Abstract
An important emerging theme is that heterogeneous nuclear ribonucleoproteins (hnRNPs) not only function in the nucleus but also control the fates of mRNAs in the cytoplasm. Here, we show that hnRNP D plays a versatile role in cytoplasmic mRNA turnover by functioning as a negative regulator in an isoform-specific and cell-type-dependent manner. We found that hnRNP D discriminates among the three classes of AU-rich elements (AREs), most effectively blocking rapid decay directed by class II AREs found in mRNAs encoding cytokines. Our experiments identified the overlapping AUUUA motifs, one critical characteristic of class II AREs, to be the key feature recognized in vivo by hnRNP D for its negative effect on ARE-mediated mRNA decay. The four hnRNP D isoforms, while differing in their ability to block decay of ARE-containing mRNAs, all potently inhibited mRNA decay directed by another mRNA cis element that shares no sequence similarity with AREs, the purine-rich c-fos protein-coding region determinant of instability. Further experiments indicated that different mechanisms underlie the inhibitory effect of hnRNP D on the two distinct mRNA decay pathways. Our study identifies a potential mechanism by which cytoplasmic mRNA turnover can be differentially and selectively regulated by hnRNP D isoforms in mammalian cells. Our results support the notion that hnRNP D serves as a key factor broadly involved in general mRNA decay.
Control of mRNA turnover is a powerful means to regulate levels of protein expression (17, 29, 34). One class of regulatory cis elements that dictates the rate of mRNA turnover comprises the AU-rich elements (AREs) found in the 3′ untranslated regions (UTRs) of many short-lived mRNAs (3). Previously, distinct sequence features and decay characteristics displayed by different AREs have led to the classification of AREs into three types (6, 32). Class I AREs, found in mRNAs like c-fos and c-myc, contain 1 to 3 scattered copies of the pentanucleotide AUUUA embedded within U-rich regions. Class II AREs, only found in cytokine mRNAs such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor alpha (TNF-α), contain multiple overlapping copies (5 to 8 copies) of the AUUUA motif. Class III AREs, such as the one in c-jun mRNA, lack the hallmark AUUUA pentanucleotide but require a U-rich sequence and possibly other unknown features for their destabilizing function. mRNA turnover mediated by AREs from all three classes is characterized by rapid shortening of the poly(A) tail followed by rapid decay of the mRNA body. Intriguingly, actinomycin D, a transcription inhibitor extensively used in mRNA turnover studies, was found to block the rapid decay directed by both class I and class II AUUUA-containing AREs but to have little effect on class III non-AUUUA AREs (6, 31). These distinct features and properties of AREs point to the possibility that, in vivo, different classes of AREs are differentially regulated, e.g., through recognition by different ARE-binding proteins (ARE-BPs).
Several in vivo observations lend support to this notion. In a monocytic tumor cell line, c-fos and c-myc 3′ UTRs, containing class I AREs, destabilized a reporter mRNA, whereas the GM-CSF 3′ UTR containing a class II ARE did not (36). A similar discrimination between the two classes of AREs has also been suggested in two other studies. Stimulation of quiescent primary T lymphocytes with antibodies to CD3/CD28 receptors specifically stabilizes lymphokine mRNAs containing class II AREs, while c-fos and c-myc mRNAs remain unstable (24). The interleukin-3 (IL-3) ARE, having class II characteristics, is required in a mast tumor cell line for the destabilization of IL-3 mRNA induced by the immunosuppressant cyclosporine A (30). Significantly, the role of IL-3 AREs cannot be substituted by class I AREs from either c-fos or c-myc. Thus, it appears that in vivo a fine-tuning mechanism is likely to exist that differentially regulates the RNA destabilizing function between class I and class II AREs.
In an effort to understand the mechanism and regulation of ARE-mediated mRNA turnover, many laboratories have used in vitro approaches to identify proteins that bind AU- or U-rich sequences (16, 21, 33; also reviewed in references 25 and 43). Among ARE-BPs, heterogeneous nuclear ribonucleoprotein (hnRNP) D, also termed AUF1 (49), has been widely studied for its potential role in ARE-mediated mRNA decay (for examples, see references 10, 25, and 41). In vitro RNA-binding studies using recombinant proteins made in Escherichia coli showed that hnRNP D displays high affinity, with dissociation constants at a nanomolar range, for a variety of AREs and a (UUAG)n sequence, as well as a stretch of 32-nucleotide (nt) uridylates (1, 10, 18, 44). It was also concluded in one of these in vitro studies that the hallmark motif of most AREs, AUUUA, is not required for hnRNP D binding (43). In vitro binding studies of other recombinant ARE-BPs, e.g., HuR (27), have often led to the similar conclusion that ARE-BPs indiscriminately bind AREs showing distinctly different sequence features and that the U-rich sequence serves as an effective competitor to abolish ARE-BP binding to native AREs.
The hnRNP D gene is also unique among other ARE-BPs in that it is transcribed into a pre-mRNA that undergoes alternative pre-mRNA splicing of two coding exons, exon 2 and exon 7, to give rise to four different protein isoforms with apparent molecular masses of 37, 40, 42, and 45 kDa (Fig. 1A) (12, 42). The hnRNP D protein exhibits structural motifs that are arranged in a similar way to hnRNP A1: an N-terminal domain followed by two consecutive RNA recognition motifs (RRMs) and a C-terminal domain containing Arg-Gly-Gly (RGG) motifs (13). Transfection studies have shown that hnRNP D functions as an mRNA destabilizing factor in human erythroleukemic K562 cells (25) and is targeted for degradation by the ubiquitin-proteasome pathway (22). More recently, the participation of hnRNP D in RNA turnover was extended to include the mRNA decay directed by the c-fos major coding determinant (15), a purine-rich sequence that shares no sequence similarity with AREs. It was found that hnRNP D is an integral component of a multiprotein complex that mediates c-fos major coding determinant-directed mRNA decay (15). These observations suggest that hnRNP D may play multiple roles in cytoplasmic mRNA turnover.
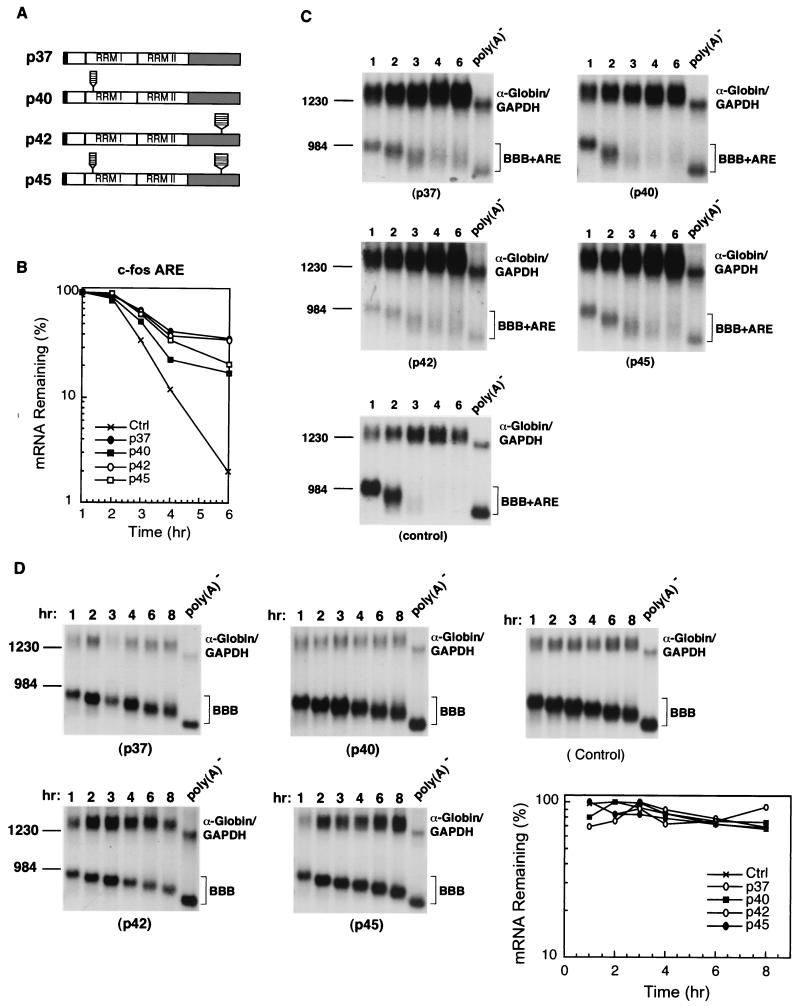
FIG. 1.
Ectopic expression of human hnRNP D isoforms in mouse NIH 3T3 cells differentially inhibits rapid mRNA decay directed by the c-fos ARE. (A) Schematic diagram of the four hnRNP D isoforms. The open box indicates the N-terminal domain. RRMI and RRMII depict RNA recognition motifs. The gray box represents the C-terminal domain. The black box at the very N terminus indicates the myc-epitope tag. The striped box represents the additional peptide sequences included as a result of alternative RNA splicing. (B) Semi-log plot showing the effects of four hnRNP D isoforms on mRNA decay directed by the c-fos ARE. Quantitation of mRNA was obtained by scanning radioactive blots with an imager (Packard) and the data were plotted as a function of time. (C) RNA blots showing deadenylation and decay of β-globin mRNA bearing the c-fos ARE (BBB+AREfos) in the absence (control) or presence of ectopically expressed individual isoforms of hnRNP D (indicated by their molecular masses). (D) Northern blots showing decay of β-globin mRNA (BBB) in NIH 3T3 B2A2 cells expressing individual isoforms of hnRNP D or vector only (control). To determine mRNA half-life, NIH 3T3 B2A2 cells were transiently cotransfected with a control plasmid (pSVα-globin/GAPDH) and one of the test plasmids as indicated under each blot. Total cytoplasmic mRNA was isolated at various time intervals after serum stimulation of quiescent cells and analyzed by Northern blot analysis. Transcription of BBB+AREfos or BBB mRNA was driven by the serum-inducible c-fos promoter. The control mRNA (α-globin/GAPDH) was expressed constitutively and served as an internal standard. The times given at the top correspond to hours after serum stimulation. Poly(A)− RNA was prepared in vitro by treating RNA samples from the 1-h time point with oligo(dT) and RNase H. The positions corresponding to 1,230 and 984 nt are indicated.
In view of these observations, several critical issues arise concerning the in vivo relationships among AREs, ARE-BPs, and molecular mechanisms controlling ARE-mediated mRNA decay. For example, what are the critical features of AREs that are recognized in vivo by different ARE-BPs? Do all ARE-BPs share similar binding specificities in vivo and thereby indiscriminately regulate the function of all AREs, or do they regulate a specific class of AREs or even a particular ARE in a cell-specific manner in response to environmental cues? What role(s) does each ARE-BP play in ARE-mediated turnover? In theory, ARE-BPs could play a destabilizing or stabilizing role or even dual roles in vivo. For instance, their association with an ARE could promote assembly of a decay complex necessary for RNA destabilization in the cytoplasm. Alternatively, their binding could prevent the formation of such a decay complex, thus leading to RNA stabilization. A relevant question is whether ARE-BPs change their RNA binding affinity or specificity, e.g., via interaction with other proteins in vivo, thus playing multiple roles in mRNA turnover involving decay pathways other than the ARE-directed pathway.
In an effort to address these issues, we investigated the roles of hnRNP D in controlling mRNA decay directed by two distinct decay pathways involving the ARE and the c-fos coding determinants. We showed that hnRNP D plays dual roles in ARE-mediated RNA turnover in a cell-type-specific manner: a stabilizing role observed in NIH 3T3 cells (this study) and a destabilizing role identified previously in K562 cells (25). In contrast to indiscriminate binding of hnRNP D to AREs observed in vitro using E. coli recombinant proteins, we found that its in vivo action on AREs is selective. hnRNP D effectively down-regulates the destabilizing function of class II AREs containing multiple clustered AUUUA motifs. The data provide a molecular basis that explains how class II ARE-containing cytokine mRNAs are differentially stabilized in lymphocytes and in lymphoid cells as described above. Moreover, there exists a significant difference among the four isoforms of hnRNP D in terms of their stabilizing effect on ARE-mediated decay with the following rank order: p37 ≥ p42 > p45 ≥ p40. It was striking to find that all hnRNP D isoforms are able to inactivate the rapid RNA decay directed by the c-fos protein-coding region that is distinct from the ARE. Our experiments further indicate that distinct mechanisms underlie the blockage by hnRNP D of these two mRNA decay pathways. Thus, our study identified a potential mechanism by which hnRNP D isoforms differentially and selectively regulate cytoplasmic mRNA turnover. Our results suggest that hnRNP D serves as a key factor broadly involved in mRNA decay directed by many different stability determinants.
MATERIALS AND METHODS
Plasmid construction.
The construction of plasmids pSVα1/GAPDH, pBBB, pBBB+AREc-fos, pBBB+AREGM-CSF, pBBB+AREc-jun, pBBB+AREc-myc, pBBB+ARESyn2, pBBB+AREGM1, pBBB+ARETNF-α, pBBB+AREfII/jIII, pTet-Myc-Ovep, and pT3AREfos has been described previously (4, 5, 31, 38, 46, 48). Plasmids expressing different isoforms of myc-tagged hnRNP D were generated in the following steps. For plasmids pTet-Myc-p40 and pTet-Myc-p45, which express the p40 and p45 isoforms, respectively, a standard PCR was performed to amplify the protein-coding region of the hnRNP D cDNA portion, using pET21(c)-cDx7His6 and pET21(c)-cDx9His6 as templates, which were kindly provided by F. Ishikawa (18). The PCR-amplified fragments were digested with SalI and EcoRV and then inserted into SalI-EcoRV-digested pTet-Myc-Ovep to create an in-frame fusion of hnRNP D downstream of the myc-tag. To construct pTet-Myc-p37 and pTet-Myc-p42, the BglI-BglII DNA region containing the C-terminal alternatively spliced sequence of hnRNP D cDNA (Fig. 1) present in pTet-Myc-p40 and pTet-Myc-p45 was replaced with the BglI-BglII DNA region from pcDNA3.AUF37, which lacks the C-terminal alternatively spliced sequence (kindly provided by G. Brewer) (49). The proper in-frame insertion of AUF1 cDNAs was confirmed by DNA sequencing. To generate the plasmid pT3AREGM63, a BstXI (fill-in)-BglII fragment covering the 63-bp ARE region from plasmid pBBB+AREGM-CSF was subcloned into the unique BamHI site of plasmid pT3/T7α-18 (Gibco BRL). Plasmid pT7fos was kindly provided by Inder Verma.
RNA blot analysis and preparation of NIH 3T3 cytoplasmic and nuclear extracts.
Cell culture, DNA transfection, isolation of total cytoplasmic RNA, Northern blot analysis, and lysate preparation were conducted as described previously (26, 39). Briefly, NIH 3T3 B2A2 cells stably harboring the tetracycline-responsive trans-activator (tTA) (47) were transfected for ≈16 h with a total of 20 μg of DNA which included 3 μg of pBBB+ARE, 3 μg of pTet-Myc-hnRNP D isoform or vector (pTet-Myc-Ovep) (31), 2 μg of pSVα1/GAPDH, and enough carrier plasmid pT3/T7α-18 (Gibco BRL) to make a final amount of 20 μg of DNA. The cells were then serum starved for 25 h followed by stimulation with 20% bovine serum (Gibco BRL). Total cytoplasmic RNA was extracted at time intervals according to time course experiments. Gene-specific DNA probes were prepared by the method of random oligonucleotide priming for Northern blot analysis. The 32P-labeled probes were produced by inclusion of [α-32P]dCTP (>6,000 Ci/mmol; DuPont). All experiments described here were repeated at least once.
For lysate preparation, two plates of transfected cells (15-mm-diameter culture dish) were pooled together and then split into two portions: one for cytoplasmic RNA extraction and the other for preparing cytoplasmic and nuclear extracts as described previously (31). Protein concentrations were analyzed by using a bicinchoninic acid protein assay reagent (Pierce). These protein samples were used in Western blot analysis and gel mobility shift and supershift assays.
Western blot analysis.
Cytoplasmic or nuclear lysates were resolved on a sodium dodecyl sulfate–12% polyacrylamide gel and analyzed by using an ECL Western blotting kit (Amersham, Arlington Heights, Ill.). The blots were probed with specific antibodies as described elsewhere (see the legends for Fig. 2 and 4). The antibody for the myc tag was obtained by collecting culture medium from hybridoma cells (9E10; American Type Culture Collection) (8) and was used at a 1:100 dilution. The purified monoclonal antibody (MAb) against α-tubulin (DM1A) was purchased from Sigma and was used at a 1:20,000 dilution as a positive control for cytoplasmic protein preparations. The antibody against the U1 70K (mouse immunoglobulin G) was kindly provided by Sue Berget and was used at a 1:100 dilution as a positive control for nuclear protein preparations.
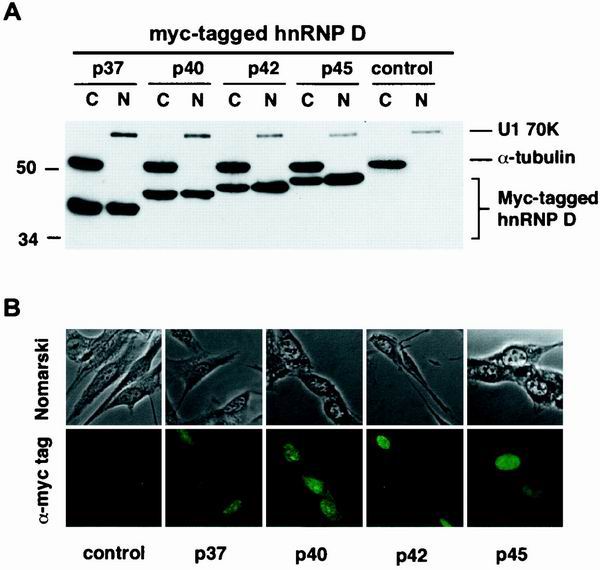
FIG. 2.
Expression levels and subcellular distributions of human hnRNP D isoforms in mouse NIH 3T3 cells. (A) Western blot analysis. Cytoplasmic (C) and nuclear (N) lysates prepared from NIH 3T3 B2A2 cells, transfected without (control) or with individual plasmids containing myc-tagged isoforms of hnRNP D, were resolved on a sodium dodecyl sulfate–12% polyacrylamide gel. The blot was probed with a MAb against myc-epitope tag (9E10) (for hnRNP D proteins) and a control MAb against α-tubulin (for cytoplasmic lysate) as well as a control polyclonal antibody against U1 70K (for nuclear lysate). (B) Indirect immunofluorescence microscopy study with the 9E10 MAb showing the subcellular distribution of hnRNP D isoforms (depicted by their molecular masses) and control. Both phase contrast (upper panels) and immunofluorescence (lower panels) views are shown.
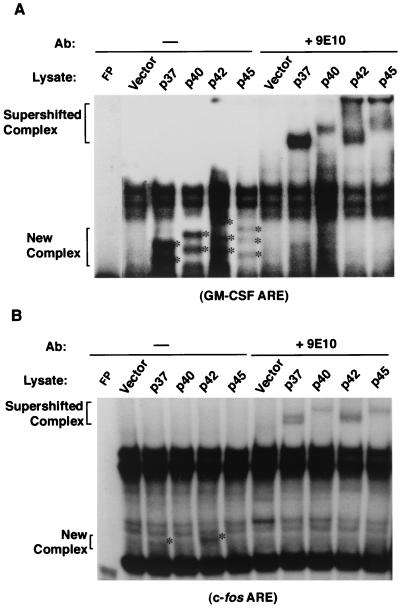
FIG. 4.
In vitro interactions of hnRNP D isoforms with different AREs correlate with the copy number of AUUUA motifs in AREs. 32P-labeled RNA transcribed in vitro from human GM-CSF ARE (A) or c-fos ARE (B) was incubated with cytoplasmic lysates from NIH 3T3 B2A2 cells transfected with individual plasmids expressing vector or myc-tagged hnRNP D isoforms. The RNA substrate was incubated with cytoplasmic lysate followed by RNase T1 digestion. The digestion mixtures were left alone (−) or further incubated in the presence of MAb against the myc-epitope tag (+9E10) as indicated on the top of each lane. The final reaction mixtures were analyzed by electrophoresis in a 6% nondenaturing polyacrylamide gel. New complexes (asterisks) and supershifted complexes (brackets) are as indicated.
Immunofluorescence microscopy study.
Indirect immunofluorescence microscopy was conducted as described previously (31). Briefly, NIH 3T3 B2A2 cells were grown on coverslips and transfected with plasmids expressing individual myc-tagged isoforms of hnRNP D or vector only. After 48 h, cells were fixed with 100% methanol, permeabilized with 0.5% Triton X-100, and then stained using a MAb against myc epitope tag (9E10) as primary antibody. The secondary antibody for anti-mouse-immunoglobulin G (purchased from Sigma) was coupled to fluorescein isothiocyanate. All images were viewed and captured by a Spot-Digital camera (Diagnostics) and processed for publication at 300 dots per in. using Adobe PhotoShop (version 4.0) software.
Analysis of RNA-protein interactions.
RNA probe synthesis, gel mobility shift assays, and antibody supershift assays were carried out as described previously (7, 48). In vitro transcription using HindIII-linearized pT3AREc-fos (48), EcoRI-linearized pT3AREGM63, or BlpI-linearized pT7fos as template was carried out to synthesize RNA probes for the c-fos ARE, GM-CSF ARE, or c-fos coding regions. Briefly, cytoplasmic lysate (8 μg of protein) and 32P-labeled RNA (1 ng) were incubated at room temperature for 15 min in a buffer containing 10 mM HEPES (pH 7.6), 3 mM MgCl2, 40 mM KCl, 2 mM dithiothreitol, 10% glycerol, and 0.5% IGAPEL CA-630. Heparin (5 μg/ml, final concentration) and yeast total RNA (200 ng/ml, final concentration) were added to reduce nonspecific binding. The volume of each reaction mixture was 10 μl. Subsequently, unbound RNA was digested by including 0.6 U of RNase T1 (Calbiochem, San Diego, Calif.) for 20 min at room temperature. RNA-protein complexes were resolved in 6% nondenaturing polyacrylamide gels. To perform gel mobility supershift analyses, following RNA-protein binding and RNase T1 digestion, 3 μl of a MAb against myc tag (9E10) was added into the binding reaction mixture. After a 15-min incubation at room temperature, RNA-protein-antibody complexes were resolved in 6% nondenaturing polyacrylamide gels.
RESULTS
Different isoforms of hnRNP D variably inhibit decay of mRNA bearing the c-fos ARE in NIH 3T3 cells.
Previously, we demonstrated in K562 cells that hnRNP D has a destabilizing role in ARE-mediated mRNA decay (25). We showed that following hemin-induced erythroid differentiation of K562 human erythroleukemia cells, ARE-containing mRNAs are stabilized. Ectopic expression of hnRNP D in hemin-treated K562 cells was able to restore rapid decay of ARE-containing mRNA. However, when hnRNP D was ectopically overexpressed in non-hemin-treated proliferating K562 cells, it had no effect on the decay of ARE-containing mRNA.
To explore the possibility that hnRNP D's function in ARE-mediated decay may be cell-type dependent, we examined the decay of β-globin mRNA bearing a 3′ UTR c-fos ARE (BBB+AREc-fos) in the presence of ectopically overexpressed hnRNP D in NIH 3T3 cells. NIH 3T3 cells were transiently cotransfected with the BBB+AREc-fos plasmid and a plasmid expressing an N-terminally myc-epitope-tagged hnRNP D isoform. Because hnRNP D cDNA is driven by the tetracycline-regulated promoter system, an NIH 3T3 stable cell line termed B2A2, which expresses the tetracycline-responsive trans-activator (tTA) in the absence of tetracycline (47), was used in our transfection experiments. BBB+AREc-fos mRNA was transiently transcribed from the c-fos promoter after serum induction of the growth-arrested B2A2 cells, which allowed determination of mRNA decay without using a transcription inhibitor to inhibit transcription (47). The decay of BBB+AREc-fos was monitored in transiently transfected B2A2 cells constitutively expressing myc-tagged hnRNP D in the absence of tetracycline. The results showed that rapid deadenylation and decay of BBB+AREc-fos mRNA in the cytoplasm is significantly impeded when the p37 or p42 isoform is overexpressed, but it is only modestly affected by p40 or p45 (Fig. 1). In contrast, BBB+AREc-fos mRNA is not affected when a cloning vector without hnRNP D cDNA is overexpressed (Fig. 1). These results showed that hnRNP D can inhibit ARE-mediated mRNA decay and that the extent of inhibition varies among different isoforms in the following rank order: p37 ≥ p42 > p45 ≥ p40. This order is consistent with the relative affinities of hnRNP D isoforms for the ARE determined by in vitro studies using recombinant proteins (10, 11, 44). These results differ markedly from our previous finding in K562 cells (25) and demonstrate that hnRNP D can exert opposite effects on ARE-mediated decay, depending on cell type and cell physiological conditions.
As a control for the specificity of hnRNP D's inhibitory effect on ARE-mediated mRNA decay, a parallel experiment measuring the decay of β-globin mRNA (BBB) bearing no specific destabilizing element was also carried out in B2A2 cells overexpressing individual isoforms. The results (Fig. 1D) showed that when β-globin mRNA does not contain any destabilizing element, its deadenylation and decay are not affected by ectopic overexpression of hnRNP D isoforms.
Characterization of expression levels and subcellular distributions of hnRNP D isoforms.
To ascertain that differential stabilization effects displayed by different isoforms are not a result of their different levels of protein expression and/or subcellular localization, cytoplasmic and nuclear extracts were prepared from B2A2 cells transiently transfected with individual hnRNP D isoforms. Western blot analysis using a MAb against myc-epitope tag (9E10) (Fig. 2A) showed no significant difference in expression levels among the four ectopically expressed isoforms in the cytoplasm or in the nucleus. No cross-contamination between the nuclear extract and cytoplasmic extract was detected, as demonstrated by exclusive detection of α-tubulin in the cytoplasmic extracts and U1 70K splicing factor in the nuclear preparations. We thus concluded that the different decay-impeding effects on the c-fos ARE displayed by hnRNP D isoforms in vivo are not due to their different levels of expression in the cytoplasm.
Indirect immunofluorescence was also performed using the 9E10 MAb against myc-epitope tag to further evaluate the subcellular distributions of hnRNP D isoforms. NIH 3T3 B2A2 cells were grown on coverslips and were transiently transfected with individual myc-tagged hnRNP D isoform constructs. The results showed that all ectopically expressed isoforms were located in both the nucleus and the cytoplasm (Fig. 2B). These results are consistent with the Western blot analysis of cell fractionation (Fig. 2A) and are also consistent with the isoforms being nucleocytoplasmic shuttling proteins.
Ectopic overexpression of hnRNP D differentially stabilizes mRNAs containing different AREs.
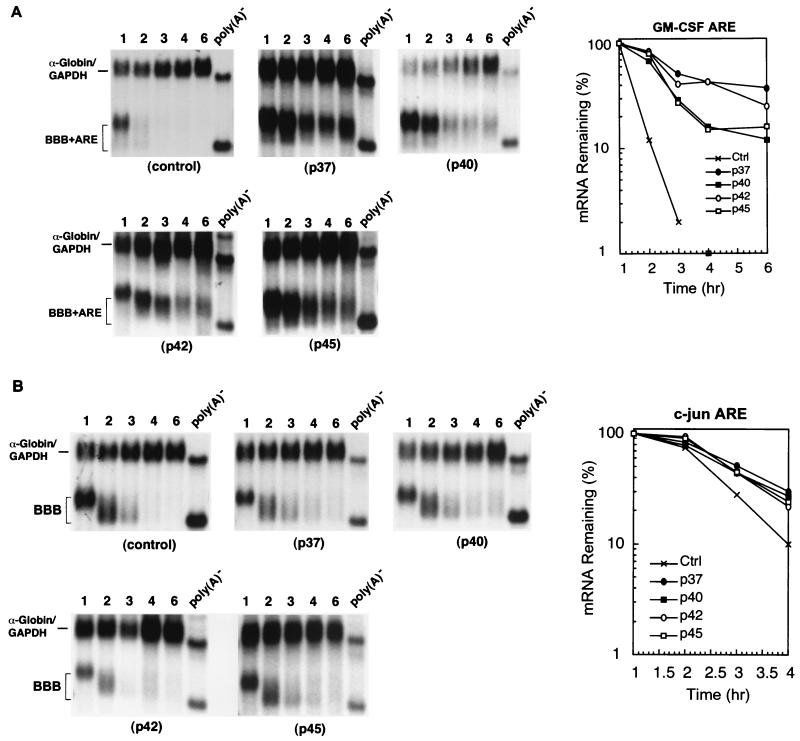
To investigate whether hnRNP D differentially regulates the mRNA decay mediated by different classes of AREs in vivo, we performed time course experiments to monitor the decay of β-globin mRNA bearing the GM-CSF ARE (class II) and the c-jun ARE (class III) in B2A2 NIH 3T3 cells that ectopically overexpress individual myc-tagged hnRNP D isoforms. The results (Fig. 3) showed that all four isoforms display a more profound impeding effect on the rapid decay of BBB+AREGM-CSF mRNA than their corresponding effect on the decay of BBB+AREc-fos mRNA. In contrast, none of the isoforms showed a significant stabilizing effect on the decay of BBB+AREc-jun. The poly(A) shortening of BBB+AREGM-CSF is significantly impaired, which then prevents decay of the RNA body. Taken together, our data show that hnRNP D differentially inhibits RNA decay mediated by the three classes of AREs in vivo with the following rank order of effect: class II ARE > class I ARE ≥ class III ARE. There is a positive correlation between the decay-impeding effect on AREs by hnRNP D and the numbers of AUUUA motifs within AREs.
FIG. 3.
Ectopic expression of human hnRNP D isoforms in mouse NIH 3T3 cells effectively blocks class II ARE- and not class III ARE-mediated mRNA decay. RNA blots show deadenylation and decay of β-globin mRNA bearing a class II ARE (BBB+AREGM-CSF) (A) or bearing a class III ARE (BBB+AREc-jun) (B) in the absence (control) or presence of ectopically expressed individual isoforms of hnRNP D (indicated by their molecular masses). Semi-log plots show the effects of four hnRNP D isoforms on mRNA decay directed by the GM-CSF ARE (A) or c-jun ARE (B). Cell culture, transfection, gene expression, RNase H treatment, and quantitation of mRNA and data plotting were as described in the legend to Fig. 1.
The presence of multiple copies of pentanucleotide AUUUA in an ARE correlates with strong in vitro interactions between an ARE and 3T3 cell-made hnRNP D.
To further address the importance of AUUUA motifs in determining the differential stabilization effects upon an ARE by hnRNP D, we conducted gel mobility shift assays using whole cytoplasmic extracts containing individual myc-tagged isoforms. Gel mobility shift assays were carried out using uniformly 32P-labeled GM-CSF ARE, c-fos ARE, or c-jun ARE RNA. To better assess RNA-protein complexes formed between the myc-tagged exogenous hnRNP D and ARE RNA substrate, antibody-supershift assays using 9E10 MAb against the myc-epitope tag were also performed in parallel. The results showed that GM-CSF ARE formed discernible new complexes with exogenous hnRNP D proteins (Fig. 4) that were readily super-shifted by MAb 9E10. In addition, c-fos ARE also supports new complex formation, albeit weakly, when lysates containing myc-tagged p37 or p42 are used. It appears that both p37 and p42 support more significant RNA-protein complex formation than either p40 or p45. In contrast, no RNA-protein complex can be detected by these assays when using a c-jun ARE probe (data not shown). The results from our in vitro studies using hnRNP D proteins made in NIH 3T3 cells are consistent with our in vivo studies showing that wild-type hnRNP D can discriminate among different classes of AREs and exerts its differential decay-impeding effect in the following rank order: GM-CSF ARE (class II) > c-fos ARE (class I) ≥ c-jun ARE (class III).
A cluster of overlapping AUUUA motifs observed in class II AREs is a key feature recognized in vivo by hnRNP D for its negative effect on mRNA decay.
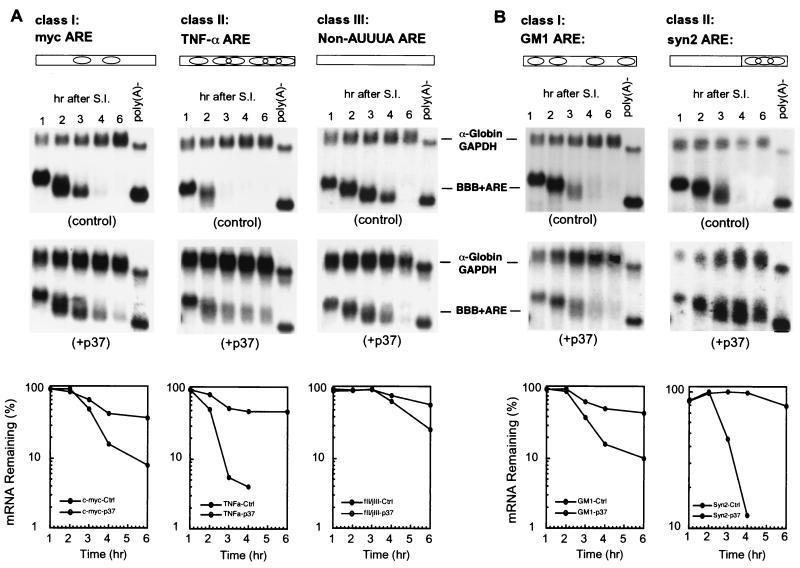
The above results suggested that multiple overlapping AUUUA motifs found in class II AREs are a key feature recognized by hnRNP D. To test this possibility, we performed two lines of experiments. First, the destabilizing function of three additional AREs, the c-myc ARE from class I, the TNF-α ARE from class II, and a synthetic non-AUUUA ARE (fII/jIII) (32) representing class III, was examined in B2A2 cells constitutively expressing the p37 isoform of hnRNP D. The results (Fig. 5A) showed that p37 has a very drastic stabilizing effect on the class II TNF-α ARE, whereas it has little effect on the non-AUUUA ARE. The class I c-myc ARE was moderately affected. This rank order of stabilization on three additional AREs by hnRNP D is consistent with the results in Fig. 1 and 3.
FIG. 5.
hnRNP D isoforms effectively inhibit class II ARE-mediated mRNA decay by recognizing multiple overlapping AUUUA motifs. (A) RNA blots and semi-log plots showing decay of BBB+ARE mRNAs representing individual classes of AREs in NIH 3T3 B2A2 cells expressing p37 isoform (+p37) or vector (control). (B) RNA blots and semi-log plots showing decay of BBB mRNAs containing a mutant GM-CSF ARE (GM1, class I ARE) and a synthetic ARE representing a class II ARE in NIH 3T3 B2A2 cells expressing the p37 isoform of hnRNP D (+p37) or vector (control). Open rectangles and oval symbols in both panels depict AREs and AUUUA motifs, respectively. Cell culture, transfection, RNase H treatment, and quantitation of mRNA and data plotting were as described in the legend to Fig. 1.
Second, to further substantiate this correlation and determine whether clustering of AUUUA motifs is critical, two additional AREs were tested. The first was a mutant GM-CSF ARE (GM1) with three point mutations that knock out the clustering of multiple AUUUA motifs (46). This manipulation transformed the GM-CSF ARE from class II, with seven copies of clustered AUUUA motifs, into class I, with four copies of scattered AUUUA motifs. The second ARE, termed syn2, is a synthetic class II ARE consisting of a 54-nt 5′ spacer and a 3′ sequence with a cluster of three AUUUA motifs. The 54-nt spacer has no destabilizing effect by itself, whereas the 3′ AUUUA cluster is the key sequence feature that constitutes the destabilizing function for syn2 ARE (46). The results (Fig. 5B) showed that the rapid decay of BBB+AREsyn2 is nearly completely blocked by p37, whereas BBB+AREGM1, like two other class I ARE-containing messages that we tested (BBB+AREc-fos and BBB+AREmyc), is only moderately stabilized by p37. Taken together, we conclude from these separate lines of evidence that hnRNP D inhibits class II ARE-mediated mRNA decay by recognizing multiple overlapping AUUUA motifs. It has only a moderate stabilization effect on class I AREs that contain a few scattered copies of the AUUUA motif.
hnRNP D isoforms inhibit the rapid decay of a reporter mRNA bearing the c-fos protein-coding region.
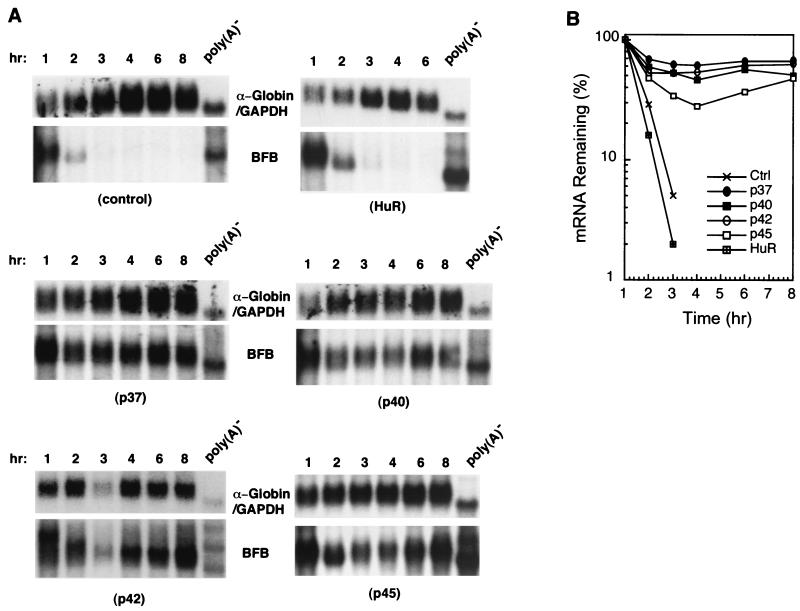
Given the above results and previous in vitro studies concerning specific binding of hnRNP D to AREs, it is intriguing that hnRNP D was recently shown to be an integral component of a complex implicated in rapid mRNA decay directed by the c-fos major coding determinant, a purine-rich sequence element in the protein-coding region (15). One implication of that study is that hnRNP D may have a general role in mRNA turnover and may participate in mRNA turnover that does not involve direct binding to a stability determinant. Therefore, we asked whether hnRNP D might regulate RNA decay directed by the entire c-fos protein-coding region, which contains multiple RNA determinants of instability including the major coding determinant (35). To test this possibility, the rapid decay of a hybrid message (BFB) consisting of the β-globin 5′ and 3′ UTRs and the entire c-fos protein-coding region (40) was monitored in B2A2 cells that constitutively overexpress individual hnRNP D isoforms. The results (Fig. 6) showed dramatic stabilization of BFB mRNA by all four isoforms, which is different from the differential effects seen with AREs. In contrast, overexpression of another ARE-binding protein, HuR, had little effect on the rapid decay of BFB mRNA. It is of particular significance that hnRNP D isoforms specifically block decay of BFB transcript, whereas they have no effect on the decay of β-globin mRNA bearing no specific decay determinant described above (Fig. 1D). These experiments demonstrated the participation of hnRNP D in mRNA turnover mediated by distinct RNA destabilizing elements.
FIG. 6.
All hnRNP D isoforms effectively block mRNA decay directed by the c-fos coding region instability determinants. (A) RNA blots showing decay of a hybrid mRNA containing the entire c-fos protein-coding region (BFB) in NIH 3T3 B2A2 cells expressing individual isoforms of hnRNP D, HuR (another ARE-BP), or vector (control). Due to comigration of BFB and α-globin control mRNAs, blots were first probed with c-fos coding region probe (for BFB message) and then stripped and reprobed with α-globin-specific probe (for control message). (B) Semi-log plot showing decay of BFB mRNA. Cell culture, transfection, RNase H treatment, and quantitation of mRNA and data plotting were as described in the legend to Fig. 1.
Different mechanisms underlie the stabilization effect of hnRNP D on the ARE-mediated and c-fos coding determinant-directed decay pathways.
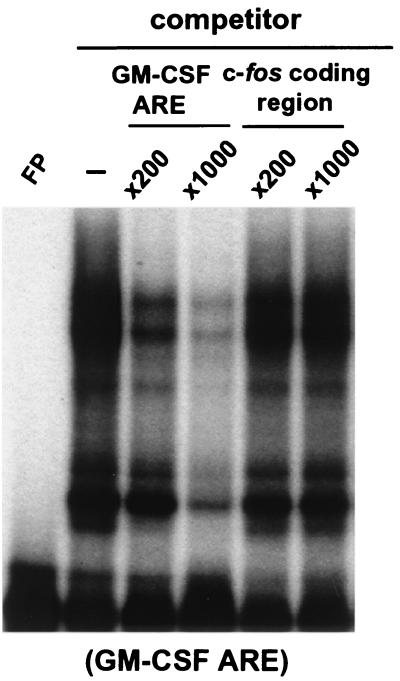
To test whether hnRNP D binds directly to the c-fos coding determinants, gel mobility and antibody super-shift assays were carried out using an RNA probe spanning the entire c-fos open reading frame and B2A2 cell lysate containing individual hnRNP D isoforms. The results (data not shown) showed that all isoforms failed to support the formation of any RNA-protein complexes that could be readily super-shifted by the anti-myc MAb (9E10). They all showed a gel-shift pattern identical to that of the control lysate (data not shown). To further substantiate that hnRNP D does not bind directly to the c-fos protein-coding region, the unlabeled c-fos protein-coding-region RNA was used as a competitor in the gel mobility shift assay. The results (Fig. 7) showed that an increasing amount of c-fos coding-region RNA does not abolish formation of RNA-protein complexes between the 32P-labeled GM-CSF ARE and myc-tagged p37 in the lysate, whereas the cold GM-CSF ARE competes well. We concluded from these two separate lines of evidence that hnRNP D synthesized in NIH 3T3 cells does not bind directly to the c-fos coding region to exert its stabilizing effect in vivo, indicating that different mechanisms underlie the stabilization effect of hnRNP D of the ARE-mediated and c-fos coding-determinant-directed decay pathways.
FIG. 7.
Ectopically expressed human hnRNP D isoforms do not directly interact with the c-fos coding region in vitro. 32P-labeled RNA transcribed in vitro from human GM-CSF ARE was incubated with cytoplasmic lysates in the presence of increasing amounts of either unlabeled GM-CSF ARE or unlabeled c-fos coding region transcript as competitor. The binding mixtures were analyzed by electrophoresis on a 6% nondenaturing polyacrylamide gel. Gel mobility shift and antibody-supershift assays were carried out as described in the legend to Fig. 4.
DISCUSSION
hnRNP D has dual roles in cytoplasmic mRNA decay in a cell-type-specific manner.
In this study, we made several findings that provide a molecular basis to explain how differential degradation of mRNAs bearing different classes of AREs may be regulated by specific ARE-BPs. The findings also have general implications for the function of ARE-BPs and their target AREs in vivo. Our experiments showed that hnRNP D has both a positive and a negative role in ARE-mediated RNA turnover: a stabilizing role observed in NIH 3T3 cells (this study) and a destabilizing role previously identified in K562 cells (25). The contrasting effects of hnRNP D exhibited in NIH 3T3 fibroblasts versus K562 erythroleukemic cells indicate that hnRNP D's function in RNA turnover is regulated in a cell-type-specific manner.
In vivo discrimination among three different classes of AREs by hnRNP D.
In contrast to a relaxed binding specificity for AREs observed in vitro using recombinant proteins synthesized in E. coli (1, 10, 44), hnRNP D discriminates among the three different classes of AREs in vivo. It most effectively down-regulates the destabilizing function of class II AREs via recognition of multiple overlapping AUUUA motifs. Consistent with our in vivo characterizations, our in vitro gel-shift assays using cytoplasmic extracts prepared from NIH 3T3 cells that overexpress individual hnRNP D isoforms also showed a similar discrimination among different classes of AREs. The discrepancy between our results and other in vitro binding studies using recombinant proteins could be due to the lack of posttranslational modifications of recombinant hnRNP D, such as methylation or phosphorylation (49), and/or the absence of specific hnRNP D-interacting proteins that modulate its ARE-binding specificity and affinity. Thus, our data suggest that in vivo these modifications and/or interactions are critical for fine-tuning hnRNP D's functions in cytoplasmic mRNA turnover.
Distinct roles for hnRNP D isoforms in the differential regulation of mRNA decay.
The differential regulation of mRNA turnover by hnRNP D isoforms observed in our experimental system defines a potential mechanism of hnRNP D isoform-specific regulation of mRNA turnover. Our data show that a significant difference exists among four hnRNP D isoforms in their ability to inhibit ARE-mediated decay in NIH 3T3 cells with the following rank order: p37 ≥ p42 > p45 ≥ p40. Surprisingly, all hnRNP D isoforms are equally capable of blocking the rapid RNA decay directed by another mRNA stability determinant, i.e., the c-fos protein-coding region, which does not share any significant sequence similarity with the AREs. Thus, p40 and p45 isoforms have the ability to down-regulate the destabilizing function of the c-fos protein-coding determinants without affecting that of the ARE. This implies that these two isoforms could be used to selectively increase the stability of c-fos mRNA without affecting other ARE-containing mRNAs. On the other hand, p37 or p42 could stabilize c-fos mRNA even more by blocking the destabilizing function of both the ARE and coding region determinant without affecting class III (non-AUUUA) ARE-containing mRNAs, such as c-jun mRNA. This point is of particular significance given that the expression of c-fos mRNA is frequently induced along with a large group of labile early-response-gene mRNAs and cytokine mRNAs that contain an ARE in their 3′ UTRs (2, 14, 37). It will be interesting for a future study to address the physiological consequence and significance of producing different isoforms of hnRNP D that display differential binding to AREs and c-fos coding determinants.
What might be the structural basis for the isoform-specific effects observed? It is interesting that both p37 and p42 lack an N-terminal peptide insertion that is alternatively included in the mRNAs encoding p40 and p45 during pre-mRNA splicing of hnRNP D (Fig. 1). In vitro studies showed that the alternatively spliced peptide inserted in the first RRM, found in p40 and p45, interferes with their ability to bind target RNA sequences (18). The significance of this structural difference observed in vitro is supported by our in vivo functional characterizations. We showed that p37 and p42 are much more effective than p40 and p45 in inhibiting ARE-mediated mRNA decay in NIH 3T3 cells (this study) or in rescuing ARE-directed decay in K562 cells that have been induced for further erythroid differentiation by hemin (25). On the other hand, the potent inhibition of the c-fos coding-determinant-directed mRNA decay by all hnRNP D isoforms may thus involve common domains present in all four isoforms. Functional dissection of hnRNP D should shed light on the relationship between the structure and function of hnRNP D.
A critical issue concerning hnRNP D is the specificity of its inhibitory effect on mRNA decay, given that hnRNP D blocks the function of two distinct RNA decay determinants. The following lines of evidence support a specific regulatory role rather than a nonspecific stabilization effect for hnRNP D. First, HuR, an ARE-BP exhibiting a binding affinity similar to that of hnRNP D in vitro (27), has no effect on the c-fos coding-determinant-mediated decay (Fig. 6). Second, the p40 isoform has essentially no effect on ARE-mediated decay but dramatically blocks the rapid decay directed by the c-fos coding region. Third, ectopic overexpression of hnRNP D in proliferating K562 cells does not inhibit ARE-mediated decay, suggesting a cell-type-specific effect. Fourth, hnRNP D's effect on AREs is class specific in that it has little effect on class III AREs. Lastly, ectopic overexpression of hnRNP D specifically impairs the deadenylation step of the β-globin mRNA bearing either an ARE or the c-fos coding region but has no such effect on the β-globin mRNA alone, demonstrating that hnRNP D's effects on deadenylation require specific RNA stability determinants.
The negative effect by hnRNP D on mRNA decay mediated by the ARE and the c-fos coding determinants involves two distinct mechanisms.
Given that hnRNP D does not bind directly to the coding sequence and there is no sequence similarity between the ARE and the c-fos coding sequence, how might p37 isoform exert its inhibitory effect on the two very distinct destabilizing elements? In the case of AREs, it is possible that stabilization is effected via direct binding of hnRNP D to an ARE, preventing the assembly of a decay complex necessary for rapid deadenylation and decay. For coding determinants whose function is coupled to translation, a few possibilities may be envisaged. For example, overexpression of hnRNP D might block translation initiation, leading to blockage of deadenylation. Recently we showed that p37 isoform is an integral component of a multiprotein complex that bridges the interaction between the major c-fos coding determinant and the 3′ poly(A) tail (15). Deadenylation and decay of the coding-determinant-containing mRNA is induced by disruption or reorganization of the complex as a result of ribosome transit following translation initiation. However, our results from sucrose gradient fractionation experiments showed no change of polysome profiles between the absence or presence of ectopically overexpressed p37 (data not shown), arguing against the notion of blockage of translation initiation. As our data also demonstrate that there was no direct binding of hnRNP D to the coding determinants, they point to a possibility of hnRNP D's direct interference with deadenylation activity. For instance, overexpression of hnRNP D affects the efficient recruitment of a critical factor, e.g., poly(A) nuclease (PARN/DAN) (9, 20), by the coding determinant. Or, overexpression of hnRNP D causes the formation of an aberrant bridging complex and/or mRNP structure that is unable to direct deadenylation. While the precise mechanism involved awaits further experimentation, our results clearly indicate that hnRNP D employs at least two distinct mechanisms for controlling mRNA turnover.
Our findings also have an important implication in that hnRNP D may serve as a global factor in controlling general mRNA turnover in mammalian cells rather than a factor only involved in ARE-mediated decay. This notion is also supported by two other recent studies. In one study, hnRNP D was found in an RNA-protein complex necessary for the stability of α-globin mRNA (19). In the other study, p45 isoform was found in RNA-protein complexes formed with a 390-nt destabilizing sequence from the 3′ UTR of cyclin D1 mRNA (23). Significantly, neither RNA stability determinant contained AUUUA motifs.
Molecular mechanism for selective stabilization of cytokine mRNAs.
Since class II AREs are primarily found in mRNAs coding for cytokines, our results provide evidence that hnRNP D, particularly the p37 and p42 isoforms, may serve as a major ARE-BP that contributes to the transient up-regulation of cytokines during a broad range of immune and stress responses. For example, one scenario for hnRNP D acting as a negative regulator is that an up-regulation of levels of p37 or p42 in the cytoplasm during, e.g., T-cell and mast cell activation (24, 30), specifically leads to binding to and then stabilization of the class II ARE-containing cytokine mRNAs, such as GM-CSF, TNF-α, and IL-3, without affecting mRNAs containing other classes of AREs, such as c-fos and c-myc mRNAs. The question then becomes how such up-regulation of hnRNP D expression may be elicited. A few recent studies showing alterations of the stability of ARE-containing mRNAs in response to the activation of specific signaling transduction pathways point to the involvement of these signaling pathways in controlling ARE and ARE-BP functions. One study showed that activation of p38 mitogen-activated protein kinase induced by proinflammatory cytokines specifically inhibited ARE-mediated mRNA decay (45). In another case, the ionomycin-induced activation of the c-Jun N-terminal kinase (JNK)-stress-activated kinase pathway in mast cells was shown to be necessary for stabilizing IL-3 mRNA, a class II ARE-containing mRNA (28). Significantly, the role of IL-3 AREs cannot be substituted by class I AREs from either c-fos or c-myc. It will be important to investigate whether the multiple yet distinct roles played by hnRNP D in mRNA turnover are controlled by phosphorylation-dephosphorylation and to identify the relevant kinase and phosphatase responsible for those direct modifications. Further light may be shed on the roles of individual isoforms of hnRNP D by using transgenic mice that lack or overly express specific isoforms of hnRNP D.
ACKNOWLEDGMENTS
We thank R. Kulmacz, C. S. Raman, F. R. Cabral, and M. Wilkinson for critical reading of the manuscript and their valuable comments, S. Berget for anti-U1 70K antiserum, G. Brewer for AUF1 plasmids, F. Ishikawa for hnRNP D0 plasmids, and I. Verma for the T7fos plasmid.
This work was supported by a grant from the National Institutes of Health (GM 46454) to A.-B.S. A.-B.S. was the recipient of an American Heart Association Established Investigator Award.
REFERENCES
- 1.Bhattacharya S, Giordano T, Brewer G, Malter J S. Identification of AUF-1 ligands reveals vast diversity of early response gene mRNAs. Nucleic Acids Res. 1999;27:1464–1472. doi: 10.1093/nar/27.6.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci USA. 1986;83:1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen A C-Y, Shyu A-B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 4.Chen C Y, Chen T M, Shyu A B. Interplay of two functionally and structurally distinct domains of the c-fos AU-rich element specifies its mRNA-destabilizing function. Mol Cell Biol. 1994;14:416–426. doi: 10.1128/mcb.14.1.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C Y, Shyu A B. Selective degradation of early-response-gene mRNAs: functional analyses of sequence features of the AU-rich elements. Mol Cell Biol. 1994;14:8471–8482. doi: 10.1128/mcb.14.12.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C Y, Xu N, Shyu A B. mRNA decay mediated by two distinct AU-rich elements from c-fos and granulocyte-macrophage colony-stimulating factor transcripts: different deadenylation kinetics and uncoupling from translation. Mol Cell Biol. 1995;15:5777–5788. doi: 10.1128/mcb.15.10.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C Y, You Y, Shyu A B. Two cellular proteins bind specifically to a purine-rich sequence necessary for the destabilization function of a c-fos protein-coding region determinant of mRNA instability. Mol Cell Biol. 1992;12:5748–5757. doi: 10.1128/mcb.12.12.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cravchik A, Matus A. A novel strategy for the immunological tagging of cDNA constructs. Gene. 1993;137:139–143. doi: 10.1016/0378-1119(93)90262-2. [DOI] [PubMed] [Google Scholar]
- 9.Dehlin E, Wormington M, Korner C G, Wahle E. Cap-dependent deadenylation of mRNA. EMBO J. 2000;19:1079–1086. doi: 10.1093/emboj/19.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMaria C T, Brewer G. AUF1 binding affinity to A+U-rich elements correlates with rapid mRNA degradation. J Biol Chem. 1996;271:12179–12184. doi: 10.1074/jbc.271.21.12179. [DOI] [PubMed] [Google Scholar]
- 11.DeMaria C T, Sun Y, Long L, Wagner B J, Brewer G. Structural determinants in AUF1 required for high affinity binding to A + U-rich elements. J Biol Chem. 1997;272:27635–27643. doi: 10.1074/jbc.272.44.27635. [DOI] [PubMed] [Google Scholar]
- 12.Dempsey L A, Li M J, DePace A, Bray-Ward P, Maizels N. The human HNRPD locus maps to 4q21 and encodes a highly conserved protein. Genomics. 1998;49:378–384. doi: 10.1006/geno.1998.5237. [DOI] [PubMed] [Google Scholar]
- 13.Dreyfuss G, Matunis M J, Pinol-Roma S, Burd C G. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg M E, Belasco J G. Control of the decay of labile protooncogene and cytokine mRNAs. In: Belasco J G, Brawerman G, editors. Control of messenger RNA stability. San Diego, Calif: Academic Press; 1993. pp. 199–218. [Google Scholar]
- 15.Grosset C, Chen C-Y A, Xu N, Sonenberg N, Jacquemin-Sablon H, Shyu A-B. A mechanism for translationally coupled mRNA turnover: interaction between the poly(A) tail and a c-fos RNA coding determinant via a protein complex. Cell. 2000;103:29–40. doi: 10.1016/s0092-8674(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 16.Gueydan C, Droogmans L, Chalon P, Huez G, Caput D, Kruys V. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor alpha mRNA. J Biol Chem. 1999;274:2322–2326. doi: 10.1074/jbc.274.4.2322. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson A, Peltz S W. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- 18.Kajita Y, Nakayama J, Aizawa M, Ishikawa F. The UUAG-specific RNA binding protein, heterogeneous nuclear ribonucleoprotein D0. Common modular structure and binding properties of the 2xRBD-Gly family. J Biol Chem. 1995;270:22167–22175. doi: 10.1074/jbc.270.38.22167. [DOI] [PubMed] [Google Scholar]
- 19.Kiledjian M, DeMaria C T, Brewer G, Novick K. Identification of AUF1 (heterogeneous nuclear ribonucleoprotein D) as a component of the alpha-globin mRNA stability complex. Mol Cell Biol. 1997;17:4870–4876. doi: 10.1128/mcb.17.8.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Körner C G, Wahle E. Poly(A) tail shortening by a mammalian poly(A)-specific 3′-exoribonuclease. J Biol Chem. 1997;272:10448–10456. doi: 10.1074/jbc.272.16.10448. [DOI] [PubMed] [Google Scholar]
- 21.Lai W S, Carballo E, Strum J R, Kennington E A, Phillips R S, Blackshear P J. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol. 1999;19:4311–4323. doi: 10.1128/mcb.19.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larola G, Guesta R, Brewer G, Schneider R J. Control of mRNA decay by heat shock-ubiquitin-proteasome pathway. Science. 1999;284:499–502. doi: 10.1126/science.284.5413.499. [DOI] [PubMed] [Google Scholar]
- 23.Lin S, Wang W, Wilson G M, Yang X, Brewer G, Holbrook N J, Gorospe M. Down-regulation of cyclin D1 expression by prostaglandin A(2) is mediated by enhanced cyclin D1 mRNA turnover. Mol Cell Biol. 2000;20:7903–7913. doi: 10.1128/mcb.20.21.7903-7913.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsten T C, June C H, Ledbetter J A, Stella G, Thompson C B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 25.Loflin P A, Chen C-Y A, Shyu A-B. Unraveling a cytoplasmic role for hnRNP D in the in vivo mRNA destabilization directed by the AU-rich element. Genes Dev. 1999;13:1884–1897. doi: 10.1101/gad.13.14.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loflin T L, Chen C-Y A, Xu N, Shyu A-B. Transcriptional pulsing approaches for analysis of mRNA turnover in mammalian cells. Methods Enzymol. 1999;17:11–20. doi: 10.1006/meth.1998.0702. [DOI] [PubMed] [Google Scholar]
- 27.Ma W J, Cheng S, Campbell C, Wright A, Furneaux H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J Biol Chem. 1996;271:8144–8151. doi: 10.1074/jbc.271.14.8144. [DOI] [PubMed] [Google Scholar]
- 28.Ming X F, Kaiser M, Moroni C. c-jun N-terminal kinase is involved in AUUUA-mediated interleukin-3 mRNA turnover in mast cells. EMBO J. 1998;17:6039–6048. doi: 10.1093/emboj/17.20.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell P, Tollervey D. mRNA stability in eukaryotes. Curr Opin Gene Dev. 2000;10:193–198. doi: 10.1016/s0959-437x(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 30.Nair A P, Hahn S, Banholzer R, Hirsch H H, Moroni C. Cyclosporin A inhibits growth of autocrine tumour cell lines by destabilizing interleukin-3 mRNA. Nature. 1994;369:239–242. doi: 10.1038/369239a0. [DOI] [PubMed] [Google Scholar]
- 31.Peng S-P, Chen C-Y, Xu N, Shyu A-B. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 1998;17:3461–3470. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng S S, Chen C Y, Shyu A B. Functional characterization of a non-AUUUA AU-rich element from the c-jun proto-oncogene mRNA: evidence for a novel class of AU-rich elements. Mol Cell Biol. 1996;16:1490–1499. doi: 10.1128/mcb.16.4.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piecyk M, Wax S, Beck A R, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, Anderson P. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 2000;19:4154–4163. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995;59:423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiavi S C, Wellington C L, Shyu A B, Chen C Y, Greenberg M E, Belasco J G. Multiple elements in the c-fos protein-coding region facilitate mRNA deadenylation and decay by a mechanism coupled to translation. J Biol Chem. 1994;269:3441–3448. [PubMed] [Google Scholar]
- 36.Schuler G D, Cole M D. GM-CSF and oncogene mRNA stabilities are independently regulated in trans in a mouse monocytic tumor. Cell. 1988;55:1115–1122. doi: 10.1016/0092-8674(88)90256-5. [DOI] [PubMed] [Google Scholar]
- 37.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 38.Shyu A-B, Belasco J G, Greenberg M G. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5:221–232. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- 39.Shyu A-B, Garcia-Sanz J A, Mullner E, editors. Analysis of mRNA decay in mammalian cells. London, United Kingdom: Academic Press; 1996. [Google Scholar]
- 40.Shyu A-B, Greenberg M E, Belasco J G. The c-fos mRNA is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989;3:60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- 41.Sirenko O I, Lofquist A K, DeMaria C T, Morris J S, Brewer G, Haskill J S. Adhesion-dependent regulation of an A+U-rich element-binding activity associated with AUF1. Mol Cell Biol. 1997;17:3898–3906. doi: 10.1128/mcb.17.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner B J, DeMaria C T, Sun Y, Wilson G M, Brewer G. Structure and genomic organization of the human AUF1 gene: alternative pre-mRNA splicing generates four protein isoforms. Genomics. 1998;48:195–202. doi: 10.1006/geno.1997.5142. [DOI] [PubMed] [Google Scholar]
- 43.Wilson G M, Brewer G. Identification and characterization of proteins binding A+U-rich elements. Methods. 1999;17:74–83. doi: 10.1006/meth.1998.0709. [DOI] [PubMed] [Google Scholar]
- 44.Wilson G M, Sun Y, Lu H, Brewer G. Assembly of AUF1 oligomers on U-rich RNA targets by sequential dimer association. J Biol Chem. 1999;274:33374–33381. doi: 10.1074/jbc.274.47.33374. [DOI] [PubMed] [Google Scholar]
- 45.Winzen R, Kracht M, Ritter B, Wilhelm A, Chen C-Y A, Shyu A-B, Muller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu N, Chen C-Y A, Shyu A-B. Modulation of the fate of cytoplasmic mRNA by AU-rich elements: key sequence features controlling mRNA deadenylation and decay. Mol Cell Biol. 1997;17:4611–4621. doi: 10.1128/mcb.17.8.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu N, Loflin P, Chen C-Y A, Shyu A-B. A broader role for AU-rich element-mediated mRNA turnover revealed by a new transcriptional pulse strategy. Nucleic Acids Res. 1998;26:558–565. doi: 10.1093/nar/26.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.You Y, Chen C Y, Shyu A B. U-rich sequence-binding proteins (URBPs) interacting with a 20-nucleotide U-rich sequence in the 3′ untranslated region of c-fos mRNA may be involved in the first step of c-fos mRNA degradation. Mol Cell Biol. 1992;12:2931–2940. doi: 10.1128/mcb.12.7.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W, Wagner B J, Ehrenman K, Schaefer A W, DeMaria C T, Crater D, DeHaven K, Long L, Brewer G. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol Cell Biol. 1993;13:7652–7665. doi: 10.1128/mcb.13.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]