The case presentation
A 31-year-old Japanese woman was admitted to her neighboring hospital for dark brown urine. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) quantitative antigen test was positive, and she was referred to our hospital. She had never been vaccinated against or infected with SARS-CoV-2. Her medical history was relevant for three episodes of thrombotic microangiopathy (TMA) at ages 3, 8, and 17 years, triggered by common cold, Campylobacter, and Influenza A infections, respectively. Accordingly, she was treated with red blood cell transfusions for the first and second episodes of TMA, and with fresh frozen plasma transfusion and plasma exchange for the last episode. She had no sequelae. Although her father, paternal uncle, and paternal grandmother had a history of TMA, genetic tests had never been performed.
Blood pressure, temperature, and SpO2 were 157/96 mmHg, 37.0 ℃, and 98%, respectively. Physical examination was unremarkable, except for abdominal tenderness and dark brown urine. Initial laboratory results demonstrated thrombocytopenia, acute kidney injury (AKI), and disseminated intravascular coagulation (DIC) (Table 1). On day 2, she was referred to the department of Kidney Disease and Hypertension. Additional analyses indicated hemolytic anemia with presence of schistocytes and a negative Coombs test. Haptoglobin was 259 mg/dL (range 19–170), but was measured after haptoglobin administration. Computed tomography performed at admission showed no evidence of pneumonia or other infections.
Table 1.
Laboratory results at admission
| Blood cell count | Coagulation | ||
| WBCs (/µL) | 6300 (3300–8600) | PT-INR | 1.2 (0.90–1.15) |
| RBCs (× 104/µL) | 434 (386–492) | APTT (sec) | 47.5 (25.7–36.8) |
| Hemoglobin (g/dL) | 13.0 (11.6–14.8) | Fibrinogen (mg/dL) | 125 (168–355) |
| Schistocytes (RBC) | 50/1,000 (< 10/1000) | FDP (µg/mL) | 281.9 (< 2.5) |
| Hematocrit (%) | 37.8 (35.1–44.4) | D-dimer (µg/mL) | 77.8 (< 0.6) |
| Platelets (× 104/µL) | 4.1 (15.8–34.8) | Immunochemistry | |
| Blood chemistry | IgG (mg/dL) | 1,141 (861–1747) | |
| Total protein (g/dL) | 6.0 (6.6–8.1) | IgA (mg/dL) | 107 (93–393) |
| Albumin (g/dL) | 3.4 (4.1–5.1) | IgM (mg/dL) | 33 (50–269) |
| Total bilirubin (mg/dL) | 1.8 (0.4–1.5) | C3 (mg/dL) | 63 (73–138) |
| Direct bilirubin (mg/dL) | 0.1 (0.0–0.2) | C4 (mg/dL) | 17.2 (11–31) |
| LDH (U/L) | 1,930 (124–222) | ANA (titer) | < × 40 (< × 40) |
| BUN (mg/dL) | 34 (8–20) | Urinalysis | |
| Cr (mg/dL) | 1.57 (0.46–0.79) | Occult blood | 3 + (−) |
| Sodium (mEq/L) | 134 (138–145) | Protein | 3 + (−) |
| Potassium (mEq/L) | 4.8 (3.6–4.8) | WBCs (/HPF) | 1–4 (< 4) |
| Chloride (mEq/L) | 104 (101–108) | RBCs (/HPF) | 10–19 (< 4) |
| Calcium (mg/dL) | 8.3 (8.8–10.1) | Protein quantity (g/gCr) | 1.89 (< 0.15) |
Normal ranges are shown in parentheses
Full list is available in Supplemental material (Table S1)
ANA, antinuclear antibodies; APTT, activated partial thromboplastin time; BUN, blood urea nitrogen; Cr, creatinine; FDP, fibrin degradation products; Ig, immunoglobulin; INR, international normalized ratio; LDH, lactate dehydrogenase; PT, prothrombin time; RBCs, red blood cells; WBCs, white blood cells
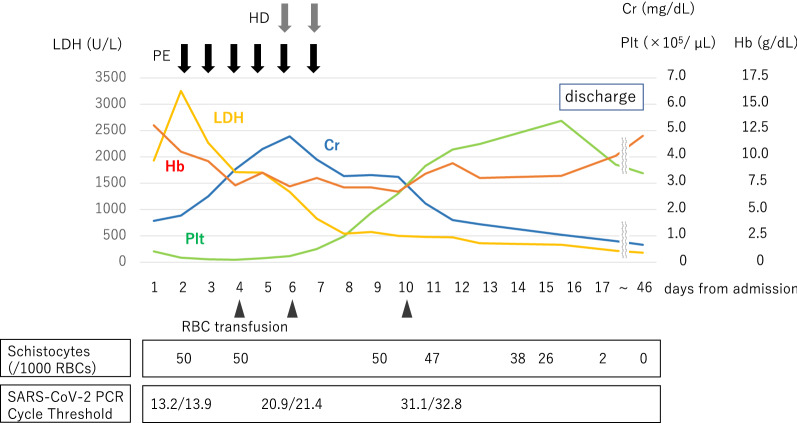
We initiated plasma exchange (Fig. 1), however we were unable to perform a renal biopsy due to patient’s refusal and severe thrombocytopenia. In the following days, the patient’s blood pressure decreased to around 120/80 mmHg without any antihypertensive medication and serum creatinine level rose to 4.78 mg/dL, and she developed oliguria and fluid overload requiring hemodialysis. Urine volume gradually increased and hemolytic anemia resolved; thus, hemodialysis and plasma exchange were stopped on day 7. The diagnoses of thrombotic thrombocytopenic purpura and Shiga toxin-producing Escherichia coli-associated hemolytic uremic syndrome were ruled out, considering normal ADAMTS13 activity (76%), low levels of ADAMTS13 inhibitor (< 0.5 BU/mL), and negative O157. Moreover, we could not identify the common causes of secondary TMA (drugs, pregnancy, autoimmune diseases, transplantation, etc.), supporting a suspicion of atypical hemolytic uremic syndrome (aHUS). Therefore, anti-C5 monoclonal antibody administration was considered. However, since the patient’s thrombocytopenia, AKI, diffuse intravascular coagulation, and hemolysis improved, and her condition stabilized, we decided to postpone this treatment. On day 17, the patient was discharged and her platelet count, hemoglobin, serum creatinine, and lactate dehydrogenase levels were 53.7 × 104/µL, 8.2 g/dL, 1.04 mg/dL, and 331 U/L, respectively.
Fig. 1.
The clinical course. We initiated plasma exchange on day 2 (plasma separator: polyethylene; membrane surface area: 0.5 m2; replacement fluid: fresh frozen plasma; exchanged plasma volume: 2,800 mL; blood flow rate: 100 ml/min; plasma removal and replacement rate: 30 ml/min). However, serum creatinine level rose to 4.78 mg/dL, and she developed oliguria and fluid overload. We started hemodialysis on day 6 (dialyzer: cellulose triacetate; membrane surface area: 0.7 m.2; blood flow rate: 100 ml/min; dialysate flow rate: 500 ml/min). Urine volume gradually increased and hemolytic anemia resolved; thus, hemodialysis and plasma exchange were stopped on day 7. Prior to anti-C5 monoclonal antibody administration, the patient’s thrombocytopenia, AKI, DIC, and hemolysis improved, and her condition stabilized. Cr, creatinine; Hb, hemoglobin; HD, hemodialysis; LDH, lactate dehydrogenase; PE, plasma exchange; Plt, Platelet; RBCs, red blood cells; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
During outpatient follow-up, the patient remained in good condition with normal laboratory indices. Genetic screening revealed a heterozygous missense variant of the C3 gene (p.I1157T), which confirmed the diagnosis of aHUS. Thus, should we suspect a further aHUS relapse, we will promptly administer or an anti-C5 monoclonal antibody.
Lessons for the clinical nephrologist
We herein report a patient with TMA as a relapse of aHUS triggered by COVID-19. To the best of our knowledge, this has previously been observed in eight other cases (Table 2) [1–4]. However, there have been no reports of patients with aHUS who developed TMA and diffuse intravascular coagulation triggered by COVID-19.
Table 2.
Characteristics of TMA as a relapse of aHUS triggered by COVID-19
| Case | Sex, Age (years) | S-Cr (mg/dL) |
Platelet (× 104/μL) | LDH (U/L) or (× ULN) | COVID-19 treatment | TMA treatment | Complement genetics | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 [1] | F, 52 | 2.9 | 31.8 | 885 | ND | HD eculizumab | CFH Cys931Tyr | ND |
| 2 [1] | F, 22 | 6.3 | 2.8 | 2,066 | ND | PE, HD eculizumab | CFH splice variant | ND |
| 3 [2] | M, 39 | 4.7 | 8 | 533 | ND | PE, HDF mPSL eculizumab | C3 Arg161Trp | ND |
| 4 [3] | F, 28 | 2.6 | 10.6 | > × 1.5 ULN | ND | ND | MCP Arg59stop | S-Cr 2.0 |
| 5 [4] | M, 66 | 10 | 5 | > ULN | – | – | CFH Ser756Thr | HD |
| 6 [4] | M, 71 | 2.3 | 1.6 | > × 1.7 ULN | Oxygen | PE eculizumab | C3 Lys155Gln | S-Cr 1.7 |
| 7 [4] | M, 35 | 7.9 | 1.1 | > × 9 ULN | – | PE eculizumab | CFI Ile416Leu | HD |
| 8 [4] | F, 26 | 7.2 | 2.2 | > ULN | – | PE eculizumab tacrolimus rituximab | CFH homozygous tgtgt haplotype | S-Cr 4.2 |
aHUS, atypical hemolytic uremic syndrome; CFH, complement factor H; CFI, complement factor I; HD, hemodialysis; HDF, hemodiafiltration; LDH, Lactate dehydrogenase; MCP, membrane cofactor protein; mPSL, methylprednisolone; ND, not described; PE, plasma exchange; S-CR, serum creatinine; TMA, thrombotic microangiopathy; ULN, upper limit normal
Patients with aHUS are at increased risk of developing TMA triggered by various environmental factors (seasonal infections such as influenza, pregnancy, certain drugs, etc.) [5]. In this case, during the patient’s childhood, TMA episodes were thought to be associated with common cold, Campylobacter, and Influenza A. The current relapse was believed to have been triggered by COVID-19.
The pathogenesis of aHUS is believed to involve loss-of-function of complement inhibitory factors and gain-of-function of complement activating factors. Yu et al. showed that the SARS-CoV-2 spike protein subunit functions as a potent activator of the complement alternative pathway, inhibiting the binding ability of complement factor H (CFH) to cell surface heparan sulfate, and significantly reducing the regulatory ability of CFH in inhibiting C3 conversion enzyme activity in vitro [6]. It has been demonstrated that the C3 gene variant is gain-of-function of complement activating factors [S1]. In this case, the patient had a heterozygous missense variant in the C3 gene. In addition to this background, the loss-of-function of the inhibitory factor associated with COVID-19 causes excessive activation of the complement alternative pathway, leading to membrane attack complex -mediated endothelial injury and capillary thrombosis, which are fundamental in the pathogenesis of TMA.
The difference between previously reported cases and this case was the presence of coagulopathy. Coagulopathy associated with COVID-19 has been widely described. The binding of SARS-CoV-2 to angiotensin-converting enzyme 2 (ACE2) reduces ACE2 activity and prevents the degradation of angiotensin II to angiotensin 1–7, resulting in vascular smooth muscle contraction, inflammation, and endothelial dysfunction related to thrombus formation [7]. Tissue factor is thought to be expressed by vascular endothelial injury, which increases thrombotic tendency. Furthermore, D-dimer is believed to increase following tissue factor degradation, and higher D-dimer levels are associated with COVID-19 severity and poor prognosis [8]. However, in this case, the clinical course of COVID-19 was not as severe as could be expected from high D-dimer level, making it difficult to explain the coagulopathy by COVID-19 alone. TMA and diffuse intravascular coagulation often occur together, and it is often difficult to distinguish them. In this case, complement activation might also have contributed to hemostatic activation, leading to coagulopathy.
In almost all of the eight cases gathered by our review [1–4], eculizumab was administered in addition to plasma exchange. Two patients remained dialysis dependent, while hemodialysis was discontinued in the six others. In our case, the patient’s condition improved with plasma exchange alone; hence, eculizumab treatment was postponed and finally not needed. Further, among the previous eight cases, two had a C3 gene variant, four had a CFH gene variant, one had a cofactor protein gene variant, and one had a complement factor I (CFI) gene variant. CFH gene variants have been well characterized in Europe and the United States, while C3 gene variants, especially the p.I1157T variant, are known to be the most frequent variants in Japan, and their prognosis is relatively better than that of CFH gene variants [9]. This was observed in our case. Furthermore, in a previous study, nineteen patients with aHUS with the C3 p.I1157T variant were investigated, and seven of them were treated with monoclonal antibodies and achieved good response [S1].
In conclusion, SARS-CoV-2 can trigger TMA through complement activation, especially in patients with aHUS. Rapid suspicion of aHUS is required to consider timely plasma exchange or an anti-C5 monoclonal antibody. Furthermore, the type of gene variant may be a prognostic indicator in patients with aHUS.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
RU wrote the manuscript. NH, RI, YU, NK, and TH contributed by reviewing and revising the manuscript. RU and MS took clinical care of the patient. NK, YT, and SM contributed to genetic interpretation.
Funding
The authors did not receive support from any organization for the submitted work.
Declarations
Conflict of interest
All the authors declare no competing interests
Ethical statement
This article does not contain any studies with human participants performed by any of the authors.
Patient protections
The authors declare that they have obtained consent from the patient reported in this article for publication of the information about her that appears within this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ryuta Uwatoko and Mayu Shindo have contributed equally to this work.
References
- 1.Kaufeld J, Reinhardt M, Schröder C, et al. Atypical hemolytic and uremic syndrome triggered by infection with SARS-CoV2. Kidney Int Rep. 2021;6(10):2709–2712. doi: 10.1016/j.ekir.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mat O, Ghisdal L, Massart A, et al. Kidney thrombotic microangiopathy after COVID-19 associated with C3 gene mutation. Kidney Int Rep. 2021;6(6):1732–1737. doi: 10.1016/j.ekir.2021.03.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ville S, Le Bot S, Chapelet-Debout A, et al. Atypical HUS relapse triggered by COVID-19. Kidney Int. 2021;99(1):267–268. doi: 10.1016/j.kint.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Sissy C, Saldman A, Zanetta G, et al. COVID-19 as a potential trigger of complement-mediated atypical HUS. Blood. 2021;138(18):1777–1782. doi: 10.1182/blood.2021012752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8(11):622–633. doi: 10.1038/nrneph.2012.195. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Ra B. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136(18):2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167–173. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujisawa M, Kato H, Yoshida Y, et al. Clinical characteristics and genetic backgrounds of Japanese patients with atypical hemolytic uremic syndrome. Clin Exp Nephrol. 2018;22(5):1088–1099. doi: 10.1007/s10157-018-1549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.