Abstract
Objective assays of human cochlear synaptopathy (CS) have been challenging to develop. It is suspected that relative summating potential (SP) changes are different in listeners with CS. In this proof-of-concept study, young, normal-hearing adults were recruited and assigned to a low/high-risk group for having CS based on their extended audiograms (9–16 kHz). SPs to paired-clicks with varying inter-click intervals isolated non-refractory receptor components of cochlear activity. Abrupt increases in SPs to paired- vs single-clicks were observed in high-risk listeners. Critically, exaggerated SPs predicted speech-in-noise and subjective hearing abilities, suggesting relative SP changes to rapid clicks might help identify putative synaptopathic listeners.
1. Introduction
Recent animal studies reveal that intense noise overexposure can lead to cochlear neuronal degeneration, even when hair cells recover and thresholds return to normal.1 Up to 50% loss of synapses between inner hair cells and cochlear nerve fibers can occur in noise-exposed or aging ears without prominent hair cell loss or auditory threshold elevation.2,3 Hence, this cochlear synaptopathy is “hidden” because it is not detected by routine behavioral or electrophysiological measures (e.g., clinical audiogram thresholds).4 Moreover, although loss of cochlear synapses can happen immediately after extreme noise exposure, the subsequent degeneration of the cochlear nerve fibers develops over a more protracted time course.1 Neural degeneration following cochlear synaptopathy is thought to manifest in poor word-recognition scores,3,5–7 particularly in noise,8–11 but not pure-tone detection in quiet. This is partly because cochlear neurons with high thresholds and low spontaneous rates are preferentially affected by cochlear synaptopathy,12,13 though this does not apply to all species.14
One common way to diagnose cochlear synaptopathy in noise-exposed or aged animals with normal-hearing thresholds is by using the suprathreshold amplitude of auditory brainstem response (ABR) wave I, which represents the summed activity of cochlear neurons and correlates with loss of cochlear synapses in corresponding frequency regions.1,2,15 However, using ABR wave I amplitude as a diagnostic tool in humans is challenging16,17 and has provided mixed results.18 The low-amplitude response is highly variable and sometimes unmeasurable at the scalp. On the other hand, the summating potential (SP), reflecting aggregated hair cell receptor activity,19 is larger in listeners with poor speech-in-noise scores7 or with greater acoustic overexposure.8 SP enhancement was also reported in listeners immediately after recreational acoustic overexposure.20 Given that the SP reflects hair cell-dendritic integrity rather than the output of the auditory nerve (cf. wave I), it may be more sensitive to synaptopathic pathology. In normal-hearing human listeners, SP amplitudes to short duration stimuli are found to be reducing as stimulus repetition rate increases.21 In contrast, as excitatory post-synaptic potentials (EPSPs) from cochlear nerve terminals under the inner hair cells may contribute to SP,8 the loss of a negative EPSP could enhance SP in cochlear synaptopathy. SP enhancement is also reported in synaptopathic mice with attenuated middle-ear-muscle reflex (MEMR).22 However, like ABR wave I, a critical limitation to overcome is the low-amplitude nature of the SP, which hinders the use of absolute measures of the response as a viable diagnostic.
Here, in this proof-of-concept study, we evaluated whether relative changes in SP to rapid auditory stimuli might serve as a new potential assay of cochlear synaptopathy. We recruited young participants with normal and similar hearing thresholds in the normal audiometric range (250–8000 Hz) but who differed in their extended high-frequency (EHF; 9–16 kHz) thresholds. We divided the sample into low- and high-risk groups based on their average EHF thresholds of both ears. We then measured SPs elicited by standard single-clicks and paired-clicks23 (in separate stimulus conditions). In this paired-click paradigm, two clicks were presented within a short interval (e.g., 0.1–4.0 ms) to boost the generation of SP. As ABR wave I is the sum of action potentials generated by auditory neurons upon activation by a stimulus, there is a refractory period in which neurons are not excitable again in response to another stimulus. Unlike ABR wave I, SP is not limited by refractoriness in response to the first click of a pair. However, SP elicited by the second click of a pair is still regulated by synaptic processes due to neurotransmitter release24 and re-uptake.25 When a click is placed within 1 ms of a preceding click (i.e., within the absolute refractory period of the auditory nerve), short-term adaptation inhibits a (neural) response to the second click, which should minimize the ABR to the second click and isolate activities of (pre-neural) afferent spiral ganglion dendrites.23 Hence, SP stimulated with double-clicks may provide a more “pure” measurement of receptor and dendritic response integrity. Instead of measuring absolute SP amplitude, we were also interested to test whether relative measures of the SP to rapid auditory stimuli, which minimize inter-subject differences, would provide a more sensitive assay of putative cochlear synaptopathy in humans.
2. Materials and methods
2.1. Participants
The sample included N = 18 young participants with age range 23–33 years [mean (M) = 25, standard deviation (SD) = 2.8 years; 10 females]. All spoke American English and had normal hearing [20 dB hearing level (HL); 250–8000 Hz] when tested by conventional audiometric standards. Each gave written informed consent in compliance with the University of Memphis institutional review board (IRB).
2.2. Auditory test battery
We obtained subjective hearing acuity and noise-exposure history of each participant by using the Lifetime Exposure to Noise and Solvents Questionnaire (LENS-Q)26 and two additional noise questionnaires (see Appendix 1 of Liberman et al.8). Moreover, we conducted otoscopy, ipsilateral acoustic reflex thresholds (ARTs), and distortion product otoacoustic emissions (DPOAEs) in participants’ right ears according to standard audiological conventions. The final ART was defined as the averaged threshold obtained across the four elicitor frequencies (0.5, 1, 2, and 4 kHz).
2.3. Audiometric thresholds and QuickSIN
We measured pure-tone air-conduction thresholds bilaterally from 0.25 to 16 kHz at octave intervals and also at 9, 10, 11.2, 12.5, and 14 kHz. For 8 kHz and below, ER-3A inserts were used for testing; for EHFs above 8 kHz, circumaural headphones were used (Sennheiser HDA 200, Wedemark, Germany) that were specialized for high-frequency audiometry. We divided participants into two groups, low- vs high-risk, based on their average EHF thresholds. High-risk participants (N = 9, 4 females) had an average EHF threshold of 12.9 ± 8.24 dB HL, while low-risk participants (N = 9, 6 females) had an average EHF threshold of −1.71 ± 2.91 dB HL.
To assess participants’ speech perception in noise, we used the Quick Speech-in-Noise (QuickSIN) test.27 Participants heard lists of six sentences, each with five target keywords spoken by a female talker embedded in four-talker babble noise. Target sentences were presented binaurally at 70 dB sound pressure level (SPL) at signal-to-noise ratios (SNRs) decreasing in 5 dB steps from 25 dB (relatively easy) to 0 dB (relatively difficult). SNR-loss scores reflect the difference between a participant’s SNR-50 (i.e., SNR required for 50% keyword recall) and the average SNR threshold for normal-hearing adults (i.e., 2 dB).27 Higher scores indicate poorer SIN performance. Participants’ scores ranged from −4 to 2 dB of SNR-loss (M = −0.03, SD = 1.74). High-risk participants’ mean score was 0.6 ± 1.19 SNR-loss, whereas low-risk participants’ mean score was −0.6 ± 2.02 SNR-loss (i.e., 1 dB better performance). The QuickSIN scores of both groups fell within the normative range for normal-hearing individuals.
2.4. Stimuli and electrophysiology
Standard single-clicks with a duration of 0.1 ms and inter-stimulus interval of 125 ms (repetition rate = 8 Hz) were presented monaurally at 80 dB SPL to participants’ right ears. Acoustic stimuli were delivered in alternating polarity via ER-3A earphones. A vertical montage (Fpz to tiptrode) was used to record ABRs. A tiptrode (ear canal) reference was used to enhance the more peripheral (i.e., cochlear) components of the electrocochleogram (ECochG) and better visualize the pre-synaptic SP.8,28 Inter-electrode impedance was kept below 2 kΩ. Electrical responses were amplified 100 000 x, sampled at 10 kHz, and filtered with a 50–3000 Hz bandpass plus 60 Hz notch filter. Total 2500 artifact-free sweeps were averaged to obtain low-noise ECochGs.
Following standard single-clicks, we also recorded ECochGs from participants using a paired-click paradigm.23 In this paradigm, paired-click stimuli with seven different inter-click intervals (ICIs) of 4.0, 2.0, 1.0, 0.8, 0.4, 0.2, and 0.1 ms were presented (in separate stimulus sessions and random condition order). Stimulus delivery and recording settings were kept the same as the single-clicks. The time intervals were chosen to be shorter than, encompass, and exceed the duration of absolute (1.0–1.2 ms) and relative (4–5 ms) refractory periods of the auditory nerve.29 This resulted in a total of eight ECochG/ABR waveforms per listener.
2.5. ECochG waveform analysis
All ECochG waveforms were processed and analysed using customized scripts in python 3.9.7. First, each waveform per participant and condition (epoched from −12 to 12 ms relative to the first click onset) was low-pass filtered at 2500 Hz to remove high-frequency noise. For waveform, we then performed baseline correction by obtaining an average amplitude at −5 to 0 ms and subtracting this average amplitude from all amplitudes within the epoch window. Next, we defined SP time range as 0.5–1.3 s after click onset as the reported mean latency of SP is 0.97 (±0.1) ms, while wave I mean latency is 1.83 (±0.1) ms.23 This SP time range and latency are also consistent with definitions in other recent studies on ECochG/ABR and synaptopathy.8,9 Subsequently, we fit a logarithmic curve [refer to Fig. 2(A)] using log*(SP time) and original amplitudes of SP time by performing a polynomial fitting (degree = 1) with “numpy” package in python,
| (1) |
where SP time is the sampled time vector within 0.5–1.3 ms, a is the fitted slope, b is the fitted constant, and f′(x) is the predicted amplitudes of SP time. The SP inflection point was estimated at 85% of the min-max rise of the fitted curve, as
| (2) |
where ŷ is the estimated SP inflection point. Finally, SP amplitudes were defined as the difference between the estimated SP inflection point and SP baseline, which was defined as the lowest amplitude within −0.5–0.5 ms (cf. Ref. 8). Meanwhile, if the fitted slope (i.e., a) was ≤0, SP amplitude was defined as the mean of original amplitudes in SP time subtracting SP baseline. In a few instances where SP amplitudes were lower than SP baseline, SP measures were excluded and considered no response (i.e., missing data). To measure relative changes in SP with increasing click ICI, SP amplitudes for each ICI condition were normalized to the SP amplitude of single-clicks per participant to obtain an SP ratio. This differential metric allowed us to assess changes in the SP within each listener, thus, avoiding confounds of absolute measures.
Fig. 2.
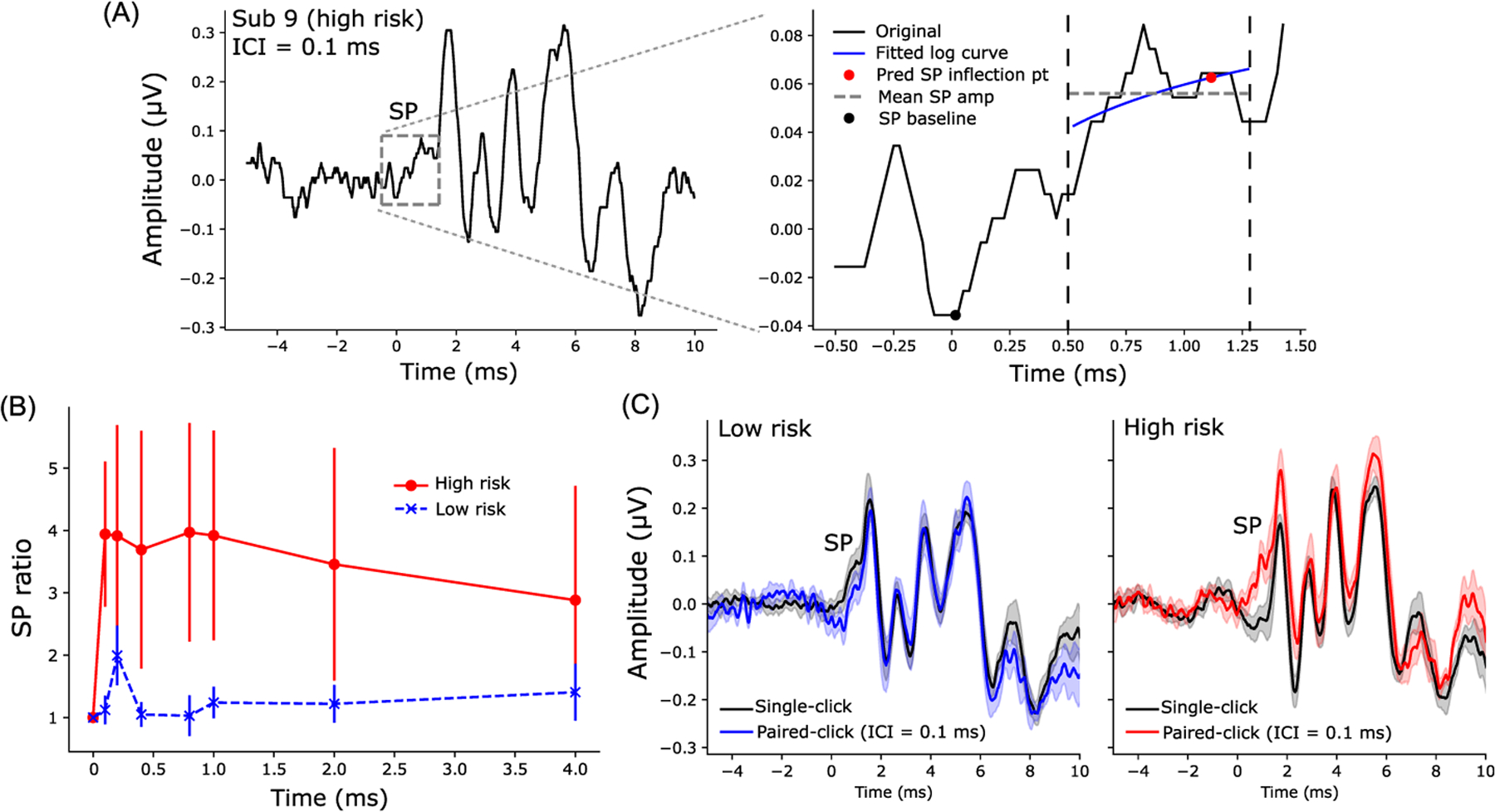
SP ratio (SP amplitudes to paired-clicks/SP amplitudes to single-clicks) were larger in the high- compared to low-risk group. (A) An example of ECochG/ABR recorded from a representative high-risk subject (left panel; paired-click response, ICI = 0.1 ms). The SP is labeled with a gray box and enlarged in the right panel. A logarithmic curve (blue line) was fit from 0.5 to 1.3 ms (the region within two dashed vertical lines) to predict the SP inflection point (red circle) at 85% of min-max amplitude. SP amplitude was measured as the difference between the predicted SP inflection point and the SP baseline (minimum amplitude within −0.5 to 0.5 ms, black circle). In some cases when the fitted curve had a slope of ≤0, SP amplitude was measured as the difference of the mean amplitude within 0.5–1.3 ms (gray dashed line) and the SP baseline. (B) Increased SP amplitudes to paired-clicks were observed in high-risk participants when normalized to the SP amplitude of single-click (within each participant). (C) Grand average ECochGs to single- and paired-clicks (ICI = 0.1 ms) in low-risk (left) and high-risk (right) groups. Error bars and shaded area = ±s.e.m.
2.6. Statistical analysis
To compare audiometric thresholds and behavioral measures between low- and high-risk groups, we used non-parametric Mann-Whitney U test (“pingouin” package in python). We performed a two-way, mixed-model analysis of variance (ANOVA) (participants = random factor) using “lme4” package in Rstudio to compare SP ratios across the two main factors (ICI; risk group). Initial diagnostics were performed using residual and Q-Q plots to assess heteroscedasticity and normality of data. Effect sizes are reported as . To assess pairwise linear relations between electrophysiological measures (i.e., normalized SP amplitudes or SP ratios) and behavioral measures (i.e., QuickSIN, LENS-Q, average EHF thresholds, average EHF threshold differences between ears, subjective acuity rating, and ARTs), we used Spearman’s correlations (“scipy” package in python).
3. Results
3.1. Audiometry, QuickSIN, and self-reported hearing acuity
Behavioral measures, including normal and EHF thresholds, ARTs, QuickSIN, LENS-Q scores, and subjective acuity ratings, are shown for low- vs high-risk listeners in Fig. 1. Average EHF thresholds of the high-risk group were worse (Mann–Whitney U test, U-val = 0, p < 0.05) than the low-risk group, although auditory thresholds of both groups fell within the clinically normal range (i.e., better than 20 dB HL). The high-risk group also showed more than 12 dB asymmetry between left and right thresholds for 9–16 kHz. Asymmetry in EHFs, however, was only 3.8 dB in the low-risk group. Asymmetry in EHFs associated strongly with average EHF thresholds (Spearman’s r = 0.59, p = 0.01). Meanwhile, differences in ipsilateral ARTs, QuickSIN, and LENS-Q scores were not significant when compared across the low- and high-risk groups [Figs. 1(B)–1(D)]. Average subjective acuity rating [Fig. 1(E)] was significantly lower (i.e., poorer hearing sensitivity, U-val = 0.69, p = 0.01) in the high-risk group.
Fig. 1.
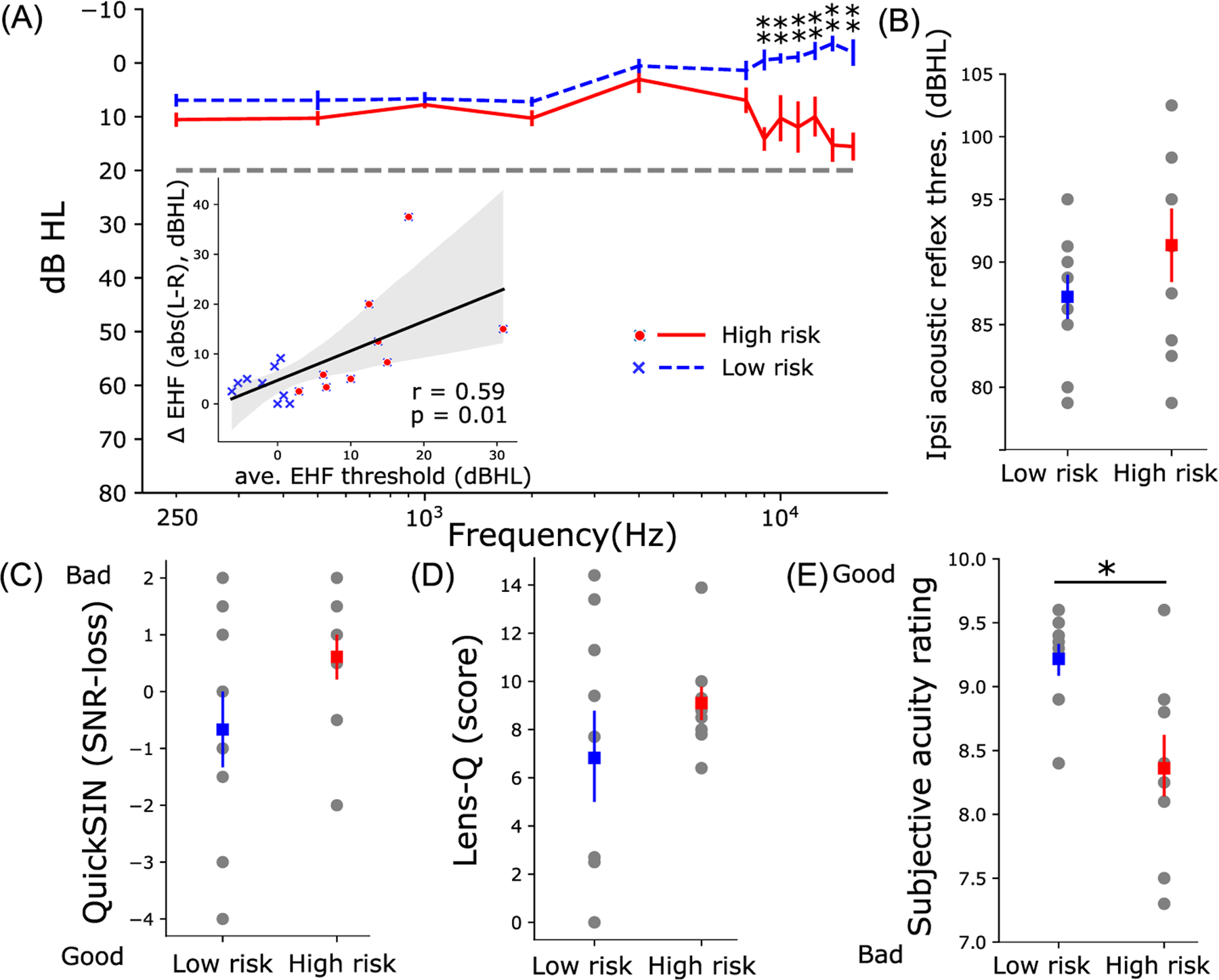
High-risk listeners have poorer and more asymmetric auditory thresholds at extended high- frequencies (EHF; 9–16 kHz) and poorer subjective acuity than low-risk listeners. (A) Low- and high-risk groups have similar auditory thresholds in the normal audiometric range (250–8000 Hz) indicative of “hidden” hearing loss (HHL). For EHFs, thresholds of high-risk participants were significantly poorer (Mann-Whitney U test) than low-risk participants. The inset in (A) shows the strong association of average EHF threshold differences between left and right ears (Δ EHF) with average EHF thresholds of both ears. Despite trends, risk groups did not differ in ipsilateral ARTs (B), QuickSIN (C), or LENS-Q (D) noise-exposure scores. (E) Subjective acuity ratings were better in the low- vs high-risk group (Mann–Whitney U test, U-val = 69, p = 0.01). Error bars = ±standard error of the mean (s.e.m.); *, p < 0.05; **, p < 0.005; r = Spearman’s correlation; shaded area indicates 95% confidence interval of the regression line.
3.2. Electrocochleography
SP amplitudes, which reflect hair cell receptor potentials,19,30 evoked by standard single- and paired-clicks (ICI = 0.1 ms) are shown in Fig. 2(C). An example of SP quantification using curve fitting (see Sec. 2) is shown in Fig. 2(A). We normalized SP amplitudes of paired-clicks to SP amplitudes of single-clicks per participant to lessen the impact of the inter-subject differences in head size, electrode contact, etc., which confound absolute measures and ECochG amplitude. SP ratios were generally larger (F1,16 = 4.47, p = 0.05, ) in the high-risk than in the low-risk group across ICI conditions [Fig. 2(B)]. Grand average waveforms clearly show the prominent increase in SP amplitude in the high-risk group when evoked by paired-clicks [right panel of Fig. 2(C)].
3.3. Brain-behavior associations
We ran correlations to determine if SP ratios across ICI conditions (0.1–4.0 ms) were related to behavior. Figures 3(A) and 3(B) show that SP ratios at ICI = 0.1 (Spearman’s r = 0.57, p = 0.03) and 0.2 ms (r = 0.6, p = 0.02) were predictive of QuickSIN scores; poorer speech-in-noise performance was associated with larger (i.e., exaggerated) SP ratios. Furthermore, SP ratios at ICI = 1.0 ms were predictive of subjective acuity ratings [Fig. 3(C)]. We also found that subjective acuity ratings had strong associations with average EHF thresholds and average EHF threshold differences between ears [Figs. 3(D) and 3(E)]. These results indicate links between poorer subjective hearing acuity and (i) poorer high-frequency hearing abilities and (ii) more asymmetric high-frequency hearing thresholds. Additional correlation analyses treating average EHF threshold or average inter-aural EHF threshold difference as a continuous variable did not reveal significant associations with SP ratios at various ICIs, though trends of positive associations were observed. Similar correlation analyses were also performed on SP ratios at all ICIs and other behavior measures (e.g., LENS-Q, ipsilateral ART, etc.), but not all correlations were significant, and only significant correlations were reported here.
Fig. 3.
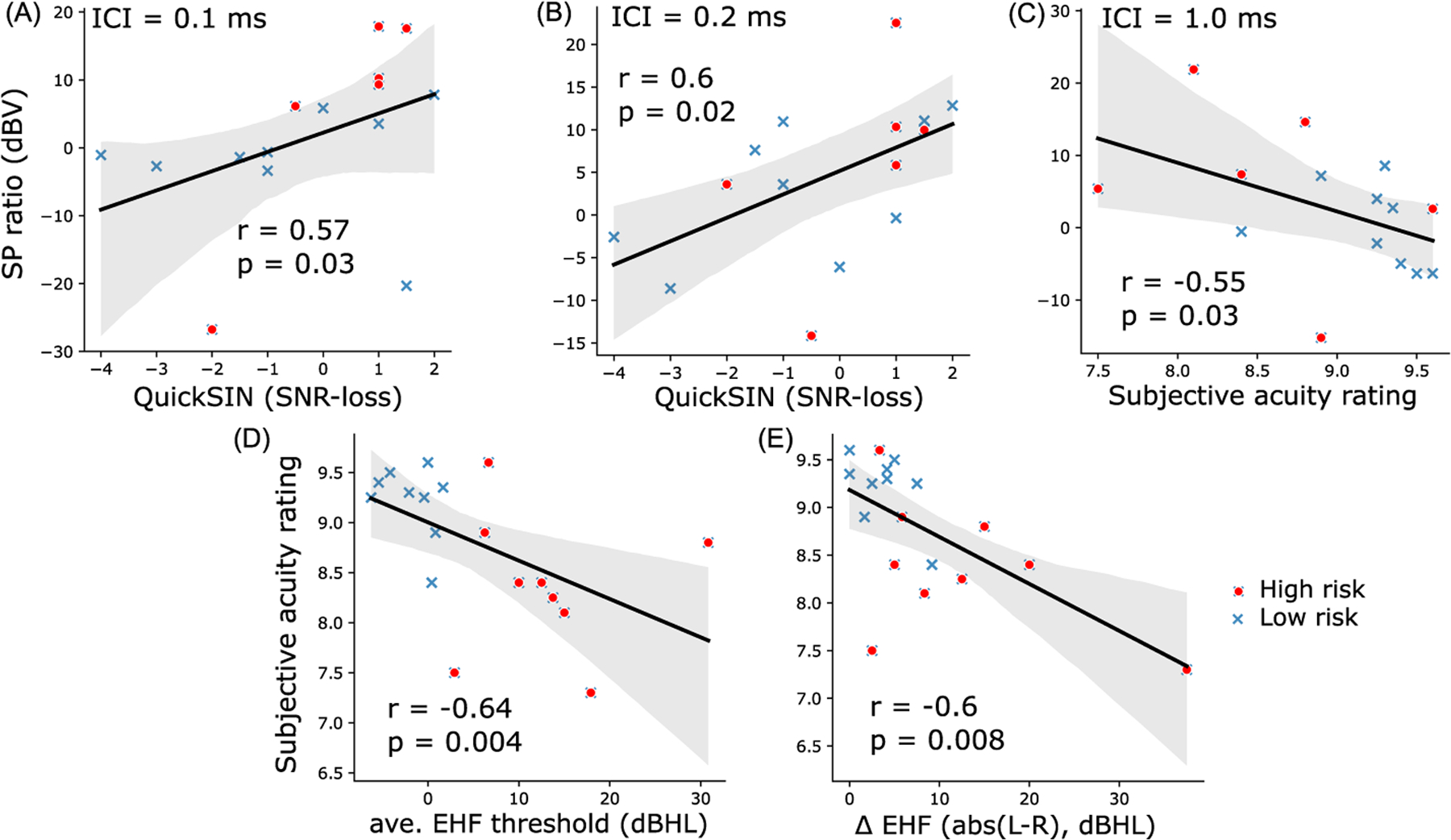
Brain-behavior correlations. [(A) and (B)] SP ratios are related to speech-in-noise perception. SP ratios (plotted in dBV scale) of ICI = 0.1 ms (A) and 0.2 ms (B) correlated significantly with QuickSIN SNR-loss. (C) Larger SP ratios for 1.0 ms predicted poorer subjective acuity rating. [(D) and (E)] EHF hearing abilities are related to subjective hearing acuity. Subjective acuity rating was strongly predictive of average EHF thresholds (D) and average EHF threshold difference between left and right ears (E). r = Spearman’s correlation, shaded area indicates 95% confidence interval of the regression line.
4. Discussion
The present study assessed behavioral and electrophysiological assays of hearing integrity in young, normal-hearing listeners with normal audiograms (i.e., 250–8000 Hz) but who varied in their EHF thresholds (9–16 kHz). Listeners with elevated EHF thresholds were considered the “high-risk” group, suspected of degeneration in the cochlear synapses, i.e., the characteristic of cochlear synaptopathy.8 We presented single- and paired-clicks to both low- and high-risk participants to test if differential changes in the cochlear-initiated SP potential with ICI would differentiate hearing groups and/or participants with degraded auditory processing as indexed by perceptual speech-in-noise measures.
Unlike most studies, which measured SP by human visual inspection,7–9 we automated our SP measurements by fitting a logarithmic curve preceding ABR wave I through the SP “pedestal” to estimate the SP inflection point [Fig. 2(A)]. This method not only saved time but also reduced human error due to inter- and intra-observers’ variability. Additionally, SP amplitudes to paired-clicks were normalized to those of single-clicks to minimize the impact of inter-subject differences in head size, tissue conductivity, etc., that normally confound absolute measures of ECochG quantification. Critically, we observed an increase in SP amplitudes evoked by paired-clicks compared to standard single-clicks in high-risk listeners [Fig. 2(B)]. However, fewer changes in SP amplitudes were found in low-risk listeners when stimulated with either single- or paired-clicks. SP ratios were also predictive of behavioral SIN perception [Figs. 3(A) and 3(B)] and subjective hearing acuity [Fig. 3(C)], confirming the behavioral relevance of our electrophysiological markers.
4.1. SP responses evoked by single- vs paired-click
ABR wave I [i.e., the action potential (AP) of the ECochG] originates from the cochlear nerve. The single-fiber AP component is an “all-or-none” response that exhibits neural refractoriness to a preceding stimulus. There are two observed refractory mechanisms: the absolute refractory period and the relative refractory period. The absolute refractory period of a human cochlear nerve AP is reported to be ~1.025 to 1.2 ms.31 Following AP generation to a first click, the auditory nerve cannot again discharge to a second click within this absolute refractory period. During the relative refractory period, it is difficult, but not impossible, for the cochlear nerve fibers to discharge and generate new APs as it is characterized by an exponentially decreasing threshold for the AP to a resting level with a time constant of 4–5 ms.25 Unlike the AP (i.e., ABR wave I), the SP is a receptor response originating from the hair cells. Consequently, it is not constrained by neural refractoriness. Two types of potentials related to hair cells can contribute to SP: (1) non-linear components in the receptor potentials from inner and/or outer hair cells that are not removed by alternating stimulus polarity and (2) EPSPs from cochlear nerve terminals under the inner hair cells.8 It is possible that the loss of a negative EPSP could contribute to SP enhancement in listeners who have putative cochlear synaptopathy and attenuated MEMR, as reported in synaptopathic mice.22 As the SP is not constrained by refractoriness, paired-clicks with ICIs within the absolute refractory period can thus elicit additional and enhanced SP responses.8 While we did not find attenuated ARTs in our listeners, we suggest the former mechanism might account for the larger SPs we found in high-risk ears.
4.2. Differential changes in SP relate to hearing acuity and speech-in-noise perception
Our results show that SP ratios of rapid auditory stimuli (ICI = 0.1 and 0.2 ms) correlated with QuickSIN [Figs. 3(A) and 3(B)], while SP ratios for ICI = 1.0 ms correlated with subjective acuity rating [Fig. 3(C)]. The rapid ICIs (0.1–0.2 ms) fall within the absolute refractory period of the auditory nerve, while an ICI of 1.0 ms is closer to the limit of absolute refractory period. In addition to not being influenced by neural refractoriness, larger SP ratios for very rapid ICIs in listeners with worse SIN perception (i.e., larger SNR-loss) could be due to unusually small synaptic adaptation in terms of neurotransmitter re-uptake. On the other hand, larger SP ratios at longer ICIs (1.0 ms) correlated with worse hearing acuity. Given that longer intervals contain neural refractory effects, this association could be related to both reduced synaptic and neural effects. It is possible our SP effects are at least partially driven by inner hair cell dysfunction in basal cochlear regions, as implied by the elevated EHF thresholds in high-risk subjects. However, hair cell impairment would tend to reduce electrophysiological responses, whereas we instead see the opposite: enlarged SPs in risk adverse ears. This argues for a neural rather than pure sensory account of our data. We speculate that, in addition to the loss of negative EPSPs, the reduction of synaptic adaptation due to synaptic loss may also contribute to larger SP ratios to very short ICIs in cochlear synaptopathy. In contrast, SP amplitudes to short stimuli were reported to be slightly reducing, because of adaptation, in normal-hearing humans as stimulus repetition rate increased.21 Proper adaptation is important in maintaining normal hearing function because it is a fundamental principle of sensory processing that enables sensory information to be represented adequately32 and remain robust to noise.33
High-risk subjects in Liberman et al.8 who had putative cochlear synaptopathy were shown to have poorer speech discrimination scores when the task was performed under conditions of degradation (e.g., noise, time-compression, reverberation). Listeners in that study were assigned to a high-risk group based on subjective responses on questionnaires related to medical history of ear and hearing function, history of noise exposure, and use of hearing protection. In contrast, in this study, we defined high-risk listeners objectively based on their average EHF thresholds and not noise-exposure history. Arguably, the stark differences in EHFs (without elevated thresholds in the normal audiometric range) suggest the hearing loss in our high-risk ears was far from “hidden.” Still, the association of SP and QuickSIN score or subjective acuity rating in this study and poorer SIN performance in the high-risk group of Liberman’s study suggest that recruiting and assigning subjects into different hearing risk groups based on more difficult SIN perceptual tests or more detailed subjective responses regarding hearing and historical noise exposure might be a better approach.
4.3. SP vs ABR measures of cochlear synaptopathy
Although ABR wave I amplitudes are usually reduced by neural damage, SP amplitudes remain robust in animals with both noise-induced and age-related synaptopathy.2 The literature is, however, highly equivocal, and some studies fail to find associations between ABR wave I amplitude and noise exposure in humans.34–38 Absolute amplitude of ABR wave I is highly variable in humans when recorded at the scalp. This is also true for the SP. However, the fact that SP is a receptor potential and not neural response might render it more sensitive to synaptopathic effects since these listeners usually have intact hair cells but loss of synapses. In addition, our use of relative (i.e., SP ratios comparing responses across stimuli) rather than absolute amplitude measures seems to make it less susceptible to confounding sources of variances and thus sensitive to putative cochlear synaptopathy.
Recently, envelope-following responses (EFRs) recorded to rectangular sinusoidal modulation (RAM) sounds were shown to predict speech-in-noise performance in normal-listening listeners with suspected cochlear synaptopathy.39 It was claimed that RAM EFRs yielded greater diagnostic utility than other metrics, including click-evoked ABRs and MEMRs, in predicting cochlear neural deficits in normal-hearing listeners. EFRs, however, may still be confounded by other unknown deficits in the auditory system since the response generators are located in the rostral brainstem rather than auditory periphery.40 As such, auditory deficits reported in EFRs might not reflect degeneration in cochlear synapses or nerve fibers, per se, but rather more central effects that are entirely independent of cochlear synaptopathy. In contrast, the source of SP is specifically localized to cochlear processes, which are the central etiology of cochlear synaptopathy. As a result, the more direct interpretation of the SP renders it a higher potential than EFRs to be used as a diagnostic tool for cochlear synaptopathy and related neural degeneration.
Last, sample size limitations of this study are worth mentioning. While the group differences and correlational effects observed here (e.g., ; r > 0.55−0.6) are considered intermediate to large effects41 and, thus, provide moderate to strong evidence favoring the alternative hypothesis, we acknowledge the limitation of our smaller sample size. Additional studies with larger populations of low- vs high-risk listeners are needed to replicate and confirm the preliminary findings of our proof-of-concept SP measures. Still, the convergence of group differences across several behavioral and electrophysiological measures is promising.
Acknowledgments
We would like to thank Kate R. Allen and Ashley A. Peeples for assistance with data collection. This work was supported by National Institutes of Health/National Institute on Deafness and Other Communication Disorders (NIH/NIDCD) Grant No. R01DC016267 (G.M.B.).
References and links
- 1.Kujawa SG and Liberman MC, “Adding insult to injury: Cochlear nerve degeneration after ‘temporary’ noise-induced hearing loss,” J. Neurosci 29(45), 14077–14085 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sergeyenko Y, Lall K, Liberman MC, and Kujawa SG, “Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline,” J. Neurosci 33(34), 13686–13694 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CACP, Santos F, Merchant SN, Liberman LD, and Liberman MC, “Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue,” Hear. Res 327, 78–88 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobarinas E, Salvi R, and Ding D, “Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas,” Hear. Res 302, 113–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felder E and Schrott-Fischer A, “Quantitative evaluation of myelinated nerve fibres and hair cells in cochleae of humans with age-related high-tone hearing loss,” Hear. Res 91(1–2), 19–32 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Wu PZ, Liberman LD, Bennett K, de Gruttola V, O’Malley JT, and Liberman MC, “Primary neural degeneration in the human cochlea: Evidence for hidden hearing loss in the aging ear,” Neuroscience 407, 8–20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mepani AM, Kirk SA, Hancock KE, Bennett K, De Gruttola V, Liberman MC, and Maison SF, “Middle ear muscle reflex and word recognition in ‘normal-hearing’ Adults: Evidence for cochlear synaptopathy?,” Ear Hear. 41(1), 25–38 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberman MC, Epstein MJ, Cleveland SS, Wang H, and Maison SF, “Toward a differential diagnosis of hidden hearing loss in humans,” PLoS One 11(9), e0162726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant KJ, Mepani AM, Wu P, Hancock KE, Gruttola VD, Liberman MC, and Maison SF, “Electrophysiological markers of cochlear function correlate with hearing-in-noise performance among audiometrically normal subjects,” J. Neurophysiol 124(2), 418–431 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kujawa SG and Liberman MC, “Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss,” Hear. Res 330(Pt. B), 191–199 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubno JR, “Effects of age and mild hearing loss on speech recognition in noise,” J. Acoust. Soc. Am 76(1), 87–96 (1984). [DOI] [PubMed] [Google Scholar]
- 12.Furman AC, Kujawa SG, and Liberman MC, “Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates,” J. Neurophysiol 110, 577–586 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmiedt RA, Mills JH, and Boettcher FA, “Age-related loss of activity of auditory-nerve fibers,” J. Neurophysiol 76(4), 2799–2803 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Suthakar K and Liberman MC, “Auditory-nerve responses in mice with noise-induced cochlear synaptopathy,” J. Neurophysiol 126(6), 2027–2038 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaheen LA, Valero MD, and Liberman MC, “Towards a diagnosis of cochlear neuropathy with envelope following responses,” J. Assoc. Res. Otolaryngol 16(6), 727–745 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorga MP, Neely ST, Ohlrich B, Hoover B, Redner J, and Peters J, “From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss,” Ear Hear. 18, 440–455 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Nikiforidis GC, Koutsojannis CM, Varakis JN, and Goumas PD, “Reduced variance in the latency and amplitude of the fifth wave of auditory brain stem response after normalization for head size,” Ear Hear. 14(6), 423–428 (1993). [DOI] [PubMed] [Google Scholar]
- 18.Bramhall N, Beach EF, Epp B, Le Prell CG, Lopez-Poveda EA, Plack CJ, Schaette R, Verhulst S, and Canlon B, “The search for noise-induced cochlear synaptopathy in humans: Mission impossible?,” Hear. Res 377, 88–103 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Durrant JD, Wang J, Ding DL, and Salvi RJ, “Are inner or outer hair cells the source of summating potentials recorded from the round window?,” J. Acoust. Soc. Am 104(1), 370–377 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Nam EC, and Park SI, “Electrocochleography is more sensitive than distortion-product otoacoustic emission test for detecting noise-induced temporary threshold shift,” Otolaryngol. Head Neck Surg 133(4), 619–624 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AE, Kaf WA, Ferraro JA, Delgado RE, and Lichtenhan JT, “Human summating potential using continuous loop averaging deconvolution: Response amplitudes vary with tone burst repetition rate and duration,” Front. Neurosci 11, 231–241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valero MD, Hancock KE, and Liberman MC, “The middle ear muscle reflex in the diagnosis of cochlear neuropathy,” Hear. Res 332, 29–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis-Gunter MJ, Löwenheim H, Gopal KV, and Moore EJ, “The I’ potential of the human auditory brainstem response to paired click stimuli,” Scand. Audiol 30(1), 50–60 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Furukawa T, Hayashida Y, and Matsuura S, “Quantal analysis of the size of excitatory post-synaptic potentials at synapses between hair cells and afferent nerve fibres in goldfish,” J. Physiol 276(1), 211–226 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eggermont JJ, “Peripheral auditory adaptation and fatigue: A model oriented review,” Hear. Res 18(1), 57–71 (1985). [DOI] [PubMed] [Google Scholar]
- 26.Griest-Hines SE, Bramhall NF, Reavis KM, Theodoroff SM and Henry JA, “Development and initial validation of the lifetime exposure to noise and solvents questionnaire in U.S. service members and veterans,” Am. J. Audiol 30(3S), 810–824 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Killion MC, Niquette PA, Gudmundsen GI, Revit LJ, and Banerjee S, “Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners,” J. Acoust. Soc. Am 116(4), 2395–2405 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Ferraro JA and Durran JD, “Electrocochleography in the evaluation of patients with Ménière’s disease/endolymphatic hydrops,” J. Am. Acad. Audiol 17(1), 45–68 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Eggermont JJ and Odenthal DW, “Electrophysiological investigation of the human cochlea: Recruitment, masking and adaptation,” Int. J. Audiol 13(1), 1–22 (1974). [DOI] [PubMed] [Google Scholar]
- 30.Pappa AK, Hutson KA, Scott WC, Wilson JD, Fox KE, Masood MM, Giardina CK, Pulver SH, Grana GD, Askew C, and Fitzpatrick DC, “Hair cell and neural contributions to the cochlear summating potential,” J. Neurophysiol 121(6), 2163–2180 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozdamar O and Dallos P, “Synchronous responses of the primary auditory fibers to the onset of tone burst and their relation to compound action potentials,” Brain Res. 155(1), 169–175 (1978). [DOI] [PubMed] [Google Scholar]
- 32.Lohse M, Bajo VM, King AJ, and Willmore BD, “Neural circuits underlying auditory contrast gain control and their perceptual implications,” Nat. Commun 11(1), 324 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabinowitz NC, Willmore BD, King AJ, and Schnupp JW, “Constructing noise-invariant representations of sound in the auditory pathway,” PLoS Biol. 11(11), e1001710 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulbright AN, Le Prell CG, Griffiths SK, and Lobarinas E, “Effects of recreational noise on threshold and suprathreshold measures of auditory function,” Semin. Hear 38(4), 298–318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grinn SK, Wiseman KB, Baker JA, and Le Prell CG, “Hidden hearing loss? No effect of common recreational noise exposure on cochlear nerve response amplitude in humans,” Front. Neurosci 11, 465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guest H, Munro KJ, Prendergast G, Millman RE, and Plack CJ, “Impaired speech perception in noise with a normal audiogram: No evidence for cochlear synaptopathy and no relation to lifetime noise exposure,” Hear. Res 364, 142–151 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prendergast G, Guest H, Munro KJ, Kluk K, Léger A, Hall DA, Heinz MG, and Plack CJ, “Effects of noise exposure on young adults with normal audiograms I: Electrophysiology,” Hear. Res 344, 68–81 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinsonnault-Skvarenina A, Moïn-Darbari K, Zhao W, Zhang M, Qiu W, and Fuente A, “No effect of occupational noise exposure on auditory brainstem response and speech perception in noise,” Front. Neurosci 16, 915211 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mepani AM, Verhulst S, Hancock KE, Garrett M, Vasilkov V, Bennett K, de Gruttola V, Liberman MC, and Maison SF, “Envelope following responses predict speech-in-noise performance in normal-hearing listeners,” J. Neurophysiol 125(4), 1213–1222 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bidelman GM, “Subcortical sources dominate the neuroelectric auditory frequency-following response to speech,” NeuroImage 175, 56–69 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Cohen J, Statistical Power Analysis for the Behavioral Sciences (Routledge, New York, 2013). [Google Scholar]


