Abstract
Acute symptomatic seizures in the term newborn are often seen after perinatal brain injury. Common etiologies include hypoxic-ischemic encephalopathy, ischemic stroke, intracranial hemorrhage, metabolic derangements, and intracranial infections. Neonatal seizures are often treated with phenobarbital, which may cause sedation and may have significant long-term effects on brain development. Recent literature has suggested that phenobarbital may be safely discontinued in some patients before discharge from the neonatal intensive care unit. Optimizing a strategy for selective early phenobarbital discontinuation would be of great value. In this study, we present a unified framework for phenobarbital discontinuation after resolution of acute symptomatic seizures in the setting of brain injury of the newborn.
Seizures are more common during the neonatal period than at any other age, occurring at a rate of 1.10–5.5 per 1,000 live births.1 These constitute a neurologic emergency and require prompt recognition because timely treatment (within 1 hour) is associated with reduced seizure burden and better seizure control.2 Acute symptomatic seizures of the newborn are by definition seizures caused by acute brain injury.3 Common etiologies include hypoxic-ischemic encephalopathy (HIE), seen in 38% of all patients presenting with neonatal seizures, ischemic stroke (18%), intracranial hemorrhage (11%) and other causes of brain injury such as intracranial infections and metabolic derangements (including hypoglycemia).4
Acute symptomatic neonatal seizures are associated with significant morbidity and mortality, including poor behavioral, cognitive, and language outcomes and an elevated risk for the development of epilepsy.5,6 Epilepsy occurs earlier and is more likely among patients with a known history of neonatal seizures, in comparison with patients with no such history.7 For patients with acute symptomatic neonatal seizures who develop postneonatal epilepsy, the latter may be associated with multiple seizure types, including focal-onset seizures and infantile spasms.3
Therapeutic hypothermia confers neuroprotection among patients with HIE with acute symptomatic neonatal seizures. Overall, targeted neuroprotective strategies beyond therapeutic hypothermia are lacking. Until now, antiseizure medications have been the mainstay of acute management of acute symptomatic neonatal seizures.
Phenobarbital
At present, phenobarbital remains first line among antiseizure medications for newborns and is superior to all other treatments, including levetiracetam.8-10 Unfortunately, phenobarbital is effective in stopping seizures in only approximately half of patients.11 It is more commonly used across neonatal intensive care units (NICUs) in the United States and Canada than fosphenytoin, with which it shares a similar efficacy profile, given its simpler pharmacokinetics. At the same time, providers are cautioned that phenobarbital may produce sedation and suppress respiratory function at higher levels (though in our experience, respiratory compromise is not typically observed at levels less than 40 mcg/mL).12
After resolution of acute symptomatic seizures, and in neonates with a low risk of subsequent seizures, it is important to discontinue phenobarbital as soon as clinically reasonable. Animal model data suggest an increased risk of apoptosis and long-term effects on synapse maturation after exposure to phenobarbital; these changes may in turn affect learning and memory.13-16 There are limited data to suggest that phenobarbital may also worsen previously existing neuronal injury, though findings are limited to animal studies.17 Meanwhile, chronic phenobarbital use for febrile seizure prophylaxis among children 8–36 months of age was associated with a decrease of 7 points of mean intelligence quotient at 2 years of age, compared with placebo18; this effect persisted 6 months after phenobarbital was stopped. In general, phenobarbital seems to be a safe option when used briefly for the treatment of acute neonatal seizures; side effects are more likely to be observed with long-term use.
When To Discontinue Phenobarbital?
Given the theoretical potential for adverse effects on long-term brain development, phenobarbital should be discontinued as soon as clinically appropriate, ideally before hospital discharge from the NICU. In practice, guidelines for discontinuation of phenobarbital, e.g., by NICU discharge vary considerably across institutions. This likely reflects that to date, there are very limited data available to guide this practice. This article aims to provide evidence-based guidance around planning of phenobarbital discontinuation in the NICU.
Original recommendations from the World Health Organization in 2011 suggested that antiseizure medications may be discontinued once a neonate with a history of acute symptomatic seizures is seizure-free for 72 hours, as long as the patient has a normal neurologic examination.19 Meanwhile, a recent prospective multicenter study following up patients with acute symptomatic seizures revealed that epilepsy is more likely among patients with a history of ≥3 days of seizures during the neonatal period.3 In addition, the degree of severity of the original brain injury contributing to seizures may also play a role in promoting long-term risk for epilepsy.20-23 While most patients with resolved acute symptomatic neonatal seizures will benefit from discontinuing all antiseizure medications during NICU discharge, there are some patients who would instead benefit from a stratified approach to discontinuing phenobarbital use, based on predictors of long-term epilepsy.
Patient-specific considerations must be taken into account when attempting to discontinue phenobarbital and other antiseizure medications among patients with differing patterns of brain injury. These are summarized in the section further.
Considerations for Specific Populations
HIE
HIE secondary to peripartum complications is the most common cause of diffuse neonatal brain injury and neonatal seizures. Anecdotally, most patients with mild-to-moderate HIE are able to safely discontinue phenobarbital use during the NICU admission because the risk of recurrent seizures or development of epilepsy is low. However, literature on the safety and tolerability of phenobarbital discontinuation in patients with mild-to-moderate HIE is lacking.
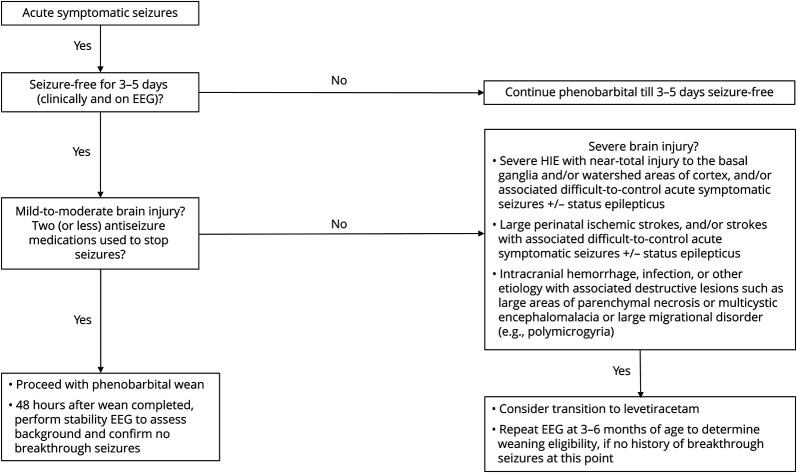
Meanwhile, epilepsy risk is increased among patients with severe HIE (Figure).21 Specifically, patients with HIE with more profound or near-total brain injuries are more likely to develop epilepsy; this includes patients with significant injury to the basal ganglia and watershed brain regions on brain MRI.20,22 Increased epilepsy risk in this population is likely a consequence of synaptic reorganization because of HIE-related neuronal injury.24 Additional imaging and neurophysiologic findings may also aid in epilepsy risk stratification, with patients with severely abnormal or inactive neonatal EEGs and bilaterally absent somatosensory evoked potentials being likelier to develop epilepsy in the long term.23 Specific guidelines on discontinuation of phenobarbital in this population is summarized in the Figure.
Figure. Framework for Phenobarbital Discontinuation After Acute Symptomatic Seizures.
While most patients with resolved acute symptomatic neonatal seizures will benefit from discontinuing all antiseizure medications during NICU discharge, there are some patients who would instead benefit from a stratified approach to discontinuing phenobarbital use, based on predictors of long-term epilepsy. Predictors of long-term epilepsy include but are not limited to severe perinatal brain injury, elevated seizure burden, and the presence of neonatal status epilepticus. NICU, neonatal intensive care unit.
Perinatal Ischemic Stroke
Perinatal ischemic strokes affect patients between ages 20 weeks gestation to 28 days of life and occur in 1/2500 live births. Thus, ischemic stroke is an even more frequent neonatal emergency than previously suspected. Subtypes of ischemic events among term newborns include neonatal arterial ischemic stroke (NAIS) and cerebral sinovenous thrombosis (CSVT). Both NAIS and CSVT are frequently associated with motor and cognitive deficits in the long term, and epilepsy may be observed in up to 40% of survivors of NAIS (Figure). Meanwhile, observational studies have shown that 25%–41% survivors of CSVT go on to develop epilepsy.25,26 Overall, up to 30% of survivors of any neonatal stroke present with breakthrough seizures in the first 6–12 months of life.27-30
There are several factors that may increase risk for epilepsy in the first year of life, including elevated neonatal seizure burden and associated status epilepticus during the neonatal period (Figure). The need for multiple antiseizure medications to control early acute symptomatic seizures may also predict future epilepsy likelihood, as does the presence of a positive family history. All should be taken into consideration before considering discontinuation of antiseizure medications at NICU discharge.28-32 Given the higher risk of postperinatal stroke epilepsy, most patients should likely be transitioned from phenobarbital to a long-term antiseizure medication such as levetiracetam (as shown in the Figure), with close monitoring postdischarge. Among patients with neonatal ischemic strokes, this practice may best benefit those patients with acute symptomatic seizures that were initially more numerous, harder to treat (i.e., requiring more than two antiseizure medications to resolve), and those patients with larger stroke sizes. Patients with punctate strokes and more limited neonatal seizures after ischemia may possibly benefit from earlier phenobarbital discontinuation, though data regarding such patients and long-term epilepsy outcomes are not readily available.
Intracranial Hemorrhage
Common types of intracranial hemorrhages encountered during the neonatal period include the following: intraparenchymal, subdural, and subpial hemorrhages. There seems to be a limited risk for developing epilepsy after perinatal subdural hemorrhages (particularly when related to birth injury) and punctate intraparenchymal hemorrhages, especially among patients with no known associated arterial malformations.33 Overall, it is believed that these patients can be discontinued off antiseizure medications after acute symptomatic seizure resolution, though prospective, randomized studies addressing this practice are lacking.
Among older children, elevated intracranial pressure in the setting of larger intraparenchymal hemorrhage requiring urgent neurosurgical intervention is a known risk factor for developing epilepsy; however, this link has not been confirmed among neonates with intracranial hemorrhage.33 Meanwhile, a recent cohort study following up patients with perinatal subpial hemorrhages revealed that at least 24% went on to develop epilepsy when monitored for over a year, particularly when there was emergence over time of significant associated encephalomalacia; we advise that these patients should be continued on long-term antiseizure therapy.34 Whether patients with neonatal subpial hemorrhages may benefit from continued antiseizure medication prophylaxis beyond the NICU is currently unknown.
Intracranial Infection
Intracranial infection is a far less common cause of acute symptomatic seizures during the neonatal period, accounting for only 4% of neonatal seizures.35 The more frequently involved infectious agents include the following: Streptococcus pneumoniae, herpes simplex virus (HSV), and cytomegalovirus (CMV), though many other infectious etiologies may also cause acute symptomatic seizures of the neonate and contribute to long-term epilepsy outcomes.36-40 Specific neuropathology secondary to infection is believed to contribute to epileptogenesis, including the presence of associated parenchymal necrosis (as seen in HSV-induced injury), multicystic encephalomalacia (as seen with various severe infectious meningitides), and migrational disruption (which can contribute to CMV-induced polymicrogyria, for example).38
Overall, cohort studies point toward an increased likelihood of epilepsy in the first few years of life, among those children with more severe associated brain injury; these patients would therefore benefit from continued antiseizure medication therapy (Figure). Prospective studies addressing the question of early antiseizure medication discontinuation in the NICU are still lacking when it comes to these specific populations; research has mostly been hindered by limited sample sizes in the past.
How Safe Is It to Discontinue Phenobarbital?
Discontinuation of phenobarbital among lower-risk populations in the NICU before hospital discharge is safe41 and has not been linked to an increased risk for seizures once the infant goes home.42 In a recent large comparative effectiveness study of 303 children with neonatal seizures from 9 centers, no difference in long-term epilepsy diagnosis or neurodevelopment was observed between patients maintained on antiseizure medications past NICU discharge and those who had antiseizure medications discontinued at NICU discharge; of note, the cohort largely consisted of individuals with seizures secondary to mild and moderate brain injury, and given that antiseizure treatment selection was at the discretion of the clinician, confounding by treatment indication is likely.41
In the case of phenobarbital, longer use has not been shown to decrease the occurrence of epilepsy long term.43 Of note, antiseizure therapies should not be stopped at NICU discharge among babies diagnosed with a neonatal epilepsy syndrome or with seizures secondary to genetic etiology because these tend to require antiseizure therapy long term.
Overall Framework for Discontinuation Recommendations
We here recommend the following targeted approach (summarized in the Figure) for phenobarbital discontinuation among patients with acute symptomatic neonatal seizures. A similar approach may also be used for patients on alternative antiseizure medications. For patients undergoing phenobarbital discontinuation around the time of NICU discharge, a time-limited EEG is recommended to assess background changes and to rule out the presence of breakthrough subclinical seizures. Stability EEGs may be collected 48 hours after phenobarbital discontinuation. For patients with seizure recurrence in the setting of phenobarbital discontinuation, transition to levetiracetam therapy may be of help.
Refractory seizures requiring multiple antiseizure medications, status epilepticus, high seizure burden, presence of brief rhythmic discharges on EEG, and significant brain lesions on MRI are risk factors against early discontinuation.3,4,20,44 Weaning within a couple of weeks of phenobarbital treatment initiation can be performed abruptly, given the short duration of phenobarbital exposure. Meanwhile, when encountering a patient who has undergone several months of phenobarbital treatment, it is advisable to wean this medication over a few weeks.
For patients with more severe brain injury during the perinatal period, and for those with difficult to control seizures early on, a safer approach may entail transitioning from phenobarbital to levetiracetam and, if possible, limiting a patient's antiseizure medication regimen to just 1 drug, followed by close outpatient monitoring. A follow-up EEG at 3–6 months of age may again reveal recurrent epileptiform activity; this finding would caution a practitioner against discontinuing all antiseizure medications, although a recent multicenter study showed that an abnormal EEG at 3 months was not necessarily associated with childhood epilepsy.3 Clinical judgment is warranted. For all patients on multiple antiseizure medications, care should be taken to schedule these patients for close pediatric neurology follow-up, so that additional medications can be discontinued as soon as possible.
Conclusions
Neonatal seizures are a common and treatable condition in NICUs. Given their potential deleterious effects on the developing brain, early recognition and appropriate treatment are necessary. But considering the potential side effects of antiseizure medications, discontinuation should be pursued as soon as it is reasonably safe. While further studies are still needed, current data show that early discontinuation of antiseizure medication is possible in many circumstances.
This article aims to address a gap in the literature, including the lack of established guidelines for discontinuing phenobarbital use among patients with neonatal seizures. The simple approach highlighted here (Figure) should apply to most patients with neonatal seizures, though patients with neonatal epileptic encephalopathies will be likelier to need long-term therapies.
New pharmacologic therapies may in time become more effective at controlling neonatal seizures. Additional treatments, including lacosamide and bumetanide, may in time become first line for treatment of neonatal seizures because new research on both medications continues to be pursued. New pharmacologic interventions would be of most value in this setting because current therapies are at best able to only decrease neonatal seizure burden by approximately 50%.
There are various promising clinical, laboratory, and neurophysiologic predictors that may help predict successful discontinuation off antiseizure medications during NICU discharge. For example, a small retrospective study showed that a normal EEG can be a helpful prognosticator for the effective discontinuation of antiseizure medications in some infants.45 Meanwhile, early changes in proinflammatory cytokine levels, including IL-6 and TNF-α, have been previously associated with long-term epilepsy, in a group of patients with neonatal seizures.46 The emergence of biomarkers for better prediction of successful antiseizure medication discontinuation remains a promising area of research.
TAKE-HOME POINTS
→ Acute symptomatic neonatal seizures are associated with significant morbidity and mortality; phenobarbital is considered the first-line treatment for neonatal seizures.
→ Phenobarbital use has the potential for significant side effects; therefore, it is important to discontinue its use as soon as clinically reasonable.
→ Guidelines for discontinuation of phenobarbital vary considerably across institutions.
→ Specific considerations must be taken into account, when attempting to discontinue phenobarbital and other antiseizure medications among patients with differing patterns of brain injury.
Acknowledgment
The authors thank the members of the Newborn Brain Society Guidelines and Publications Committee, including: Pia Wintermark (Pediatrics/Newborn Medicine, Montreal Children's Hospital, McGill University, Montreal, QC, Canada), Andrea Pardo (Pediatrics, Feinberg School of Medicine, Northwestern University, Chicago, IL), Courtney Wusthoff (Child Neurology, Stanford University, Stanford, CA), Eric Peeples (Pediatrics, University of Nebraska Medical Center, Omaha, NE), Hannah Glass (Pediatrics and Neurology, University of California, San Francisco, CA), Hany Aly (Neonatalogy, Cleveland Clinic Children's Hospital, Bratenahl, OH), and Monica Lemmon (Pediatrics and Population Health Sciences, Duke University School of Medicine, Durham, NC).
Appendix. Authors

Study Funding
V. Chau is supported by the SickKids Foundation.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Pisani F, Facini C, Bianchi E, Giussani G, Piccolo B, Beghi E. Incidence of neonatal seizures, perinatal risk factors for epilepsy and mortality after neonatal seizures in the province of Parma, Italy. Epilepsia. 2018;59(9):1764-1773. doi: 10.1111/epi.14537 [DOI] [PubMed] [Google Scholar]
- 2.Pavel AM, Rennie JM, de Vries LS, et al. Neonatal seizure management: is the timing of treatment critical? J Pediatr. 2022;243:61-68.e2. doi: 10.1016/j.jpeds.2021.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shellhaas RA, Wusthoff CJ, Numis AL, et al. Early-life epilepsy after acute symptomatic neonatal seizures: a prospective multicenter study. Epilepsia. 2021;62(8):1871-1882. doi: 10.1111/epi.16978 [DOI] [PubMed] [Google Scholar]
- 4.Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr. 2016;174:98-103.e1. doi: 10.1016/j.jpeds.2016.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69(19):1816-1822. doi: 10.1212/01.wnl.0000279335.85797.2c [DOI] [PubMed] [Google Scholar]
- 6.Glass HC, Grinspan ZM, Shellhaas RA. Outcomes after acute symptomatic seizures in neonates. Semin Fetal Neonatal Med. 2018;23(3):218-222. doi: 10.1016/j.siny.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 7.Andreolli A, Turco EC, Pedrazzi G, Beghi E, Pisani F. Incidence of epilepsy after neonatal seizures: a population-based study. Neuroepidemiology. 2019;52(3-4):144-151. doi: 10.1159/000494702 [DOI] [PubMed] [Google Scholar]
- 8.Sewell EK, Hamrick SEG, Patel RM, Bennett M, Tolia VN, Ahmad KA. Association between anti-seizure medication and outcomes in infants. J Perinatol. 2022;42(3):359-364. doi: 10.1038/s41372-021-01240-1 [DOI] [PubMed] [Google Scholar]
- 9.Sharpe C, Reiner GE, Davis SL, et al. Levetiracetam versus phenobarbital for neonatal seizures: a randomized controlled trial. Pediatrics. 2020;145(6):e20193182. doi: 10.1542/peds.2019-3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slaughter LA, Patel AD, Slaughter JL. Pharmacological treatment of neonatal seizures: a systematic review. J Child Neurol. 2013;28(3):351-364. doi: 10.1177/0883073812470734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sankar R, Painter MJ. Neonatal seizures: after all these years we still love what doesn't work. Neurology. 2005;64(5):776-777. doi: 10.1212/01.wnl.0000157320.78071.6d [DOI] [PubMed] [Google Scholar]
- 12.Gilman JT, Gal P, Duchowny MS, Weaver RL, Ransom JL. Rapid sequential phenobarbital treatment of neonatal seizures. Pediatrics. 1989;83(5):674-678. doi: 10.1542/peds.83.5.674 [DOI] [PubMed] [Google Scholar]
- 13.Noguchi KK, Fuhler NA, Wang SH, et al. Brain pathology caused in the neonatal macaque by short and prolonged exposures to anticonvulsant drugs. Neurobiol Dis. 2021;149:105245. doi: 10.1016/j.nbd.2020.105245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Muhtasib N, Sepulveda-Rodriguez A, Vicini S, Forcelli PA. Neonatal phenobarbital exposure disrupts GABAergic synaptic maturation in rat CA1 neurons. Epilepsia. 2018;59(2):333-344. doi: 10.1111/epi.13990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaushal S, Tamer Z, Opoku F, Forcelli PA. Anticonvulsant drug-induced cell death in the developing white matter of the rodent brain. Epilepsia. 2016;57(5):727-734. doi: 10.1111/epi.13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forcelli PA, Janssen MJ, Vicini S, Gale K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann Neurol. 2012;72(3):363-372. doi: 10.1002/ana.23600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torolira D, Suchomelova L, Wasterlain CG, Niquet J. Phenobarbital and midazolam increase neonatal seizure-associated neuronal injury. Ann Neurol. 2017;82(1):115-120. doi: 10.1002/ana.24967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures--effects on intelligence and on seizure recurrence. N Engl J Med. 1990;322(6):364-369. doi: 10.1056/nejm199002083220604 [DOI] [PubMed] [Google Scholar]
- 19.Guidelines on neonatal seizures. apps.who.int/iris/bitstream/handle/10665/77756/9789241548304_eng.pdf;jsessionid=F003F8404F212EE834A319EB499F78B3?sequence=1
- 20.Glass HC, Hong KJ, Rogers EE, et al. Risk factors for epilepsy in children with neonatal encephalopathy. Pediatr Res. 2011;70(5):535-540. doi: 10.1203/pdr.0b013e31822f24c7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisani F, Orsini M, Braibanti S, Copioli C, Sisti L, Turco EC. Development of epilepsy in newborns with moderate hypoxic-ischemic encephalopathy and neonatal seizures. Brain Dev. 2009;31(1):64-68. doi: 10.1016/j.braindev.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 22.Xu Q, Chau V, Sanguansermsri C, et al. Pattern of brain injury predicts long-term epilepsy following neonatal encephalopathy. J Child Neurol. 2019;34(4):199-209. doi: 10.1177/0883073818822361 [DOI] [PubMed] [Google Scholar]
- 23.Nevalainen P, Metsaranta M, Toiviainen-Salo S, et al. Neonatal neuroimaging and neurophysiology predict infantile onset epilepsy after perinatal hypoxic ischemic encephalopathy. Seizure. 2020;80:249-256. doi: 10.1016/j.seizure.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 24.Pisani F, Spagnoli C, Falsaperla R, Nagarajan L, Ramantani G. Seizures in the neonate: a review of etiologies and outcomes. Seizure. 2021;85:48-56. doi: 10.1016/j.seizure.2020.12.023 [DOI] [PubMed] [Google Scholar]
- 25.Mineyko A, Kirton A, Billinghurst L, et al. Seizures and outcome one year after neonatal and childhood cerebral sinovenous thrombosis. Pediatr Neurol. 2020;105:21-26. doi: 10.1016/j.pediatrneurol.2019.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald KC, Williams LS, Garg BP, Carvalho KS, Golomb MR. Cerebral sinovenous thrombosis in the neonate. Arch Neurol. 2006;63(3):405-409. doi: 10.1001/archneur.63.3.405 [DOI] [PubMed] [Google Scholar]
- 27.Ferriero DM, Fullerton HJ, Bernard TJ, et al. Management of stroke in neonates and children: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2019;50(3):e51–e96. doi: 10.1161/str.0000000000000183 [DOI] [PubMed] [Google Scholar]
- 28.Billinghurst LL, Beslow LA, Abend NS, et al. Incidence and predictors of epilepsy after pediatric arterial ischemic stroke. Neurology. 2017;88(7):630-637. doi: 10.1212/wnl.0000000000003603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beslow LA, Helbig I, Fox CK. Long-term risk of epilepsy after pediatric stroke and potential genetic vulnerabilities. Stroke. 2021;52(11):3541-3542. doi: 10.1161/strokeaha.121.036376 [DOI] [PubMed] [Google Scholar]
- 30.Sundelin HEK, Tomson T, Zelano J, Soderling J, Bang P, Ludvigsson JF. Pediatric ischemic stroke and epilepsy: a nationwide cohort study. Stroke. 2021;52(11):3532-3540. doi: 10.1161/strokeaha.121.034796 [DOI] [PubMed] [Google Scholar]
- 31.Fox CK, Jordan LC, Beslow LA, Armstrong J, Mackay MT, deVeber G. Children with post-stroke epilepsy have poorer outcomes one year after stroke. Int J Stroke. 2018;13(8):820-823. doi: 10.1177/1747493018784434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suppiej A, Mastrangelo M, Mastella L, et al. Pediatric epilepsy following neonatal seizures symptomatic of stroke. Brain Dev. 2016;38(1):27-31. doi: 10.1016/j.braindev.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 33.Beslow LA, Abend NS, Gindville MC, et al. Pediatric intracerebral hemorrhage: acute symptomatic seizures and epilepsy. JAMA Neurol. 2013;70(4):448-454. doi: 10.1001/jamaneurol.2013.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dabrowski AK, Carrasco M, Gatti JR, et al. Neonatal subpial hemorrhage: clinical factors, neuroimaging, and outcomes in a Quaternary Care Children's Center. Pediatr Neurol. 2021;120:52-58. doi: 10.1016/j.pediatrneurol.2021.04.011 [DOI] [PubMed] [Google Scholar]
- 35.Soul JS. Acute symptomatic seizures in term neonates: etiologies and treatments. Semin Fetal Neonatal Med. 2018;23(3):183-190. doi: 10.1016/j.siny.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fister P, Pecek J, Jeverica S, Primec ZR, Paro-Panjan D. Neonatal group B streptococcal meningitis: predictors for poor neurologic outcome at 18 months. J Child Neurol. 2022;37(1):64-72. doi: 10.1177/08830738211053128 [DOI] [PubMed] [Google Scholar]
- 37.Murthy JMK, Prabhakar S. Bacterial meningitis and epilepsy. Epilepsia. 2008;49(suppl 6):8-12. doi: 10.1111/j.1528-1167.2008.01750.x [DOI] [PubMed] [Google Scholar]
- 38.Malm G, Forsgren M, el Azazi M, Persson A. A follow-up study of children with neonatal herpes simplex virus infections with particular regard to late nervous disturbances. Acta Paediatr. 1991;80(2):226-234. doi: 10.1111/j.1651-2227.1991.tb11838.x [DOI] [PubMed] [Google Scholar]
- 39.Suzuki Y, Toribe Y, Mogami Y, Yanagihara K, Nishikawa M. Epilepsy in patients with congenital cytomegalovirus infection. Brain Develop. 2008;30(6):420-424. doi: 10.1016/j.braindev.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 40.Lin CH, Chou IC, Lee IC, Hong SY. Cytomegalovirus infection in infancy may increase the risk of subsequent epilepsy and autism spectrum disorder in childhood. Children (Basel). 2021;8(11):1040. doi: 10.3390/children8111040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glass HC, Soul JS, Chang T, et al. Safety of early discontinuation of antiseizure medication after acute symptomatic neonatal seizures. JAMA Neurol. 2021;78(7):817-825. doi: 10.1001/jamaneurol.2021.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fitzgerald MP, Kessler SK, Abend NS. Early discontinuation of antiseizure medications in neonates with hypoxic-ischemic encephalopathy. Epilepsia. 2017;58(6):1047-1053. doi: 10.1111/epi.13745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Natarajan N, Beatty CW, Gust J, Hamiwka L. Provider practices of phenobarbital discontinuation in neonatal seizures. J Child Neurol. 2018;33(2):153-157. doi: 10.1177/0883073817745990 [DOI] [PubMed] [Google Scholar]
- 44.Nagarajan L, Palumbo L, Ghosh S. Brief electroencephalography rhythmic discharges (BERDs) in the neonate with seizures: their significance and prognostic implications. J Child Neurol. 2011;26(12):1529-1533. doi: 10.1177/0883073811409750 [DOI] [PubMed] [Google Scholar]
- 45.Brod SA, Ment LR, Ehrenkranz RA, Bridgers S. Predictors of success for drug discontinuation following neonatal seizures. Pediatr Neurol. 1988;4(1):13-17. doi: 10.1016/0887-8994(88)90018-5 [DOI] [PubMed] [Google Scholar]
- 46.Numis AL, Foster-Barber A, Deng X, et al. Early changes in pro-inflammatory cytokine levels in neonates with encephalopathy are associated with remote epilepsy. Pediatr Res. 2019;86(5):616-621. doi: 10.1038/s41390-019-0473-x [DOI] [PMC free article] [PubMed] [Google Scholar]