1213, project A06, A08, B08, CP02. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimminger J, Richter M, Tello K, et al. Thin air resulting in high pressure: mountain sickness and hypoxia-induced pulmonary hypertension. Can Respir J 2017; 2017: 8381653. doi: 10.1155/2017/8381653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sommer N, Ghofrani HA, Pak O, et al. Current and future treatments of pulmonary arterial hypertension. Br J Pharmacol 2021; 178: 6–30. doi: 10.1111/bph.15016 [DOI] [PubMed] [Google Scholar]
- 4.Takashio S, Sugiyama S, Yamamuro M, et al. Significance of low plasma levels of brain-derived neurotrophic factor in patients with heart failure. Am J Cardiol 2015; 116: 243–249. doi: 10.1016/j.amjcard.2015.04.018 [DOI] [PubMed] [Google Scholar]
- 5.Fukushima A, Kinugawa S, Homma T, et al. Serum brain-derived neurotropic factor level predicts adverse clinical outcomes in patients with heart failure. J Card Fail 2015; 21: 300–306. doi: 10.1016/j.cardfail.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 6.Kwapiszewska G, Chwalek K, Marsh LM, et al. BDNF/TrkB signaling augments smooth muscle cell proliferation in pulmonary hypertension. Am J Pathol 2012; 181: 2018–2029. doi: 10.1016/j.ajpath.2012.08.028 [DOI] [PubMed] [Google Scholar]
- 7.Helan M, Aravamudan B, Hartman WR, et al. BDNF secretion by human pulmonary artery endothelial cells in response to hypoxia. J Mol Cell Cardiol 2014; 68: 89–97. doi: 10.1016/j.yjmcc.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartman W, Helan M, Smelter D, et al. Role of hypoxia-induced brain derived neurotrophic factor in human pulmonary artery smooth muscle. PLoS One 2015; 10: e0129489. doi: 10.1371/journal.pone.0129489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss A, Boehm M, Egemnazarov B, et al. Kinases as potential targets for treatment of pulmonary hypertension and right ventricular dysfunction. Br J Pharmacol 2021; 178: 31–53. doi: 10.1111/bph.14919 [DOI] [PubMed] [Google Scholar]
- 10.Numakawa T, Odaka H, Adachi N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int J Mol Sci 2018; 19: 3650. doi: 10.3390/ijms19113650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng L, Liu B, Ji R, et al. Targeting the BDNF/TrkB pathway for the treatment of tumors. Oncol Lett 2019; 17: 2031–2039. doi: 10.3892/ol.2018.9854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyileten C, Kaplon-Cieslicka A, Mirowska-Guzel D, et al. Antidiabetic effect of brain-derived neurotrophic factor and its association with inflammation in type 2 diabetes mellitus. J Diabetes Res 2017; 2017: 2823671. doi: 10.1155/2017/2823671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kermani P, Hempstead B. BDNF actions in the cardiovascular system: roles in development, adulthood and response to injury. Front Physiol 2019; 10: 455. doi: 10.3389/fphys.2019.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada S, Yokoyama M, Toko H, et al. Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arterioscler Thromb Vasc Biol 2012; 32: 1902–1909. doi: 10.1161/ATVBAHA.112.248930 [DOI] [PubMed] [Google Scholar]
- 15.Halade GV, Ma Y, Ramirez TA, et al. Reduced BDNF attenuates inflammation and angiogenesis to improve survival and cardiac function following myocardial infarction in mice. Am J Physiol Heart Circ Physiol 2013; 305: H1830–H1842. doi: 10.1152/ajpheart.00224.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tello K, Dalmer A, Vanderpool R, et al. Impaired right ventricular lusitropy is associated with ventilatory inefficiency in pulmonary arterial hypertension. Eur Respir J 2019; 54: 1900342. doi: 10.1183/13993003.00342-2019 [DOI] [PubMed] [Google Scholar]
- 17.Tello K, Seeger W, Naeije R, et al. Right heart failure in pulmonary hypertension: diagnosis and new perspectives on vascular and direct right ventricular treatment. Br J Pharmacol 2021; 178: 90–107. doi: 10.1111/bph.14866 [DOI] [PubMed] [Google Scholar]
- 18.Brimioulle S, Wauthy P, Ewalenko P, et al. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol 2003; 284: H1625–H1630. doi: 10.1152/ajpheart.01023.2002 [DOI] [PubMed] [Google Scholar]
- 19.Tello K, Dalmer A, Axmann J, et al. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail 2019; 12: e005512. doi: 10.1161/CIRCHEARTFAILURE.118.005512 [DOI] [PubMed] [Google Scholar]
- 20.Veith C, Neghabian D, Luitel H, et al. FHL-1 is not involved in pressure overload-induced maladaptive right ventricular remodeling and dysfunction. Basic Res Cardiol 2020; 115: 17. doi: 10.1007/s00395-019-0767-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heitmeier T, Sydykov A, Lukas C, et al. Altered proteasome function in right ventricular hypertrophy. Cardiovasc Res 2020; 116: 406–415. doi: 10.1093/cvr/cvz103 [DOI] [PubMed] [Google Scholar]
- 22.Pak O, Scheibe S, Esfandiary A, et al. Impact of the mitochondria-targeted antioxidant MitoQ on hypoxia-induced pulmonary hypertension. Eur Respir J 2018; 51: 1701024. doi: 10.1183/13993003.01024-2017 [DOI] [PubMed] [Google Scholar]
- 23.Weissmann N, Dietrich A, Fuchs B, et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA 2006; 103: 19093–19098. doi: 10.1073/pnas.0606728103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahls M, Könemann S, Markus MRP, et al. Brain-derived neurotrophic factor is related with adverse cardiac remodeling and high NTproBNP. Sci Rep 2019; 9: 15421. doi: 10.1038/s41598-019-51776-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barman HA, Şahin I, Atıcı A, et al. Prognostic significance of brain-derived neurotrophic factor levels in patients with heart failure and reduced left ventricular ejection fraction. Anatol J Cardiol 2019; 22: 309–316. doi: 10.14744/AnatolJCardiol.2019.37941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lommatzsch M, Zingler D, Schuhbaeck K, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging 2005; 26: 115–123. doi: 10.1016/j.neurobiolaging.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 27.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 2017; 69: 236–243. doi: 10.1016/j.jacc.2016.10.047 [DOI] [PubMed] [Google Scholar]
- 28.Donovan MJ, Lin MI, Wiegn P, et al. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 2000; 127: 4531–4540. doi: 10.1242/dev.127.21.4531 [DOI] [PubMed] [Google Scholar]
- 29.Kreusser MM, Buss SJ, Krebs J, et al. Differential expression of cardiac neurotrophic factors and sympathetic nerve ending abnormalities within the failing heart. J Mol Cell Cardiol 2008; 44: 380–387. doi: 10.1016/j.yjmcc.2007.10.019 [DOI] [PubMed] [Google Scholar]
- 30.Stephan JS, Sleiman SF. Exercise factors released by the liver, muscle, and bones have promising therapeutic potential for stroke. Front Neurol 2021; 12: 600365. doi: 10.3389/fneur.2021.600365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polyakova M, Stuke K, Schuemberg K, et al. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord 2015; 174: 432–440. doi: 10.1016/j.jad.2014.11.044 [DOI] [PubMed] [Google Scholar]
- 32.Rothman SM, Mattson MP. Activity-dependent, stress-responsive BDNF signaling and the quest for optimal brain health and resilience throughout the lifespan. Neuroscience 2013; 239: 228–240. doi: 10.1016/j.neuroscience.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yajima H, Amano I, Ishii S, et al. Absence of thyroid hormone induced delayed dendritic arborization in mouse primary hippocampal neurons through insufficient expression of brain-derived neurotrophic factor. Front Endocrinol 2021; 12: 629100. doi: 10.3389/fendo.2021.629100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baek JH, Kang ES, Fava M, et al. Thyroid stimulating hormone and serum, plasma, and platelet brain-derived neurotrophic factor during a 3-month follow-up in patients with major depressive disorder. J Affect Disord 2014; 169: 112–117. doi: 10.1016/j.jad.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 35.Le Blanc J, Fleury S, Boukhatem I, et al. Platelets selectively regulate the release of BDNF, but not that of its precursor protein, proBDNF. Front Immunol 2020; 11: 575607. doi: 10.3389/fimmu.2020.575607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noren Hooten N, Ejiogu N, Zonderman AB, et al. Protective effects of BDNF against C-reactive protein-induced inflammation in women. Mediators Inflamm 2015; 2015: 516783. doi: 10.1155/2015/516783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basu S, Kumar D, Anupurba S, et al. Effect of maternal iron deficiency anemia on fetal neural development. J Perinatol 2018; 38: 233–239. doi: 10.1038/s41372-017-0023-5 [DOI] [PubMed] [Google Scholar]
- 38.Brigadski T, Lessmann V. The physiology of regulated BDNF release. Cell Tissue Res 2020; 382: 15–45. doi: 10.1007/s00441-020-03253-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00230-2022.SUPPLEMENT (511.3KB, pdf)