Summary
Introduction
The COVID-19 pandemic is having a huge impact on human health with high morbidity and mortality rates worldwide. Healthcare Workers (HCWs) are one of the most at risk categories to contract the infection. Effective anti-COVID-19 vaccines were approved in a very short time. Making the 1st booster dose is essential to induce a good protection against the infection.
Methods
We conducted a retrospective sero-epidemiological survey of already existing data concerning the antibody response of a HCWs sample vaccinated with the primary cycle and the 1st booster dose of the Pfizer-BioNTech COVID-19 mRNA vaccine and, specifically, after three weeks from the third dose of vaccination.
Results
In our analysis, after the primary cycle, a 95.15% efficacy was detected. Among the non-responders, women were significantly more frequent (69.56%). Moreover, we found a significant reverse correlation between the immune response and the age of the sample, especially in women. However, the 1st booster dose completely cancelled these differences.
Conclusions
Our data are perfectly in line with what has been declared by the conducted studies in terms of efficacy. However, it is important to highlight that people with only the primary cycle are at high risk to contract the COVID-19 infection. Therefore, it is necessary to not consider people vaccinated with the primary cycle completely risk-free and to stress the importance to perform the 1st booster dose.
Keywords: COVID-19 infection, COVID-19 Vaccination, Booster dose, Seroprevalence, Prevention
Introduction
The COVID-19 (Coronavirus Disease-19) pandemic with its huge impact on human health in terms of morbidity and mortality and on the environmental issues, shocked the community and gave a huge boost to the scientific research worldwide [1, 2]. The cause of this pandemic is the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a new member of Coronavirus family (CoVs), responsible for high-risks pathological respiratory diseases [3]. This virus is surrounded by an external envelope containing a glycoprotein, called “spike” or “S” protein, forming spicules that allows it to recognize the cellular target [4].
Vaccination is undoubtedly one of the most effective preventive measures towards infectious diseases [5]. Traditionally, it takes between 4 to 15 years to develop a safe, efficacious, and cost-effective human vaccine. In comparison to these times, COVID-19 vaccine development has progressed at an unprecedented rate. On January 11, 2020, less than a month after the first documented cases in Wuhan, the complete viral genome sequence of SARS-CoV-2 was obtained [6]. Due to this crucial information, several laboratories and biotechnology companies globally have tried to produce an effective SARS-CoV-2 vaccine. Due to the knowledge obtained from previous vaccine development efforts against SARS and MERS, the initial step of target identification was remarkably facilitated as, for both SARS-CoV and MERS-CoV, the S protein had been demonstrated as the dominant antigen responsible for the production of neutralizing antibodies and an important target of protective T cell responses [7]. Several vaccine candidates for SARS-CoV-2 were then planned rapidly using the SARS-CoV-2 S protein as the primary immunogen.
mRNA vaccines are a new platform consisting of an mRNA, including a 5′ cap, regulatory elements in the 5′ and/or 3′ regions, a poly(A) tail, and modified nucleosides to increase RNA stability, that is elaborated to encode specific viral antigens and delivered by polymer-based or lipid nanoparticles [8]. Once entered into the cell cytoplasm, the mRNA is translated by ribosomes as an endogenous mRNA, with production of the antigen that is presented to the immune system. Two mRNA vaccines, Pfizer/BioNtech’s BNT162b2 and Moderna/NIAID’s mRNA-1273, received the Emergency Use Authorization (EUA) status from the U.S. Food and Drug Administration (FDA) and other countries [9, 10] among which that from the European Medicines Agency (EMA) for the European Union (EU) [11, 12]. Specifically, BNT162b2 is an mRNA-lipid nanoparticle-formulated vaccine encoding a membrane-bound, stabilized form of the full-length SARS-CoV-2 S protein [13]. mRNA-1273 encodes a prefusion stabilized form of the SARS-CoV-2 S protein and is delivered by lipid-encapsulated nanoparticles [14].
In natural infection, seroconversion for SARS-CoV-2 was described to occur 7–14 days after the onset of symptomatology, 100% within 19 days after clinical onset [15]. Several serologic investigations suggest that, in affected areas, SARS-CoV-2 infection has been acquired by a much higher number of persons compared to the number of infected people resulted positive to PCR analysis of nasopharyngeal swab specimens [15, 16].
In Italy, at 28 September 2022, from the beginning of the outbreak 22,567,577 cases were reported with a median age of 43 years and 173,368 deaths [17]. The vaccination campaign in Italy began on December 27, 2020. As of 28 September 2022, a total of 140,732,854 doses were administered (47,320,682 first doses, 49,975,928 second/single doses, 40,152,811 third doses). COVID-19 infection can be a problem especially for vulnerable and/or particularly at risk people such as people affected by comorbidities, obesity and pregnant women [18-20]. Among people particularly at risk to contract the infection, Healthcare Workers (HCWs) are surely one of the most exposed category. A meta-analysis assessed that the overall proportion of HCWs testing positive for SARS-CoV-2 was 8.7% among all enrolled subjects. Moreover, it has been reported that the values of seroprevalence among HCWs ranges from 0% to 45.3% [21]. These remarkable differences could be explained by different settings, observation period, and government strategies adopted with the aim of reducing viral transmission (e.g., lockdown, quarantine measures, etc.) [22]. In Italy, during the first wave of the infection, 421,521 (1.85% of the total reported cases) HCWs, of which 70.3% women and 29.7% men, with a median age of 47 years, have been reported to be infected since the beginning of the pandemic. In this category, 328 (0.2%) deaths were reported of which 226 (68.9%) were men and 102 (31.1%) were women with lethality rates of 0.6% and 0.1% in the two sexes respectively [23].
The purpose of this study was to evaluate the seroconversion rate of a population of HCWs vaccinated with the primary cycle and the 1st booster doses of COVID-19 mRNA vaccine, highlighting the role of the booster and the individual variables in the antibody response elicited by the vaccine.
Methods
DATA COLLECTION
The sample was made up by HCWs of the Messina University Hospital “G. Martino”, Italy, to whom the primary cycle and the 1st booster of BNT162b2 COVID-19 mRNA vaccine (Pfizer/Biontech) were administered. As exclusion criterion, a previous COVID-19 infection was considered. Therefore, people that stated to have contracted the infection and/or already underwent only a single dose of vaccine were excluded. Aiming to evaluate the efficacy of the vaccination cycle, we carried out a survey, in the period April-May 2022, evaluating the antibody response rate of the HCWs three weeks after the administration of the second dose of vaccine and three weeks after the administration of the 1st booster dose. To this aim, after obtaining the informed consent of all the participants including all the information about the study, we collected blood samples that were centrifuged at 4,000 rpm for 10 minutes and a CLIA (ChemiLuminescence ImmunoAssay) test (LIAISON SARS-CoV-2 S1/S2 IgG - DIASORIN S.p.A., Saluggia, Italia), consisting in a quantitative assay for the detection of IgG antibodies against S1/S2 antigens of SARS-CoV-2, was used. Specifically, values < 12 AU/ml were considered negative. The results were correlated with the individual variables (age and gender) to evaluate their potential role played influenced the immune response.
STATISTICAL ANALYSES
Descriptive statistics were used to find the percentages. Correlations were determined using the standard Pearson correlation coefficient. Significance was assessed at the p < 0.05 levels. All analyses were performed using Prism 4.0 software.
Results
The characteristics of the sample, concerning the total number of people, their gender, occupation and hospital areas, are shown in Table I.
Tab. I.
Personal details and features of the sample.
| Total sample | ||
|---|---|---|
| 480 | ||
| Men | Women | |
| Absolute Number (%) | 286 (59.58%) | 194 (40.42%) |
| Average Age (±SD) | 45.97 ± 12.39 (min 25; max 67) | 48.01 ± 12.01(min 25; max 67) |
| Occupation (%) | ||
| Physicians | 39.23% | |
| Nurses | 36.92% | |
| Technicians | 6.15% | |
| Administrative personnel | 4.62% | |
| Biologists | 3.08% | |
| Pharmacists | 3.08% | |
| Others | 2.31% | |
| Hospital areas (%) | ||
| Medical | 36.93% | |
| Surgical | 19.32% | |
| Emergency | 9.09% | |
| Services | 34.66% | |
The immune response to the primary vaccination cycle was 95.15% with a mean antibody response of 214.62 AU/mL (min 3.8; max ≥ 400). Dividing according the gender, the mean antibody response was 224.53 AU/mL (min 3.8; max ≥ 400) in men and 205.27 AU/mL (min 3.8; max ≥ 400) in women. To the 1st booster does the immune response was 100% (68.75% of the sample reached the maximum value ≥ 400) with a mean antibody response of 352.21 (min. 251; max ≥ 400).
According to the antibody titre of the primary cycle, we divided the sample in four groups, specifically <12 = no response, 12-99 = low response, 100-299 = intermediate response and > 300 = high response, in order to better characterised the different responses to the vaccine evaluating for each group the role played by some individual variables such as age and gender (Fig. 1).
Fig. 1.
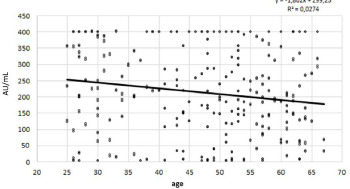
Statistical correlation between the immune response and the age of the sample.
The figure shows that 4.85% of the sample was negative to the antibody search. Among these negative people, women were significantly more frequent than men with percentages of 69.56% and 30.44% respectively (P < 0.05). In general, about these non-responder people, the mean age was 46.26 ± 10.81, specifically 51.43 ± 11.59 in men and 44.88 ± 9.61 in women. Moreover, 78.48% of the entire sample shows an intermediate-high response, with an average age of 46.76 ± 12.62. Specifically, 61.29% were women and 38.71% were men, with a mean age of 46.33 ± 12.58 and 47.44 ± 12.66 years, respectively. For the three groups of responders, there were no significant statistical differences by gender and age.
A significant reverse correlation (P < 0.05) was found between the immune response and the age of the sample (Fig. 2).
Fig. 2.
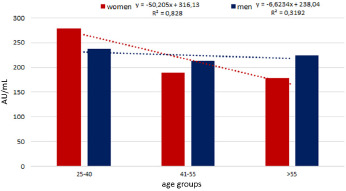
Different antibody response according to gender and age groups.
No correlation was found between immune response and gender. However, dividing according the gender, this significance was found only for the women group (Fig. 3).
Fig. 3.
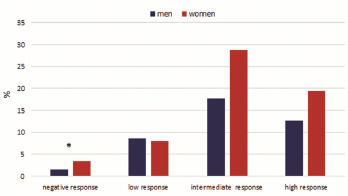
Percentage results of antibody response levels divided per gender.
As shown by the figure, an important percentage decrease (35.97) was found in the immune response between the youngest and oldest women while in men such percentage decrease was 5.59. Moreover, among men, a slight percentage increase (4.74%) was found in the oldest compared to the second group.
In the first age group the immune response was higher in the women with a percentage increase of 17.78 compared to men. An opposite result was found in the others two age groups in which the response was higher in men with a percentage increase of 11.39 and 20.12 in the two age groups respectively.
No statistical correlation between the individual variables and the immune response was observed after the 1st booster dose.
Discussion
HCWs are surely one of the most important categories at risk to contract COVID-19 [24, 25] and infections in general due to the exposure with potential infected people during their working activity [26, 27]. For this reason, according to the current Italian National Vaccination Plan, it is important to protect these at high risk people with the active offer of some strongly recommended vaccinations [28]. This issue applies also and above all concerning the COVID-19 infection. According to a recent meta-analysis of studies evaluating the positivity of SARS-CoV-2 antibodies on HCWs after natural infection, an overall result of 8.7%, ranging from 0% to 45.3%, was found [21].
Indeed, the occurrence of nosocomial transmission of SARS-CoV-2 has been reported, highlighting the necessity for HCWs of a strict adherence to infection control measures in order to protect themselves and avoid the transmission to inpatients and the onset of nosocomial outbreaks [29]. In this light, vaccination is undoubtedly the cornerstone of the control measures. Data from phase 3 clinical trials showed a 95% efficacy for the prevention of the infection at 7 days after the second dose of the BNT162b2 vaccine [30].
Unfortunately, vaccine hesitancy is an attitude more and more present in general population, linked to several wrong beliefs about vaccines among which fear of potential side effects and lack of trust towards health providers are the most declared [31]. This attitude is even present among HCWs, as showed by previous studies [32]. However, some reviews reported in this category a high rate of acceptance of COVID-19 vaccination [33, 34].
In our study, the antibody response of HCWs to vaccine was closely similar to that reported for general population [17]. After primary cycle, a significant correlation was found between the immune response and age especially in women. Specifically, the response decreased with the increasing of the age suggesting the important role played by the physiological process of immunosenescence [35]. The immune changes linked to the senescence explain the higher severity of some viral and bacterial infections (e.g., influenza, herpes zoster, pneumococcal disease) among elderly compared to younger individuals, and of the onset of more acute and long-term sequelae [36]. Moreover, in this category of people, vaccine responses are often lower and frequently failing to induce long-term immunity, placing these individuals at risk for subsequent disease [37]. These findings in elderly people have been largely linked to the failure of the adaptive immune response. Specifically, in elderly a lower antibody response is elicited by diphtheria [38], hepatitis A [39], Hepatitis B [40] and pneumococcal polysaccharide (PPV23) [41] vaccinations.
However, this result was more marked in women compared to men and this could be explained by the presence, in aged women, of some variables influencing negatively the antibody response. Particularly, it has long been proven that, in women, menopause could negatively impact on the immune system and the response to vaccination. Previous evidences showed that in postmenopausal and with surgical menopause women, a reduced number of total lymphocytes, mainly B and CD4+ T lymphocytes, is present and that the CD4+/CD8+ ratio and the numbers of circulating B cells are decreasing, while NK cells are increasing [42]. These changes in immune system could explain the difference found between genders in our study. On the contrary, in younger group, the response was higher in women compared to men. This result can be explained by the difference existing between sexes in the antibody production. It has been reported that females produce more elevated circulating levels of antibodies than males following the positive influence exerted on the humoral immune responses by estrogens [43, 44]. On the other hand, androgens play a very important role in modulating negatively the immune responses by affecting both the innate and the adaptive immune system (immunosuppressive action) [45]. These hormonal differences can account for the discrepancies between females and males found on the onset of different kind of diseases among which autoimmune diseases, but also in response to infectious diseases and vaccination [46].
It is important to emphasize that the 1st booster dose completely annulled the amount of non-responders to the primary cycle even if no statistical correlation was found between individual variables and immune response probably because the majority of the sample (68.75%) had a response ≥ 400 AU/mL and, therefore, hypothetical correlations were masked by this limit. Therefore, it is important to highlight the essential role played by the 1st booster dose in the complete protection against COVID-19 infection. This is important not only for HCWs but especially for general population. Indeed, it has been estimated that about 20% of general population in Italy has not yet received the 1st booster dose and is, therefore, at risk to get the infection with more severe clinical outcomes [47].
Conclusions
In conclusion, our data are perfectly in line with what has been declared by the conducted studies in terms of vaccine efficacy. Considering that HCWs are at first line in the fight against COVID-19 and, therefore, at high risk to contract and, eventually, spread the infection in nosocomial settings, it is important to highlight that, even if only a very little portion of the sample did not produce antibodies after the primary vaccination cycle, this risk was completely cancelled by the 1st booster dose. Moreover, individual variables as age and gender played an important role in the immune response to the primary cycle putting potentially at risk to get the infection and to have negative clinical outcomes more vulnerable people. However, the 1st booster dose produced a good immune response in all the sample independently from the individual variables. This finding is valid also for the general population on which the importance of the 1st booster dose should be stressed through a general and correct information.
Conflict of interest
The authors declare no conflict of interest.
Funding
None.
Ethics approval
The study has been performed in accordance with the Declaration of Helsinki and has been approved by the Ethics Committee of the University Hospital “G. Martino”, Messina, Italy (protocol number 1860 of 05/10/2022).
Authors’ contributions
Conceptualization: ADP, SC and DLG. Methodology: AF and GV. Formal analysis, data curation and writing - original draft: GV and AF. Resources: AB and AL. All Authors revised the manuscript and gave their contribution to improve the paper. All authors read and approved the final manuscript.
Figures and tables
References
- [1].Miyah Y, Benjelloun M, Lairini S, Lahrichi A. COVID-19 Impact on Public Health, Environment, Human Psychology, Global Socioeconomy, and Education. Sci World J 2022;2022:5578284. https://doi.org/10.1155/2022/5578284 10.1155/2022/5578284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Facciolà A, Laganà P, Caruso G. The COVID-19 pandemic and its implications on the environment. Environ Res 2021;201:111648. https://doi.org/10.1016/j.envres.2021.111648 10.1016/j.envres.2021.111648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burki T. Outbreak of coronavirus disease 2019. Lancet Infect Dis 2020;20:292-3. https://doi.org/10.1016/S1473-3099(20)30076-1 10.1016/S1473-3099(20)30076-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Malik YS, Sircar S, Bhat S, Sharun K, Dhama K, Dadar M, Tiwari R, Chaicumpa W. Emerging novel coronavirus (2019-nCoV) –current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q 2020;40:68-76. https://doi.org/10.1080/01652176.2020.1727993 10.1080/01652176.2020.1727993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].World Health Organization (WHO). Immunization. Available at: https://www.who.int/news-room/facts-in-pictures/detail/immunization#:~:text=Immunization%20prevents%20deaths%20every%20year,cost%2Deffective%20public%20health%20interventions (Accessed on: 07/07/2022).
- [6].Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270-3. https://doi.org/10.1038/s41586-020-2012-7 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yong CY, Ong HK, Yeap SK, Ho KL, Tan WS. Recent advances in the vaccine development against Middle East respiratory syndrome-coronavirus. Front Microbiol 2019;10:1999. https://doi.org/10.3389/fmicb.2019.01781 10.3389/fmicb.2019.01781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov 2018;17:261-79. https://doi.org/10.1038/nrd.2017.243 10.1038/nrd.2017.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cohen J. “Absolutely remarkable”: No one who got Moderna’s vaccine in trial developed severe COVID-19. Available at: https://www.sciencemag.org/news/2020/11/absolutely-remarkable-no-one-who-got-modernas-vaccine-trial-developed-severe-covid-19 (Accessed on: 30/07/2022).
- [10].World Health Organization (WHO). DRAFT Landscape of COVID-19. Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (Accessed on: 04/08/2022).
- [11].European Medicines Agency (EMA). EMA recommends COVID-19 Vaccine Moderna for authorisation in the EU. Available at: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-moderna-authorisation-eu. Accessed on 05/08/2022.
- [12].European Medicines Agency (EMA). EMA recommends first COVID-19 vaccine for authorisation in the EU. Available at: https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu (Accessed on: 10/08/2022).
- [13].Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Şahin U, Gruber WC. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med 2020;383:2439-50. https://doi.org/10.1056/nejmoa2027906 10.1056/nejmoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O’Dell S, Schmidt SD, Swanson PA, 2nd, Padilla M, Mascola JR, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS, Beigel JH; mRNA-1273 Study Group. An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med 2020;383:1920-31. https://doi.org/10.1056/NEJMoa2022483 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, Liao P, Qiu JF, Lin Y, Cai XF, Wang DQ, Hu Y, Ren JH, Tang N, Xu YY, Yu LH, Mo Z, Gong F, Zhang XL, Tian WG, Hu L, Zhang XX, Xiang JL, Du HX, Liu HW, Lang CH, Luo XH, Wu SB, Cui XP, Zhou Z, Zhu MM, Wang J, Xue CJ, Li XF, Wang L, Li ZJ, Wang K, Niu CC, Yang QJ, Tang XJ, Zhang Y, Liu XM, Li JJ, Zhang DC, Zhang F, Liu P, Yuan J, Li Q, Hu JL, Chen J, Huang AL. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020;26:845-8. https://doi.org/10.1038/s41591-020-0897-1 10.1038/s41591-020-0897-1 [DOI] [PubMed] [Google Scholar]
- [16].Percivalle E, Cambiè G, Cassaniti I, Nepita EV, Maserati R, Ferrari A, Di Martino R, Isernia P, Mojoli F, Bruno R, Tirani M, Cereda D, Nicora C, Lombardo M, Baldanti F. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill 2020;25:25. https://doi.org/10.2807/1560-7917.ES.2020.25.24.2001031 10.2807/1560-7917.ES.2020.25.24.2001031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Epicentro. Sorveglianza integrata COVID-19: i principali dati nazionali. Available at: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_28-settembre-2022.pdf (Accessed on: 20/08/2022).
- [18].Balik M, Svobodova E, Porizka M, Maly M, Brestovansky P, Volny L, Brozek T, Bartosova T, Jurisinova I, Mevaldova Z, Misovic O, Novotny A, Horejsek J, Otahal M, Flaksa M, Stach Z, Rulisek J, Trachta P, Kolman J, Sachl R, Kunstyr J, Kopecky P, Romaniv S, Huptych M, Svarc M, Hodkova G, Fichtl J, Mlejnsky F, Grus T, Belohlavek J, Lips M, Blaha J. The impact of obesity on the outcome of severe SARS-CoV-2 ARDS in a high volume ECMO centre: ECMO and corticosteroids support the obesity paradox. J Crit Care 2022;72:154162. https://doi.org/10.1016/j.jcrc.2022.154162 10.1016/j.jcrc.2022.154162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Facciolà A, Micali C, Visalli G, Venanzi Rullo E, Russotto Y, Laganà P, Laganà A, Nunnari G, Di Pietro A. COVID-19 and pregnancy: clinical outcomes and scientific evidence about vaccination. Eur Rev Med Pharmacol Sci 2022;26:2610-26. https://doi.org/10.26355/eurrev_202204_28499 10.26355/eurrev_202204_28499 [DOI] [PubMed] [Google Scholar]
- [20].Morrison FJ, Su M, Turchin A. COVID-19 outcomes in patients taking cardioprotective medications. PLoS One 2022;17:e0275787. https://doi.org/10.1371/journal.pone.0275787 10.1371/journal.pone.0275787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in health care workers: A systematic review and meta-analysis. J Hosp Infect 2021;108:120-34. https://doi.org/10.1016/j.jhin.2020.11.008 10.1016/j.jhin.2020.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lai CC, Wang CY, Wang YH, Hsueh SC, Ko WC, Hsueh PR. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents 2020;55:105946. https://doi.org/10.1016/j.ijantimicag.2020.105946 10.1016/j.ijantimicag.2020.105946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Epicentro. Comirnaty (BNT162b2), il primo vaccino contro il COVID-19 approvato in Europa e in Italia (2021). Available at: https://www.epicentro.iss.it/vaccini/covid-19-vaccino-pfizer-biontech (Accessed on: 21/08/2022).
- [24].Center for Disease Control and Prevention (CDC). Interim Guidance for Managing Healthcare Personnel with SARS-CoV-2 Infection or Exposure to SARS-CoV-2 (2021). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html (Accessed on: 21/08/2022).
- [25].European Center for Disease Control and Prevention (ECDC). Infection prevention and control and preparedness for COVID-19 in healthcare settings – sixth update (2021). Available at: https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-and-preparedness-covid-19-healthcare-settings (Accessed on: 22/08/2022).
- [26].Rule AM, Apau O, Ahrenholz SH, Brueck SE, Lindsley WG, de Perio MA, Noti JD, Shaffer RE, Rothman R, Grigorovitch A, Noorbakhsh B, Beezhold DH, Yorio PL, Perl TM, Fisher EM. Healthcare personnel exposure in an emergency department during influenza season. PLoS One 2018;13:e0203223. https://doi.org/10.1371/journal.pone.0203223 10.1371/journal.pone.0203223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Calimeri S, La Fauci V, Squeri R, Lo Giudice D. Susceptibility to measles among health workers in a university hospital in southern Italy. Clin Ter 2020;171:e486-e489. https://doi.org/10.7417/CT.2020.2262 10.7417/CT.2020.2262 [DOI] [PubMed] [Google Scholar]
- [28].Di Pietro A, Visalli G, Antonuccio GM, Facciolà A. Today’s vaccination policies in Italy: The National Plan for Vaccine Prevention 2017-2019 and the Law 119/2017 on the mandatory vaccinations. Ann Ig 2019;31(2 Suppl 1):54-64. https://doi.org/10.7416/ai.2019.2277 10.7416/ai.2019.2277 [DOI] [PubMed] [Google Scholar]
- [29].Gómez-Ochoa SA, Franco OH, Rojas LZ, Raguindin PF, Roa-Díaz ZM, Wyssmann BM, Guevara SLR, Echeverría LE, Glisic M, Muka T. COVID-19 in healthcare workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol 2021;190:161-75. https://doi.org/10.1093/aje/kwaa191 10.1093/aje/kwaa191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WCC4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-15. https://doi.org/10.1056/NEJMoa2034577 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Facciolà A, Visalli G, Orlando A, Bertuccio MP, Spataro P, Squeri R, Picerno I, Di Pietro A. Vaccine hesitancy: An overview on parents’ opinions about vaccination and possible reasons of vaccine refusal. J Public Health Res 2019;8:1436. https://doi.org/10.4081/jphr.2019.1436 10.4081/jphr.2019.1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Maltezou HC, Theodoridou K, Ledda C, Rapisarda V, Theodoridou M. Vaccination of healthcare workers: is mandatory vaccination needed? Expert Rev Vaccines 2019;18:5-13. https://doi.org/10.1080/14760584.2019.1552141 10.1080/14760584.2019.1552141 [DOI] [PubMed] [Google Scholar]
- [33].Gagneux-Brunon A, Detoc M, Bruel S, Tardy B, Rozaire O, Frappe P, Botelho-Nevers E. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross-sectional survey. J Hosp Infect 2021;108:168-73. https://doi.org/10.1016/j.jhin.2020.11.020 10.1016/j.jhin.2020.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Verger P, Scronias D, Dauby N, Adedzi KA, Gobert C, Bergeat M, Gagneur A, Dubé E. Attitudes of healthcare workers towards COVID-19 vaccination: a survey in France and French-speaking parts of Belgium and Canada, 2020. Euro Surveill 2021;26:2002047. https://doi.org/10.2807/1560-7917.ES.2021.26.3.2002047 10.2807/1560-7917.ES.2021.26.3.2002047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zimmermann P, Curtis N. Factors That Influence the Immune Response to Vaccination. Clin Microbiol Rev 2019;32:e00084-18. https://doi.org/10.1128/CMR.00084-18 10.1128/CMR.00084-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gordon A, Reingold A. The burden of influenza: a complex problem. Curr Epidemiol Rep 2018;5:1-9. https://doi.org/10.1007/s40471-018-0136-1 10.1007/s40471-018-0136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wagner A, Garner-Spitzer E, Jasinska J, Kollaritsch H, Stiasny K, Kundi M, Wiedermann U. Age-related differences in humoral and cellular immune responses after primary immunisation: indications for stratified vaccination schedules. Sci Rep 2018;8:9825. https://doi.org/10.1038/s41598-018-28111-8 10.1038/s41598-018-28111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bayas JM, Vilella A, Bertran MJ, Vidal J, Batalla J, Asenjo MA, Salleras LL. Immunogenicity and reactogenicity of the adult tetanusdiphtheria vaccine. How many doses are necessary? Epidemiol Infect 2001;127:451-60. https://doi.org/10.1017/s095026880100629x 10.1017/s095026880100629x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].D’Acremont V, Herzog C, Genton B. Immunogenicity and safety of a virosomal hepatitis A vaccine (Epaxal) in the elderly. J Travel Med 2006;13:78-83. https://doi.org/10.1111/j.1708-8305.2006.00001.x 10.1111/j.1708-8305.2006.00001.x [DOI] [PubMed] [Google Scholar]
- [40].Yang S, Tian G, Cui Y, Ding C, Deng M, Yu C, Xu K, Ren J, Yao J, Li Y, Cao Q, Chen P, Xie T, Wang C, Wang B, Mao C, Ruan B, Jiang T, Li L. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep 2016;6:27251. https://doi.org/10.1038/srep27251 10.1038/srep27251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sankilampi U, Honkanen PO, Bloigu A, Herva E, Leinonen M. Antibody response to pneumococcal capsular polysaccharide vaccine in the elderly. J Infect Dis 1996;173:387-93. https://doi.org/10.1093/infdis/173.2.387 10.1093/infdis/173.2.387 [DOI] [PubMed] [Google Scholar]
- [42].Kumru S, Godekmerdan A, Yilmaz B. Immune effects of surgical menopause and estrogen replacement therapy in peri-menopausal women. J Reprod Immunol 2004;63:31-8. https://doi.org/10.1016/j.jri.2004.02.001 10.1016/j.jri.2004.02.001 [DOI] [PubMed] [Google Scholar]
- [43].Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis 2010;10:338-49. https://doi.org/10.1016/S1473-3099(10)70049-9 10.1016/S1473-3099(10)70049-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Klein SL, Roberts CW, eds. Sex hormones and immunity to infection. Berlin: Springer Verlag; 2010. https://doi.org/10.1007/978-3-642-02155-8. 10.1007/978-3-642-02155-8 [DOI] [Google Scholar]
- [45].Trigunaite A, Dimo J, Jørgensen TN. Suppressive effects of androgens on the immune system. Cell Immunol 2015;294:87-94. https://doi.org/10.1016/j.cellimm.2015.02.004 10.1016/j.cellimm.2015.02.004 [DOI] [PubMed] [Google Scholar]
- [46].Migliore L, Nicolì V, Stoccoro A. Gender specific differences in disease susceptibility: the role of epigenetics. Biomedicines 2021;9:652. https://doi.org/10.3390/biomedicines9060652 10.3390/biomedicines9060652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ministero della Salute. Report Vaccini Anti COVID-19. 2022. Available at: https://www.governo.it/it/cscovid19/report-vaccini/ (Accessed on: 05/09/2022).


