Abstract
BACKGROUND
Asymptomatic atrial fibrillation (AF) is associated with an increased risk of stroke. The yield of serial electrocardiographic (ECG) screening for AF is unknown.
OBJECTIVES
The aim of this study was to determine the frequency of AF detected by serial, 7-day ECG patch screenings in older women identified as having an elevated risk of AF according to the CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology)-AF clinical prediction score.
METHODS
Postmenopausal women with a 5-year predicted risk of new-onset AF ≥5% according to CHARGE-AF were recruited from the ongoing WHISH (Women’s Health Initiative Strong and Healthy) randomized trial of a physical activity intervention. Participants with AF at baseline by self-report or medical records review were excluded. Screening with 7-day ECG patch monitors was performed at baseline, 6 months, and 12 months from study enrollment.
RESULTS
On baseline monitoring, 2.5% of the cohort had AF detected, increasing to 3.7% by 6 months and 4.9% cumulatively by 12 months. Yield of patch screening was higher among participants with a higher (≥10%) CHARGE-AF score: 4.2% had AF detected at baseline, 5.9% at 6 months, and 7.2% at 12 months. Most participants with patch-identified AF never had a clinical diagnosis of AF (36 of 46 [78%]).
CONCLUSIONS
Older women with an elevated CHARGE-AF score had a high prevalence of AF on 7-day ECG patch screening. Serial screening over 12 months substantially increased the detection of AF. These data can be useful in helping identify high-risk participants for enrollment in future studies of the management of asymptomatic AF.(Women’s Health Initiative Silent Atrial Fibrillation Recording Study [WHISH STAR]; NCT05366803.)
Keywords: arrhythmias, atrial fibrillation, screening, Women’s Health Initiative
Longitudinal cohort studies have shown that more than one-third of individuals with atrial fibrillation (AF) may have asymptomatic, silent AF.1,2 In a significant proportion of patients, AF is identified only after the development of complications such as stroke or heart failure,3 and up to one-third of strokes are associated with AF.4 Short episodes of paroxysmal AF early in the natural course of the disease may be missed by standard electrocardiograms (ECGs) and physical examinations. Data from implantable loop recorders suggest that AF can be detected in up to 30% of high-risk individuals, such as those who have experienced an ischemic stroke within the preceding 3 years.5 Atrial tachyarrhythmias detected on cardiovascular implantable electronic devices, which often represent AF, are associated with an increased risk of stroke and death.6–8
Current guidelines do not recommend screening for AF beyond pulse palpation or an ECG rhythm strip in those aged ≥65 years, and they consider systematic screening only in those aged ≥75 years and at high risk.9–11 The United States Preventive Services Task Force concluded that the current evidence is insufficient to assess the risks and benefits of AF screening by ECG.12 Population-based and pragmatic screening studies for silent AF13–17 have consistently shown a substantial rate of AF detection; none of these studies, however, has used a clinical prediction tool to identify high-risk subgroups to increase the yield of screening. The identification of high-risk populations for AF screening could improve clinical care and outcomes.18
Clinical risk prediction tools have been developed to estimate the risk for developing clinical AF. The CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology)-AF score, which was derived and validated from 3 large multiethnic population-based cohorts in the United States,19,20 is particularly promising. In the present study, we used the CHARGE-AF score to select participants for AF screening among participants enrolled in the ongoing WHISH (Women’s Health Initiative Strong and Healthy) clinical trial for AF screening, which is testing whether a public health–oriented physical activity intervention reduces major clinical cardiovascular events in older women. Here, we report the yield of 7-day ECG patch screening for AF at baseline, 6 months, and 12 months using ambulatory ECG patch monitoring among all participants with a predicted AF risk ≥5% at 5 years.
METHODS
STUDY POPULATION.
The WHI (Women’s Health Initiative) was designed to assess the impact of hormone therapy and lifestyle on clinical outcomes in 161,808 postmenopausal women who were enrolled into 1 of 3 randomized trials or a prospective observation study.21,22 Women between the ages of 50 and 79 years were recruited at 40 clinical centers across the United States between 1993 and 1998 and continue to be followed up for long-term clinical outcomes.
The WHISH trial is an ongoing, pragmatic randomized controlled trial of physical activity intervention conducted within the WHI and funded by the National Institutes of Health (NCT02425345). WHISH enrolled 49,331 women who were between 68 and 99 years of age in 2015 and being followed up in WHI, and then randomized them in WHISH using a Zelen design opt-out consent process.23 The study was designed to determine the impact of an intervention to increase physical activity vs a control group to evaluate the impact of physical activity on cardiovascular and noncardiovascular outcomes. The trial design, recruitment and consent procedures, and baseline characteristics of participants have been detailed elsewhere.24 The trial is ongoing, and the blind has not yet been broken.
WHISH participants without a history of clinical AF (n = 33,861) were eligible for the WHISH STAR (Women’s Health Initiative Silent Atrial Fibrillation Recording Study) ancillary study (NCT05366803). Women were excluded if they had a diagnosis of clinical AF on: self-report at baseline WHI enrollment, self-report on an annual follow-up questionnaire, AF on the baseline ECG in a randomized WHI trial, or a hospitalization for AF confirmed by review of medical records (Internal Classification of Diseases-9th Revision code 427.31 or Internal Classification of Diseases-10th Revision code I48.X). Participants who were not enrolled in Medicare Fee-for-Service were also excluded (n = 9,967), as preceding diagnoses of clinical AF could not be fully ascertained. WHISH participants with a ≥5% estimated risk of developing AF within 5 years, based on the CHARGE-AF score, were eligible for enrollment. CHARGE-AF estimates the 5-year risk for incident AF based on an individual’s age, race, height, weight, smoking history, systolic and diastolic blood pressure levels, hypertension treatment, diabetes, history of heart failure, and history of stroke and myocardial infarction.19 Eligible participants were selected to be invited to participate in systematic screening using an ECG monitoring patch.
The research protocol for this ancillary study was reviewed and approved by the Institutional Review Board at Stanford University.
AMBULATORY RHYTHM PATCH MONITOR AND AF ASCERTAINMENT.
Enrolled participants were mailed a single-lead Cardea SOLO ECG patch monitor (Cardiac Insight)25,26 to be self-applied to the chest, worn for up to 7 days and returned by mail for analysis. Patch monitoring was performed at baseline, 6 months, and 12 months. The first ECG patch monitor returned with interpretable ECG data was considered the baseline monitor, regardless of when the patch was performed.
AF was defined as at least 30 seconds of AF or atrial flutter on an ECG patch monitor recording.11,13 Episodes of AF identified by the ECG patch screening were over-read by 2 clinical cardiologists (V.F., M.V.P.), and disagreements in classification were resolved by a third cardiologist (M.W.). Episodes were adjudicated based only on ECG patch recording data. Persistent AF was defined as having AF throughout the entire monitoring period. Arrhythmia burden was defined as percentage of time (AF) or beats (premature atrial or ventricular beats, supraventricular tachycardia [SVT] episodes) spent in the abnormal rhythm over the monitoring period.
Participants with clinically urgent incidental findings (including ventricular tachycardia ≥6 beats, pauses ≥6 seconds, high-grade atrioventricular block, or ≥16% premature ventricular complexes) were notified of the abnormal finding and encouraged to speak with their physician for further management. Discovery of urgent incidental findings did not preclude participation with subsequent patch wears.
STATISTICAL ANALYSES.
Baseline characteristics in those with and without AF detected were compared by using 2-tailed Student’s t-tests for continuous demographic variables; chi-square tests were used for categorical variables for values >5 and Fisher’s exact tests for values ≤5. Logistic regression models adjusted for patch wear times were used to estimate odds ratios (95% CIs) for detected AF as a function of premature atrial complex burden, SVT episode burden, and alcohol use. Multivariate regression was then used to further adjust these for age. Statistical significance was defined as a 2-tailed P value <0.05.
For the primary WHISH-STAR study aim, we sought to enroll 1,054 participants with ECG patch monitor data to provide 80% power based on an alpha of 0.05 to detect a 3% difference in rates of AF between the intervention arms of the WHISH trial of physical activity intervention. Here, we report on initial analysis on AF prevalence in all participants, combined over intervention groups, with completed cardiac patch wears. All analyses were conducted by using SAS 9.4 for Windows (SAS Institute, Inc.).
RESULTS
BASELINE CHARACTERISTICS.
Of the 14,290 active participants in the WHISH trial, 4,791 women were randomly selected and invited to participate, and 1,257 (51.8%) of those who consented to be contacted were then consented to be enrolled in the study (Figure 1). A total of 1,067 (85%) participants successfully returned a baseline ECG patch monitor with readable data. Of those who returned a readable baseline monitor, 866 (81%) completed a second, 6-month monitor and 777 (73%) completed a third, 12-month monitor.
FIGURE 1. Study Enrollment.

Consort diagram for WHISH-STAR (Women’s Health Initiative Strong and Healthy Study) enrollment. Participants were excluded if they were deceased, had known atrial fibrillation (AF), or were not enrolled in Medicare Fee-for-Service at the time of selection. Women with a CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology)-AF 5-year predicted risk ≥5% for AF and participating in the WHISH trial were then randomly selected for recruitment into WHISH STAR. ECG = electrocardiogram.
The mean age of all participants who returned a readable baseline monitor was 79 years, and 92% were white, 4.6% were African American, and 1.5% were Hispanic (Table 1). Most (84%) received an education beyond high school, 70% had treated hypertension, 19% had treated diabetes, 5.8% had a history of coronary artery disease, and 0.7% had a history of heart failure. Most participants had a CHARGE-AF 5-year predicted risk between 5% and <10% (57.2% of the screened cohort), whereas 26.4% had a CHARGE-AF predicted risk between 10% and <15%, and the remaining 16.4% had a CHARGE-AF predicted risk ≥15%.
TABLE 1.
Baseline Characteristics According to Presence of Detected AF
| All Participants (N = 1,067) |
AF Detected (n = 52) |
No AF Detected (n = 1,015) |
P Value | |
|---|---|---|---|---|
|
| ||||
| Characteristic | ||||
| Age, y | 78.8 4.3 | 80.0 4.1 | 78.8 4.3 | 0.04 |
| Race/ethnicity | 0.42 | |||
| Non-Hispanic White | 981 (91.9) | 51 (98.1) | 930 (91.6) | |
| Non-Hispanic Black/African American | 49 (4.6) | 0 | 49 (4.8) | |
| Hispanic/Latina | 16 (1.5) | 1 (1.9) | 15 (1.5) | |
| American Indian/Alaskan Native | 4 (0.1) | 0 | 4 (0.4) | |
| Asian/Pacific Islander | 17 (1.6) | 0 | 17 (1.7) | |
| Education | 0.98 | |||
| High school or less/GED | 168 (15.7) | 8 (15.4) | 160 (15.8) | |
| School after high school | 395 (37.0) | 19 (36.5) | 376 (37.0) | |
| College degree or advanced degree | 501 (47.0) | 25 (48.1) | 476 (46.9) | |
| Region | 0.84 | |||
| Northeast | 259 (24.3) | 12 (23.1) | 247 (24.3) | |
| South | 289 (27.1) | 16 (30.8) | 273 (26.9) | |
| Midwest | 295 (27.6) | 12 (23.1) | 283 (27.9) | |
| West | 224 (21.0) | 12 (23.1) | 212 (20.9) | |
| Body mass index, kg/m2 | 28.6 5.9 | 29.8 7.2 | 28.5 5.9 | 0.15 |
| Medical history | ||||
| Treated hypertension | 745 (69.8) | 38 (73.1) | 707 (69.7) | 0.60 |
| Heart failure | 7 (0.7) | 0 | 7 (0.7) | >0.99 |
| Treated diabetes | 202 (18.9) | 10 (19.2) | 192 (18.9) | 0.96 |
| Stroke/TIA | 72 (6.7) | 2 (3.8) | 70 (6.9) | 0.57 |
| Peripheral artery disease | 14 (1.3) | 0 | 14 (1.4) | >0.99 |
| Treated hyperlipidemia | 566 (53.0) | 35 (67.3) | 531 (52.3) | 0.04 |
| Coronary artery disease | 62 (5.8) | 3 (5.8) | 59 (5.8) | >0.99 |
| Myocardial infarction | 30 (2.8) | 1 (1.9) | 29 (2.9) | >0.99 |
| CABG/PTCA | 50 (4.7) | 3 (5.8) | 47 (4.6) | 0.73 |
| Lifestyle | ||||
| Alcohol use, drinks/wk | 0.19 | |||
| <1 | 621 (58.2) | 24 (46.2) | 597 (58.8) | |
| 1 to <7 | 296 (27.7) | 19 (36.5) | 277 (27.3) | |
| ≥7 | 150 (14.1) | 9 (17.3) | 141 (13.9) | |
| Smoking | 0.62 | |||
| Never | 557 (52.2) | 25 (48.1) | 532 (52.4) | |
| Past | 480 (45.0) | 27 (51.9) | 453 (44.6) | |
| Current | 24 (2.2) | 0 | 24 (2.4) | |
| CHARGE-AF score, % | 10.8 6.1 | 13.4 7.2 | 10.7 6.0 | 0.002 |
Values are mean ± SD, or n (%) unless otherwise indicated. Baseline characteristics at the time of enrollment are shown. P values based on chi-square tests for variables with values >5, and Fisher’s exact tests for values ≤5.
AF = atrial fibrillation; CHARGE-AF = Cohorts for Heart and Aging Research in Genomic Epidemiology–AF; GED = general education development; CABG = coronary artery bypass graft; PTCA = percutaneous transluminal coronary angioplasty; TIA = transient ischemic attack.
AF AND ECG PATCH MONITORING.
Women wore the baseline ECG patch monitor for an average of 173.4 ± 39.1 hours, the 6-month monitor for 177.2 ± 33.7 hours, and the 12-month monitor for 176.4 ± 35.1 hours. AF was found in 27 women (2.5%) on baseline monitoring; additional patch monitoring at 6 months and 12 months increased the cumulative total of women with AF detected to 52 (4.9%) (Figure 2). Of the 846 participants who completed a 6-month monitor and did not have AF detected at baseline, 12 (1.4%) had AF detected on the second monitor. Of the 746 participants who completed both a 6- and 12-month monitor without AF detected on the baseline or 6-month monitor, 13 (1.7%) had AF detected on the third monitor. By two-sample Student’s t-test, there was no significant difference in analyzable patch hours between participants with silent AF detected (mean 406.8 ± 119.1 hours) and those with no silent AF detected (mean 377.6 ± 143.5 hours; P = 0.15). No episodes detected were adjudicated as atrial flutter.
FIGURE 2. Cumulative Rate of AF Detection During Patch Monitoring According to Age and CHARGE-AF Score.
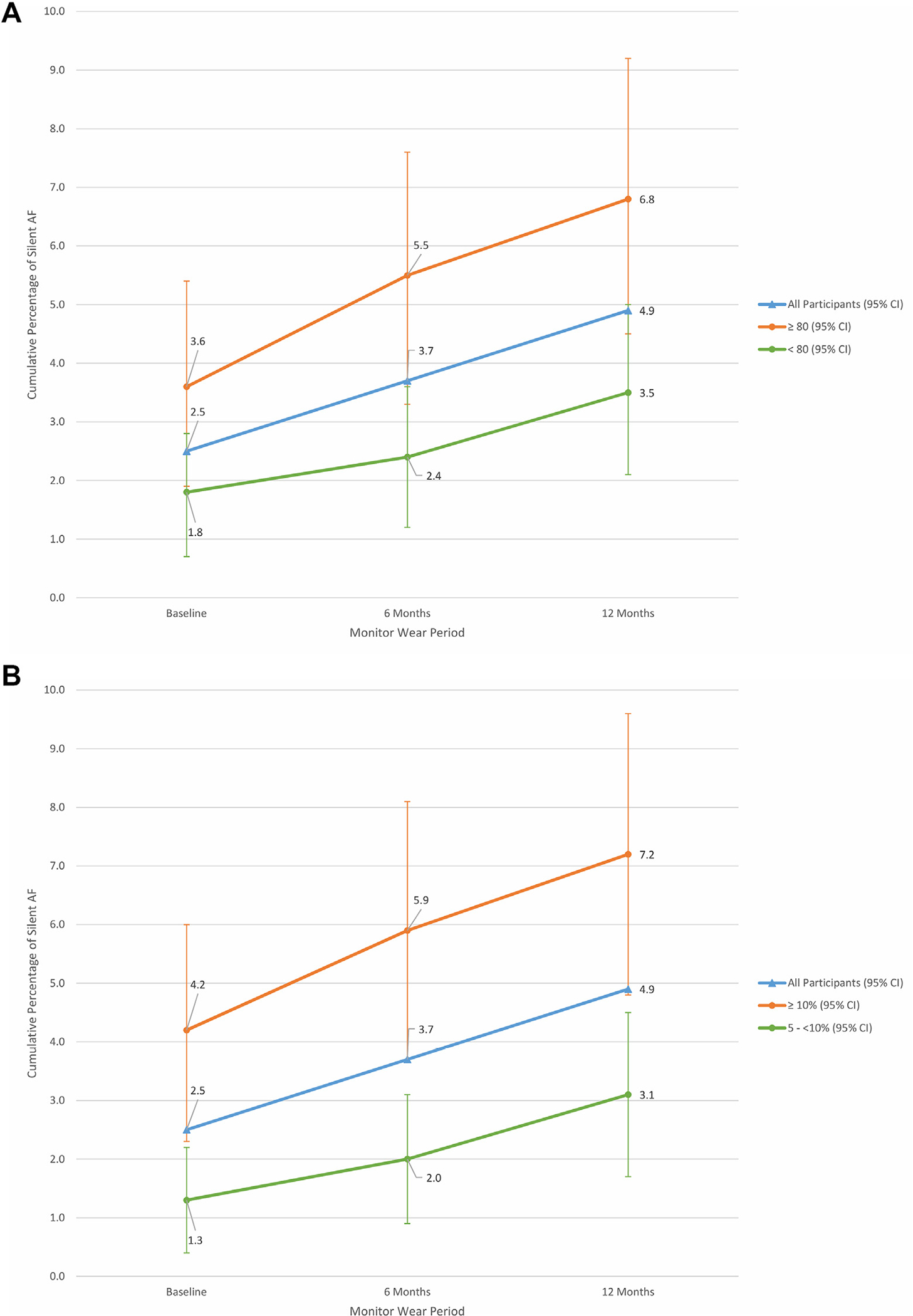
(A) Cumulative AF detection rates over 3 monitoring periods stratified according to age. Significantly more AF was detected in participants aged ≥80 years compared with those aged <80 years. Error bars represent 95% CIs. (B) Cumulative AF detection rates over 3 monitoring periods stratified according to CHARGE-AF scores. Significantly more AF was detected in participants with a CHARGE-AF estimated risk ≥10% compared with those with an estimated risk between 5% and <10%. Abbreviations as in Figure 1.
Participants with AF detected by ECG patch monitoring had very similar clinical profiles at baseline compared with participants without AF (Table 1), apart from slight differences in age (80 years vs 78.8 years; P = 0.04) and treated hyperlipidemia (67% vs 52%; P = 0.04). The mean CHARGE-AF 5-year risk estimate was significantly higher in those with AF detected on monitoring (13.4% vs 10.7%; P = 0.002), even after adjusting for age (P = 0.02), although the SD for age in the cohort was small. There was no significant difference in AF detection rates according to alcohol use defined as <1 drink per week (reference), 1 to 7 drinks per week, or ≥7 drinks per week.
AF was detected more often in participants aged ≥80 years compared with those aged <80 years: 6.8% vs 3.5% by 12 months (Figure 2A). Participants with higher CHARGE-AF scores (≥10% estimated 5-year risk) had a 7.2% rate of AF detected by 12 months, compared with 3.1% in those with an estimated 5-year risk between 5% and <10% (Figure 2B, Central Illustration).
CENTRAL ILLUSTRATION. Atrial Fibrillation Detection by CHARGE-AF Score in the Women’s Health Initiative.
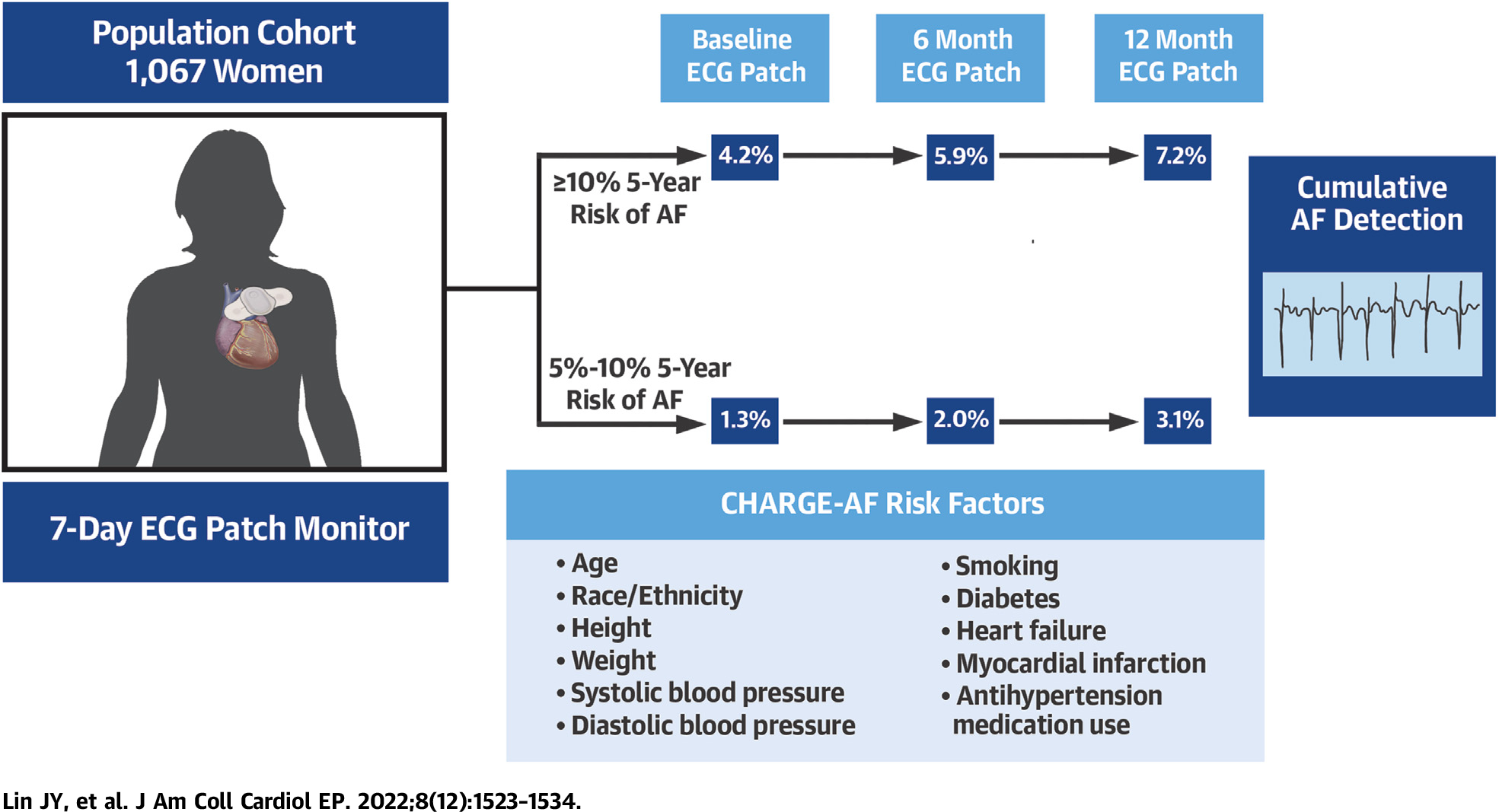
AF = atrial fibrillation; CHARGE-AF = Cohorts for Heart and Aging Research in Genomic Epidemiology-AF.
All participants with AF had at least one episode lasting >6 minutes (Figure 3). Of all the participants with detected AF, 24 (46%) had at least one episode that lasted >6 hours, and 13 (25%) had an episode that lasted >24 hours. Only one participant had an episode duration of ≤24 hours at baseline that increased to >24 hours on subsequent monitors.
FIGURE 3. Longest Episode of AF Detected.
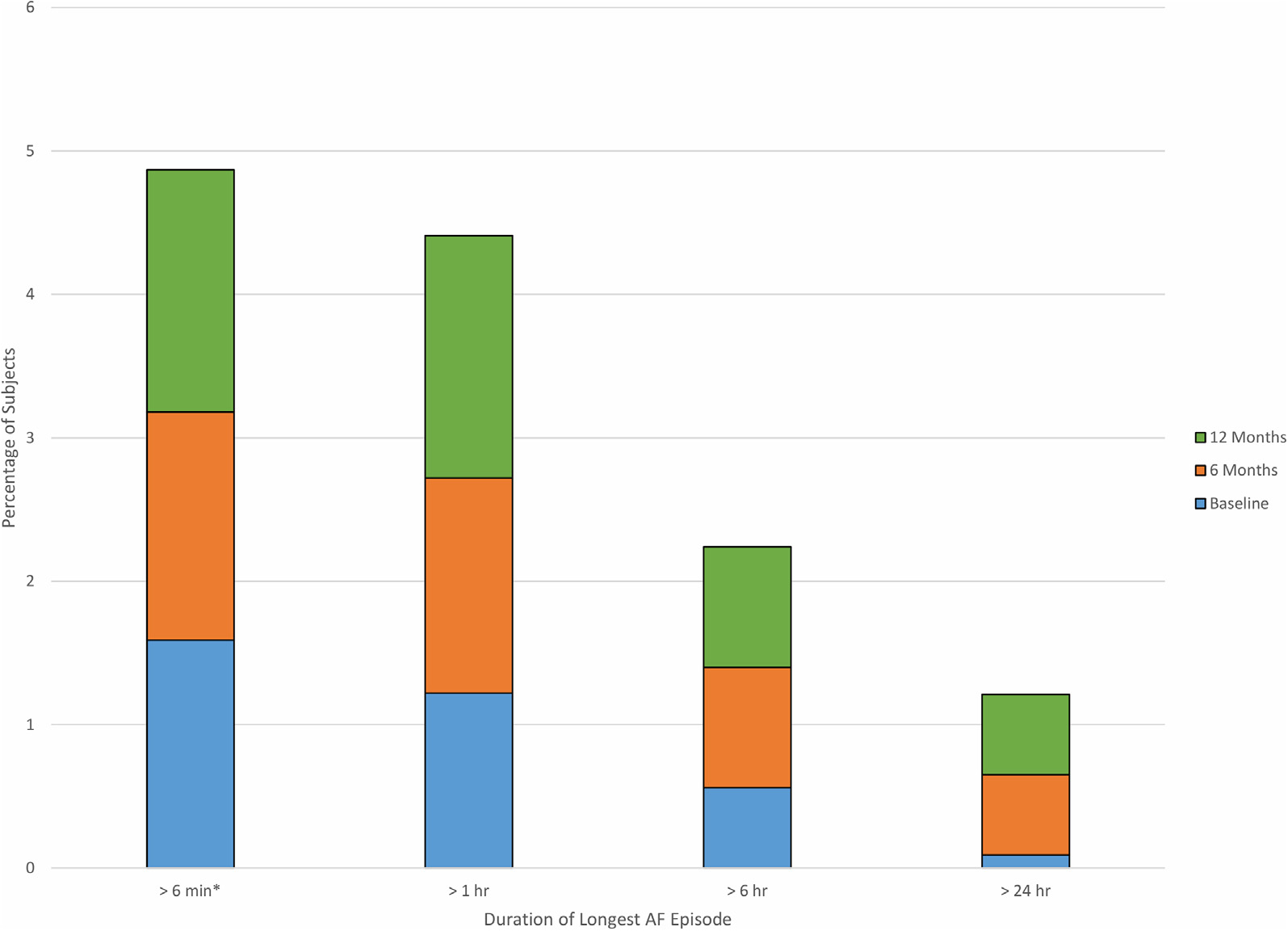
The longest episode of detected atrial fibrillation (AF) in any monitoring period for a single participant is shown, stratified according to the monitoring period in which the event occurred. Bars reflect the percentage of the total cohort, including those without AF detected. All AF episodes found were >6 minutes in duration.
The maximum burden of AF varied widely: 10 participants (19%) had a <1% AF burden, 14 participants (27%) had a 1% to <6% burden of AF, 18 participants (35%) had a 6% to 90% burden of AF, and 10 participants (19%) had >90% burden or persistent AF. Among the 52 participants with silent AF, there was no significant difference in longest AF episode (hours) between participants with CHARGE-AF ≥10% (mean 51.7 ± 77.7%) and those with CHARGE-AF <10% (mean 26.7 ± 59.8%; P = 0.23).
Of the 52 participants with AF detected, 46 were still enrolled in Medicare at the time of their first detected AF event. By a comparison with diagnosis codes in linked data from the Centers for Medicare & Medicaid Services, 3 (6.5%) of the 46 had clinical AF diagnosed after enrollment in our study but before AF detection by ECG patch. Seven (15.2%) had their first clinical AF event after AF detection by ECG patch. The remaining 36 (78.3%) participants had no clinical AF detected at any time during the study.
ECG PATCH MONITORING PREDICTORS OF SUBSEQUENT AF.
The risk of AF detection on subsequent monitors (ie, second and third monitors) based on baseline patch findings was also explored. Of the 885 participants without AF found on baseline patch monitoring, and with at least one subsequent monitor, 25 (2.8%) had AF identified on subsequent monitoring. Premature atrial complex (PAC) burden on baseline monitoring was associated with development of AF on a subsequent 6- or 12-month monitoring (Figure 4A). Compared with participants with <75 PACs per 24 hours, participants with ≥1,000 PACs per 24 hours had a higher likelihood of having AF on a subsequent patch monitor (odds ratio: 2.82 [95% CI: 1.08–7.38]; age-adjusted odds ratio: 2.70 [95% CI: 1.02–7.10]).
FIGURE 4. Burden of PACs and SVTs and the Likelihood of Detected AF.

(A) A high average premature atrial complex (PAC) burden of ≥1,000 per 24 hours on the baseline monitor was associated with a significantly higher risk of atrial fibrillation (AF) on the subsequent 6- or 12-month monitors compared with <75 per 24 hours (odds ratio: 2.82; 95% CI: 1.08–7.38). This association was not seen in those with an average PAC burden <1,000. (B) A high average supraventricular tachycardia (SVT) burden of ≥25 episodes per week on the baseline monitor was associated with a significantly higher risk of AF on the subsequent 6- or 12-month monitors compared with no episodes per week (odds ratio: 7.32; 95% CI: 2.11–25.45). For both panels, the odds ratios for the risk of having AF on any monitor (including baseline) are also shown. The numbers of participants in each category are reported below.
Episodes of SVTs lasting <30 seconds on the baseline patch monitor were similarly associated with AF on subsequent patch monitors (Figure 4B). Compared vs those with no SVT episodes, participants with ≥25 episodes per week had a significantly higher likelihood of later AF (odds ratio: 7.32 [95% CI: 2.11–25.45]; age-adjusted odds ratio: 7.13 [95% CI: 2.04–24.90]).
SKIN RASHES AND URGENT NOTIFICATIONS.
The patch monitors were generally well tolerated, but 4 (0.3%) patients had an immediate allergic reaction to the adhesive, and 44 (4.1%) had a reaction later during the baseline monitoring that resulted in discontinuation of the patch. An additional 20 (2.2%) participants had a reaction to the 6-month ECG patches significant enough to result in withdrawal from subsequent monitoring (Figure 1). None of the skin reactions required medical attention.
Of the 2,710 ECG patch monitors completed during the 3 time periods, 20 (0.7%) recordings had findings requiring notification in 17 unique participants. Fourteen patch monitors exhibited nonsustained ventricular tachycardia, with the longest episode lasting 24 seconds. Eight patch monitors showed a premature ventricular contraction burden of >15%, with the highest burden recorded at 36%. One patch monitor documented high-grade atrioventricular block. None of the 15 participants who were successfully contacted via telephone were symptomatic. The remaining 2 participants who could not be reached by telephone were sent a notification letter.
DISCUSSION
The value of systematic screening for AF has not been established. A recent National Institutes of Health workshop on research priorities for AF screening18 identified the key first steps to be: 1) establishing the population prevalence of asymptomatic, undiagnosed AF; and 2) identification of the populations in which asymptomatic AF is most likely to be found. The present study contributes data to address these questions. We found that, among older community-dwelling, higher risk women, previously undiagnosed episodes of AF were found in 2.5% on a single 7-day ECG patch monitor and in up to 4.9% after 3 screenings over 12 months. Participants with a higher CHARGE-AF clinical risk score19 had a higher yield of AF on screening, with twice the risk among participants with 5-year CHARGE-AF risk estimate of AF ≥10% compared with participants with a risk estimate between 5% and <10% (Central Illustration). Our study used a duration of at least 30 seconds to define AF, in line with older guidelines11,27 and other AF screening studies.13,14 All episodes of AF detected were >6 minutes in duration.
Several population cohort studies have recently explored the prevalence of silent AF in at-risk individuals.5,13,14,17,28 The ARIC (Atherosclerosis Risk in Communities) study found subclinical AF in 2.5% of participants on a single 2-week patch monitor, increasing to 4.1% in the subset who wore a second 2-week monitor.14 A recent study of older adults recruited from primary care clinics found a 5.3% subclinical AF burden in those randomized to screening with sequential 14-day monitors.29 Our study is the largest study to use serial monitoring to assess the incremental yield of ECG patches in a population selected on the basis of an elevated CHARGE-AF risk score.
In addition, we found that serial ECG patch monitoring spaced every 6 months over the course of a year increased the yield of identifying prolonged episodes of AF. It is notable that there was no clear leveling off in the yield of newly detected AF between the second and third monitors, implying that additional monitoring could result in similar additional yield. This dose-response relationship should be explored further in future studies. We also found evidence that the burden of AF increased over follow-up. Only one participant was found to have >24-hour episodes of AF at baseline, but 6 had prolonged AF by the third monitor. Similarly, the percentage of participants with AF that had episodes >6 hours at baseline increased from 12% to 17% by the third monitor. Although the overall prevalence of these prolonged AF episodes was low (2.2% for >6 hours and 1.2% for >24 hours), the evidence suggests that those with prolonged episodes of AF are at greatest risk of stroke.30,31
Our finding that the burden of PACs and of SVT is a predictor of subsequent development of AF is consistent with prior reports that atrial ectopy is associated with a subsequent development of AF.32–35 The use of a risk prediction model such as CHARGE-AF in combination with baseline PAC/SVT burden may support a more cost-effective strategy in AF screening and should be studied further. The data we present suggest that it may be most efficient, for example, to screen high-risk populations for AF with a baseline monitor but then offer subsequent monitoring only to those with frequent PACs or SVTs.
STUDY LIMITATIONS.
The WHI reflects a long-standing, well-characterized community-dwelling cohort that has maintained a high level of commitment to participation. Although the successful ECG patch completion of at least one monitor from all participants who were contacted for the study was only 22%, this is higher than in similar population-based screening studies.13,31 In addition, the retention rate of those who completed 6- and 12-month screening (81% and 73%, respectively) is noteworthy but may exceed follow-up screening rates attainable in clinical practice. Generalizability to other demographic characteristics may also be limited; although attempts were made to oversample Hispanic and African-American populations, the enrolled WHISH-STAR cohort consisted of predominantly older, white postmenopausal women. The study population was sampled from an ongoing randomized clinical trial, and we remain blinded to the intervention assignment; thus, the analyses presented here cannot take into account randomization status. Additional multivariate analyses were also not reported due to the small sample size with detected AF. Pre-existing AF was ascertained at the time of study enrollment, and therefore some participants with AF detected may have manifested clinical AF over the course of the study. The presence of valvular heart disease is also not considered in the CHARGE-AF prediction score. Most importantly, the threshold for the burden of AF necessary to result in benefit from long-term anticoagulation has not yet been determined. The recently published LOOP (Implantable loop Recorder Detection of Atrial Fibrillation to Prevent Stroke) study,36 for example, showed no significant reduction in stroke or arterial embolism in study participants who underwent AF screening using an implantable loop recorder and started on anticoagulation for AF episodes over 6 minutes. However, these findings may not translate to those with AF detected by serial, intermittent short-term monitoring as in our study. Other randomized clinical trials (eg, NOAH [Non-Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes], ARTESiA [Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation]) are ongoing to help determine whether anticoagulation could be beneficial at different AF duration cutoffs.31 Finally, 0.7% of ECG patches completed revealed other findings that prompted participant notification according to our study protocol; the risks of downstream testing and treatment for incidental findings should be acknowledged when considering long-term rhythm monitoring for AF screening. In the mSToPS (mHealth Screening to Prevent Strokes) trial, for example, pacemaker and/or defibrillator implantation occurred in 0.9% of the screened cohort vs none in the control group.37
CONCLUSIONS
This large-scale study found that ECG patch monitoring is an effective screening tool for AF and that its yield can be increased by use of a clinical risk prediction score. Repeated monitoring identifies additional individuals with asymptomatic AF, particularly among those with evidence of PACs and SVT on the initial monitor. Long-term outcomes of this population are not yet known, but our findings suggest that ECG patch monitoring combined with clinical risk scoring can identify high-risk participants for enrollment in future studies of the management of asymptomatic AF.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
In this prospectively screened cohort of 1,257 women at-risk for AF in the WHI, significantly more AF was detected in those with an elevated CHARGE-AF score.
TRANSLATIONAL OUTLOOK:
Prospective trials are of paramount importance to determine whether clinical intervention in asymptomatic AF improves outcomes.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services, through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, and 75N92021D00005. This ancillary study was funded through 1R01HL136390. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- ECG
electrocardiogram
- PAC
premature atrial complex
- SVT
supraventricular tachycardia
- WHI
Women’s Health Initiative
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Kerr C, Boone J, Connolly S, et al. Follow-up of atrial fibrillation: the initial experience of the Canadian Registry of Atrial Fibrillation. Eur Heart J. 1996;17(suppl C):48–51. [DOI] [PubMed] [Google Scholar]
- 2.Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation. 30-Year follow-up in the Framingham Study. JAMA. 1985;254:3449–3453. [PubMed] [Google Scholar]
- 3.Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol. 2000;4:369–382. [DOI] [PubMed] [Google Scholar]
- 4.Hannon N, Sheehan O, Kelly L, et al. Stroke associated with atrial fibrillation—incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc Dis. 2010;29:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanna T, Diener H-C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 6.Healey JS, Connolly SJ, Gold MR, et al. Sub-clinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 7.Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614–1619. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez M, Keating RJ, Markowitz SM, et al. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm. 2014;11:2214–2221. [DOI] [PubMed] [Google Scholar]
- 9.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke. Stroke. 2014;45:3754–3832. 10.1161/str.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 11.Mairesse GH, Moran P, Van Gelder IC, et al. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). Europace. 2017;19:1589–1623. [DOI] [PubMed] [Google Scholar]
- 12.US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for atrial fibrillation: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327:360–367. [DOI] [PubMed] [Google Scholar]
- 13.Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA. 2018;320:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rooney MR, Soliman EZ, Lutsey PL, et al. Prevalence and characteristics of subclinical atrial fibrillation in a community-dwelling elderly population: the ARIC Study. Circ Arrhythm Electrophysiol. 2019;12:e007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation. 2015;131:2176–2184. [DOI] [PubMed] [Google Scholar]
- 16.Philippsen TJ, Christensen LS, Hansen MG, Dahl JS, Brandes A. Detection of subclinical atrial fibrillation in high-risk patients using an insertable cardiac monitor. J Am Coll Cardiol EP. 2017;3:1557–1564. [DOI] [PubMed] [Google Scholar]
- 17.Heckbert SR, Austin TR, Jensen PN, et al. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: the Multi-Ethnic Study of Atherosclerosis. J Electrocardiol. 2018;51:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamin EJ, Go AS, Desvigne-Nickens P, et al. Research priorities in atrial fibrillation screening: a report from a National Heart, Lung, and Blood Institute Virtual Workshop. Circulation. 2021;143:372–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulman E, Kargoli F, Aagaard P, et al. Validation of the Framingham Heart Study and CHARGE-AF risk scores for atrial fibrillation in Hispanics, African-Americans, and Non-Hispanic Whites. Am J Cardiol. 2016;117:76–83. [DOI] [PubMed] [Google Scholar]
- 21.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S78–S86. [DOI] [PubMed] [Google Scholar]
- 22.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 23.Zelen M A new design for randomized clinical trials. N Engl J Med. 1979;300:1242–1245. 10.1056/nejm197905313002203 [DOI] [PubMed] [Google Scholar]
- 24.Stefanick ML, King AC, Mackey S, et al. Women’s Health Initiative Strong and Healthy Pragmatic Physical Activity Intervention Trial for cardiovascular disease prevention: design and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2021;76:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christle JW, Hershman SG, Soto JT, Ashley EA. Mobile health monitoring of cardiac status. Ann Rev Biomedic Data Sci. 2020;3:243–263. 10.1146/annurev-biodatasci-030220-105124 [DOI] [Google Scholar]
- 26.Cardea SOLO ECG System. 2020. Accessed September 10, 2021. https://www.cardiacinsightinc.com/cardea-solo-2/
- 27.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Healey JS, Alings M, Ha A, et al. Subclinical atrial fibrillation in older patients. Circulation. 2017;136:1276–1283. [DOI] [PubMed] [Google Scholar]
- 29.Gladstone DJ, Wachter R, Schmalstieg-Bahr K, et al. Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol. 2021;6:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Gelder IC, Healey JS, Crijns HJGM, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 31.Noseworthy PA, Kaufman ES, Chen LY, et al. Subclinical and device-detected atrial fibrillation: pondering the knowledge gap: a scientific statement from the American Heart Association. Circulation. 2019;140:e944–e963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dewland TA, Vittinghoff E, Mandyam MC, et al. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nortamo S, Kinta TV, Ukkola O, Huikuri HV, Poriomania JS. Supraventricular premature beats and risk of new-onset atrial fibrillation in coronary artery disease. J Cardiovasc Electrophysiol. 2017;28:1269–1274. [DOI] [PubMed] [Google Scholar]
- 34.Acharya T, Tringali S, Bhullar M, et al. Frequent atrial premature complexes and their association with risk of atrial fibrillation. Am J Cardiol. 2015;116:1852–1857. [DOI] [PubMed] [Google Scholar]
- 35.Binici Z, Intzilakis T, Nielsen OW, Køber L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. 10.1161/circulationaha.109.874982 [DOI] [PubMed] [Google Scholar]
- 36.Svendsen JH, Diederichsen SZ, Højberg S, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet. 2021;398:1507–1516. [DOI] [PubMed] [Google Scholar]
- 37.Waalen J, Edwards AM, Sanyal A, et al. Healthcare resource utilization following ECG sensor patch screening for atrial fibrillation. Heart Rhythm O2. 2020;1:351–358. 10.1016/j.hroo.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


