Abstract
Purpose of Review
To provide relevant background of the Integrated Alzheimer's Disease Rating Scale (iADRS), with examples, to assist the reader with the interpretation of iADRS findings from the TRAILBLAZER-ALZ study.
Recent Findings
The iADRS is an integrated measure of global Alzheimer disease (AD) severity for use in the clinical trial environment. It provides a single score that captures commonalities across cognitive and functional ability domains, reflecting disease-related impairment, while minimizing noise not related to disease progression that may exist within each domain. In AD, disease-modifying therapies (DMTs) are expected to slow the rate of clinical decline, changing the trajectory of disease progression. The overall percent slowing of disease progression with treatment is a more informative outcome of effect than absolute point differences between treatment and placebo groups at any given time point because the latter is influenced by treatment period and disease severity. The TRAILBLAZER-ALZ trial was a phase 2 study designed to evaluate the safety and efficacy of donanemab in participants with early symptomatic AD; the primary outcome measure was the change from baseline to 76 weeks on the iADRS. In the TRAILBLAZER-ALZ study, donanemab slowed disease progression by 32% at 18 months (p = 0.04 vs placebo), demonstrating clinical efficacy. At the patient level, one can assess whether the DMT effect is clinically meaningful by estimating the threshold of change consistent with clinically meaningful worsening; based on the TRAILBLAZER-ALZ findings, treatment with donanemab would delay reaching this threshold by approximately 6 months.
Summary
The iADRS is capable of accurately describing clinical changes associated with disease progression and detecting treatment effects and is an effective assessment tool for use in clinical trials of individuals with early symptomatic AD.
Alzheimer disease (AD) is an irreversible neurodegenerative disease that advances over decades and is characterized by underlying amyloid and tau pathology and neurodegeneration and progressive loss of cognitive and functional abilities. Finding effective treatments for AD remains a critical unmet need worldwide. Until recently, symptomatic treatments were the only available therapies; they are designed to manage cognitive and behavioral symptoms, but their effects do not alter the underlying course of the disease.
Current drug development efforts are focused on disease-modifying therapies (DMTs) in early AD, where the intent of treatment is to target the underlying pathology of disease and thus slow cognitive and functional decline and delay loss of quality of life and independence.
As a result, there has also been increased interest in identifying appropriate clinical outcome measures to capture disease progression, and the effects of DMT, in populations with less advanced disease. Regulators historically required both a cognitive measure and a global or functional measure in the assessment of treatment in the AD trial setting. With the shift in focus to intervene earlier in the disease continuum, regulatory guidance for clinical trials has evolved from requiring coprimary outcome measures that assess cognition and function separately to supporting the use of a single outcome measure that captures both cognition and function in an integrated manner.1
Over the past decade, considerable effort has been invested in developing instruments that accurately track the clinical progression of early AD and are sensitive to treatment effects.2-5 This investment was driven by the inconsistent performance of outcome measures at this stage of disease.6,7
One measure of global AD severity that has been used as a prespecified outcome measure in the clinical trial setting is the Integrated Alzheimer's Disease Rating Scale (iADRS), comprising items that assess both cognition and the ability to perform activities of daily living. The iADRS was the primary outcome measure in the TRAILBLAZER-ALZ trial, a phase 2 study designed to evaluate the safety and efficacy of donanemab, developed by Eli Lilly and Company, in participants with early symptomatic AD (defined as mild cognitive impairment [MCI] or mild dementia due to AD).8 The TRAILBLAZER-ALZ trial was the first completed study of a DMT in AD to have successfully met its prespecified primary endpoint (the change in the iADRS total score from baseline to 76 weeks), demonstrating statistically significant slowing of decline on an integrated measure that assesses the effect of cognitive loss on the ability to perform daily activities.
The aim of this study was to provide the relevant background to assist with the understanding of the iADRS and findings from the TRAILBLAZER-ALZ trial. Specifically, we review the natural disease progression of AD and its measurement using clinical outcome tools; discuss the relevant properties of the iADRS and provide guidance for the interpretation of an iADRS score change; and, with this foundation of understanding, review the iADRS findings from the TRAILBLAZER-ALZ trial.
Clinical Outcomes in AD Trials
Assessment of disease modification through therapeutic intervention necessitates an understanding of the natural disease progression trajectory along the AD disease continuum. Progression of AD occurs in a nonlinear manner over the spectrum of disease severity, although it may be nearly linear over shorter periods (e.g., over 1 year); the full disease trajectory appears sigmoid shaped, both for pathologic (including biomarker) changes and clinical presentation.9 Temporally, changes in biomarkers of AD pathology are detected first (amyloid and then tau), followed by clinical decline (cognitive then functional).
Impairment in cognition and function progresses irreversibly, with cognitive decline both preceding and predicting functional decline.10 In a very early AD population, cognitive impairment may be subtle, and functional impairment may not be present, or well masked by accommodations. With disease progression, both cognitive and functional decline become more apparent and increasingly correlated. Functional decline initially affects the instrumental activities of daily living (iADLs) ( i.e., complex activities required for independent living, such as housekeeping, managing own finances, shopping, and meal preparation). Later in the disease, basic ADLs, a person's fundamental functioning skills (basic self-care tasks such as bathing, toileting, and feeding oneself), are also affected.
Based on our understanding of AD progression and the general requirements for clinical outcomes, important desirable properties for an AD clinical outcome scale are as follows:
It exhibits construct validity. That is, the scale assesses the core aspects of the disease it intends to measure (cognition and function), which are meaningful to the patients, care partners, and their health care providers.
It has a large enough range to capture divergent presentations and progression over time. That is, no item in the scale exhibits a floor effect (with individuals performing at a minimum score) or ceiling effect (individuals performing at a maximum score). This allows for the measurement of disease severity across an enrolled trial population both at baseline and trial endpoint.
It is discriminatory. That is, the scale is granular enough to be able to measure change accurately.
It comprises question and scoring methodologies that are objective (because subjectivity in scoring can lead to an increased variability in scores).
It minimizes response and rating bias (e.g., bias due to education, gender, rural/urban, cultural, and rater expectations).
The iADRS
In 2015, Eli Lilly and Company developed the iADRS for use as a study outcome in trials of DMTs in symptomatic AD. The iADRS was not developed for use in clinical practice. Accordingly, discussion of the iADRS is in relation to its use in clinical trials unless noted otherwise.
Development was both theoretically and empirically informed; that is, it was based on understanding of the core disease processes (cognitive and functional decline), and data were used to establish which tools or items most comprehensively measure those concepts. Further evidence for content validity of the iADRS has been established through work completed through the What Matters Most (WMM) research initiative,11 designed to assess treatment-related needs, preferences, and priorities for individuals with or at risk of AD and their care partners. Through qualitative interviews, individuals with, or are at risk of, AD and their care partners were asked what treatment-related outcomes matter to them12; subsequently, existing outcome measures were mapped to these items deemed important. Among outcome measures studied, the iADRS directly mapped to the most WMM concepts.9
Figure 1 shows the conceptual framework of the iADRS. The iADRS is a measure that comprises 2 underlying domains, “cognitive ability” and “functional ability”; the iADRS score serves to integrate the items that make up both domains into a single score that is conceptually distinct from either domain assessed individually.
Figure 1. The iADRS Conceptual Framework.
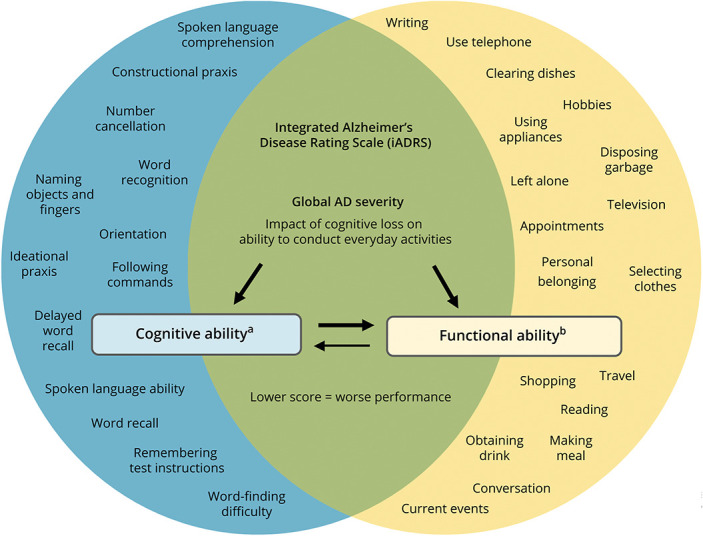
aMeasured using ADAS-Cog13 (hybrid of performance-based (standardized task) and clinician-reported outcome measure). bMeasured using ADCS-iADL (observer-reported outcome measure). AD = Alzheimer disease; ADAS-Cog13 = Alzheimer Disease Assessment Scale–13-item Cognitive subscale; ADCS iADL = Alzheimer Disease Cooperative Study–Instrumental Activities of Daily Living subscale.
The combination score of the iADRS captures commonalities across the cognitive and functional ability domains and minimizes noise that exists within each domain individually. This “noise” is the result of aspects of change that are not specifically related to AD and occurs in both domains. For example, in the cognitive domain, distractions during the cognitive testing can affect performance, while in the functional ability domain, physical impairment (other than those directly due to dementia) can temporarily affect daily functioning. The extent of noise reflected in the score is dependent on the domain affected, the assessment point in time, and features of the assessment interaction. Those components of the cognitive and functional ability domains that change concurrently and to a similar degree reflect disease-specific change. Changes in these components drive the integrated score and tend to negate random noise to a greater degree than when each domain is analyzed separately (data on file, Eli Lilly and Company). The iADRS score captures disease progression, as measured by aligned changes in cognitive impairment and the ability to conduct everyday activities, to provide a measure of global AD severity as a single summary score.
To obtain the iADRS score (i.e., to obtain responses to items for each domain), the AD Assessment Scale-Cognitive subscale (ADAS-Cog13)13,14 and the AD Cooperative Study-Activities of Daily Living subscale (ADCS-ADL)15,16 are administered to the study participant and care partner, respectively. The ADAS-Cog is a performance-rated/clinician-rated outcome measure, composed of items that assess cognitive function areas most typically impaired in AD; it is these items that make up the “cognitive ability” domain within the iADRS score. For the ADCS-ADL, the participant's performance level over the last 4 weeks for various activities is rated by the care partner; the ADCS-iADL is a subset of the 23-item ADCS-ADL (items 6a and 7–23) and assesses higher-level activities that place greater demand on cognitive resources. This subset of higher-level activity items constitute the “functional ability” domain within the iADRS score.
The iADRS has been validated, with well-described statistical properties,17,18 and has been used or is currently in use, as a clinical outcome measure in phase 2 and 3 clinical trials in AD, across MCI, mild dementia, and moderate dementia due to AD.19 Data from the validation study and clinical trials demonstrated that the iADRS was effective in capturing clinical progression from MCI through moderate dementia due to AD and treatment effects across MCI and mild dementia. In addition, the iADRS has been demonstrated to effectively capture changes in clinically meaningful outcomes associated with AD progression (e.g., quality of life, care partner support including supervision time, and health care costs).20 This body of evidence supports the use of the iADRS in clinical trials to measure clinically meaningful outcomes of AD.
Measuring Natural Disease Progression Using the iADRS
The iADRS has a score range of 0–144 points, with lower scores indicating a greater global AD severity. The large score range avoids floor and ceiling effects, permitting both the capture of disease progression over extended periods (years) from MCI through moderate dementia due to AD and the detection of treatment effect over short periods.
Because clinical trials in AD invariably focus on a specific severity (e.g., early AD or AD with mild dementia), it is not expected that within these severities, participants will score at the upper or lower extremities of the iADRS scale. The dynamic range is defined as the score range that captures approximately 70% of the datapoints of a population at a given disease severity (i.e., mean ± 1 SD). In MCI due to AD, the dynamic range of the iADRS is 20 points centered around a mean score of 114; in mild dementia due to AD, the dynamic range is 25 points, centered around a mean score of 104.
The iADRS domains of cognition and function are moderately correlated, suggesting that each domain measures distinct but related concepts. Each iADRS domain has a similar dynamic range and rate of change over time. Figure 2 shows the change in iADRS score over time with natural disease progression along the clinical continuum. The expected iADRS point change increases as the disease progresses; that is, the rate of progression increases from MCI to mild dementia to moderate dementia due to AD.
Figure 2. Expected iADRS Baseline Scores and Rate of Change by Clinical Presentation of AD.
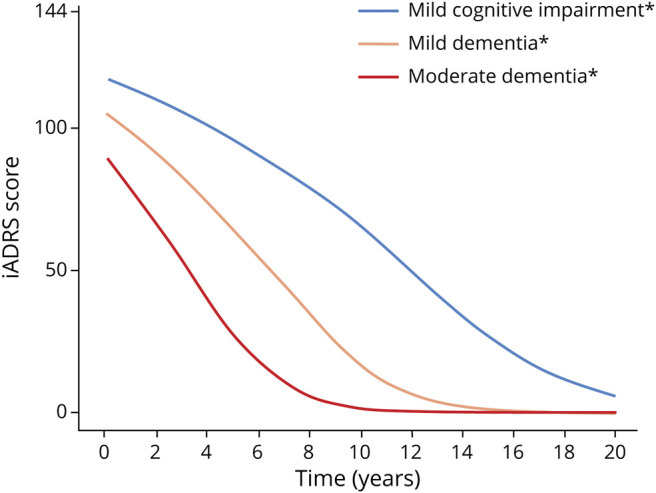
*Due to Alzheimer disease. Figure created using placebo data EXPEDITION-1, -2, and -3; AMARANTH; and DAYBREAK-ALZ; for details see Gueorguieva et al., Alz & Dem 2022; doi:10.1002/alz.12876. iADRS = Integrated Alzheimer's Disease Rating Scale.
Understanding Disease Modification Through the iADRS
Expression of DMT Effect
In the evaluation of the magnitude of a treatment effect in a progressive disease such as AD, it is more helpful to interpret treatment difference as a percent slowing with active treatment vs placebo rather than the absolute point difference between the treatment groups. This is because, unlike percent change, an absolute point difference is dependent on the duration of observation and stage of disease. This is illustrated in Figure 3 in a theoretical example demonstrating expected iADRS changes with a hypothetical DMT that slows progression by 25%. In a placebo group (i.e., untreated), the expected iADRS point change over 18 months differs by disease severity with a consistent 25% slowing of decline with treatment; the iADRS point change difference between the DMT and placebo groups increases as the disease advances (1.75 points for MCI due to AD and 5 points for moderate AD dementia).
Figure 3. Change in iADRS Score at 18 Months With Placebo vs a Hypothetical DMT With a 25% Treatment Effect.
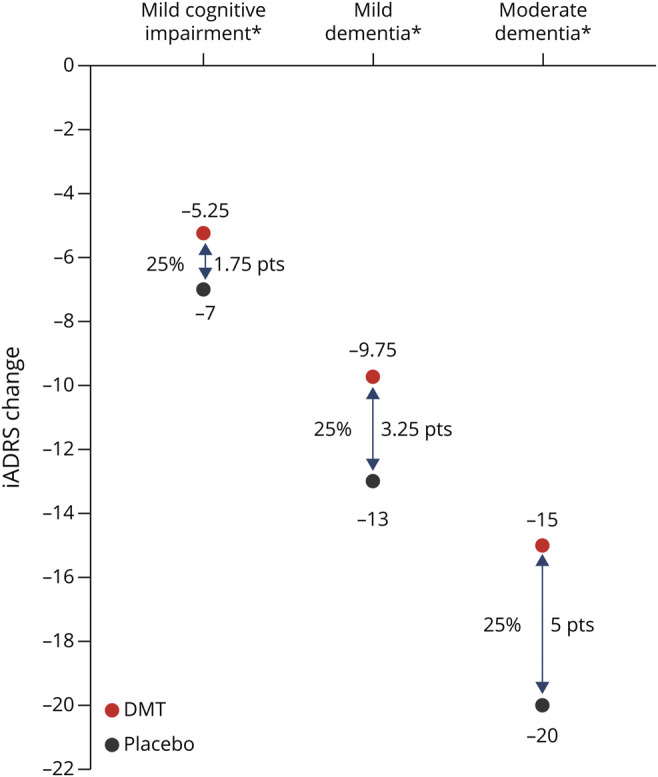
*Due to Alzheimer disease. Assumptions: (1) Change over 18 months with placebo is- 7 for MCI, -13 for mild dementia, and -20 for moderate dementia (calculated using placebo data EXPEDITION-1, -2 and -3; AMARANTH; and DAYBREAK-ALZ; for details see Gueorguieva et al., Alz & Dem 2022; doi:10.1002/alz.12876. (2) Disease progression portrayed as linear for simplicity of presentation. (3) DMT effect maintained over 18 months. iADRS = Integrated Alzheimer's Disease Rating Scale; DMT = disease-modifying therapy; MCI = mild cognitive impairment; pts = points.
Disease Modification Changes the Disease Trajectory
By definition, a DMT will change the disease trajectory (i.e., course over time) (Figure 4). More specifically, disease modification delays clinical decline. With a hypothetical DMT that slows disease progression by 25%, the placebo group will experience an additional 3.25 points of worsening on the iADRS compared with the DMT treatment group at 18 months. This equates to an approximately 4.5-month delay in disease progression with treatment. If this effect continues in a linear manner, at 36 months, there will be an approximately 9-month delay in disease progression with treatment.
Figure 4. Change in iADRS Score Over 36 Months With Placebo vs a Hypothetical DMT With a 25% Treatment Effect in Mild Dementia Due to AD.
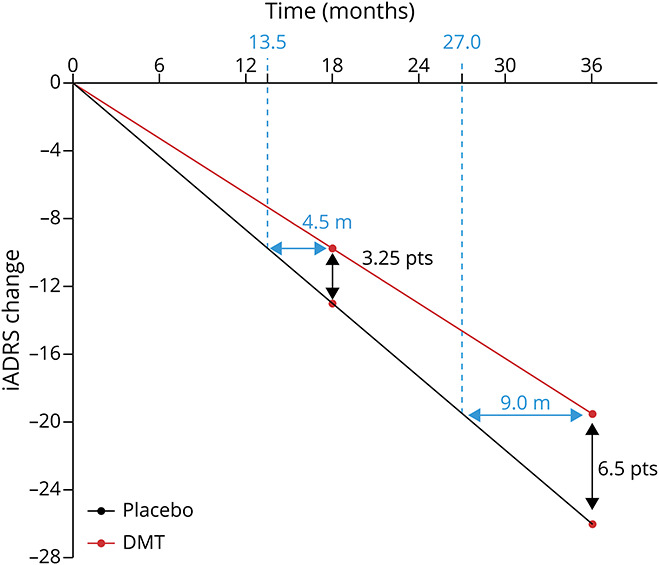
Assumptions: (1) Change over 18 months with placebo is −7 for MCI, −13 for mild dementia, and −20 for moderate dementia (calculated using placebo data EXPEDITION-1, -2, and -3; AMARANTH; and DAYBREAK-ALZ; for details see Gueorguieva et al., Alz & Dem 2022; doi:10.1002/alz.12876. (2) Disease progression portrayed as linear for simplicity of presentation. (3) DMT effect maintained over 36 months. AD = Alzheimer disease; DMT = disease-modifying therapy; iADRS = Integrated Alzheimer's Disease Rating Scale; MCI = mild cognitive impairment; pts = points.
Reviewing the TRAILBLAZER-ALZ Findings
In TRAILBLAZER-ALZ trial, the study population comprised individuals with MCI or mild dementia due to AD at baseline (i.e., early symptomatic disease, based on baseline Mini-Mental State Examination [MMSE] score); key enrollment criteria included MMSE scores between 20 and 28 and confirmed AD pathology (evidence of amyloid and tau on PET). The primary findings from the TRAILBLAZER-ALZ study were published in 20218 (Figure 5).
Figure 5. Schematic of Primary Efficacy Findings in the TRAILBLAZER-ALZ Trial8.
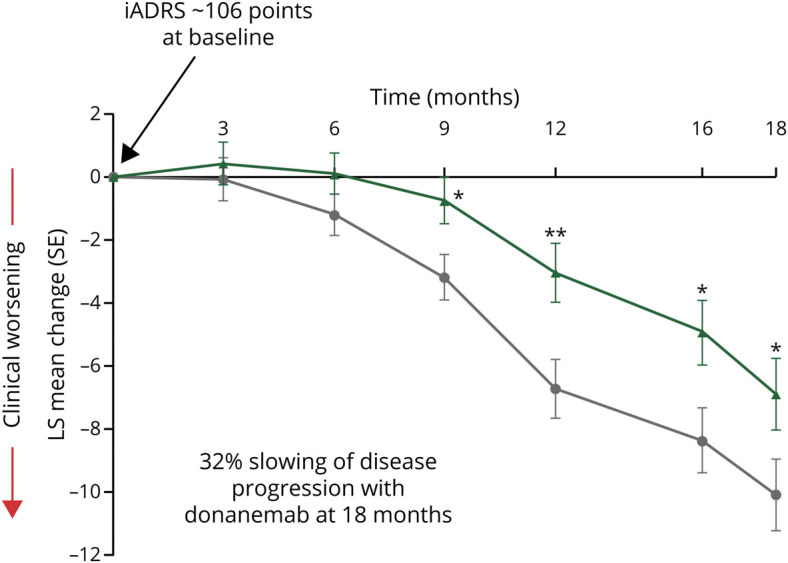
*p < 0.05; **p < 0.005. At 18 months, p = 0.04. Placebo (gray line), N = 126; Donanemab (green line), N = 131. iADRS = Integrated Alzheimer's Disease Rating Scale; LS = least sum of squares; SE = standard error.
Baseline iADRS Score
At baseline, the mean iADRS score was 106.2. This is as expected for the enrolled population (MCI or mild dementia due to AD). Of note, the iADRS minimum score was 60 and the maximum score was 139 at baseline, and they were 27 and 136, respectively, for study completers at 18 months. Thus, no participant reached the floor (0 points) or ceiling (144 points).
The iADRS dynamic range at baseline was 26 points, approximately 70% of study participants' baseline scores fell within a 26-point range around the mean of 106.2. Both domains contributed similarly to the iADRS total score at baseline (52% contribution from cognition and 48% contribution from function and equal dynamic range of both domains) and to the treatment group difference (44% contribution from cognition and 56% contribution from function).
Effect of Donanemab
Although both groups worsened on the iADRS over the study period, as expected with a progressive disease, less decline was observed in the donanemab group. At 76 weeks, the change from baseline in the iADRS score was −6.86 in the donanemab group and −10.06 in the placebo group (difference, 3.20; 95% confidence interval, 0.12–6.27; p = 0.04). This equates to a 32% slowing of disease progression with donanemab, which is generally viewed as a clinically meaningful difference in an early symptomatic AD population (20%–30% slowing).21,22
What Do DMT Study Findings Mean for the Individual Patient?
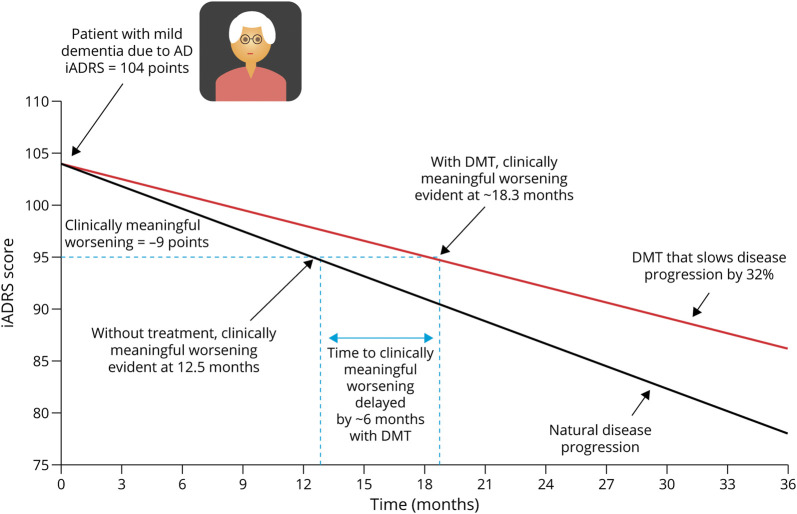
A statistically significant difference between the DMT and placebo group at the study endpoint provides a measure of drug efficacy. This is not necessarily synonymous with meaningful clinical change, which is of relevance to the individual patient. The minimal clinically meaningful change is the threshold at which the well-being of a patient and/or their care partner is notably affected (i.e., clinically meaningful worsening of symptoms). The change threshold on the iADRS associated with clinically meaningful worsening has been estimated to be 5 points for patients with MCI due to AD and 9 points for patients with AD with mild dementia.23
For example, clinically meaningful worsening will be evident at approximately 12.5 months for a typical untreated patient with mild dementia due to AD. With a DMT that slows disease progression by 32%, clinically meaningful worsening will be evident at approximately 18.3 months, the point at which clinically meaningful worsening of symptoms is evident has been delayed by 6 months with treatment (Figure 6).
Figure 6. Potential DMT Effect on Disease Progression in an Individual Patient, Using iADRS Findings From the TRAILBLAZER-ALZ Trial (32% Slowing of Disease Progression).
*Not advocating for using in clinical practice. Assumptions: (1) Change over 18 months with placebo is - 7 for MCI, -13 for mild dementia, and -20 for moderate dementia (calculated using placebo data EXPEDITION-1, -2, and -3; AMARANTH; and DAYBREAK-ALZ; for details see Gueorguieva et al, Alz & Dem 2022; DOI:10.1002/alz.12876. (2) Disease progression portrayed as linear for simplicity of presentation. (3) DMT effect is that seen in TRAILBLAZER-ALZ and is maintained over 36 months. DMT = disease-modifying therapy; iADRS = Integrated Alzheimer's Disease Rating Scale; MCI = mild cognitive impairment.
Health outcome measures (e.g., patient quality of life, caregiver burden, and costs) are recognized as being meaningful to patients, care partners, and society at large.24-26 Recently, it has been demonstrated that worsening on the iADRS is associated with meaningful change in these health outcome measures.20 As an example, a patient with mild dementia due to AD who experiences a decline on the iADRS of 10.1 points over 18 months would require approximately 164–236 hours of caregiver supervision time. If that same patient instead received treatment with a DMT that slows progression by 32%, the patient would require approximately 112–161 caregiver hours; this equates to savings of approximately 52–75 hours of caregiver time over 18 months with treatment. Because a DMT slows disease progression and changes the slope of the disease trajectory, one can expect that a follow-up after 18 months would result in an even greater savings in caregiver time.
Concluding Remarks
In this study, we have provided background and examples to assist the reader in the interpretation of iADRS findings from the TRAILBLAZER-ALZ trial (see eFigure 1, links.lww.com/CPJ/A397 for summary). This included discussion of clinically meaningful change in the iADRS (i.e., the magnitude of the change, i.e., consequential to patients and their partners).
The iADRS is a validated scale intended for use as an outcome measure in the assessment of meaningful treatment benefit in AD clinical studies. The iADRS was constructed using items that capture cognitive and functional domains relevant to in early AD. Arguably, human cognition is perhaps the most complex measure in all of medicine and has many influences that are beyond an actual disease process (e.g., learning effects, distractions, attention, mood, pain, acute illness, sleep, and medicines). Similarly, function can be altered by internal and environmental events unrelated to cognition. The iADRS total score reflects aligned changes in cognitive impairment and the ability to conduct everyday activities, filtering out noise unrelated to disease. The resultant measure of global AD severity captures the impact of cognitive impairment because of disease progression on the ability to conduct everyday activities.
The iADRS has a large score range (0–144), permitting the capture of disease progression over extended periods (years) from MCI through moderate dementia due to AD and the detection of treatment effect over short periods. The iADRS is not intended for use in a population with preclinical AD because individuals at this stage show only subtle cognitive deficits, detectable on sensitive measures of neuropsychological performance, and no evidence of functional impairment.
Disease modification using a DMT will change the disease trajectory, slowing decline. This is captured as less worsening (smaller point change) on the iADRS in the DMT group vs placebo. The absolute point change difference is dependent on the disease severity and rate of disease progression, while the percent slowing with treatment remains relatively constant and is thus most relevant for describing the magnitude of a DMT effect.
Using a hypothetical DMT that delays disease progression by 25% and assuming disease progresses in a linear manner (for simplicity), we illustrated that treatment delays meaningful worsening, as measured by a decline on the iADRS, by approximately 4.5 months after 18 months of treatment and approximately 9.0 months after 36 months of treatment. The effect of the DMT will increase further over time as the trajectories of untreated and treated groups diverge. Slowing the loss of cognitive and functional abilities will preserve patient quality of life, maintain independence, and minimize the impact of the disease on families and society.
In the TRAILBLAZER-ALZ trial, the difference between placebo and donanemab in the change in iADRS score at 18 months was statistically significant (p = 0.04); donanemab slowed disease progression by 32%. While a statistically significant difference between a DMT and placebo in the study endpoint provides a measure of drug efficacy, what is perhaps of greater importance to the patients and their families is whether this observed difference is clinically meaningful. In this study, we showed that in a patient with mild dementia due to AD, treatment with a DMT that slows disease progression by 32% would delay clinically meaningful worsening by approximately 6 months. We also calculated that this treatment could save approximately 52–75 hours of caregiver time. Given that dementia due to AD currently affects approximately 6 million individuals in the United States, one can appreciate the potential impact of a treatment that slows progression could have on society.
TAKE-HOME POINTS
→ The Integrated Alzheimer's Disease (AD) Rating Scale is an integrated measure of global AD severity. It provides a single score that captures commonalities across cognitive and functional ability domains, reflecting disease-related impairment, while minimizing noise not related to disease progression. It is capable of accurately describing clinical changes associated with disease progression and detecting treatment effects and is an effective assessment tool for use in clinical trials of individuals with early symptomatic AD.
→ In AD, disease-modifying therapies (DMTs) are expected to slow the rate of clinical decline, changing the trajectory of disease progression. The overall percent slowing of disease progression with treatment is a more informative outcome of effect than absolute point differences between treatment and placebo groups at any given time point because the latter is influenced by treatment period and disease severity. In the TRAILBLAZER-ALZ trial, donanemab slowed disease progression by 32% at 18 months (p = 0.04 vs placebo), demonstrating clinical efficacy.
→ At the patient level, one can assess whether the DMT effect is clinically meaningful by estimating the threshold of change consistent with clinically meaningful worsening; based on the TRAILBLAZER-ALZ findings, treatment with donanemab would delay reaching this threshold by approximately 6 months.
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
A.M. Wessels, E.B. Dennehy, and S.A. Dowsett are full-time employees and minor stockholders at Eli Lilly and Company; S.P. Dickson and S.B. Hendrix report no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Early Alzheimer's Disease: Developing Drugs for Treatment Guidance for Industry. 2018. Accessed June 1, 2022. fda.gov/media/110903/download. [Google Scholar]
- 2.Skinner J, Carvalho JO, Potter GG, et al. The Alzheimer's disease assessment scale-cognitive-plus (ADAS-Cog-Plus): an expansion of the ADAS-Cog to improve responsiveness in MCI. Brain Imaging Behav. 2012;6(4):489-501. doi: 10.1007/s11682-012-9166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghavan N, Samtani MN, Farnum M, et al. The ADAS-Cog revisited: novel composite scales based on ADAS-Cog to improve efficiency in MCI and early AD trials. Alzheimers Dement. 2013;9(suppl l):S21-S31. doi: 10.1016/j.jalz.2012.05.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y, Ito K, Billing CB Jr, Anziano RJ. Development of a straightforward and sensitive scale for MCI and early AD clinical trials. Alzheimers Dement. 2015;11(4):404-414. doi: 10.1016/j.jalz.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Logovinsky V, Hendrix SB, et al. ADCOMS: a composite clinical outcome for prodromal Alzheimer's disease trials. J Neurol Neurosurg Psychiatry. 2016;87(9):993-999. doi: 10.1136/jnnp-2015-312383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. New Engl J Med. 2014;370(4):311-321. doi: 10.1056/nejmoa1312889. [DOI] [PubMed] [Google Scholar]
- 7.Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197-210. doi: 10.14283/jpad.2022.30. [DOI] [PubMed] [Google Scholar]
- 8.Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384(18):1691-1704. doi: 10.1056/nejmoa2100708. [DOI] [PubMed] [Google Scholar]
- 9.Hartry A, Menne H, Wronski S, et al. Evaluation of What Matters Most in Existing Clinical Outcomes Assessments in Alzheimer's Disease; 2020. Accessed June 1, 2022. usagainstalzheimers.org/sites/default/files/2020-11/40100_Evaluation%20of%20What%20Matters%20Most_0.pdf. [Google Scholar]
- 10.Liu-Seifert H, Siemers E, Price K, et al. Cognitive impairment precedes and predicts functional impairment in mild Alzheimer's disease. J Alzheimers Dis. 2015;47(1):205-214. doi: 10.3233/jad-142508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Us Against Alzheimer's. Alzheimer's Disease Patient and Caregiver Engagement. Accessed June 1, 2022. usagainstalzheimers.org/networks/ad-pace. [Google Scholar]
- 12.DiBenedetti DB, Slota C, Wronski SL, et al. Assessing what matters most to patients with or at risk for Alzheimer's and care partners: a qualitative study evaluating symptoms, impacts, and outcomes. Alzheimers Res Ther. 2020;12(1):90. doi: 10.1186/s13195-020-00659-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141(11):1356-1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 14.Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. Alzheimer Dis Associated Disord. 1997;11(suppl 2):S13-S21. doi: 10.1097/00002093-199700112-00003. [DOI] [PubMed] [Google Scholar]
- 15.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer Dis Assoc Disord. 1997;11(11):S33-S39. doi: 10.1097/00002093-199700112-00005. [DOI] [PubMed] [Google Scholar]
- 16.Galasko D, Kershaw PR, Schneider L, Zhu Y, Tariot PN. Galantamine maintains ability to perform activities of daily living in patients with Alzheimer's disease. J Am Geriatr Soc. 2004;52(7):1070-1076. doi: 10.1111/j.1532-5415.2004.52303.x. [DOI] [PubMed] [Google Scholar]
- 17.Wessels AM, Siemers ER, Yu P, et al. A combined measure of cognition and function for clinical trials: the integrated Alzheimer's disease rating scale (iADRS). J Prevent Alzheimers Dis. 2015;2(4):227-241. doi: 10.14283/jpad.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu-Seifert H, Andersen S, Case M, et al. Statistical properties of continuous composite scales and implications for drug development. J Biopharm Stat. 2017;27(6):1104-1114. doi: 10.1080/10543406.2017.1315819. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health. US National Library of Medicine. Accessed June 1, 2022. clinicaltrials.gov. [Google Scholar]
- 20.Wessels AM, Belger M, Johnston JA, et al. Demonstration of clinical meaningfulness of the integrated Alzheimer's Disease Rating Scale (iADRS): association between change in iADRS scores and patient and caregiver health outcomes. J Alz Dis. 2022;88(2):577-588. doi: 10.3233/jad-220303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vellas B, Andrieu S, Sampaio C, Wilcock G. Disease-modifying trials in Alzheimer's disease: a European task force consensus. Lancet Neurol. 2007;6(1):56-62. doi: 10.1016/s1474-4422(06)70677-9. [DOI] [PubMed] [Google Scholar]
- 22.Insel PS, Weiner M, Mackin RS, et al. Determining clinically meaningful decline in preclinical Alzheimer disease. Neurology. 2019;93(4):e322-e333. doi: 10.1212/wnl.0000000000007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wessels AM, Rentz DM, Case M, Lauzon S, Sims JR. Integrated Alzheimer's disease rating scale: clinically meaningful change estimates. Alzheimers Dement. 2022;8(1):e12312. doi: 10.1002/trc2.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haro JM, Kahle-Wrobleski K, Bruno G, et al. Analysis of burden in caregivers of people with Alzheimer's disease using self-report and supervision hours. J Nutr Health Aging. 2014;18(7):677-684. doi: 10.1007/s12603-014-0500-x. [DOI] [PubMed] [Google Scholar]
- 25.Deb A, Thornton JD, Sambamoorthi U, Innes K. Direct and indirect cost of managing Alzheimer's disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res. 2017;17(2):189-202. doi: 10.1080/14737167.2017.1313118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tochel C, Smith M, Baldwin H, et al. What outcomes are important to patients with mild cognitive impairment or Alzheimer's disease, their caregivers, and health-care professionals? A systematic review. Alzheimers Demen Diagn Assess Dis Monit. 2019;11(1):231-247. doi: 10.1016/j.dadm.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]