Abstract
Background
The purpose of our study is to investigate the expression level and prognostic value of serum α-hydroxybutyrate dehydrogenase (α-HBDH) in lung cancer (LC) patients.
Method
LC patients treated in the Department of Oncology, Shaanxi Provincial Cancer Hospital from January 2014 to December 2016 were included in this study, all of whom underwent serological detection of α-HBDH prior to admission, and were enrolled in follow-up 5-year survival. Comparing the differences between high group and normal groups based on α-HBDH and LDH expression via clinicopathological parameters and laboratory data. Univariate and multivariate regression and overall survival (OS) were analyzed to explore whether elevated α-HBDH was an independent risk factor for LC, compared to LDH.
Results
Multivariate regression analysis showed that age (P = 0.018), liver metastasis (P = 0.011), α-HBDH (P = 0.015), and neutrophil-to-lymphocyte ratio (NLR) (P = 0.031) were independent prognostic factors affecting OS in LC patients. The overall diagnostic efficacy of α-HBDH (AUC = 0.887) was higher than that of LDH (AUC = 0.709) in the ROC curve. The sensitivity was significantly higher of α-HBDH (sensitivity: 76.06%, specificity: 94.87%) compared with LDH (sensitivity: 49.30%, specificity: 94.87%). The median of OS was more significant in the high-α-HBDH group (6.4 months) than in the normal-α-HBDH group (12.7 months) (P = 0.023). The median of OS was significant in the high-LDH (> 245 U/L) group at 5.8 months and 12.0 months in the normal-LDH (≤ 245 U/L) group (P = 0.068).
Conclusions
Elevated expression of α-HBDH may indicate a poor prognosis of LC patients. It has a higher sensitivity than LDH and can be used as a potential early biomarker and an independent risk factor predicting the prognosis of LC survival.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-023-02965-3.
Keywords: α-HBDH, Lung cancer, Survival time, Prognosis
Introduction
Serum α-hydroxybutyrate dehydrogenase (α-HBDH) is an isoenzyme of lactate dehydrogenase (LDH), and its acts by catalyzing the oxidation of α-hydroxybutyrate to α-ketobutyric acid [1]. In the human body, α-HBDH is found in a variety of tissues, with the highest concentration in the cardiac tissue, followed by the red blood cells, white blood cells, and kidneys [2–4]. Studies have reported and confirmed that α-HBDH is significantly upregulated in many diseases and is associated with severe adverse prognoses, such as core-binding factor-related acute myeloid leukemia [5], acute pancreatitis [6], liver injury [7], and AIDS [8], which can be used as a potential early biomarker and an early prognostic indicator.
When cardiomyocytes are damaged, hydroxybutyrate dehydrogenase is released into the serum in large quantities, so it can be used as an indicator of myocardial damage [9]. Subsequently, researchers found that some patients with acute and chronic malignant tumors had abnormally elevated serum α-HBDH levels. It has been observed that serum α-HBDH is significantly elevated in patients with some hematological tumors [10] and malignant ovarian tumors [11]. Early scholars found that intracranial tumors can also lead to the abnormal elevation of α-HBDH and concluded that it may be used as an indicator of poor prognosis [12]. Khanolkar et al. study found that serum α-HBDH levels were elevated in patients with testicular tumors than in healthy subjects [13]. Therefore, we believe that this index may also be increased in malignant tumors, but there are few studies in this area.
Lung cancer (LC) is still one of the major malignant tumors that seriously endanger human health. Few studies have assessed the value of serological α-HBDH as a predictor and prognostic indicator in LC patients [14]. Therefore, we hypothesized that serum α-HBDH is associated with LC prognosis and explored the value of α-HBDH for prognosis in LC.
However, there are some studies on the abnormal expression of LDH in tumors, and many scholars take it as one of the prognostic indicators [15, 16]. Existing studies have demonstrated that elevated LDH in tumor tissue is associated with the clinical outcomes of multiple cancers, including renal cell carcinoma, melanoma, prostate cancer, lung cancer, and colorectal cancer [17, 18]. Could α-HBDH also be a biomarker for the prognosis and diagnosis of lung cancer? How are its sensitivity and specificity compared to LDH? There are also few comparing studies on the specificity and sensitivity of α-HBDH and LDH expression as biomarkers in the serum of tumor patients [13, 19]. So this study also compared the specificity and sensitivity of the α-HBDH and LDH, providing data support for the biological indicators of tumor prediction and prognosis of LC patients.
Materials and methods
Study population
This study was approved by the Ethics Committee of Shaanxi Provincial Cancer Hospital (No. 2021072), and the ethics committee approved an exemption from informed consent for this study. All methods were carried out in accordance with relevant guidelines and regulations. Collected LC patients with serum α-HBDH and LDH test results were hospitalized in Shaanxi Provincial Cancer Hospital from January 2014 to December 2016, excluded other clinical diseases associated with abnormal α-HBDH and LDH, such as cardiac disease, muscular dystrophy, vitamin B12 deficiency, hemolytic anemia, renal infarction, renal vascular embolization, and cachexia. Finally, we obtained 71 eligible LC patients, including 52 males and 19 females, aged between 39 and 87 years. Clinic pathological data were obtained from patients’ electronic medical records, which included age, gender, smoking history, tumor stage, lymph nodes, and metastasis of other sites (liver, kidney, brain, and bone), clinical routine, and biochemical and tumor markers.
Follow-up
This study logged into the medical record system of Shaanxi Cancer Hospital and obtained the basic information and general information of patients by consulting the inpatient history. In addition, medical records were screened according to the aforementioned inclusion and exclusion criteria. The screened cases were followed up. Through telephone follow-up and consulting the follow-up table, we obtained the main contents of the follow-up, involving the time of death, cause of death, and current living conditions. The study follow-up deadline was December 31, 2021. Cases affecting the results of the study, such as deaths due to accidents or other diseases, were excluded based on the exclusion criteria and the cause of death obtained. All the families included in the study were informed that their cases had been included in a retrospective study and had passed ethical review.
Data collection
General data including gender, age, tumor type, stage, and metastasis were collected. Hematological data were collected at pre-admission examination, including α-HBDH, LDH, homocysteine (HCY), fibrinogen (FIB), carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), total protein (TP), albumin (ALB), total calcium (Ca), leukocyte (WBC), neutrophil-to-lymphocyte ratio (NLR), and platelets (PLT) values. All patients’ electronic medical records and laboratory test results were reviewed by an independent physician.
The α-HBDH, LDH, HCY, TP, ALB, and Ca were analyzed by AU5800 Fully Automatic Biochemical analyzer from Beckman Coulter (American) Co., Ltd., WBC and PLT were analyzed by BC-6800 Fully Automatic Serum Cell analyzer from Mindray Medical International (Shenzhen) Co., Ltd., AFP and CEA were analyzed by E411 Automatic Immunoanalyzer from Roche Diagnostic Products (Shanghai) Co., Ltd., and FIB was analyzed by STA-R Fully Automatic Hemagglutator from Stago Diagnostic Technology (Tianjin) Co., Ltd. During the test, quality control was carried out with the supporting quality control materials of the instruments.
Statistics
In this study, categorical variables were described as proportions and were compared by using χ2 tests. Continuous variables were described as mean (x ± SD) or median (IQR). Comparisons of continuous variables between groups were done using t tests, one-way ANOVA, or equivalent non-parametric tests. The Cox proportional hazards regression model was used to perform the analysis α-HBDH with overall survival (OS). The ROC curves were evaluated for the diagnostic efficacy of the patient α-HBDH and LDH. The application SPSS 23.0 was used for all statistical comparisons, and the significant statistical level was set at the threshold of p < 0.05. The comparison of ROC curves and OS by GraphPad Prism 9.0 software (GraphPad Software, Inc.). The cutoff level of α-HBDH was set to a normal upper limit of 220 U/L, and the cutoff level of LDH was set to a normal upper limit of 245 U/L according to the reference range established in our laboratory.
Results
Clinical characteristics and baseline demographics of LC patients
Seventy-one patients with lung cancer were included in this study sequence. As shown in Table 1, the mean age of the study cohort was 64.2 years. Of the 71 patients, 52 were male and 19 were female. Twenty-five of the 71 patients (35%) had a history of smoking. Of the 71 patients, 45% (32) had lymph node metastases, 27% (19) had bone metastases, 18% (13) had brain metastases, 8 (11%) had liver metastases, and 3 (4%) had renal metastases. Among the 71 patients, 12 (17%) were in the low stage (stages I and II) and 59 (83%) were in the high stage (stages III and IV). Of the 71 patients, 17 (24%) were small cell lung cancer, 36 (51%) were lung adenocarcinoma, and 18 (25%) were squamous cell carcinoma. The average α-HBDH was 307 ± 152 U/L before admission.
Table 1.
Comparison of clinical characteristics and laboratory data of serum α-HBDH in LC patients
| Variables | Cases(n = 71) | α-HBDH > 220U/L (n = 44) | α-HBDH ≤ 220U/L (n = 27) | p value |
|---|---|---|---|---|
| Clinical characteristic | ||||
| Age (mean ± SD), years | 64.2 ± 11.0 | 63.5 ± 11.8 | 65.3 ± 10.1 | 0.699 |
| Gender | ||||
| Male | 52 (73%) | 28 (64%) | 24 (89%) | 0.021* |
| Female | 19 (27%) | 16 (36%) | 3 (11%) | |
| Smoking | 25 (35%) | 15 (34%) | 10 (37%) | 0.802 |
| Metastasis | ||||
| Liver | 8 (11%) | 6 (14%) | 2 (7%) | 0.640 |
| Lymphnodes | 32 (45%) | 24 (55%) | 8 (30%) | 0.042* |
| Kindey | 3 (4%) | 2 (5%) | 1 (4%) | 0.865 |
| Brain | 13 (18%) | 10 (23%) | 3 (11%) | 0.222 |
| Bone | 19 (27%) | 15 (34%) | 4 (15%) | 0.077 |
| Staging (I + II/III + IV) | ||||
| Low (I + II) | 12 (17%) | 5 (11%) | 7 (26%) | 0.096 |
| High (III + IV) | 59 (83%) | 39 (89%) | 20 (74%) | |
| Classification | ||||
| Small-cell carcinoma | 17 (24%) | 12 (27%) | 5 (19%) | 0.109 |
| Adenocarcinoma | 36 (51%) | 24 (55%) | 12 (44%) | |
| Squamous cell carcinoma | 18 (25%) | 8 (18%) | 10 (37%) | |
| Laboratory data | ||||
| LDH, U/L | 284 ± 135 | 351 ± 132 | 176 ± 32 | < 0.001*** |
| HCY, umol/L | 27.6 ± 52.5 | 31.6 ± 66.3 | 21.2 ± 9.2 | 0.644 |
| FIB, g/L | 4.27 ± 1.51 | 4.34 ± 1.57 | 4.16 ± 1.42 | 0.603 |
| CEA, ng/ml | 22.3 ± 20.6 | 25.3 ± 23.3 | 17.3 ± 14.3 | 0.079 |
| AFP, IU/ml | 10.4 ± 6.3 | 11.2 ± 7.4 | 9.2 ± 3.5 | 0.141 |
| TP, g/L | 66.2 ± 8.01 | 65.5 ± 7.54 | 67.2 ± 8.75 | 0.404 |
| ALB, g/L | 36.1 ± 6.39 | 35.6 ± 5.79 | 36.9 ± 7.31 | 0.451 |
| Ca, mmol/L | 2.26 ± 0.25 | 2.24 ± 0.20 | 2.31 ± 0.30 | 0.319 |
| WBC, × 109/L | 8.57 ± 5.37 | 9.05 ± 6.10 | 7.78 ± 3.87 | 0.284 |
| NLR | 8.30 ± 11.64 | 10.13 ± 14.14 | 5.32 ± 4.37 | 0.040* |
| PLT, × 109/L | 226 ± 106 | 235 ± 113 | 211 ± 94 | 0.335 |
Data are presented as the means ± standard deviation or interquartile range. *p < 0.05, **p < 0.01, and ***p < 0.001. p values were determined by Student’s t test for continuous variables and the chi-square test for categorical variables
Abbreviations: LDH Lactate dehydrogenase, HCY Homocysteine, FIB Fibrinogen, CEA Carcinoembryonic antigen, AFP Alpha-fetoprotein, TP Total protein, ALB Albumin, Ca Total calcium, WBC Leukocyte, NLR Neutrophil-to-lymphocyte ratio, PLT Platelets
Reference ranges: α-HBDH: 95–220U/L, LDH: 109–245U/L, HCY: 5–20umol/L, FIB: 2–4 g/L, CEA: 0–5.5 ng/ml, AFP: 0–6.05 IU/ml, TP: 60–82 g/L, ALB: 35–50 g/L, Ca: 2–3 mmol/L, WBC: 4–10 × 109/L, PLT: 100–300 × 109/L
Based on α-HBDH level, of the 71 patients, 27 were assigned to the normal-α-HBDH group (≤ 220 U/L), while 44 were assigned to the high-α-HBDH group (> 220 U/L). Table 1 Statistical calculation results showed that there were statistically significant differences in gender (P = 0.021) and lymph node metastasis (P = 0.042) time between the high-α-HBDH group and the normal-α-HBDH group in general clinical data. LDH (P < 0.001) and NLR (P = 0.04) were statistically different between the two groups.
α-HBDH was an independent factor for overall survival in univariate and multivariate cox regression
As shown in Table 2, the univariate analysis found that age, liver metastasis, α-HBDH, ALB, WBC, and NLR were associated with overall survival with a statistical difference (P < 0.05). In order to include as many clinical indicators as possible, we included all parameters of P < 0.1 in the multivariate cox analysis, the result showed that age, liver metastasis, α-HBDH, and NLR levels were still major factors affecting survival.
Table 2.
Univariate and multivariate analysis of clinical characteristics and laboratory data of in LC patients
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| p | HR | 95% Cl | p | HR | 95%Cl | |
| Age | 0.004 | 2.168 | 1.266–3.713 | 0.018* | 1.966 | 1.123–3.440 |
| Gender | 0.173 | 1.485 | 0.839–2.629 | |||
| Smoking | 0.258 | 1.337 | 0.807–2.213 | |||
| Metastasis | ||||||
| Liver | 0.002 | 3.278 | 1.501–7.161 | 0.011* | 2.911 | 1.277–6.636 |
| Lymphnodes | 0.471 | 1.193 | 0.738–1.926 | |||
| Kidney | 0.356 | 0.519 | 0.125–2.146 | |||
| Brain | 0.172 | 0.645 | 0.342–1.216 | |||
| Bone | 0.505 | 1.199 | 0.703–2.042 | |||
| Staging | 0.754 | 1.044 | 0.799–1.363 | |||
| Classification | 0.680 | 1.080 | 0.749–1.556 | |||
| α-HBDH (U/L) | 0.023 | 1.774 | 1.075–2.926 | 0.015* | 1.892 | 1.129–3.170 |
| LDH (U/L) | 0.068 | 1.559 | 0.964–2.521 | 0.895 | ||
| HCY (umol/L) | 0.299 | 1.288 | 0.798–2.080 | |||
| FIB g/L | 0.075 | 1.551 | 0.954–2.522 | 0.151 | ||
| CEA ng/ml | 0.062 | 0.250 | 0.058–1.072 | 0.933 | ||
| AFP IU/ml | 0.170 | 0.655 | 0.356–1.204 | |||
| TP g/L | 0.227 | 0.687 | 0.372–1.268 | |||
| ALB g/L | 0.021 | 0.556 | 0.336–0.922 | 0.566 | ||
| A/G | 0.368 | 0.780 | 0.453–1.342 | |||
| Ca mmol/L | 0.671 | 0.832 | 0.355–1.947 | |||
| WBC × 109/L | 0.013 | 1.896 | 1.144–3.145 | 0.153 | ||
| NLR | 0.009 | 1.965 | 1.174–3.290 | 0.031* | 1.787 | 1.054–3.029 |
| PLT × 109/L | 0.629 | 0.894 | 0.567–1.409 | |||
HR Hazards ratio
*p < 0.05, **p < 0.01, and ***p < 0.001
ROC of α-HBDH and LDH for LC
In addition, ROC analysis of LC showed a higher AUC value in α-HBDH (AUC = 0.887, 95%Cl 0.827–0.947, P < 0.0001) than LDH (AUC = 0.709, 95%Cl 0.615–0.803, P = 0.0003). The optimal clinical cutoff value of α-HBDH for distinguishing LC patients was 197 U/L, which provided a 76.06% sensitivity and a 94.87% specificity, which was higher than the sensitivity (49.30%) of LDH. The results showed a higher overall diagnostic efficacy of α-HBDH than LDH (Fig. 1).
Fig. 1.
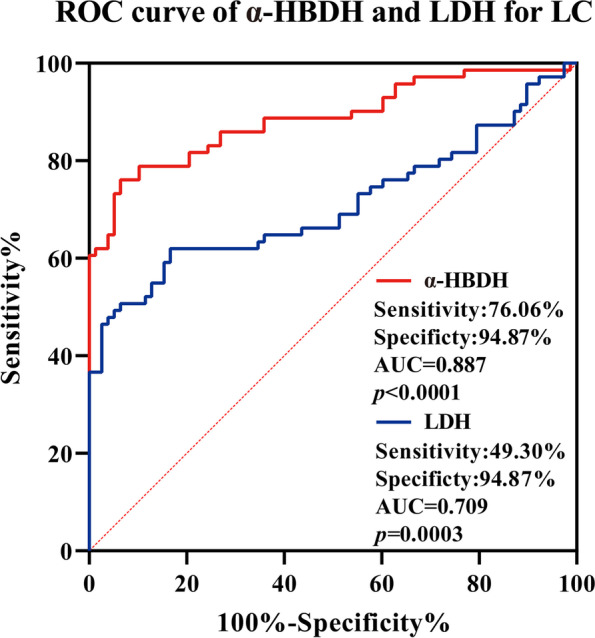
ROC curve of α-HBDH and LDH for LC
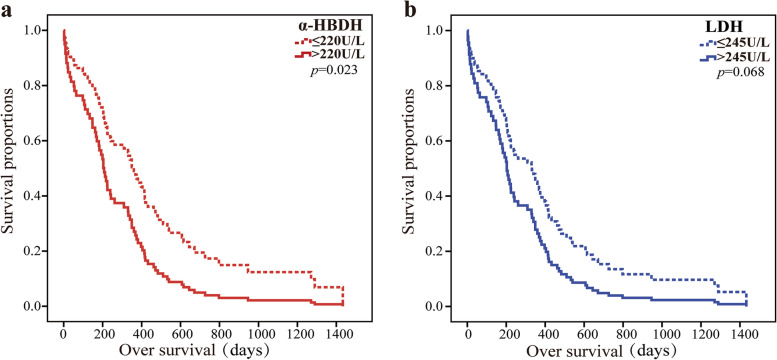
OS of α-HBDH and LDH for LC
In this study, compared with the normal group of α-HBDH (12.7 months, 95%Cl 0.8–46.4), the group of high-α-HBDH had a significantly lower median OS (6.4 months, 95%Cl 0.2–26.0), and the difference was statistically significant (P = 0.023). The median OS of the LDH > 245 U/L group was 5.8 (95%Cl 0.1–34.4) months, and the LDH ≤ 245 U/L group was 12.0 (95%Cl 0.3–44.7) months; however, the difference was not statistically significant (P = 0.068) (Fig. 2a, b).
Fig. 2.
OS of α-HBDH (a) and LDH (b) for LC
Discussion
Lung cancer (LC) is one of the most common malignant tumors with high morbidity and mortality and seriously endangers human life and health [20]. Early assessment of the severity of LC is a key factor in determining treatment strategies. At present, the diagnosis of LC is mainly based on low-dose spiral CT and serological tumor markers [21]. Some serological tumor markers have poor sensitivity and specificity, so we need to find more biomarkers to improve the diagnostic efficacy and make a more accurate judgment of the severity and prognosis of the disease. Usually, serum α-HBDH and LDH are common biochemical index in the laboratory, and α-HBDH was generally detected by the α-ketobutyrate method and LDH was lactate method, which was simple and quick detection method by using a fully automated biochemical analyzer. In this study, we found that the level of α-HBDH was elevated easier compared with the other conventional biochemical indexes in LC patients. This is consistent with some studies in intracranial and testicular tumors [12, 13, 19]. This indicates that the serum level of this index is also abnormally elevated in patients with lung cancer.
The male-to-female ratio in this cohort was nearly 2.73, which is 1.5 times the world average for lung cancer incidence [22]. We also found a significantly higher proportion of males in the normal-α-HBDH group and the high-α-HBDH group, and the male with a female ratio was significantly different (χ2 = 4.232, P = 0.021). Since this study is a retrospective study and there are standard screening cases, there may be biases such as selection bias and confounding bias leading to errors, and a large sample is still needed to avoid these biases. However, α-HBDH levels were not affected by the higher proportion of men in the normal-α-HBDH group. The result showed that smoking also was not affected the level of α-HBDH, So the difference in gender between the two groups did not significantly affect the study results.
In this study, among all 33 cases with lymph node metastasis, 24 cases had a high serum value of this index, accounting for 55% of all high-value cases. This indicates that the elevation of α-HBDH is probably associated with lymph node metastasis. The reason may be that in the tumor microenvironment, the glycolysis of malignant tumor tissues is higher than that of normal tissues, and tumor cells preferentially use lactic acid as an energy source, which reduces the pH value in vivo and promotes the invasion and metastasis of tumor cells [23]. Scholars studied that elevated serum LDH has the potential to predict early metastasis, according to an analysis of serum biomarkers in 267 patients with gastrointestinal cancer [24]. As we all know, lymph node metastasis is associated with poor prognosis, which indirectly suggests that elevated lymph node metastasis may be associated with poor prognosis.
In the serum of small-cell carcinoma patients, it was determined that the enzymes LDH and α-HBDH were shown with the highest share of abnormal values by Warnecke et al. study [14]. Compared with the laboratory data of the two groups, there was a correlation between this index and LDH, and the value of LDH in the high-α-HBDH group was significantly higher than that in the normal-α-HBDH group. This indicates that these two indexes have similar effects, and the poor prognosis of other tumors may be negatively correlated. Consistent with the results of this study, Khanolkar et al. found that serum LDH and α-HBDH levels were elevated in both seminomatous and non-seminomatous germ cell tumors in 1990, and α-HBDH was being more specific in monitoring therapy as compared to serum LDH [19].
NLR is the ratio of neutrophil to lymphocyte counts in serum, which can be used as a prognostic indicator in inflammation and some tumors [25]. It is also a putative measure of the balance between neutrophil-associated tumor-pro-inflammatory response and lymphocyte-dependent antitumor immune function and has been proposed as a prognostic factor in a variety of cancers [25, 26]. In this study, the serum NLR value of patients in the high-α-HBDH group was significantly higher than that in the normal-α-HBDH group, indicating that the prognosis of patients in the high-value group was poor. α-HBDH is not an independent specific enzyme, but rather the LDH isoenzyme total term of LDH-1 and LDH-2 containing the H subunits. Metabolic changes in rapidly dividing cancer cells are closely associated with increased uptake of glucose and abnormal activity of LDH, which regulates the processing of glucose to lactic acid [27, 28]. The authors suggest a possible mechanism which is the α-HBDH, expressed by malignant cells, significantly increases lactate formation, and lactate induces the proliferation of oxygenated malignant cells and angiogenesis and inhibits the innate and adaptive immune responses [29]. This process results that α-HBDH and LDH activities were elevated in malignant cells, and the value of NLR was downgraded in serum.
The prognosis and 5-year survival of cancer patients may be influenced by some factors, such as tumor classification, early postoperative chemotherapy [30, 31], and metastasis. In the survival analysis of this study, it was confirmed that the elevated α-HBDH levels, NLR, WBC, liver metastasis, and age were the risk factors affecting OS, and the association was positive between α-HBDH and the increased risk of OS (HR 1.892, 95%Cl 1.129–3.170, P = 0.015). Studies have shown that elevated α-HBDH in some acute heart and lung diseases suggests poor survival [8, 32]. As in other diseases, an increase in this index predicted poor overall survival in LC patients. So we have explored the correlation between α-HBDH elevation and the prognosis of LC and found that baseline serum α-HBDH elevation correlates with shorter survival, and α-HBDH can be identified as an independent risk factor for LC.
LDH has been incorporated into the Ranson score system to assess tumor severity and predict cancer prognosis. Currently, elevated serum LDH in solid tumors is associated with clinical outcomes in a variety of cancers, and tumor prognosis is a risk factor for outcomes [17, 33]. Sun et al. found that serum LDH and α-HBDH levels were significantly increased in patients with malignant tumors, and these levels can be used as reference indicators for the diagnosis and treatment of digestive system malignant tumors [34]. The result showed that the high-α-HBDH group was more significant in predicting OS than the high LDH group (0.023 vs 0.068). In addition, ROC analysis showed that α-HBDH had a predictive value (AUC = 0.887) better than LDH (AUC = 0.709) for LC, mainly manifested in α-HBDH has a high sensitivity value (76.06% vs 49.30%) than LDH. This is perhaps the reason why we found that α-HBDH is more valuable than LDH in LC.
In summary, as serum α-HBDH levels were found to be commonly increased in LC patients and correlated with poor clinical outcomes in this study. The determination of α-HBDH may become a supportive tool in prognosis cancers. This study excluded other factors and proved the important value of serum α-HBDH in the prognosis of lung cancer, but there are still some deficiencies in this study. First of all, the amount of data in this study is small. This may be because α-HBDH is not tested as a routine testing indicator, especially in tumor patients with the non-cardiac disease. Secondly, the data of this study came from a single center, and there may be biases such as selective and confounding bias leading to errors, and a large sample is still needed to avoid these biases. What is more, the AUC difference between models. The AUC has been criticized for its insensitivity in model comparisons where the baseline model is better, and NRI (net reclassification improvement) and IDI (integrated discrimination improvement) have been proposed as alternatives to the increase in the AUC for evaluating improvement in the performance of risk assessment algorithms. However, NRI and IDI are also not without problems [35]. In addition, this study was a retrospective prognostic study and the prognostic validation set also took longer, and it was not completed in the short term. In the future, a multi-center study of medical record tracking will be more helpful to prove whether α-HBDH can be used as a basis for the prognosis of lung cancer. So, a single serological laboratory index cannot fully be a key evaluation index of the tumor prognosis, but it can provide a powerful auxiliary tool for disease severity and prognosis for LC patients.
Conclusion
In conclusion, this study suggested that α-HBDH is an independent risk factor for poor prognosis in LC patients. And also showed that serum α-HBDH was more prognostic sensitive than LDH.
Supplementary Information
Additional file 1: Figure 1. ROC curve of α-HBDH and LDH for LC. Figure 2. OS of α-HBDH (a) and LDH (b) for LC.
Acknowledgements
Not applicable.
Authors’ contributions
Zm Y. and Lh W. wrote the main manuscript text and Zm Y. prepared figures 1 and 2. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the.preparation of this manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Shaanxi Provincial Cancer Hospital (No. 2021072), and the ethics committee approved an exemption from informed consent of this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhi-Min Yuan and Long-Hao Wang contributed equally to this work.
References
- 1.Bruschke AV, van der Laarse A, van der Wall EE. Assessment of the size of acute myocardial infarction. I: Biochemical methods. Cleve Clin J Med. 1990;57(6):547–50. doi: 10.3949/ccjm.57.6.547. [DOI] [PubMed] [Google Scholar]
- 2.Zołnierowicz S, Swierczyński J, Zelewski L. Short communication: beta-hydroxybutyrate dehydrogenase activity in human placenta. Placenta. 1984;5(2):183–187. doi: 10.1016/S0143-4004(84)80061-2. [DOI] [PubMed] [Google Scholar]
- 3.Fast JH, Kubat K, van Haelst UJ, et al. The usefulness of an endomyocardial biopsy in heart disease of unknown etiology. Int J Cardiol. 1986;11(3):317–328. doi: 10.1016/0167-5273(86)90036-7. [DOI] [PubMed] [Google Scholar]
- 4.González Buitrago JM, Pastor I, Corrales JJ. Cytoplasmic enzyme activities in human hypernephroma compared with normal renal cortical tissue. Urol Int. 1988;43(1):32–34. doi: 10.1159/000281299. [DOI] [PubMed] [Google Scholar]
- 5.Fan LF, Xu J, Chen XH, et al. Effect of signal transduction pathway gene mutations on the one- course induced remission rate and analysis of clinical characteristics in patients with CBF-AML. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28(3):781–788. doi: 10.19746/j.cnki.issn.1009-2137.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Xiao W, Liu W, Yin L, et al. Serum hydroxybutyrate dehydrogenase as an early predictive marker of the severity of acute pancreatitis: a retrospective study. BMC Gastroenterol. 2020;20(1):393. doi: 10.1186/s12876-020-01521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H, Han H, Li J, et al. Alpha-hydroxybutyrate dehydrogenase as a biomarker for predicting systemic lupus erythematosus with liver injury. Int Immunopharmacol. 2019;77:105922. doi: 10.1016/j.intimp.2019.105922. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Su J, Xie Y et al. Plasma IL-6/IL-10 ratio and IL-8, LDH, and HBDH level predict the severity and the risk of death in AIDS patients with pneumocystis pneumonia. J Immunol Res. 2016;2016(3):1583951–60. [DOI] [PMC free article] [PubMed]
- 9.Dissmann R, Linderer T, Schröder R. Estimation of enzymatic infarct size: direct comparison of the marker enzymes creatine kinase and alpha-hydroxybutyrate dehydrogenase. Am Heart J. 1998;135(1):1–9. doi: 10.1016/S0002-8703(98)70335-7. [DOI] [PubMed] [Google Scholar]
- 10.Trapani S, Grisolia F, Simonini G, et al. Incidence of occult cancer in children presenting with musculoskeletal symptoms: a 10-year survey in a pediatric rheumatology unit. Semin Arthritis Rheum. 2000;29(6):348–359. doi: 10.1053/sarh.2000.5752. [DOI] [PubMed] [Google Scholar]
- 11.Kawai M, Yamashita H, Hayashi T, et al. Serum CA125 in malignant ovarian tumor–periodical monitoring and correlation with other tumor markers. Nihon Sanka Fujinka Gakkai Zasshi. 1986;38(3):402–410. [PubMed] [Google Scholar]
- 12.Zukiel R, Nowak S, Jankowski R, et al. Enzymatic activity in cerebrospinal fluid in the monitoring of the brain lesions following intracranial tumors. Neurol Neurochir Pol. 1998;32(2):359–365. [PubMed] [Google Scholar]
- 13.Khanolkar MM, Sirsat AV. Comparative study on determination of alpha-hydroxybutyrate dehydrogenase, lactate dehydrogenase isoenzyme LDH-1 by adsorption on DEAE Sephadex A-50 & electrophoresis. Indian J Med Res. 1992;96:136–138. [PubMed] [Google Scholar]
- 14.Warnecke R, Wetzer K, Preisler B. Biochemical and immunological examinations in patients with lung diseases under particular consideration of bronchial carcinoma (author’s transl) Z Erkr Atmungsorgane. 1980;154(3):345–349. [PubMed] [Google Scholar]
- 15.Zhang X, Guo M, Fan J, et al. Prognostic significance of serum LDH in small cell lung cancer: a systematic review with meta-analysis. Cancer Biomark. 2016;16(3):415–423. doi: 10.3233/CBM-160580. [DOI] [PubMed] [Google Scholar]
- 16.Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017;19(4):353–363. doi: 10.3233/CBM-160336. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Lu C, Pan N, et al. Serum lactate dehydrogenase activities as systems biomarkers for 48 types of human diseases. Sci Rep. 2021;11(1):12997. doi: 10.1038/s41598-021-92430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serpa MJ, Franco S, Repolho D, et al. An unusual case of primary retroperitoneal germ cell tumour in a young man. Eur J Case Rep Intern Med. 2018;5(7):000900. doi: 10.12890/2018_000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanolkar MM, Sirsat AV, Deshmane VH, et al. Serum lactate dehydrogenase, alpha-hydroxybutyrate dehydrogenase and ratio of alpha-hydroxybutyrate dehydrogenase to lactate dehydrogenase in testicular tumours. Indian J Cancer. 1990;27(4):243–249. [PubMed] [Google Scholar]
- 20.Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103(3):463–473. doi: 10.1016/j.mcna.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Villalobos P, Wistuba II. Lung cancer biomarkers. Hematol Oncol Clin North Am. 2017;31(1):13–29. doi: 10.1016/j.hoc.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sung H, Ferlay J, Siegel RL. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed]
- 23.Brown NJ, Higham SE, Perunovic B, et al. Lactate dehydrogenase-B is silenced by promoter methylation in a high frequency of human breast cancers. PLoS ONE. 2013;8(2):e57697. doi: 10.1371/journal.pone.0057697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X, Fan Y, Chen Z, et al. Blood biomarkers of bone metastasis in digestive tract malignant tumors. Future Oncol. 2021;17(12):1507–1518. doi: 10.2217/fon-2020-0509. [DOI] [PubMed] [Google Scholar]
- 25.Cupp MA, Cariolou M, Tzoulaki I et al. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18(1):360. [DOI] [PMC free article] [PubMed]
- 26.Mouchli M, Reddy S, Gerrard M, et al. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma. Review Article Ann Hepatol. 2021;22:100249. doi: 10.1016/j.aohep.2020.08.067. [DOI] [PubMed] [Google Scholar]
- 27.Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett. 2015;358(1):1–7. doi: 10.1016/j.canlet.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 28.Forkasiewicz A, Dorociak M, Stach K et al. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell Mol Biol Lett. 2020;25:35. [DOI] [PMC free article] [PubMed]
- 29.Deme D, Telekes A. Prognostic importance of lactate dehydrogenase (LDH) in oncology. Orv Hetil. 2017;158(50):1977–1988. doi: 10.1556/650.2017.30890. [DOI] [PubMed] [Google Scholar]
- 30.Gamucci T, Zampa G. Cardiotoxicity of 5-fluorouracil. Tumori. 1980;66(5):635–636. doi: 10.1177/030089168006600511. [DOI] [PubMed] [Google Scholar]
- 31.Franken NA, Strootman E, Hollaar L, et al. Myocardial enzyme activities in plasma after whole-heart irradiation in rats. J Cancer Res Clin Oncol. 2000;126(1):27–32. doi: 10.1007/PL00008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinellu A, Paliogiannis P, Carru C et al. Serum hydroxybutyrate dehydrogenase and COVID-19 severity and mortality: a systematic review and meta-analysis with meta-regression. Clin Exp Med. 2022;22(4):499–508. [DOI] [PMC free article] [PubMed]
- 33.Zhang J, Yao YH, Li BG, et al. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: a systematic review and meta-analysis. Sci Rep. 2015;5:9800. doi: 10.1038/srep09800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Z, Qi S, Qi X, et al. Clinical value of LDH and α-HBDH levels detecting in the diagnosis of malignant tumors. Chinese J Coal Ind Med. 2017;20(1):1–3. [Google Scholar]
- 35.Pencina MJ, D’agostino RB, Pencina KM, et al. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176(6):473–481. doi: 10.1093/aje/kws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure 1. ROC curve of α-HBDH and LDH for LC. Figure 2. OS of α-HBDH (a) and LDH (b) for LC.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.