Abstract
While physiological levels of IL-7 are essential for T cell proliferation, survival and co-stimulation, its escalated concentration has been associated with autoimmune diseases such as Rheumatoid arthritis (RA). Expression of IL-7 and IL-7R in RA monocytes is linked to disease activity score and TNF transcription. TNF stimulation can modulate IL-7 secretion and IL-7R frequency in myeloid cells, however, only IL-7R transcription levels are downregulated in anti-TNF responsive patients. Elevated levels of IL-7 in RA synovial tissue and fluid are involved in attracting RA monocytes into the inflammatory joints and remodeling them into proinflammatory macrophages and mature osteoclasts. Further, IL-7 amplification of RA Th1 cell differentiation and IFNγ secretion, can directly prime myeloid IL-7R expression and thereby exacerbate IL-7-mediated joint inflammatory and erosive imprints. In parallel, IL-7 accentuates joint angiogenesis by expanding the production of proangiogenic factors from RA macrophages and endothelial cells. In preclinical models, blockade of IL-7 or IL-7R can effectively impair joint inflammation, osteoclast formation, and neovascularization primarily by impeding monocyte and endothelial cell infiltration as well as inhibition of pro-inflammatory macrophage and Th1/Th17 cell differentiation. In conclusion, disruption of IL-7/IL-7R signaling can uniquely intercept the crosstalk between RA myeloid and lymphoid cells in their ability to trigger neovascularization.
Keywords: IL-7, IL-7R, RA, macrophages, T cells and autoimmunity
1. INTRODUCTION
IL-7 also known as lymphopoietin belongs to the IL-2/IL-15 cytokine family [1]. IL-7 is secreted from bone marrow and thymus stromal cells, liver and gut epithelial cells, endothelial cells, fibroblasts smooth muscles, keratinocytes, and activated dendritic cells [2–5]. IL-7 binds to IL-7R which consists of a high-affinity α-subunit (CD127) and the common γ-chain. IL-7R is physiologically expressed on CD4+ and CD8+ T cells and myeloid cells, but not on human B cells [6]. Murine bone marrow lymphoid progenitor cells express IL-7R, and mice deficient in IL-7R display disruption in B and T cell development leading to SCID syndrome [7].
It is widely known that lymphopenia predisposes humans to autoimmune disease, and murine models substantiate a link between lymphocyte deregulation and colitis, gastritis, and diabetes [8, 9]. Notably, IL-7 has emerged as a cofactor for autoimmune development as its levels are escalated in lymphopenic hosts that are predisposed to autoimmunity [8]. In RA patients, IL-7 levels are enriched in circulating monocytes and synovial fluid macrophages leading to accelerated monokine release [10, 11]. Extending these observations, a polymorphism in IL-7R has been linked to an increased risk for multiple sclerosis, ulcerative colitis, and sarcoidosis [12–14]. More recently, a unique subset of myeloid CD127high has been distinguished in COVID-19 infected lungs that have STAT5-coordinated transcriptional programming [15]. Nonetheless, expanded levels of IL-7R on monocytes/macrophages are a hallmark of human inflammatory condition that is detected in COVID-19 patients as well as RA synovial myeloid cells [15]. Notably, the inflammatory response mediated by TLR2/4/7 signaling potentiated cell surface IL-7R expression in myeloid cells [10, 11].
In RA monocytes, IL-7 and IL-7R expression is closely linked to TNF and disease activity score (DAS28) and IL-7R was characterized as a marker for TNF response [11]. In line with these findings, RA patients responsive to anti-TNF therapy displayed markedly lower levels of circulating IL-7 [16]. In contrast, others have shown that IL-7R overexpression in colon tissues of severe Crohn’s patients is indicative of non-anti-TNF responsiveness [17].
Hence in this review, we will elucidate the mechanism of IL-7/IL-7R action in immune cells and further define the significance of their function in RA (Fig. 1) and other autoimmune diseases.
Figure 1. IL-7/IL-7R mechanism of action in immune cells and autoimmune diseases.
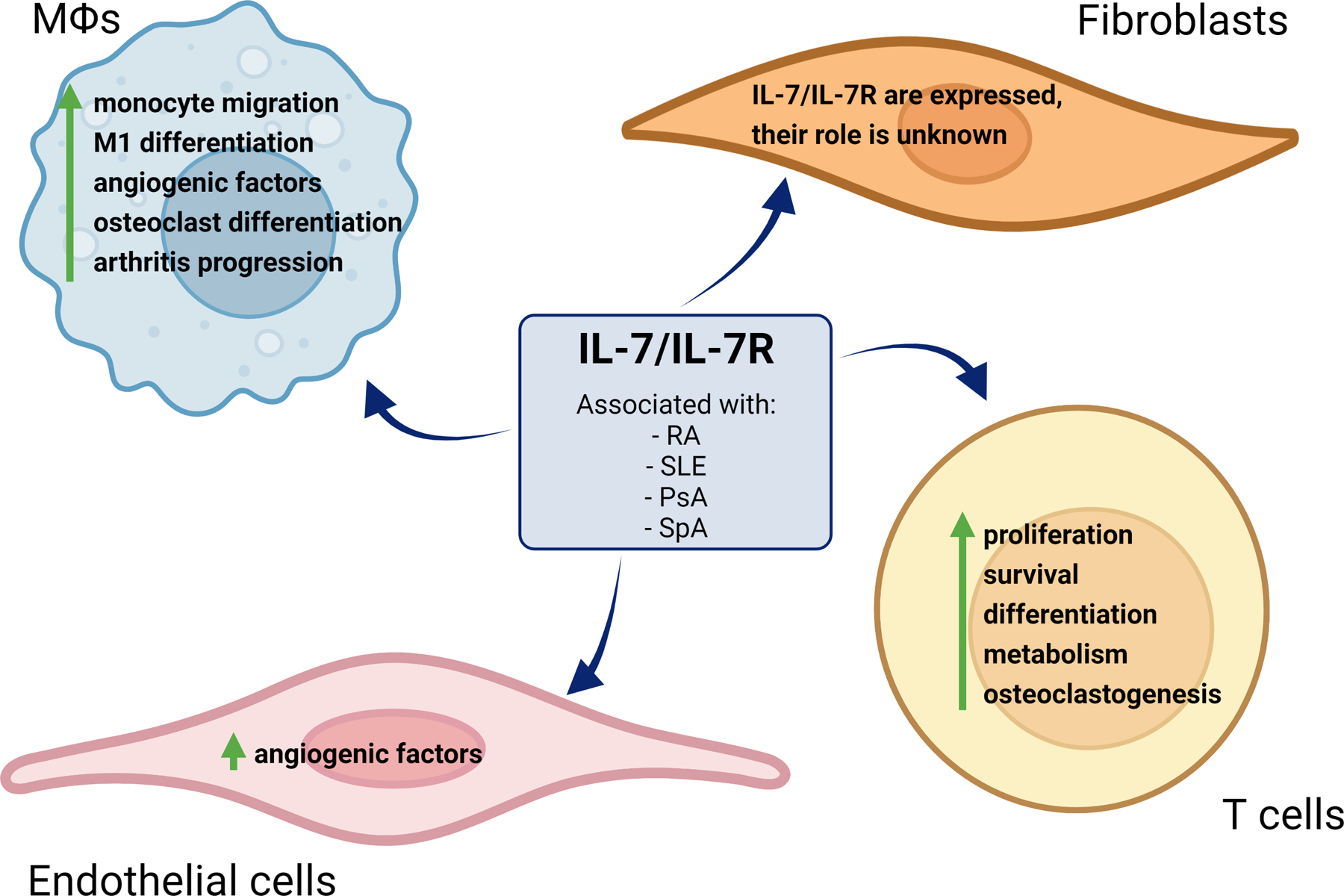
IL-7/IL-7R is associated with several autoimmune diseases like Rheumatoid Arthritis (RA), Systemic Lupus Erythematosus (SLE), Psoriatic Arthritis (PsA), and Spondyloarthritis (SpA). Furthermore, it plays a pivotal role in different cell types such as monocytes/macrophages (Mϴs), RA fibroblasts (FLS), endothelial cells, and T cells. The IL-7/IL-7R pathway is involved in monocyte migration, pro-inflammatory Mϴ, osteoclast reprogramming, and the elevation of pro-angiogenic factors, that result in arthritis progression. Moreover, IL-7/IL-7R physiologically promotes T cell proliferation and prolonged survival as well as pathologically influencing Th1/Th17 cell differentiation, potentiated glycolysis, and expansion of osteoclast maturation. In endothelial cells, angiogenic factors are produced upon IL-7/IL-7R signaling. To date, the impact of IL-7/IL-7R pathology is undescribed in RA FLS.
2. Impact of IL-7 on T cell function
IL-7 is involved in various stages of T cell proliferation as well as positive and negative selection [18]. IL-7 synergizes with other growth factors to expand T-cell precursors and supports early thymocyte survival at the triple-negative stage through BCL2 family members [19, 20]. Moreover, T cell co-stimulation by IL-7 is facilitated via proliferation and cytokine secretion [21, 22]. IL-7-mediated T cell co-stimulation is partially dependent on IL-2 and modulation of IL-2Rα [23, 24]. Furthermore, the expansion of CXCR4 on CD4+ T cells by IL-7 enhances their migration in response to CXCL12/SDF1 enriched lymphoid tissues [25]. Interestingly, IL-7 also promotes memory T-cell differentiation through co-stimulation and cell death inhibition [26].
It was shown that while the proliferative effect of IL-7 was comparable in healthy and RA peripheral blood mononuclear cells, this function was exacerbated in RA cells activated by IL-7 and phytohaemagglutinin (PHA) [27]. Interestingly, IL-7 co-stimulation of PHA-mediated proliferation was greater in RA peripheral blood mononuclear cells (PBMCs) compared to RA synovial fluid mononuclear cells (SFMCs) [27]. In contrast, IL-7-activated RA SFMCs show a stronger proliferation compared to matched PBMCs [28, 29]. In RA, T-cell and macrophage crosstalk are responsible for IL-7-potentiated cell proliferation, which is fostered by lymphoid MHCII and CD25 upregulation as well as myeloid CD80 and CD40 cell marker induction [28].
IL-7 plays a critical role in Th1 differentiation and IL-2 secretion, in part by increasing IL-12R on mature T cells [23, 30]. In RA, Th1 polarization promotes IL-2 secretion, and these differentiated cells are double-positive for IL-2 and IFNγ [23]. Interestingly, the Th1-associated transcription factor, T-bet was lower in early RA patients compared to normal individuals, and T-bet levels were positively linked to IFNγ and negatively to CRP concentration [31]. On the contrary, circulating IL-7 is closely connected to T-bet and IFNγ expression, while being disassociated from BCL2 and BAX concentration in RA patients [32]. Extending these results, IL-7 potentiated TNF secretion from RA SFMCs and expanded IFNγ production from RA SF CD4+ T cells in part through an IL-12 induction [28, 31]. In contrast, normal and RA PB T cells were nonresponsive to the physiological concentration of IL-7, indicating that IL-7R is primarily expressed on joint cells. It was also demonstrated that PHA and IL-7 co-stimulation shifts the CD4+CD25+FOXP3+ Treg balance to effector CD4+ T cells [32, 33].
Interestingly, although some studies have shown that IL-23/IL-23R signaling influences IL-7R expression in Th17 cell differentiation and expansion [34], others revealed that the sustained IL-7R in IL-23R−/− mice did not contribute to Th17 cell polarization in experimental autoimmune encephalomyelitis (EAE) [35]. Although IL-7R frequency was amplified on Th17 cells in EAE mice, IL-7 switched polarization of Th17 to Th1 cells that secreted IFNγ and GM-CSF [35]. In line with these observations, CXCR3-expressing Th1 cells were enriched by IL-7 exposure, while Th17 cells were unaffected in EAE development in wildtype or IL-23R−/− mice [35]. Moreover, IL-7 activates STAT5 signaling that is involved in re-establishing IL-7R on Th1 cells for survival and memory cell generation [36, 37]. Meanwhile, STAT5 activation downstream of IL-2R signaling suppresses Th17 polarization. More recent studies reveal that IL-7-activated STAT5 cultivates GM-CSF-producing CD4+ T cells which are diverse from Th1 or Th17 cells and display a distinct transcriptional landscape [38]. Nonetheless, in patients with Sjogren’s syndrome, IL-7 instigates Th1 and Th17 cell polarization despite IL-7R reduction in CD4+ T cells [39, 40].
IL-7-mediated T cell survival is dependent on anti-apoptotic BCL2 and MCL1 [41]. However, because IL-7 enhances cell survival in BCL2−/− cells, alternative strategies may be responsible for its mechanism of action [42]. Intriguingly, it has come to light that IL-7 maintains T cell glucose uptake via GLUT1 induction [43]. Regulation of glucose metabolism plays an important role in T homeostasis, survival, and effector cell polarization. Consistently, the removal of IL-7R in mature T cells resulted in a significant reduction in cell size, rate of survival, growth, and glycolytic flux [43, 44]. Since both PI3K/AKT and STAT5 signaling are involved in glucose uptake it is postulated that both pathways are implicated in IL-7-mediated glycolysis [45–47]. GLUT1−/− T cells and glucose deprivation dysregulate Th1 and Th17 cell differentiation, while Tregs utilize alternative metabolic strategies [48]. In agreement with the importance of PI3K/AKT/mTORC1 signaling in IL-7- activated T cell differentiation, deletion of mTOR disrupts Th1 and Th17 polarization without affecting Tregs [49, 50]. Overall, previous studies reveal that IL-7 and IL-7R play an important role in T cell development and survival in normal individuals as well as being involved in T effector cell differentiation and immunometabolism in autoimmune patients.
3. Role of IL-7 in myeloid cells
IL-7 and IL-7R are co-expressed on RA synovial tissue lining and sub-lining macrophages [10]. IL-7R transcription is highly elevated in RA synovial fluid macrophages, while RA monocyte-differentiated macrophages and monocytes express elevated levels of this receptor compared to normal counterparts [10]. IL-7 and IL-7R were similarly enriched by TLR4 ligation as well as TNF and IL-1β stimulation in RA monocyte-differentiated macrophages [10]. Additionally, priming of normal human CD14+ myeloid cells or bone marrow differentiated macrophages (BMMϴs) by LPS or IFNγ expands IL-7R cell surface expression and their inflammatory response to IL-7 by accentuating TNF and IL-6 production [51]. Authenticating these findings, IL-7R deficiency in bone marrow differentiated macrophages blunted LPS or IFNγ-induced priming and their impact on IL-7-enhanced inflammatory profile [51]. Intriguingly, local expression of IL-7 promotes arthritis via expansion of F480+iNOS+ macrophages which could be maintained by repeated injection [51]. Meanwhile, intra-articular LPS injection stabilizes IL-7-mediated joint inflammation in part through IL-7R induction and IFNγ expression [51]. IL-7-induced arthritis was responsive to anti-TNF therapy and joint F480+iNOS+IL-7R+CCL5+ macrophage reprogramming was dysregulated by this therapeutic strategy [51]. Likewise, in the phase 4 clinical study (NCT02451748), responsiveness to anti-TNF therapy was primarily due to the reduction of IL-7R transcription without affecting IL-7 expression in RA PBMCs [51].
IL-7 was markedly higher in RA compared to OA and normal synovial tissues or synovial fluids [10, 11]. Early in the disease, IL-7 enrichment in RA synovial fluid attracted circulating IL-7R+ monocytes as blockade of IL-7 in synovial fluid or IL-7R on monocytes restrains their trafficking [11]. It was established that IL-7-activated PI3K and ERK signaling was responsible for the infiltration of monocytes into the joints [11].
IL-7 reprograms naïve RA macrophages into classical M1 cells that express elevated levels of TNF, IL-6, CCL2, CCL5, and iNOS [51]. The proinflammatory profile of IL-7 in RA macrophages can be reversed by IL-7R knockdown [51]. Murine BMMϴs exposed to IL-7 are also remodeled into pro-inflammatory cells that exhibit higher expression of monokines, NOS2, and CD80, in contrast, IL-7R−/− cells are unresponsive to IL-7 stimulation [51]. Corroborating these findings, IL-7 can trigger T cell-dependent co-stimulatory factors on monocytes/macrophages via cell-to-cell contact and trigger the expression of IL-1α, IL-1β, IL-6, IL-8, and CCL4 [52]. These observations suggest that T cell and macrophage cross-regulation play a critical for IL-7-induced Th1 cell differentiation and IFNγ secretion, which will, in turn, expand the frequency of myeloid IL-7R or co-signaling molecules [51, 52].
Results from our lab and others have illustrated that IL-7 is involved in RA inflammation in addition to osteoclastic bone erosion [5, 51, 53, 54]. IL-7 inhibits basal and BMP2-induced bone formation in calvarial organ culture as well as in vivo in mice [55]. Earlier investigations have demonstrated that RA synovial fluid CD14+IL-7R+ macrophages can mature into osteoclasts and do not require T cells for this process [56]. We found that IL-7 can dose-dependently differentiate RA PBMCs (T cells and monocytes) through induction of RANK, RANKL, and TNF [51]. However, in the absence of T cells, RA monocytes cultured in suboptimal conditions stimulated with IL-7 are transformed into osteoclasts via TNF, IL-6, and RANK transcriptional upregulation [51]. Nonetheless priming of RA monocytes with IFNγ, advanced IL-7-induced TNF and IL-6 expression as well as the number of TRAP+ osteoclasts compared to non-primed cells [51]. Interestingly, while blockade of TNF played a pivotal role in suppressing IL-7-mediated osteoclastogenesis, IL-6R Ab therapy was ineffective in this phenomenon [51]. Consistently, earlier studies have shown that IL-7-driven TNF induction has an important impact on its osteoclast formation [57, 58]. Other groups document that RA circulating monocytes or synovial fluid macrophages stimulated with IL-7 mature into osteoclasts via STAT5 activation which is independent of RANKL [54].
Collectively, the reported findings indicate that IL-7 is involved in different facets of RA myeloid cell-mediated pathology including monocyte migration, inflammatory macrophage differentiation, and remodeling of RA monocyte/macrophages into mature osteoclasts.
4. Myeloid and T cell signaling
In RA monocytes, IL-7 activates ERK, AKT1, STAT3, and STAT5 phosphorylation, while STAT1 was unaffected [11] (Fig. 2). We found that inhibition or knockdown of PI3K/AKT or ERK pathway intercepts IL-7-mediated monocyte homing, conversely, STAT3 and STAT5 were uninvolved [11] (Fig. 2). It was also illustrated that while other monocyte chemoattractants utilize the p38 signaling pathway to recruit monocyte, IL-7 can uniquely advance monocyte infiltration through PI3K/AKT signaling [11, 59–61]). Nevertheless, IL-7-instigated reprogramming of RA naïve cells into pro-inflammatory phenotype is fostered through STAT5 activation [15]. Moreover, RA osteoclast maturation by IL-7 was also triggered by STAT5 signaling [62] (Fig. 2).
Figure 2. IL-7/IL7-R signaling in RA monocytes/Mϴs.
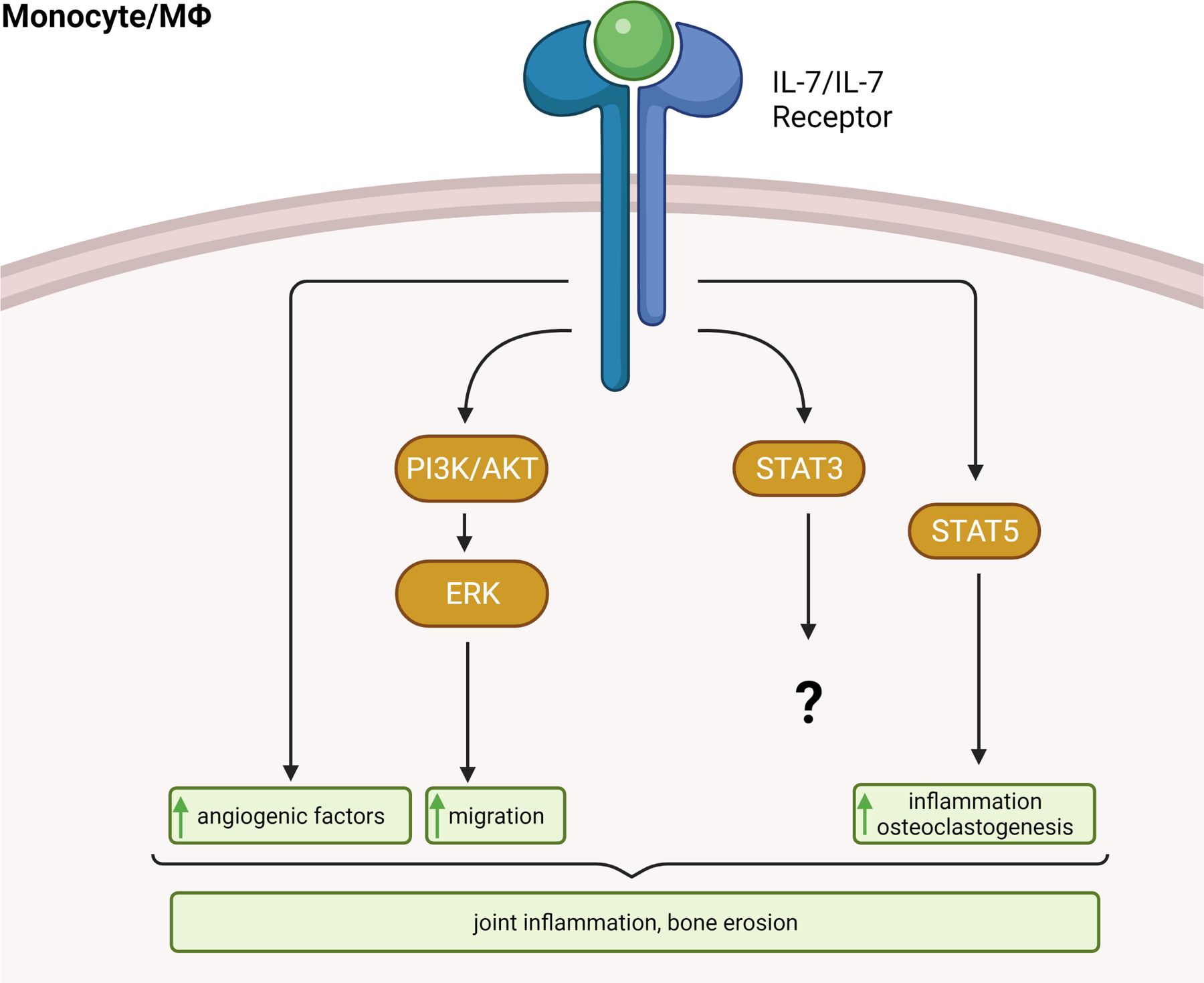
In RA monocytes, IL-7 activates PI3K/AKT, ERK, STAT3, and STAT5 phosphorylation. While PI3K/AKT and ERK signaling amplify monocytes infiltration, STAT5 phosphorylation facilitates RA inflammation and osteoclast formation. Further, activation of IL-7/IL7R results in the production of angiogenic factors. Taken together, signaling through the IL-7/IL-7R cascade results in exacerbated joint inflammation and bone erosion. The illustration was generated from data reported in [11, 15, 62].
IL-7 ligation to IL-7R activates JAK1 and JAK3/STAT5 signaling pathways, resulting in BCL2 induction and T cell proliferation [63] (Fig. 3). Moreover, the IL-7/NFATc1 pathway was identified as an alternative signaling pathway that has a cross-regulation with IL-7/STAT5 and orchestrates the thymocyte development [64, 65]. The IL-7Rγc receptor is recruited following IL-7 binding to IL-7Rα to develop a high-affinity complex via JAK1/JAK3/STAT5 and PI3K-AKT signaling pathways that are shared with IL-2 [66]. Notably, the PI3K/AKT pathway is an essential signaling pathway that manipulates T cell proliferation, survival, and glucose metabolism [67]. Consistently, PI3K inhibitors disrupt murine T cell proliferation and activation in response to IL-7 [68] (Fig. 3).
Figure 3. IL-7 signaling in T cells.
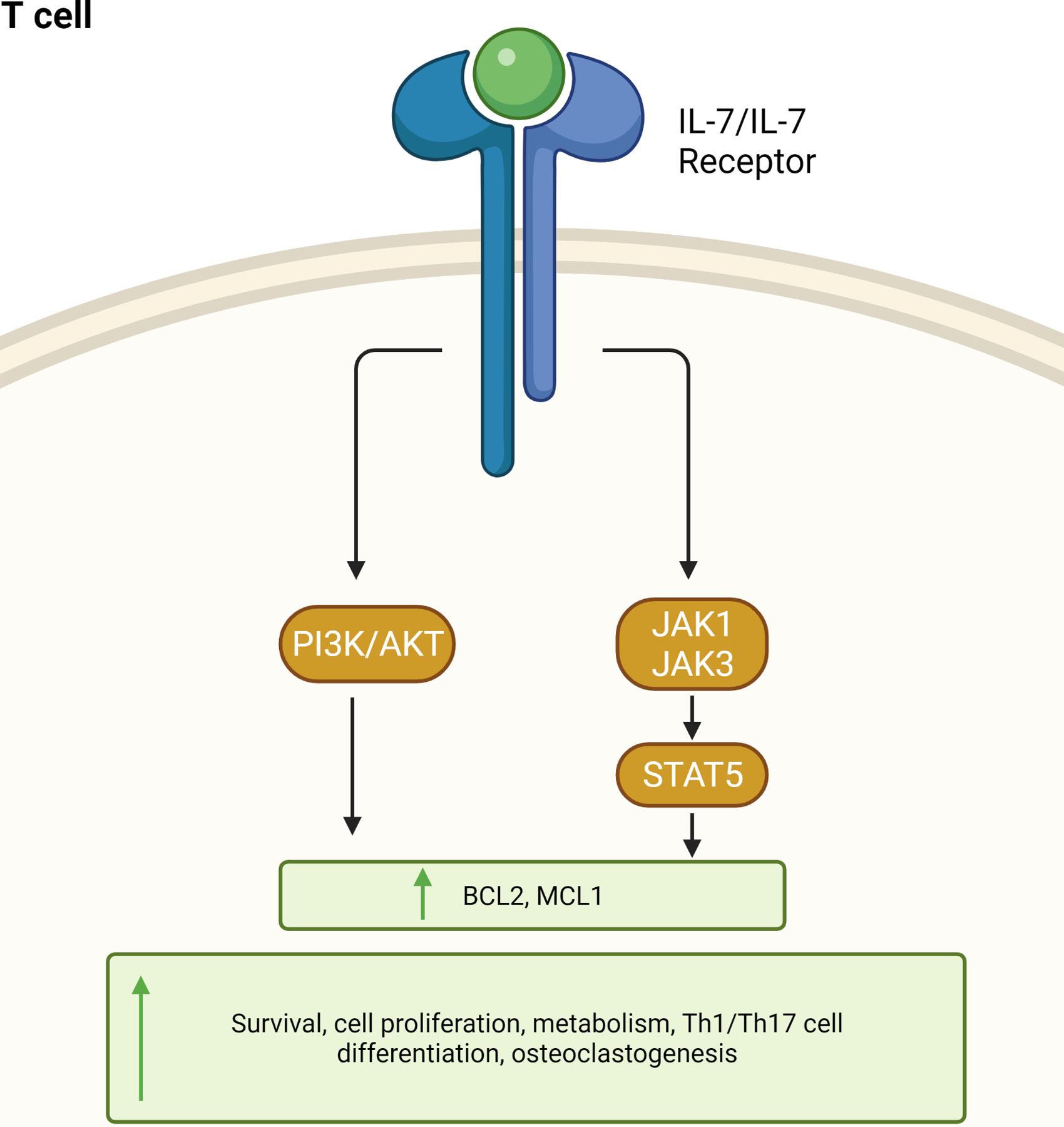
Upon binding of IL-7 to the IL-7R on T cells, multiple intracellular phosphorylation cascades, such as JAK-STAT and PI3 Kinase are activated, resulting in activation or inhibition of specific target genes. In detail, IL-7 triggers JAK1, JAK3, and PI3K signaling which contributes to STAT5 or AKT phosphorylation and increased expression of the anti-apoptotic genes BCL-2 (B cell lymphoma 2) and MCL1 (myeloid cell leukemia sequence). While physiological levels of IL-7 promote T cell survival and proliferation, its pathogenic effect fosters T cell hypermetabolism, Th1 and Th17 cell polarization in addition to osteoclast formation. This schema is adapted from [63].
Overall, these findings suggest that PI3K/AKT and STAT5 pathways regulate IL-7-mediated pathogenicity in myeloid and lymphoid cells while ERK signaling is uniquely activated in RA myeloid cells.
5. Effect IL-7 on fibroblast-like synoviocytes (FLS) or endothelial cell-mediated arthritogenicity
Expression of IL-7 and IL-7R is elevated in RA compared to normal FLS and IL-7R levels are potentiated by LPS and TNF stimulation [10]. Other groups have shown soluble IL-7R can be secreted from RA FLS in response to TNF, IL-1β, and/or IL-17 individually or in combination [69]. It is however unclear how IL-7 affects RA FLS pathology.
Notably, both IL-7 and IL-7R expression are enriched in endothelial cells in response to LPS and TNF exposure [10]. While IL-7R levels are upregulated by IL-1β stimulation, IL-7 concentration is unaffected by this inflammatory mediator in endothelial cells [10]. Activation of endothelial cells by IL-7, like RA macrophages, results in the production of proangiogenic factors like Ang1, suggesting a role for IL-7 in RA vascularization [10]. Extending these data, IL-7 systemic injection in CIA led to exacerbated joint angiogenesis due to enhanced sera FGF2, VEGF, and VCAM1 levels [70]. Consistently, CIA mice systemically treated with IL-7R Ab manifested a reduction in joint VWF and VCAM1 protein levels [71]. Taken together, the impact of IL-7/IL-7R on RA FLS or endothelial cell pathogenesis may be restricted compared to those propagated by RA macrophages and T cells.
6. Significance of IL-7/IL-7R blockade in RA preclinical models
Three earlier studies have shown that CIA disease activity and bone erosion were alleviated by IL-7R Ab therapy [62, 71, 72]. IL-7R Ab therapy disrupted IFNγ and T-bet or IL-17 and RORγT transcription levels when CIA CD4+ splenocytes were cocultured in presence of CD11b cells and challenged with collagen type II [72]. These investigators demonstrated that while the number of CD3+ and CD4+ splenocytes were suppressed by IL-7R Ab therapy in CIA, CD11b+ cells remained unaffected [72]. Supporting this notion, IL-7 systemic administration in CIA increases spleen Th1 and Th17 differentiation and stimulated the lymph node IFNγ and IL-17 production [70, 72].
However, morphological studies authenticated that anti-IL-7 Ab therapy attenuated CIA inflammation by markedly repressing the number of joint F480+ macrophage and VWF+ blood vessel infiltration as well as a lower tend of CD3+ T cell homing [11]. Suppression of myeloid cell joint migration as a result of IL-7 Ab therapy can be due to restrained IL-7 function or downregulation of joint monocyte chemoattractant, CCL2 in CIA joints or sera [11]. Consistent with the ability of IL-7 to potently reprogram naïve RA cells in M1 macrophages, dysregulation of IL-7 in CIA mice resulted in joint TNF reduction [11].
Interestingly, IL-7 advances angiogenesis by activating IL-8 and Ang1 secretion from RA macrophages and endothelial cells [10]. CIA vascularization was counteracted by IL-7 Ab therapy via CXCL2 (IL-8 analog) suppression, however, joint protein levels of Ang1, VEGF, CXCL1, or bFGF were unaffected [11]. In contrast, the protein concentration of joint IL-6, IL-1β, and IL-17, or the ankle CD3+ T cell immunostaining were unchanged by IL-7 Ab therapy in CIA [11]. Data inconsistency between groups may be due to the evaluation of T cells in CIA ex vivo activated splenocytes compared to the cells infiltrated in the joints [11, 72].
Nevertheless, all studies illustrated that blockade of IL-7 or IL-7R pathway could effectively alleviate CIA bone erosion by reducing joint TRAP+ osteoclasts in part by decreasing RANKL, CTSK, and MMP9 [11, 62, 71]. In contrast to more recent studies [54], IL-7-mediated osteoclast differentiation through RANKL was shown to be in part due to STAT5 signaling [62]. Collectively, blockade of the IL-7/IL-7R pathway efficiently mitigates CIA swelling and bone destruction by manipulating joint myeloid cell migration and proinflammatory reprogramming and neovascularization as well as T effector cell polarization in ex vivo splenocytes.
7. The implication of IL-7 and IL-7R in autoimmune diseases
7.1. Spondyloarthritis (SpA)
Excessive levels of IL-7 were detected in Spondyloarthritis (SpA) compared to osteoarthritis synovial fluid and tissue [73]. Differentiation of Th17 cells or production of IL-17 plays a critical role in the inflammatory and proliferative imprints of SpA. In addition to triggering Th17 cell differentiation, IL-7 can advance innate immune cells like γδLT and mucosa-associated invariant T (MAIT) cell polarization that is responsible for IL-17 secretion [74–77]. Interestingly, in SpA patients, innate-like T cells (T γδ, MAIT, and ILC3) are postulated to be the primary source of IL-17 rather than Th17 cells [77–79]. The number of MAIT cells was markedly reduced in SpA compared to normal sera [77, 80, 81]. Extending these findings, MAIT cells were enriched in SpA synovial fluid rather than sera, contributing to exacerbated IL-17 phenotype [77]. It was also noted that IL-7 priming accentuated IL-17 expansion in SpA MAIT cells, while IL-23 stimulation was ineffective [82]. Taken together, in SpA patients IL-17 secretion is reprogrammed by activation of innate-like T cells and effector T cells exposed to synovial fluid IL-7.
7.2. Systemic Lupus Erythematosus (SLE)
Soluble (s)IL-7R has been found in higher levels in the sera and urine of patients with Systemic Lupus Erythematosus (SLE) [83, 84], as well as in kidney tissues where TNF is expressed [85]. sIL-7R levels also correlate with SLE disease activity and anti-dsDNA antibody concentration and could potentially predict flare [86]. While the response to therapy reduces sera IL-7R levels, patients with enriched IL-7-related genes display worse prognoses [87]. Studies have postulated that the IL-7/IL-7R pathway helps to destabilize SLE immune tolerance and creates the conditions to override the endogenous checkpoints against autoimmunity. Therefore IL-7/IL-7R blockade may suppress the ability for immune cascades to spiral out of control and instigate an inflammatory response.
IL-7, with its manipulation of T cells, seems to play a particular role in the generation and persistence of CD8+ effector T cells, as these cells are detected in higher quantities in SLE patients [88]. Others have suggested that IL-7Rlow CD8+ T cell expansion in SLE patients may provide upregulation of unbound IL-7 to stimulate other autoimmune and pro-inflammatory mechanisms [89]. Moreover, IL-7 contributes to Th17 cell polarization in SLE [90]. In short, while some studies indicated that the IL-7/IL-7R cascade has an indirect impact on SLE, others suggest that the involvement is directly escalated through pathogenic T effector cells.
7.3. Psoriasis (Ps) and Psoriatic Arthritis (PsA)
IL-7 levels were markedly enriched in Psoriasis (Ps) biopsy taken from lesional skin compared to non-lesional or normal skin, suggesting that keratinocytes are responsible for this phenotype [91]. Consistently, sera IL-7 concentration was potentiated in Ps relative to normal individuals [91]. Interestingly sera IL-7 levels were unaffected by therapy and its concentration did not correlate with Psoriasis Area and Severity Index (PASI) scores; in contrast, a positive relationship was established between sera IL-6 levels and PASI scores [92]. Furthermore, IL-7 expressed from PsA PBMCs, or SF was responsible for osteoclast maturation as its immunosuppression dysregulated osteoclastogenesis [93]. Taken together these findings suggest that IL-7 is associated with Ps and PsA active disease and bone erosion through an IL-6 independent pathway.
8. Conclusion and Future Direction
Enrichment of IL-7 and IL-7R has been observed in several autoimmune diseases. Nevertheless, its mechanism of action is mostly investigated in RA immune cells and experimental models compared to other autoimmune conditions. Given that the current biotherapies are ineffective in negating IL-7/IL-7R-mediated arthritogenicity, innovative strategies are much needed.
PI3K and STAT5 signaling is coactivated by IL-7 in RA macrophages and T cells and is linked to cell migration, inflammatory cell differentiation, and bone erosion. Perhaps one idea may be to block the activation of PI3K/mTOR or STAT5 signaling in RA patients. Alternatively, since the expression of myeloid IL-7 and IL-7R is modulated by TLR4 and IL-1β, inhibiting the common transcription factors such as IRF5/IRF7 or downstream shared signaling intermediates such as IRAK1/4 will disrupt IL7/IL-7R-activated pathology. While complete blockade of the IL-7/IL-7R pathway may not be feasible as it is required for T cell survival, proliferation and metabolism. Hence future studies should be designed to identify components of the IL-7 pathway that can be targeted without impacting the normal physiological function.
HIGHLIGHTS.
Early in the disease, IL-7 promotes RA monocyte migration by activating ERK and PI3K pathways
During active disease, naive cells are reprogramed by IL-7 into inflammatory macrophages that produce proangiogenic factors to expand joint vascularization
IL-7-mediated Th1 cell differentiation can prime cell surface IL-7R expression in RA myeloid cells, resulting in potentiated inflammatory and erosive responses
The crosstalk between macrophages and T cells in RA patients plays an important role in IL-7-driven osteoclastogenesis
Authenticating these findings, blockade of IL-7 or IL-7R mitigates collagen-induced arthritis joint swelling and bone erosion by dysregulating joint leukocyte migration and differentiation as well as impeding neovascularization
ACKNOWLEDGEMENTS
This work was supported in part by awards from the Department of Veteran’s Affairs MERIT Award BX002286, the Innovative Research Award from the Rheumatology Research Foundation (RRF), the National Institutes of Health NIH AI147697, Pfizer Investigator-Initiated Research (IIR) Program and Chicago Biomedical Consortium (CBC) Accelerator Award. We would like to apologize to colleagues whose work was not cited due to space limitations. Figures were created with BioRender.com.
ABBREVIATIONS
- RA
rheumatoid arthritis
- TLR
Toll-like receptor
- Th
T helper cells
- TNF
tumor necrosis factor
- IFN
interferon
- SCID
Severe Combined Immunodeficiency
- DAS28
disease activity score assessed by 28 joint scores
- PHA
phytohaemagglutinin
- PBMCs
peripheral blood mononuclear cells
- SFMCs
synovial fluid mononuclear cells
- BCL2
B-cell lymphoma
- BAX
Bcl-2 Associated X-protein
- EAE
experimental autoimmune encephalomyelitis
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- STAT
Signal Transducer and Activator of Transcription
- mTOR
Mechanistic Target Of Rapamycin Kinase
- BMMϴs
bone marrow differentiated macrophages
- RANK
Receptor activator of nuclear factor κ B
- RANKL
Receptor activator of nuclear factor-kappa-Β ligand
- TRAP
Tartrate-resistant acid phosphatase
- NFATc1
Nuclear Factor of Activated T-Cells
- FLS
fibroblast-like synoviocytes
- FGF2
Fibroblast growth factor 2
- VEGF
vascular endothelial growth factor
- VCAM
Vascular Cell Adhesion Molecule
- CIA
collagen-induced arthritis
- SpA
Spondyloarthritis
- MAIT
mucosa-associated invariant T
- CTSK
cathepsin K
- MMP
Matrix metalloproteinases
- SLE
Systemic Lupus Erythematosus
- Ps
psoriasis
- PsA
psoriatic arthritis
- IRF
Interferon regulatory factor
- IRAK
interleukin 1 receptor associated kinase
Footnotes
DISCLOSURE
No conflict of interest was disclosed.
INTRODUCTION
- [1].Ariel A, Hershkoviz R, Cahalon L, Williams DE, Akiyama SK, Yamada KM et al. , Induction of T cell adhesion to extracellular matrix or endothelial cell ligands by soluble or matrix-bound interleukin-7, Eur. J. Immunol 27 (1997) 2562–70. [DOI] [PubMed] [Google Scholar]
- [2].Golden-Mason L, Kelly AM, Traynor O, McEntee G, Kelly J, Hegarty JE et al. , Expression of interleukin 7 (IL-7) mRNA and protein in the normal adult human liver: implications for extrathymic T cell development, Cytokine 14 (2001) 143–51. [DOI] [PubMed] [Google Scholar]
- [3].Kroncke R, Loppnow H, Flad HD, Gerdes J, Human follicular dendritic cells and vascular cells produce interleukin-7: a potential role for interleukin-7 in the germinal center reaction, Eur. J. Immunol 26 (1996) 2541–4. [DOI] [PubMed] [Google Scholar]
- [4].de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S et al. , The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation, J. Immunol 160 (1998) 1666–76. [PubMed] [Google Scholar]
- [5].Churchman SM, Ponchel F, Interleukin-7 in rheumatoid arthritis, Rheumatology (Oxford) 47 (2008) 753–9. [DOI] [PubMed] [Google Scholar]
- [6].Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR et al. , Cell biology of IL-7, a key lymphotrophin, Cytokine Growth Factor Rev 16 (2005) 513–33. [DOI] [PubMed] [Google Scholar]
- [7].Buckley RH, Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution, Annu. Rev. Immunol 22 (2004) 625–55. [DOI] [PubMed] [Google Scholar]
- [8].Lundstrom W, Fewkes NM, Mackall CL, IL-7 in human health and disease, Semin. Immunol 24 (2012) 218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Capitini CM, Chisti AA, Mackall CL, Modulating T-cell homeostasis with IL-7: preclinical and clinical studies, J. Intern. Med 266 (2009) 141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pickens SR, Chamberlain ND, Volin MV, Pope RM, Talarico NE, Mandelin AM 2nd et al. , Characterization of interleukin-7 and interleukin-7 receptor in the pathogenesis of rheumatoid arthritis, Arthritis Rheum 63 (2011) 2884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen Z, Kim SJ, Chamberlain ND, Pickens SR, Volin MV, Volkov S et al. , The novel role of IL-7 ligation to IL-7 receptor in myeloid cells of rheumatoid arthritis and collagen-induced arthritis, J. Immunol 190 (2013) 5256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gregory SG, Schmidt S, Seth P, Oksenberg JR, Hart J, Prokop A et al. , Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis, Nat. Genet 39 (2007) 1083–91. [DOI] [PubMed] [Google Scholar]
- [13].Anderson CA, Boucher G, Lees CW, Franke A, D’Amato M, Taylor KD et al. , Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47, Nat. Genet 43 (2011) 246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heron M, Grutters JC, van Moorsel CH, Ruven HJ, Huizinga TW, van der Helm-van Mil AH et al. , Variation in IL7R predisposes to sarcoid inflammation, Genes Immun 10 (2009) 647–53. [DOI] [PubMed] [Google Scholar]
- [15].Zhang B, Zhang Y, Xiong L, Li Y, Zhang Y, Zhao J et al. , CD127 imprints functional heterogeneity to diversify monocyte responses in inflammatory diseases, J. Exp. Med 219 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].van Roon JA, Hartgring SA, Wenting-van Wijk M, Jacobs KM, Tak PP, Bijlsma JW et al. , Persistence of interleukin 7 activity and levels on tumour necrosis factor alpha blockade in patients with rheumatoid arthritis, Annals of the Rheumatic Diseases 66 (2007) 664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Belarif L, Danger R, Kermarrec L, Nerriere-Daguin V, Pengam S, Durand T et al. , IL-7 receptor influences anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease, J. Clin. Invest 129 (2019) 1910–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fry TJ, Mackall CL, Interleukin-7: from bench to clinic, Blood 99 (2002) 3892–904. [DOI] [PubMed] [Google Scholar]
- [19].Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P, Lipopolysaccharide interferes with the induction of peripheral T cell death, Immunity 2 (1995) 261–70. [DOI] [PubMed] [Google Scholar]
- [20].Boise LH, Minn AJ, June CH, Lindsten T, Thompson CB, Growth factors can enhance lymphocyte survival without committing the cell to undergo cell division, Proc. Natl. Acad. Sci. U. S. A 92 (1995) 5491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chazen GD, Pereira GM, LeGros G, Gillis S, Shevach EM, Interleukin 7 is a T-cell growth factor, Proc. Natl. Acad. Sci. U. S. A 86 (1989) 5923–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Costello R, Brailly H, Mallet F, Mawas C, Olive D, Interleukin-7 is a potent co-stimulus of the adhesion pathway involving CD2 and CD28 molecules, Immunology 80 (1993) 451–7. [PMC free article] [PubMed] [Google Scholar]
- [23].Mehrotra PT, Grant AJ, Siegel JP, Synergistic effects of IL-7 and IL-12 on human T cell activation, J. Immunol 154 (1995) 5093–102. [PubMed] [Google Scholar]
- [24].Borger P, Kauffman HF, Postma DS, Vellenga E, IL-7 differentially modulates the expression of IFN-gamma and IL-4 in activated human T lymphocytes by transcriptional and post-transcriptional mechanisms, J. Immunol 156 (1996) 1333–8. [PubMed] [Google Scholar]
- [25].Jourdan P, Vendrell JP, Huguet MF, Segondy M, Bousquet J, Pene J et al. , Cytokines and cell surface molecules independently induce CXCR4 expression on CD4+ CCR7+ human memory T cells, J. Immunol 165 (2000) 716–24. [DOI] [PubMed] [Google Scholar]
- [26].Bradley LM, Haynes L, Swain SL, IL-7: maintaining T-cell memory and achieving homeostasis, Trends Immunol 26 (2005) 172–6. [DOI] [PubMed] [Google Scholar]
- [27].Natsumeda M, Nishiya K, Ota Z, Stimulation by interleukin-7 of mononuclear cells in peripheral blood, synovial fluid and synovial tissue from patients with rheumatoid arthritis, Acta Med. Okayama 47 (1993) 391–7. [DOI] [PubMed] [Google Scholar]
- [28].van Roon JA, Verweij MC, Wijk MW, Jacobs KM, Bijlsma JW, Lafeber FP, Increased intraarticular interleukin-7 in rheumatoid arthritis patients stimulates cell contact-dependent activation of CD4(+) T cells and macrophages, Arthritis Rheum 52 (2005) 1700–10. [DOI] [PubMed] [Google Scholar]
- [29].Timmer TC, Baltus B, Vondenhoff M, Huizinga TW, Tak PP, Verweij CL et al. , Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis, Arthritis Rheum 56 (2007) 2492–502. [DOI] [PubMed] [Google Scholar]
- [30].Gringhuis SI, de Leij LF, Verschuren EW, Borger P, Vellenga E, Interleukin-7 upregulates the interleukin-2-gene expression in activated human T lymphocytes at the transcriptional level by enhancing the DNA binding activities of both nuclear factor of activated T cells and activator protein-1, Blood 90 (1997) 2690–700. [PubMed] [Google Scholar]
- [31].van Roon JA, Glaudemans KA, Bijlsma JW, Lafeber FP, Interleukin 7 stimulates tumour necrosis factor alpha and Th1 cytokine production in joints of patients with rheumatoid arthritis, Annals of the Rheumatic Diseases 62 (2003) 113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Churchman SM, El-Jawhari JJ, Burska AN, Parmar R, Goeb V, Conaghan PG et al. , Modulation of peripheral T-cell function by interleukin-7 in rheumatoid arthritis, Arthritis Res. Ther 16 (2014) 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR, A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells, J. Immunol 181 (2008) 225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM et al. , The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo, Nat. Immunol 10 (2009) 314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Arbelaez CA, Glatigny S, Duhen R, Eberl G, Oukka M, Bettelli E, IL-7/IL-7 Receptor Signaling Differentially Affects Effector CD4+ T Cell Subsets Involved in Experimental Autoimmune Encephalomyelitis, J. Immunol 195 (2015) 1974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B, TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells, Immunity 24 (2006) 179–89. [DOI] [PubMed] [Google Scholar]
- [37].Stockinger B, Veldhoen M, Martin B, Th17 T cells: linking innate and adaptive immunity, Semin. Immunol 19 (2007) 353–61. [DOI] [PubMed] [Google Scholar]
- [38].Sheng W, Yang F, Zhou Y, Yang H, Low PY, Kemeny DM et al. , STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation, Cell Res 24 (2014) 1387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bikker A, Kruize AA, Wenting M, Versnel MA, Bijlsma JW, Lafeber FP et al. , Increased interleukin (IL)-7Ralpha expression in salivary glands of patients with primary Sjogren’s syndrome is restricted to T cells and correlates with IL-7 expression, lymphocyte numbers and activity, Ann. Rheum. Dis 71 (2012) 1027–33. [DOI] [PubMed] [Google Scholar]
- [40].Bikker A, van Woerkom JM, Kruize AA, van der Wurff-Jacobs KM, Bijlsma JW, Lafeber FP et al. , Clinical efficacy of leflunomide in primary Sjogren’s syndrome is associated with regulation of T-cell activity and upregulation of IL-7 receptor alpha expression, Ann. Rheum. Dis 71 (2012) 1934–41. [DOI] [PubMed] [Google Scholar]
- [41].Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ, Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1, Nature 426 (2003) 671–6. [DOI] [PubMed] [Google Scholar]
- [42].Nakayama K, Nakayama K, Dustin LB, Loh DY, T-B cell interaction inhibits spontaneous apoptosis of mature lymphocytes in Bcl-2-deficient mice, J. Exp. Med 182 (1995) 1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jacobs SR, Michalek RD, Rathmell JC, IL-7 is essential for homeostatic control of T cell metabolism in vivo, J. Immunol 184 3461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rathmell JC, Farkash EA, Gao W, Thompson CB, IL-7 enhances the survival and maintains the size of naive T cells, J. Immunol 167 (2001) 6869–76. [DOI] [PubMed] [Google Scholar]
- [45].Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA, Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells, J. Exp. Med 200 (2004) 659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC, IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival, Blood 111 (2008) 2101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Carrette F, Surh CD, IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis, Semin. Immunol 24 (2012) 209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gerriets VA, Rathmell JC, Metabolic pathways in T cell fate and function, Trends Immunol 33 (2012) 168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Reading JL, Vaes B, Hull C, Sabbah S, Hayday T, Wang NS et al. , Suppression of IL-7-dependent Effector T-cell Expansion by Multipotent Adult Progenitor Cells and PGE2, Mol. Ther 23 (2015) 1783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Buck MD, O’Sullivan D, Pearce EL, T cell metabolism drives immunity, J. Exp. Med 212 (2015) 1345–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kim SJ, Chang HJ, Volin MV, Umar S, Van Raemdonck K, Chevalier A et al. , Macrophages are the primary effector cells in IL-7-induced arthritis, Cell. Mol. Immunol 17 (2020) 728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hartgring SA, Bijlsma JW, Lafeber FP, van Roon JA, Interleukin-7 induced immunopathology in arthritis, Annals of the Rheumatic Diseases 65 Suppl 3 (2006) iii69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Toraldo G, Roggia C, Qian WP, Pacifici R, Weitzmann MN, IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells, Proc. Natl. Acad. Sci. U. S. A 100 (2003) 125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kim JH, Sim JH, Lee S, Seol MA, Ye SK, Shin HM et al. , Interleukin-7 Induces Osteoclast Formation via STAT5, Independent of Receptor Activator of NF-kappaB Ligand, Front. Immunol 8 (2017) 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R, Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency, J. Clin. Invest 110 (2002) 1643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Toyosaki-Maeda T, Takano H, Tomita T, Tsuruta Y, Maeda-Tanimura M, Shimaoka Y et al. , Differentiation of monocytes into multinucleated giant bone-resorbing cells: two-step differentiation induced by nurse-like cells and cytokines, Arthritis Res 3 (2001) 306–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Roato I, Brunetti G, Gorassini E, Grano M, Colucci S, Bonello L et al. , IL-7 up-regulates TNF-alpha-dependent osteoclastogenesis in patients affected by solid tumor, PLoS One 1 (2006) e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R, Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines, Blood 96 (2000) 1873–8. [PubMed] [Google Scholar]
- [59].Kim HS, Ullevig SL, Zamora D, Lee CF, Asmis R, Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment, Proc. Natl. Acad. Sci. U. S. A 109 (2012) E2803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shahrara S, Pickens SR, Dorfleutner A, Pope RM, IL-17 induces monocyte migration in rheumatoid arthritis, J. Immunol 182 (2009) 3884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ayala JM, Goyal S, Liverton NJ, Claremon DA, O’Keefe SJ, Hanlon WA, Serum-induced monocyte differentiation and monocyte chemotaxis are regulated by the p38 MAP kinase signal transduction pathway, J. Leukoc. Biol 67 (2000) 869–75. [PubMed] [Google Scholar]
- [62].Xu H, Cai L, Li Z, Zhang L, Wang G, Xie R et al. , Dual effect of IL-7/IL-7R signalling on the osteoimmunological system: a potential therapeutic target for rheumatoid arthritis, Immunology 164 (2021) 161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mackall CL, Fry TJ, Gress RE, Harnessing the biology of IL-7 for therapeutic application, Nat. Rev. Immunol 11 (2011) 330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Niu N, Qin X, New insights into IL-7 signaling pathways during early and late T cell development, Cell. Mol. Immunol 10 (2013) 187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Iwanami N, Mateos F, Hess I, Riffel N, Soza-Ried C, Schorpp M et al. , Genetic evidence for an evolutionarily conserved role of IL-7 signaling in T cell development of zebrafish, J. Immunol 186 (2011) 7060–6. [DOI] [PubMed] [Google Scholar]
- [66].Read KA, Powell MD, McDonald PW, Oestreich KJ, IL-2, IL-7, and IL-15: Multistage regulators of CD4(+) T helper cell differentiation, Exp. Hematol 44 (2016) 799–808. [DOI] [PubMed] [Google Scholar]
- [67].Xie Y, Shi X, Sheng K, Han G, Li W, Zhao Q et al. , PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review), Mol Med Rep 19 (2019) 783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lali FV, Crawley J, McCulloch DA, Foxwell BM, A late, prolonged activation of the phosphatidylinositol 3-kinase pathway is required for T cell proliferation, J. Immunol 172 (2004) 3527–34. [DOI] [PubMed] [Google Scholar]
- [69].Badot V, Durez P, Van den Eynde BJ, Nzeusseu-Toukap A, Houssiau FA, Lauwerys BR, Rheumatoid arthritis synovial fibroblasts produce a soluble form of the interleukin-7 receptor in response to pro-inflammatory cytokines, J. Cell. Mol. Med 15 (2011) 2335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hartgring SA, Willis CR, Bijlsma JW, Lafeber FP, van Roon JA, Interleukin-7-aggravated joint inflammation and tissue destruction in collagen-induced arthritis is associated with T-cell and B-cell activation, Arthritis Res. Ther 14 (2012) R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hartgring SA, Willis CR, Alcorn D, Nelson LJ, Bijlsma JW, Lafeber FP et al. , Blockade of the interleukin-7 receptor inhibits collagen-induced arthritis and is associated with reduction of T cell activity and proinflammatory mediators, Arthritis Rheum 62 (2010) 2716–25. [DOI] [PubMed] [Google Scholar]
- [72].Cai L, Xu H, Zhang H, Zhang L, Wang G, Nie H, Blockade of IL-7Ralpha alleviates collagen-induced arthritis via inhibiting Th1 cell differentiation and CD4(+) T cell migration, Mol. Immunol 79 (2016) 83–91. [DOI] [PubMed] [Google Scholar]
- [73].Rihl M, Kellner H, Kellner W, Barthel C, Yu DT, Tak PP et al. , Identification of interleukin-7 as a candidate disease mediator in spondylarthritis, Arthritis Rheum 58 (2008) 3430–5. [DOI] [PubMed] [Google Scholar]
- [74].Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA et al. , Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids, J. Exp. Med 211 (2014) 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC, Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing gammadelta cells, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 17549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC et al. , IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells, J. Immunol 190 (2013) 3142–52. [DOI] [PubMed] [Google Scholar]
- [77].Goncalves RSG, Duarte A, IL-7 is a Key Driver Cytokine in Spondyloarthritis?, Journal of immunology research 2019 (2019) 7453236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Debusschere K, Lories RJ, Elewaut D, MAIT cells: not just another brick in the wall, Ann. Rheum. Dis 75 (2016) 2057–9. [DOI] [PubMed] [Google Scholar]
- [79].Venken K, Elewaut D, IL-23 responsive innate-like T cells in spondyloarthritis: the less frequent they are, the more vital they appear, Curr. Rheumatol. Rep 17 (2015) 30. [DOI] [PubMed] [Google Scholar]
- [80].Sugimoto C, Konno T, Wakao R, Fujita H, Fujita H, Wakao H, Mucosal-associated invariant T cell is a potential marker to distinguish fibromyalgia syndrome from arthritis, PLoS One 10 (2015) e0121124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].O’Brien-Gore C, Gray EH, Durham LE, Taams LS, Kirkham BW, Drivers of Inflammation in Psoriatic Arthritis: the Old and the New, Curr. Rheumatol. Rep 23 (2021) 40. [DOI] [PubMed] [Google Scholar]
- [82].Gracey E, Qaiyum Z, Almaghlouth I, Lawson D, Karki S, Avvaru N et al. , IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis, Ann. Rheum. Dis 75 (2016) 2124–32. [DOI] [PubMed] [Google Scholar]
- [83].Chi S, Xue J, Li F, Zhu C, Yu Y, Li H et al. , Correlation of Serum Soluble Interleukin-7 Receptor and Anti-C1q Antibody in Patients with Systemic Lupus Erythematosus, Autoimmune Dis 2016 (2016) 8252605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Stanley S, Mok CC, Vanarsa K, Habazi D, Li J, Pedroza C et al. , Identification of Low-Abundance Urinary Biomarkers in Lupus Nephritis Using Electrochemiluminescence Immunoassays, Arthritis Rheumatol 71 (2019) 744–55. [DOI] [PubMed] [Google Scholar]
- [85].Badot V, Luijten RK, van Roon JA, Depresseux G, Aydin S, Van den Eynde BJ et al. , Serum soluble interleukin 7 receptor is strongly associated with lupus nephritis in patients with systemic lupus erythematosus, Ann. Rheum. Dis 72 (2013) 453–6. [DOI] [PubMed] [Google Scholar]
- [86].Lauwerys BR, Husson SN, Maudoux AL, Badot V, Houssiau FA, sIL7R concentrations in the serum reflect disease activity in the lupus kidney, Lupus science & medicine 1 (2014) e000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].McKinney EF, Lyons PA, Carr EJ, Hollis JL, Jayne DR, Willcocks LC et al. , A CD8+ T cell transcription signature predicts prognosis in autoimmune disease, Nat. Med 16 (2010) 586–91, 1p following 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kim JS, Cho BA, Sim JH, Shah K, Woo CM, Lee EB et al. , IL-7Ralphalow memory CD8+ T cells are significantly elevated in patients with systemic lupus erythematosus, Rheumatology (Oxford) 51 (2012) 1587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gonzalez-Quintial R, Lawson BR, Scatizzi JC, Craft J, Kono DH, Baccala R et al. , Systemic autoimmunity and lymphoproliferation are associated with excess IL-7 and inhibited by IL-7Ralpha blockade, PLoS One 6 (2011) e27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Leng RX, Pan HF, Chen GM, Feng CC, Fan YG, Ye DQ et al. , The dual nature of Ets-1: focus to the pathogenesis of systemic lupus erythematosus, Autoimmunity reviews 10 (2011) 439–43. [DOI] [PubMed] [Google Scholar]
- [91].Bonifati C, Trento E, Cordiali-Fei P, Carducci M, Mussi A, D’Auria L et al. , Increased interleukin-7 concentrations in lesional skin and in the sera of patients with plaque-type psoriasis, Clin. Immunol. Immunopathol 83 (1997) 41–4. [DOI] [PubMed] [Google Scholar]
- [92].Szepietowski JC, Bielicka E, Nockowski P, Noworolska A, Wasik F, Increased interleukin-7 levels in the sera of psoriatic patients: lack of correlations with interleukin-6 levels and disease intensity, Clin. Exp. Dermatol 25 (2000) 643–7. [DOI] [PubMed] [Google Scholar]
- [93].Colucci S, Brunetti G, Cantatore FP, Oranger A, Mori G, Quarta L et al. , Lymphocytes and synovial fluid fibroblasts support osteoclastogenesis through RANKL, TNFalpha, and IL-7 in an in vitro model derived from human psoriatic arthritis, J. Pathol 212 (2007) 47–55. [DOI] [PubMed] [Google Scholar]


