Abstract
Transcription factor EB, as a component of the microphthalmia family of transcription factors, has been demonstrated to be a key controller of autophagy–lysosomal biogenesis. Transcription factor EB is activated by stressors such as nutrition and deprivation of growth factors, hypoxia, lysosomal stress, and mitochondrial injury. To achieve the ultimate functional state, it is controlled in a variety of modes, such as in its rate of transcription, post-transcriptional control, and post-translational alterations. Due to its versatile role in numerous signaling pathways, including the Wnt, calcium, AKT, and mammalian target of rapamycin complex 1 signaling pathways, transcription factor EB—originally identified to be an oncogene—is now well acknowledged as a regulator of a wide range of physiological systems, including autophagy–lysosomal biogenesis, response to stress, metabolism, and energy homeostasis. The well-known and recently identified roles of transcription factor EB suggest that this protein might play a central role in signaling networks in a number of non-communicable illnesses, such as cancer, cardiovascular disorders, drug resistance mechanisms, immunological disease, and tissue growth. The important developments in transcription factor EB research since its first description are described in this review. This review helps to advance transcription factor EB from fundamental research into therapeutic and regenerative applications by shedding light on how important a role it plays in human health and disease at the molecular level.
Keywords: Transcription factor EB, signaling pathway, autophagy–lysosomal system
Introduction
Transcription factor EB (TFEB) is a component of basic helix–loop–helix (bHLH) leucine zipper containing microphthalmia/transcription factor E family (Mit/TFE), which also contains TFE3, TFEC, and microphthalmia-associated TF (MITF) as members. When first discovered, TFEB was thought to be an oncogene in some cases of juvenile renal carcinoma cells, and it is now known to have a more complex role.1 It regulates the expression of genes involved in a wide range of cellular activities, including autophagy–lysosomal biogenesis (ALB), cellular energy balance, angiogenesis, inflammation, and metabolic functions.2–6 It regulates the activity of genes that affect how lysosomal and non-lysosomal enzymes involved in the breakdown of cellular macromolecules such as glycosaminoglycans (GAGs), proteins, glycogen, lipids, and hemoglobin are expressed, located, function, and influx.7–9 Therefore, it plays a role in keeping bodily homeostasis, especially in the neurological, immunological, metabolic, cancer, and cardiovascular systems.10 TFEB is also subject to a highly complicated but poorly understood regulatory network that controls its activity and function. It is normally found in the cytosol of cells in a phosphorylated and inactive state. Nevertheless, stress-related circumstances like lysosomal malfunction or starvation cause TFEB to move to the nuclei of cells, in which it stimulates the expression of the target genes.8,11–13
TFEB first came to light and became well known when it was shown to be the main regulator of lysosomal and mitochondrial biogenesis. A gene transcriptional network called the coordinating lysosomal expression and regulation (CLEAR) network, with TFEB serving as the key controller, has been identified as being involved in lysosomal biogenesis because of systems biology investigations. TFEB controls the expression of targeted genes by binding precisely to the target promoters’ CLEAR motif.7,9,14 TFEB links ALB, and regulates the expression of genes involved lysosomal biogenesis and autophagy to ensure adequate TFEB-mediated intracellular clearance of cytotoxic protein aggregates under tissue-specific regulation.8 TFEB can also enhance lysosomal exocytosis, which enables cargo (undegraded lysosomal content) secretion by fusion of lysosomes to the cell membrane.15,16
All eukaryotic cell types engage in autophagy, which is a key cellular mechanism for the destruction of long-lived cytoplasmic organelles and proteins.17 Maintaining cell homeostasis and adapt to certain environmental stress, autophagy, the body’s natural process for breaking down proteins and organelles, is crucial.18 The ineffectiveness of autophagy control systems is strongly associated with a variety of diseases, including microbial diseases, inflammation, immune system changes, cancer, neurological disorders, muscle disorders, and cardiac diseases.19,20 Hence, inducing autophagy can help in treating these diseases.21–23
Numerous studies have taken use of TFEB’s advantages in increasing cellular clearance in a number of cellular and murine models of human disorders associated with the buildup of un-degraded chemicals because of its crucial involvement in ALB.4,8,24–29 Lysosomal storage disorders (LSDs), metabolic illnesses,30 malignancies,31 and neurodegenerative diseases like Alzheimer’s disease and Parkinson’s disease are a few examples of human diseases brought on by dysregulation of TFEB activity. TFEB has gained interest as a potential therapeutic target for diseases because of its capacity to enhance disease state in animal models.32 The objective of this review is to discuss TFEB as a key molecular factor in human health and its implication in diseases.
Structure and molecular attributes of TFEB
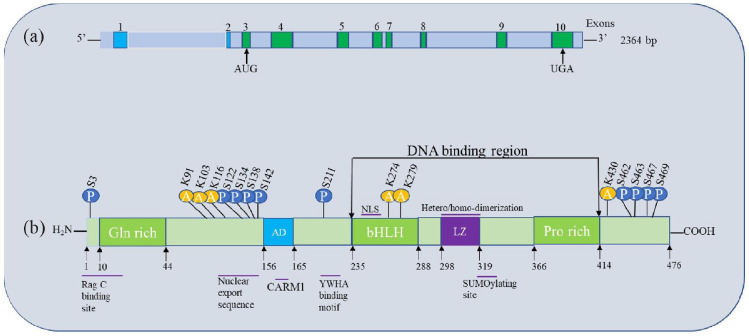
The human TFEB gene, which is found at chromosome 6 (6p21.1), generates a 2364-bp mRNA transcript that has eight coding exons and two non-coding exons. It also has a 302-bp 5′ untranslated region (UTR), a start codon at exon 3, and a stop codon at exon 10, as well as a 621-bp 3′ UTR. There are at least seven distinct mRNAs with alternate 5′ exons that have been identified, and their distribution in different tissues varies.33 (Figure 1).
Figure 1.
The TFEB (a) gene transcript, the numbers indicate exons, the two noncoding exons and the eight coding exons. In addition, the start codon AUG at exon 3 and the stop codon UGA at exon 10 are shown; (b) the protein is shown schematically, together with the pertinent domains (AD), bHLH region, LZ, and proline-rich domain (Pro-rich), regions, and amino acid residues that are undergoing posttranslational modifications. The numbers show the amino acid location in TFEB.
AD, activation domain; bHLH, basic helix–loop–helix; CARM1, co-activator-associated arginine methyltransferase 1; LZ, leucine zipper; NLS, nuclear localization signal; SUMO, small ubiquitin-like modifier; TFEB, transcription factor EB.
There are 476 amino acids in TFEB. The bHLH/ZIP structure, an acidic transcription activation domain, a glutamic acid-rich domain, a serine-rich domain, and other domains are among its constituents. A basic region upstream capable of recognizing the E-box sequence (CAYGTG) in the targeted gene promoter regions flanks the helix–loop–helix (HLH) and Zip domains that make up the DNA-binding region.
A palindromic consensus sequence known as the coordinated lysosomal expression and regulation motif (GTCACGTGAC; CLEAR), which overlaps the E-Box, was described to be a determinant of promoters of lysosomal gene controlled by TFEB.7–9 Numerous research have been conducted in response to this discovery to test the hypothesis that TFEB controls autophagy and lysosome processes and may be a potential drug target in lysosome storage illnesses.3,34 It must become homodimerized or form a heteromer with MiT/TFE genes to bind with DNA effectively.35–37 Homo- and heterodimers’ biological significance is yet unclear, though. The Zip domain is important for hetero-oligomerization or homodimerization with other MiT/TFE genes. In addition, TFEB interacts with the co-activator-associated arginine methyltransferase 1 (CARM1) methyltransferase domain, and CARM1 binds to the TFEB transcriptional activation domain to operate as a transcriptional co-activator38 (Figure 1).
Molecular processes that control TFEB activity and TFEB signaling pathways
Phosphorylation, acetylation, ubiquitination, and small ubiquitin-like modifier/SUMOylation are examples of post-translational modifications (PTMs) that can change the activity, subcellular distribution, and protein–protein interactions of a protein.
TFEB cytoplasmic-nuclear shuttling
Cytoplasmic TFEB retention
The TFEB phosphorylation and acetylation sites have been found to control TFEB cytoplasmic-nuclear shuttling and its transcriptional activity (Figures 1 and 2). Mounting evidence demonstrates that the subcellular localization of TFEB, which mostly depends on its phosphorylating state, regulates its activity.39 Under basal conditions, TFEB is localized in the cytoplasm resting on the lysosomal surface, and it is relocated from the cytoplasm to the nucleus in response to different stimuli and upstream signaling pathways. Food deprivation, physical activity,40 endoplasmic reticulum (ER) stress,41 mitochondrial damage,42 infections,43–45 and inflammation45 have been discovered to affect the nuclear localization of TFEB. PTMs and protein–protein interactions are the primary regulators of TFEB activation and cellular localization. Notably, phosphorylation strongly controls the subcellular location of TFEB.
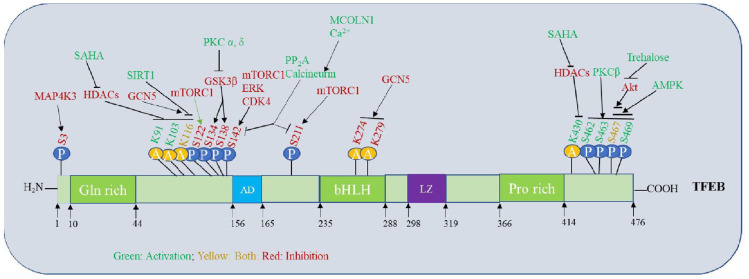
Figure 2.
Phosphorylation and acetylation sites in the TFEB domain structure and their regulatory role. Post-translational changes, such as phosphorylation and acetylation, tightly regulate the function of TFEB. Small compounds that affect TFEB or the kinases and phosphatases that come before TFEB as well as the phosphorylated serine sites on TFEB, its structural domains, and the kinases and phosphatases that control them are all indicated.
AD, activation domain; AMPK, adenosine monophosphate-activated protein kinase; bHLH, basic helix–loop–helix; CDK4, cyclin-dependent kinase 4; ERK, extracellular signal-regulated kinase; GCN5, general control non-repressed protein 5; GSK3β, glycogen synthase kinase 3 beta; MAP4K3, mitogen-activated protein kinase kinase kinase 3; mTORC1, mammalian target of rapamycin complex 1; PKC, protein kinase; SAHA, suberoylanilide hydroxamic acid; TFEB, transcription factor EB.
The mammalian target of rapamycin complex 1 (mTORC1) phosphorylates TFEB at S122, S142, and S211 to regulate its activity. The retention of TFEB in the cytoplasm is brought on by mTORC1-mediated phosphorylation of TFEB at S211, which binds to the cytosolic chaperone protein 14-3-3.29,46–48 Extracellular signal-regulated kinase 2 (ERK2) and mTORC1 both have a role in the phosphorylation of S142 TFEB8,47 (Figure 2).
Other kinases can also phosphorylate TFEB at other serine residues. These include glycogen synthase kinase 3 beta (GSK3β; S134 and S138), ERK2 (S142), AKT (S467), MAPK4 (S3), and protein kinase Cβ (PKCβ; S462, S463, S467, and S469). In addition, PKCβ increases the stability and activity of the TFEB protein by phosphorylating it49 (Figures 1 and 2).
Nuclear localization of TFEB by dephosphorylation
For TFEB activation and nuclear transport, the conversion between phosphorylation and dephosphorylation state is important. During nutrient deficiency, mTORC1 is deactivated and separates from the lysosome leading to increased unphosphorylated and active form of TFEB which then moves into the nucleus after separating from YWHA/14-3-3 to increase the transcription of the targeted genes.46
The stimulation and nuclear import of TFEB is similarly triggered by the inactivation of AKT, GSK3β, ERK2, and mitogen-activated protein kinase kinase kinase 3.50–52 In addition, through blocking the mTORC1 pathway, adenosine monophosphate-activated protein kinase (AMPK) stimulation encourages the activation of TFEB. Independent of mTORC1, AMPK can also promote the dephosphorylation and nuclear import of TFEB.53 However, it is not well understood how AMPK dephosphorylates and activates TFEB independent of mTORC1.53 The process will be inhibited by pharmacologically activating AMPK or inhibiting Folliculin (FLCN), which is AMPK’s negative regulator,54–56.
Protein phosphatase 2A dephosphorylates TFEB and stimulates its nuclear import.57 The dephosphorylation of TFEB is also hypothesized to be caused by a phosphatase calcineurin, which can be triggered by lysosomal Ca2+.15 Through mucolipin 1 (MCOLN1), a lysosomal Ca2+ channel, ER stress as well as reactive oxygen species (ROS) might encourage calcineurin’s actions on TFEB either directly or indirectly.58,59 In addition, a recent study found that proteasome inhibition considerably increased the level of TFEB dephosphorylation and nuclear translocation.60 This suggested a critical role for the ubiquitin proteasome pathway in TFEB activation, but the exact mechanism and discrepancy between different studies still need to be clarified.
TFEB re-phosphorylation and nuclear export
It has been made clear that TFEB’s intracellular location, namely its ability to shuttle between the cytoplasm and nucleus, is determined by whether or not it is activated. The intracellular stability may also be jeopardized by delayed or altered nuclear TFEB export. According to recent investigations, re-phosphorylation of nuclear TFEB is the primary mode causing its cytoplasmic re-localization following re-feeding, which causes phosphorylation of S142 and S138.61,62
The N-terminal region of TFEB contains a highly evolutionarily conserved sequence known as the nuclear export signal (NES), which is essential for the cytoplasmic re-localization of TFEB and is significantly compromised by NES mutation.62 TFEB nuclear export is also mediated by chromosomal maintenance 1, a significant export protein which makes it easier for proteins to cross the nuclear membrane and enter the cytoplasm.61,63 It is intriguing that the NES is close to both the S142 and the S138, suggesting that NES may have a function in the re-phosphorylation of TFEB at both locations. Notably, it has been found that blocking the nuclear export protein Exportin 1 (XPO1) can help TFEB localize to the nucleus while being unaffected by mTOR activity.63 In addition, it has also been found that XPO1 potently increases the nuclear enrichment of HLH-30, the TFEB ortholog in C. elegans.63 However, research into TFEB’s nuclear export is yet to uncover the precise mechanism and it is still in its infancy. Also, cyclin-dependent kinase 4 phosphorylates TFEB at S142, allowing its nuclear export and inhibition of transcriptional activity.64
Additional TFEB activity regulation
Other post-translational changes, in addition to phosphorylation, are involved in the control of TFEB. For instance, TFEB’s activity is influenced by the acetylation of its K91, K103, K116, and K430.65 In addition, general control non-repressed protein 5 (GCN5) increased acetylation of TFEB at K116, K274, and K279 and lowered TFEB’s transcriptional activity by preventing dimerization and its capacity to bind target gene promoter regions.66 Deacetylase Sirtuin-1 (SIRT1) has the ability to bind to and deacetylate TFEB at K116 in microglia, enhancing TFEB transcriptional activity.67 Suberoylanilide hydroxamic acid, an established histone deacetylase inhibitor, is interestingly able to increase TFEB acetylation at K91, K103, K116, and K430 to activate lysosomal activity in human cancer cells.68
The ubiquitination and subsequent destruction of phosphorylated TFEB will enable TFEB activation via the ubiquitin-proteasome pathway. To resynthesize new TFEB, cells degrade inactive TFEB. The accumulation of phosphorylated TFEB is inactive, and it further decreases TFEB activity because it forms heterodimers with unphosphorylated and active TFEB. This results in less TFEB translocation to the nucleus.69 However, small ubiquitin-like modifier (SUMOylation), a reversible post-translational modification, of TFEB at a lysine residue could result in a reduction in transcriptional activity.13,49,70 The expression of TFEB can also be self-regulated.49
In addition, methylation and glycosylation are common posttranslational modifications related to TFEB.38 A protein methyltransferase is also responsible for controlling TFEB activity. According to studies, when nutrients are few, TFEB and CARM1 combine, which raises the levels of histone H3 Arg17 dimethylation and autophagy. While TFEB interacts with the methyltransferase domain of CARM1, CARM1 binds to the transcriptional activation domain of TFEB to perform transcriptional co-activator action.13,38,71 The discovery that TFEB is a key upstream activator of certain glycoproteins suggests that pancreatic ductal adenocarcinoma (PDAC) may have higher amounts of N-glycosylation and altered lysosomal protein activity.72 The precise method by which TFEB controls glycosylation and how variations in the amount of glycosylation in certain glycoproteins impact TFEB activity, however, is yet unknown.
Furthermore, various transcription factors including peroxisome proliferator-activated receptor alpha (PPAR-α), peroxisome proliferator-activated receptor gamma coactivator-1 α (PGC-1α), cAMP response element-binding protein (CREB) and its co-activator, CREB-regulated transcription co-activator 2 (CRTC2), Kruppel-like factor 2, and Forkhead box O1 that controls TFEB at the transcriptional level have been discovered in particular cells under various circumstances.30,73,74
Finally, it has been discovered that histone deacetylase may bind to the TFEB promoter in human cell models to halt the expression of the gene.75 However, the findings are contradictory and may imply that the outcomes of these posttranslational alterations depend on the cellular context.76
TFEB signaling pathways
The mTORC1, the most widely studied signaling for mediating phosphorylation of TFEB, is attracted to the lysosomal membrane under nutrient-rich circumstances, such as amino stimulation. The lysosomal membrane allows amino acids to readily pass and accumulate inside the lysosomes. By way of the v-ATPase-Ragulator complex, the amino acids are recognized by the lumen of the lysosome and communicate with the Rag GTP: guanosine triphosphate (GTP)ases.77 Ras homolog that is bound to GTP and abundant in the brain (Rheb) activates mTORC1 at the lysosomal surface.77–79 Notably, active Rag GTPases attract TFEB to the lysosomal surface,80 where the mTORC1 complex phosphorylates it29,46–48 at a number of serine (S) residues, including S122, S142, and S211 (Figure 2).
ERK2 through MEK ½ is also another signaling pathway affecting TFEB.8,47 Recent research has shown that the chaperone-dependent E3 ubiquitin ligase STUB1 regulates TFEB activity by choosing phosphorylated TFEB (S142 and S211) for ubiquitin-mediated proteasomal destruction.69 This implies that TFEB’s phosphorylation state affects both its intracellular location and stability. In addition, PGC1α, a crucial controller of mitochondrial biogenesis and, lipid metabolism is transcriptionally activated by TFEB. PGC1α is a co-activator of the nuclear receptor PPAR α whose expression is also activated by TFEB30,69 (Figure 3). AKT51 and GSK3β also phosphorylate TFEB, causing cytoplasmic retention.81
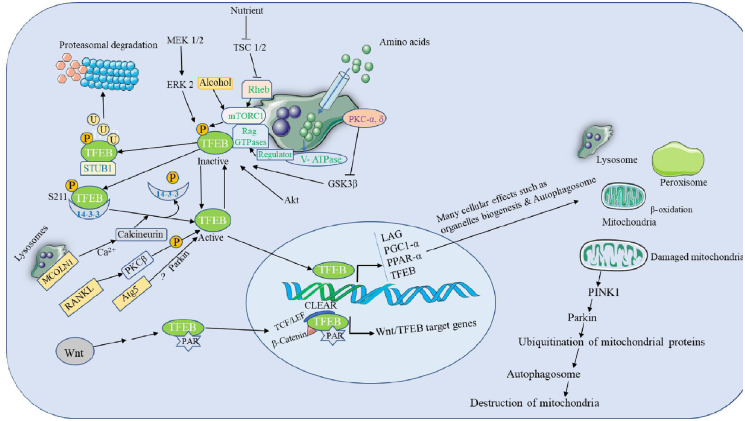
Figure 3.
The upstream regulators of TFEB and its downstream signaling pathways. For example, mTORC1 is attracted to the lysosomal membrane under nutrient-rich circumstances, such as amino stimulation. The lysosomal membrane allows amino acids to readily pass and accumulate inside the lysosomes. This signals the TFEB’s deactivation. However, when nutrients are few, TFEB becomes unphosphorylated and active. The expression of target genes, including as those involved in ALB, is subsequently increased as a result of active TFEB’s subsequent nuclear translocation, CLEAR motif binding, and activation of gene expression.
Akt, AKT serine/threonine kinase; ALB, autophagy–lysosomal biogenesis; CLEAR, coordinating lysosomal expression and regulation; ERK, extracellular signal-regulated kinase; LAG, lysosomal–autophagic genes; mTORC1, mammalian target of rapamycin complex 1; PAR, poly ADP-ribosyl; PGC1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; PKC, PKC, protein kinase; PPAR-α, peroxisome proliferator-activated receptor alpha; RANKL, receptor activator of nuclear factor-κB ligand; TCF/LEF, T-cell factor/lymphoid enhancer factor; TSC, tuberous sclerosis complex; p, phosphate; TFEB, transcription factor EB.
More specifically, in the nucleus accumbens and orbitofrontal cortex of mice and rats, excessive alcohol consumption activates mTORC1.82,83 Also, by acting as a GTPase-activating protein for Rheb, TSC1/2 reduces Rheb activity. Rheb may bind to mTORC1 directly and promote its activity.84,85
As a result of phosphatase and tensin (PETN)-induced kinase 1 (PINK1)’s attraction to the outer mitochondrial membrane (OMM) and auto-phosphorylation, the OMM is activated when mitochondria are damaged and their membrane potential is depleted (Figure 3). PINK1 initiates mitophagy by phosphorylating the ubiquitin attached to the unstructured and broken OMM proteins.86 In addition, Parkin, an E3 ligase, is phosphorylated by PINK1 and activated as a result. The signal is amplified even more by activated Parkin, which further ubiquitinates misfolded OMM proteins. In addition, phosphorylated ubiquitin engages autophagy receptors to cause the creation of autophagosomes (APs), which are used to degrade damaged mitochondria. Nuclear TFEB transcriptional activity is crucial for the induction of genes involved in autophagy biogenesis throughout this process. Whereas TFEB nuclear localization is dependent on Parkin, ATG5 activates TFEB through an unidentified mechanism.42 The expression of PGC1, an important regulator of mitochondrial biogenesis, is likewise induced by TFEB.
Recently, it was discovered that Yes-associated protein (YAP)/TFEB signaling is in charge of the development of cardiomyopathy and autophagic cell death in LSDs.87 In a mouse model of LSD with cardiomyopathy, the oncogene YAP physically interrelates with TFEB to generate high AP buildup. Rag A/B conditional knockout mice develop nuclear YAP buildup in their hearts. Nuclear endogenous YAP physically interacts with TFEB to promote the transcription of TFEB target genes, which are involved in the synthesis of APs. Due to the buildup of this undigested APs, patients with LSDs develop hypertrophic cardiomyopathy.87
The receptor activator of nuclear factor-κB ligand enhances one of the various PKC isoforms, PKCβ, which then phosphorylates several serine residues (S462, S463, S467, and S469) in the carboxy terminal region of TFEB, increasing TFEB stability and nuclear localization. This causes osteoclasts to secrete lysosomal hydrolases and induce lysosomal biogenesis.88,89 The PKC isoforms PKC-α and PKC-δ, in turn, cause TFEB to become activated and go into the nucleus by blocking GSK3’s phosphorylation of TFEB81 (Figure 3).
MCOLN1 channel protein allows calcium to be released from lysosomes under starving circumstances.90,91 TFEB is dephosphorylated as a result of calcium ions activated phosphatase, calcineurin. Dephosphorylated TFEB reaches the nucleus to trigger ALB after separating from the 14-3-3 chaperone proteins.16 The post-translational change known as poly-ADP-ribosylation (PARsylation) which is catalyzed by Tankyrase 1, a poly (ADP-ribose) polymerase (PARP), occurs on TFEB when Wnt is activated.92 ADP-ribosylation is a reversible change that happens when an ADP-ribose molecule is transferred from NAD+ to certain residues of proteins including lysine, arginine, glutamate, aspartate, cysteine, phosphoserine, and asparagine.93 Together with β-catenin- T-cell factor/lymphoid enhancer factor 1, the PARsylated TFEB imports to the nucleus forming a transcription factor trimeric complex. The TFEB mediated Wnt target genes such as those for mediating ion transport are a subset of the canonical β-catenin target genes that are regulated by this complex.94 This shows that the transcription of autophagy and lysosomal genes is not the exclusive function of TFEB activity. The nuclear localization of TFEB induced by starvation is different from the nuclear TFEB mediated by Wnt signaling.95
FLCN, which is a recognized tumor suppressor gene for Birt–Hogg–Dubé (BHD) syndrome, regulates TFEB activity.96,97 The skin, kidneys, and lungs are all impacted by BHD syndrome, which was initially linked to the FLCN gene.97 Bilateral multifocal renal tumors, lung cysts, fibrofolliculomas, and spontaneous pneumothorax are all brought on by BHD.98 Folliculin interacting protein (FNIP)1/2 and FLCN often combine to form a complex, and this FLCN/FNIP complex functions as a GTPase-activating protein (GAP) for the small GTPases Rag A, Rag B, Rag C, and Rag D.79 Rag A/B and Rag C/D are two pairs that make up the RagGTPase complex. Rag A/B binds to guanosine diphosphate (GDP) and Rag C/D binds to GTP when they are both inactive. But when Rags are activated, such as when they are stimulated by growth factors or amino acids, the FLCN/FNIP complex activates its GAP activity toward Rag C/D, which causes Rag C/D to bind to GDP and Rag A/B to bind to GTP, activating Rags.79 FLCN is necessary for mTORC1 to be attracted to the lysosomal membrane and for the Rheb protein to then activate it. Then, TFEB is phosphorylated and rendered inactive by active mTORC1, which prevents it from localizing to the nucleus.99–102
Drugs, pathogens, crystals, and silicas are just a few examples of exogenous and endogenous substances that can cause lysosomal membrane permeabilization (LMP) or rupture. A crucial mediator for lysosomal homeostasis following lysosomal injury has recently been identified as TFEB.103 The activation of TFEB, which is brought on by lysosomal damage, requires light chain 3 (LC3) lipidation, which is mediated by the ATG conjugation system. LC3 is drawn to the lysosomal surface and goes through lipidation after lysosomal injury. Lipidated LC3 interacts with the calcium channel transient receptor potential mucolipin 1, causing a significant amount of calcium efflux that is necessary for TFEB activation. In addition, cells missing LC3 lipidation factors (LC3A, LC3B, LC3C, and GABARAP) and ATG3, ATG7, and ATG5 exhibit TFEB inactivation upon lysosomal injury.103 To stop the development of lysosomal damage, lipidated LC3 must activate TFEB.
As a crucial PTM, acetylation affects the enzymatic activity, solubility, stability, and subcellular localization of proteins.104 E1A binding protein p300, which is expressed by the EP300 gene, is one example of a lysine acetyltransferase that acetylates proteins at the lysine residues’ ε-amino group.105 Although p300 is primarily found in the nucleus, intracellular signals may cause it to migrate to the cytoplasm. Many ATG proteins that have numerous functions in controlling autophagy appear to be acetylated by p300. While p300 overexpression inhibits autophagy, p300 knockdown can promote it.105 P300 is activated as a result of an interaction between active mTORC1 and p300, which phosphorylates it. As a result, lipogenesis is encouraged, whereas starvation-induced autophagy is suppressed.106 Interesting evidence suggests that the mTOR pathway modifies p300 activity to regulate TFEB subcellular localization since p300 increases TFEB acetylation.107–110
The function of TFEB
By increasing the synthesis of several organelles and autophagic activities, activated TFEB that transport to the nucleus and activate the transcription of different genes contributes to maintaining cellular structure and function.
Role on the function and biogenesis of organelles
Lysosome can serve as a hub for signals that control the primary regulatory system for nutrient sensing.111 In addition to controlling lysosomal biosynthesis, studies have shown that TFEB also promotes the expression of several genes controlling lysosomal-related activities such phagocytosis, autophagy, and exocytosis.7,112 TFEB controls nearly all of the receptors involved in lysosome biogenesis at the transcriptional level.113 It was discovered that the fusion of lysosome with the plasma membrane, and exocytosis are facilitated by TFEB by inducing MCOLN1 to become activated in a Ca2+-dependent manner. Furthermore, it has been suggested that TFEB can control where lysosomes are situated by increasing transmembrane protein 55b (TMEM55B) expression.15,114
Numerous disorders have been linked to mitochondrial malfunction, and treating these conditions remains difficult. Recent research reveals that TFEB is crucial for maintaining the morphology, function, and number of mitochondria—the quality control of mitochondria.115 This is essentially accomplished by triggering mitophagy, controlling mitochondrial biogenesis, and clearing ROS. By stimulating the expression of PGC1α, the main factor controlling mitochondrial biogenesis, TFEB functions in mitochondrial biosynthesis.116,117 In mechanisms unrelated to PGC1α, TFEB mediates the increasing expression of several mitochondrial proteins. Nuclear respiratory factor (NRF)1 and NRF2 can activate mitochondrial transcription factor A (TFAM), which is essential for directly controlling mtDNA transcription.118
In the skeletal muscle of PGC1α knockout mice, overexpression of TFEB was found to simultaneously upregulate the expression of NRF1, NRF2, and TFAM as well as mitochondrial density and volume.40 In addition, by decreasing mitochondrial division, TFEB promotes mitochondrial stability in mitochondrial dynamics.119 Functionally, TFEB activation enhances mitochondrial substrate use, which is beneficial for respiratory function and speeds up the synthesis of ATP, whereas TFEB inhibition may result in the buildup of defective mitochondria.40,58
Recently, it was shown that TFEB is capable of regulating peroxisome synthesis at the transcriptional level, as demonstrated by noticeably lower amounts of peroxisome when TFEB is inhibited.117 In addition, it was claimed that mTORC1 suppression reduced the aberrant peroxisome biogenesis, which may have been partially reversed by TFEB inhibition. The synthesis of APs and the subsequent fusion with lysosomes were also increased by TFEB.47
Role in lysosomal dynamics
TFEB is required for the completion of all types of autophagy, including macro-autophagy, micro-autophagy, and chaperone-mediated autophagy, by regulating the activity and number of lysosomes. To encourage autophagy, TFEB also upregulates autophagy genes. For cells to adapt to nutrient deprivation and stress, autophagy and lysosomal activity must be improved.8,10 In addition, the serine-threonine kinase RIP1, a controller of cell survival and cell death, adversely controls autophagic flux through TFEB to lessen sensitivity to cell death.120 Lysosomal exocytosis is also transcriptionally controlled by TFEB.15 Lysosome trafficking and fusion to the plasma membrane are enhanced by TFEB overexpression. TFEB activates MCOLN1, one of its target genes, to cause Ca2+ elevation. The number of lysosomes in the area of the plasma membrane rises when MCOLN1 is activated.15
The TFEB/TMEM55B/JIP4 pathway, which coordinates lysosome movement in response to stress circumstances, has recently been discovered to mediate the possibility that lysosomal location may affect lysosomal functioning.114 Briefly stated, in response to food deprivation, TFEB dynamically modifies the location of the lysosomes by controlling the expression of TMEM55B, which, in turn, attracts JIP4 to the lysosomes.114 Lysosomes disperse toward the cell periphery when JIP4 or TMEM55B are depleted, but TMEM55B overexpression causes lysosomes to collapse into the cell center. Following TFEB activation by starvation or cholesterol-induced lysosomal stress, TMEM55B levels are transcriptionally increased. When a cell is starved, APs and lysosomes travel toward the cell’s center, which promotes their fusion and the breakdown of the AP contents. Depletion of TMEM55B or JIP4 inhibits AP–lysosome fusion and reverses the effects of starvation on retrograde lysosomal transport.121,122
TFEB in autophagy-dependent cell death
Autophagy-dependent cell death (ADCD) is the name given to cell death caused by elements of the autophagy system.123–125 By inducing ADCD in melanoma and multiple myeloma proliferating cells, TFEB activation has anticancer effects.126,127 Depletion of NAD+ intracellular reserves enhances ADCD in various myeloma cells via enhancing nuclear import of TFEB and expression of genes related to autophagy.126 Dendrogenin A, a mammalian metabolite, interacts to the transcription factor NR1H2 to activate ADCD and stimulate the production of TFEB, which has anticancer effects in mice.127
Signal transducer and activator of transcription 3 (STAT3) interaction with nuclear TFEB causes a rise in lysosome-dependent cell death (LDCD) in breast cancer, an anticancer effect.128 The lysosomal enzymes are released by L-leucyl-L-leucine methyl ester (LLME), resulting in LMP and then LDCD. TFEB targets the genes BHLHE40/41. Particularly in LLME-induced LDCD, BHLHE40/41 sensitizes the cells while TFEB protects the cells.129 As a result of the DNA damage response suppression of mTORC1, TFEB/TFE3 activation occurs as an initial reaction. Upon sustained DNA damage, TFE3/TFEB activates genes linked to LMP and apoptosis.130 In addition, it has also been found that the interaction between YAP and TFEB in the nucleus, which resulted in cardiomyopathy in RagA/B KO mice, enhances ADCD.87
TFEB in lysophagy
Usually, LLME is utilized to cause lysosomal injury, which causes Galectin-3 puncta to accumulate on the damaged lysosomes. Cytosolic lectins like Galectin-3 play a crucial role in the onset of the autophagy response because they identify the galactoside present on the luminal surface of organelles and draw in autophagic receptors like TRIM16.131 TRIM16, a protein with a tripartite domain, interacts with ATG16L1, Beclin-1, and ULK1 to control autophagy. In Galectin-3 KO cells that had undergone lysosomal injury, there was an improvement in TFEB nuclear entry. According to several studies, lysosomal membrane degradation stimulates TFEB nuclear translocation and encourages lysosomal biogenesis.131,132 Lysosome replenishment is facilitated by TRIM16’s role in mTOR suppression and its effects on TFEB during autophagy.131
TFEB activation in response to lysosomal damage is reportedly dependent on lipidated LC3, according to a recent study by Nakamura and colleagues.103,133 Calcium leaks from lysosomes in mammalian cells as a result of lysosomal injury. The calcium that has been released localizes to lysosomes and causes LC3 lipidation (LC-3II). The subsequent association of the MCOLN1 with lipidated LC3 speeds up the release of large amounts of calcium into the cytoplasm which is required for TFEB activation.103
The role of TFEB that is independent autophagy–lysosomal system
Although many studies concentrated on the effects of TFEB that were dependent on autophagy in various cells and tissues, new evidence suggests that TFEB affects a wide range of other genes and signaling pathways in addition to autophagy. By blocking activating transcription factor 4 and CCAAT/enhancer-binding protein homologous protein, TFEB promotes cell differentiation in osteoblasts.134 By controlling the transcription of the muscle-abundant E3 ubiquitin ligase, muscle RING finger-1 (MuRF1), TFEB promotes Ang II-induced skeletal muscle atrophy.135 In addition, in the tumor microenvironment, TFEB controls macrophage polarization. Through decreasing suppressor of cytokine signaling 3 (SOCS3) and stimulating STAT3, TFEB downregulation in tumor-associated macrophages increases M2 polarization.136 Conversely, upregulation of TFEB induces SOCS3 during infection, and SOCS3 then prevents STAT3 from acting on M2 macrophage polarization.137
While the antioxidant genes heme oxygenase-1 and superoxide dismutase 2 (SOD2) are upregulated by TFEB in vascular endothelial cells (ECs), it suppresses the nuclear factor kappa B pathway, thereby inhibiting vascular inflammation.73,138 In addition, TFEB interacts to the promoter of B-cell lymphoma 2, a strong anti-apoptotic gene, in vascular smooth muscle cells (VSMCs). When considered together, TFEB not only improves lysosome- and autophagy-mediated cellular clearance, but it also controls a variety of cellular activities that are heavily dependent on the transcriptional regulation of targeted genes.139
Notably, TFEB performs a unique, lysosome-independent role during DNA damage in triple-negative breast cancer (TNBC), a kind of breast cancer that lacks three commonly found breast cancer receptors (estrogen, progesterone, and human epidermal growth factor receptors).140 TNBC cell viability is decreased and caspase-3-dependent apoptosis is promoted by TFEB reduction. When lysosomal inhibitors are present, however, TFEB continues to be able to prevent apoptosis in TNBC cells.140 Similar to how TFEB activation is unrelated to high baseline levels of autophagy in pancreatic cancer cells, TFEB knockdown has no effect on autophagic flux under normal circumstances.141
The PARsylation of TFEB is brought on by the activation of Wnt signaling. However, TFEB-mediated lysosomal target genes are not activated by PARsylated TFEB.94 In addition, independent of ALB, TFEB controls the network of transcription for pluripotency in mouse embryonic stem cells (mESCs). The expression of TFEB lysosome–autophagy targeted genes was not affected by changes in TFEB levels in mESCs.142
In a different study, TFEB was shown to enhance the development of murine liver stem/progenitor cells into the progenitor/cholangiocyte lineage while appearing to restrict hepatocyte differentiation in a way that was independent of autophagy and lysosomes. Sox9, a precursor and biliary cell marker, is directly activated by TFEB.142 In addition to controlling the fate of liver cell, TFEB also offers non-autophagy-dependent defense against obesity and insulin resistance. Particularly, TFEB activation of growth differentiation factor 15 expression is essential for controlling metabolic disorders brought on by obesity.143
Function of TFEB specific to tissues
TFEB role in lipid metabolism of the liver
The lipid droplets that have collected in the cytoplasm are transported to the lysosome during the specific process of autophagy called lipophagy, where they are broken down by acid lipase to produce free cholesterol and free fatty acids.144 Cellular lipid homeostasis requires the redistribution and recycling of intracytoplasmic lipid droplets by lipophagy.
Induction of free fatty acid oxidation-related genes in the mitochondria and peroxisomes occurs as a result of TFEB-mediated activation of the PGC1α-PPAR-α program. Enzymes of the mitochondria and peroxisomes break down the free fatty acids produced from the lysosome to produce ATP.30 High-fat diet (HFD)-TFEB-injected mice were noticeably thinner than controls when given a conventional chow diet. The lowered lipid content seen as a normal red color and normal weight of the livers in HFD-fed TFEB-injected mice as compared to the wild type controls suggested that TFEB overexpression reduced the harmful effects of the HFD by promoting lipid breakdown.30 Notably, malnutrition causes TFEB (HLH-30) to take the position of the repressor Max-like 3 and boost the production of lysosomal lipase.145
In both human and mouse hepatocytes, TFEB is concentrated in the promoter region of the CYP7A1 gene, which codes for cholesterol 7-hydroxylase. Thus, in a Western diet mice fed, TFEB causes the production of hepatic bile acids, which prevents the buildup of cholesterol and metabolic problems.146 In addition, acid lipase in the lysosome and fatty acid oxidation mitochondria were promoted as a result of TFEB activation in diet-induced atherogenesis mimicked using APOE KO mice with myeloid cell-specific sorting nexin 10 impairment.147 Lee et al. discovered that organelle stress also induces TFEB. Adipose tissue macrophages (ATMs) activation of TFEB, a sign of lysosomal stress brought on by obesity, was induced by inadequate buildup of the lysosomal degradation fragment lipofuscin in ATMs of humans or obese mice.143
Recent research has shown that the fasting-induced hormone Fgf21-TFEB axis orchestrates lipid metabolism during fasting by integrating extracellular hormonal signaling and lysosome homeostasis.148 By preventing TFEB nuclear shuttling, Fgf21 depletion impairs hepatic lysosomal activity and increases lipid buildup in the liver tissue. As a result, TFEB target genes that are involved in lipid metabolism, such as lipid oxidation and intracellular lipid breakdown, are suppressed.148
TFEB in skeletal muscle function
Contraction-mediated adaptive responses are known to be coordinated by the AMPK-PGC1 pathway; nevertheless, TFEB has recently come to light as a novel metabolic coordinator.149 A study demonstrated that TFEB is a critical modulator of muscle metabolic adaptability during exercise by using TFEB muscle-specific loss-of-function and gain of-function techniques.40 In the nucleus during exercise, TFEB translocates and boosts the expression of genes involved in mitochondrial homeostasis and glucose oxidative metabolism, both of which are essential for the health and endurance of muscles. It is important to evaluate if individuals with metabolic problems have lower TFEB levels because of its role in metabolic flexibility.
The discovery of the FGF/TFEB/FAM134B signaling is the first research to link TFEB to ER-phagy and its physiological significance during in vivo skeletal development.150 Lysosomal genes and FAM134B, an ER-phagy receptor and recently discovered target gene of TFEB, are both enhanced by nuclear TFEB.
Inflammatory and immune reactions
By controlling both innate and adaptive immunity, TFEB affects how the host responds.54,151 It is recognized that TFEB takes part in the activation of macrophages through a number of different ways, including enhancing bactericidal activity and encouraging the synthesis of numerous proinflammatory mediators.136,152 It has been shown that TFEB mediates xenophagy, a kind of selective autophagy that targets microorganisms that have infiltrated a cell.65 For instance, sustained activation of nuclear TFEB caused functioning lysosomes to engage in scavenging activity, which further inhibited Salmonella reproduction by concentrating on Salmonella-containing vacuoles.153
In addition, TFEB was necessary for dendritic cells to function when they came into contact with certain foreign antigens. Major histocompatibility complex (MHC) class I’s ability to deliver antigens is known to be inhibited by TFEB activation, whereas MHC class II production is upregulated.154,155 It is most likely that TFEB collaborates with TFE3 to counteract CD40 activation on the surface of T cells, which plays a crucial role in T-cell-dependent antibiotic action. Additionally, TFEB promotes the lysosomal breakdown of anti-inflammatory cytokines, acting as a proinflammatory mediator.155
Ca2+ signaling activates TFEB, causing lysosomal endocytosis and further promoting IL-1β production in human monocytes.156 In addition, it works with TFE3 to activate macrophages and microglia and cause the gene transcription of proinflammatory cytokines. The production of important inflammatory mediators, such as CSF2, IL1, IL2, and IL27, was considerably reduced in TFEB and TFE3 defective cells, indicating the significance of TFEB in controlling inflammatory response.45 In addition, TFEB can activate PPAR-α and PGC1α to stimulate mitochondrial biogenesis and hence indirectly lower ROS and inflammatory levels.157
The regulation of energy metabolism, including that of fat, amino acids, and glucose, has also been linked to TFEB.158 By controlling lipid transport and lipophagy, TFEB contributes to the metabolism of lipids. However, TFEB overexpression disrupts the expression of several genes involved in fatty acid binding, transport, and oxidation. Furthermore, transcriptome analysis of TFEB-depleted mouse cardiomyocytes showed that TFEB controls a gene network involved in carbohydrate and lipid metabolism apart from macroautophagy.56,159 When there is an imbalance in the energy system, TFEB also reacts. Exercise’s higher energy demands can cause TFEB expression and activation, which can enhance energy metabolism by promoting mitochondrial biogenesis and mitophagy.56 The cellular response to amino acid signal is primarily via RAGs, heterodimers formed by the combination of RagA/B and RagC/D. RagC mutant cardiomyopathy can only be treated by overexpressing TFEB, not by inhibiting mTOR, as it has been shown that faulty mTOR-TFEB signaling contributes to its development. Although it needs further research to confirm, TFEB is purported to regulate RagC and affect amino acid metabolism.160 Regarding glucose metabolism, TFEB has the ability to regulate the expression of glycolytic enzymes, glucose transporters, and pathways that regulate glucose homeostasis.40 Also, TFEB can boost insulin receptor substrates 1 and 2 (IRS1 and IRS2) and activate AKT to promote glucose absorption.161
TFEB and cardiovascular homeostasis
It has been suggested that TFEB is essential for preserving vascular and cardiac homeostasis and has positive effects on cardiovascular diseases, making it a viable molecular target for the prevention and treatment of these conditions.10,76
Dynamic integrin-mediated EC adhesion to the surrounding extracellular matrix (ECM) is crucial for angiogenesis in both healthy and pathological circumstances, such as the growth of an embryo and the progression of cancer.162 The control of integrin trafficking and conformational activation is essential for maintaining the dynamics of EC-to-ECM adhesions. According to a research, TFEB regulates a transcriptional program that favors ECM adhesion turnover by encouraging the transcription of genes that produce cholesterol, which, in turn, increases caveolin-1 aggregation and integrin 1 endocytosis that is caveolin dependent.162
TFEB shows pro-angiogenic effects, anti-atherosclerotic, and anti-inflammatory in ECs.73,138,163 TFEB increases insulin transport across ECs and increases uptake of glucose in ECs. In ECs, It stimulates the AKT serine/threonine kinase (Akt) signaling pathway and upregulates IRS1 and IRS2.161 By activating AMPK signaling and enhancement of autophagy, endothelial TFEB enhances postischemic angiogenesis.164 Although the functions of TFEB in VSMC biology and vascular disorders have not yet been thoroughly investigated, it has been discovered that TFEB affects vascular smooth muscle migration, proliferation, and apoptosis.165 Also, growing body of research demonstrates that TFEB improves cardiomyocyte survival in diseased states.119
TFEB in human diseases
As was previously mentioned, TFEB is essential for preserving the constancy of cellular function and structure. Many human illnesses, including LSDs, neurodegenerative diseases, inflammatory diseases, and cancers, appear to be linked to dysregulated TFEB activity, indicating that TFEB modulation is a potential therapeutic target.49,166
TFEB in LSDs
LSDs are often brought on by mutations in the genes that produce the enzymes necessary for the breakdown of macromolecules and other functions of lysosomal homeostasis.34 TFEB is linked to LSDs such as multiple sulfatase deficiency (MSD), Pompe disease, and Batten disease.
Sulfatase-modifying factor-1 gene (SUMF1) mutations are the root cause of MSD.167,168 Sulfatases must have their catalytic site modified post-translationally by SUMF1 in order for them to be activated. The concurrent functional loss of 17 sulfatases, the majority of which are lysosomal, is caused by a deficiency of active SUMF1.169 The fundamental characteristic of MSD is the buildup of sulfated GAGs, which ultimately lead to neurodegeneration in people. In adult MSD mice, systemic adenovirus-mediated overexpression of TFEB reduced GAGs in skeletal muscles and the liver and caused inflammation of tissues and cell death.15 Lysosomal exocytosis contributes to the GAG clearance brought on by TFEB.15
Lack of the lysosomal enzyme acid α-glucosidase (GAA; acid maltase), which converts glycogen to glucose in acidic lysosomes, results in Pompe disease. The almost total absence of GAA activity causes lysosome enlargement, glycogen accumulation, and the appearance of abnormal autophagic waste in skeletal muscle fibers. Muscle weakness, trouble swallowing, respiratory infections, and hypertrophic cardiomyopathy are among the disease’s symptoms.170 In an GAA KO mouse model (GFP-LC3:GAA/), the pathophysiology of muscle injury in Pompe disease has been investigated.24 In Pompe muscle, TFEB was overexpressed using an adenovirus, which decreased autophagic accumulation and removed the glycogen burden.24 In addition, TFEB treatment increased the number of lysosomes, and lysosomal exocytosis mostly mediated the effect on glycogen clearance.
A series of 13 deadly diseases known as hereditary neurodegenerative disorders with genetically unique origins make up Batten disease. Each of the 13 illnesses is denoted by the abbreviation CLN followed by a numeral. Patients with Batten disease exhibit neuronal accumulation of auto-fluorescent ceroid lipo-pigments at the cellular level.171,172 CLN3’s biochemical role is yet unclear, though. Cln3Δex7/8 mice, a well-known model of Batten disease, were given oral trehalose to increase endogenous TFEB levels, and this treatment was effective.51 The electron-dense material indicative of Batten disease in cortical neurons was likewise removed by trehalose therapy. Trehalose inhibited Akt biochemically, increasing TFEB nuclear translocation without the assistance of mTORC1. The mechanism by which TFEB activation reduces ceroid lipo-pigment storage in lysosomes in CLN3 KO neurons and the molecular connection between CLN3 and the buildup of ceroid lipo-pigment, however, require more investigation.
TFEB in neurodegenerative diseases
The buildup of abnormal proteins is one of the important pathological characteristics of neurodegenerative diseases, including amyloid β (Aβ) and tau of AD, α-synuclein of Parkinson’s disease, huntingtin (HTT) of Huntington’s disease, polyglutamine-expanded androgen receptor (polyQ-AR) of X-linked spinal and bulbar muscular atrophy (SBMA), and mutant SOD of amyotrophic lateral sclerosis (ALS). According to reports, the activity of TFEB is intimately linked to the disruption of autophagy–lysosomal function, which is the main cause of these aberrant aggregates.49
Extracellular Aβ plaques and cellular Tau tangles are the pathological features of AD, an age-related neurodegenerative disease.173,174 Exogenous TFEB expression was widely seen in astrocytes in the mouse model of AD with tau spreading, which was capable of trapping and degrading tau, hence significantly reducing tau-associated degenerative changes.67 Exogenous TFEB supplementation improved the degenerative alterations in a mouse model of AD,175 which was accomplished by enhancing lysosomal exocytosis and triggering autophagy to reduce tau.176 TFEB efficiently decreases neurofibrillary tangle pathology and restores behavioral and synaptic impairments as well as neurodegeneration in the rTg4510 animal model of tauopathy. Tau species that are present in both soluble and aggregated fractions are preferentially targeted by TFEB, whereas regular Tau is left unaffected. By increasing lysosomal activity and deacetylating TFEB in microglia, the Aβ was broken down.177 It is also found that CLEAR elements in the promoters of crucial autophagy–lysosomal pathway (ALP) genes, such as MAP1LC3B, SQSTM1, and lysosomal-associated membrane protein 2, were apparently competed for binding by apolipoprotein E4 (APOE4) with TFEB. A significant genetic risk factor for sporadic AD is the APOE 4 allele.178
Also, results from a thorough investigation of the autophagic process in the human hippocampus from the early to late stages of AD revealed that autophagy is increased throughout the AD disease. Notably, glia showed stronger TFEB activity than neurons.179 But several studies have also discovered decreased TFEB activity in AD. TFEB mRNA is a target of the microRNA (miR)-128, which lowers its expression.9 MiR-128 is expressed more often in the hippocampi of AD patients,180 which reduces TFEB expression and Aβ clearance.180 Results demonstrating that PSEN deficiency suppresses the activation of TFEB showed a distinct link between TFEB function and AD pathogenesis. In line with this, Aβ and p-Tau accumulated in the mouse brain after neuron-specific TFEB ablation.181 Another study found that overexpressing TFEB significantly decreased the amounts of paired-helical filament Tau in the P301S tauopathy model mice.182
The fact that levels of nuclear TFEB were dramatically decreased in the midbrain of Parkinson’s disease (PD) patients provides compelling evidence for the involvement of TFEB in the pathophysiology of the disease.28,174,183 Lewy bodies accumulate in PD, which is brought on by the death of dopaminergic neurons in the substantia nigra.184 According to reports, PD-associated molecules including PINK1, Parkin, and α-synuclein (A53T and A30P mutants) control the autophagy process.184 Excessive synthesis of α-synuclein in the nigral dopaminergic neurons in a model of α-synuclein toxicity was strongly associated with the reduction in lysosomal function brought on by cytoplasmic retention of TFEB.28 Also, α-synuclein-induced neurodegeneration and subsequent disease progression were prevented by activation of TFEB activity via suppression of mTORC1.28 Dopaminergic neurons in PD patients’ postmortem brain samples had an accumulation of APs, which was followed by autophagic cell death.185 In PD patients, TFEB is not present in the dopaminergic neurons’ nuclei.28 Through genetically induced TFEB overexpression, lysosomal depletion is prevented, and caused cell death is prevented.
The cytosol retaining of TFEB caused by phosphorylation of TFEB on S211 strengthens its interaction with YWHA/14-3-3 proteins in atypical patterns.46 In addition, Decressac et al.28 demonstrated that in midbrain tissue overexpressing α-synuclein, both 14-3-3 and α-synuclein interacted with TFEB. Overexpression of α-synuclein causes TFEB to remain in the cytoplasm, preventing the ALP and, consequently, its own clearance. Importantly, parkin interacting substrate (PARIS)-mediated inhibition of PGC1 also lowers the expression of TFEB, suggesting that TFEB and PGC1 are linked in a positive transcriptional feedback loop.186
The HTT gene’s abnormally expanded cytosine–adenine–guanine (CAG) repeats are the primary cause of HD, an autosomal-dominant neurological disease.187 In vitro HD models have proven the therapeutic benefits of TFEB. In the rat HD43 striatal cell model, it has been demonstrated that TFEB overexpression decreases the accumulation of polyglutamine (polyQ)-expanded HTT.9 PGC1α is a regulator of TFEB in the clearance of mutant HTT aggregates, according to follow-up research.186 In addition, the amount of TFEB and the expression of its target gene were significantly reduced in the mouse model of Huntington disease. Restoring PGC1α is enough to activate TFEB and minimize HTT aggregation and neurotoxicity.186 The production of TFEB was stimulated by increased PGC1α, which then cleared the mutant HTT protein clumps and brought back mitochondrial function.186 Exogenous TFEB expression also increased lysosome activity, autophagy, and the effective clearance of mutant HTT protein aggregates in the striatum of zQ175 HD-mutant mice.188 It is also intriguing that mHTT levels were decreased by 21–35% in membrane fractions but not WT HTT levels by TFEB-HA expression. Since autophagy frequently targets proteins that are prone to aggregate, like mHTT, TFEB expression may selectively accelerate autophagy-dependent destruction of mHTT relative to WT HTT.188
Trehalose can also activate TFEB by suppressing AKT activity, which improves protein clearance and lessens neuropathologic symptoms.189 Conversely, silencing TFEB dramatically impairs the pro-degradation activity of trehalose.189 Because of the aberrant buildup of polyQ-AR, which causes the deterioration of the lower motor neurons in the brain stem and the spinal cord, SBMA is characterized by proximal muscular weakness.55 Normal AR shows TFEB activation, but polyQ-AR interferes with it, further compromising autophagy and accelerating the pathogenesis of SBMA.190 TFEB overexpression through nuclear factor-YA upregulation significantly increased the removal of pathogenic AR protein in muscles and motor neurons of SBMA animal model, hence minimizing the pathological and behavioral deficits.191 The motor neuron condition ALS is characterized by an aberrant buildup of mutant SOD1.55 In a research with ALS patients brain samples, distinct phases of ALS transgenic mice’s spinal cords and an NSC-34 cell culture with the SOD1-G93A mutation showed changed expression of TFEB and Beclin-1. Beclin-1 mRNA and protein levels rose in NSC-34 cells with the SOD1-G93A mutation in response to overexpression of TFEB, and LC3-II protein levels also rose. The MTS experiment demonstrated that TFEB overexpression improved NSC-34 cells with the SOD1-G93A mutation’s ability to proliferate and survive. The results imply that TFEB stimulates Beclin-1 expression to induce autophagy. TFEB is a very attractive target for the development of new medicines and new gene treatments for ALS because the altered autophagy mediated by TFEB is a crucial component in the pathogenesis of ALS.192
As a result of its crucial function in regulating lysosomal function, TFEB is crucially involved in the pathophysiological process of many neurodegenerative diseases. Also, TFEB enhanced mesenchymal stem cell therapy enhances autophagy and decreases spinocerebellar ataxia type 3 defects in the neuron cells model.193 Therefore, maintaining TFEB activity might be seen as a successful strategy to hasten the removal of improperly accumulating proteins.
TFEB in inflammatory diseases
One of the main causes of inflammatory illnesses is the dysregulation of TFEB, which results in an imbalanced inflammatory response. It has been demonstrated that TFEB protects ECs by diffusing oxidative stress and boosting the expression of several antioxidant genes, which slows the development of atherosclerosis.138 Macrophages are brought into the expanding plaques when atherosclerosis worsens to sweep up accumulated lipids and apoptotic cells. While TFEB can improve macrophage lysosomal function and trigger a phenotypic switch to an anti-inflammation subtype, this lessens the load on atherosclerotic plaques. While modification of the stearyl coenzyme A desaturase 1 (SCD1)/TFEB machinery and promotion of TFEB-mediated lipo-phagy can both prevent the production of foam cells, SCD1 may also provide innovative treatment methods for atherosclerosis.147,163,194,195
As is well known, autophagy is regarded as a crucial self-defense process for cell survival with significant potential for preserving immunological homeostasis and reducing multiorgan failure in response to a septic assault.196 TFEB has a lot of potential for reducing a deadly septic response since it regulates the beginning of autophagy. The clear benefit of TFEB-mediated autophagy for migrating myocardial damage was seen in the mouse model of sepsis-induced cardiac dysfunction.197
Both human and animal chondrocytes with osteoarthritis (OA) displayed a substantial reduction of TFEB expression and nuclear transport.198 According to research, TFEB overexpression via lentiviral transfection enhanced cartilage degradation and decreased chondrocyte apoptosis and senescence by boosting autophagy, suggesting that this therapy may be a potential option for treating OA.198 A growing body of data suggests that TFEB may play a new function in the initiation and progression of additional inflammatory illnesses. For instance, ERK phosphorylation by reduced TFEB expression causes lung tissue and alveolar epithelial cells to become inflamed and suffer mitochondrial damage, which can lead to pneumonia.50 Pancreatitis results from increased pancreatic proteasome activity and inhibited lysosomal autophagy pathway caused by increased phosphorylated level and cytosolic retention of TFEB.199 In addition, TFEB deficiency might result in lipoprotein ApoA1, which is connected to colitis susceptibility, being downregulated.200 As a result, TFEB may be a novel target for therapy and management of several inflammatory illnesses.
TFEB in cancers
TFEB has multifaceted activities in cancer onset and progression. Recent research has demonstrated that TFEB participates in the control of biological processes in the tumor microenvironment, which may promote or inhibit the growth of cancer cells and influence the emergence of therapy resistance. Recent research has demonstrated that TFEB participates in the control of biological processes in the tumor microenvironment, which may promote or inhibit the growth of cancer cells and influence the development of therapy resistance.201 In addition, independent of its functions in the regulation of autophagic flow, TFEB may be essential for cell proliferation and motility.166 According to a research, TFEB facilitates Wnt signaling to promote cancer growth.94
According to reports, TFEB may help tumors develop and spread by increasing autophagy and endocytosis in prostate and pancreatic cancer cells to meet their metabolic demands.202,203 By promoting DNA repair, TFEB can also prevent breast cancer cells death.140 By increasing the release of cathepsins and the production of lysosomes, TFEB increased the metastasis in a mouse model of lung cancer with liver metastasis.204 TFEB was seen to inhibit cell apoptosis in fibroblasts.205
On the basis of recent research, it is thought that cancer cells use TFEB-induced transcriptional ALB activation to ensure their survival as oncogenic cells.203 A specific kind of renal cell cancer (RCC), known as translocation RCC, is brought on by chromosomal translocations involving the MiT/TFE family that lead to the fusion of TFEB and TFE3 genes. The DNA binding domains and TFEB/TFE3 open reading frame are regularly preserved in TFE3 gene fusions, however less commonly in TFEB gene fusions.206 In addition, alveolar soft part sarcoma207 and juvenile renal cancer206,208,209 have been linked to TFEB and TFE3 chromosomal translocations.201
A small number of juvenile kidney neoplasms have also been linked to the TFEB fusion partner metastasis-associated lung adenocarcinoma transcript 1 (MALAT1). The coding region of the gene for TFEB and the regulatory region of the non-coding gene for MALAT1 are fused as a consequence of the exceedingly unusual 6p21/11q13 chromosomal translocation (MALAT1-TFEB).206,210 As a result of the addition of a new active promoter, the TFEB oncogene becomes strongly expressed, which has the potential to increase its inherent pro-oncogenic potential.211 Furthermore, TFEB amplification may potentially cause kidney cancer. For example, the 6p21.1 region, which contains the nearby vascular endothelial growth factor A and TFEB genes and is amplified as a result, shows genomic amplification in a collection of aggressive RCCs with similar morphological traits.212 Furthermore, kidney-specific TFEB overexpression in transgenic mice resulted in severe cystic pathology, renal cysts, renal clear cells, and papillary cancers metastasizing to the liver.213
Carcinogenesis of PDAC and proliferation of pancreatic cancer cell are both linked to altered TFEB expression.203,214 According to the decreased nuclear levels of these proteins in PDAC cell lines with IPO7 and IPO8 knockdown, TFEB, MITF, and TFE3 are localized in the nucleus of PDAC cells and remain constitutively active regardless of nutrient status because of their interaction with the nucleocytoplasmic transporters importin 7 (IPO7) and importin 8 (IPO8).203 Under starvation circumstances, TFEB nuclear localization in primary PDAC cell lines was also found.215
Cell lines of oral squamous cell carcinoma and non-small-cell lung cancer with TFEB depletion showed lower migratory and invasive characteristics, indicating that it may govern cellular migration.216,217 High TFEB and ALB expression in tumors is associated with increased metastasis and regrowth.216 In a different investigation, it was discovered that the tumor suppressor p53 controls the TFEB-induced ALP in lung malignant cells. In particular, TFEB nuclear translocation and TFEB-induced autophagic activity were boosted by p53 inhibition.218
Kidney cancer is the defining feature of BHD, a hereditary condition brought on by mTORC1 regulator loss-of-function mutations, FLCN.98 This phenotype is very similar to that brought on by the loss of FLCN expression, according to recent research which found that TFEB overexpression in mice produced kidney cysts and renal cancer.213 This suggested that mTORC1 hyperactivity is related to BHD syndrome and that TFEB activation is a major cause of the renal phenotype. RagC and RagD GTPases are activated by amino acids, which causes the phosphorylation of S6K, 4E-BP1, and TFEB. This prevents TFEB from translocating into the nucleus. FLCN favorably regulates RagC/D, which is necessary for mTORC1-mediated phosphorylation of TFEB. The nuclear localization of TFEB and overactivation of mTORC1 are symptoms of BHD syndrome resulted in by the loss of FLCN function.219
Limitation of the review
This review has a limitation in that it primarily concentrates on how TFEB-related human disorders are caused by its relations to the lysosome and autophagy system. Other disease progression mechanisms could have also been explicated. In addition, because it is a narrative review, it is more descriptive and does not objectively address a specific question by thorough and in-depth literature searches as found in systematic reviews and meta-analyses, which are backed by statistical analysis. Therefore, this review offers the writers’ subjective viewpoints on a wider issue.
Conclusion
TFEB is an essential transcription factor for cellular homeostasis. By encouraging the “CLEAR” network, TFEB acts as a key controller of ALB. TFEB is regulated by a number of upstream pathways, including as nutrition availability and stress, and is implicated in several signaling pathways such as ERK and mTOR. Numerous diseases, including LSDs, malignancies, and metabolic diseases, are associated with impaired autophagy–lysosomal processes as a result of faulty TFEB. Considering the biological and pathophysiological importance of TFEB, it is beneficial to keep exploring novel yet effective treatment approaches for human diseases.
Acknowledgments
Not applicable.
Footnotes
Abbreviations***: MSH: Melanocyte stimulating hormones
Authors’ contributions: AG: Conception of research review, literature review, interpretation, and write up of the manuscript.
Availability of data and materials: Not applicable.
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Consent for publication: Not applicable.
Ethics approval and consent to participate: Not applicable.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Not applicable.
ORCID iD: Alemu Gebrie  https://orcid.org/0000-0001-5575-6823
https://orcid.org/0000-0001-5575-6823
References
- 1. Haq R, Fisher DE. Biology and clinical relevance of the micropthalmia family of transcription factors in human cancer. J Clin Oncol 2011; 29(25): 3474–3482. [DOI] [PubMed] [Google Scholar]
- 2. Steingrímsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet 2004; 38: 365–411. [DOI] [PubMed] [Google Scholar]
- 3. Napolitano G, Ballabio A. TFEB at a glance. J Cell Sci 2016; 129(13): 2475–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martini-Stoica H, Xu Y, Ballabio A, et al. The autophagy–lysosomal pathway in neurodegeneration: a TFEB perspective. Trends Neurosci 2016; 39(4): 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Song T, Cai R, Hu R, et al. The important role of TFEB in autophagy-lysosomal pathway and autophagy-related diseases: a systematic review. Eur Rev Med Pharmacol Sci 2021; 25: 1641–1649. [DOI] [PubMed] [Google Scholar]
- 6. Sardiello M. Transcription factor EB: from master coordinator of lysosomal pathways to candidate therapeutic target in degenerative storage diseases. Ann N Y Acad Sci 2016; 1371: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmieri M, Impey S, Kang H, et al. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum Mol Genet 2011; 20(19): 3852–3866. [DOI] [PubMed] [Google Scholar]
- 8. Settembre C, Di Malta C, Polito VA, et al. TFEB links autophagy to lysosomal biogenesis. Science 2011; 332: 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sardiello M, Palmieri M, Di Ronza A, et al. A gene network regulating lysosomal biogenesis and function. Science 2009; 325: 473–477. [DOI] [PubMed] [Google Scholar]
- 10. Lu H, Sun J, Hamblin MH, et al. Transcription factor EB regulates cardiovascular homeostasis. EBioMedicine 2021; 63: 103207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu H, Ren D. Lysosomal physiology. Annu Rev Physiol 2015; 77: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan Y, Lu H, Liang W, et al. Endothelial TFEB (transcription factor EB) positively regulates postischemic angiogenesis. Circ Res 2018; 122(7): 945–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li M, Wang Z, Wang P, et al. TFEB: a emerging regulator in lipid homeostasis for atherosclerosis. Front Physiol 2021; 12: 639920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang W, Li X, Wang S, et al. Regulation of TFEB activity and its potential as a therapeutic target against kidney diseases. Cell Death Discov 2020; 6(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Medina DL, Fraldi A, Bouche V, et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev cell 2011; 21(3): 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Medina DL, Di Paola S, Peluso I, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 2015; 17: 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci 2005; 118(1): 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun 2004; 313(2): 453–458. [DOI] [PubMed] [Google Scholar]
- 19. Saha S, Panigrahi DP, Patil S, et al. Autophagy in health and disease: a comprehensive review. Biomed Pharmacother 2018; 104: 485–495. [DOI] [PubMed] [Google Scholar]
- 20. Corà D, Bussolino F, Doronzo G. TFEB signalling-related MicroRNAs and autophagy. Biomolecules 2021; 11(7): 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka M, Machida Y, Niu S, et al. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med 2004; 10(2): 148–154. [DOI] [PubMed] [Google Scholar]
- 22. Forlenza OV, de Paula VJ, Machado-Vieira R, et al. Does lithium prevent Alzheimer’s disease? Drugs Aging 2012; 29(5): 335–342. [DOI] [PubMed] [Google Scholar]
- 23. Vingtdeux V, Giliberto L, Zhao H, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J Biol Chem 2010; 285(12): 9100–9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spampanato C, Feeney E, Li L, et al. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol Med 2013; 5(5): 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siddiqui A, Bhaumik D, Chinta SJ, et al. Mitochondrial quality control via the PGC1α-TFEB signaling pathway is compromised by parkin Q311X mutation but independently restored by rapamycin. J Neurosci 2015; 35(37): 12833–12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan B, Zhang H, Cui T, et al. TFEB activation protects against cardiac proteotoxicity via increasing autophagic flux. J Mol Cell Cardiol 2017; 113: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pastore N, Ballabio A, Brunetti-Pierri N. Autophagy master regulator TFEB induces clearance of toxic SERPINA1/α-1-antitrypsin polymers. Autophagy 2013; 9(7): 1094–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Decressac M, Mattsson B, Weikop P, et al. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci 2013; 110(19): E1817–E1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roczniak-Ferguson A, Petit CS, Froehlich F, et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal 2012; 5(228): ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Settembre C, De Cegli R, Mansueto G, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol 2013; 15(6): 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bahrami A, Bianconi V, Pirro M, et al. The role of TFEB in tumor cell autophagy: diagnostic and therapeutic opportunities. Life Sci 2020; 244: 117341. [DOI] [PubMed] [Google Scholar]
- 32. Yan S. Role of TFEB in autophagy and the pathogenesis of liver diseases. Biomolecules 2022; 12(5): 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuiper RP, Schepens M, Thijssen J, et al. Regulation of the MiTF/TFE bHLH-LZ transcription factors through restricted spatial expression and alternative splicing of functional domains. Nucleic Acids Res 2004, 32(8): 2315–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parenti G, Andria G, Ballabio A. Lysosomal storage diseases: from pathophysiology to therapy. Annu Rev Med 2015; 66: 471–486. [DOI] [PubMed] [Google Scholar]
- 35. Fisher D, Carr C, Parent L, et al. TFEB has DNA-binding and oligomerization properties of a unique helix-loop-helix/leucine-zipper family. Genes Dev 1991; 5(12a): 2342–2352. [DOI] [PubMed] [Google Scholar]
- 36. Muhle-Goll C, Gibson T, Schuck P, et al. The dimerization stability of the HLH-LZ transcription protein family is modulated by the leucine zippers: a CD and NMR study of TFEB and c-Myc. Biochemistry 1994; 33(37): 11296–11306. [DOI] [PubMed] [Google Scholar]
- 37. Hemesath TJ, Steingrímsson E, McGill G, et al. Microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev 1994; 8(22): 277–02780. [DOI] [PubMed] [Google Scholar]
- 38. Shin H-JR, Kim H, Oh S, et al. AMPK–SKP2–CARM1 signalling cascade in transcriptional regulation of autophagy. Nature 2016; 534(7608): 553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Puertollano R, Ferguson SM, Brugarolas J, et al. The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. EMBO J 2018; 37(11): e98804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mansueto G, Armani A, Viscomi C, et al. Transcription factor EB controls metabolic flexibility during exercise. Cell Metab 2017; 25(1): 182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martina JA, Diab HI, Brady OA, et al. TFEB and TFE 3 are novel components of the integrated stress response. EMBO J 2016; 35(5): 479–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nezich CL, Wang C, Fogel AI, et al. MiT/TFE transcription factors are activated during mitophagy downstream of Parkin and Atg5. J Cell Biol 2015; 210: 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Visvikis O, Ihuegbu N, Labed SA, et al. Innate host defense requires TFEB-mediated transcription of cytoprotective and antimicrobial genes. Immunity 2014; 40(6): 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Campbell GR, Rawat P, Bruckman RS, et al. Human immunodeficiency virus type 1 Nef inhibits autophagy through transcription factor EB sequestration. PLoS Pathog 2015; 11(6): e1005018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pastore N, Brady OA, Diab HI, et al. TFEB and TFE3 cooperate in the regulation of the innate immune response in activated macrophages. Autophagy 2016; 12(8): 1240–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martina JA, Chen Y, Gucek M, et al. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 2012; 8(6): 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Settembre C, Zoncu R, Medina DL, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J 2012; 31(5): 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vega-Rubin-de-Celis S, Peña-Llopis S, Konda M, et al. Multistep regulation of TFEB by MTORC1. Autophagy 2017; 13(3): 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu S-y, Yao R-q, Li Y-x, et al. The role and regulatory mechanism of transcription factor EB in health and diseases. Front Cell Dev Biol 2021; 9: 667750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu W, Li C-C, Lu X, et al. Overexpression of transcription factor EB regulates mitochondrial autophagy to protect lipopolysaccharide-induced acute lung injury. Chin Med J 2019; 132(11): 1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palmieri M, Pal R, Nelvagal HR, et al. MTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat Commun 2017; 8(1): 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hsu CL, Lee EX, Gordon KL, et al. MAP4K3 mediates amino acid-dependent regulation of autophagy via phosphorylation of TFEB. Nat Commun 2018; 9(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Collodet C, Foretz M, Deak M, et al. AMPK promotes induction of the tumor suppressor FLCN through activation of TFEB independently of mTOR. FASEB J 2019; 33(11): 12374–12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brady OA, Martina JA, Puertollano R. Emerging roles for TFEB in the immune response and inflammation. Autophagy 2018; 14(2): 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cortes CJ, La Spada AR. TFEB dysregulation as a driver of autophagy dysfunction in neurodegenerative disease: molecular mechanisms, cellular processes, and emerging therapeutic opportunities. Neurobiol Dis 2019; 122: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Erlich AT, Brownlee DM, Beyfuss K, et al. Exercise induces TFEB expression and activity in skeletal muscle in a PGC-1α-dependent manner. Am J Physiol-Cell Physiol 2018; 314(1): C62–C72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Martina JA, Puertollano R. Protein phosphatase 2A stimulates activation of TFEB and TFE3 transcription factors in response to oxidative stress. J Biol Chem 2018; 293(32): 12525–12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang Z, Yan J, Bowman AB, et al. Dysregulation of TFEB contributes to manganese-induced autophagic failure and mitochondrial dysfunction in astrocytes. Autophagy 2020; 16(8): 1506–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martina JA, Diab HI, Lishu L, et al. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci Signal 2014; 7(309): ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li C, Wang X, Li X, et al. Proteasome inhibition activates autophagy-lysosome pathway associated with TFEB dephosphorylation and nuclear translocation. Front Cell Dev Biol 2019; 7: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li L, Friedrichsen HJ, Andrews S, et al. A TFEB nuclear export signal integrates amino acid supply and glucose availability. Nat Commun 2018; 9(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Napolitano G, Esposito A, Choi H, et al. MTOR-dependent phosphorylation controls TFEB nuclear export. Nat Commun 2018; 9(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Silvestrini MJ, Johnson JR, Kumar AV, et al. Nuclear export inhibition enhances HLH-30/TFEB activity, autophagy, and lifespan. Cell Rep 2018; 23(7): 1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yin Q, Jian Y, Xu M, et al. CDK4/6 regulate lysosome biogenesis through TFEB/TFE3. J Cell Biol 2020, 219(8): e201911036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang Z, Li C. Xenophagy in innate immunity: a battle between host and pathogen. Dev Comp Immunol 2020; 109: 103693. [DOI] [PubMed] [Google Scholar]
- 66. Wang Y, Huang Y, Liu J, et al. Acetyltransferase GCN5 regulates autophagy and lysosome biogenesis by targeting TFEB. EMBO Rep 2020; 21(1): e48335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bao J, Zheng L, Zhang Q, et al. Deacetylation of TFEB promotes fibrillar Aβ degradation by upregulating lysosomal biogenesis in microglia. Protein Cell 2016; 7(6): 417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang J, Wang J, Zhou Z, et al. Importance of TFEB acetylation in control of its transcriptional activity and lysosomal function in response to histone deacetylase inhibitors. Autophagy 2018; 14(6): 1043–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sha Y, Rao L, Settembre C, et al. STUB 1 regulates TFEB-induced autophagy–lysosome pathway. EMBO J 2017; 36(17): 2544–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miller AJ, Levy C, Davis IJ, et al. Sumoylation of MITF and its related family members TFE3 and TFEB. J Biol Chem 2005; 280(1): 146–155. [DOI] [PubMed] [Google Scholar]
- 71. Shin H-JR, Kim H, Kim KI, et al. Epigenetic and transcriptional regulation of autophagy. Autophagy 2016; 12(11): 2248–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pan S, Chen R, Tamura Y, et al. Quantitative glycoproteomics analysis reveals changes in N-glycosylation level associated with pancreatic ductal adenocarcinoma. J Proteome Res 2014; 13(3): 1293–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Song W, Zhang C-L, Gou L, et al. Endothelial TFEB (transcription factor EB) restrains IKK (IκB kinase)-p65 pathway to attenuate vascular inflammation in diabetic db/db mice. Arterioscler Thromb Vasc Biol 2019; 39(4): 719–730. [DOI] [PubMed] [Google Scholar]
- 74. Ghosh A, Jana M, Modi K, et al. Activation of peroxisome proliferator-activated receptor α induces lysosomal biogenesis in brain cells: implications for lysosomal storage disorders. J Biol Chem 2015; 290(16): 10309–10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Annunziata I, van de Vlekkert D, Wolf E, et al. MYC competes with MiT/TFE in regulating lysosomal biogenesis and autophagy through an epigenetic rheostat. Nat Commun 2019; 10(1): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Doronzo G, Astanina E, Bussolino F. The oncogene transcription factor EB regulates vascular functions. Front Physiol 2021; 12: 640061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zoncu R, Bar-Peled L, Efeyan A, et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science 2011; 334(6056): 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sancak Y, Peterson TR, Shaul YD, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008; 320(5882): 1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sancak Y, Bar-Peled L, Zoncu R, et al. Ragulator-rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010; 141(2): 290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Martina JA, Puertollano R. Rag GTPases mediate amino acid–dependent recruitment of TFEB and MITF to lysosomes. J Cell Biol 2013; 200(4): 475–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li Y, Xu M, Ding X, et al. Protein kinase C controls lysosome biogenesis independently of mTORC1. Nat Cell Biol 2016; 18(10): 1065–1077. [DOI] [PubMed] [Google Scholar]
- 82. Neasta J, Ben Hamida S, Yowell Q, et al. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci 2010; 107(46): 20093–20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Laguesse S, Morisot N, Phamluong K, et al. Region specific activation of the AKT and mTORC1 pathway in response to excessive alcohol intake in rodents. Addict Biol 2017; 22(6): 1856–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Long X, Lin Y, Ortiz-Vega S, et al. Rheb binds and regulates the mTOR kinase. Curr Biol 2005, 15(8): 702–713. [DOI] [PubMed] [Google Scholar]
- 85. Yang Q, Inoki K, Kim E, et al. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc Natl Acad Sci 2006; 103(18): 6811–6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Narendra D, Kane LA, Hauser DN, et al. P62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 2010; 6(8): 1090–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ikeda S, Nah J, Shirakabe A, et al. YAP plays a crucial role in the development of cardiomyopathy in lysosomal storage diseases. J Clin Invest 2021; 131(5): e143173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ferron M, Settembre C, Shimazu J, et al. A RANKL–PKCβ–TFEB signaling cascade is necessary for lysosomal biogenesis in osteoclasts. Genes Dev 2013; 27(8): 955–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Peña-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, et al. Regulation of TFEB and V-ATPases by mTORC1. EMBO J 2011, 30(16): 3242–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hogan PG, Li H. Calcineurin. Curr Biol 2005; 15(12): R442–R443. [DOI] [PubMed] [Google Scholar]
- 91. Creamer TP. Calcineurin. Cell Commun Signal 2020; 18(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Páhi ZG, Borsos BN, Pantazi V, et al. PARylation during transcription: insights into the fine-tuning mechanism and regulation. Cancers 2020; 12(1): 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Simonet NG, Rasti G, Vaquero A. The histone code and disease: posttranslational modifications as potential prognostic factors for clinical diagnosis. In: García-Giménez JL. (eds.), Epigenetic biomarkers and diagnostics. Amsterdam: Elsevier, 2016, pp. 417–445. [Google Scholar]
- 94. Kim S, Song G, Lee T, et al. PARsylated transcription factor EB (TFEB) regulates the expression of a subset of Wnt target genes by forming a complex with β-catenin-TCF/LEF1. Cell Death & Differ 2021; 28(9): 2555–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Franco-Juárez B, Coronel-Cruz C, Hernández-Ochoa B, et al. TFEB; beyond its role as an autophagy and lysosomes regulator. Cells 2022; 11(19): 3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977; 113(12): 1674–1677. [PubMed] [Google Scholar]
- 97. Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell 2002; 2(2): 157–164. [DOI] [PubMed] [Google Scholar]
- 98. Schmidt LS, Linehan WM. FLCN: the causative gene for Birt-Hogg-Dubé syndrome. Gene 2018; 640: 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ramirez Reyes JM, Cuesta R, Pause A. Folliculin: a regulator of transcription through AMPK and mTOR signaling pathways. Front Cell Dev Biol 2021; 9: 667311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang S, Tsun Z-Y, Wolfson RL, et al. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015; 347(6218): 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lawrence RE, Fromm SA, Fu Y, et al. Structural mechanism of a Rag GTPase activation checkpoint by the lysosomal folliculin complex. Science 2019; 366(6468): 971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fromm SA, Lawrence RE, Hurley JH. Structural mechanism for amino acid-dependent Rag GTPase nucleotide state switching by SLC38A9. Nat Struct Mol Biol 2020; 27(11): 1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nakamura S, Shigeyama S, Minami S, et al. LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nat Cell Biol 2020; 22(10): 1252–1263. [DOI] [PubMed] [Google Scholar]
- 104. Drazic A, Myklebust LM, Ree R, et al. The world of protein acetylation. Biochim Biophys Acta (BBA)-Proteins Proteom 2016; 1864(10): 1372–1401. [DOI] [PubMed] [Google Scholar]
- 105. Son SM, Park SJ, Fernandez-Estevez M, et al. Autophagy regulation by acetylation—implications for neurodegenerative diseases. Exp Mol Med 2021; 53(1): 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wan W, You Z, Xu Y, et al. mTORC1 phosphorylates acetyltransferase p300 to regulate autophagy and lipogenesis. Mol Cell 2017; 68(2): 323–335. [DOI] [PubMed] [Google Scholar]
- 107. Louphrasitthiphol P, Siddaway R, Loffreda A, et al. Tuning transcription factor availability through acetylation-mediated genomic redistribution. Mol Cell 2020; 79(3): 472–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Price ER, Horstmann MA, Wells AG, et al. α-Melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome. J Biol Chem 1998; 273(49): 33042–33047. [DOI] [PubMed] [Google Scholar]
- 109. Sato S, Roberts K, Gambino G, et al. CBP/p300 as a co-factor for the Microphthalmia transcription factor. Oncogene 1997; 14(25): 3083–3092. [DOI] [PubMed] [Google Scholar]
- 110. Tan A, Prasad R, Lee C, et al. Past, present, and future perspectives of transcription factor EB (TFEB): mechanisms of regulation and association with disease. Cell Death Differ 2022; 29(8): 1433–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lawrence RE, Zoncu R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat Cell Biol 2019; 21(2): 133–142. [DOI] [PubMed] [Google Scholar]
- 112. Gray MA, Choy CH, Dayam RM, et al. Phagocytosis enhances lysosomal and bactericidal properties by activating the transcription factor TFEB. Curr Biol 2016; 26(15): 1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen M, Dai Y, Liu S, et al. TFEB Biology and Agonists at a Glance. Cells 2021; 10(2): 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Willett R, Martina JA, Zewe JP, et al. TFEB regulates lysosomal positioning by modulating TMEM55B expression and JIP4 recruitment to lysosomes. Nat Commun 2017; 8(1): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang S, Chen Y, Li X, et al. Emerging role of transcription factor EB in mitochondrial quality control. Biomed Pharmacother 2020; 128: 110272. [DOI] [PubMed] [Google Scholar]
- 116. Ma X, Liu H, Murphy JT, et al. Regulation of the transcription factor EB-PGC1α axis by beclin-1 controls mitochondrial quality and cardiomyocyte death under stress. Mol Cell Biol 2015; 35(6): 956–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Eun SY, Lee JN, Nam I-K, et al. PEX5 regulates autophagy via the mTORC1-TFEB axis during starvation. Exp Mol Med 2018; 50(4): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Farge G, Falkenberg M. Organization of DNA in mammalian mitochondria. Int J Mol Sci 2019; 20(11): 2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Santin Y, Sicard P, Vigneron F, et al. Oxidative stress by monoamine oxidase-A impairs transcription factor EB activation and autophagosome clearance, leading to cardiomyocyte necrosis and heart failure. Antioxid Redox Signal 2016; 25(1): 10–27. [DOI] [PubMed] [Google Scholar]
- 120. Yonekawa T, Gamez G, Kim J, et al. RIP 1 negatively regulates basal autophagic flux through TFEB to control sensitivity to apoptosis. EMBO Rep 2015; 16(6): 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kimura S, Noda T, Yoshimori T. Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct Funct 2008; 33(1): 109–122. [DOI] [PubMed] [Google Scholar]
- 122. Korolchuk VI, Saiki S, Lichtenberg M, et al. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol 2011; 13(4): 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 2018; 25(3): 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Green DR, Galluzzi L, Kroemer G. Metabolic control of cell death. Science 2014; 345(6203): 1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kriel J, Loos B. The good, the bad and the autophagosome: exploring unanswered questions of autophagy-dependent cell death. Cell Death Differ 2019; 26(4): 640–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cea M, Cagnetta A, Patrone F, et al. Intracellular NAD+ depletion induces autophagic death in multiple myeloma cells. Autophagy 2013; 9(3): 410–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Silvente-Poirot S, Segala G, Poirot MC, et al. Ligand-dependent transcriptional induction of lethal autophagy: a new perspective for cancer treatment. Autophagy 2018; 14(3): 555–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li L, Sun B, Gao Y, et al. STAT3 contributes to lysosomal-mediated cell death in a novel derivative of riccardin D-treated breast cancer cells in association with TFEB. Biochem Pharmacol 2018; 150: 267–279. [DOI] [PubMed] [Google Scholar]
- 129. Carey KL, Paulus GL, Wang L, et al. TFEB transcriptional responses reveal negative feedback by BHLHE40 and BHLHE41. Cell Rep 2020; 33(6): 108371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Brady OA, Jeong E, Martina JA, et al. The transcription factors TFE3 and TFEB amplify p53 dependent transcriptional programs in response to DNA damage. Elife 2018; 7: e40856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chauhan S, Kumar S, Jain A, et al. TRIMs and galectins globally cooperate and TRIM16 and galectin-3 co-direct autophagy in endomembrane damage homeostasis. Dev Cell 2016; 39(1): 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Leow SM, Chua SXS, Venkatachalam G, et al. Sub-lethal oxidative stress induces lysosome biogenesis via a lysosomal membrane permeabilization-cathepsin-caspase 3-transcription factor EB-dependent pathway. Oncotarget 2017; 8(10): 16170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nakamura S, Akayama S, Yoshimori T. Autophagy-independent function of lipidated LC3 essential for TFEB activation during the lysosomal damage responses. Autophagy 2021; 17(2): 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yoneshima E, Okamoto K, Sakai E, et al. The transcription factor EB (TFEB) regulates osteoblast differentiation through ATF4/CHOP-dependent pathway. J Cell Physiol 2016; 231(6): 1321–1333. [DOI] [PubMed] [Google Scholar]
- 135. Du Bois P, Pablo Tortola C, Lodka D, et al. Angiotensin II induces skeletal muscle atrophy by activating TFEB-mediated MuRF1 expression. Circ Res 2015; 117(5): 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Fang L, Hodge J, Saaoud F, et al. Transcriptional factor EB regulates macrophage polarization in the tumor microenvironment. Oncoimmunology 2017; 6(5): e1312042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122(3): 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lu H, Fan Y, Qiao C, et al. TFEB inhibits endothelial cell inflammation and reduces atherosclerosis. Sci Signal 2017; 10(464): eaah4214. [DOI] [PubMed] [Google Scholar]
- 139. Lu H, Sun J, Liang W, et al. Cyclodextrin prevents abdominal aortic aneurysm via activation of vascular smooth muscle cell transcription factor EB. Circulation 2020; 142(5): 483–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Slade L, Biswas D, Ihionu F, et al. A lysosome independent role for TFEB in activating DNA repair and inhibiting apoptosis in breast cancer cells. Biochem J 2020; 477: 137–160. [DOI] [PubMed] [Google Scholar]
- 141. Kim JH, Lee J, Cho Y-R, et al. TFEB supports pancreatic cancer growth through the transcriptional regulation of glutaminase. Cancers 2021; 13(3): 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tan A, Prasad R, Jho E-h. TFEB regulates pluripotency transcriptional network in mouse embryonic stem cells independent of autophagy–lysosomal biogenesis. Cell Death Dis 2021; 12(4): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kim J, Kim SH, Kang H, et al. TFEB–GDF15 axis protects against obesity and insulin resistance as a lysosomal stress response. Nat Metab 2021; 3(3): 410–427. [DOI] [PubMed] [Google Scholar]
- 144. Khawar MB, Abbasi MH, Rafiq M, et al. A decade of mighty lipophagy: what we know and what facts we need to know? Oxid Med Cell Longev 2021; 2021: 5539161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. O’Rourke EJ, Ruvkun G. MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability. Nat Cell Biol 2013; 15(6): 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wang Y, Gunewardena S, Li F, et al. An FGF15/19-TFEB regulatory loop controls hepatic cholesterol and bile acid homeostasis. Nat Commun 2020; 11(1): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. You Y, Bao W-L, Zhang S-L, et al. Sorting Nexin 10 mediates metabolic reprogramming of macrophages in atherosclerosis through the Lyn-dependent TFEB signaling pathway. Circ Res 2020; 127(4): 534–549. [DOI] [PubMed] [Google Scholar]
- 148. Chauhan S, Ahmed Z, Bradfute SB, et al. Pharmaceutical screen identifies novel target processes for activation of autophagy with a broad translational potential. Nat Commun 2015; 6(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Markby GR, Sakamoto K. Transcription factor EB and TFE3: new metabolic coordinators mediating adaptive responses to exercise in skeletal muscle? Am J Physiol – Endocrinol Metab 2020; 319(4): E763–E768. [DOI] [PubMed] [Google Scholar]
- 150. Cinque L, De Leonibus C, Iavazzo M, et al. MiT/TFE factors control ER-phagy via transcriptional regulation of FAM 134B. EMBO J 2020; 39(17): e105696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ivankovic D, Chau KY, Schapira AH, et al. Mitochondrial and lysosomal biogenesis are activated following PINK 1/parkin-mediated mitophagy. J Neurochem 2016; 136(2): 388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Liu X, Zhang N, Liu Y, et al. MPB, a novel berberine derivative, enhances lysosomal and bactericidal properties via TGF-β–activated kinase 1-dependent activation of the transcription factor EB. FASEB J 2019; 33(1): 1468–1481. [DOI] [PubMed] [Google Scholar]
- 153. Ammanathan V, Mishra P, Chavalmane AK, et al. Restriction of intracellular Salmonella replication by restoring TFEB-mediated xenophagy. Autophagy 2020; 16(9): 1584–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Samie M, Cresswell P. The transcription factor TFEB acts as a molecular switch that regulates exogenous antigen-presentation pathways. Nat Immunol 2015; 16(7): 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. He Y, Xu Y, Zhang C, et al. Identification of a lysosomal pathway that modulates glucocorticoid signaling and the inflammatory response. Sci Signal 2011; 4(180): ra44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tseng HHL, Vong CT, Kwan YW, et al. Lysosomal Ca2+ signaling regulates high glucose-mediated interleukin-1β secretion via transcription factor EB in human monocytic cells. Front Immunol 2017; 8: 1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kim YS, Lee H-M, Kim JK, et al. PPAR-α activation mediates innate host defense through induction of TFEB and lipid catabolism. J Immunol 2017; 198(8): 3283–3295. [DOI] [PubMed] [Google Scholar]
- 158. Pastore N, Vainshtein A, Klisch TJ, et al. TFE 3 regulates whole-body energy metabolism in cooperation with TFEB. EMBO Mol Med 2017; 9(5): 605–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Trivedi PC, Bartlett JJ, Mercer A, et al. Loss of function of transcription factor EB remodels lipid metabolism and cell death pathways in the cardiomyocyte. Biochim Biophys Acta (BBA)-Mol Basis Dis 2020; 1866(10): 165832. [DOI] [PubMed] [Google Scholar]
- 160. Kim M, Lu L, Dvornikov AV, et al. Tfeb overexpression, not mtor inhibition, ameliorates ragcs75y cardiomyopathy. Int J Mol Sci 2021, 22(11): 5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sun J, Lu H, Liang W, et al. Endothelial TFEB (transcription factor EB) improves glucose tolerance via upregulation of IRS (insulin receptor substrate) 1 and IRS2. Arterioscler Thromb, Vasc Biol 2021; 41(2): 783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ariano C, Riganti C, Corà D, et al. TFEB controls integrin-mediated endothelial cell adhesion by the regulation of cholesterol metabolism. Angiogenesis 2022; 25: 471–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sergin I, Evans TD, Zhang X, et al. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat Commun 2017; 8(1): 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Doronzo G, Astanina E, Corà D, et al. TFEB controls vascular development by regulating the proliferation of endothelial cells. EMBO J 2019; 38(3): e98250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wang Y-T, Li X, Chen J, et al. Activation of TFEB ameliorates dedifferentiation of arterial smooth muscle cells and neointima formation in mice with high-fat diet. Cell Death Dis 2019; 10(9): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Astanina E, Bussolino F, Doronzo G. Transcription factor EB controls both motogenic and mitogenic cell activities. FEBS Lett 2022; 596: 1973–1980. [DOI] [PubMed] [Google Scholar]
- 167. Cosma MP, Pepe S, Annunziata I, et al. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell 2003; 113(4): 445–456. [DOI] [PubMed] [Google Scholar]
- 168. Dierks T, Schmidt B, Borissenko LV, et al. Multiple sulfatase deficiency is caused by mutations in the gene encoding the human Cα-formylglycine generating enzyme. Cell 2003; 113(4): 435–444. [DOI] [PubMed] [Google Scholar]
- 169. Diez-Roux G, Ballabio A. Sulfatases and human disease. Annu Rev Genomics Hum Genet 2005; 6: 355–379. [DOI] [PubMed] [Google Scholar]
- 170. Kishnani PS, Hwu W-L, Mandel H, et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 2006; 148(5): 671–676. [DOI] [PubMed] [Google Scholar]
- 171. Palmer DN, Fearnley IM, Walker JE, et al. Mitochondrial ATP synthase subunit c storage in the ceroid-lipofuscinoses (Batten disease). Am J Med Genet 1992; 42(4): 561–567. [DOI] [PubMed] [Google Scholar]
- 172. Anderson GW, Goebel HH, Simonati A. Human pathology in NCL. Biochim Biophys Acta (BBA)-Mol Basis Dis 2013; 1832(11): 1807–1826. [DOI] [PubMed] [Google Scholar]
- 173. Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci 2008; 9(10): 768–778. [DOI] [PubMed] [Google Scholar]
- 174. Rai SN, Tiwari N, Singh P, et al. Therapeutic potential of vital transcription factors in alzheimer’s and parkinson’s disease with particular emphasis on transcription factor eb mediated autophagy. Front Neurosci 2021; 15: 777347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Xu Y, Du S, Marsh JA, et al. TFEB regulates lysosomal exocytosis of tau and its loss of function exacerbates tau pathology and spreading. Mol Psychiatry 2021; 26(10): 5925–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Polito VA, Li H, Martini-Stoica H, et al. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol Med 2014; 6(9): 1142–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Martini-Stoica H, Cole AL, Swartzlander DB, et al. TFEB enhances astroglial uptake of extracellular tau species and reduces tau spreading. J Exp Med 2018; 215(9): 2355–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Parcon PA, Balasubramaniam M, Ayyadevara S, et al. Apolipoprotein E4 inhibits autophagy gene products through direct, specific binding to CLEAR motifs. Alzheimers Dement 2018; 14(2): 230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Bordi M, Berg MJ, Mohan PS, et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy 2016; 12(12): 2467–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Tiribuzi R, Crispoltoni L, Porcellati S, et al. miR128 up-regulation correlates with impaired amyloid β (1-42) degradation in monocytes from patients with sporadic Alzheimer’s disease. Neurobiol Aging 2014; 35(2): 345–356. [DOI] [PubMed] [Google Scholar]
- 181. Reddy K, Cusack CL, Nnah IC, et al. Dysregulation of nutrient sensing and CLEARance in presenilin deficiency. Cell Rep 2016; 14(9): 2166–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wang H, Wang R, Carrera I, et al. TFEB overexpression in the P301S model of tauopathy mitigates increased PHF1 levels and lipofuscin puncta and rescues memory deficits. eNeuro 2016; 3(3): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Song J-X, Liu J, Jiang Y, et al. Transcription factor EB: an emerging drug target for neurodegenerative disorders. Drug Discov Today 2021; 26(1): 164–172. [DOI] [PubMed] [Google Scholar]
- 184. Kalia LV, Lang AE. Evolving basic, pathological and clinical concepts in PD. Nat Rev Neurol 2016; 12(2): 65–66. [DOI] [PubMed] [Google Scholar]
- 185. Anglade P, Vyas S, Javoy-Agid F, et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol 1997; 12: 25–31. [PubMed] [Google Scholar]
- 186. Tsunemi T, Ashe TD, Morrison BE, et al. PGC-1α rescues Huntington’s disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci Trans Med 2012; 4(142): 142ra197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Bar-Yosef T, Damri O, Agam G. Dual role of autophagy in diseases of the central nervous system. Front Cell Neurosci 2019; 13: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Vodicka P, Chase K, Iuliano M, et al. Autophagy activation by transcription factor EB (TFEB) in striatum of HD Q175/Q7 mice. J Huntington’s Dis 2016; 5(3): 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Rusmini P, Cortese K, Crippa V, et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019; 15(4): 631–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Cortes CJ, Miranda HC, Frankowski H, et al. Polyglutamine-expanded androgen receptor interferes with TFEB to elicit autophagy defects in SBMA. Nat Neurosci 2014; 17(9): 1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Tohnai G, Adachi H, Katsuno M, et al. Paeoniflorin eliminates a mutant AR via NF-YA-dependent proteolysis in spinal and bulbar muscular atrophy. Hum Mol Genet 2014; 23(13): 3552–3565. [DOI] [PubMed] [Google Scholar]
- 192. Chen Y, Liu H, Guan Y, et al. The altered autophagy mediated by TFEB in animal and cell models of amyotrophic lateral sclerosis. Am J Trans Res 2015; 7(9): 1574. [PMC free article] [PubMed] [Google Scholar]
- 193. Han X, de Dieu Habimana J, Li AL, et al. Transcription factor EB-mediated mesenchymal stem cell therapy induces autophagy and alleviates spinocerebellar ataxia type 3 defects in neuronal cells model. Cell Death Dis 2022, 13(7): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Pi H, Wang Z, Liu M, et al. SCD1 activation impedes foam cell formation by inducing lipophagy in oxLDL-treated human vascular smooth muscle cells. J Cell Mol Med 2019; 23(8): 5259–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Evans TD, Jeong S-J, Zhang X, et al. TFEB and trehalose drive the macrophage autophagy-lysosome system to protect against atherosclerosis. Autophagy 2018; 14(4): 724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ren C, Zhang H, Wu T-t, et al. Autophagy: a potential therapeutic target for reversing sepsis-induced immunosuppression. Front Immunol 2017; 8: 1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Li F, Lang F, Zhang H, et al. Role of TFEB mediated autophagy, oxidative stress, inflammation, and cell death in endotoxin induced myocardial toxicity of young and aged mice. Oxid Med Cell Longev 2016; 2016: 5380319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zheng G, Zhan Y, Li X, et al. TFEB, a potential therapeutic target for osteoarthritis via autophagy regulation. Cell Death Dis 2018; 9(9): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Wang S, Ni H-M, Chao X, et al. Impaired TFEB-mediated lysosomal biogenesis promotes the development of pancreatitis in mice and is associated with human pancreatitis. Autophagy 2019; 15(11): 1954–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Murano T, Najibi M, Paulus GL, et al. Transcription factor TFEB cell-autonomously modulates susceptibility to intestinal epithelial cell injury in vivo. Sci Rep 2017; 7(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Astanina E, Bussolino F, Doronzo G. Multifaceted activities of transcription factor EB in cancer onset and progression. Mol Oncol 2021; 15(2): 327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Blessing AM, Rajapakshe K, Reddy Bollu L, et al. Transcriptional regulation of core autophagy and lysosomal genes by the androgen receptor promotes prostate cancer progression. Autophagy 2017; 13(3): 506–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Perera RM, Stoykova S, Nicolay BN, et al. Transcriptional control of autophagy–lysosome function drives pancreatic cancer metabolism. Nature 2015; 524(7565): 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Kundu ST, Grzeskowiak CL, Fradette JJ, et al. TMEM106B drives lung cancer metastasis by inducing TFEB-dependent lysosome synthesis and secretion of cathepsins. Nat Commun 2018; 9(1): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Zhou L, Liu Z, Chen S, et al. Transcription factor EB‑mediated autophagy promotes dermal fibroblast differentiation and collagen production by regulating endoplasmic reticulum stress and autophagy‑dependent secretion. Int J Mol Med 2021; 47(2): 547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Kuiper RP, Schepens M, Thijssen J, et al. Upregulation of the transcription factor TFEB in t (6; 11)(p21; q13)-positive renal cell carcinomas due to promoter substitution. Hum Mol Genet 2003; 12(14): 1661–1669. [DOI] [PubMed] [Google Scholar]
- 207. Ladanyi M, Lui MY, Antonescu CR, et al. The der (17) t (X; 17)(p11; q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 2001; 20(1): 48–57. [DOI] [PubMed] [Google Scholar]
- 208. Argani P, Lui MY, Couturier J, et al. A novel CLTC-TFE3 gene fusion in pediatric renal adenocarcinoma with t (X; 17)(p11. 2; q23). Oncogene 2003; 22(34): 5374–5378. [DOI] [PubMed] [Google Scholar]
- 209. Clark J, Lu Y-J, Sidhar SK, et al. Fusion of splicing factor genes PSF and NonO (p54nrb) to the TFE3 gene in papillary renal cell carcinoma. Oncogene 1997; 15(18): 2233–2239. [DOI] [PubMed] [Google Scholar]
- 210. Davis IJ, Hsi B-L, Arroyo JD, et al. Cloning of an Alpha-TFEB fusion in renal tumors harboring the t (6; 11)(p21; q13) chromosome translocation. Proc Natl Acad Sci 2003; 100(10): 6051–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kauffman EC, Ricketts CJ, Rais-Bahrami S, et al. Molecular genetics and cellular features of TFE3 and TFEB fusion kidney cancers. Nat Rev Urol 2014; 11(8): 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Gupta S, Johnson SH, Vasmatzis G, et al. TFEB-VEGFA (6p21. 1) co-amplified renal cell carcinoma: a distinct entity with potential implications for clinical management. Mod Pathol 2017; 30(7): 998–1012. [DOI] [PubMed] [Google Scholar]
- 213. Calcagnì A, Verschuren E, De Cegli R, et al. Modelling TFE renal cell carcinoma in mice reveals a critical role of WNT signaling. Elife 2016; 5: e17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Marchand B, Arsenault D, Raymond-Fleury A, et al. Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells. J Biol Chem 2015; 290(9): 5592–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Klein K, Werner K, Teske C, et al. Role of TFEB-driven autophagy regulation in pancreatic cancer treatment. Int J Oncol 2016; 49(1): 164–172. [DOI] [PubMed] [Google Scholar]
- 216. Giatromanolaki A, Kalamida D, Sivridis E, et al. Increased expression of transcription factor EB (TFEB) is associated with autophagy, migratory phenotype and poor prognosis in non-small cell lung cancer. Lung Cancer 2015; 90(1): 98–105. [DOI] [PubMed] [Google Scholar]
- 217. Sakamoto H, Yamashita K, Okamoto K, et al. Transcription factor EB influences invasion and migration in oral squamous cell carcinomas. Oral Dis 2018; 24(5): 741–748. [DOI] [PubMed] [Google Scholar]
- 218. Zhang Z, Wang H, Ding Q, et al. The tumor suppressor p53 regulates autophagosomal and lysosomal biogenesis in lung cancer cells by targeting transcription factor EB. Biomed Pharmacother 2017; 89: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 219. Napolitano G, Di Malta C, Esposito A, et al. A substrate-specific mTORC1 pathway underlies Birt–Hogg–Dubé syndrome. Nature 2020; 585(7826): 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]