Abstract
Exercise promotes functional improvements in aged tissues, but the extent to which it simulates partial molecular reprogramming is unknown. Using transcriptome profiling from 1) a skeletal muscle-specific in vivo Oct3/4, Klf4, Sox2, and Myc (OKSM) reprogramming-factor expression murine model, 2) an in vivo inducible muscle-specific Myc induction murine model, 3) a translatable high-volume hypertrophic exercise training approach in aged mice, and 4) human exercise muscle biopsies, we collectively defined exercise-induced genes that are common to partial reprogramming. Late-life exercise training lowered murine DNA methylation age according to several contemporary muscle-specific clocks. A comparison of the murine soleus transcriptome after late-life exercise training to the soleus transcriptome after OKSM induction revealed an overlapping signature that included higher JunB and Sun1. Also, within this signature, downregulation of specific mitochondrial and muscle-enriched genes was conserved in skeletal muscle of long-term exercise-trained humans; among these was muscle-specific Abra/Stars. Myc is the OKSM factor most induced by exercise in muscle and was elevated following exercise training in aged mice. A pulse of MYC rewired the global soleus muscle methylome, and the transcriptome after a MYC pulse partially recapitulated OKSM induction. A common signature also emerged in the murine MYC-controlled and exercise adaptation transcriptomes, including lower muscle-specific Melusin and reactive oxygen species-associated Romo1. With Myc, OKSM, and exercise training in mice as well habitual exercise in humans, the complex I accessory subunit Ndufb11 was lower; low Ndufb11 is linked to longevity in rodents. Collectively, exercise shares similarities with genetic in vivo partial reprogramming.
Keywords: DNA Methylation, Ageing, MYC, Yamanaka Factors
Graphical Abstract

Diverse forms of exercise training improve muscle function and whole-body health, even if initiated late in life. Information on conserved exercise-controlled molecular cues that underpin a younger muscle phenotype in aged muscle has potential utility in the development of anti-ageing therapies. Induction of the Yamanaka factors Oct3/4, Klf4, Sox2, and Myc are known to ameliorate ageing hallmarks. Comparison of transcriptomic data from aged exercise-trained mice and humans to muscle fibre-specific genetically driven models of epigenetic reprogramming (e.g. Yamanaka factor or Myc expression) unearthed conserved biomarkers associated with molecular age mitigation. Considering reduced biological age according to DNA methylome analysis, high-volume exercise training can be classified as an epigenetic reprogramming stimulus. Chronic exercise should be considered alongside and/or as a method to inform healthspan-extending longevity approaches such as pharmacologic and dietary interventions.
Introduction
The Yamanaka transcription factors Oct3/4, Klf4, Sox2, and Myc (OKSM) epigenetically revert somatic cells to a stem cell-like state (Takahashi & Yamanaka, 2006). Cellular reprogramming via OKSM is in part mediated by remodeling of the DNA methylome (Nishino et al., 2011; Gao et al., 2013; Lee et al., 2014; Chondronasiou et al., 2022). The expression of OKSM facilitates cellular plasticity that is characteristic of more youthful cells (Gill et al., 2022). The finding that OKSM drive molecular and cellular ageing mitigation (Ocampo et al., 2016b) prompted an interest in exploiting these factors to improve human health and lifespan (Ocampo et al., 2016a). In vivo expression of OKSM in mice combats several aspects of ageing (Ocampo et al., 2016b) including DNA methylation clock age (mDNAge) (Horvath, 2013; Olova et al., 2019), transcriptional perturbations (Chondronasiou et al., 2022), impaired tissue regeneration (Ocampo et al., 2016b; Chondronasiou et al., 2022), and mortality (Ocampo et al., 2016b; Alle et al., 2022). Phenotypes and transcriptomes associated with ameliorated ageing hallmarks are similarly observed with transient OKSM-mediated reprogramming of cells derived from aged humans (Sarkar et al., 2020; Gill et al., 2022). Potential issues with epigenetic reprogramming to alleviate ageing phenotypes are a loss of cellular identity (Olova et al., 2019; Roux et al., 2022) and neoplastic transformation (Friedmann‐Morvinski & Verma, 2014). In skeletal muscle, high levels of cell cycle inhibitors and expression of tumor-repressing genes provides terminally-differentiated muscle fibres resistance to tumorigenesis (Seely, 1980; Keckesova et al., 2017). Muscle has therefore been used as a model tissue to identify cancer-fighting strategies (Keckesova et al., 2017; Crist et al., 2022). Also due to its unique ability to resist primary and secondary tumor growth, skeletal muscle is an ideal tissue for studying the effects of epigenetic reprogramming via Yamanaka factors.
Mechanistic studies place skeletal muscle health at the center of whole-body health throughout the lifespan (Demontis et al., 2013; Rai et al., 2021). Exercise is considered the most effective and inexpensive strategy to improve muscle health and by extension whole-body health. Pharmacologic and dietary methods might extend healthspan and ameliorate ageing hallmarks as ageing progresses, but exercise training naturally elicits many of the same benefits and more (Furrer & Handschin, 2022). Despite this, exercise is often overlooked as a frontline approach to combat ageing hallmarks, restore cellular resiliency, and attenuate biological ageing (Zhang et al., 2022a). A popular drug-based therapy (metformin) used to combat ageing (Xenos et al., 2022) blunts various beneficial effects of endurance or resistance exercise training in the muscle of healthy non-diabetic humans (Konopka et al., 2019; Konopka & Miller, 2019; Walton et al., 2019). Another drug touted as “anti-ageing”, rapamycin (Blagosklonny, 2018), prevents hypertrophic muscle adaptation to loading (Bodine et al., 2001; Goodman et al., 2011). All-cause mortality and reduced quality of life are linked to reductions in muscle mass, strength, and/or power (Metter et al., 2002; Newman et al., 2006; Reid & Fielding, 2012; Li et al., 2018; de Santana et al., 2021; López-Bueno et al., 2022; Losa-Reyna et al., 2022; Winger et al., 2022). Exercise, and specifically resistance training, is a key countermeasure against ageing-associated sarcopenia (Talar et al., 2021; Grosicki et al., 2022). Preventing exercise-mediated muscle adaptation via supplementation with current anti-ageing drugs may therefore be objectionable. If exercise is a “polypill” (Fiuza-Luces et al., 2013; Hawley et al., 2021), understanding shared features of training adaptations and longevity interventions could be a prudent strategy for identifying molecular targets capable of conferring a “younger” phenotype.
To study the benefits of exercise during ageing, we applied a voluntary murine exercise training approach called progressive weighted wheel running (PoWeR) (Dungan et al., 2019; Murach et al., 2020) to aged mice (Dungan et al., 2022a). PoWeR in aged mice promotes adaptations in soleus muscle mass, cellular phenotype, and myonuclear DNA methylation reminiscent of what occurs in younger muscle with training (Wen et al., 2021; Dungan et al., 2022a). The current investigation leveraged PoWeR to identify exercise-associated genes in muscle that are linked to partial epigenetic reprogramming, a benchmark pre-clinical longevity intervention (Ocampo et al., 2016a; Ocampo et al., 2016b; Zhang et al., 2022a). We compared the in vivo gene expression landscape after PoWeR in skeletal muscle of aged mice to that of in vivo muscle-specific OKSM expression. Of OKSM, Myc mRNA and protein is highly exercise-responsive in human skeletal muscle (Trenerry et al., 2007; Broholm et al., 2011; Brook et al., 2016; Figueiredo et al., 2016; Stec et al., 2016; Townsend et al., 2016; Popov et al., 2019; Figueiredo et al., 2021). We recently reported that Myc induction specifically in muscle fibres is central to the acute mechanical loading-induced transcriptome in the plantaris of young mice (Murach et al., 2022). A corroborating result in muscle tissue was reported with frequent resistance-type training in rats (Viggars et al., 2022). Furthermore, the induction of Myc was recently linked to “biological immortality” in hydrozoans (Pascual-Torner et al., 2022). We therefore deployed a genetically driven muscle fibre-specific doxycycline-inducible model of in vivo Myc expression to explore shared features of Myc, OKSM partial reprogramming, and exercise adaptation transcriptomes in mice. We then compared these features to long-term exercise-trained human muscle biopsy transcriptomic data. Finally, we performed DNA methylome profiling after a pulse of MYC in muscle. Our findings point to new biomarkers of ageing-mitigation in skeletal muscle.
Methods
Ethical Approval
IACUCs at the University of Kentucky (UK, 2020–3535) and University of Arkansas (UA, AUP 21037) approved all animal procedures. Mice were housed in a temperature and humidity-controlled room, maintained on 12:12-h (UK) and 14:10-h(UA) light-dark cycles, and food and water were provided ad libitum throughout experimentation. All animals were sacrificed via cervical dislocation under deep anesthesia with inhaled isoflurane (UA) or lethal dosage of 0.1 ml of sodium pentobarbital injected intraperitoneally (UK).
Animals and Animal Procedures
Approximately 22-month-old female C57BL/6N mice were obtained from the Charles River Laboratories National Institute of Aging research colony; exact ages are not recorded for this colony, but the birth month is documented. 22 months in mice is estimated to correspond to ~73 human years (Dutta & Sengupta, 2016). Female mice were used for these experiments since they tend to run more in our hands. The C57BL/6N mice were used for the DNA methylation ageing experiments (mDNAge) and RNA-sequencing experiments. The mDNAge experiments were part of a related but separate study and were subcutaneously injected daily with a 0.9% NaCl sterile saline solution (Fisher Scientific, cat. 50–843-141) throughout the eight weeks of PoWeR. For all murine exercise training experiments, aged female mice were subjected to voluntary progressive weighted wheel running (PoWeR) as previously described by us (Murach et al., 2021; Dungan et al., 2022a). Mice in the PoWeR group were singly housed in cages with running wheels to allow monitoring of individual running distance using ClockLab software (Actimetrics, v6.1.01), and mice in the sedentary group were group housed in cages without running wheels. Following an introductory week with an unweighted wheel, 8 weeks of PoWeR training commenced with the following weight progression: 2g in week 1, 3g in week 2, 4g in week 3, and 5g in weeks 4–8. One-gram magnets (product no. B661, K&J Magnetics) were affixed to one side of the wheel to allow for the progressive increase in weight. Mice were approximately 24 months old upon completion of the experiment. PoWeR mDNAge mice ran 4.3±2.9 km/d (mean±standard deviation); running volume for PoWeR RNA-seq mice was reported previously (Dungan et al., 2022a). Following 8 weeks of PoWeR or 8 weeks as sedentary controls, mice were humanely euthanized by cervical dislocation under deep isoflurane anesthesia after a 24-hour wheel lock and overnight fast. Hindlimb muscles were then rapidly dissected and flash frozen.
For the Myc expression experiments, male HSA-rtTA+/−-;TRE-Myc+/− (HSA-Myc) were generated by crossing homozygous HSA-rtTA mice (Iwata et al., 2018) with heterozygous TRE-Myc mice (Felsher & Bishop, 1999); HSA-rtTA littermate mice were used as controls. All mice were genotyped as previously described (Iwata et al., 2018; Rutledge et al., 2020). HSA-Myc and littermate control mice were given access to doxycycline water overnight (0.5 mg/ml with 2% sucrose for 12 hours) at 2.5 months of age, and this water was replaced with un-supplemented water for 12 hours before being euthanized. Tissue was stored at −80°C until the time of RNA extraction.
DNA Methylation Age (mDNAge) Analysis
Samples were blinded until statistical analysis required unblinding. DNA was extracted from flash frozen gastrocnemius tissue by the Clock Foundation using a Qiagen DNeasy Blood & Tissue kit (cat. 69504 or 69506, Qiagen, Hilden, North Rhine-Westphalia, Germany) following standard protocols, and eluted into ultrapure, nuclease-free milliQ water. The Clock Foundation quantified the DNA and assessed purity via 260/280 absorption ratio. The isolated DNA then underwent bisulfite conversion at AKESOGen, Inc. (AKESOgen, Peachtree Corners, GA USA) using the Zymo EZ DNA methylation kit (Zymo, Irvine CA, USA). A minimum of 150 ng of total bisulfite-converted DNA was processed using a customized Infinium HD iSelect Methyl Custom 48-sample BeadChip termed the Illumina Horvath Mammalian Methylation Chip array kit (Clock Foundation, Los Angeles, CA USA), and scanned with an iScan system (Illumina, San Diego, CA, USA); samples from each treatment group were present on each chip to help eliminate chip-related artifacts. This custom methylation array targets more than 37,000 CpG methylation loci that are conserved across several mammalian species, the majority of which are found in mice (Arneson et al., 2022). Array output were normalized by the Clock Foundation using the R package SeSAMe, then values were input through an R pipeline with several clocks developed by the Horvath laboratory, including the Muscle Clock and the Developmental Muscle Clock (Figure 1A), each generated by the Horvath Laboratory using elastic net regression (Mozhui et al., 2022).
Figure 1.
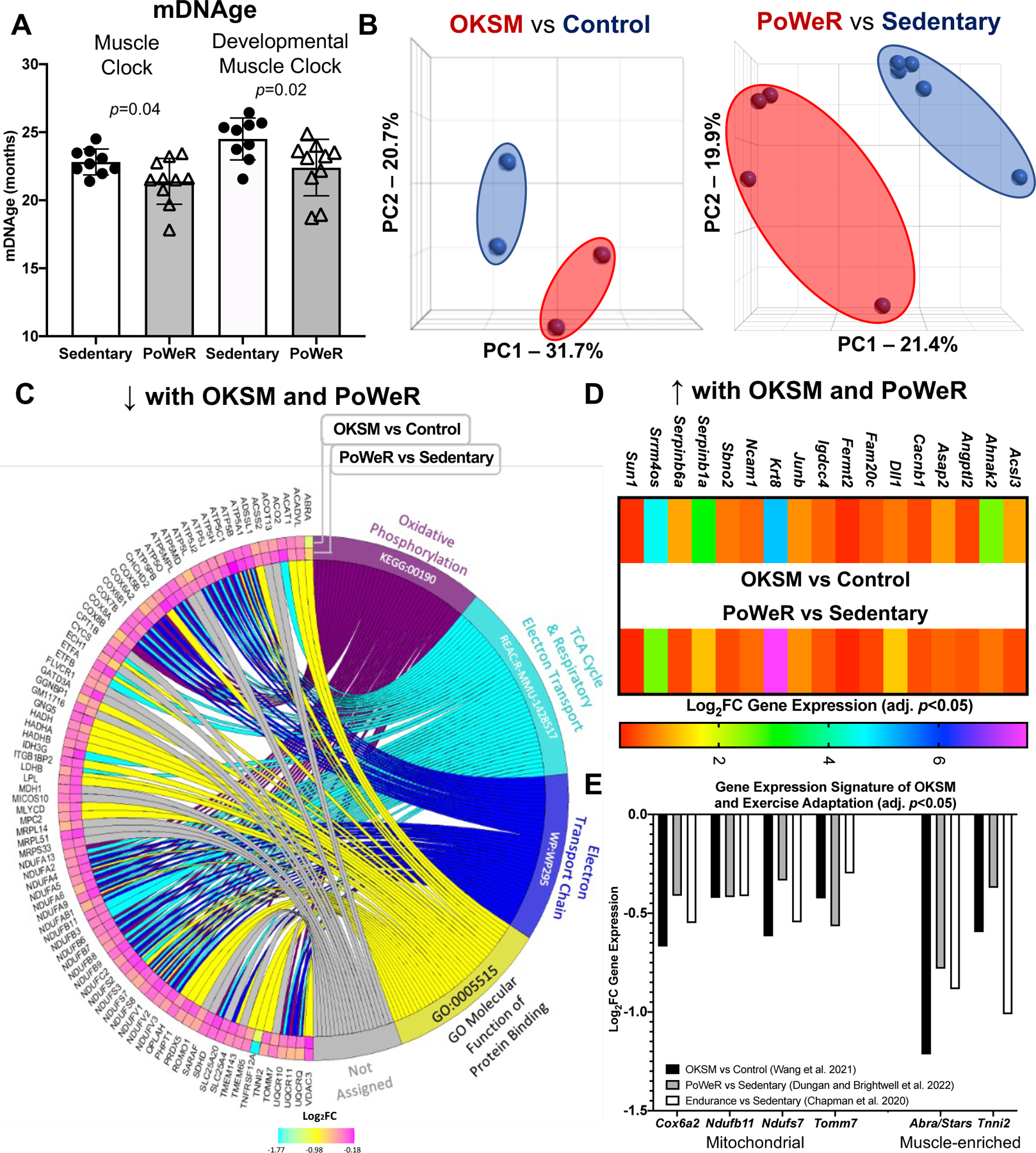
Comparison of Oct3/4, Klf4, Sox2, and Myc (OKSM) expression to late-life progressive weighted wheel running (PoWeR) in mouse muscle A DNA methylation age (mDNAge) derived from clocks developed by the Horvath laboratory applied to late-life PoWeR (n=10) and sedentary control (n=9) mouse gastrocnemius muscle B Principal component analysis (PCA) plots showing DESeq2-normalized gene count transcriptome data from OKSM expression (n=2) versus control (n=2) in mouse soleus muscle fibres (Wang et al., 2021) and the late-life PoWeR (n=4) versus the sedentary (n=5) transcriptome in soleus muscle (Dungan et al., 2022a) C Chord diagram showing significantly downregulated genes common across OKSM and late-life PoWeR in the soleus D Upregulated genes common across OKSM and late-life PoWeR in the soleus E Downregulated genes common across OKSM, late-life PoWeR, and long-term exercise training. All data reported as mean ± standard deviation
Western Blotting for MYC
Western blots were carried out as previously described (Lim et al., 2022). Briefly, 20 mg of frozen quadriceps and heart muscle samples were powdered and homogenized in Laemmli buffer. Following RC/DC assay (BioRad, Hercules, CA, USA, cat. 500–0119), 40μg of total protein was subjected to SDS-PAGE using a 10% gel. Membranes were blocked with 5% of milk. Primary antibody incubation was conducted at 4C overnight using anti-MYC (D84C12 cat. 5605, Cell Signaling, Danvers, MA, USA) diluted 1:500. Secondary antibody (IRDye 800CW/680RD, LI-COR Biosciences, Lincoln, NE) was diluted 1:10,000 and membranes were imaged on LI-COR Odyssey FC using IR detection. All bands were normalized to the 45-kDa actin band of Ponceau S stain as a loading control.
RNA Isolation, Sequencing, and Analyses
Soleus RNA was isolated using TRI Reagent (Sigma-Aldrich, St Louis, MO, USA). Tissue was homogenized using beads and the Fisher Bead Mill (Fisher, Hanover Park, IL, USA). Following homogenization, RNA was isolated via phase separation by addition of bromochloropropane or chloroform then centrifugation. The aqueous phase was transferred to a new tube and further processed on columns using the Direct-zol Kit (Zymo Research, Irvine, CA, USA) (Figueiredo et al., 2021). concentration and purity were determined using a BioTek Take3 micro-volume microplate with a BioTek PowerWave XS microplate reader (BioTek Instruments Inc., Winooski, VT). Following library preparation with Poly A enrichment, RNA was sequenced by Novogene on an Illumina HiSeq using 150 bp paired-end sequencing, as we have performed previously (Wen et al., 2021; Dungan et al., 2022a).
FASTQ files (PoWeR and Myc experiments) or processed files from GEO (OKSM experiments) were aligned using STAR in Partek, then normalized and analyzed using DESeq2 (minimum read cutoff of ≤20 across all samples). P values were adjusted using the Benjamini-Hochberg false discovery rate (FDR) step-up method. Pathway over-representation analysis was performed using ConsensusPathDB (Kamburov & Herwig, 2022) with non-ordered query and up- or-downregulated genes with FDR adj. p<0.05; all KEGG and Reactome pathways reported had adjusted p values <0.05. PoWeR data were from soleus muscles of mice aged 24 months that was published previously (Dungan et al., 2022a) (GSE198652). Muscle was harvested 24 hours after the last exercise bout. OKSM data were from Wang et al. (Wang et al., 2021) (GSE148911) using male and female mice that were at least two months of age (<4.5 months old). OKSM induction in soleus muscle was 2.5 days. The human exercise data from Chapman et al. (43.5±5.2 y sedentary [n=8] and 41.8±5.9 y trained females [n=9], to match the female PoWeR mice) were already processed and presented in supplemental tables (Chapman et al., 2020). The samples were from biopsies of the vastus lateralis after a 72-hour cessation of activity and standardized dietary conditions. The online MetaMEx analysis tool from the Zierath laboratory was used to determine Yamanaka factor gene expression levels with exercise in skeletal muscle of humans (Pillon et al., 2020).
Reduced Representation Bisulfite Sequencing (RRBS)
DNA was extracted from soleus muscle using the Qiagen DNA MicroKit (Hilden, Germany). Following standard quality control, Msp1 RRBS was performed by Zymo Research (Irvine, California, USA) using 40 ng of genomic DNA. The tissue for RRBS analysis was the same as that used for RNA-seq. RRBS data were processed using MethylSig (Park et al., 2014) with a minimum cutoff of 10x coverage per CpG site in each sample, as we have previously described (Murach et al., 2021; Wen et al., 2021). Promoters were defined as within 1 kb upstream of the transcription start site (Wen et al., 2021). Differentially methylated sites were determined using a beta binomial distribution and p value <0.0005; Benjamini-Hochberg false discovery rate adjusted p values are reported for significant p value findings. Results are presented at the gene level, and site-specific CpG data are in a supplemental table.
Statistical Considerations
For mDNAge analysis, data were determined to be normal (Shapiro-Wilk test) and a two-tailed t-test was employed. The chord plot was generated using GOplot and in R version 4.1.1. The 87 genes significantly downregulated in both the OKSM versus Control comparison and the PoWeR versus Sedentary comparisons were input into gProfiler. The top KEGG, Reactome, and Wikipathway were input into GOplot for these genes (defined by the smallest adjusted p value, as calculated within the gProfiler platform), along with the GO molecular function protein binding since it contained many of the proteins not in the other top pathways. PCA plots were generated in Partek Genomic Suite. All other figures were generated using GraphPad Prism version 7.00 for Mac OS X (GraphPad Software, La Jolla, CA) and BioRender. Landscape In Silico deletion Analysis (Lisa, also called Cistrome) was run according to recommended procedures (Qin et al., 2020). In brief, differentially expressed gene lists (adj. p<0.05) from the PoWeR RNA-seq experiment were input into the online graphical user interface. The Cauchy combination p-value test was used to determine the overall influence of MYC. Data presented as mean ± standard deviation of mean unless otherwise stated.
Results
Late-life exercise training associates with lower skeletal muscle DNA methylation age using contemporary muscle-specific epigenetic clocks
To corroborate our previously-described exercise training-mediated shift in methylation age toward a younger status in muscle, female C57BL/6N mice (n=10) completed PoWeR from 22–24 months. 22-month-old mice are estimated to be equivalent to ~73 year old humans (Dutta & Sengupta, 2016). Age-matched untrained mice were sedentary controls (n=9). Muscle methylation was quantified with the Horvath Mammalian Methylation Chip that focuses on conserved cytosines (Arneson et al., 2022) and analyzed with contemporary murine mDNAge clocks (Mozhui et al., 2022). After PoWeR, skeletal muscle mDNAge was lower relative to sedentary using the Horvath Muscle Clock (−5.7 weeks, p=0.04) and Developmental Muscle Clock (−7.1 weeks, p=0.02) (Figure 1A). The magnitude of epigenetic age mitigation by late-life exercise in muscle was similar to our previous report using an independent cohort of mice and a different mDNAge analysis (8 weeks, n=5 per group, p=0.07) (Murach et al., 2021). Running volume was slightly lower in the current investigation (4.3 km/d vs 6.4 km/d). Consistently lower mDNAge with exercise motivated us to compare transcriptome profiles of a partial reprogramming stimulus (OKSM expression) versus exercise adaptation in muscle to identify a common gene expression signature.
The transcriptome resulting from OKSM expression in muscle fibres overlaps with the late-life exercise adaptation transcriptome in mouse muscle
RNA-seq data from the soleus muscle of adult mice after 2.5 days of genetic OKSM expression (Wang et al., 2021) was compared to RNA-sequencing data from the soleus of late-life PoWeR mice (Dungan et al., 2022a). The soleus adapts more substantially to PoWeR than other hindlimb muscles in young and old mice (Englund et al., 2020; Murach et al., 2020), with similar gene expression patterns regardless of age (Dungan et al., 2022a). The soleus also contains similar proportions of the primary myosin fibre types found in human vastus lateralis (Murach et al., 2019; Murach et al., 2020; Dungan et al., 2022a), further justifying our focus on this muscle.
OKSM induction and PoWeR revealed stark effects on gene expression for each intervention (Figure 1B). With OKSM, 412 genes were upregulated and 523 genes were downregulated (adj. p<0.05) (Supplemental Table 1). Pathway analysis of OKSM induction revealed repression of genes associated with oxidative phosphorylation, in agreement with the analysis from the Belmonte laboratory (Wang et al., 2021). We compared the list of genes downregulated by OKSM with those downregulated by PoWeR (377 genes, adj. p<0.05) (Supplemental Table 2). About one quarter of downregulated genes overlapped (87 genes, Figure 1C). Pathway analysis of shared downregulated genes revealed oxidative phosphorylation (KEGG, adj. p=9.77×10−54), the citric acid cycle (Reactome, adj. p=8.31×10−56), and electron transport chain (Wikipathways, adj. p=2.08×10−44) as the top pathways (Figure 1C). The muscle-enriched genes fast skeletal muscle troponin (Tnni2) (Sheng & Jin, 2016) and striated muscle activator of Rho signaling (Stars, or Abra) (Arai et al., 2002; Wallace et al., 2012) were also lower with OKSM and PoWeR; this suggested a repression of muscle cellular identity, consistent with partial reprogramming effects. Seventeen upregulated genes were common between OKSM and PoWeR datasets (Figure 1D), including the potent muscle mass-regulator JunB (Raffaello et al., 2010) and myonuclear anchor Sun1 (Lei et al., 2009). Perhaps alteration to Sun1 could be related to myonuclear shape changes that occur with exercise training in rodents (Murach et al., 2020, Rader and Baker, 2022). Collectively, our analysis unearthed a canonical skeletal muscle gene expression signature common between OKSM and exercise adaptation.
Gene expression alterations with OKSM and exercise adaptation in mouse muscle are conserved in human muscle after prolonged exercise training
To validate our murine findings in humans we queried an RNA-sequencing dataset from muscle biopsy samples of women with at least 15 years of mixed-modality endurance training history (n=9, ~42 years old) (Chapman et al., 2020). Comparing our murine PoWeR data to long-term training in humans seemed appropriate as 8 weeks of high-volume PoWeR corresponds to ~10% of the mouse lifespan and could thus be characterized as prolonged training. Several mitochondrial-related genes downregulated by OKSM and PoWeR were significantly lower in muscle of long-term endurance-trained athletes relative to sedentary age-matched controls (COX6A2, adj. p=0.046; NDUFB11, adj. p=0.017; NDUFS7, adj. p=0.041; TOMM7, adj. p=0.028). Downregulation of mitochondrial genes with endurance training seems counterintuitive. To further explore this observation, we compared genes that were altered in female endurance trained muscle to the human MitoCarta 3.0 database (Rath et al., 2021). While 100 genes that were higher with training overlapped with MitoCarta, 52 genes that were lower also overlapped; these included NDUFs such as NDUFAF3 and NDUFS7 as well as COX6A1 and COX6A2 (Supplemental Table 3). Thus, endurance training adaptations in skeletal muscle are not characterized by unanimously elevated mitochondrial gene levels. In addition to mitochondrial genes, muscle-enriched TNNI2 and ABRA (STARS) were lower in exercise-trained human muscle (adj. p=0.002 and 0.003, respectively), consistent with OKSM expression and late-life PoWeR in mice (Figure 1E).
Myc is an exercise-responsive transcription factor in muscle that influences the gene expression landscape with PoWeR in aged mice
The OKSM genetic mouse model was designed to induce all four Yamanaka factors, but Klf4 gene expression was not increased significantly in the soleus in the OKSM experiment (Wang et al., 2021); we corroborate this in our analysis (1.05 FC OKSM vs Sedentary, adj. p=0.95). All four factors can contribute to muscle adaptation via a change in activity independent of expression levels, but partial reprogramming was likely most attributable to Oct3/4, Sox2, and Myc. Of OKSM, only MYC is significantly induced by a bout of exercise in skeletal muscle from healthy humans according to a transcriptome meta-analysis (n=64–187 subjects, acute resistance and endurance exercise) (Pillon et al., 2020) (Figure 2A). Worth noting is that the KLF4 response is quite variable but may be induced in the first few hours after endurance exercise. Using up- and downregulated differentially expressed gene lists from our PoWeR RNA-seq data in the soleus, we performed Landscape in silico deletion analysis (Lisa/Cistrome) for insight on the potential contribution of MYC in the control of gene expression (Qin et al., 2020; Murach et al., 2022). Lisa incorporates transcriptome input data with an extensive library of publicly-available transcription factor ChIP-seq and global chromatin accessibility profiles to infer transcriptional regulators. According to Lisa, MYC is in the top 2% of transcription factors (TFs) predicted to influence upregulated genes in the PoWeR-trained transcriptome of aged mice (8 of 536 TFs) (Figure 2B), and in the top 5% for downregulated genes (19 of 536) (Supplemental Table 4). MYC was the highest ranked among Yamanaka factors. MYC as a primary TF controlling exercise adaptation in muscle is consistent with human biopsy data (Popov et al., 2019). MYC transcript and protein localizes in myonuclei during developmental and hypertrophic muscle growth in muscle of young animals (Alway, 1997; Veal & Jackson, 1998; Armstrong & Esser, 2005; Murach et al., 2022). Myc was 60% higher after late-life exercise in the soleus of PoWeR versus sedentary mice (24 hours after the final exercise bout, p=0.013, adj. p=0.14, Figure 2C). We therefore sought to explore the specific effects of a pulse of MYC in muscle fibres on muscle gene expression and compare it to the effects of OKSM and exercise training.
Figure 2.
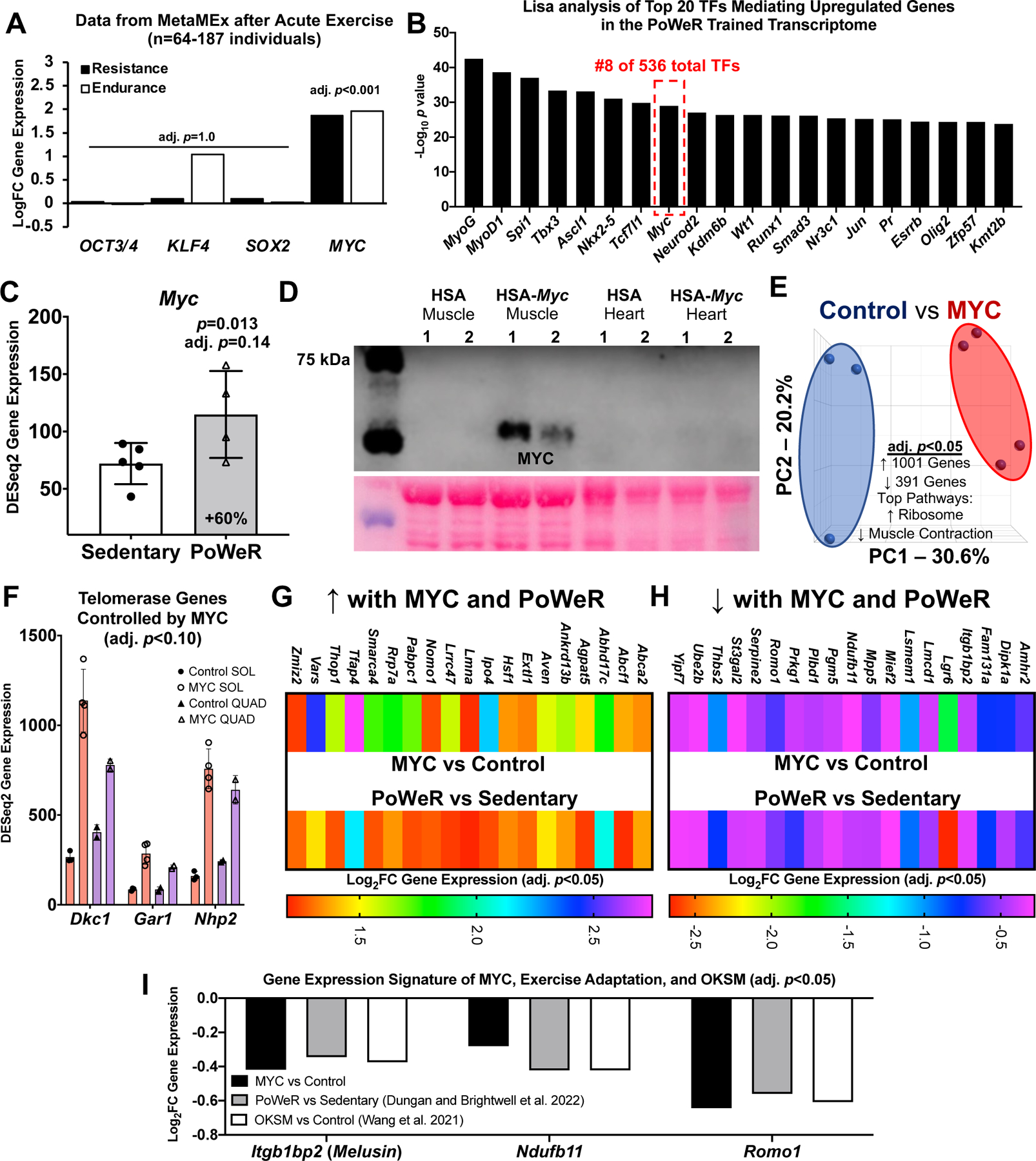
Identification of MYC-controlled genes in soleus muscle that also associate with late-life exercise and OKSM induction A Data from the MetaMEx human muscle and exercise meta-analysis tool from the Zierath laboratory (Pillon et al., 2020) showing the responsiveness of Oct3/4, Klf4, Sox2, and Myc to endurance and resistance exercise in humans. Input parameters: Male/Female, Young, Sedentary/Active/Athlete, Lean, Vastus Lateralis, Healthy, Immediate/0/3/4/5/6/8/18/24 hours. Adjusted p values are for each individual gene and condition. B Lisa analysis of transcription factors (TFs) controlling upregulated genes in the PoWeR-trained transcriptome C Myc gene expression in the soleus after 8 weeks of PoWeR from 22–24 months in mice (Dungan et al., 2022a) D Western blot showing MYC protein in skeletal muscle and heart 12 hours after removal of overnight doxycycline in biological replicate MYC and control mice, with corresponding Ponceau S stain beneath E PCA plot of full soleus DESeq2 normalized gene count transcriptome dataset from MYC (n=3) and control (n=4) mice F Telomerase complex genes controlled by MYC in the soleus (SOL) (n=3 control, n=4 MYC) and quadriceps (QUAD) (n=2 control, n=2 MYC) muscles G Upregulated genes in the soleus common to MYC and late-life PoWeR H Downregulated genes in the soleus common to MYC and late-life PoWeR I Gene expression signature common to MYC, PoWeR, and OKSM in the murine soleus. All data reported as mean ± standard deviation
Genetically controlled muscle-specific Myc induction recapitulates aspects of OKSM expression and exercise training adaptation
We generated a doxycycline-controlled muscle fibre-specific Myc expression model by crossing the HSA-rtTA mouse (Iwata et al., 2018) with the TetO-Myc mouse (Felsher & Bishop, 1999). This mouse is a tool for understanding the temporal control of MYC target genes in skeletal muscle fibres in vivo. Young adult mice had access to doxycycline in drinking water overnight resulting in Myc induction. Doxycycline was removed in the morning and soleus muscle was collected 12 hours later (n=3 HSA-rtTA littermate controls, n=4 HSA-Myc). MYC protein was elevated at 12 hours in muscle and global soleus gene expression was markedly altered at this time (Figure 2D and E). Inputting the MYC-controlled genes (adj. p<0.05) from the soleus into Lisa (Qin et al., 2020), MYC is predicted as the highest ranked transcription factor regulating induced genes (data not shown); this confirms the veracity of Lisa and agrees with our previous findings in the plantaris muscle (Murach et al., 2022). MYC induced 1001 genes and repressed 391 genes in the soleus (adj. p<0.05) (Supplemental Table 5). Ribosomal genes were the most upregulated (adj. p=3.3×10−29, KEGG) and muscle contraction genes were downregulated (adj. p=0.0009, Reactome, Figure 2E). MYC can transiently repress cellular identity (Sullivan et al., 2022), and downregulation of muscle-enriched genes corresponds with OKSM partial reprogramming in myofibres (see Figure 1). We previously reported that a pulse of MYC downregulated Nr1d2 (Reverbβ) and upregulated Rpl3 in the plantaris muscle of the mice used here (Murach et al., 2022). These findings were corroborated in the soleus and we also report a significant repression of Rpl3l by MYC (adj. p=0.041) (Supplemental Table 5). This inverse pattern of Rpl3 (induction) and Rpl3l (repression) is indicative of a developmental- and growth-oriented gene expression program in skeletal muscle (Zhang et al.; Chaillou et al., 2016; Chaillou, 2019; Kao et al., 2021). OKSM expression versus MYC induction revealed 39 upregulated genes and 31 downregulated genes in common. Ribosome biogenesis-related genes including Akt1, Mdn1, and Wdr3 were commonly upregulated and mitochondrial genes including Apoo, Ndufa1, Ndufb11, Romo1, and Pdcd5 were commonly downregulated (Supplemental Table 6). MYC recapitulated several aspects of OKSM-mediated partial reprogramming.
To further define the gene expression profile induced by MYC, we performed RNA-seq on the quadriceps muscles from a subset of HSA-Myc mice (n=2 control and n=2 Myc). The quadriceps are considerably larger than the soleus and are also involved in murine wheel training. Quadriceps were less affected by MYC and fewer genes were altered (40 upregulated and 26 downregulated at adj. p<0.10) (Supplemental Table 7) relative to the soleus and the plantaris, the latter of which we reported previously (Murach et al., 2022). Thus, the magnitude of MYC regulation may be muscle-specific, and could depend on muscle function, fibre type distribution, and/or metabolic profile. Pathway analysis of genes altered by MYC induction in the quadriceps revealed downregulation of muscle contraction genes (e.g. various myosins and troponins) consistent with what is observed in the soleus (current study) and plantaris (Murach et al., 2022). MYC induced Dkc1, Gar1, and Nhp2 in the quadriceps which were also markedly upregulated by MYC in the soleus (Figure 2F). These genes are members of the telomerase complex and associated with telomere maintenance (Gu et al., 2008; Vulliamy et al., 2008). Telomere shortening is associated with ageing (López-Otín et al., 2013). MYC is known to activate telomerase (Wang et al., 1998) which facilitates peripheral nervous system regeneration (Ma et al., 2019). Alterations to telomerase genes further implicate MYC as a potentially age-mitigating reprogramming factor.
We next asked whether gene expression in the soleus after PoWeR in aged mice matched the MYC-controlled soleus transcriptome. Thirty-eight genes overlapped (19 up- and 19 down-regulated, adj. p<0.05, Figure 2G and H). Itgb1bp2 (Melusin), Ndufb11, and Romo1 were downregulated by MYC, PoWeR, and OKSM (Figure 2I). Repression of the mitochondrial complex I accessory subunit Ndufb11 was common to all datasets including long-term human exercise training (adj. p=0.02) (Chapman et al., 2020) (Figure 2E).
A pulse of MYC reshapes the global muscle methylome
Using the same soleus tissue from the RNA-seq experiment above, we performed RRBS to assess the global DNA methylome. At twelve hours after overnight exposure to doxycycline in HSA-Myc mice, the muscle methylome underwent global CpG remodeling (>1,000 distinct differentially methylated CpGs across promoters, exons, and introns at p<0.0005 and 10x coverage across all samples) relative to controls. MYC caused slight hypomethylation of promoter regions, and hypermethylation of exons and introns relative to controls (Figure 3A) (Supplemental Table 8). There was some agreement between promoter methylation status and gene expression mediated by MYC. Three genes (Cfap298, Marchf5, and Mettl1) had a hypomethylated promoter CpG and higher gene expression with MYC induction (Figure 3B and C). Cfap298 controls cilia motility and polarization (Jaffe et al., 2016), but its role in skeletal muscle is not understood. Marchf5 (also called March5 or Mitol) is an E3 ubiquitin ligase required for mitochondrial fission and regulates mitochondrial dynamics (Nie et al., 2021). Mettl1 is an RNA methyltransferase, which is noteworthy since MYC was recently identified as a regulator of RNA methylation (Jansson et al., 2021). Perhaps global gene expression and methylation status would align more closely after a longer and/or more frequent period of MYC induction, or if we used a more comprehensive DNA methylation analysis. We previously published low-input RRBS from soleus myonuclei of late-life PoWeR mice versus sedentary controls (Dungan et al., 2022a). There was modest agreement in gene-level regulation of promoter and exon regions when comparing the soleus MYC methylome to the myonuclear methylome after PoWeR (Supplemental Table 9). A larger number of common genes with broadly intersecting regulation was apparent in introns (Figure 3D and E). Collectively, these data suggest that MYC regulates DNA methylation in skeletal muscle and could be sufficient to induce epigenetic reprogramming, even after brief exposure.
Figure 3.
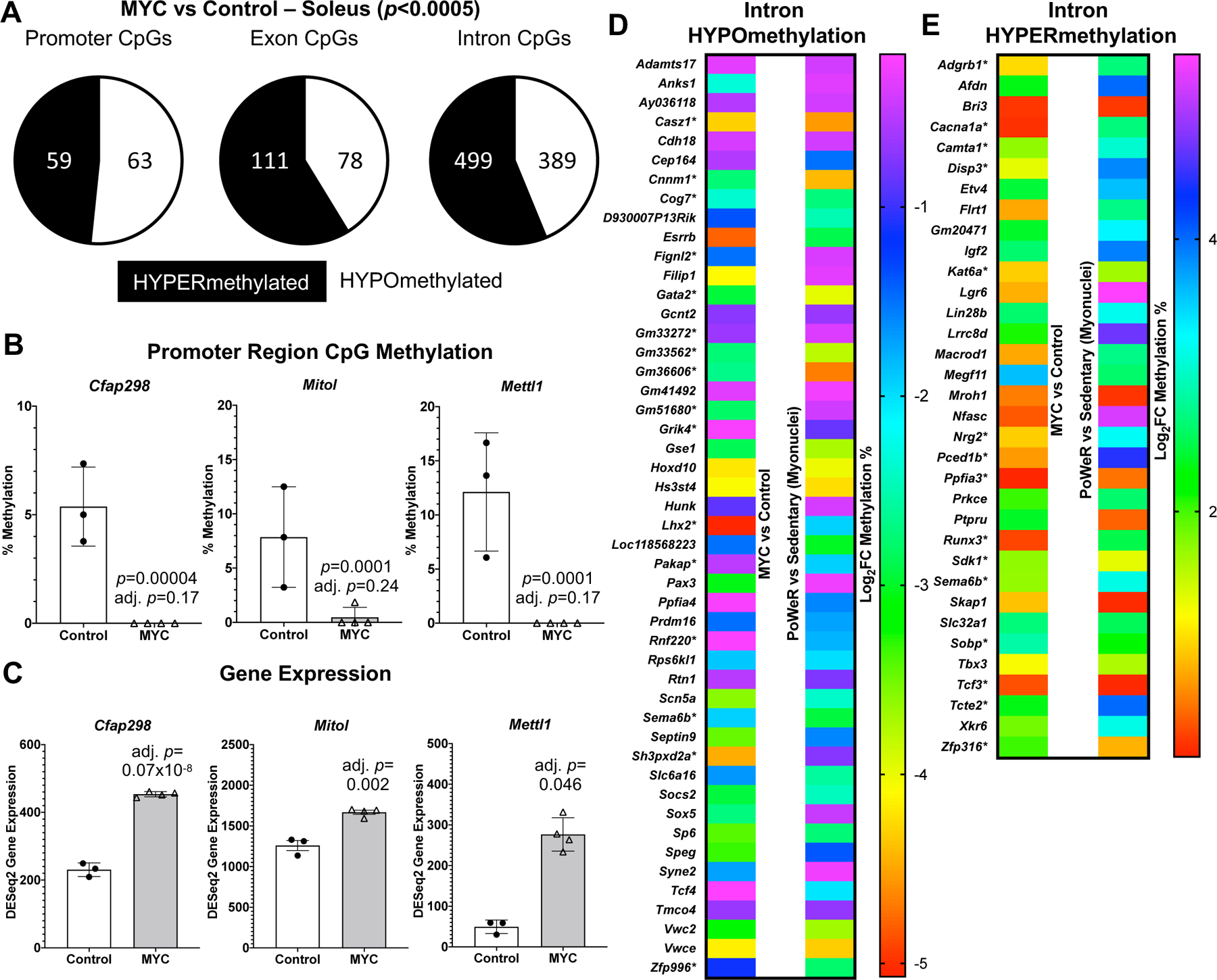
A pulse of MYC alters the DNA methylome in skeletal muscle A Soleus muscle DNA methylation in promoter, exon, and intron regions of HSA-Myc (n=4) relative to control mice (n=3), p<0.0005 and 10x coverage per CpG site for MYC methylation data B Promoter CpG DNA methylation and C gene expression of Cfap298, Mitol (Marchf5), and Mettl1 after a pulse of MYC, p<0.0005 and 10x coverage per CpG site for MYC methylation data D Common gene-level intron hypo- and E hyper-methylation with MYC induction in the soleus compared to soleus myonuclear DNA methylation after 8 weeks of late-life PoWeR (Dungan et al., 2022a).*=multiple sites of regulation in one or both conditions, but only data from one site is shown. All data reported as mean ± standard deviation
Discussion
In the current investigation, we report: 1) a biological age-mitigating effect on the epigenetic landscape by late-life exercise-training in murine skeletal muscle, 2) a common gene expression signature of partial reprogramming by OKSM and exercise training in muscle of humans and aged mice, and 3) that Myc is an exercise-responsive factor that contributes to a rewired molecular profile at the transcriptome and methylome levels.
Lower muscle epigenetic age using updated and customized mDNAge clocks with exercise training corroborates and expands on our initial report in mice (Murach et al., 2021). Studies examining the muscle methylome after chronic exercise in aged humans also support our findings (Sailani et al., 2019; Blocquiaux et al., 2021; Ruple et al., 2021). Lower mDNAge in muscle appears to be a feature of the molecular environment with exercise adaptation that could have practical consequences for biological ageing and muscle performance. Indeed, emerging evidence suggests that blood mDNAge inversely associates with grip strength and frailty in ageing humans (Verschoor et al., 2021; Peterson et al., 2022), and is lower following a dietary/physical activity intervention in postmenopausal women (Fiorito et al., 2021). Exercise training and OKSM share substantial gene expression overlap. Given the known phenotypic and functional benefits of exercise adaptation in aged animals (Dungan et al., 2022a), an altered transcriptome after brief OKSM-mediated epigenetic partial reprogramming in skeletal muscle could have functional consequences. Prior work shows that OKSM induction in muscle enhances regeneration after injury (Ocampo et al., 2016b; Wang et al., 2021), but more work is needed in the context of muscle mass, strength, quality, fatigue resistance, and senescent cell abundance with ageing. Exploration into the interaction of epigenetic reprogramming via OKSM in combination with exercise training is also warranted.
Both OKSM and late-life exercise adaption in mice as well as chronic exercise training in humans results in lower levels of Abra/Stars in muscle. Exploratory proteomics recently found that low ABRA/STARS in human skeletal muscle is strongly associated with high physical activity throughout the lifespan (Ubaida-Mohien et al., 2019). ABRA/STARS has numerous functions in muscle (Lamon et al., 2014), but knockdown in myotubes enhances insulin signaling and sensitivity (Jin et al., 2011). Since insulin resistance associates with ageing (Fink et al., 1983; Barzilai & Ferrucci, 2012), lower ABRA/STARS in muscle could be beneficial for improving muscle health throughout the lifespan. Itgb1bp2 (Melusin), Ndufb11, and Romo1 were downregulated by OKSM expression, late-life exercise training, as well as a pulse of MYC in the muscle of mice. Melusin is specific to striated muscle and increases during regeneration (Brancaccio et al. 1999). Conversely, Melusin decreases during atrophy (Vitadello et al. 2020) but its role in exercise adaptation is unclear. ROMO1 generates reactive oxygen species (Chung et al., 2006) and induces senescence (Chung et al., 2008). Downregulation of Romo1 may be beneficial in ageing muscle where senescent cells can manifest and impair adaptation (Dungan et al., 2021; Dungan et al., 2022b; Zhang et al., 2022b; Zhang et al., 2022c). ROMO1 is also a negative feedback regulator of MYC (Lee et al., 2011), so its downregulation by MYC induction seems intuitive. Repressed gene expression of the complex I accessory subunit Ndufb11 with OKSM, Myc, and exercise training in mice is conserved with chronic exercise in humans. Reduced complex I activity prevents reactive oxygen species production which enhances cellular fitness and longevity (Rodríguez-Nuevo et al., 2022). Low abundance of specific complex I components including NDUFB11 is a characteristic of tissue from long-lived animals (Miwa et al., 2014; Sahm et al., 2018). Lower Nudfb11 with chronic exercise, likely mediated by MYC, could be part of a larger adaptive response that defends trained skeletal muscle against oxidative damage (Criswell et al., 1993; Powers et al., 1999; Radak et al., 1999; Parise et al., 2005a; Parise et al., 2005b; Smuder et al., 2011). On balance, studies with ageing and exercise in muscle of humans report a disconnect between mitochondrial gene and protein abundance (Robinson et al., 2017; Tumasian III et al., 2021). Our results should be interpreted with this in mind. Future Ndufb11 gain- and loss-of-function experiments in muscle may be warranted to elucidate its potential role in combatting an aged muscle phenotype.
Myc gene and protein expression is induced in the muscle of young adult humans (Trenerry et al., 2007; Broholm et al., 2011; Brook et al., 2016; Figueiredo et al., 2016; Stec et al., 2016; Townsend et al., 2016; Popov et al., 2019; Figueiredo et al., 2021) and rodents (Whitelaw & Hesketh, 1992; Chen et al., 2002; Armstrong & Esser, 2005; Lai et al., 2010; von Walden et al., 2012; Goodman et al., 2015; Kirby et al., 2016; West et al., 2016; Murach et al., 2022) in response to loading. MYC participates in epigenetic reprogramming in concert with Oct3/4, Klf4, and Sox2 (Takahashi & Yamanaka, 2006), but can also facilitate epigenome remodeling on its own (Brenner et al., 2005; Gartel, 2006; Lin et al., 2009; Nakagawa et al., 2010; Poole et al., 2017; Pang et al., 2018; Poole et al., 2019). Consistent with work in other cell types, there was global remodeling of the muscle DNA methylome following MYC induction. We speculate this could be due to MYC’s interactions with DNA methyltransferases (DNMTs) and ten eleven translocases (TETs) (Brenner et al., 2005; Pang et al., 2018; Poole et al., 2019). It is striking that <24 h of MYC induction can change muscle DNA methylation status, but a single bout of exercise also remodels DNA methylation in human muscle tissue (Barres et al., 2012; Seaborne et al., 2018; Maasar et al., 2021). The mechanisms of methylation regulation in muscle by exercise are unclear (Small et al., 2021; Villivalam et al., 2021), but MYC could be a central factor. Recent evidence suggests that overexpression of MYC in aged oligodendrocyte progenitors is sufficient to restore regenerative remyelination in vivo (Neumann et al., 2021); this is consistent with MYC’s role in regulating in vivo sensory nerve regeneration (Ma et al., 2019). Short-term MYC expression recapitulates numerous aspects of myonuclear gene expression at the onset of rapid overload-mediated muscle hypertrophy (Murach et al., 2022). Two weeks of MYC overexpression also mimics the protein synthesis response to exercise-like high-intensity muscle contractions (Mori et al., 2020). Since MYC-controlled gene expression in muscle fibres overlaps with OKSM partial reprogramming and the exercise training-mediated transcriptomes of aged mice, we propose that pulses of Myc could serve to enhance muscle function.
A 60% induction of Myc in the soleus after late-life PoWeR is intriguing since whole-organism MYC knockdown may enhance longevity and healthspan in mice (Hofmann et al., 2015). By contrast, MYC induction in specific cell populations can attenuate cellular ageing and restore regenerative potential (Neumann et al., 2021). Myc gene and protein in muscle tissue may increase in rodents (Hofmann et al., 2014; Mobley et al., 2017) but not humans during ageing (Drummond et al., 2011; Stec et al., 2015). The behavior of MYC in muscle with ageing is likely complex and could be differentially affected in mononuclear proliferative cells versus multinuclear post-mitotic muscle fibres within muscle tissue. Single cell RNA-sequencing in muscle revealed Myc enrichment specifically in fibro/adipogenic progenitors, satellite cells, and tenocytes but not myonuclei of aged mice (Zhang et al., 2022c). Elevated Myc in satellite cells of aged mice has been described previously (Price et al., 2014). Cell type-specific upregulation of Myc may explain differing results at the tissue level. Aspects of Myc’s gene expression network, which is operative after muscle contraction when young (Popov et al., 2019; Murach et al., 2022), may be alternatively regulated after resistance training in human muscle when aged (Phillips et al., 2013). MYC gene expression in vastus lateralis muscle samples four hours after a bout of resistance exercise in ~80 year old men and women is blunted (logFC=0.64 and −0.54, respectively, adj. p>0.90) relative to ~24 year old men and women (logFC=1.84 and 1.73, respectively, adj. p<0.05, data extracted from MetaMEx) (Raue et al., 2012; Pillon et al., 2020). Blunted MYC gene and protein responsiveness to acute resistance exercise in muscle of younger, albeit still aged humans (~70 y) has also been observed (Rivas et al., 2014; Brook et al., 2016). Perhaps an attenuated MYC response in muscle fibres of very old humans and animals (Alway, 1997) explains diminished adaptability to exercise, specifically in glycolytic Type 2 muscle fibres (Slivka et al., 2008; Raue et al., 2009; Grosicki et al., 2022). We speculate that higher Myc levels in the oxidative soleus muscle after high-volume PoWeR in aged mice is favorable and could contribute to the notable cellular adaptations observed previously (Dungan et al., 2022a).
Tighter control over the duration of OKSM and Myc dosing, the timing of sampling after exercise and genetically-induced epigenetic partial reprogramming, the mode of exercise and training status, the potential effects of biological sex, and several other factors may identify a larger gene expression signature of exercise-associated reprogramming-linked genes in muscle. Importantly, understanding whether epigenetic reprogramming, specifically by MYC, induces functional consequences in skeletal muscle is essential. Such insight will help drive healthspan-extending therapeutic efforts forward and advance the ageing field beyond biomarker discovery. Since DNA demethylation alterations are required for cellular reprogramming (Simonsson & Gurdon, 2004), the precise mechanisms by which MYC reshapes the methylome and global epigenome in muscle also deserves further exploration. Ultimately, the mechanisms of muscle molecular reprogramming required to fully recapitulate a youthful phenotype remain elusive, but our data provide a roadmap for further examination of how exercise training combats aspects of ageing. Our data also implicate MYC as an exercise-induced reprogramming factor in skeletal muscle.
Supplementary Material
Supplemental Table 1 Gene expression in the soleus muscle of OKSM versus control mice (Wang et al., 2021, GEO GSE148911)
Supplemental Table 2 Gene expression in the mouse soleus after 8 weeks of PoWeR from 22–24 months of age versus sedentary controls (Dungan and Brightwell et al. 2022, GEO GSE198652)
Supplemental Table 3 Genes altered by long-term endurance training in human females that overlap with MitoCarta 3.0 (DEGs from Chapman et al. 2020)
Supplemental Table 4 Lisa/Cistrome analysis using up- and downregulated DEGs (adj. p<0.05) in the soleus muscle after late-life PoWeR
Supplemental Table 5 Gene expression in the mouse soleus of MYC versus controls
Supplemental Table 6 Overlap of genes altered by MYC and OKSM expression in the mouse soleus
Supplemental Table 7 Gene expression in the mouse quadriceps of MYC versus controls
Supplemental Table 8 DNA methylation in the mouse soleus muscle DNA after a pulse of MYC
Supplemental Table 9 Genes with CpGs that overlap between MYC induction in the soleus muscle and myonuclear DNA following 8 weeks of PoWeR in aged mice (Dungan and Brightwell et al. 2022)
Key Points.
Advances in the last decade related to cellular epigenetic reprogramming (e.g. DNA methylome remodeling) toward a pluripotent state via the Yamanaka transcription factors Oct3/4, Klf4, Sox2, and Myc (OKSM) provide a window into potential mechanisms for combatting the deleterious effects of cellular ageing
Using global gene expression analysis, we compared the effects of in vivo OKSM-mediated partial reprogramming in skeletal muscle fibres of mice to the effects of late-life murine exercise training in muscle
Myc is the Yamanaka factor most induced by exercise in skeletal muscle, so we compared the MYC-controlled transcriptome in muscle to Yamanaka factor-mediated and exercise adaptation gene landscapes in mice and humans
A single pulse of MYC is sufficient to remodel the muscle methylome
We identify partial reprogramming-associated genes that are innately altered by exercise training and conserved in humans, and propose that MYC contributes to some of these responses
Acknowledgements
The TRE-Myc mouse was a generous gift from Dr. Andrew McMahon at the University of Southern California. We would like to thank Dr. Ferdinand von Walden of the Karolinska Institute for thoughtful discussion of our work. Thank you to Sabrina Kozel of The Epigenetic Clock Development Foundation. The Graphical Summary was created using BioRender.
Funding Statement
This work was supported by National Institutes of Health R00 AG063994, and a Glenn Foundation/American Federation for Aging Research (AFAR) Junior Investigator Award, and startup funds from the University of Arkansas Vice Chancellor for Research and Innovation to KAM.
Biography

Ronald G. Jones III is a first-year doctoral student working with Dr. Kevin A. Murach in the Molecular Muscle Mass Regulation (M3R) Laboratory at the University of Arkansas. Ronald received his undergraduate degree in Exercise Science from Towson University in Baltimore, Maryland. He completed a master’s degree in Clinical Exercise Physiology at the University of Delaware. Ronald is a Certified Clinical Exercise Physiologist under the American College of Sports Medicine and worked in the Baltimore VA Medical Center as a Research Physiologist. His research focus includes the molecular and cellular mechanisms of skeletal muscle physiology and has a particular interest in -omics.
Footnotes
Conflict of Interest: YW is the founder of MyoAnalytics LLC. SJW is the founder of Ridgeline Therapeutics. The authors have no other conflicts to declare.
Data Availability
All unpublished data will be deposited in GEO upon publication; processed data are provided in Supplemental Tables.
References
- Alle Q, Le Borgne E, Bensadoun P, Lemey C, Béchir N, Gabanou M, Estermann F, Bertrand-Gaday C, Pessemesse L, Toupet K, Desprat R, Vialaret J, Hirtz C, Noël D, Jorgensen C, Casas F, Milhavet O & Lemaitre J-M (2022). A single short reprogramming early in life initiates and propagates an epigenetically related mechanism improving fitness and promoting an increased healthy lifespan. Aging Cell 21, e13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alway SE. (1997). Overload-induced C-Myc oncoprotein is reduced in aged skeletal muscle. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 52, B203–B211. [DOI] [PubMed] [Google Scholar]
- Arai A, Spencer JA & Olson EN. (2002). STARS, a striated muscle activator of Rho signaling and serum response factor-dependent transcription. Journal of Biological Chemistry 277, 24453–24459. [DOI] [PubMed] [Google Scholar]
- Armstrong DD & Esser KA. (2005). Wnt/β-catenin signaling activates growth-control genes during overload-induced skeletal muscle hypertrophy. American Journal of Physiology-Cell Physiology 289, C853–C859. [DOI] [PubMed] [Google Scholar]
- Arneson A, Haghani A, Thompson MJ, Pellegrini M, Kwon SB, Vu H, Maciejewski E, Yao M, Li CZ & Lu AT. (2022). A mammalian methylation array for profiling methylation levels at conserved sequences. Nature Communications 13, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O’Gorman DJ & Zierath JR. (2012). Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metabolism 15, 405–411. [DOI] [PubMed] [Google Scholar]
- Barzilai N & Ferrucci L. (2012). Insulin resistance and aging: a cause or a protective response? Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 67, 1329–1331. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. (2018). Disease or not, aging is easily treatable. Aging 10, 3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocquiaux S, Ramaekers M, Van Thienen R, Nielens H, Delecluse C, De Bock K & Thomis M (2022). [Google Scholar]; Recurrent training rejuvenates and enhances transcriptome and methylome responses in young and older human muscle. JCSM Rapid Communications 5, 10–32. [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC & Glass DJ. (2001). Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology 3, 1014. [DOI] [PubMed] [Google Scholar]
- Brenner C, Deplus R, Didelot C, Loriot A, Viré E, De Smet C, Gutierrez A, Danovi D, Bernard D & Boon T. (2005). Myc represses transcription through recruitment of DNA methyltransferase corepressor. The EMBO Journal 24, 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broholm C, Laye MJ, Brandt C, Vadalasetty R, Pilegaard H, Pedersen BK & Scheele C. (2011). LIF is a contraction-induced myokine stimulating human myocyte proliferation. Journal of Applied Physiology 111, 251–259. [DOI] [PubMed] [Google Scholar]
- Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ, Greenhaff PL, Smith K & Atherton PJ. (2016). Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. The Journal of Physiology 594, 7399–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillou T. (2019). Ribosome specialization and its potential role in the control of protein translation and skeletal muscle size. Journal of Applied Physiology 127, 599–607. [DOI] [PubMed] [Google Scholar]
- Chaillou T, Zhang X & McCarthy JJ. (2016). Expression of muscle‐specific Ribosomal Protein L3‐Like impairs myotube growth. Journal of Cellular Physiology 231, 1894–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MA, Arif M, Emanuelsson EB, Reitzner SM, Lindholm ME, Mardinoglu A & Sundberg CJ. (2020). Skeletal muscle transcriptomic comparison between long-term trained and untrained men and women. Cell Reports 31, 107808. [DOI] [PubMed] [Google Scholar]
- Chen YW, Nader GA, Baar KR, Fedele MJ, Hoffman EP & Esser KA. (2002). Response of rat muscle to acute resistance exercise defined by transcriptional and translational profiling. The Journal of Physiology 545, 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondronasiou D, Gill D, Mosteiro L, Urdinguio RG, Berenguer‐Llergo A, Aguilera M, Durand S, Aprahamian F, Nirmalathasan N, Abad M, Martin‐Herranz DE, Stephan‐Otto Attolini C, Prats N, Kroemer G, Fraga MF, Reik W & Serrano M (2022). Multi‐omic rejuvenation of naturally aged tissues by a single cycle of transient reprogramming. Aging Cell 21, e13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YM, Kim JS & Do Yoo Y. (2006). A novel protein, Romo1, induces ROS production in the mitochondria. Biochemical and Biophysical Research Communications 347, 649–655. [DOI] [PubMed] [Google Scholar]
- Chung YM, Lee SB, Kim HJ, Park SH, Kim JJ, Chung JS & Do Yoo Y. (2008). Replicative senescence induced by Romo1-derived reactive oxygen species. Journal of Biological Chemistry 283, 33763–33771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist SB, Nemkov T, Dumpit RF, Dai J, Tapscott SJ, True LD, Swarbrick A, Sullivan LB, Nelson PS, Hansen KC & Ghajar CM (2022). Unchecked oxidative stress in skeletal muscle prevents outgrowth of disseminated tumour cells. Nature Cell Biology 24, 538–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell D, Powers S, Dodd S, Lawler J, Edwards W, Renshler K & Grinton S. (1993). High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Medicine and Science in Sports and Exercise 25, 1135–1140. [PubMed] [Google Scholar]
- de Santana FM, Premaor MO, Tanigava NY & Pereira RMR (2021). Low muscle mass in older adults and mortality: A systematic review and meta-analysis. Experimental Gerontology 152, 111461. [DOI] [PubMed] [Google Scholar]
- Demontis F, Piccirillo R, Goldberg AL & Perrimon N. (2013). The influence of skeletal muscle on systemic aging and lifespan. Aging Cell 12, 943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, McCarthy JJ, Sinha M, Spratt HM, Volpi E, Esser KA & Rasmussen BB. (2011). Aging and microRNA expression in human skeletal muscle: a microarray and bioinformatics analysis. Physiological Genomics 43, 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan CM, Brightwell CR, Wen Y, Zdunek CJ, Latham CM, Thomas NT, Zagzoog AM, Brightwell BD, VonLehmden GL, Keeble AR, Watowich SJ, Murach KA & Fry CS (2022). Muscle-specific cellular and molecular adaptations to late-life voluntary concurrent exercise. Function 3, zqac027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan CM, Figueiredo VC, Wen Y, VonLehmden GL, Zdunek CJ, Thomas NT, Mobley CB, Murach KA, Brightwell CR, Long DE, Fry CS, Kern PA, McCarthy JJ & Peterson CA (2022). Senolytic treatment rescues blunted muscle hypertrophy in old mice. Geroscience 44, 1925–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan CM, Murach KA, Frick KK, Jones SR, Crow SE, Englund DA, Vechetti IJ Jr, Figueiredo VC, Levitan BM & Satin J. (2019). Elevated myonuclear density during skeletal muscle hypertrophy in response to training is reversed during detraining. American Journal of Physiology-Cell Physiology 316, C649–C654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan CM, Murach KA, Zdunek CJ, Tang ZJ, VonLehmden GL, Brightwell CR, Hettinger Z, Englund DA, Liu Z, Fry CS, Filareto A, Franti M & Peterson CA (2022). Deletion of SA β-Gal+ cells using senolytics improves muscle regeneration in old mice. Aging Cell 21, e13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S & Sengupta P. (2016). Men and mice: relating their ages. Life Sciences 152, 244–248. [DOI] [PubMed] [Google Scholar]
- Englund D, Figueiredo V, Dungan C, Murach K, Peck B, Petrosino J, Brightwell C, Dupont A, Neal A, Fry C, Accornero F, McCarthy J & Peterson C. (2020). Satellite cell depletion disrupts transcriptional coordination and muscle adaptation to exercise. Function 2, zqaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsher DW & Bishop JM. (1999). Reversible tumorigenesis by MYC in hematopoietic lineages. Molecular Cell 4, 199–207. [DOI] [PubMed] [Google Scholar]
- Figueiredo VC, Roberts LA, Markworth JF, Barnett MP, Coombes JS, Raastad T, Peake JM & Cameron‐Smith D. (2016). Impact of resistance exercise on ribosome biogenesis is acutely regulated by post‐exercise recovery strategies. Physiological Reports 4, e12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo VC, Wen Y, Alkner B, Fernandez-Gonzalo R, Norrbom J, Vechetti IJ Jr, Valentino T, Mobley CB, Zentner GE, Peterson CA, McCarthy JJ, Murach KA & von Walden F. (2021). Genetic and epigenetic regulation of skeletal muscle ribosome biogenesis with exercise. Journal of Physiology 599, 3363–3384. [DOI] [PubMed] [Google Scholar]
- Fink RI, Kolterman OG, Griffin J & Olefsky JM. (1983). Mechanisms of insulin resistance in aging. The Journal of Clinical Investigation 71, 1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito G, Caini S, Palli D, Bendinelli B, Saieva C, Ermini I, Valentini V, Assedi M, Rizzolo P, Ambrogetti D, Ottini L. (2021). DNA methylation‐based biomarkers of aging were slowed down in a two‐year diet and physical activity intervention trial: the DAMA study. Aging Cell 20, e13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiuza-Luces C, Garatachea N, Berger NA & Lucia A (2013). Exercise is the real polypill. Physiology 28, 330–358. [DOI] [PubMed] [Google Scholar]
- Friedmann‐Morvinski D & Verma IM. (2014). Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO Reports 15, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrer R & Handschin C (n.d.). Drugs, clocks and exercise in ageing: hype and hope, fact and fiction. The Journal of Physiology; DOI: 10.1113/JP282887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Chen J, Li K, Wu T, Huang B, Liu W, Kou X, Zhang Y, Huang H & Jiang Y. (2013). Replacement of Oct4 by Tet1 during iPSC induction reveals an important role of DNA methylation and hydroxymethylation in reprogramming. Cell Stem Cell 12, 453–469. [DOI] [PubMed] [Google Scholar]
- Gartel A. (2006). A new mode of transcriptional repression by c-myc: methylation. Oncogene 25, 1989–1990. [DOI] [PubMed] [Google Scholar]
- Gill D, Parry A, Santos F, Okkenhaug H, Todd CD, Hernando-Herraez I, Stubbs TM, Milagre I & Reik W. (2022). Multi-omic rejuvenation of human cells by maturation phase transient reprogramming. eLife 11, e71624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CA, Dietz JM, Jacobs BL, McNally RM, You J-S & Hornberger TA. (2015). Yes-Associated Protein is up-regulated by mechanical overload and is sufficient to induce skeletal muscle hypertrophy. FEBS Letters 589, 1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS & Hornberger TA. (2011). The role of skeletal muscle mTOR in the regulation of mechanical load‐induced growth. The Journal of Physiology 589, 5485–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosicki GJ, Zepeda CS & Sundberg CW (2022). Single muscle fibre contractile function with ageing. The Journal of Physiology; DOI: 10.1113/JP282298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B-W, Bessler M & Mason PJ. (2008). A pathogenic dyskerin mutation impairs proliferation and activates a DNA damage response independent of telomere length in mice. Proceedings of the National Academy of Sciences 105, 10173–10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley JA, Joyner MJ & Green DJ. (2021). Mimicking exercise: what matters most and where to next? The Journal of Physiology 599, 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JW, McBryan T, Adams PD & Sedivy JM. (2014). The effects of aging on the expression of Wnt pathway genes in mouse tissues. Age 36, 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JW, Zhao X, De Cecco M, Peterson AL, Pagliaroli L, Manivannan J, Hubbard GB, Ikeno Y, Zhang Y & Feng B. (2015). Reduced expression of MYC increases longevity and enhances healthspan. Cell 160, 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. (2013). DNA methylation age of human tissues and cell types. Genome Biology 14, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Englund DA, Wen Y, Dungan CM, Murach KA, Vechetti IJ, Mobley CB, Peterson CA & McCarthy JJ (2018). A novel tetracycline-responsive transgenic mouse strain for skeletal muscle-specific gene expression. Skeletal Muscle 8, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe KM, Grimes DT, Schottenfeld-Roames J, Werner ME, Ku T-SJ, Kim SK, Pelliccia JL, Morante NF, Mitchell BJ & Burdine RD. (2016). c21orf59/kurly controls both cilia motility and polarization. Cell Reports 14, 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson MD, Häfner SJ, Altinel K, Tehler D, Krogh N, Jakobsen E, Andersen JV, Andersen KL, Schoof EM & Ménard P. (2021). Regulation of translation by site-specific ribosomal RNA methylation. Nature Structural & Molecular Biology 28, 889–899. [DOI] [PubMed] [Google Scholar]
- Jin W, Goldfine AB, Boes T, Henry RR, Ciaraldi TP, Kim E-Y, Emecan M, Fitzpatrick C, Sen A & Shah A. (2011). Increased SRF transcriptional activity in human and mouse skeletal muscle is a signature of insulin resistance. The Journal of Clinical Investigation 121, 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamburov A & Herwig R. (2022). ConsensusPathDB 2022: molecular interactions update as a resource for network biology. Nucleic Acids Research 50, D587–D595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao BR, Malerba A, Lu-Nguyen NB, Harish P, McCarthy JJ, Dickson G & Popplewell LJ. (2021). Knockdown of muscle-specific Ribosomal Protein L3-Like enhances muscle function in healthy and dystrophic mice. Nucleic Acid Therapeutics 31, 457–464. [DOI] [PubMed] [Google Scholar]
- Keckesova Z, Donaher JL, De Cock J, Freinkman E, Lingrell S, Bachovchin DA, Bierie B, Tischler V, Noske A & Okondo MC. (2017). LACTB is a tumour suppressor that modulates lipid metabolism and cell state. Nature 543, 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby TJ, Patel RM, McClintock TS, Dupont-Versteegden EE, Peterson CA & McCarthy JJ. (2016). Myonuclear transcription is responsive to mechanical load and DNA content but uncoupled from cell size during hypertrophy. Molecular Biology of the Cell 27, 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, Musci RV, Safairad OD, Linden MA & Biela LM. (2019). Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 18, e12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR & Miller BF. (2019). Taming expectations of metformin as a treatment to extend healthspan. GeroScience 41, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai RY, Ljubicic V, D’souza D & Hood DA. (2010). Effect of chronic contractile activity on mRNA stability in skeletal muscle. American Journal of Physiology-Cell Physiology 299, C155–C163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamon S, Wallace MA & Russell AP. (2014). The STARS signaling pathway: a key regulator of skeletal muscle function. Pflügers Archiv-European Journal of Physiology 466, 1659–1671. [DOI] [PubMed] [Google Scholar]
- Lee D-S, Shin J-Y, Tonge PD, Puri MC, Lee S, Park H, Lee W-C, Hussein SM, Bleazard T & Yun J-Y. (2014). An epigenomic roadmap to induced pluripotency reveals DNA methylation as a reprogramming modulator. Nature Communications 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Kim JJ, Chung JS, Lee M-S, Lee K-H, Kim BS & Do Yoo Y. (2011). Romo1 is a negative-feedback regulator of Myc. Journal of Cell Science 124, 1911–1924. [DOI] [PubMed] [Google Scholar]
- Lei K, Zhang X, Ding X, Guo X, Chen M, Zhu B, Xu T, Zhuang Y, Xu R & Han M. (2009). SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proceedings of the National Academy of Sciences 106, 10207–10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Xia J, Zhang X, Gathirua-Mwangi WG, Guo J, Li Y, McKenzie S & Song Y. (2018). Associations of muscle mass and strength with all-cause mortality among US older adults. Medicine and Science in Sports and Exercise 50, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Deaver JW, Rosa-Caldwell ME, Lee DE, Morena da Silva F, Cabrera AR, Schrems ER, Saling LW, Washington TA & Fluckey JD. (2022). Muscle miR-16 deletion results in impaired insulin sensitivity and contractile function in a sex-dependent manner. American Journal of Physiology-Endocrinology and Metabolism 322, E278–E292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-H, Lin C, Tanaka H, Fero ML & Eisenman RN. (2009). Gene regulation and epigenetic remodeling in murine embryonic stem cells by c-Myc. PloS One 4, e7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Xian M, Ren T, Mo G, Zhang li & Zhang X (2022). Mining of chicken muscle growth genes and the function of important candidate gene RPL3L in muscle development. Frontiers in Physiology; DOI: 10.3389/fphys.2022.1033075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bueno R, Andersen LL, Calatayud J, Casaña J, Smith L, Jacob L, Koyanagi A, López-Gil JF & Del Pozo Cruz B (2022). Longitudinal association of handgrip strength with all-cause and cardiovascular mortality in older adults using a causal framework. Experimental Gerontology 168, 111951. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M & Kroemer G. (2013). The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losa-Reyna J, Alcazar J, Carnicero J, Alfaro-Acha A, Castillo-Gallego C, Rosado-Artalejo C, Rodríguez-Mañas L, Ara I & García-García FJ. (2022). Impact of relative muscle power on hospitalization and all-cause mortality in older adults. The Journals of Gerontology: Series A 77, 781–789. [DOI] [PubMed] [Google Scholar]
- Ma J-J, Ju X, Xu R-J, Wang W-H, Luo Z-P, Liu C-M, Yang L, Li B, Chen J-Q & Meng B. (2019). Telomerase reverse transcriptase and p53 regulate mammalian peripheral nervous system and CNS axon regeneration downstream of c-Myc. Journal of Neuroscience 39, 9107–9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maasar M-F, Turner DC, Gorski PP, Seaborne RA, Strauss JA, Shepherd SO, Cocks M, Pillon NJ, Zierath JR & Hulton AT. (2021). The comparative methylome and transcriptome after change of direction compared to straight line running exercise in human skeletal muscle. Frontiers in Physiology 12, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metter EJ, Talbot LA, Schrager M & Conwit R. (2002). Skeletal muscle strength as a predictor of all-cause mortality in healthy men. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 57, B359–B365. [DOI] [PubMed] [Google Scholar]
- Miwa S, Jow H, Baty K, Johnson A, Czapiewski R, Saretzki G, Treumann A & von Zglinicki T. (2014). Low abundance of the matrix arm of complex I in mitochondria predicts longevity in mice. Nature Communications 5, 3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley CB, Mumford PW, Kephart WC, Haun CT, Holland AM, Beck DT, Martin JS, Young KC, Anderson RG & Patel RK. (2017). Aging in rats differentially affects markers of transcriptional and translational capacity in soleus and plantaris muscle. Frontiers in Physiology 8, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Ato S, Knudsen JR, Henriquez-Olguin C, Li Z, Wakabayashi K, Suginohara T, Higashida K, Tamura Y, Nakazato K, Jensen TE & Ogasawara R (2021). c-Myc overexpression increases ribosome biogenesis and protein synthesis independent of mTORC1 activation in mouse skeletal muscle. American Journal of Physiology Endocrinology and Metabolism 321, E551–E559. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Lu AT, Li CZ, Haghani A, Sandoval-Sierra JV, Wu Y, Williams RW & Horvath S. (2022). Genetic loci and metabolic states associated with murine epigenetic aging. eLife 11, e75244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach KA, Dimet-Wiley AL, Wen Y, Brightwell CR, Latham CM, Dungan CM, Fry CS & Watowich SJ (2022). Late-life exercise mitigates skeletal muscle epigenetic aging. Aging Cell 21, e13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach KA, Dungan CM, Kosmac K, Voigt TB, Tourville TW, Miller MS, Bamman MM, Peterson CA & Toth MJ. (2019). Cores of reproducibility in physiology (CORP): fiber typing human skeletal muscle with fluorescent immunohistochemistry. Journal of Applied Physiology 127, 1632–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach KA, Liu Z, Jude B, Figueiredo VC, Wen Y, Khadgi S, Lim S, Silva FM da, Greene NP, Lanner JT, McCarthy JJ, Vechetti IJ & Walden F von (2022). Multi-transcriptome analysis following an acute skeletal muscle growth stimulus yields tools for discerning global and MYC regulatory networks. Journal of Biological Chemistry; DOI: 10.1016/j.jbc.2022.102515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach KA, Mobley CB, Zdunek CJ, Frick KK, Jones SR, McCarthy JJ, Peterson CA & Dungan CM. (2020). Muscle memory: myonuclear accretion, maintenance, morphology, and miRNA levels with training and detraining in adult mice. Journal of Cachexia, Sarcopenia and Muscle 11, 1705–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Takizawa N, Narita M, Ichisaka T & Yamanaka S. (2010). Promotion of direct reprogramming by transformation-deficient Myc. Proceedings of the National Academy of Sciences 107, 14152–14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann B, Segel M, Ghosh T, Zhao C, Tourlomousis P, Young A, Förster S, Sharma A, Chen CZ-Y & Cubillos JF. (2021). Myc determines the functional age state of oligodendrocyte progenitor cells. Nature Aging 1, 826–837. [DOI] [PubMed] [Google Scholar]
- Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM & Harris TB. (2006). Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 61, 72–77. [DOI] [PubMed] [Google Scholar]
- Nie T, Tao K, Zhu L, Huang L, Hu S, Yang R, Xu P, Mao Z & Yang Q. (2021). Chaperone-mediated autophagy controls the turnover of E3 ubiquitin ligase MARCHF5 and regulates mitochondrial dynamics. Autophagy 17, 2923–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino K, Toyoda M, Yamazaki-Inoue M, Fukawatase Y, Chikazawa E, Sakaguchi H, Akutsu H & Umezawa A. (2011). DNA methylation dynamics in human induced pluripotent stem cells over time. PLoS Genetics 7, e1002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo A, Reddy P & Belmonte JCI. (2016a). Anti-aging strategies based on cellular reprogramming. Trends in Molecular Medicine 22, 725–738. [DOI] [PubMed] [Google Scholar]
- Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M & Beyret E. (2016b). In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167, 1719–1733. e1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olova N, Simpson DJ, Marioni RE & Chandra T. (2019). Partial reprogramming induces a steady decline in epigenetic age before loss of somatic identity. Aging Cell 18, e12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Liu J, Li X, Xiao G, Wang H, Yang G, Li Y, Tang SC, Qin S & Du N. (2018). MYC and DNMT 3A‐mediated DNA methylation represses micro RNA‐200b in triple negative breast cancer. Journal of Cellular and Molecular Medicine 22, 6262–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise G, Brose AN & Tarnopolsky MA. (2005a). Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Experimental Gerontology 40, 173–180. [DOI] [PubMed] [Google Scholar]
- Parise G, Phillips SM, Kaczor JJ & Tarnopolsky MA. (2005b). Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radical Biology and Medicine 39, 289–295. [DOI] [PubMed] [Google Scholar]
- Park Y, Figueroa ME, Rozek LS & Sartor MA. (2014). MethylSig: a whole genome DNA methylation analysis pipeline. Bioinformatics 30, 2414–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Torner M, Carrero D, Pérez-Silva JG, Álvarez-Puente D, Roiz-Valle D, Bretones G, Rodríguez D, Maeso D, Mateo-González E & Español Y. (2022). Comparative genomics of mortal and immortal cnidarians unveils novel keys behind rejuvenation. Proceedings of the National Academy of Sciences 119, e2118763119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MD, Collins S, Meier HCS, Brahmsteadt A & Faul JD (2022). Grip strength is inversely associated with DNA methylation age acceleration. Journal of Cachexia, Sarcopenia and Muscle; DOI: 10.1002/jcsm.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BE, Williams JP, Gustafsson T, Bouchard C, Rankinen T, Knudsen S, Smith K, Timmons JA & Atherton PJ. (2013). Molecular networks of human muscle adaptation to exercise and age. PLoS Genetics 9, e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon NJ, Gabriel BM, Dollet L, Smith JA, Puig LS, Botella J, Bishop DJ, Krook A & Zierath JR. (2020). Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nature Communications 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CJ, Lodh A, Choi J-H & Van Riggelen J. (2019). MYC deregulates TET1 and TET2 expression to control global DNA (hydroxy) methylation and gene expression to maintain a neoplastic phenotype in T-ALL. Epigenetics & Chromatin 12, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CJ, Zheng W, Lodh A, Yevtodiyenko A, Liefwalker D, Li H, Felsher DW & van Riggelen J. (2017). DNMT3B overexpression contributes to aberrant DNA methylation and MYC-driven tumor maintenance in T-ALL and Burkitt’s lymphoma. Oncotarget 8, 76898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov DV, Makhnovskii PA, Shagimardanova EI, Gazizova GR, Lysenko EA, Gusev OA & Vinogradova OL. (2019). Contractile activity-specific transcriptome response to acute endurance exercise and training in human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism 316, E605–E614. [DOI] [PubMed] [Google Scholar]
- Powers SK, Ji LL & Leeuwenburgh C. (1999). Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Medicine and Science in Sports and Exercise 31, 987–997. [DOI] [PubMed] [Google Scholar]
- Price FD, Von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J & Rudnicki MA. (2014). Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nature Medicine 20, 1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader EP and Baker BA. (2022). Elevated muscle mass accompanied by transcriptional and nuclear altearations several months following cessation of resistance training in rats. Physiological Reports 10, e15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Fan J, Zheng R, Wan C, Mei S, Wu Q, Sun H, Brown M, Zhang J & Meyer CA. (2020). Lisa: inferring transcriptional regulators through integrative modeling of public chromatin accessibility and ChIP-seq data. Genome Biology 21, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Kaneko T, Tahara S, Nakamoto H, Ohno H, SASVari M, Nyakas C & Goto S. (1999). The effect of exercise training on oxidative damage of lipids, proteins, and DNA in rat skeletal muscle: evidence for beneficial outcomes. Free Radical Biology and Medicine 27, 69–74. [DOI] [PubMed] [Google Scholar]
- Raffaello A, Milan G, Masiero E, Carnio S, Lee D, Lanfranchi G, Goldberg AL & Sandri M. (2010). JunB transcription factor maintains skeletal muscle mass and promotes hypertrophy. Journal of Cell Biology 191, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M, Coleman Z, Curley M, Nityanandam A, Platt A, Robles-Murguia M, Jiao J, Finkelstein D, Wang Y-D & Xu B. (2021). Proteasome stress in skeletal muscle mounts a long-range protective response that delays retinal and brain aging. Cell Metabolism 33, 1137–1154. e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath S, Sharma R, Gupta R, Ast T, Chan C, Durham TJ, Goodman RP, Grabarek Z, Haas ME & Hung WH. (2021). MitoCarta3. 0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Research 49, D1541–D1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raue U, Slivka D, Minchev K & Trappe S. (2009). Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. Journal of Applied Physiology 106, 1611–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC & Trappe S. (2012). Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. Journal of Applied Physiology 112, 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KF & Fielding RA. (2012). Skeletal muscle power: a critical determinant of physical functioning in older adults. Exercise and Sport Sciences Reviews 40, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas DA, Lessard SJ, Rice NP, Lustgarten MS, So K, Goodyear LJ, Parnell LD & Fielding RA. (2014). Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF‐1 signaling. The FASEB Journal 28, 4133–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, & Nair KS. (2017). Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metabolism 25, 581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Nuevo A, Torres-Sanchez A, Duran JM, De Guirior C, Martínez-Zamora MA & Böke E (2022). Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature 607, 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux AE, Zhang C, Paw J, Zavala-Solorio J, Malahias E, Vijay T, Kolumam G, Kenyon C & Kimmel JC (2022). Diverse partial reprogramming strategies restore youthful gene expression and transiently suppress cell identity. Cell Systems 13, 574–587.e11. [DOI] [PubMed] [Google Scholar]
- Ruple BA, Godwin JS, Mesquita PHC, Osburn SC, Vann CG, Lamb DA, Sexton CL, Candow DG, Forbes SC, Frugé AD, Kavazis AN, Young KC, Seaborne RA, Sharples AP & Roberts MD (2021). Resistance training rejuvenates the mitochondrial methylome in aged human skeletal muscle. The FASEB Journal 35, e21864. [DOI] [PubMed] [Google Scholar]
- Rutledge EA, Lindström NO, Michos O & McMahon AP. (2020). Genetic manipulation of ureteric bud tip progenitors in the mammalian kidney through an Adamts18 enhancer driven tet-on inducible system. Developmental Biology 458, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm A, Bens M, Szafranski K, Holtze S, Groth M, Görlach M, Calkhoven C, Müller C, Schwab M & Kraus J. (2018). Long-lived rodents reveal signatures of positive selection in genes associated with lifespan. PLoS Genetics 14, e1007272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailani MR, Halling JF, Møller HD, Lee H, Plomgaard P, Pilegaard H, Snyder MP & Regenberg B. (2019). Lifelong physical activity is associated with promoter hypomethylation of genes involved in metabolism, myogenesis, contractile properties and oxidative stress resistance in aged human skeletal muscle. Scientific Reports 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar TJ, Quarta M, Mukherjee S, Colville A, Paine P, Doan L, Tran CM, Chu CR, Horvath S & Qi LS. (2020). Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nature Communications 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaborne RA, Strauss J, Cocks M, Shepherd S, O’Brien TD, van Someren KA, Bell PG, Murgatroyd C, Morton JP, Stewart CE & Sharples AP. (2018). Human skeletal muscle possesses an epigenetic memory of hypertrophy. Scientific Reports 8, 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely S. (1980). Possible reasons for the high resistance of muscle to cancer. Medical Hypotheses 6, 133–137. [DOI] [PubMed] [Google Scholar]
- Sheng J-J & Jin J-P. (2016). TNNI1, TNNI2 and TNNI3: Evolution, regulation, and protein structure–function relationships. Gene 576, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsson S & Gurdon J. (2004). DNA demethylation is necessary for the epigenetic reprogramming of somatic cell nuclei. Nature Cell Biology 6, 984–990. [DOI] [PubMed] [Google Scholar]
- Slivka D, Raue U, Hollon C, Minchev K & Trappe S. (2008). Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. American journal of physiology Regulatory, Integrative and Comparative Physiology 295, R273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small L, Ingerslev LR, Manitta E, Laker RC, Hansen AN, Deeney B, Carrié A, Couvert P & Barrès R. (2021). Ablation of DNA-methyltransferase 3A in skeletal muscle does not affect energy metabolism or exercise capacity. PLoS Genetics 17, e1009325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuder AJ, Kavazis AN, Min K & Powers SK. (2011). Exercise protects against doxorubicin-induced oxidative stress and proteolysis in skeletal muscle. Journal of Applied Physiology 110, 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC & Bamman MM. (2016). Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. American Journal of Physiology-Endocrinology and Metabolism 310, E652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec MJ, Mayhew DL & Bamman MM. (2015). The effects of age and resistance loading on skeletal muscle ribosome biogenesis. Journal of Applied Physiology 119, 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DK, Deutzmann A, Yarbrough J, Krishnan MS, Gouw AM, Bellovin DI, Adam SJ, Liefwalker DF, Dhanasekaran R & Felsher DW (2022). MYC oncogene elicits tumorigenesis associated with embryonic, ribosomal biogenesis, and tissue-lineage dedifferentiation gene expression changes. Oncogene 41, 4960–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K & Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Talar K, Hernández-Belmonte A, Vetrovsky T, Steffl M, Kałamacka E & Courel-Ibáñez J. (2021). Benefits of resistance training in early and late stages of frailty and sarcopenia: a systematic review and meta-analysis of randomized controlled studies. Journal of Clinical Medicine 10, 1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JR, Stout JR, Jajtner AR, Church DD, Beyer KS, Oliveira LP, La Monica MB, Riffe JJ, Muddle TW & Baker KM. (2016). Resistance exercise increases intramuscular NF-κb signaling in untrained males. European Journal of Applied Physiology 116, 2103–2111. [DOI] [PubMed] [Google Scholar]
- Trenerry MK, Carey KA, Ward AC & Cameron-Smith D. (2007). STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. Journal of Applied Physiology 102, 1483–1489. [DOI] [PubMed] [Google Scholar]
- Tumasian RA, et al. (2021). Skeletal muscle transcriptome in healthy aging. Nature Communications 12, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubaida-Mohien C, Gonzalez-Freire M, Lyashkov A, Moaddel R, Chia CW, Simonsick EM, Sen R & Ferrucci L. (2019). Physical activity associated proteomics of skeletal muscle: being physically active in daily life may protect skeletal muscle from aging. Frontiers in Physiology 10, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal E & Jackson M. (1998). C-myc is expressed in mouse skeletal muscle nuclei during post-natal maturation. The International Journal of Biochemistry & Cell Biology 30, 811–821. [DOI] [PubMed] [Google Scholar]
- Verschoor CP, Lin DT, Kobor MS, Mian O, Ma J, Pare G, Ybazeta G. (2021) Epigenetic age is associated with baseline and 3-year change in frailty in the Canadian Longitudinal Study on Aging. Clinical Epigenetics 30, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggars MR, Sutherland H, Lanmüller H, Schmoll M, Bijak M, Jarvis JC. (2022) Adaptation of the transcriptional response to resistance exercise over 4 weeks of daily training. The FASEB Journal 37, e22686. [DOI] [PubMed] [Google Scholar]
- Villivalam S, Ebert SM, Lim HW, Kim J, You D, Jung BC, Palacios HH, Tcheau T, Adams CM & Kang S (2021). A necessary role of DNMT3A in endurance exercise by suppressing ALDH1L1-mediated oxidative stress. The EMBO Journal 40, e106491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Walden F, Casagrande V, Östlund Farrants A-K & Nader GA. (2012). Mechanical loading induces the expression of a Pol I regulon at the onset of skeletal muscle hypertrophy. American Journal of Physiology-Cell Physiology 302, C1523–C1530. [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A & Dokal I. (2008). Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proceedings of the National Academy of Sciences 105, 8073–8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MA, Lamon S & Russell AP. (2012). The regulation and function of the striated muscle activator of rho signaling (STARS) protein. Frontiers in Physiology 3, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton RG, Dungan CM, Long DE, Tuggle SC, Kosmac K, Peck BD, Bush HM, Villasante Tezanos AG, McGwin G & Windham ST. (2019). Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: A randomized, double‐blind, placebo‐controlled, multicenter trial: The MASTERS trial. Aging Cell 18, e13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ros RR, Martinez-Redondo P, Ma Z, Shi L, Xue Y, Guillen-Guillen I, Huang L, Hishida T & Liao H-K. (2021). In vivo partial reprogramming of myofibers promotes muscle regeneration by remodeling the stem cell niche. Nature Communications 12, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xie LY, Allan S, Beach D & Hannon GJ. (1998). Myc activates telomerase. Genes & Development 12, 1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Dungan CM, Mobley CB, Valentino T, von Walden F & Murach KA (2021). Nucleus type-specific DNA methylomics reveals epigenetic “memory” of prior adaptation in skeletal muscle. Function 2, zqab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DW, Baehr LM, Marcotte GR, Chason CM, Tolento L, Gomes AV, Bodine SC & Baar K. (2016). Acute resistance exercise activates rapamycin‐sensitive and‐insensitive mechanisms that control translational activity and capacity in skeletal muscle. The Journal of Physiology 594, 453–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw PF & Hesketh JE. (1992). Expression of c-myc and c-fos in rat skeletal muscle. Evidence for increased levels of c-myc mRNA during hypertrophy. Biochemical Journal 281, 143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger ME, Caserotti P, Cauley JA, Boudreau RM, Piva SR, Cawthon PM, Orwoll ES, Ensrud KE, Kado DM, Strotmeyer ES, & Osteoporotic Fractures in Men (MrOS) Research Group (2022). Lower leg power and grip strength are associated with increased fall injury risk in older men: the ssteoporotic fractures in men study. The Journals of Gerontology: Series A, glac122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenos D, Mecocci P & Boccardi V (2022). A blast from the past: To tame time with metformin. Mechanisms of Ageing and Development 208, 111743. [DOI] [PubMed] [Google Scholar]
- Zhang B, Trapp A, Kerepesi C & Gladyshev VN. (2022a). Emerging rejuvenation strategies—Reducing the biological age. Aging Cell 21, e13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Englund DA, Aversa Z, Jachim SK, White TA & LeBrasseur NK (2022). Exercise counters the age- related accumulation of senescent cells. Exercise and Sport Sciences Reviews 50, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X et al. (2022). Characterization of cellular senescence in aging skeletal muscle. Nature Aging 2, 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 Gene expression in the soleus muscle of OKSM versus control mice (Wang et al., 2021, GEO GSE148911)
Supplemental Table 2 Gene expression in the mouse soleus after 8 weeks of PoWeR from 22–24 months of age versus sedentary controls (Dungan and Brightwell et al. 2022, GEO GSE198652)
Supplemental Table 3 Genes altered by long-term endurance training in human females that overlap with MitoCarta 3.0 (DEGs from Chapman et al. 2020)
Supplemental Table 4 Lisa/Cistrome analysis using up- and downregulated DEGs (adj. p<0.05) in the soleus muscle after late-life PoWeR
Supplemental Table 5 Gene expression in the mouse soleus of MYC versus controls
Supplemental Table 6 Overlap of genes altered by MYC and OKSM expression in the mouse soleus
Supplemental Table 7 Gene expression in the mouse quadriceps of MYC versus controls
Supplemental Table 8 DNA methylation in the mouse soleus muscle DNA after a pulse of MYC
Supplemental Table 9 Genes with CpGs that overlap between MYC induction in the soleus muscle and myonuclear DNA following 8 weeks of PoWeR in aged mice (Dungan and Brightwell et al. 2022)


