Abstract
Objective:
To examine the value of data obtained outside of regular healthcare visits (clinical communications) to detect problematic opioid use in electronic health records (EHRs).
Design:
A retrospective cohort study.
Participants:
Chronic pain patient records in a large academic medical center.
Interventions:
We compared evidence for problematic opioid use in (1) clinic notes, (2) clinical communications, and (3) full EHR data. We analyzed keyword counts and calculated concordance and Cohen’s κ between data sources.
Main outcome measure:
Evidence of problematic opioid use in EHR defined as none, some, or high.
Results:
Twenty-six percent of records were discordant in determination of problematic opioid use between clinical communications and clinic notes. Of these, 54 percent detected more evidence in clinical communications, and 46 percent in clinic notes. Compared to full EHR review, clinic notes exhibited higher concordance (78 percent; κ = 0.619) than clinical communications (60 percent; κ = 0.290).
Conclusion:
Clinical communications are a valuable addition to opioid EHR research.
Keywords: opioid use disorder, electronic health records, patient communications, opioid prescriptions, chronic pain
BACKGROUND
Despite substantial efforts to curb the opioid epidemic, opioid-related deaths continue to rise globally.1 Electronic health records (EHRs) represent a rich source of longitudinal data, collected during routine delivery of healthcare, that can be used to facilitate clinical research, support observational studies, and improve clinical care quality.2 As such, EHR data can provide rich multidimensional insight for retrospectively investigating problematic opioid use; however, their use is stymied for a variety of reasons. Whether for research or patient care, detecting problematic opioid use through the EHR is challenging. Most EHR data collection focuses on structured data, eg, International Classification of Disease (ICD) codes, laboratory values, and vital signs.3 However, providers demonstrate reluctance to use diagnostic labels associated with problematic opioid use, eg, within ICD codes or problem lists, for various reasons including fear of stigmatizing the patient, the potential for future pain management barriers, and lack of clearly defined diagnostic criteria.4,5 Additionally, prescription data, which could be valuable for detecting opioid use trajectories, are often fragmented or not captured in EHRs.6 These standard EHR data sources are, therefore, insufficient to capture opioid concerns. It is common to circumvent the problem of inadequate structured data sources by supplementing with clinical note data, but provider reluctance, and lack of standardized nomenclature also limit the utility of these data sources.4,7,8 Therefore, it is understood that current methods to identify problematic opioid use in EHR data that rely only on structured data or clinician documentation within clinic notes likely significantly underrepresent the problem.9
While many EHR studies focus on notes generated from a patient visit (notes), this study examined the value of EHR communications occurring outside of a patient visit, which we term clinical communications. These data are records of provider and staff communications about patient phone calls/texts/electronic portal messages, often quoting patient or family comments (direct transcripts of calls/texts are generally not available). We hypothesize that clinical communications will provide different, but valuable data in the quest to detect problematic opioid use in EHRs.
OBJECTIVE
To examine the value of including clinical communication data in efforts to detect problematic opioid use in EHRs.
METHODS
This study was conducted in two stages:
Fifty records of chronic pain patients from Vanderbilt University Medical Center’s de-identified data warehouse were searched using keywords associated with problematic opioid use developed in a previous study (Figure 1).10 Two reviewers (SK and AS) independently examined medical records (12 training, 23 inter-rater assessment, and 50 test records, all of which had both clinical communications and notes) for level of evidence of problematic opioid use: no evidence, some evidence, or high evidence. One reviewer was restricted to clinical communications, and the other reviewer was restricted to notes. To minimize inter-rater biases, reviewers exchanged document types halfway through reviews. To assess inter-rater reliability, Reviewers 1 and 2 reviewed both clinical communications and notes for the same participant record, with 83 percent concordance, and Cohen’s κ = .559 (95 percent confidence interval (CI): .253, .865).
Two experts in addiction (SSR) and opioid (LS) research manually reviewed the full cohort using all EHR data (including diagnoses codes and problem lists) and categorized records for problematic opioid use evidence. Manual review of these records is described in detail elsewhere.10 Briefly, records were independently reviewed and given a score of no evidence, some evidence, or high evidence of problematic opioid use based upon the same keyword template used by Reviewers 1 and 2 in the first stage of this study. Record discordance between SSR and LS was resolved via rereview and consensus.
Figure 1.
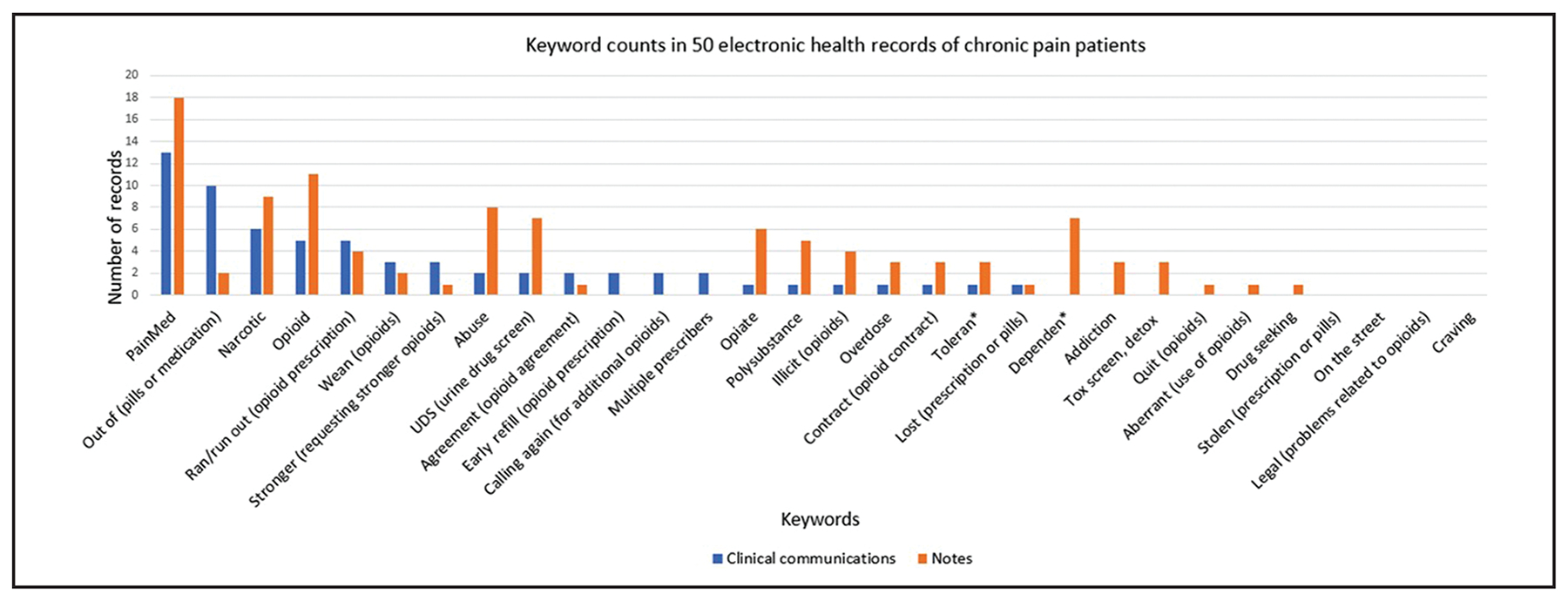
Keyword counts in 50 electronic health records of chronic pain patients.
Analyses
Keyword counts were calculated for clinical communications and notes. Percent of concordance and Cohen’s κ was calculated to determine concordance of problematic opioid use evidence classification between (a) review of clinical communications versus notes, (b) Reviewers 1 and 2, and (c) restricted and full record reviews.
RESULTS
The most common keywords identified in EHRs were “opioid” and “pain med” (Figure 1). However, keywords involving patient requests for prolonged or stronger opioid prescription were more common in clinical communications over notes. Within the 50 test records, concordance between Reviewers 1 and 2 was 74 percent (reviewers were not reviewing the same data: one reviewed clinical communications and one reviewed notes), and Cohen’s κ = 0.418 (95 percent CI: 0.206–0.630). We identified 13 (26 percent) records where the review of clinical communications resulted in a different category of evidence than only reviewing notes. Of the 13 discordant records, seven were rated higher in the clinical communication review, and six in the notes review. When compared to full EHR manual review, those restricted to notes exhibited higher concordance (78 percent) than clinical communications (60 percent) (Table 1). Similarly, Cohen’s κ was highest in comparing notes to full EHR manual review (κ = 0.619, 95 percent CI: 0.433–0.805) and lowest in comparing clinical communications to full EHR review (κ = 0.290, 95 percent CI: 0.104–0.478).
Table 1.
Comparison of unrestricted manual electronic record review for evidence of problematic opioid use to reviews restricted to clinical notes or clinical communications
| All records | Review concordance (percent) | κ (95 percent CI) |
| Comparison notes to clinical communication | 74 | 0.413 (0.195–0.630) |
| Comparison Reviewers 1 and 2 | 74 | 0.418 (0.206–0.630) |
| Comparison notes to full record review | 78 | 0.619 (0.433–0.805) |
| Comparison clinical communication to full record review | 60 | 0.290 (0.104–0.478) |
Note: Clinical Communication represents record review restricted to clinical communications. Notes represent record review restricted to clinical notes. Full record review represents all records reviewed, including billing codes and problem lists. κ represents Cohen’s κ.
Notably, in five of the 50 records, the clinical communications classification of evidence was rated higher than notes. Of these, three records had No Evidence of problematic opioid use in the notes, but Some (two records) or High (one record) Evidence was detected in clinical communications.
DISCUSSION
Clinical communications, which are readily available in most EHR systems, offer a valuable additional EHR data source for the detection of problematic opioid use, though they are clearly not exhaustive. More opioid-related keywords were present in clinical notes; however, potential “red flag” keywords related to need for prolonged or stronger opioid prescriptions were more prevalent in clinical communications. Taking opioids in larger amounts or over longer periods of time than originally intended is a major determinant of problematic opioid use11 that may not be recorded in patient visit records.
Data recorded during a patient visit (notes) are commonly used to augment structured EHR data, although as this study highlights, it often contains similar data to that found in a manual record review (78 percent concordance). Information that characterizes a patient’s health status, such as diagnoses (ICD codes and problem lists), or laboratory values and vital signs, would logically be contained in a clinical note. However, our findings suggest clinical communications contains novel information that is not contained in clinical notes and should be included in EHR evaluations of opioid use.
Additionally, clinical communications is one of the few EHR locations that offers documentation from the patient perspective (Figure 2), and previous research suggests patients may be more willing than providers to raise concerns with prescription drug use.12 While we recognize concerns that classification of patients based on opioid use may lead to medical discrimination,13 the risk of development of problematic opioid use for chronic opioid users is a major concern for clinicians. The most effective way to assist individuals who may be at risk for developing opioid use disorders is through early detection, before the condition can progress.14 This research highlights the need to include clinical communications as a data source to improve problematic opioid use detection.
Figure 2.

Clinical communication examples.
There are several limitations to this work. Importantly, although patient and provider communications portals (clinical communications) are a standard feature of most modern EHR systems, variation in how these features are utilized between clinical settings may affect the utility of this data source in the detection of problematic opioid use. Additionally, although some individuals in this study displayed putative evidence of problematic opioid use in clinical communications, there is no objective measure of problematic opioid use associated with these records; therefore, validity of using clinical communications for this purpose is not established.
CONCLUSION
Data that represent the patient perspective and voice will complement data sources routinely captured in EHR studies, with the potential to accelerate opioid research. Providers might immediately benefit by reviewing clinical communications to personalize pain management strategies. A long-term goal is to create decision support tools that automatically extract relevant information from clinical communications to improve pain management safety and effectiveness.
Clinical relevance
Problematic opioid use is a prevalent clinical concern that is difficult to detect in EHRs, and standard EHR data sources likely significantly underrepresent the problem. Provider and staff communications about patient phone calls/texts/electronic portal messages contain important opioid use information not found in standard EHR data sources, and inclusion of these data will improve problematic opioid use detection.
ACKNOWLEDGMENTS
Funding:
Dr. Sanchez-Roige—California Tobacco-Related Disease Research Program (TRDRP; Grant Number T29KT0526), National Institutes of Health, and National Institute of Drug Abuse (NIDA DP1DA054394). Dr. Jeffery—Healthcare Research and Quality (AHRQ) and the Patient-Centered Outcomes Research Institute (PCORI) (K12 HS026395).
The data used for this publication were supported by CTSA Award No. UL1 TR002243 from the National Center for Advancing Translational Sciences.
Contributor Information
Lori Schirle, Vanderbilt University School of Nursing, Nashville, Tennessee..
Shinwho Kwun, Vanderbilt University, Blair School of Music, Nashville, Tennessee..
Ashley Suh, Department of Medicine, Health, and Society, Vanderbilt University, Nashville, Tennessee..
Sandra Sanchez-Roige, Department of Psychiatry, University of California, Oakland, California; Vanderbilt University Medical Center, Division of Genetic Medicine, Nashville, Tennessee..
Alvin D. Jeffery, Department of Biomedical Informatics, Vanderbilt University School of Nursing, Vanderbilt University Medical Center, Nashville, Tennessee..
David C. Samuels, Department of Molecular Physiology and BioPhysics, Vanderbilt University School of Medicine, Vanderbilt Genetics Institute, Nashville, Tennessee..
REFERENCES
- 1.Imtiaz S, Nafeh F, Russell C, et al. : The impact of the novel coronavirus disease (COVID-19) pandemic on drug overdose-related deaths in the United States and Canada: A systematic review of observational studies and analysis of public health surveillance data. Subst Abuse Treat Prev Policy. 2021; 16(1): 87. DOI: 10.1186/s13011-021-00423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowie MR, Blomster JI, Curtis LH, et al. : Electronic health records to facilitate clinical research. Clin Res Cardiol. 2017; 106(1): 1–9. DOI: 10.1007/s00392-016-1025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrenstein V, Kharrazi H, Lehmann H: Obtaining data from electronic health records. In Gliklich RE, Leavy MB, Dreyer NA (eds.): Tools and Technologies for Registry Interoperability, Registries for Evaluating Patient Outcomes: A User’s Guide, 3rd ed. Addendum 2. Rockville, MD: Agency for Healthcare Research and Quality (US), 2019, Chapter 4. [PubMed] [Google Scholar]
- 4.Campbell G, Bruno R, Lintzeris N, et al. : Defining problematic pharmaceutical opioid use among people prescribed opioids for chronic noncancer pain: Do different measures identify the same patients? Pain. 2016; 157(7): 1489–1498. DOI: 10.1097/j.pain.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 5.Manhapra A, Sullivan MD, Ballantyne JC, et al. : Complex persistent opioid dependence with long-term opioids: A gray area that needs definition, better understanding, treatment guidance, and policy changes. J Gen Intern Med. 2020; 35(Suppl. 3): 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastarache L, Brown JS, Cimino JJ, et al. : Developing real-world evidence from real-world data: Transforming raw data into analytic datasets. Learn Health Syst. 2022; 6(1): e10293. DOI: 10.1002/lrh2.10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovatch M, Feingold D, Elkana O, et al. : Evaluation and comparison of tools for diagnosing problematic prescription opioid use among chronic pain patients. Int J Methods Psychiatr Res. 2017; 26(4): e1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer RE, Carrell DS, Cronkite D, et al. : The prevalence of problem opioid use in patients receiving chronic opioid therapy: Computer-assisted review of electronic health record clinical notes. Pain. 2015; 156(7): 1208–1214. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan MD, Edlund MJ, Fan MY, et al. : Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and Medicaid insurance plans: The TROUP study. Pain. 2010; 150(2): 332–339. DOI: 10.1016/j.pain.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schirle L, Jeffery A, Yaqoob A, et al. : Two data-driven approaches to identifying the spectrum of problematic opioid use: A pilot study within a chronic pain cohort. Int J Med Inform. 2021; 156: 104621. DOI: 10.1016/j.ijmedinf.2021.104621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Psychiatric Association (APA): Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition DSM-5. 5th ed. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 12.Ballantyne JC, Sullivan MD, Kolodny A: Opioid dependence vs addiction: A distinction without a difference? Arch Intern Med. 2012; 172(17): 1342–1343. DOI: 10.1001/archinternmed.2012.3212. [DOI] [PubMed] [Google Scholar]
- 13.Kimmel SD, Rosenmoss S, Bearnot B, et al. : Rejection of patients with opioid use disorder referred for post-acute medical care before and after an anti-discrimination settlement in Massachusetts. J Addict Med. 2021; 15(1): 20–26. DOI: 10.1097/ADM.0000000000000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Substance Abuse and Mental Health Services Administration (SAMSA): Prevention programs and policies. In Office of the Surgeon General. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Department of Health and Human Services, 2016: Chapter 3. Available at https://addiction.surgeongeneral.gov/. Accessed June 27, 2022. [Google Scholar]


