Abstract
Background and Objective
Pectus excavatum is the most common congenital chest wall anomaly, the hallmark of which is the caved-in appearance of the anterior chest. A growing body of literature exists surrounding methods of surgical correction, though considerable variability in management remains. The primary objectives of this review are to outline the current practices surrounding the care of pediatric patients with pectus excavatum and present emerging trends in the field that continue to impact the care of these patients.
Methods
Published material in English was identified utilizing the PubMed database using multiple combinations of the keywords: pectus excavatum, pediatric, management, complications, minimally invasive repair of pectus excavatum, MIRPE, surgery, repair, and vacuum bell. Articles from 2000–2022 were emphasized, though older literature was included when historically relevant.
Key Content and Findings
This review highlights contemporary management principles of pectus excavatum in the pediatric population, comprising preoperative evaluation, surgical and non-surgical treatment, postoperative considerations including pain control, and monitoring strategies.
Conclusions
In addition to providing an overview of pectus excavatum management, this review highlights areas that remain controversial including the physiologic effects of the deformity and the optimal surgical approach, which invite future research efforts. This review also features updated content on non-invasive monitoring and treatment approaches such as three-dimensional (3D) scanning and vacuum bell therapy, which may alter the treatment landscape for pectus excavatum in order to reduce radiation exposure and invasive procedures when able.
Keywords: Pectus excavatum, minimally invasive repair of pectus excavatum (MIRPE), Nuss procedure, vacuum bell
Introduction
Pectus excavatum is the most common congenital chest wall deformity, with an incidence of 1/400 births (0.25%) (1). Though a definite pathologic mechanism has not been established, disproportionate overgrowth of costal cartilages, histopathologic changes of collagen content in costal cartilages, and abnormal posterior tethering of the diaphragm to the sternum may be contributing factors (2). The defect may also be asymmetric, which is often associated with sternal rotation and concomitant scoliosis. Family history of chest wall deformity and connective tissue disorders such as Marfan’s syndrome in up to 5% of patients are additional factors associated with pectus excavatum (3). Pectus excavatum is usually well-tolerated in younger children; however, symptoms such as pain in the affected costal cartilages, exercise intolerance and shortness of breath may develop as the deformity often worsens with rapid vertical growth during adolescence. The decision to treat is ultimately guided by the patient’s perception of cosmetic discomfort and supported by measures such as severity index and markers of physiologic compromise (4). A surge in referrals for pectus excavatum repair has facilitated large-scale clinical analyses that have led to modified treatment strategies and technical improvements to enhance patient safety. This review addresses components of pectus excavatum management, including preoperative considerations, surgical and non-surgical techniques, postoperative pain control, and patient monitoring strategies, with emphasis on novel or emerging approaches when relevant. We also highlight various contemporary modifications in procedure techniques including pectus bar configuration, treatment strategies and practice patterns that have sought to optimize patient safety and clinical outcomes over time. We present the following article in accordance with the Narrative Review reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-361/rc).
Methods
This manuscript concerns a narrative review, which was assembled through the study, analysis, and discussion of previously published journal articles specific to the management of pectus excavatum in pediatric patients. An emphasis was placed on recent literature over the past 22 years, 2000–2022, however, older studies were included when necessary. Published material was identified utilizing the PubMed® (National Center for Biotechnology Information, United States National Library of Medicine, National Institutes of Health) database using multiple combinations of the keywords: pectus excavatum, pediatric, management, complications, minimally invasive repair of pectus excavatum, MIRPE, surgery, repair, cardiopulmonary and vacuum bell. The initial literature search was conducted by a certified librarian. The articles were then examined for relevancy and the references reviewed for additional citations; the search strategy is summarized in Table 1. Importance of the articles was based on previously described levels of evidence (5). Article inclusion within this review was determined by the authors after their evaluation, analysis and interpretation.
Table 1. The search strategy summary.
| Items | Specification |
|---|---|
| Date of search | The first search was conducted on 3/1/2022. The second search was conducted on 7/20/2022. The last search was conducted on 12/5/2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Pectus excavatum, pediatric, management, complications, minimally invasive repair of pectus excavatum, MIRPE, pectus bar removal, surgery, repair, cardiopulmonary, and vacuum bell |
| Timeframe | Emphasis on 2000–2022, older studies included when historically relevant |
| Inclusion and exclusion criteria | Included English language review articles, clinical trials, randomized control trials, meta analyses, and book chapters |
| Selection process | Initial search conducted by librarian, authors later refined and added additional references when all authors agreed on relevance |
Pectus excavatum management
Introduction and preoperative evaluation
The pectus excavatum deformity has been associated with various cardiorespiratory implications, though the precise relationship has been debated. Though pectus excavatum is generally well tolerated in children, symptoms such as palpitations (presumably due to transient atrial arrhythmias), and exertional dyspnea or precordial pain with exercise have been reported. Reports of improved exercise tolerance following surgical repair of pectus excavatum, including a decrease in dyspnea and tachycardia, have supported an underlying physiologic abnormality associated with the physical deformity. A systolic ejection murmur may also be heard in patients with pectus excavatum, as a result of the close proximity of the sternum to the pulmonary artery. Patients with history or exam findings suspicious for a connective tissue disorder such as Marfan’s syndrome should also be referred for genetic counseling, as twenty unique genetic disorders have been associated with pectus excavatum (6). Cardiac anomalies have been shown to co-occur in up to 84% of patients with Marfan’s syndrome and pectus deformities (3). All patients with significant pectus excavatum should undergo an echocardiogram, especially those who are being considered for surgical intervention, as this may detect mitral valve prolapse, compression of the right ventricular outflow tract, or aortic root dilation, which may require long term monitoring (7,8).
The improvement in exercise tolerance following pectus excavatum repair remains an area of controversy and has been attributed to improvement in respiratory mechanics as well as resolution of cardiac compression, which has been shown to be the more clinically relevant parameter. In patients with pectus excavatum, the right atrium is compressed by the depressed sternum, which ultimately results in a reduced stroke volume. Corrective surgery has been shown to result in an increase in stroke volume and oxygen consumption, which was attributed to an increase in anterior-posterior thoracic dimensions (9). With regard to pulmonary parameters, resting pulmonary function tests (PFTs) evaluating forced vital capacity (FVC) and forced expired volume in one second (FEV1) show an expected initial deterioration after Nuss bar implantation due to chest rigidity that improves after bar removal (10). In a subset of 66 patients who underwent PFTs prior to surgical repair and one year after bar removal, there were significant improvements in median FVC (88% to 92% of predicted) and FEV1 (83 to 88% of predicted) (11). This improvement in function has not been consistently demonstrated in the literature, including a meta-analysis of 12 studies that failed to show a statistically significant change in pulmonary function, or in a recent prospective study that also included cardiopulmonary exercise testing and echocardiography (12,13).
Several methods of quantifying the severity of pectus excavatum defects have been proposed, the most common of which is the Haller index (HI), which is the ratio of the maximum transverse diameter of the chest to the minimum anteroposterior distance from the sternum to the spine at the deepest point of the deformity (14). Chest computed tomography (CT) was the imaging modality used to initially calculate the HI and remains the primary method of preoperative imaging, with a measurement of 3.25 or greater used as a requisite for insurance coverage of surgical correction. St. Peter et al. later proposed the Correction Index, which expresses the pectus defect as a percentage of chest depth, and better differentiates patients with significant pectus excavatum compared to the HI without the strong influence of the transverse width of the chest (15,16). Chest CT may also be used to assess for cardiac compression or the presence of other thoracoabdominal abnormalities and may be performed with a low-dose radiation protocol. There is evidence suggest to that plain film chest radiographs are equivalent to CT with regard to measuring HI, with some groups proposing their use as the primary tool for the preoperative assessment of pectus excavatum in average-risk patients due to reduced radiation dosing (17,18). Ultimately, CT provides a more comprehensive evaluation of factors impacting the operative approach such as sternal torsion, which may guide Nuss bar configuration, especially for older or female patients (15). Conventional photography is also often used to document the appearance of pectus excavatum defects and chest wall changes over time in response to growth or treatment. Photographic documentation was previously standardized by van Dijk et al. and includes five photographs acquired from different angles and two recordings used to determine the pectus excavatum depth as an objective measure of severity to assess evolution. Though commonplace in some clinical practices, simple photography is labor-intensive and lacks three-dimensional (3D) information that is essential to objectively track chest wall deformities (19,20).
Non-radiation based imaging modalities are being explored to objectively assess and monitor patients with pectus deformities. Poncet et al. conducted one of the first pilot studies that demonstrated a strong correlation (r=0.94) between an optical 3D image-derived external HI with the CT-derived HI in 10 patients with pectus excavatum or pectus carinatum (21). Glinowski et al. developed a novel 3D scan index which was strongly correlated to CT-derived HI (r=0.876) and 3D scan index based on CT (r=0.9939) in a limited cohort of 12 patients (22). Beyond demonstrating the correlation between 3D imaging and conventional indices, recent reports have evaluated the capacity for such measures to determine surgical eligibility by establishing cut-off values for the external HI (>1.83) and correction index (>15.2%), with excellent discrimination observed (23). With regard to physiologic impact of pectus excavatum, 3D imaging has been employed to predict the presence of cardiac compression using a combination of external pectus depth and anteroposterior distance; identification of compression also has implications on surgical selection (24). 3D surface imaging of the chest will be further discussed in the context of monitoring strategies for pectus deformities.
Surgical treatment
Surgical correction of pectus excavatum is typically recommended if a patient experiences cosmetic discomfort with their chest, and meets two or more of the following criteria: chest CT or MRI showing cardiac or pulmonary compression with HI of 3.25 or greater, restrictive lung disease by PFT evaluation, cardiac conduction abnormalities, performance abnormalities, or recurrence of pectus excavatum following open or closed surgical intervention (25). Despite repair being more technically feasible in a younger child with a more malleable chest wall, the potential for long-term recurrence of pectus excavatum or impairment in chest wall growth has led to a delay in surgery until the pubertal years in the authors’ practice. Because recurrence was found to occur more frequently during the pubertal period, Fonkalsrud and colleagues also advocated for delaying surgical repair of pectus excavatum until at least 10 years of age (26). Young patients may also be referred for vacuum bell therapy as a bridge to potential surgical correction or definitive therapy, as described later.
Minimally invasive repair of pectus excavatum (MIRPE)
Nuss first described a technique to elevate the sternum without division of the costal cartilages with the use of a retrosternal bar in 1998. MIRPE—which is now the most common operative approach used—involves insertion of a convex metal bar through small bilateral thoracic incisions under the sternum and anterior to the heart that aligns with the curvature of the deformity. Nuss described the advancement of a long clamp across the mediastinum to the opposite side of the chest, followed by using umbilical tape pulled through the tract to guide the clamp in from the opposite direction. The tape was then used for traction to pull the steel bar through the pleural cavity. Once in position, the bar was rotated with the convex side facing posteriorly to correct the sternum and rib depressions (27). The original series included 42 patients under 15 years of age, without specification of an ideal upper limit for age, and the bar was left in place for two years prior to removal. While thoracoscopy was not used by Nuss in his first series, it is now considered standard of care to thoracoscopically visualize the creation of the retrosternal tunnel and passage of the bar. The use of stronger suture such as Fiberwire or suction tubing to guide the bar across the posterior sternum have been described to replace the umbilical tape originally used by Nuss (28,29). Further modifications of this original technique have been employed to account for the increased chest wall rigidity of older adolescent or adult patients (30,31). In particular, forced external sternal elevation prior to bar passage has been employed through various means including a mechanical crane technique, manual elevation, and use of the vacuum bell with the intent of optimizing visibility and preventing cardiac injury (32). Goretsky et al. described the use of sternal elevation as an adjunct to avoid cardiac perforation. The technique should be mandatory particularly in patients who have had previous cardiac surgery with opening of the pericardium and are therefore at the highest risk for injury (33). An example of the MIRPE intraoperative setup with pectus introducer in place and crane mechanism for sternal elevation is shown in Figure 1.
Figure 1.
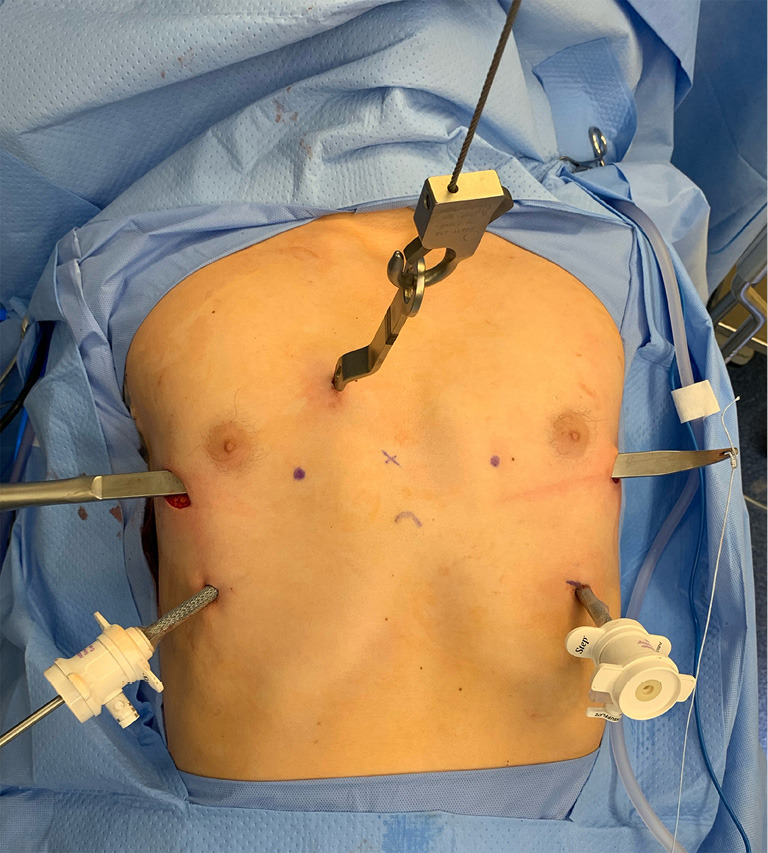
Intraoperative view of pectus introducer tunneled through subcutaneous space in the same horizontal plane as the level of the deepest depression. The use of a crane mechanism for sternal elevation and thoracoscopy for visualization facilitate retrosternal passage of the introducer prior to bar placement.
Kelly et al. presented a large cohort of 1,215 MIRPE cases over 21 years, including interval modifications to the procedure, with an update released in 2022 (11,34). At the time of bar removal, good or excellent surgical outcomes were reported in 96% of patients. The median age at surgery increased from 6 to 14 years during the study period. Various modifications of the technique during the study interval included routine use of thoracoscopy, techniques such as sternal elevation to minimize risk of dissection between the sternum and heart in patients with severe depressions, use of titanium bars in cases where a metal allergy is identified, more frequent use of two bars in older patients or those with severe depressions, and use of a metal stabilizer to decrease bar rotation.
Postoperative complications to consider include metal allergy, infection, and bar displacement. Most current Nuss bars are made of stainless steel, which raises the concern of metal allergy. Allergic reaction to the nickel-containing bars occurred in 2.2% of patients who underwent MIRPE by the Nuss group (35). It is important to distinguish an allergic presentation from an infectious one, and many practices have instituted preoperative patch allergy testing to identify patients that would require a custom titanium bar as an alternative. With regard to infectious complications, several authors have demonstrated the preservation of hardware with antibiotic treatment alone in cases of infection (36-38). Obermeyer and colleagues recently performed a single-center analysis of risk factors for Nuss bar infections; the cohort consisted of 781 patients, of which 25 (2.5%) developed a postoperative infection. The authors concluded that the rate of deep bar infection increased after perioperative clindamycin rather than cefazolin, while the rate of superficial infection increased with the use of peri-incisional subcutaneous catheters for analgesia (39).
An abundance of articles exist describing technical modifications of MIRPE since the original Nuss publication. With regard to bar configuration, the use of multiple bars and the cross-bar technique were introduced to correct complex chest-wall defects. While Nuss placed up to two bars in select patients in his original series, the frequency of multiple bar insertion has increased in modern pectus excavatum repair to facilitate more uniform pressure distribution and improved defect correction and chest wall remodeling, especially in older patients with rigid and stiff chest walls (40). Pilegaard reported on a series of 383 patients in which they used up to 3 bars in parallel and left several bar introducers in place to elevate the sternum while positioning the additional bars; in an updated series by the same author, multiple bar insertions were increasingly common among adult patients (41,42). Park et al. first described the cross-bar technique in 2016 for a rigid, steep, and focal (“Grand Canyon”) depression with the advantages of decreased bar dislocation, ability to cover the entire anterior chest wall, and more precise targeting of the bars to the exact point of depression while avoiding residual depression from unsupported ribs. The two bars are inserted diagonally, cross in the center, and are attached using bridge fixation to prevent rotation (43). The same group recently published a series comparing cross-bar and parallel-bar techniques among 247 patients and demonstrated the cross-bar technique to be as safe and effective as parallel bar with no difference in complication rates despite patients in the cross-bar group overall being older with a higher depression index, and generally with more rigid and complex pectus defects (44).
Methods of bar fixation including stabilizing bars were introduced to mitigate bar flipping and migration, which has presented significant challenges in MIRPE. The lateral stabilizing bar was introduced as a separate piece that slides onto the Nuss bar. In a series of 303 patients, Croitoru and colleagues applied lateral stabilizers in 70% of patients and wired them for additional stability in 65%; bar shifts were reduced from 15% to 6% with the introduction of stabilizers and to 5% when the stabilizers were wired (25). Disadvantages of this technique include increased thickness of the metal (which is relevant for the typically slender pectus excavatum patient), risk for separation of the components, and potential increased incidence of seroma with dermatitis due to pressure damage as reported by Watanabe et al. (45). Other variations have been described such as 3-point fixation using a suture deployed thoracoscopically adjacent to the sternum and encircling the bar and a rib to further minimize the risk of bar shifting (46). One of the most promising approaches is the bridge technique proposed by Park et al. in patients who required 2 parallel bars, and involves connecting the bars laterally with plate-screws at the ends, rendering the bars essentially unmovable. In a series of 80 patients who underwent bridge fixation, they reported minimal rotational movement of the bars 4 months postoperatively, with no dislocation or reoperation required (47). The Park group has developed additional fixation innovations including the claw fixator which attaches to a rib by hooking it with metal blades to avoid pericostal suturing, and is then fastened to the end hole of the bar with a screw and nut to prevent bar flipping and lateral sliding (48).
One of the primary determinants of pectus bar stability and dislocating forces is its shape, with the lengths of the vertical lateral segments accounting for the tendency to flip and the curvature at the point of contact with the chest wall as the primary contributor to lateral instability. Pérez et al. described a shorter, flat bar as preferable to the standard U-shaped bar to avoid displacement while maintaining similar cosmetic outcomes, operative times, and hospital stays (49). Shorter bars are often coupled with medial stabilizers, which restrict bar movement and early rotation compared to lateral stabilizers, and may also allow for unilateral stabilization (50). For mixed pectus carinatum and excavatum defects, Park and Kim introduced a sandwich technique using internal and external pectus bars to bring the carinatum down and the excavatum up. In the same report, they describe variations of the approach using suture to tie down the flared ribs to the internal bar to treat the lower costal flare and focal protuberances, termed the flare buster and magic string techniques (51). In addition to modified bar length, innovations in bar shape have been developed beyond the typical strategy of bending to a desired contour. This includes computer systems that bend the bars according to a CT-derived shape prediction have been described to provide customized correction (52,53).
When evaluating outcomes of MIRPE, it is critical to consider the learning curve associated with the operation. Hebra and colleagues reported on life threatening complications of MIRPE in 59 cases, which included cardiac perforation, hemothorax, major vessel injury, lung, liver, and diaphragm injury. Though such complications are fortunately very rare, the authors advocate for recognizing risk factors such as prior chest surgery, and utilizing proper training and sternal elevation techniques to reduce these complications (54). A retrospective series by Fonkalsrud and colleagues comparing MIRPE with open repair reported a higher incidence of reoperations and re-hospitalizations in the Nuss group; however, it was noted that 90% of the MIRPE complications occurred in the first 25 cases performed, reflecting the role of surgeon experience (26). Efforts have been made to define the learning process of the minimally invasive repair, which may have implications for the training of surgeons involved in a Nuss program. In an analysis of 222 Nuss procedures performed by 3 surgeons, de Loos and colleagues found the operation to be safe to perform at least once every 35 days with a complication rate under 10% after undergoing proctoring by an experienced thoracic surgeon during the initial 10 procedures (55). Another group similarly reported that MIRPE postoperative complications are less dependent on learning curve; however the work was limited by retrospective analysis and lack of standardized technical details (56).
Pectus bar removal (PBR) is the final stage of MIRPE. This is typically done as an outpatient procedure with low complication rates (57). It is generally agreed that a pectus bar should be removed two to four years following MIRPE, depending on patient age and severity of the chest wall deformity; removing the bar too soon may increase recurrence, while keeping it in place beyond four years may increase difficulty of removal due to bar ossification (58).
Several technical modifications have been described for PBR. It is generally recommended to re-open both lateral incisions used for initial bar placement and to straighten the bar prior to removal, though some authors have proposed techniques that do not require unbending of the bar (58-60). Other protective techniques such as covering the serrated edge of the bar to reduce injury, and tying an umbilical tape and sponge to the end of the bar as it is pulled through the tract to tamponade any bleeding have been proposed (61,62).
Despite the lack of sufficient evidence-based data on complication rates during PBR, several studies have shown the most common complications are wound seroma and pneumothorax (58). For example, in a cohort of 1,821 patients with mean duration of bar maintenance of 2.57 years, Park et al. reported wound seroma, including infection, was the most common complication after bar removal in 2.36% of patients (57). In rare instances, PBR may be associated with major complications including hemorrhage from intercostal or internal mammary artery injury, cardiac or lung injury. In total, 116 members of the Chest Wall International Group (CWIG) were surveyed on their complications with PBR; bleeding from intercostal artery injury (34.9%) and severe adhesions in the bar tunnel (26.9%) were the most common acute intraoperative complications (58,63).
Regardless of technique, pediatric patients should be followed postoperatively until they reach full stature to ensure a satisfactory result without unwanted secondary effects of the pectus bar including secondary pectus carinatum or increased costal flaring.
Open surgical repair
Though the majority of pectus excavatum repairs are now done by the minimally invasive Nuss procedure, open repair is used by some surgeons in cases of severely asymmetrical chest wall, mixed pectus deformities, or in those patients who do not wish to have a substernal bar in place for over two years (4). The open repair—as popularized by Ravitch—uses an anterior chest wall incision to perform a subperichondrial resection of the deformed costal cartilages. A transverse wedge osteotomy is created through the sternum, which is then closed with elevation by struts. Strut fixation to stabilize the position of the fractured sternum during cartilage reformation is employed using an anterior or a retrosternal approach (64). Limited variations on the open technique exist and include various means of sternal elevation such as with a sheathed wire placed behind the sternum and attached to an external brace postoperatively (termed the Leonard procedure), or using a synthetic mesh hammock support (65). Though both minimally invasive and open techniques were shown to be effective in correcting pectus excavatum when performed by an experienced surgeon, there lacks a consensus as to the superior technique for all patients. Long-term follow-up after bar removal is ultimately needed, and the choice of operation should be individualized to the patient.
Non-surgical treatment
Though MIRPE is considered the gold standard treatment for pectus excavatum, there is a need for less invasive treatment modalities that are effective in defect correction and pain management. The first nonoperative management of pectus excavatum was described by Schier and colleagues in 2005 as the vacuum bell, shown in Figure 2. The device is a suction cup placed over the anterior chest and connected to a patient-activated hand pump used to reduce pressure up to 15% below atmospheric pressure, resulting in sternal elevation (66). The initial cohort consisted of 60 patients (median 14.8 years) who used the vacuum bell for a minimum of 30 minutes twice per day up to five hours per day (median 90 minutes). Sternal elevation of 1 cm was demonstrated in 85% of patients after one month. A recent report from Haecker et al. included a subset of 140 vacuum bell patients with a mean pectus excavatum depth of 2.7 cm. 44% of patients had complete correction of their pectus excavatum deformity after an average of 21.8 months wearing the vacuum bell (67). The ideal vacuum bell candidate is relatively young with a flexible chest wall, has a mild to moderate chest wall deformity, and is motivated to be compliant with therapy. Patients who are able to transiently correct their pectus deformity with a Valsalva maneuver are classified as having flexible chest walls. Obermeyer et al. reported an excellent outcome was more likely in patients 11 years of age or younger, with a sternal depth 1.5 cm or less, and a flexible chest wall (68). Adverse effects associated with the vacuum bell include sternal pain, skin irritation and hematoma, while contraindications to therapy include musculoskeletal disorders, vasculopathies and coagulopathies (69). As the vacuum bell is an emerging conservative therapy, consistent data is lacking regarding a standardized treatment algorithm with ideal parameters for duration, frequency, and pressure. For example, Schier and colleagues allowed patients to apply pressure according to individual comfort level, while Lopez et al. utilized a protocol with gradually increasing suction and reported improved outcomes with sternal flattening at 6 months in patients who wore the vacuum bell for over 4 hours daily (70). It should be noted that a general disadvantage of non-surgical therapy for pectus excavatum is patient non-compliance as a primary contributor to treatment failure. In most series, a substantial proportion of patients abandon treatment, for reasons that have yet to be studied systematically (71). Further research into long-term outcomes, including series with higher rates of treatment completion, is warranted to further define the ideal candidates and treatment regimen for vacuum bell therapy.
Figure 2.

A patient with pectus excavatum fitted with the vacuum bell device.
Postoperative pain management
Pain control following surgical repair of pectus excavatum is often challenging and is a significant contributor to length of stay (LOS). This is especially true for MIRPE, where the postoperative pain may be disproportionate to the size of the incisions, though there is overall a lower incidence of chronic postoperative pain in the pediatric population compared to adults (72). Recent advances in protocolized pain management and multimodal pain control have significantly reduced postoperative LOS and narcotic usage after MIRPE. Initial strategies included thoracic epidural and patient-controlled analgesia (PCA), often used as a bridge to oral pain medications (73). A prospective, randomized trial comparing epidural and PCA reported more favorable pain scores for the epidural approach in the early postoperative days and for PCA in the later period. Epidurals, however, were associated with longer operative times, greater hospital charges, and more calls to anesthesia providers (74). As an alternative regional anesthesia approach to epidurals, the use of ultrasound-guided erector spinae plane blocks has recently been shown to be effective while reducing LOS (75).
The use of intercostal nerve cryoablation (INC) has recently been employed as a means to reduce postoperative pain after MIRPE. Broadly, cryoanalgesia is the use of cold temperature to disrupt peripheral nerve function. The INC technique involves thoracoscopically placing a “cryoprobe” on the target intercostal nerve through the same incision used for bar placement, as demonstrated in Figure 3. The device reduces the temperature of the tissue to the specific temperature that causes Wallerian degeneration of nerve axons to temporarily prevent pain transmission, with regeneration occurring within 4–6 weeks (76). Kim and colleagues were the first to describe the use of INC in MIRPE using a transthoracic technique that facilities access to the posterolateral intercostal nerves (77). Later that year, Keller and colleagues described INC via a subcutaneous tunnel approach, and demonstrated a significant reduction in opioid use and LOS when compared to epidural analgesia, with a mean LOS of 3.5 days in the cryo group (78). A recent randomized trial found a 2-day reduction in hospital LOS (3 versus 5 days) and reduced opioid requirements in patients who received cryoablation when compared to thoracic epidural analgesia (76). The trend of reduced LOS was supported in a recent systematic review and meta-analysis of five studies comparing cryoablation with thoracic epidural analgesia, though no significant difference in pain scores were reported among the groups (79). The use of INC in the context of multimodal pain control protocols including intercostal nerve blocks has recently been used to allow same- and next-day discharge after MIRPE. The wide adoption of INC was limited by concerns of long term neuropathy after axon regeneration (80,81). In a recent retrospective study of 43 patients who underwent INC during MIRPE, none of the patients under 21 years of age experienced neuropathic pain, while 3 of 13 adults in the study experienced neuropathic pain or prolonged numbness (82). This may be partially attributed to pediatric patients generally experiencing less chronic postoperative pain than adults as previously mentioned. Despite the retrospective nature and small sample sizes of recent studies, cryoablation is a promising adjunct for pain control and should be considered in enhanced recovery protocols after MIRPE. It may also be considered for open repair of pectus excavatum (83). Further studies are needed to assess the long-term implications of this treatment.
Figure 3.
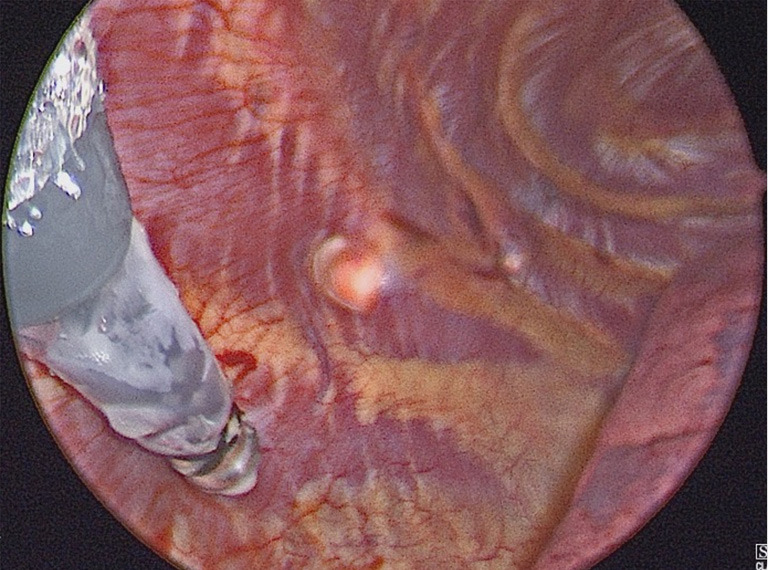
Thoracoscopic view of a cryoprobe in the thoracic cavity after being tunneled subcutaneously and placed on the target intercostal nerve to perform cryoablation.
Recurrent pectus excavatum
A dreaded complication after surgical correction of pectus excavatum is recurrence of the deformity, which is reported to occur in 2–10% of cases (37). Various factors are thought to be responsible for recurrence, including timing of the operation during development, duration of retrosternal strut maintenance, and inherent connective tissue dysfunction as in patients with Marfan syndrome (84). There is a high risk of recurrence reported in Marfan’s patients without strut fixation; it is therefore recommended that all children with Marfan’s and pectus excavatum be repaired with strut fixation (85). To decrease the risk of recurrence, the authors wait at least 3 years before removing the retrosternal bar after MIRPE, and 4 or more years for those with connective tissue disorders. For patients who do experience recurrent pectus excavatum, the MIRPE technique has been utilized safely and effectively for correction of recurrent cases. In a series of 100 patients, Redlinger et al. showed a 95% success rate of MIRPE regardless of whether the initial repair was open or minimally invasive. Patients with prior MIRPE may be prone to more extensive pleural adhesions and require decortication during their subsequent repair, while patients with a prior open repair may have acquired thoracic chondrodystrophy with stiff chests. This may require a greater number of bars for repair and predispose to additional complications (86).
Monitoring
There is currently no diagnostic method in routine use that is both safe for longitudinal monitoring and accurate enough to assess preoperative or pre-intervention severity of pectus excavatum. As previously discussed, radiographic evaluation with thoracic CT scan is the most widely used method to measure pectus excavatum severity via HI, and may be required to support the medical necessity for corrective surgery (87). Other modalities may be superior for monitoring of response to therapy. A manual measure of pectus excavatum depth is commonly performed by providers and involves inserting a ruler into the depression at its deepest point and recording the distance from this point to the surface of the depression by placing a straight edge horizontally across the chest (87). Though non-invasive and easily accessible, this method is overall less precise, highly provider-dependent, and unreliable in cases of asymmetric defects. A novel external landmark-based approach using calipers—termed modified percent depth—may provide improved inter-rater reliability and account for asymmetry (88). CT-derived HI remains the standard that is often necessary for surgical clearance, though X-ray and MRI have demonstrated comparable measurements.
An ideal method to quantify and monitor the physical exam of pectus excavatum patients is to map the chest wall deformity without ionizing radiation given the target population is often evaluated in childhood or early adolescence—this can be optimally achieved with 3D surface imaging and automated measurements of the deformity. As discussed in the preoperative evaluation section of this review, 3D imaging has a role in diagnosis, recommendation for surgical treatment, and prediction of physiologic compromise. To cite an example of such a modality, recent work from Hebal et al. has assessed the use of a handheld white light scanner (WLS) as a non-invasive, non-ionizing means to obtain 3D measurements and indices of pectus excavatum deformities in a larger cohort of 127 patients (87). A severity index, termed the Hebal-Malas index (HMI), was derived from mediolateral and anteroposterior chest wall diameters; this index was shown to have a strong correlation (r=0.87) to CT-derived HI for patients with severe pectus excavatum. The WLS was also shown to be an effective means of quantifying the extent of pectus excavatum correction following the Nuss procedure (89). Though unlikely to completely replace CT scan for evaluation of pectus deformities, high fidelity non-radiation based imaging such as WLS should be incorporated into the clinical workflow as an assessment tool, particularly for treatments such as vacuum bell therapy or external bracing for pectus carinatum, that require a high degree of compliance (90). Barriers to widespread adoption include the variety, cost, and availability of a standardized system to implement on a large scale and thereby develop institutional expertise (91).
Conclusions
The care of children with pectus excavatum continues to evolve with nuances in preoperative evaluation, operative and non-operative techniques, and postoperative management. Despite the breadth of literature on the topic, there is a lack of international consensus guidelines regarding the preoperative evaluation and treatment of these patients, which presents an opportunity for future research efforts in these areas.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-361/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-361/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-361/coif). Farokh R. Demehri serves as an unpaid editorial board member of Translational Pediatrics from February 2021 to January 2023. The other author has no conflicts of interest to declare.
References
- 1.Obermeyer RJ, Goretsky MJ. Chest wall deformities in pediatric surgery. Surg Clin North Am 2012;92:669-84, ix. 10.1016/j.suc.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 2.Feng J, Hu T, Liu W, et al. The biomechanical, morphologic, and histochemical properties of the costal cartilages in children with pectus excavatum. J Pediatr Surg 2001;36:1770-6. 10.1053/jpsu.2001.28820 [DOI] [PubMed] [Google Scholar]
- 3.Behr CA, Denning NL, Kallis MP, et al. The incidence of Marfan syndrome and cardiac anomalies in patients presenting with pectus deformities. J Pediatr Surg 2019;54:1926-8. 10.1016/j.jpedsurg.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 4.Frantz FW. Indications and guidelines for pectus excavatum repair. Curr Opin Pediatr 2011;23:486-91. 10.1097/MOP.0b013e32834881c4 [DOI] [PubMed] [Google Scholar]
- 5.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 2011;128:305-10. 10.1097/PRS.0b013e318219c171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billar RJ, Manoubi W, Kant SG, et al. Association between pectus excavatum and congenital genetic disorders: A systematic review and practical guide for the treating physician. J Pediatr Surg 2021;56:2239-52. 10.1016/j.jpedsurg.2021.04.016 [DOI] [PubMed] [Google Scholar]
- 7.Shamberger RC, Welch KJ, Sanders SP. Mitral valve prolapse associated with pectus excavatum. J Pediatr 1987;111:404-7. 10.1016/s0022-3476(87)80465-1 [DOI] [PubMed] [Google Scholar]
- 8.Coln E, Carrasco J, Coln D. Demonstrating relief of cardiac compression with the Nuss minimally invasive repair for pectus excavatum. J Pediatr Surg 2006;41:683-6; discussion 683-6. 10.1016/j.jpedsurg.2005.12.009 [DOI] [PubMed] [Google Scholar]
- 9.Malek MH, Fonkalsrud EW, Cooper CB. Ventilatory and cardiovascular responses to exercise in patients with pectus excavatum. Chest 2003;124:870-82. 10.1378/chest.124.3.870 [DOI] [PubMed] [Google Scholar]
- 10.Jaroszewski DE, Velazco CS, Pulivarthi VSKK, et al. Cardiopulmonary Function in Thoracic Wall Deformities: What Do We Really Know? Eur J Pediatr Surg 2018;28:327-46. 10.1055/s-0038-1668130 [DOI] [PubMed] [Google Scholar]
- 11.Kelly RE, Goretsky MJ, Obermeyer R, et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg 2010;252:1072-81. 10.1097/SLA.0b013e3181effdce [DOI] [PubMed] [Google Scholar]
- 12.Malek MH, Berger DE, Marelich WD, et al. Pulmonary function following surgical repair of pectus excavatum: a meta-analysis. Eur J Cardiothorac Surg 2006;30:637-43. 10.1016/j.ejcts.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 13.Del Frari B, Sigl S, Schwabegger AH, et al. Impact of surgical treatment of pectus carinatum on cardiopulmonary function: a prospective study. Eur J Cardiothorac Surg 2021;59:382-8. 10.1093/ejcts/ezaa335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haller JA, Jr, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 1987;22:904-6. 10.1016/s0022-3468(87)80585-7 [DOI] [PubMed] [Google Scholar]
- 15.Sarwar ZU, DeFlorio R, O'Connor SC. Pectus excavatum: current imaging techniques and opportunities for dose reduction. Semin Ultrasound CT MR 2014;35:374-81. 10.1053/j.sult.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 16.St Peter SD, Juang D, Garey CL, et al. A novel measure for pectus excavatum: the correction index. J Pediatr Surg 2011;46:2270-3. 10.1016/j.jpedsurg.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 17.Mueller C, Saint-Vil D, Bouchard S. Chest x-ray as a primary modality for preoperative imaging of pectus excavatum. J Pediatr Surg 2008;43:71-3. 10.1016/j.jpedsurg.2007.09.023 [DOI] [PubMed] [Google Scholar]
- 18.Khanna G, Jaju A, Don S, et al. Comparison of Haller index values calculated with chest radiographs versus CT for pectus excavatum evaluation. Pediatr Radiol 2010;40:1763-7. 10.1007/s00247-010-1681-z [DOI] [PubMed] [Google Scholar]
- 19.van Dijk H, Höppener PF, Siebenga J, et al. Medical photography: a reliable and objective method for documenting the results of reconstructive surgery of pectus excavatum. J Vis Commun Med 2011;34:14-21. 10.3109/17453054.2011.550869 [DOI] [PubMed] [Google Scholar]
- 20.Daemen JHT, Loonen TGJ, Coorens NA, et al. Photographic documentation and severity quantification of pectus excavatum through three-dimensional optical surface imaging. J Vis Commun Med 2020;43:190-7. 10.1080/17453054.2020.1784711 [DOI] [PubMed] [Google Scholar]
- 21.Poncet P, Kravarusic D, Richart T, et al. Clinical impact of optical imaging with 3-D reconstruction of torso topography in common anterior chest wall anomalies. J Pediatr Surg 2007;42:898-903. 10.1016/j.jpedsurg.2006.12.070 [DOI] [PubMed] [Google Scholar]
- 22.Glinkowski W, Sitnik R, Witkowski M, et al. Method of pectus excavatum measurement based on structured light technique. J Biomed Opt 2009;14:044041. 10.1117/1.3210782 [DOI] [PubMed] [Google Scholar]
- 23.Daemen JHT, Coorens NA, Hulsewé KWE, et al. Three-dimensional Surface Imaging for Clinical Decision Making in Pectus Excavatum. Semin Thorac Cardiovasc Surg 2022;34:1364-73. 10.1053/j.semtcvs.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 24.Daemen JHT, Heuts S, Rezazadah Ardabili A, et al. Development of Prediction Models for Cardiac Compression in Pectus Excavatum Based on Three-Dimensional Surface Images. Semin Thorac Cardiovasc Surg 2021. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Croitoru DP, Kelly RE, Jr, Goretsky MJ, et al. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg 2002;37:437-45. 10.1053/jpsu.2002.30851 [DOI] [PubMed] [Google Scholar]
- 26.Fonkalsrud EW, Beanes S, Hebra A, et al. Comparison of minimally invasive and modified Ravitch pectus excavatum repair. J Pediatr Surg 2002;37:413-7. 10.1053/jpsu.2002.30852 [DOI] [PubMed] [Google Scholar]
- 27.Nuss D, Kelly RE, Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. 10.1016/s0022-3468(98)90314-1 [DOI] [PubMed] [Google Scholar]
- 28.McMahon LE, Johnson KN, Jaroszewski DE, et al. Experience with FiberWire for pectus bar attachment. J Pediatr Surg 2014;49:1259-63. 10.1016/j.jpedsurg.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 29.Messineo A, Ghionzoli M, Lo Piccolo R, et al. A simplified method to pass the bar through the mediastinum in the Nuss technique. Ann Thorac Surg 2015;99:717-8. 10.1016/j.athoracsur.2014.09.072 [DOI] [PubMed] [Google Scholar]
- 30.Jaroszewski DE, Johnson K, McMahon L, et al. Sternal elevation before passing bars: a technique for improving visualization and facilitating minimally invasive pectus excavatum repair in adult patients. J Thorac Cardiovasc Surg 2014;147:1093-5. 10.1016/j.jtcvs.2013.09.049 [DOI] [PubMed] [Google Scholar]
- 31.Velazco CS, Arsanjani R, Jaroszewski DE. Nuss procedure in the adult population for correction of pectus excavatum. Semin Pediatr Surg 2018;27:161-9. 10.1053/j.sempedsurg.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 32.Haecker FM, Krebs T, Kocher GJ, et al. Sternal elevation techniques during the minimally invasive repair of pectus excavatum. Interact Cardiovasc Thorac Surg 2019;29:497-502. 10.1093/icvts/ivz142 [DOI] [PubMed] [Google Scholar]
- 33.Goretsky MJ, McGuire MM. Complications associated with the minimally invasive repair of pectus excavatum. Semin Pediatr Surg 2018;27:151-5. 10.1053/j.sempedsurg.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 34.Kelly RE, Jr, Obermeyer RJ, Goretsky MJ, et al. Recent Modifications of the Nuss Procedure: The Pursuit of Safety During the Minimally Invasive Repair of Pectus Excavatum. Ann Surg 2022;275:e496-502. 10.1097/SLA.0000000000003877 [DOI] [PubMed] [Google Scholar]
- 35.Rushing GD, Goretsky MJ, Gustin T, et al. When it is not an infection: metal allergy after the Nuss procedure for repair of pectus excavatum. J Pediatr Surg 2007;42:93-7. 10.1016/j.jpedsurg.2006.09.056 [DOI] [PubMed] [Google Scholar]
- 36.Van Renterghem KM, von Bismarck S, Bax NM, et al. Should an infected Nuss bar be removed? J Pediatr Surg 2005;40:670-3. 10.1016/j.jpedsurg.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 37.Calkins CM, Shew SB, Sharp RJ, et al. Management of postoperative infections after the minimally invasive pectus excavatum repair. J Pediatr Surg 2005;40:1004-7; discussion 1007-8. 10.1016/j.jpedsurg.2005.03.017 [DOI] [PubMed] [Google Scholar]
- 38.Obermeyer RJ, Godbout E, Goretsky MJ, et al. Risk factors and management of Nuss bar infections in 1717 patients over 25 years. J Pediatr Surg 2016;51:154-8. 10.1016/j.jpedsurg.2015.10.036 [DOI] [PubMed] [Google Scholar]
- 39.Obermeyer RJ, Cohen NS, Gaffar S, et al. Multivariate analysis of risk factors for Nuss bar infections: A single center study. J Pediatr Surg 2018;53:1226-9. 10.1016/j.jpedsurg.2018.02.090 [DOI] [PubMed] [Google Scholar]
- 40.Notrica DM. Modifications to the Nuss procedure for pectus excavatum repair: A 20-year review. Semin Pediatr Surg 2018;27:133-50. 10.1053/j.sempedsurg.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 41.Pilegaard HK, Licht PB. Early results following the Nuss operation for pectus excavatum--a single-institution experience of 383 patients. Interact Cardiovasc Thorac Surg 2008;7:54-7. 10.1510/icvts.2007.160937 [DOI] [PubMed] [Google Scholar]
- 42.Pilegaard HK. Nuss technique in pectus excavatum: a mono-institutional experience. J Thorac Dis 2015;7:S172-6. 10.3978/j.issn.2072-1439.2015.04.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park HJ. A technique for complex pectus excavatum repair: the cross-bar technique for grand canyon type deformity (Park classification). Ann Cardiothorac Surg 2016;5:526-7. 10.21037/acs.2016.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hyun K, Park HJ. The Cross-Bar Technique for Pectus Excavatum Repair: A Key Element for Remodeling of the Entire Chest Wall. Eur J Pediatr Surg 2022. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe A, Watanabe T, Obama T, et al. The use of a lateral stabilizer increases the incidence of wound trouble following the Nuss procedure. Ann Thorac Surg 2004;77:296-300. 10.1016/s0003-4975(03)01335-3 [DOI] [PubMed] [Google Scholar]
- 46.Hebra A, Gauderer MW, Tagge EP, et al. A simple technique for preventing bar displacement with the Nuss repair of pectus excavatum. J Pediatr Surg 2001;36:1266-8. 10.1053/jpsu.2001.25791 [DOI] [PubMed] [Google Scholar]
- 47.Park HJ, Kim KS, Moon YK, et al. The bridge technique for pectus bar fixation: a method to make the bar un-rotatable. J Pediatr Surg 2015;50:1320-2. 10.1016/j.jpedsurg.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 48.Park HJ, Kim KS, Lee S, et al. A next-generation pectus excavatum repair technique: new devices make a difference. Ann Thorac Surg 2015;99:455-61. 10.1016/j.athoracsur.2014.08.026 [DOI] [PubMed] [Google Scholar]
- 49.Pérez D, Martel O, Yánez A, et al. Does the modelling of the pectus bar affect its stability? Rationale for using a short flat bar. Interact Cardiovasc Thorac Surg 2020;30:11-7. 10.1093/icvts/ivz217 [DOI] [PubMed] [Google Scholar]
- 50.Pilegaard HK. Single centre experience on short bar technique for pectus excavatum. Ann Cardiothorac Surg 2016;5:450-5. 10.21037/acs.2016.09.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park HJ, Kim KS. The sandwich technique for repair of pectus carinatum and excavatum/carinatum complex. Ann Cardiothorac Surg 2016;5:434-9. 10.21037/acs.2016.08.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilaça JL, Rodrigues PL, Soares TR, et al. Automatic prebent customized prosthesis for pectus excavatum minimally invasive surgery correction. Surg Innov 2014;21:290-6. 10.1177/1553350613506299 [DOI] [PubMed] [Google Scholar]
- 53.Xie L, Cai S, Xie L, et al. Development of a computer-aided design and finite-element analysis combined method for customized Nuss bar in pectus excavatum surgery. Sci Rep 2017;7:3543. 10.1038/s41598-017-03622-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hebra A, Kelly RE, Ferro MM, et al. Life-threatening complications and mortality of minimally invasive pectus surgery. J Pediatr Surg 2018;53:728-32. 10.1016/j.jpedsurg.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 55.de Loos ER, Daemen JHT, Pennings AJ, et al. Minimally invasive repair of pectus excavatum by the Nuss procedure: The learning curve. J Thorac Cardiovasc Surg 2022;163:828-837.e4. 10.1016/j.jtcvs.2020.11.154 [DOI] [PubMed] [Google Scholar]
- 56.Torre M, Guerriero V, Wong MCY, et al. Complications and trends in minimally invasive repair of pectus excavatum: A large volume, single institution experience. J Pediatr Surg 2021;56:1846-51. 10.1016/j.jpedsurg.2020.11.027 [DOI] [PubMed] [Google Scholar]
- 57.Park HJ, Kim KS. Pectus bar removal: surgical technique and strategy to avoid complications. J Vis Surg 2016;2:60. 10.21037/jovs.2016.02.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haecker FM, Hebra A, Ferro MM. Pectus bar removal - why, when, where and how. J Pediatr Surg 2021;56:540-4. 10.1016/j.jpedsurg.2020.11.001 [DOI] [PubMed] [Google Scholar]
- 59.Nuss D, Obermeyer RJ, Kelly RE, Jr. Pectus excavatum from a pediatric surgeon's perspective. Ann Cardiothorac Surg 2016;5:493-500. 10.21037/acs.2016.06.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu W, Kong D, Yu F, et al. A simple technique for pectus bar removal using a modified Nuss procedure. J Pediatr Surg 2013;48:1137-41. 10.1016/j.jpedsurg.2013.01.052 [DOI] [PubMed] [Google Scholar]
- 61.de Campos JR, Das-Neves-Pereira JC, Lopes KM, et al. Technical modifications in stabilisers and in bar removal in the Nuss procedure. Eur J Cardiothorac Surg 2009;36:410-2. 10.1016/j.ejcts.2009.03.061 [DOI] [PubMed] [Google Scholar]
- 62.Toselli L, Bellía Munzón G, Martinez J, et al. Safety-string: A handy maneuver to control pectus bar removal bleeding complications. J Pediatr Surg 2020;55:1162-4. 10.1016/j.jpedsurg.2020.01.058 [DOI] [PubMed] [Google Scholar]
- 63.Alvarez-Garcia N, Ardigo L, Bellia-Munzon G, et al. Close Examination of the Bar Removal Procedure: The Surgeons' Voice. Eur J Pediatr Surg 2018;28:406-12. 10.1055/s-0037-1606842 [DOI] [PubMed] [Google Scholar]
- 64.Robicsek F, Watts LT, Fokin AA. Surgical repair of pectus excavatum and carinatum. Semin Thorac Cardiovasc Surg 2009;21:64-75. 10.1053/j.semtcvs.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 65.Antonoff MB, Erickson AE, Hess DJ, et al. When patients choose: comparison of Nuss, Ravitch, and Leonard procedures for primary repair of pectus excavatum. J Pediatr Surg 2009;44:1113-8; discussion 118-9. 10.1016/j.jpedsurg.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 66.Schier F, Bahr M, Klobe E. The vacuum chest wall lifter: an innovative, nonsurgical addition to the management of pectus excavatum. J Pediatr Surg 2005;40:496-500. 10.1016/j.jpedsurg.2004.11.033 [DOI] [PubMed] [Google Scholar]
- 67.Haecker FM, Zuppinger J, Sesia SB. Die konservative Therapie der Trichterbrust mittels Vakuumtherapie. Swiss Med Forum 2014;14:842-9. Available online: https://doi.emh.ch/smf.2014.02088
- 68.Obermeyer RJ, Cohen NS, Kelly RE, Jr, et al. Nonoperative management of pectus excavatum with vacuum bell therapy: A single center study. J Pediatr Surg 2018;53:1221-5. 10.1016/j.jpedsurg.2018.02.088 [DOI] [PubMed] [Google Scholar]
- 69.Haecker FM, Sesia S. Vacuum bell therapy. Ann Cardiothorac Surg 2016;5:440-9. 10.21037/acs.2016.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopez M, Patoir A, Costes F, et al. Preliminary study of efficacy of cup suction in the correction of typical pectus excavatum. J Pediatr Surg 2016;51:183-7. 10.1016/j.jpedsurg.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 71.Toselli L, Chinni E, Nazar-Peirano M, et al. Determinants of success associated with vacuum bell treatment of pectus excavatum. J Pediatr Surg 2022;57:550-4. 10.1016/j.jpedsurg.2022.04.010 [DOI] [PubMed] [Google Scholar]
- 72.de Loos ER, Pennings AJ, van Roozendaal LM, et al. Nuss Procedure for Pectus Excavatum: A Comparison of Complications Between Young and Adult Patients. Ann Thorac Surg 2021;112:905-11. 10.1016/j.athoracsur.2020.10.017 [DOI] [PubMed] [Google Scholar]
- 73.Dekonenko C, Dorman RM, Duran Y, et al. Postoperative pain control modalities for pectus excavatum repair: A prospective observational study of cryoablation compared to results of a randomized trial of epidural vs patient-controlled analgesia. J Pediatr Surg 2020;55:1444-7. 10.1016/j.jpedsurg.2019.09.021 [DOI] [PubMed] [Google Scholar]
- 74.St Peter SD, Weesner KA, Weissend EE, et al. Epidural vs patient-controlled analgesia for postoperative pain after pectus excavatum repair: a prospective, randomized trial. J Pediatr Surg 2012;47:148-53. 10.1016/j.jpedsurg.2011.10.040 [DOI] [PubMed] [Google Scholar]
- 75.Bliss DP, Jr, Strandness TB, Derderian SC, et al. Ultrasound-guided erector spinae plane block versus thoracic epidural analgesia: Postoperative pain management after Nuss repair for pectus excavatum. J Pediatr Surg 2022;57:207-12. 10.1016/j.jpedsurg.2021.10.030 [DOI] [PubMed] [Google Scholar]
- 76.Graves CE, Moyer J, Zobel MJ, et al. Intraoperative intercostal nerve cryoablation During the Nuss procedure reduces length of stay and opioid requirement: A randomized clinical trial. J Pediatr Surg 2019;54:2250-6. 10.1016/j.jpedsurg.2019.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim S, Idowu O, Palmer B, et al. Use of transthoracic cryoanalgesia during the Nuss procedure. J Thorac Cardiovasc Surg 2016;151:887-8. 10.1016/j.jtcvs.2015.09.110 [DOI] [PubMed] [Google Scholar]
- 78.Keller BA, Kabagambe SK, Becker JC, et al. Intercostal nerve cryoablation versus thoracic epidural catheters for postoperative analgesia following pectus excavatum repair: Preliminary outcomes in twenty-six cryoablation patients. J Pediatr Surg 2016;51:2033-8. 10.1016/j.jpedsurg.2016.09.034 [DOI] [PubMed] [Google Scholar]
- 79.Daemen JHT, de Loos ER, Vissers YLJ, et al. Intercostal nerve cryoablation versus thoracic epidural for postoperative analgesia following pectus excavatum repair: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2020;31:486-98. 10.1093/icvts/ivaa151 [DOI] [PubMed] [Google Scholar]
- 80.DiFiore JW, Robertson JO, Chhabada S, et al. Next day discharge after the Nuss procedure using intercostal nerve cryoablation, intercostal nerve blocks, and a perioperative ERAS pain protocol. J Pediatr Surg 2022;57:213-8. 10.1016/j.jpedsurg.2021.10.034 [DOI] [PubMed] [Google Scholar]
- 81.Rettig RL, Rudikoff AG, Annie Lo HY, et al. Same-day discharge following the Nuss repair: A comparison. J Pediatr Surg 2022;57:135-40. 10.1016/j.jpedsurg.2021.09.023 [DOI] [PubMed] [Google Scholar]
- 82.Zobel MJ, Ewbank C, Mora R, et al. The incidence of neuropathic pain after intercostal cryoablation during the Nuss procedure. Pediatr Surg Int 2020;36:317-24. 10.1007/s00383-019-04602-1 [DOI] [PubMed] [Google Scholar]
- 83.Pilkington M, Harbaugh CM, Hirschl RB, et al. Use of cryoanalgesia for pain management for the modified ravitch procedure in children. J Pediatr Surg 2020;55:1381-4. 10.1016/j.jpedsurg.2019.09.016 [DOI] [PubMed] [Google Scholar]
- 84.Arn PH, Scherer LR, Haller JA, Jr, et al. Outcome of pectus excavatum in patients with Marfan syndrome and in the general population. J Pediatr 1989;115:954-8. 10.1016/s0022-3476(89)80749-8 [DOI] [PubMed] [Google Scholar]
- 85.Scherer LR, Arn PH, Dressel DA, et al. Surgical management of children and young adults with Marfan syndrome and pectus excavatum. J Pediatr Surg 1988;23:1169-72. 10.1016/s0022-3468(88)80335-x [DOI] [PubMed] [Google Scholar]
- 86.Redlinger RE, Jr, Kelly RE, Jr, Nuss D, et al. One hundred patients with recurrent pectus excavatum repaired via the minimally invasive Nuss technique--effective in most regardless of initial operative approach. J Pediatr Surg 2011;46:1177-81. 10.1016/j.jpedsurg.2011.03.048 [DOI] [PubMed] [Google Scholar]
- 87.Hebal F, Port E, Hunter CJ, et al. A novel technique to measure severity of pediatric pectus excavatum using white light scanning. J Pediatr Surg 2019;54:656-62. 10.1016/j.jpedsurg.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 88.Bludevich BM, Kauffman JD, Litz CN, et al. External caliper-based measurements of the modified percent depth as an alternative to cross-sectional imaging for assessing the severity of pectus excavatum. J Pediatr Surg 2020;55:1058-64. 10.1016/j.jpedsurg.2020.02.053 [DOI] [PubMed] [Google Scholar]
- 89.Port E, Hebal F, Hunter CJ, et al. Measuring the impact of surgical intervention on pediatric pectus excavatum using white light scanning. J Pediatr Surg 2019;54:2261-7. 10.1016/j.jpedsurg.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 90.Hussain A, Patel A, Hunt I. Are non-radiation-based imaging modalities effective for objectively assessing and monitoring patients with pectus deformities? Interact Cardiovasc Thorac Surg 2020;31:536-9. 10.1093/icvts/ivaa134 [DOI] [PubMed] [Google Scholar]
- 91.Wei B, Gleason F. Commentary: In With The New: Three-Dimensional Surface Imaging For Pectus Excavatum. Semin Thorac Cardiovasc Surg 2022;34:1376-7. 10.1053/j.semtcvs.2021.08.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


