Abstract
Background:
Rotavirus vaccine (RV) coverage levels for U.S. infants are <80%.
Methods:
We surveyed nationally representative networks of pediatricians by internet/mail from April-June, 2019. Multivariable regression assessed factors associated with difficulty administering the first RV dose (RV#1) by the maximum age.
Results:
Response rate was 68% (303/448). 99% of providers reported strongly recommending RV. The most common barriers to RV delivery overall (definite/somewhat of a barrier) were: parental concerns about vaccine safety overall (27%), parents wanting to defer (25%), parents not thinking RV was necessary (12%) and parent concerns about RV safety (6%). The most commonly reported reasons for non-receipt of RV#1 by 4–5 months (often/always) were parental vaccine refusal (9%), hospitals not giving RV at discharge from nursery (7%), infants past the maximum age when discharged from NICU/nursery (6%), and infant not seen before maximum age for well care visit (3%) or seen but no vaccine given (4%). Among respondents 4% strongly agreed and 25% somewhat agreed that they sometimes have difficulty giving RV#1 before the maximum age. Higher percentage of State Child Health Insurance Program/Medicaid-insured children in the practice and reporting that recommendations for timing of RV doses are too complicated were associated with reporting difficulty delivering the RV#1 by the maximum age.
Conclusions:
U.S. pediatricians identified multiple, actionable issues that may contribute to suboptimal RV immunization rates including lack of vaccination prior to leaving nurseries after prolonged stays, infants not being seen for well care visits by the maximum age, missed opportunities at visits and parents refusing/deferring.
Keywords: Rotavirus vaccine, ACIP recommendations, neonatal vaccination, vaccination delay, vaccine hesitancy
INTRODUCTION
Prior to the rotavirus vaccination program, rotavirus gastroenteritis in the U.S. was responsible for approximately 410,000 physician visits and 55,000–70,000 hospitalizations among children younger than five years of age, with total annual costs of approximately $1 billion.1–5 In 2006, the Advisory Committee on Immunization Practices (ACIP) recommended rotavirus vaccine (RV) for all eligible infants.6 Since then, numerous evaluations in the U.S. and globally have documented the effectiveness of the vaccines in preventing rotavirus disease and the resulting dramatic reduction in rotavirus hospitalizations and emergency department (ED) visits.7–9 However, although vaccination coverage levels increased quickly after recommendations, they have plateaued since 2013 in the U.S., with 2019 rates of only 76.3% for series completion, a level 15–20 percentage points lower than other routine childhood vaccines.7,10 U.S. rates are significantly lower than those achieved in several European, African and Latin American countries that have achieved rates close to or exceeding 90%.11,12
Two rotavirus vaccines are currently licensed for routine use among U.S. infants; pentavalent human-bovine reassortant RV [RV5 (RotaTeq®)], given in three doses at 2, 4 and 6 months of age, and monovalent human RV [RV1 (Rotarix®)], given in two doses at 2 and 4 months of age. In addition to contraindications to vaccination [history of severe allergic reaction or intussusception or severe combined immunodeficiency (SCID)],13 ACIP identifies groups subject to “precautions” for which practitioners should consider the potential risks and benefits before recommending RV, including infants with altered immunocompetence (other than SCID), moderate or severe acute illness and pre-existing chronic gastrointestinal disease.6 ACIP also highlights several “special situations,” (preterm infants and infants living in households with persons with impaired immunity or with pregnant women) for whom the vaccine is recommended because the benefits of RV vaccination outweigh any potential risks 6,14.
Unlike other infant vaccines, the recommendations for RV include a maximum age for the first dose (14 weeks 6 days) and for the last dose (8 months 0 days).14 These age restrictions were implemented because they were the maximum ages used in the pre-licensure trials and there were insufficient data on the safety of the first dose of RV vaccine in older infants.6 Some infants that were born prematurely or have other serious health conditions at birth necessitating hospital stays through age 14 weeks 6 days may not start the RV series because ACIP recommends that RV not be administered to infants in the neonatal intensive care unit (NICU) or hospital nursery due to concern of possible transmission of shed live vaccine virus to other ill infants.6 For this group, the ACIP recommends that eligible infants receive the vaccine at the time of discharge from the hospital, or after discharge at the primary care site, if age <15 weeks 0 days.
Given the maximum age restrictions for RV administration, some degree of lower vaccination coverage compared with other routine infant vaccines was expected. Data from six Immunization Information System (IIS) Sentinel Sites in 2016 demonstrated that approximately one-third of the difference in coverage between ≥1 dose of DTAP and ≥1 dose of RV among infants could be due to the maximum age restriction of the first dose of RV.15 However, there are limited data on potential other reasons for the current suboptimal RV rates and if any of these are remediable to interventions. Infants who have prolonged hospitalizations may not be vaccinated at the time of discharge and may not be seen for primary care before reaching the maximum age for RV. Although the number of infants who have a true contraindication for RV is very small, the groups that are singled out for “precaution” and those fall into the ACIP’s “special situation,” groups who some providers may not always opt to vaccinate might make a larger contribution. Finally, given the withdrawal of the first licensed RV due to an association between the vaccine and intussusception,16,17 as well as other potential safety issues publicized in the past, concerns on the part of parents and providers about the safety of RV may be leading providers to recommend the vaccine less strongly or to parental refusal.
To better understand the contribution of these and potentially other factors affecting RV series completion rates, we assessed in a nationally representative sample of pediatricians: 1) the strength of physician recommendation for RV compared with other vaccines received at 2 months of age; 2) physician attitudes about RV and experiences with administering RV; 3) perceived barriers to administering RV; 4) frequency of non-receipt of first RV dose by 4–5 month visit and perceived reasons for non-receipt of RV; and 5) factors associated with difficulty administering the first RV dose by the maximum age.
METHODS
We conducted a survey from April to June 2019 among U.S. pediatricians who were part of a sentinel network developed by the Vaccine Policy Collaborative Initiative. The multi-institution review board at the University of Colorado Anschutz Medical Campus approved this study as exempt research.
Study Population
We developed a national network of pediatricians by recruiting from the American Academy of Pediatrics (AAP) and then performing post-stratification quota sampling to create a network of physicians similar to AAP membership with respect to region, practice location, and practice setting.18 Physicians were excluded if they spent <50% of their time in primary care, did not practice in the U.S., or were in training. In previous work, survey responses from network physicians compared to those of physicians randomly sampled from American Medical Association physician databases have demonstrated similar demographic characteristics, practice attributes, and attitudes about vaccination issues.18
Survey Design
The survey was developed collaboratively with content experts at the Centers for Disease Control and Prevention (CDC) and with input from AAP leadership. Questions were based on templates used in many previous surveys with changes to reflect different content. Four-point Likert scales were used for questions assessing 1) strength of vaccine recommendations; 2) level of agreement with statements regarding attitudes and delivery experiences; 3) perceived barriers to RV administration in the office; and 4) perceived reasons for non-receipt of first RV dose by the 4–5 month visit. We pre-tested the survey within a national advisory panel of pediatricians. The survey was then piloted among 48 pediatricians nationally and further modified based on their feedback.
Survey Administration
We surveyed physicians by Internet (www.qualtrics.com) or by mail based on the physician’s preference. The Internet group received an initial e-mail with up to eight email reminders, and the mail group received an initial mailing and up to two additional mailed reminders. Internet survey non-respondents were sent a mail survey in case of problems with e-mail correspondence. The mail protocol was based on Dillman’s tailored design method.19
Statistical Analysis
We combined Internet and mail surveys analytically because studies have shown that physician attitudes are similar when obtained by either method.1,19–21 We compared respondents with non-respondents using t-test, Wilcoxon, and chi-squared tests, as appropriate. We conducted a multivariable analysis with the dependent variable of a response of “strongly agree” or “somewhat agree” to the statement: “I sometimes have difficulty giving the first dose of rotavirus vaccine before the maximum age.” Independent variables included practice setting, practice location, age of the provider, proportion of patient population with Medicaid or State Child Health Insurance Plan (SCHIP) insurance, percent minority and number of providers in the practice. In addition, we included whether or not the respondent agreed (strongly or somewhat) with the statement “The recommendations for timing of doses of rotavirus vaccination are too complicated” because we hypothesized that this perception would be associated with having difficulty complying with the recommendations. To account for the high prevalence of the outcome, we used Poisson regression with robust standard errors to obtain risk ratios and 95% confidence intervals. Analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
The response rate for the survey was 68% (303/448). Four providers reported not providing care to infants <6 months of age and were excluded from analysis. Respondents did not differ from non-respondents with respect to gender, age, practice type or setting, number of providers in the practice or whether vaccine decisions are made at the practice or at a larger system level (Table 1). Responders were more likely to be from the Midwest and Northeast regions and less likely to be from the South or Western regions of the country. All survey questions had a response rate of ≥95%, therefore we do not provide separate respondent numbers for each question.
Table 1.
Comparison of Respondents and Non-respondents and Additional Characteristics of Respondents and their Practices
| Characteristic | Total Respondents (N=303) | Total Non-Respondents (N=145) | P-value, chi-squared test, respondents vs. non-respondents |
|---|---|---|---|
| Provider gender, % (n) | |||
| Female | 64 (195) | 66 (96) | 0.70 |
| Male | 36 (108) | 34 (49) | |
| Mean (SD) provider age in years | 53.0 (10.4) | 51.8 (10.9) | 0.24* |
| Practice setting, % (n) | |||
| Private practice | 79 (240) | 81 (117) | |
| Hospital or clinic | 17 (52) | 15 (22) | |
| HMO | 4 (11) | 4 (6) | 0.85 |
| Practice location, % (n) | |||
| Rural/Suburban | 49 (147) | 43 (62) | 0.25 |
| Urban | 52 (156) | 57 (83) | |
| Practice region, % (n) | |||
| South | 34 (104) | 44 (64) | |
| Midwest | 23 (70) | 18 (26) | |
| West | 19 (58) | 23 (34) | |
| Northeast | 23 (71) | 15 (21) | 0.04 |
| Practice-level decision-making about vaccines, % (n) | |||
| Larger System Level | 29 (86) | 31 (44) | |
| Independent | 71 (208) | 69 (99) | 0.74 |
| Median (IQR) number of providers in practice | 6.0 (3.5–9.0) | 5.0 (3.0–10.5) | 0.74† |
| Proportion of privately insured patients in practice, % (n) | |||
| 0–24 | 24 (72) | N/A | |
| 25–49 | 23 (48) | N/A | |
| ≥50 | 53 (159) | N/A | |
| Proportion of Medicaid or your state’s Children’s Health Insurance patients in practice, % (n) | |||
| 0–24 | 40 (117) | N/A | |
| 25–49 | 27 (79) | N/A | |
| ≥50 | 33 (97) | N/A | |
| Patient race/ethnicity in practice, % (n) | |||
| ≥10% Black/African American | 58 (171) | N/A | |
| ≥10% Hispanic | 55 (164) | N/A | |
| ≥10% Asian/Pacific Islander | 32 (96) | N/A |
IQR: interquartile range; SD: standard deviation.
t-test,
Fisher’s Exact test,
Wilcoxon Test
Strength of recommendation for RV compared with other vaccines received at 2 months of age
All physicians reported strongly recommending Diphtheria, Tetanus and acellular Pertussis (DTaP), Pneumococcal Conjugate vaccine (PCV13) and Haemophilus influenzae type B (HiB) vaccines to infants 2 month of age and 99% recommended RV strongly, with 1% recommending but not strongly.
Attitudes about RV and vaccine delivery experiences
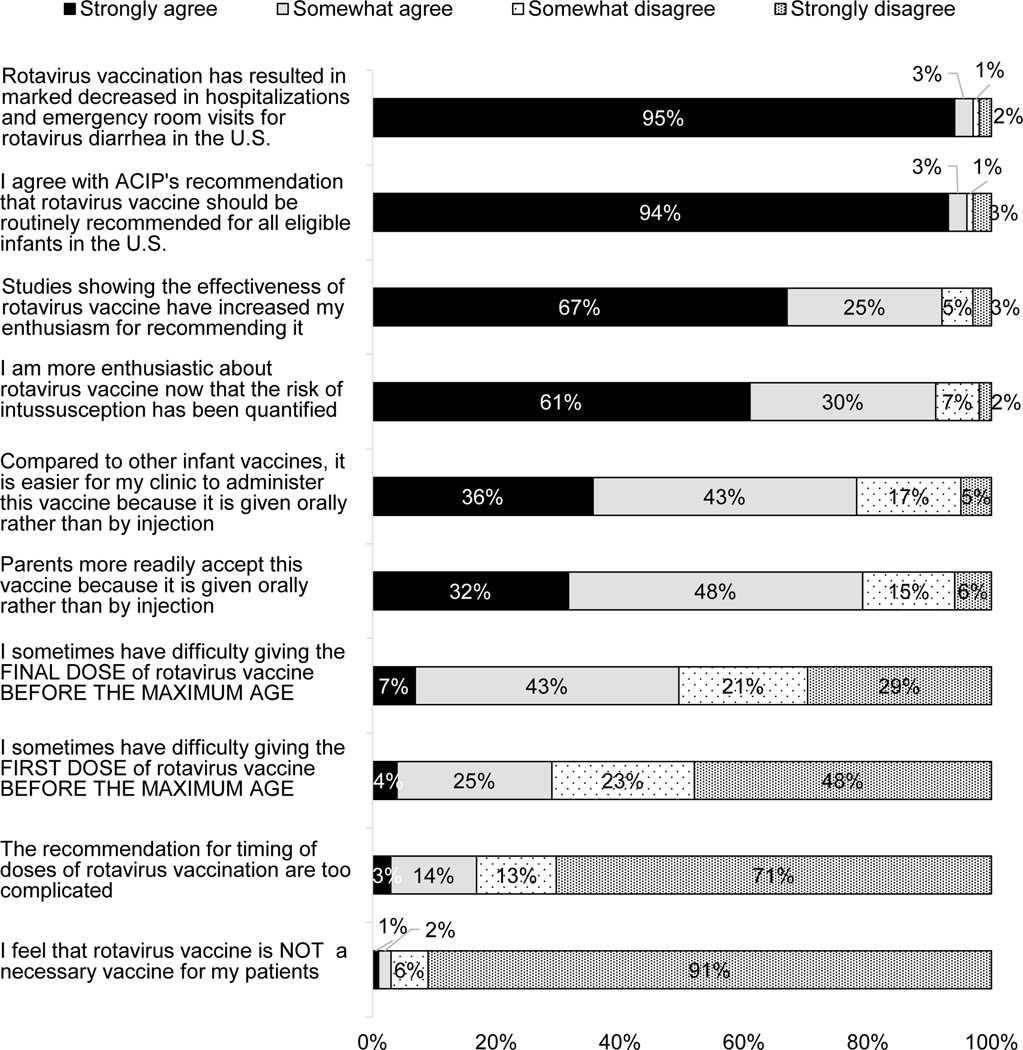
Almost all physicians strongly agreed that RV has resulted in marked decreases in ED visits and hospitalizations and that the vaccine should be routinely recommended for U.S. infants (Figure 1). The majority strongly agreed that they were more enthusiastic about RV now that its effectiveness has been well-demonstrated and the risk of intussusception has been quantified. The majority either strongly or somewhat agreed that RV is easier to administer and more acceptable to parents than other vaccines because it is given orally. Fifty percent (7% strongly) agreed that it was sometimes difficult completing the final dose before the maximum age, while 29% (4% strongly) agreed with sometimes having difficulty giving the first dose before the maximum age. Less than 20% agreed “the recommendations for timing of RV doses are too complicated.”
Figure 1: Attitudes about RV and Vaccine Delivery Experiences (n=299).
Percentages may not add up to 100% due to rounding.
Perceived barriers to administering RV at the practice
There were few perceived barriers to administering RV (Figure 2). The only barriers endorsed by ≥10% as either definitely or somewhat a barrier were parents wanting to defer the RV, parental concerns about vaccine safety in general, and parents thinking RV was not necessary for their children. Few providers endorsed barriers such as the complexity of the recommendations for timing of the RV doses, lack of adequate reimbursement for RV, the time it takes to discuss RV safety, and parents’ beliefs that RV is not effective.
Figure 2: Perceived Barriers to Administering RV in Practice (n=299).
Percentages may not add up to 100% due to rounding.
Frequency of non-receipt of first RV vaccine by 4–5 month well care visit and perceived reasons for non-receipt
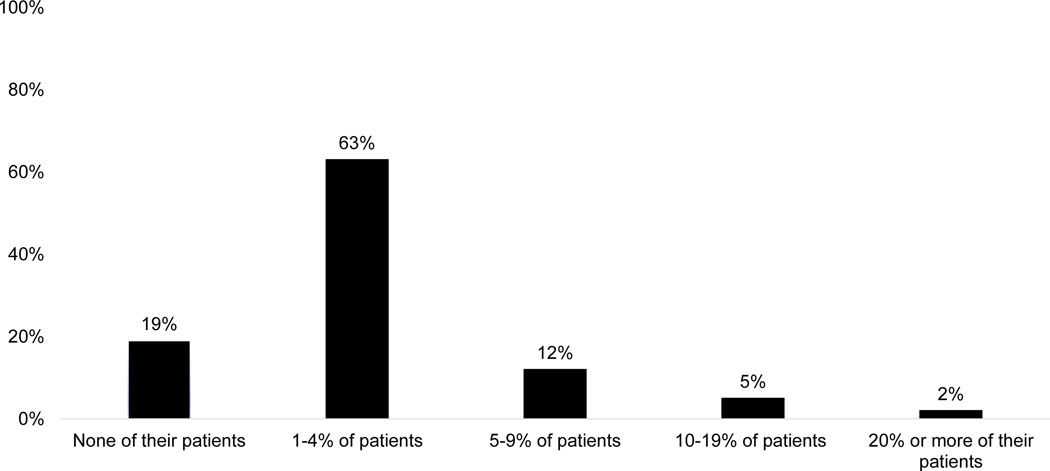
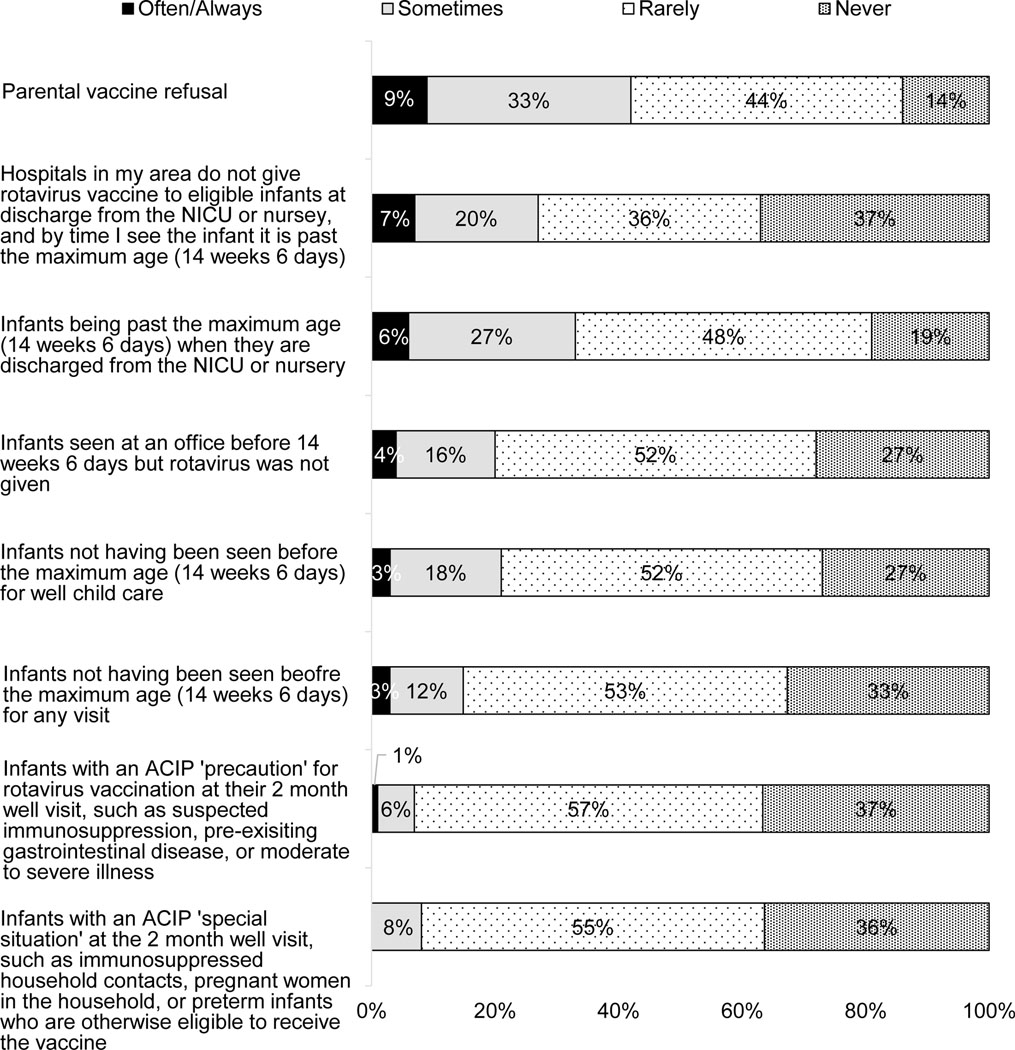
The majority of physicians reported that <5% of the infants 4–5 months old that they had seen in the past six months had not received the first dose of RV; 12% reported 5–9% of infants 4–5 months old had not received the first dose; and 7% reported ≥10% had not received the first dose (Figure 3). The most commonly reported perceived reasons for non-receipt (Figure 4) were parental vaccine refusal, infants being past the maximum age when discharged from NICU or nursery, hospitals not giving RV at discharge from NICU or nursery, and infant not seen before maximum age for RV. Infants with ACIP precaution or special situation were reported as a perceived reason (often/always) by ≤1%.
Figure 3.
Percentage of 4–5 Month Old Infants Seen for a Well Visit in the Past 6 Months Who Had Not Previously Received a Dose of Rotavirus Vaccine
Figure 4: Perceived Reasons for Non-Receipt of First RV Dose by 4–5 Month Visit (n=299).
Percentages may not add up to 100% due to rounding.
Factors associated with finding it difficult to give 1st RV dose by maximum age
Having a higher percentage of Medicaid- or SCHIP-insured children in the practice and reporting that recommendations for timing of RV doses are too complicated were significantly associated with finding it difficult to initiate the RV series before the maximum age (Table 2).
Table 2:
Characteristics Associated with Agreeing (Strongly/Somewhat) that it is Difficult to Give First Dose of RV by Maximum Age (n=284)
| Difficult to give first dose by maximum age | ||||||
|---|---|---|---|---|---|---|
| Variable | Category | Yes (N=84) | No (N=200) | Biv. P-value | MV RR (95% CI) | MV p-value |
| Practice setting | Community or Hospital based/HMO | 25% (21) | 17% (34) | 0.12 | 0.98 (0.63–1.53) | 0.93 |
| Private practice | 75% (63) | 83% (166) | Ref. | |||
| Census Location | Rural/Suburban | 49% (41) | 47% (94) | 0.78 | Ref. | 0.99 |
| Urban | 51% (43) | 53% (106) | 1.00 (0.70–1.45) | |||
| Proportion of patients that have Medicaid or your state’s CHIP | 0–49% | 55% (46) | 73% (140) | 0.02 | Ref. | 0.03 |
| 50–74% | 24% (20) | 17% (32) | 1.51 (0.97–2.34) | |||
| 75–100% | 20% (17) | 11% (21) | 1.90 (1.16–3.09) | |||
| Practice is <10% Percent Minority (Black, Hispanic, Asian) | Yes | 87% (72) | 81% (158) | 0.22 | Ref. | 0.68 |
| No | 13% (11) | 19% (38) | 0.89 (0.52–1.55) | |||
| The recommendations for timing of doses of rotavirus vaccination are too complicated | S/S agree | 24% (20) | 14% (28) | 0.04 | 1.63 (1.10–2.40) | 0.04 |
| S/S disagree | 76% (63) | 86% (171) | Ref. | |||
| Mean (sd) Provider Age in Years | 52.9 (10.9) | 52.9 (10.2) | 0.96 | 0.99 (0.91–1.08) (Per 5 years) | 0.88 | |
| Median (IQR) Number of providers in practice | 5.0 (4.0–12.0) | 6.0 (4.0–9.0) | 0.77^ | 1.01 (0.98–1.04) (Per 10 providers) | 0.67 | |
Wilcoxon Test
Discussion
This survey examined causes of the persistently lower rates of RV vaccination compared with other pediatric vaccines in the United States. Although it was anticipated that the maximum age restrictions would result in lower rates, our data demonstrate that, from the pediatricians’ perspective, a variety of other issues, originating at the health system, practice, and individual levels are also playing a part. Importantly, our data suggest that parental vaccine hesitancy, potentially related to safety concerns, is playing a notable role.
Importantly, our study demonstrates that among pediatricians there is very strong belief in the effectiveness of RV and recognition of its impact on improving health outcomes for children. There was strong agreement with recommendations for its routine use and many physicians reported it was easier to administer and more acceptable to parents because it is given orally with less than five percent thinking RV was not a necessary vaccine. Virtually all (99%) reported recommending RV strongly, up from a previous national survey in 2010 that showed 85% of pediatricians strongly recommended.22 Therefore, our data do not support a low level of enthusiasm on the part of U.S. pediatricians as a reason for suboptimal rates.
Approximately a third of pediatricians agreed that they sometimes have difficulty administering the first dose of RV before the maximum age. Additionally, for almost 20% of pediatricians, the complexity of recommendations for the timing of the RV doses was at least a minor barrier to its delivery. Reported causes of non-receipt of the first RV dose by the 4–5 month well care visit included infants being past the maximum age for the first dose when they were discharged from the NICU or nursery and the fact that hospitals were not giving RV to infants leaving the NICU or nursery at the time of discharge; these infants then failed to present for well care visits prior to the maximum age. These data are in line with a study of infants enrolled in the New Vaccine Surveillance Network23 that demonstrated an odds ratio of 0.32 for receipt of the first dose of RV among infants born at <37 weeks gestation, the group of infants most likely to be discharged after a longer than normal nursery or NICU stay. The prevalence of hospitals not providing RV at the time of discharge for infants who are discharged after weeks of hospitalization is unknown, but this presents a clear option for intervention.
Also prominent among the perceived reasons for non-receipt were eligible infants having been seen but not vaccinated or infants not being seen at all before the maximum age for RV. These findings are consistent with past data from the 2014 National Immunization Survey of U.S. children 19–35 months old, showing that among the 14% of children who had received no doses of RV, 72% had ≥1 ACIP-defined missed opportunity, defined as a visit where DTaP was given and the child was eligible for but did not receive RV.24 This study estimated a 10 percentage point increase in completed rotavirus vaccination (from 71% to 81%) if all identified missed opportunities were eliminated. Similarly, data from the IIS Sentinel Sites indicated that two-thirds of the difference in coverage between receipt of ≥1 DTaP and ≥1 RV among 5 month old infants was not related to the maximum age restriction for the first dose and likely represented missed opportunities of various etiologies.15 Although numerous studies identify delays in receipt of other childhood vaccines in the first 4–5 months,25–28 the implications of delays in RV vaccination are very different from delays for other routine vaccines since a delay past 14 weeks 6 days effectively precludes any RV vaccination. Therefore, strong recommendations with a focus on timeliness of vaccination are especially crucial for increasing RV vaccination rates.
There were relatively few significant barriers to administering RV reported by physicians in this survey, and most barriers had decreased since a similar national survey using the same methodology in 2010.22 For example, the percentage reporting “parents perceiving that RV is not necessary” as definitely or somewhat of a barrier fell from 24% in 2010 to 12% in the present survey; “parental concerns about safety of RV” fell from 15% to 6%; “lack of adequate reimbursement for RV” fell from 9% to 3% as did physician perception that “the complexity of the timing of doses” is a barrier. Level of parental “concerns about vaccine safety in general” (definitely or somewhat of a barrier) was also perceived to have decreased from 42% to 27%, but the 2010 survey did not ask about parents wanting to decide on their own RV schedule or deferring the vaccine which was one of the prominent barriers in the present survey.
The only remaining barriers to RV delivery noted by ≥5% of respondents were perceived to be on the parental side, including parents wanting to defer the RV, having safety concerns about vaccines in general or with RV specifically and thinking RV was not a necessary vaccine. Remaining safety concerns about RV may continue to be related to historical reasons or could be related to interpretation of risk of the current vaccine. Parents may be aware of the withdrawal from the market of the first licensed RV and, therefore, may be concerned about the current RV. Additionally, given the known risk of intussusception for the two current RVs (1 excess case of intussusception per an estimated 20,000 to 100,000 vaccinated infants)29 it is possible that some parents and providers believe the risk of intussusception for an individual infant outweighs the benefit of receiving RV. Another possible safety concern about RV in the recent past was the detection of porcine circovirus DNA in the formulations of both vaccines,30 which resulted in a two month suspension of Rotarix in 2010. The Food and Drug Administration ultimately concluded that there is no known safety risk from this. Although one qualitative study performed in 2010, shortly after the suspension, suggested pediatricians considered the porcine DNA a “non-issue”, for some mothers in the same study, this information eroded their confidence in the vaccine.31
Parental vaccine refusal was the most frequently perceived reason for non-receipt of the first dose of RV by the 4–5 month well care visit. Other than discussions about the two potential safety concerns that were previously publicized, there has been little examination of vaccine hesitancy related to RV, and its prevalence nationally is unknown. Indeed, other than rates of vaccine refusal or deferral, there is little U.S. national data on the prevalence of parental vaccine hesitancy, as measured by a hesitancy scale, for any individual vaccines in the childhood series.
The fact that high percentages of patients with Medicaid or SCHIP in the physician’s practice were associated with difficulty giving the first dose of RV by the maximum age may speak to the types of practices or delivery systems that are serving such patients, to access issues, or to health beliefs or behaviors of these patient populations. Previous studies have shown that lower educational status, public insurance, or no insurance are factors that are related to lack of receipt of RV.23,24 Our data also show an association between difficulty administering the first RV dose and feeling the recommendations for timing of RV doses are too complicated. It is not clear whether this identifies practitioners who, by virtue of practice or community characteristics or the patients they serve, are truly experiencing more challenges or whether this simply identifies physicians who perceive that the recommendations are problematic.
There are important strengths and limitations to our data. We surveyed a large, nationally representative sample of pediatricians and achieved a relatively high response rate. Previous research by our group has demonstrated the sentinel survey method described yield similar responses to the most commonly employed method of sampling physicians nationally.18 However, responses of our network may not be fully generalizable to all pediatricians and survey data may be subject to reporting bias. Our response rates differed by region of the country which could introduce bias. Our data are also based on self-report rather than direct observation and may not reflect actual practice. Finally, our data focus exclusively on pediatric practices and do not include the opinions of family physicians, who care for approximately 15% of infants younger than two years of age.
Our study provides actionable results in guiding efforts to further increase RV rates at the public health, practice, and individual levels. At the health care system level, there is a need to identify infants who remain unvaccinated for RV, or who have received no vaccines, before age 15 weeks and attempt to get them in for vaccination. This could be accomplished with the use of state or regional immunization information systems (IIS) to identify infants who are not vaccinated and a targeted centralized reminder-recall effort to bring these children in for vaccination. Such a centralized IIS-based approach has been shown to be effective for other childhood vaccines and could be implemented by a state public health department to recall those infants not yet associated with a practice. For those infants who are associated with a practice32,33, reminder-recall efforts could also be implemented at the practice level.34 Working with neonatal providers to implement policies that encourage RV at the time of discharge for infants who have had prolonged NICU or nursery stays is an important objective. When this is not possible, discussion with the family about the importance of RV and an appointment and warm hand-off to a primary care provider to assure infants are seen for vaccination, preferably on the day of discharge, but in all cases prior to age 15 weeks should be routine for discharging nurseries. Missed opportunities should also be addressed at the practice level, using evidence-based methods such as reminder-recall, provider prompts, and standing orders.35 The prominence of parental RV refusal or deferral as an issue driving lack of vaccination may reflect vaccine hesitancy driven by general vaccine concerns or specific safety concerns about RV that are inadequately appreciated by providers. Vaccine hesitancy has recently been identified by the WHO as one of the ten top threats to health internationally36 and, based on our data, may be having a more substantial impact on RV rates than previously realized. In addition to the need to test interventions targeting parental vaccine hesitancy, our data would suggest the need to better measure its contribution to individual vaccine rates.
What’s New:
This survey of U.S. pediatricians identified issues contributing to suboptimal rotavirus vaccination rates including prolonged nursery stays, infants not seen for well care visits or having missed opportunities prior to the maximum age for initiating vaccination and parents refusing/deferring vaccination.
Acknowledgements:
The authors would like to thank Lynn Olson, PhD and Anne Edwards, MD from the AAP for collaborating in the establishment of the sentinel network in pediatrics. We would also like to thank all pediatricians in the network for participating and responding to this survey.
Funding Source:
Centers for Disease Control and Prevention, grant 1U01IP000849-02
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Co-authors from the CDC contributed to identifying the survey topic, critically reviewing the data, and contributing to manuscript review.
Footnotes
Financial Disclosure: No financial disclosures were reported by the authors of this paper
CONFLICT OF INTEREST: None of the authors has any conflict of interests.
References
- 1.Widdowson MA, Meltzer MI, Zhang X, et al. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics. 2007;119(4):684–697. [DOI] [PubMed] [Google Scholar]
- 2.Charles MD, Holman RC, Curns AT, et al. Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993–2002. Pediatr Infect Dis J. 2006;25(6):489–493. [DOI] [PubMed] [Google Scholar]
- 3.Malek MA, Curns AT, Holman RC, et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117(6):1887–1892. [DOI] [PubMed] [Google Scholar]
- 4.Parashar UD, Holman RC, Clarke MJ, et al. Hospitalizations associated with rotavirus diarrhea in the United States, 1993 through 1995: surveillance based on the new ICD-9-CM rotavirus-specific diagnostic code. J Infect Dis. 1998;177(1):13–17. [DOI] [PubMed] [Google Scholar]
- 5.Tucker AW, Haddix AC, Bresee JS, et al. Cost-effectiveness analysis of a rotavirus immunization program for the United States. Jama. 1998;279(17):1371–1376. [DOI] [PubMed] [Google Scholar]
- 6.Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009;58(Rr-2):1–25. [PubMed] [Google Scholar]
- 7.Pindyck T, Tate JE, Parashar UD. A decade of experience with rotavirus vaccination in the United States - vaccine uptake, effectiveness, and impact. Expert Rev Vaccines. 2018;17(7):593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonesteller CL, Burnett E, Yen C, et al. Effectiveness of Rotavirus Vaccination: A Systematic Review of the First Decade of Global Postlicensure Data, 2006–2016. Clin Infect Dis. 2017;65(5):840–850. [DOI] [PubMed] [Google Scholar]
- 9.Burnett E, Jonesteller CL, Tate JE, et al. Global Impact of Rotavirus Vaccination on Childhood Hospitalizations and Mortality From Diarrhea. J Infect Dis. 2017;215(11):1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Supplementary figures and table for Vaccination Coverage by Age 24 Months Among Children Born in 2016 and 2017 – National Immunization Survey-Child, United States, 2017–2019. https://www.cdc.gov/vaccines/imz-managers/coverage/childvaxview/pubs-presentations/NIS-child-vac-coverage-2016-2017-tables.html. Published 2020. Accessed February 23, 2021.
- 11.de Hoog MLA, Vesikari T, Giaquinto C, Huppertz HI, Martinon-Torres F, Bruijning-Verhagen P. Report of the 5th European expert meeting on rotavirus vaccination (EEROVAC). Hum Vaccin Immunother. 2018;14(4):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global Impact of Rotavirus Vaccination on Childhood Hospitalizations and Mortality From Diarrhea. J Infect Dis. 2017;215(11):1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Addition of History of Intussusception as a Contraindication for Rotavirus Vaccination. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6041a5.htm. Published 2011. Accessed June 24, 2020.
- 14.Centers for Disease Control and Prevention. Prevention of Rotavirus Gastroenteritis Among Infants and Children Recommendations of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5802a1.htm. Published 2009. Accessed June 25, 2020.
- 15.Armah G, Pringle K, Enweronu-Laryea CC, et al. Impact and Effectiveness of Monovalent Rotavirus Vaccine Against Severe Rotavirus Diarrhea in Ghana. Clin Infect Dis. 2016;62 Suppl 2:S200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344(8):564–572. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48(43):1007. [PubMed] [Google Scholar]
- 18.Crane LA, Daley MF, Barrow J, et al. Sentinel Physician Networks as a Technique for Rapid Immunization Policy Surveys. Evaluation & the Health Professions. 2007;31(1):43–64. [DOI] [PubMed] [Google Scholar]
- 19.Dillman DA, J S, LM C. Internet, Mail and Mixed-Mode Surveys: The Tailored Desgin Method, 3rd Edition. 3rd ed. New York, NY: John Wiley Co.; 2009. [Google Scholar]
- 20.Atkeson LR AA, Bryant LA, Ziberman L, Saunders KL. Considering Mixed Mode Surveys for Questions in Political Behavior: Using the Internet and Mail to Get Quality Data at Reasonable Costs. Political Behavior. 2011;33(1):161–178. [Google Scholar]
- 21.McMahon SR, Iwamoto M, Massoudi MS, et al. Comparison of e-mail, fax, and postal surveys of pediatricians. Pediatrics. 2003;111(4 Pt 1):e299-e303. [DOI] [PubMed] [Google Scholar]
- 22.O’Leary ST, Parashar UD, Crane LA, et al. Adoption of rotavirus vaccine by U.S. physicians: progress and challenges. American journal of preventive medicine. 2013;44(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aliabadi N, Wikswo ME, Tate JE, et al. Factors Associated With Rotavirus Vaccine Coverage. Pediatrics. 2019;143(2):e20181824. [DOI] [PubMed] [Google Scholar]
- 24.Sederdahl BK, Orenstein WA, Yi J, et al. Missed Opportunities for Rotavirus Vaccination. Pediatrics. 2019;143(5). [DOI] [PubMed] [Google Scholar]
- 25.Kiely M, Boulianne N, Talbot D, et al. Impact of vaccine delays at the 2, 4, 6 and 12 month visits on incomplete vaccination status by 24 months of age in Quebec, Canada. BMC Public Health. 2018;18(1):1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazan G, Dagan R, Friger M. Maternal Education Is Inversely Related to Vaccination Delay among Infants and Toddlers. J Pediatr. 2019;205:120–125 e122. [DOI] [PubMed] [Google Scholar]
- 27.Hargreaves AL, Nowak G, Frew P, et al. Adherence to Timely Vaccinations in the United States. Pediatrics. 2020;145(3). [DOI] [PubMed] [Google Scholar]
- 28.Hough-Telford C, Kimberlin DW, Aban I, et al. Vaccine Delays, Refusals, and Patient Dismissals: A Survey of Pediatricians. Pediatrics. 2016;138(3). [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Questions & Answers about Intussusception and Rotavirus Vaccine. Vaccines and Preventable Diseases Web site. https://www.cdc.gov/vaccines/vpd/rotavirus/about-intussusception.html. Published 2017. Accessed August 20, 2020. [Google Scholar]
- 30.Victoria JG, Wang C, Jones MS, et al. Viral nucleic acids in live-attenuated vaccines: detection of minority variants and an adventitious virus. J Virol. 2010;84(12):6033–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Payne DC, Humiston S, Opel D, et al. A multi-center, qualitative assessment of pediatrician and maternal perspectives on rotavirus vaccines and the detection of Porcine circovirus. BMC Pediatr. 2011;11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kempe A, Saville AW, Dickinson LM, et al. Collaborative centralized reminder/recall notification to increase immunization rates among young children: a comparative effectiveness trial. JAMA Pediatr. 2015;169(4):365–373. [DOI] [PubMed] [Google Scholar]
- 33.Hurley LP, Beaty B, Lockhart S, et al. RCT of Centralized Vaccine Reminder/Recall for Adults. Am J Prev Med. 2018;55(2):231–239. [DOI] [PubMed] [Google Scholar]
- 34.Pich J.Patient reminder and recall interventions to improve immunization rates: A Cochrane review summary. Int J Nurs Stud. 2019;91:144–145. [DOI] [PubMed] [Google Scholar]
- 35.The Community Guide. Vaccination. https://www.thecommunityguide.org/topic/vaccination. Accessed August 19, 2020.
- 36.World Health Organization. Ten threats to global health in 2019. https://www.who.int/news-room/feature-stories/ten-threats-to-global-health-in-2019. Published 2019. Accessed August 19, 2020.