Abstract
Prostate cancer is a leading cause of death in men worldwide. For over 30 years, growing interest has focused on the development of vaccines as treatments for prostate cancer, with the goal of using vaccines to activate immune cells capable of targeting prostate cancer to either eradicate recurrent disease or at least delay disease progression. This interest has been prompted by the prevalence and long natural history of the disease and by the fact that the prostate is an expendable organ. Thus, an immune response elicited by vaccination might not need to target the tumour uniquely but could theoretically target any prostate tissue. To date, different vaccine approaches and targets for prostate cancer have been evaluated in clinical trials. Overall, five approaches have been assessed in randomized phase III trials and sipuleucel-T was approved as a treatment for metastatic castration-resistant prostate cancer, being the only vaccine approved to date by the FDA as a treatment for cancer. Most vaccine approaches showed safety and some evidence of immunological activity but had poor clinical activity when used as monotherapies. However, increased activity has been observed when these vaccines were used in combination with other immune-modulating therapies. This evidence suggests that, in the future, prostate cancer vaccines might be used to activate and expand tumour-specific T cells as part of combination approaches with agents that target tumour-associated immune mechanisms of resistance.
Subject terms: Vaccines, Tumour immunology
In this Review, current vaccine-based approaches to treat prostate cancer are described. The authors discuss results from clinical trials in which overall vaccine safety and biological activity were shown, albeit with modest clinical activity, suggesting that the future of these approaches will be as part of combination therapies with agents targeting tumour-associated immune mechanisms of resistance.
Key points
Over 175 clinical trials to assess antitumour vaccines as treatments for prostate cancer have been conducted over the past 30 years.
A total of five vaccine approaches have been evaluated in randomized phase III trials.
One vaccine approach, sipuleucel-T, has been approved by the FDA as a treatment for advanced prostate cancer.
Most vaccine approaches showed safety and biological activity, yet little clinical activity was observed when these vaccines were used as monotherapies.
Future exploration of prostate cancer vaccines will focus on the use of these agents to activate and expand tumour-specific T cells as part of combination therapies with agents targeting tumour-associated immune mechanisms of resistance.
Introduction
Prostate cancer is a substantial health concern worldwide and, currently, the most commonly diagnosed malignancy in men in the USA1. Surgery and/or radiation therapy is available for localized disease, but approximately one-third of patients will experience disease recurrence and approximately one-third of these individuals will ultimately die from prostate cancer2. Androgen deprivation has been the cornerstone of treatment for metastatic disease for over 60 years; however, castration-resistant prostate cancer (CRPC) typically occurs within 3 years from the beginning of androgen deprivation therapy and metastatic CRPC (mCRPC) is the lethal stage of the disease3. Over the past 15 years, substantial improvements have been observed in the treatment of prostate cancer but most of these treatments involved non-specific chemotherapy agents or agents that further suppress androgen receptor signalling4–7. Notable exceptions include two poly(ADP-ribose) polymerase (PARP) inhibitors that have been approved by the FDA for a subset of patients with specific mutations in DNA repair genes8,9, 223Ra for patients with bone-restricted mCRPC10, 177Lu-PSMA-617 for patients with prostate-specific membrane antigen (PSMA)-expressing mCRPC11, PD1 inhibitors for a small number of patients with microsatellite instability-high tumours or high tumour-mutational burden12, and sipuleucel-T, a prostate antigen-specific autologous cellular vaccine approved for all patients with early, asymptomatic mCRPC13. Sipuleucel-T is the only FDA-approved tumour vaccine used to treat existing cancers.
Antitumour vaccines are agents designed to be delivered to patients with cancer to elicit an immune response that can recognize and eliminate tumour cells14. Antitumour vaccine approaches are generally based on the delivery of tumour cells that have been inactivated or, in some instances, modified to increase the recognition of these cells by the immune system; alternatively, tumour vaccine strategies use the delivery of tumour components to activate immune responses towards specific antigenic targets15–17. These approaches rely on the use of whole proteins, cancer-specific carbohydrates, peptides derived from target proteins, or genetic vaccines (such as viral, bacterial, DNA or RNA vaccines) encoding tumour-associated antigen targets18–22.
The prostate is a non-essential organ and, particularly in patients with prostate cancer who have undergone prostatectomy, any remaining prostate tissue is unwanted. This feature prompted investigation of antitumour vaccines as potential treatments for prostate cancer, with the rationale of using vaccines to create a 'tissue-rejection' response to eliminate any remaining microscopic residual cancer cells. The targets of these vaccines would not necessarily need to be cancer specific, in contrast to vaccines targeting cancers in vital organs, for which progress in the identification of cancer-specific target antigens has stalled. Additionally, prostate cancer typically has a long natural history, with the majority of patients living over 15 years from the time of diagnosis23. This evidence encouraged the development of therapies such as vaccines, which might take time to begin working or might simply alter the trajectory of the disease. Results from a study in which the growth rates of prostate tumours were modelled suggested that vaccine treatments might slow prostate cancer growth and produce better long-term outcomes and survival than therapies such as chemotherapies, which might only temporarily halt cancer growth during the period of treatment24. Moreover, differently from most cancer types for which a serum biomarker is not available, the availability of prostate-specific antigen (PSA) as a serum biomarker of prostate cancer has enabled the detection of micro-metastatic disease and PSA has been used to identify patients at risk for disease progression and reduced survival25. Lastly, in the 1970s, several reports showed that some patients experienced regression of prostate cancer metastases following treatment with cryotherapy, and that this treatment led to the development of prostate cancer-specific antibodies26,27; these findings are anecdotal but support the concept that vaccines used to generate prostate-specific immunity might have a therapeutic benefit.
In this Review, we describe the history of vaccines as treatments for prostate cancer and discuss vaccines that have been assessed in phase III clinical trials (Table 1) as well as other vaccines and combination approaches that have been tested or are currently being evaluated in clinical trials (Supplementary Table 1).
Table 1.
Phase III trials to assess antitumour vaccines in patients with prostate cancer
| Antigen specificity | Vaccine type | Vaccine | Antigen | Combination agents | Patients enrolled | Study title | Results and/or comments | Refs. |
|---|---|---|---|---|---|---|---|---|
| Non-antigen specific | Cellular vaccines | GVAX | NA | NA | 626 | GVAX vaccine for prostate cancer versus docetaxel and prednisone in patients with metastatic hormone-refractory prostate cancer | Terminated (based on futility analysis showing that the chance of meeting the primary end point was <30%) | 38 |
| GVAX | NA | Docetaxel | 408 | Docetaxel in combination with GVAX immunotherapy versus docetaxel and prednisone in patients with prostate cancer | Terminated (accrual and treatment with GVAX stopped owing to IDMC recommendation) | 39 | ||
| Dendritic cell vaccines | DCVAC/PCa | NA | Docetaxel and prednisone | 1,182 | A randomized, double-blind, multicentre, parallel group, phase III study to evaluate efficacy and safety of DCVAC/PCa versus placebo in men with mCRPC eligible for first-line chemotherapy (VIABLE) | No improvement in overall survival of patients with mCRPC | 45 | |
| Antigen specific | Peptide vaccines | Personalized peptide vaccines | HLA-A24-restricted peptide epitopes from several proteins | NA | 306 | A randomized phase III trial of personalized peptide vaccination for CRPC progressing after docetaxel | No improvement in overall survival | 66 |
| Antigen-loaded dendritic cell or antigen-presenting cell vaccines | Sipuleucel-T | PAP | NA | 176 | PROvenge treatment and early cancer treatment | In randomized trials conducted in patients with recurrent prostate cancer after prostatectomy, no difference in time to biochemical failure was observed | 13,84 | |
| Sipuleucel-T | PAP | NA | 127 | Vaccine therapy in treating patients with metastatic prostate cancer that has not responded to hormone therapy | In a randomized trial, no evidence of increased time to progression was observed but prolonged overall survival was shown in patients receiving sipuleucel-T | 77 | ||
| Sipuleucel-T | PAP | NA | 512 | A randomized, double-blind, placebo-controlled phase III trial of immunotherapy with autologous antigen-presenting cells loaded with PA2024 (Provenge(R), APC8015) in men with metastatic androgen-independent prostatic adenocarcinoma | Prolonged overall survival in patients treated with the vaccine compared with patients receiving placebo led to FDA approval in 2010 | 13 | ||
| Viral, bacterial and fungal vaccines | PROSTVAC-VF/TRICOM | PSA | GM-CSF | 1,297 | Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic patients with mCRPC | PROSTVAC was safe and well tolerated, but no improvement in overall survival was reported | 115 |
CRPC, castration-resistant prostate cancer; GM-CSF, granulocyte–macrophage colony-stimulating factor; IDMC, Independent Data Monitoring Committee; mCRPC, metastatic castration-resistant prostate cancer; NA, not available; PAP, prostatic acid phosphatase; PSA, prostate-specific antigen; TRICOM, B7-1, ICAM1 and leukocyte function-associated antigen 3.
Non-antigen-specific vaccines
Vaccines can be broadly classified as non-antigen specific, typically whole-cell vaccines, for which the specific antigenic target is not known, or antigen specific (Fig. 1). Whole-cell vaccine approaches initially leveraged knowledge of early microbial vaccines, in which the entire pathogen was inactivated and then readministered into the host in an attempt to generate protective immunity to one or more antigens presented by the pathogen15. This approach was favoured in the absence of known tumour-specific target antigens, which are possibly different among individuals. In early studies, irradiated prostate cancer cells, chemically coupled to rabbit γ-globulin as a foreign protein, were used as vaccines28,29. The use of this approach showed modest induction of tumour-associated antibodies, and further efforts focused on increasing the immunogenicity of these cellular vaccines. In a phase I/IIb trial, an autologous prostate cancer vaccine consisting of prostate cancer tissue obtained at the time of prostatectomy and treated ex vivo to upregulate major histocompatibility complex class I (MHC-I) and MHC-II expression was used to immunize patients at risk for prostate cancer recurrence30. In this study, after 5 years, a reduction in disease recurrence was observed in patients receiving the vaccine (PSA undetectable in 17 of 20 patients, 85%), compared with a non-randomized control group consisting of untreated patients (PSA undetectable in 10 of 21 patients, 48%). A new trial is being planned to confirm these results.
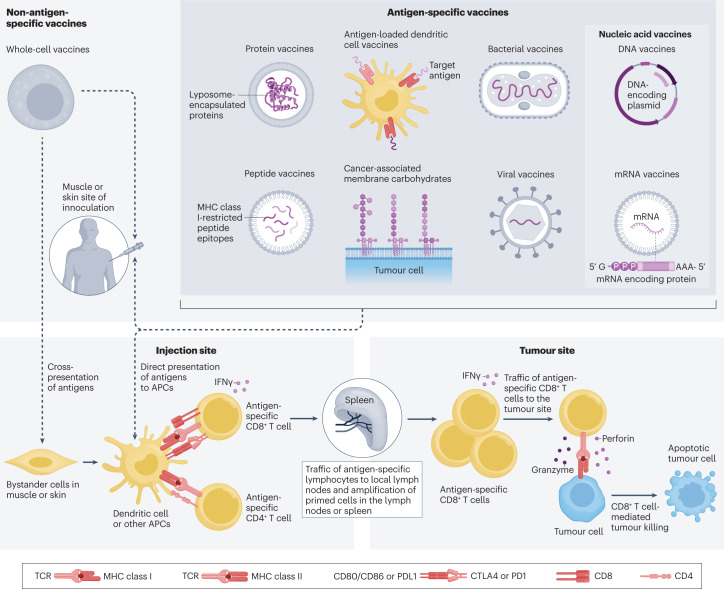
Fig. 1. Antitumour vaccine approaches in prostate cancer.
The types of vaccine platforms that have been explored for the treatment of prostate cancer using antigen-specific or non-antigen-specific approaches are shown. Vaccines have been delivered intradermally and intramuscularly alone or in combination with dendritic cells. In principle, antigens are present in whole-cell vaccines, are delivered as proteins or peptides, or are encoded by genetic vectors that activate CD4+ and CD8+ T cells within local lymph nodes and spleen, which can then traffic to tumour sites and mediate killing of antigen-expressing tumour cells. APC, antigen-presenting cell; CTLA4, cytotoxic T lymphocyte antigen 4; MHC, major histocompatibility complex; TCR, T cell receptor.
Another approach to increase the immunogenicity of tumour cells is transfection with genes encoding different cytokines. This approach was tested in the Dunning rat prostate cancer model, in which orthotopic injection of the syngeneic MatLyLu prostate cancer cell line formed anaplastic androgen-independent tumours that spontaneously metastasized31. Immunization of rats with irradiated MatLyLu cells generated no protection towards subsequent injection of live tumour cells unless these irradiated cells were transfected to express IL-2 or granulocyte–macrophage colony-stimulating factor (GM-CSF), suggesting that cellular vaccines engineered to secrete these cytokines might have antitumour efficacy32. Results from similar studies in a murine melanoma model indicated that the greatest stimulation of long-lasting antitumour immunity was observed with cellular vaccines transfected to express GM-CSF33. This approach, broadly known as GVAX, was then translated to human clinical trials. In the first clinical trial in which autologous prostate tumour cells were transfected to express GM-CSF16, the authors reported that the expansion of autologous cells treated ex vivo with a retrovirus to express GM-CSF could be accomplished in 8 of 11 (73%) of tumour samples. However, this approach of transfecting autologous tumour cells as a vaccine was not further pursued owing to the volume of tumour needed at surgery (>90%) to establish vaccine cell lines. Consequently, in subsequent studies, allogeneic prostate cancer cell lines transfected to express GM-CSF were used. This approach was assessed in five phase I/II trials including patients with prostate cancer at different stages and using different allogeneic cell lines34–37. In a trial including patients with recurrent, castration-sensitive prostate cancer, the investigators observed a significant decrease in the rate of serum PSA rise (PSA slope) in 16 of 21 patients (76%; P < 0.001) treated with GVAX37. In a separate dose-escalation phase I/II trial including 80 patients with mCRPC, 19% of patients showed PSA level stabilization after receiving GVAX vaccination36. Similar findings were observed in the other trials. On the basis of these results suggesting that GVAX vaccines might slow the progression of prostate cancer, two randomized phase III trials were initiated to evaluate the efficacy of GVAX versus docetaxel chemotherapy in patients with mCRPC (VITAL-1), and of docetaxel chemotherapy with or without GVAX in patients with mCRPC (VITAL-2)38. Results from an interim analysis of the VITAL-1 trial showed no improvement in overall survival (OS) in patients treated with GVAX compared with patients receiving docetaxel39. Results from an interim analysis of the VITAL-2 trial showed a greater death rate in patients treated with the combination therapy than in patients receiving docetaxel alone39. Thus, both trials were halted and the clinical development of GVAX for prostate cancer was suspended. Retrospectively, the design of these trials has been criticized, as little prior data were available to suggest the optimal dose and sequence of docetaxel to be used in combination with GVAX, which might have negatively affected the outcome of the VITAL-2 trial39. Additionally, in VITAL-1, a control group was absent; thus, GVAX might have provided some clinical benefit at least as good as — or even better than — docetaxel chemotherapy39.
GVAX was also assessed in combination with other agents. In a murine transgenic model of prostate cancer in which mice express the SV40 large T antigen under a prostate-specific promoter and develop spontaneous prostate tumours by 20 weeks of age, GVAX vaccination or cytotoxic T lymphocyte antigen 4 (CTLA4) blockade alone did not reduce tumour incidence, yet the combination of the two agents led to a reduction in prostate tumour incidence and inflammation within the prostate40. This approach was evaluated in a phase I dose-escalation trial, in which adverse events were more frequent with the combination therapy than those reported in clinical trials in which the single agents were used; however, 25% of patients had PSA declines of >50%, a higher frequency than previously reported for GVAX alone or CTLA4 blockade alone41. GVAX was also assessed in combination with androgen deprivation and cyclophosphamide in a trial including patients with newly diagnosed prostate cancer treated before prostatectomy42. In this trial, 29 patients were randomized to receive androgen deprivation using degarelix alone versus degarelix preceded by treatment with cyclophosphamide and GVAX (both given 2 weeks before degarelix). CD8+ T cell and CD4+ regulatory T cell tumour infiltration was observed in both treatment arms. Overall, 69% of patients treated with GVAX (plus cyclophosphamide and degarelix) were free of PSA recurrence at 2 years compared with 40% of patients treated with degarelix alone (not statistically significant, P value not reported). Unfortunately, GVAX either in combination with CTLA4 blockade or with degarelix has not moved forward to subsequent trials owing to the decision of Cell Genesys to suspend further clinical development of prostate GVAX following the results of the phase III trials.
Another type of whole-cell vaccine involves autologous dendritic cells (DCs) cultured ex vivo with either RNA from autologous tumour cells or with allogeneic prostate tumour cell lines. Specifically, DCVAC/PCa consists of autologous DCs cultured ex vivo with poly(I:C) and LNCaP prostate cancer cells, subsequently delivered subcutaneously as a vaccine43. This approach was tested in several early-phase clinical trials, alone or with radiotherapy or chemotherapy44. In a phase III trial (VIABLE trial), 1,182 men with mCRPC were randomized to receive DCVAC/PCa or placebo in combination with docetaxel chemotherapy45. No significant difference was observed between patients in the two groups with regard to the primary trial end point of OS (P = 0.6), or radiographic progression-free survival (PFS) (P = 0.89), time to PSA progression (P = 0.39) or time to skeletal-related events (P = 0.73) — all secondary end points of the trial. Considering these findings, DCVAC/PCa plus docetaxel has not been further studied. This outcome might have been anticipated based on the results from the VITAL-2 trial, in which GVAX had been similarly evaluated in combination with docetaxel. Moreover, the phase III trial in which DCVAC/PCa was used in combination with docetaxel was designed based on the results of a non-randomized, phase I/II trial including only 25 patients with progressive mCRPC44. This trial was almost certainly insufficient to identify the optimal treatment sequence and/or to justify conducting a large phase III trial in which DCVAC/PCa was combined with docetaxel. Based on the negative results from the phase III trial, DCVAC/PCa is not being pursued further.
Antigen-specific vaccines
A major disadvantage of non-antigen-specific vaccines was the absence of a defined target as a measure of immune response. Indeed, only clinical responses could be evaluated, and no known antigens were available to measure whether vaccination led to a biological effect. Additionally, this approach led to the co-administration of thousands of antigens that might be theoretically harmful, irrelevant or diminish the response to actually favourable antigens. In the development of infectious disease vaccines, such as for hepatitis B, using vaccines focused on a particular antigen was preferable compared with using an entire virus, particularly if the virus could not be cultured46. This antigen-specific vaccine led to a highly potent protective immunity and enabled measurement of whether an individual had been effectively immunized. These principles have favoured the development of antigen-specific vaccines, in most cases with the ultimate goal of developing multi-valent vaccines targeting multiple defined antigens.
Cancer-associated membrane carbohydrates
Membrane-bound carbohydrate moieties, including ganglioside (GM2), mucin 1 (MUC1), globo H and Thompson–Friedenreich antigen, have been found to be expressed preferentially on the surface of a variety of different tumour cells, suggesting that these factors might be candidate targets for immunotherapeutic approaches for prostate cancer, similarly to what was observed for other cancer types47. In a study in which human prostate tissues from patients with primary or metastatic disease were screened by immunohistochemistry for the presence of cancer-specific membrane-bound carbohydrate moieties and compared with tissues from healthy individuals, several membrane-bound carbohydrate antigens were found to be overexpressed in malignant prostate tissue compared with normal tissues48. The same investigators initiated two phase I vaccine trials including patients with biochemically recurrent, non-metastatic prostate cancer targeting the globo H hexasaccharide49 as well as the Thompson–Friedenreich antigen, each conjugated to the immunogenic foreign protein keyhole limpet haemocyanin (KLH) and delivered with a saponin-containing adjuvant known as QS-21 (ref. 50). In the trial in which globo H was targeted, two of five patients were observed to have a decrease in the PSA slope from pretreatment to post-treatment49. Similar changes in PSA slopes were observed in the trial targeting the Thompson–Friedenreich antigen, suggesting that vaccination targeting these antigens might slow prostate cancer progression50. Consequently, a phase II trial including 30 patients with castration-sensitive non-metastatic prostate cancer with rising PSA within 2 years of definitive local therapy was conducted, in which patients received subcutaneous injection with six carbohydrate antigens in QS-21 adjuvant for up to 1 year51; however, no changes in PSA slopes were observed (P = 0.89) and this approach was not further developed. The authors of this study questioned whether the discordant results compared with the previous phase I trials50,52 might have been ascribed to the small sample size of this phase II trial or to the inclusion of multiple antigens, which potentially distorted the immune response, leading to a reduction of the therapeutic response51.
Protein vaccines
Results from studies in which recombinant protein vaccines were used to treat infectious diseases (for example, vaccines targeting the hepatitis B surface antigen) showed that immunogenicity could be augmented using a cytokine adjuvant such as GM-CSF53. Based on this approach, in a small phase I trial including patients with prostate cancer at any stage after initial local therapy, PSA protein, formulated in liposomes, was given as a vaccine with or without GM-CSF as an adjuvant. In this study, 8 of 10 patients developed low frequencies of T cells specific for PSA but no clinical results were reported. Using GM-CSF as an adjuvant showed no benefit in terms of increasing the magnitude of immune response to the PSA target18; this approach has not been pursued further. In a similar approach, named Proscavax, PSA protein was used with IL-2 and GM-CSF as adjuvants. A phase II trial to assess the efficacy of this approach in patients with localized prostate cancer randomized to receive vaccination versus surveillance is ongoing; results from this trial have not yet been reported54.
Protein vaccines continue to be explored as anti-prostate cancer vaccines, but whether this approach provides advantages over simpler vaccine methods, such as peptide or genetic vaccines, is unknown.
Peptide vaccines
Several groups have identified MHC-I-restricted peptide epitopes derived from multiple prostate-specific proteins, including PSA55,56, prostatic acid phosphatase (PAP)57–59 and PSMA60. Phase I clinical trials have been conducted with these and other peptides, in which peptides were delivered directly, with or without various adjuvants, in patients with metastatic prostate cancer19,61,62. In general, results from these trials have shown no toxicity and some evidence of immune response being elicited towards the immunizing peptide but no substantial clinical benefit in terms of PSA decline, objective radiographic response or delays in disease progression. Thus, most of these trials were not further explored beyond phase I trials. Another group developed personalized peptide vaccines, in which 14 HLA-matched peptides derived from multiple prostate cancer-associated antigens — each chosen based on the patient’s pre-existing immunity to specific peptides before vaccination — were used as vaccines in patients with mCRPC63. This treatment was shown to be safe in early-phase clinical trials64,65. However, results from a phase III trial in which 310 patients with mCRPC who had previously received taxane chemotherapy were randomized to receive vaccination or placebo showed no difference in OS (P = 0.77)66. In this trial, patients receiving vaccination had been selected for the expression of peptide-specific IgG to a panel of 12 peptides and received up to 4 peptides for which pre-existing immunity had been shown. The reason for this failure is not clear but can be potentially related to the diversity of peptides used, not all of which would be predicted to expand cytotoxic T cells in all treated individuals. Indeed, the presence of pre-existing immunity, and IgG immunity specifically, does not necessarily mean that this immunity is therapeutic and, consequently, the choice of peptides used for immunization might not have led to the expansion of T cells with antitumour efficacy. However, considering these results, strategies using peptides altered to change the binding affinity to MHC or TCR as well as combinations of peptide vaccines with other immunomodulatory therapies are being explored in preclinical research as tools to increase the immunological and therapeutic benefit of peptide vaccines.
Antigen-loaded DC vaccines
The ability of DCs to take up exogenous antigens and prime T cells has led to a multitude of trials in which a target antigen was directly delivered using DCs loaded with proteins, peptides or nucleic acids67. In 1996, autologous DCs from patients with prostate cancer were cultured ex vivo for the first time with either autologous tumour cell lysates or HLA-A2-restricted peptide epitopes from PSMA to generate cytotoxic T lymphocytes in vitro60. Subsequently, the same group conducted trials using patient autologous DCs cultured with putative HLA-A2-restricted MHC-I epitopes from the PSMA protein68,69. In a phase II trial including 37 patients with prostate cancer at different stages who experienced recurrence after local therapy, the investigators reported complete response in 1 patient and partial response in 10 patients, 3 of whom had at least a 50% reduction in serum PSA68. However, in this study, Prostascint scans, which detect prostate cancer on the basis of PSMA expression, were used to identify radiographic responses. The high variability of this method, which is no longer used in clinical practice and also detects the target of the vaccine, probably confounded the interpretation of clinical response. A randomized phase III trial to assess this approach in patients with mCRPC was planned but never completed, primarily owing to funding constraints. The company developing this vaccine opted to pursue a similar approach using autologous DCs as a vaccine but loaded ex vivo with autologous tumour lysates rather than peptides based on results from a non-randomized clinical trial in which prolonged OS (18.3 months) had been observed in patients with glioblastoma70. In another approach, DCs isolated from patients with prostate cancer and transfected with mRNA encoding PSA were shown to stimulate a potent antigen-specific cytotoxic T cell response71. Similarly, in a small study including 13 patients with metastatic prostate cancer, autologous DCs were loaded with mRNA encoding PSA72, with the results showing a decreased PSA slope and increased PSA-specific T cell responses in all patients; unfortunately, this approach has not advanced further, for unclear reasons. In another trial, patients with mCRPC were treated with autologous DCs that had been cultured ex vivo with the mouse homologue of PAP, in principle using the xenoantigen form of the protein to circumvent immune tolerance to the native antigen73. T cell immune responses to the native protein were elicited by this approach, and 6 of 21 patients had stable disease following treatment. However, this approach was not pursued further owing to the results from concurrent trials in which a similar approach, sipuleucel-T, was evaluated. In the production of sipuleucel-T, autologous peripheral blood cells obtained through leukapheresis were enriched for monocyte populations and cultured ex vivo with a protein conjugate of human PAP and GM-CSF74. The concept was that GM-CSF would permit the uptake of the fusion construct and lead to the differentiation of monocyte populations to increase the efficacy of these antigen-presenting cells to present the PAP antigen when re-infused back into patients74. Sipuleucel-T was evaluated as a single agent in separate phase I/II clinical trials. In a trial in which patients with CRPC received three sipuleucel-T doses at 4-week intervals75, all patients developed T cell responses to the PAP–GM-CSF fusion protein (PA2024) and 38% of patients developed reactivity to the native PAP protein. Overall, 6 of 31 (19%) patients developed a PSA decline of at least 25%. In a separate trial in which patients with mCRPC received two sipuleucel-T doses followed by subcutaneous booster immunizations with the PA2024 protein, PSA decreases were observed in 3 of 21 (14%) individuals, with the decrease persisting for several years in 1 individual, suggesting that this treatment might lead to prolonged stable disease76.
These trials served as the basis for randomized phase III trials to evaluate sipuleucel-T in patients with early mCRPC. In the first phase III trial (D9901), 127 men with asymptomatic mCRPC, with tumours expressing PAP, were enrolled77. Patients were randomized 2:1 to receive three biweekly infusions of sipuleucel-T or a placebo (consisting of autologous cells obtained through leukapheresis that were re-infused without being cultured with PA2024). In this study, the primary end point was time to progression (TTP). Serum PSA decreases of >25% were detected in 6.8% of patients receiving sipuleucel-T; however, differences in the primary end point of TTP did not reach statistical significance (P = 0.052) as patients receiving sipuleucel-T had a median TTP of 11.7 weeks (95% CI 9.1–16.6) compared with 10.0 weeks for patients receiving placebo (95% CI 8.7–13.1)77. While the D9901 trial was ongoing, a second companion phase III trial (D9902A) was also under way, in which patients with asymptomatic mCRPC were accrued. This trial was designed as a second, confirmatory study for D9901, with the same primary end point. Considering that the results from the D9901 trial did not show any difference in TTP, the D9902A trial was abandoned before full accrual was reached.
Results from a subset analysis of the D9901 trial showed that patients treated with sipuleucel-T who had a Gleason score of ≤7 had a significant increase in TTP (16.0 weeks) compared with patients receiving placebo (9.0 weeks; P = 0.001)78,79. This evidence led to the design of another phase III trial (D9902B) that, similarly to the D9901 trial, had TTP as the primary end point; however, in this trial, only patients with asymptomatic mCRPC who had tumours with a Gleason score of ≤7 (n = 127) were enrolled. When this trial was starting, results from a further analysis of the previous D9901 trial showed a significantly higher 3-year OS in patients receiving sipuleucel-T (25.9 months) than in patients receiving placebo (21.4 months, HR 1.43; P = 0.01)80. Moreover, results from data integration of D9901 and the partially completed D9902A trial, including a total of 147 patients treated with sipuleucel-T and 78 patients treated with a placebo, showed that patients receiving sipuleucel-T had a median OS of 23.2 months (95% CI 19.0–31.0) versus 18.9 months (95% CI 13.5–25.3) in the placebo group (HR 1.5; P = 0.011)80. Considering these findings, the D9902B trial was expanded to become the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial13. The primary end point was changed from TTP to OS, and the accrual goal was expanded to 512 participants, with no exclusions based on Gleason score. Results from the IMPACT trial confirmed that treatment with sipuleucel-T provided a substantial increase in OS (25.8 and 21.7 months in patients receiving treatment and placebo, respectively), which was consistent with results from the previous trials and reflected a significant difference in the risk of death between the two groups (HR 0.78, 95% CI 0.61–0.96; P = 0.03)13. No significant difference in TTP was observed between patients in the two arms (14.6 weeks in the sipuleucel-T group versus 14.4 weeks in the placebo group; P = 0.63). Based on this trial and on the totality of the trials preparatory to the IMPACT study, sipuleucel-T was approved by the FDA in 2010 as a treatment for patients with asymptomatic or minimally symptomatic mCRPC81. Sipuleucel-T was the first vaccine approved in the USA as a treatment for existing human cancer, rather than a vaccine that might prevent the development of cancer. Notably, in all the trials with sipuleucel-T, very few adverse events were observed. In the IMPACT trial, grade 3 or higher events were observed in 31.7% of patients receiving sipuleucel-T and in 35% of patients receiving placebo. Low-grade adverse events that occurred most frequently in patients receiving sipuleucel-T included chills, fever, headache, myalgias and flu-like symptoms13.
Since the approval of sipuleucel-T, this agent has been evaluated in other stages of disease and in combination with other standard agents used for the treatment of prostate cancer. In a phase II trial, sipuleucel-T was given to 37 patients in the neoadjuvant setting, 6–7 weeks before prostatectomy, and treatment led to an increase in tumour-infiltrating T cells; however, no reports of whether the treatment improved pathological responses or resulted in delays in disease recurrence are available82,83. In a randomized trial including 117 patients with evidence of biochemical recurrence following prostatectomy, patients underwent androgen deprivation for 3–4 months and were subsequently randomized to receive sipuleucel-T or placebo. No difference in time to PSA recurrence (P = 0.74) was observed between patients in the two groups; however, patients treated with sipuleucel-T had a 48% longer PSA doubling time after testosterone recovery than patients treated with placebo (P = 0.04), suggesting that sipuleucel-T might be combined with androgen deprivation to slow disease progression84. In another trial, sipuleucel-T was given either before or after standard androgen deprivation therapy to patients with early PSA-recurrent prostate cancer without evidence of metastases. In this trial, the time to PSA recurrence after stopping therapy was similar between the two groups, but the sequence of treatments seemed to affect the generation of immune responses, as patients who received sipuleucel-T before androgen deprivation had greater T cell responses to the immunizing antigen than patients receiving the treatment after androgen deprivation85. Another trial included 69 patients with mCRPC who received sipuleucel-T in combination or in sequence with abiraterone. In this study, the feasibility of using this combination was confirmed but clinical end points, such as PFS or OS, were not reported. PSA decreases of ≥50% were observed in 65.7% of patients receiving the combination therapy and in 58.8% of patients receiving the agents sequentially (P = 0.624)86. The authors concluded that sipuleucel-T could be safely given with abiraterone, that sipuleucel-T production was not affected by the concurrent use of prednisone with abiraterone and that the sequence of therapy might not matter. Similarly, results from a trial including 51 patients with mCRPC who received sipuleucel-T with or without a priming dose of radiation to a single metastatic lesion87 showed that radiotherapy did not have a negative effect on the generation of immune responses; however, the combination therapy did not induce prolonged PFS (3.65 months) compared with sipuleucel-T alone (2.46 months; P = 0.06). The combination of sipuleucel-T with radiotherapy was assessed in another randomized phase II trial in which 32 patients with bone-metastatic CRPC received sipuleucel-T with or without 223Ra (ref. 88). Patients treated with the combination therapy had greater clinical responses in terms of PSA decline than patients in the sipuleucel-T-alone arm, with a PSA reduction of at least 50% observed in 5 of 15 patients in the combination arm and in 0 of 14 patients receiving sipuleucel-T alone (P = 0.04). Patients treated with the combination therapy also had prolonged TTP compared with patients receiving only sipuleucel-T (median 39 weeks versus 12 weeks; P < 0.01), although reduced rates of immune response were detected in the combination arm88. Taken together, these results suggest that the combination of sipuleucel-T with radiotherapy is feasible but does not seem to affect antitumour response.
In some studies, sipuleucel-T was combined with other immune-active agents. Trials in which patients with mCRPC received sipuleucel-T in combination with IL-7 (ref. 89), atezolizumab90, ipilimumab91, indoximod92 or a DNA vaccine encoding the same PAP target93 have been conducted. In none of these trials was substantial differences observed in clinical outcomes, such as TTP or OS, in patients receiving the combination therapy compared with sipuleucel-T alone. A possible explanation for this evidence is that the 6-week treatment course used for sipuleucel-T delivery might not be optimal for the use of this agent in combination with other agents that might affect the generation or activity of the antitumour immune response. Although not yet published in a peer-reviewed journal, results from a study presented at the AUA meeting in 2022 have begun to detail the immune subsets of T cells present in the sipuleucel-T product, including the expression of immune-checkpoint molecules94. Further similar preclinical studies are needed to understand the mechanisms of action and resistance to this therapy to identify better potential treatment combinations than those investigated to date and to evaluate whether the timing and sequence of these different combinations will result in improved efficacy.
Viral and bacterial vaccines
A major function of the immune system is to recognize and respond to microbial infections. This feature has led to the engineering of viral and bacterial vectors to encode tumour-associated antigens as a tool to elicit antitumour immunity95,96. Perhaps the best-studied approach has been the one using vaccinia and other poxvirus vectors. Vaccinia virus is a DNA orthopoxvirus that replicates in the cytoplasm97. Delivery of poxvirus vectors results in infected cells expressing peptides from virally derived proteins in MHC-I molecules, stimulating a vigorous cell-mediated response against the antigenic proteins98. Additionally, vaccinia virus has a large genome and, therefore, is an ideal vector for deliveries in which transduction of multiple genes or genes encoding large proteins are involved99. Results from early studies showed that immunization with recombinant vaccinia virus expressing a target antigen, including viruses expressing PSA, could elicit antigen-specific cytotoxic T cell responses in murine and primate models98,100–103. In the first trial in which the vaccinia virus encoding PSA was used104, the approach showed safety and the ability to elicit antibody responses to PSA. The effect of multiple immunizations with vaccina-PSA was evaluated in subsequent trials. In a study in which 33 patients with PSA-recurrent prostate cancer received 3 doses of vaccinia-PSA at 4-week intervals, the treatment was shown to be safe and to elicit PSA-specific T cells105. Another dose-escalation trial involved 42 patients with mCRPC. In this study, no objective tumour responses were observed and vaccination with vaccinia-PSA did not elicit detectable antibody responses to PSA. However, using enzyme-linked immunosorbent spot (ELISPOT) assay to evaluate cells secreting IFNγ in response to antigen challenge, a more than twofold increase in PSA-specific T cells was observed in 3 of 5 patients20. Taken together, results from these trials showed that the use of vaccinia-PSA vaccines was safe and could elicit a T cell immune response to the PSA ‘self’ antigen but did not elicit objective tumour responses.
Results from studies in murine models showed that repetitive immunization with vaccinia could lead to a diminished T cell response to the encoded target antigen owing to an overwhelming response to the vaccinia vector itself103,106. This evidence led to studies in which heterologous prime–boost approaches using other poxvirus vectors, specifically fowlpox virus, were used. Differently from vaccinia, few patients have pre-vaccination exposure and pre-existing immunity to fowlpox virus107. Additionally, fowlpox does not replicate and reinfect other primate cells — as vaccinia does103 — which probably reduces the risk of sensitization to the fowlpox vector. In a multi-site phase II study, tolerability and efficacy of different prime–boost sequences using vaccinia and fowlpox viruses encoding PSA were assessed in patients with early PSA-recurrent prostate cancer108. Specifically, patients received either four vaccinations with recombinant fowlpox-PSA (rF-PSA), three rF-PSA vaccinations followed by one vaccinia-PSA (rV-PSA) vaccination, or one rV-PSA followed by three rF-PSA vaccinations. The trial was not powered to detect differences in progression rates among the study arms. However, at a 19-month follow-up time, 45% of patients in all treatment groups were free of PSA progression and 78% of all patients were free of objective disease progression. The median time to PSA progression was greatest in patients receiving rV-PSA followed by rF-PSA, suggesting that this treatment sequence would be the preferred sequence for subsequent trials108.
In addition to studying the use of adjuvants such as GM-CSF, the incorporation of other immune-modulating agents directly into the vectors was also explored. Specifically, results from preclinical studies in mice showed that poxvirus vectors encoding three T cell costimulatory molecules — B7-1, ICAM1 and leukocyte function-associated antigen 3 (LFA3) (TRICOM) — along with a target antigen were superior to viral vectors encoding the antigen alone in eliciting antigen-specific T cell responses109. The safety and feasibility of this approach using vaccinia (PROSTVAC-V) and fowlpox (PROSTVAC-F) vectors encoding PSA and TRICOM were first shown in a phase I trial including 10 patients with CRPC110. Evidence from several other small trials showed that this vaccine could be safely given to patients with newly diagnosed prostate cancer111 and in combination with standard therapies such as androgen deprivation112 or docetaxel chemotherapy113. Subsequently, a multicentre phase II trial including 127 patients with mCRPC randomized to receive treatment with PROSTVAC-VF (a priming immunization with PROSTVAC-V followed by six booster immunizations with PROSTVAC-F) or placebo (using empty viral vectors) was conducted. In this trial, no increase in PFS, which was the primary end point, was observed (P = 0.6); however, an increase in OS was reported in patients receiving PROSTVAC-VF (25.1 months) compared with patients receiving placebo (16.6 months; P = 0.0061), similarly to what had been observed with sipuleucel-T114. Based on these results, a phase III trial was conducted to evaluate PROSTVAC-VF in patients with asymptomatic or minimally symptomatic mCRPC — the same population that was approved for the treatment with sipuleucel-T — with OS as the primary end point. The treatment was well tolerated but no increase in OS was observed in patients receiving PROSTVAC-VF treatment compared with patients receiving placebo115. The reason for this failure is not clear; however, between the conduct of phase II and phase III trials for PROSTVAC-VF, several agents had been approved for mCRPC, including abiraterone and enzalutamide, and ~70% of patients received subsequent therapies following treatment during the phase III trial116. Thus, a treatment benefit might have been lost owing to subsequent therapies that could have positively affected OS and, therefore, negated any difference between the study arms115. In subsequent exploratory trials, the PROSTVAC vaccine was given in combination with other immune-modulating agents, such as nivolumab117, or before prostatectomy118. In both trials, PROSTVAC showed biological activity, with PSA declines observed in patients treated with nivolumab plus PROSTVAC and increased T cell infiltration in patients receiving PROSTVAC in the neoadjuvant setting118. However, the clinical development of this vaccine was discontinued following the results of the phase III trial115.
Listeria monocytogenes is an intracellular bacterium that has been investigated as a vaccine vector, in part owing to the predilection of this bacterium to be uptaken by DCs119. In murine models of colorectal cancer and prostate cancer, a modified strain with deletions in two virulence factors, which limits L. monocytogenes infection of non-phagocytic cells and cell-to-cell spread, was shown to lead to potent antitumour activity120,121. This strain engineered to express four prostate cancer-associated antigens (PAP, NKX3.1, synovial sarcoma X breakpoint 2 (SSX2) and PSMA), was used in a phase I trial including 26 patients with mCRPC122. This trial was terminated early owing to the absence of sufficient evidence of immune response to the target antigens and the absence of objective clinical responses122. In another trial including 50 patients with mCRPC, a different attenuated strain of Listeria encoding PSA (ADXS31-142) was used. Patients with mCRPC received ADXS31-142 alone (n = 13) or with pembrolizumab (n = 37). No objective radiographic responses were observed and one (13%) patient in the monotherapy arm and five (17%) patients in the combination arm had a PSA reduction of ≥50% from baseline. However, the median OS was 7.5 months for patients in the vaccine-alone arm and 33.7 months for patients in the combination arm (P value not reported)123. Taken together, these results suggest that combination treatments, particularly combinations with PD1-blocking agents, are preferred approaches for future trials to assess bacterial or viral vaccines.
Nucleic acid vaccines
Nucleic acid vaccines, in which plasmid DNA or mRNA are used to encode defined target antigens, are similar to viral vector vaccines in terms of mechanism of action but have the advantage of not expressing any foreign viral genes124. Thus, nucleic acid vaccines are safer than viral and bacterial vaccines, have a low likelihood of genome integration, and do not elicit an immune response towards the vectors. Plasmid DNA derived from bacterial DNA encodes an antigen but might also provide TLR9 agonist signals and activate other cytoplasmic DNA-sensing molecules such as AIM2, RIG-I and STING125; similarly, RNA might activate other TLR sensors such as TLR3 and TLR7 (refs. 126,127). The activation of different innate-sensing pathways through these approaches could potentially lead to different immune responses. Currently, much enthusiasm exists around the development of mRNA vaccines as cancer vaccines considering the success of this vaccine type against SARS-CoV-2; however, little comparison between DNA versus RNA vaccines in terms of potential differences in immunogenicity or antitumour efficacy exists128. Conceptually, RNA vaccines have a potential advantage over plasmid DNA vaccines as mRNA does not need to pass the nuclear membrane for transcription to take place within transfected cells and, therefore, higher amounts of protein can be rapidly generated from transfected mRNA than those obtained with DNA. However, RNA is less stable than DNA owing to tissue RNases. Thus, historically, DNA vaccines have been explored the most.
Nucleic acid vaccines — plasmid DNA
The first target for DNA vaccines to be explored for prostate cancer treatment was PSA. In a phase I dose-escalation study, a plasmid encoding full-length PSA was given intramuscularly and intradermally to patients with advanced CRPC in monthly cycles for 5 months. The highest dose of vaccine (900 μg of DNA) elicited PSA-specific cellular and humoral antibody responses as measured by enzyme-linked immunosorbent assays (ELISA) for either PSA-specific antibodies or IFNγ release following PSA stimulation of lymphocytes129. To further improve the immunogenicity of this PSA vaccine, researchers from the same group assessed the efficacy of a DNA vaccine encoding the rhesus PSA gene given intradermally and with electroporation; electroporation was added following plasmid intradermal DNA delivery to increase the transfection of antigen-presenting cells130. Patients with non-metastatic castration-sensitive prostate cancer were treated with androgen deprivation followed by the vaccine, and immune responses to PSA were detected in at least 4 of 15 patients, and 4 of 15 patients had a ≥50% increase in PSA doubling time. Immune responses to PSA were also detected in patients receiving androgen deprivation alone before vaccination, complicating the interpretation of the findings from this trial. Consequently, this approach has not been pursued further.
The most studied antigen in DNA vaccine trials for prostate cancer has been PAP, the same target used for the sipuleucel-T vaccine. In a phase I dose-escalation study, a plasmid encoding PAP (pTVG-HP) was given to 22 patients with PSA-recurrent, non-metastatic prostate cancer, and was shown to be safe and to elicit CD8+ T cell immune responses at each dose level tested21. In a separate phase I trial, two different treatment schedules of pTVG-HP (six biweekly immunizations followed by quarterly immunizations versus an individualized schedule determined by real-time immunological monitoring) were assessed in 17 patients with non-metastatic CRPC131. Results from this study showed that multiple DNA immunizations were required to elicit and maintain PAP-specific immune responses. In both studies, increases in PSA doubling time were observed131,132. Based on these findings, a randomized phase II study was conducted to determine whether vaccination could delay the development of metastatic disease in patients with non-metastatic, castration-sensitive prostate cancer133. Overall, 99 patients with a pretreatment PSA doubling time of <12 months were randomized to receive pTVG-HP with GM-CSF adjuvant or GM-CSF alone. No difference in overall 2-year metastasis-free survival was observed between the two cohorts (41.8% versus 42.3%; P = 0.97). However, the subset of patients with the most rapidly progressing disease (PSA doubling time of <3 months) treated with pTVG-HP did have increased metastasis-free survival (12.0 months versus 6.1 months in patients receiving GM-CSF alone; P = 0.03). Additionally, sodium fluoride uptake in micro-metastatic bone disease detected by 18F-NaF PET–CT was decreased in patients receiving the vaccine treatment, suggesting that biological activity exists but is not sufficient to delay disease progression133.
Together, these findings suggest that the pTVG-HP vaccine showed immunological activity but should not be further pursued as a single agent, and that DNA vaccines should be evaluated in combination with other vaccines or other immune-activating agents. As a result of these findings, in a subsequent trial, the pTVG-HP vaccine was given following sipuleucel-T as a prime–boost approach targeting the same target antigen (PAP)93. This trial was small, but DNA booster immunizations were shown to augment the antibody response to PAP that was elicited by sipuleucel-T; however, no differences in T cell responses or in TTP were observed between patients in the two groups. Results from preclinical studies in which murine models were used showed that DNA vaccination led to an increase in PD1 expression on activated CD8+ T cells and that combining vaccination with PD1 or PDL1 blockade led to superior antitumour response, as measured by tumour growth rates and tumour eradication, than treatment with either agent alone, suggesting that DNA vaccination should be used with concurrent PD1 blockade134,135. The efficacy of DNA vaccination with PD1 blockade was initially shown in a pilot trial including patients with mCRPC. Patients were vaccinated with pTVG-HP either concurrently with pembrolizumab over 12 weeks or receiving pembrolizumab after a 12-week course of vaccination136. In this trial, decreases in tumour volumes were observed in 4 of 5 patients with measurable disease treated with the agents in combination versus 1 of 3 patients treated sequentially. Similarly, any PSA decline from baseline was identified in 8 of 13 patients receiving the agents in combination compared with 1 of 12 patients receiving the agents sequentially (P = 0.01)136. PSA declines of >50% occurred in 2 of 13 patients receiving the agents in combination but not in any patient receiving the agents sequentially. Thus, this trial was expanded to include a total of 66 patients with mCRPC to evaluate the effect of treatment on TTP using pTVG-HP in combination with pembrolizumab. Overall, 10 of 25 patients with measurable disease experienced decreases in tumour volume, but only 1 patient had a partial response and no complete radiographic responses were observed. The overall radiographic PFS rate at 6 months was 47%, the overall median time on treatment was 5.6 months (95% CI 5.4–10.8 months) and 32% of patients remained on trial beyond 6 months without progression137; the median OS was 22.9 months (95% CI 16.2–25.6 months). Considering these findings, pTVG-HP in combination with PD1-blocking agents is being pursued in trials including patients with early recurrent prostate cancer receiving pTVG-HP in combination with nivolumab138 and in trials including patients with mCRPC receiving pTVG-HP in combination with a DNA vaccine encoding another antigen (pTVG-AR) and pembrolizumab139.
PSMA has also been evaluated as a target antigen for DNA vaccines. A DNA vaccine encoding an HLA-A2-binding epitope of the PSMA gene fused to a fragment of the tetanus toxin was tested in a phase I/II dose-escalation trial140. Patients received the vaccine with or without electroporation, and a significant increase in PSA doubling time (from 11.97 months pretreatment to 16.82 months after treatment; P = 0.04) was observed in patients receiving the vaccine compared with unvaccinated patients140. The investigators deemed this approach worthy of evaluation in a randomized trial; however, to our knowledge, no further clinical development of this vaccine has been conducted. Another approach consisted of a DNA vaccine encoding fragments of PSMA and preferentially expressed antigen in melanoma (PRAME). In a multicentre phase I trial, patients with several cancer types were immunized by intra-lymph node injection with DNA followed by immunization with peptides derived from PSMA and PRAME141. This approach was safe and induced immunological activity but has not been further pursued in patients with prostate cancer, probably owing to evidence indicating that only 1 of 10 (10%) patients experienced a PSA decline of >50% and no partial or complete radiographic responses being detected. However, in another trial, a DNA vaccine consisting of plasmids encoding both PSMA and PSA (INO-5150) was delivered by electroporation with or without a plasmid DNA encoding IL-12 (INO-9012) in 62 patients with biochemically recurrent prostate cancer142. No safety concerns were shown in this study and patients experienced an increase in PSA doubling time (from 6.4 months to 17.3 months; P < 0.001), which was associated with the development of CD8+ T cells specific for the target antigens and expressing CD38, perforin and PD1 (ref. 142). This vaccine approach is currently being evaluated in combination with PD1 blockade (nivolumab) and FLT3 ligand (CDX-301), an agent used to specifically expand DCs, in a multi-arm trial including patients with mCRPC143.
The androgen receptor (AR), the primary pharmacological target of prostate cancer, has also been investigated as a vaccine antigen. Results from preclinical studies in mouse models of prostate cancer showed the safety and antitumour efficacy of a DNA vaccine encoding the ligand-binding domain of AR (pTVG-AR)144,145. Specifically, antitumour efficacy was observed when pTVG-AR was used with androgen deprivation (the primary treatment for prostate cancer that can lead to overexpression of AR), suggesting that vaccination with pTVG-AR might be combined with androgen deprivation146. In a phase I trial including patients with newly metastatic prostate cancer who had begun androgen deprivation within 6 months of trial entry, pTVG-AR was assessed in four treatment arms in which patients received one of two treatment schedules, with or without GM-CSF given as a vaccine adjuvant147. This vaccination was shown to be safe, and one schedule elicited more AR-specific IFNγ-secreting T cells than the other schedule; GM-CSF addition was not shown to augment immune responses. Patients who experienced immune response to the AR ligand-binding domain target had a longer time to development of castration-resistant disease (median time not reached) than patients who did not develop immune responses (median time 9.2 months; P = 0.003)147. The pTVG-AR vaccine is being further explored in ongoing trials in combination with pTVG-HP and pembrolizumab in patients with mCRPC139 and in patients with high-risk newly diagnosed prostate cancer in sequence with androgen deprivation therapy, with or without PD1 blockade (nivolumab)148.
Prostate cancer has traditionally been associated with a low mutational burden, suggesting a low number of potential tumour-specific mutation-associated neoantigens (MANA)149. This evidence, together with the availability of prostate cancer-specific targets, impeded the development of personalized vaccines for prostate cancer targeting MANA, similarly to vaccine antigens explored in other cancer types150. However, in a pilot clinical trial, a personalized vaccine approach using a DNA vaccine encoding MANA in combination with ipilimumab and nivolumab following treatment with the PROSTVAC vaccine is being assessed in patients with metastatic castration-sensitive prostate cancer151.
Plasmid DNA vaccines have also been used as part of heterologous immunization approaches152,153. An approach known as vaccine-based immunotherapy regimen (VBIR), in which a chimpanzee adenovirus vector encoding a target was used for priming followed by vaccination with plasmid DNA encoding the same antigen, was tested in murine and non-human primate preclinical models and shown to induce potent T cell responses, which could be further augmented using antibodies targeting PD1 and CTLA4 delivered subcutaneously. This approach was used in a large phase I trial including patients with different stages of prostate cancer, and three antigens were targeted (PSA, PSMA and prostate stem cell antigen). Although not yet published in a peer-reviewed journal, results from this trial showed few objective tumour responses and grade 3 or 4 adverse events in 38% of patients154. Consequently, this approach is not being further pursued. However, collectively, DNA vaccines have shown evidence of eliciting T cell immune response to the encoded antigen and, in combination with T cell-checkpoint blockade, have shown clinical activity as measured by changes in serum PSA. Randomized clinical trials in which DNA vaccines will be used in combination with T cell-checkpoint blockade and other immunotherapy approaches are anticipated.
Nucleic acid vaccines — mRNA
To date, only one vaccine approach using mRNA through direct injection has been reported. In a phase I/IIa study, an mRNA vaccine, known as CV9103, encoding PSA, prostate stem cell antigen, PSMA and six-transmembrane epithelial antigen of the prostate 1 (STEAP1) was given by intradermal injection to 76 patients with CRPC155. A total of 26 of 33 evaluable patients developed an immune response to one or more antigens, and 15 of 33 patients developed an immune response to multiple antigens. Based on this evidence of immunogenicity, and on the observation that the estimated OS of patients with metastatic disease was favourable at 31.4 months, the vaccine was modified to include two additional antigens, PAP and MUC1, for further clinical evaluation. This second-generation vaccine, named CV9104, was assessed in a randomized phase I/IIb trial including 197 patients with mCRPC; however, no increase in OS was observed in patients treated with the vaccine (35.5 months) compared with patients receiving placebo (33.7 months; P = 0.33)156. Similarly to what was observed in PROSTVAC trials, this failure might be ascribed to the development of other therapies for prostate cancer that also affected OS. The initial phase I trial155 was conducted in 2009, before the approval of several life-prolonging therapies, including enzalutamide, abiraterone, cabazitaxel and 223Ra. The subsequent use of these therapies in patients included in the phase I/IIb trial to assess CV9104 (ref. 156) could have negated any positive clinical benefit from CV9104, particularly considering the relatively small sample size; however, this approach has not been further pursued. Considering the success of mRNA vaccines against SARS-CoV-2, renewed interest has grown in mRNA vaccines as anticancer vaccines. We anticipate that multiple new mRNA vaccines will be evaluated over the next 5 years and that the efficacy of these vaccines might be increased in combination with T cell-checkpoint blockade as observed with DNA vaccines.
Lessons learned to guide future antitumour vaccine approaches
Therapeutic cancer vaccines targeting single or multiple target antigens through different approaches have been extensively studied as treatments for prostate cancer over the past 20 years. Most of these approaches have been limited to phase I studies, and nearly all vaccines were safe and showed some evidence of immunological activity. Overall, five approaches — GVAX, DCVAC/PCa, a multi-epitope peptide vaccine, sipuleucel-T and PROSTVAC — have been investigated in randomized phase III trials (Table 1). Among these vaccines, sipuleucel-T was the only agent to prolong OS in patients with mCRPC and is the only therapeutic cancer vaccine approach currently approved by the FDA for the treatment of advanced prostate cancer.
Different lessons can be learnt from the experience of vaccine trials. First, all vaccine approaches showed rather modest clinical activity when used as single agents, with objective tumour responses or large PSA declines occurring only in a few patients. Results from several studies have shown more subtle effects on disease growth, as exemplified by changes in PSA doubling time, rather than changes in tumour volumes detected by radiographic imaging, suggesting that vaccines might have increased efficacy in the treatment of patients with minimal disease burden24. However, the fact that only one vaccine approach (sipuleucel-T) was able to improve OS in phase III trials suggests that the mechanism of action of this agent could be different from the others. The fact that sipuleucel-T is an unpurified cellular immunotherapy product suggests that perhaps this product contains other effector cell populations or other factors able to maintain T cell effector function rather than simply serving to amplify patient antigen-specific T cell responses74. This conclusion is supported by the fact that sipuleucel-T did not elicit detectable target antigen-specific immunity in the majority of patients, and this was certainly not higher than the response observed with PROSTVAC or by using a plasmid DNA vaccine targeting the same antigen (PAP)13,157. Moreover, the fact that a variety of vaccine approaches (GVAX, tumour lysate-loaded DCs, peptide vaccines, viral vaccines) assessed in other phase III trials did not improve patient OS suggests that using vaccines alone with the goal of amplifying and/or activating tumour-specific T cells is not sufficient to treat advanced prostate cancer.
Additionally, experience with vaccines suggests that the ‘failure’ of these tools as single-agent antitumour therapies is not ascribable to a deficiency of the vaccine approach, as no single vaccine seemed markedly different from another in terms of clinical efficacy or success in eliciting T cell responses to the target antigen. This evidence is of high relevance because a renewed interest in developing RNA-based antitumour vaccines is currently growing. However, this approach, if used as a monotherapy, will be unlikely to yield markedly better clinical results in patients with cancer than other vaccine approaches. Similarly, multiple different antigens have been used as targets in prostate cancer vaccines; thus, whether some individual antigens, or multiple targets in the case of whole-cell vaccines, serve as better targets than others is unclear. This issue is of current relevance as considerable interest has also been growing in the identification of mutation-associated neoantigens to be included in tumour vaccines as theoretically superior antigens158. Unfortunately, the low frequency of tumour mutations in prostate cancer precludes this approach for most patients with prostate cancer, and the collective experience of trials for other cancer types showed that objective tumour responses following single-agent vaccination targeting neoantigens remain infrequent and not substantially different from those following vaccination targeting shared antigens159,160.
The general 'failure' of antitumour vaccines as single-agent therapies for prostate cancer and other cancers is, perhaps, not surprising. Antimicrobial vaccines have only been used in the setting of disease prophylaxis and not in the treatment of existing infections. Similarly, in preclinical models, many antitumour vaccines have shown greater efficacy in preventing the establishment of tumours than in treating existing tumours33. These observations serve as proof that vaccines can elicit immune responses with antitumour activity but suggest that mechanisms present within tumours can dysregulate the immune effector functions activated by vaccines. Indeed, increasing evidence indicates that tumours avoid immune detection and destruction through multiple means161. Activation of CD8+ T cells by vaccination was shown to lead to increased expression of PD1 on these CD8+ T cells; additionally, CD8+ T cells secreting IFNγ can increase the expression of the ligand PDL1 within the tumour microenvironment, leading to the decreased antitumour activity of these PD1-expressing CD8+ T cells134. Similarly, advanced prostate tumours are infiltrated by regulatory cell populations, including myeloid-derived suppressor cells (MDSCs) or tumour-associated macrophages (TAMs)162. These populations can be recruited specifically to prostate tumours through the release of IL-8 and potentially other chemokines by these tumours163. These myeloid populations have a direct suppressive activity on the function of CD8+ T cells and can also secrete factors, including indoleamine 2,3-dioxygenase, which can suppress the function of CD8+ T cells and lead to the recruitment of regulatory CD4+ T cells164,165. Similarly, prostate cancers can secrete large amounts of TGFβ, which, in turn, can also lead to reduced T cell function and recruitment of MDSCs or TAMs166. Considering the presence of all these, and potentially other, immunosuppressive mechanisms within prostate tumours, the activation and expansion of tumour-targeted T cells through vaccines are likely to be circumvented or rendered free of consequences by numerous tumour-associated mechanisms of resistance and immune evasion. Directly providing increased numbers of activated tumour-specific T cells using chimeric antigen receptor (CAR) T cell approaches or activating tumour-specific T cells through bispecific antibodies could potentially be promising alternatives to vaccines; however, to date, these approaches have not been shown to induce sustained antitumour efficacy, similarly to what was observed with vaccines, and will likely be limited by the same tumour-associated mechanisms of resistance as vaccines167–169.
Owing to the multiple mechanisms of tumour-associated resistance and immune evasion (Fig. 2), the optimal use of prostate cancer vaccines — and the focus of vaccine trials over the next 5–10 years — will likely be in combination with agents targeting these resistance mechanisms. Increased antitumour activity of vaccines has already been observed by combining plasmid DNA vaccines with PD1 blockade136,137, and this approach is being further investigated in several trials138,139,148. Additionally, a trial in which an RNA vaccine encoding five prostate tumour antigens is being used in combination with cemiplimab is currently under way170 as are two trials to assess viral vaccines in combination with PD1 blockade171,172. Together, results from these trials should provide evidence about the efficacy of these combination approaches with immune-checkpoint blockade plus vaccination using different vaccine delivery methods. The expression of immune-checkpoint receptors other than PD1 is also increased on activated T cells; thus, in future studies, vaccination approaches will probably target multiple immune-checkpoint receptors173. In an ongoing trial, PROSTVAC is being used in combination with an antibody targeting PDL1 and TGFβ signalling with the aim of targeting these two separate mechanisms of resistance172. Trials to assess vaccines in combination with agents that can deplete or alter the function of MDSCs or TAMs are eagerly awaited as this approach will likely target a major mechanism of resistance used by prostate tumours. With regard to vaccine approaches, nucleic acid vaccines offer considerable advantages over other approaches in terms of cost, ease of manufacturing and safety; moreover, differently from peptide vaccines, DNA vaccines are not restricted to certain MHC types. The success of both RNA and DNA vaccines in quickly addressing the worldwide need for SARS-CoV-2 prophylaxis underscores the safety and feasibility of genetic vaccines. Additionally, the reduced cost and the safety of these types of vaccines will be of high importance considering that the best use of antitumour vaccines will almost certainly be as components in combination treatment approaches.
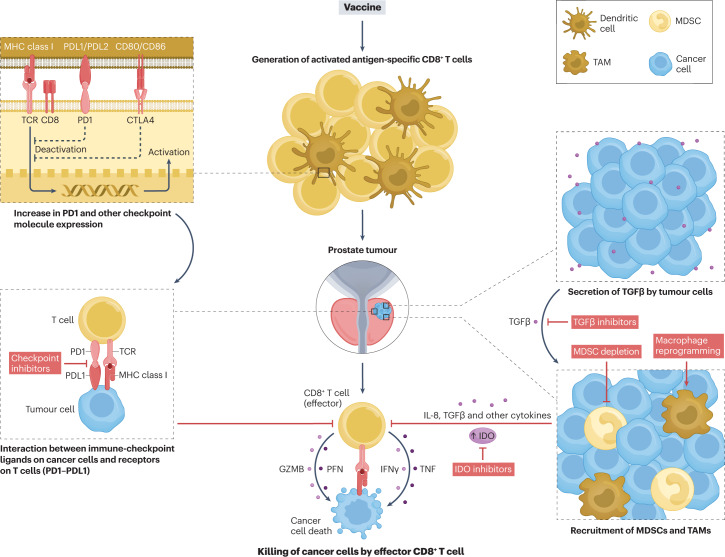
Fig. 2. Mechanisms of antitumour vaccine resistance and potential combination approaches in prostate cancer.
Antitumour vaccination leads to the activation of T cells that can express T cell-checkpoint molecules, such as PD1 and cytotoxic T lymphocyte antigen 4 (CTLA4), which can inhibit the effector function of these cells. Tumour cells can also secrete immune-inhibitor molecules, such as TGFβ, or recruit inhibitory immune populations that decrease the efficacy of vaccine-activated T cells. These mechanisms of resistance indicate pathways that can be targeted as parts of combination therapies using antitumour vaccines. GZMB, granzyme B; IDO, indoleamine 2,3-dioxygenase; MDSC, myeloid-derived suppressor cell; MHC, major histocompatibility complex; PFN, perforin; TAM, tumour-associated macrophage; TCR, T cell receptor.
Conclusions
The aim of using antitumour vaccines is to elicit tumour-specific immune responses that can eliminate tumour cells. For over 30 years, interest has been growing in the development of vaccines as treatments for prostate cancer, prompted by the prevalence and long natural history of the disease and by the fact that the prostate is an expendable organ. Thus, a therapeutic immune response against any prostate tissue might be beneficial in patients following prostatectomy. Over 175 clinical trials have been conducted using vaccines as treatments for prostate cancer using a variety of approaches, including whole-cell vaccines and non-antigen-specific vaccines as well as antigen-specific vaccines using proteins, peptides, viruses, bacteria or nucleic acids to deliver a specific target. To date, five approaches (GVAX, DCVAC/PCa, a multi-epitope peptide vaccine, sipuleucel-T and PROSTVAC) have been evaluated in randomized phase III trials, and one of these approaches, sipuleucel-T, improved patient OS compared with patients receiving placebo, leading to the approval of this agent by the FDA as a treatment for mCRPC. Indeed, sipuleucel-T is the only vaccine approved by the FDA as a treatment for any existing cancer. Most vaccine approaches showed safety and some evidence of immunological activity but clinical activity was not sufficient to advance to randomized clinical trials. Increased clinical activity in terms of PSA declines and objective responses has been observed in studies in which vaccines were used in combination with other immune-modulating therapies. This evidence suggests that, in the future, prostate cancer vaccines will be explored as part of combination therapies. Specifically, in the next 5–10 years, vaccines can be expected to be used to activate and expand tumour-specific T cells in combination with agents that target tumour-associated immune mechanisms of resistance.
Supplementary information
Author contributions
All authors researched data for the article. All authors contributed substantially to the discussion of content. D.G.M. and I.R. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Urology thanks R. Pachynski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
D.G.M. has ownership interest, has received research support and serves as consultant to Madison Vaccines, Inc., which has licensed material described in this manuscript. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41585-023-00739-w.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Oefelein MG, Smith ND, Grayhack JT, Schaeffer AJ, McVary KT. Long-term results of radical retropubic prostatectomy in men with high grade carcinoma of the prostate. J. Urol. 1997;158:1460–1465. doi: 10.1016/S0022-5347(01)64243-5. [DOI] [PubMed] [Google Scholar]
- 3.Garcia JA, Rini BI. Castration-resistant prostate cancer: many treatments, many options, many challenges ahead. Cancer. 2012;118:2583–2593. doi: 10.1002/cncr.26582. [DOI] [PubMed] [Google Scholar]
- 4.Scher HI, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tannock IF, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 7.de Bono JS, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 8.de Bono J, et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 9.Abida W, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J. Clin. Oncol. 2020;38:3763–3772. doi: 10.1200/JCO.20.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker C, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 11.Sartor O, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 2019;25:3753–3758. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 13.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 14.Pardoll DM. Cancer vaccines. Nat. Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 15.Euhus DM, Gupta RK, Morton DL. Induction of antibodies to a tumor-associated antigen by immunization with a whole melanoma cell vaccine. Cancer Immunol. Immunother. 1989;29:247–254. doi: 10.1007/BF00199212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simons JW, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 17.McNeel DG, Disis ML. Tumor vaccines for the management of prostate cancer. Arch. Immunol. Ther. Exp. 2000;48:85–93. [PubMed] [Google Scholar]
- 18.Meidenbauer N, Harris DT, Spitler LE, Whiteside TL. Generation of PSA-reactive effector cells after vaccination with a PSA-based vaccine in patients with prostate cancer. Prostate. 2000;43:88–100. doi: 10.1002/(SICI)1097-0045(20000501)43:2<88::AID-PROS3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Perambakam S, et al. Induction of specific T cell immunity in patients with prostate cancer by vaccination with PSA146-154 peptide. Cancer Immunol. Immunother. 2006;55:1033–1042. doi: 10.1007/s00262-005-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulley J, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–117. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 21.McNeel DG, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J. Clin. Oncol. 2009;27:4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rausch S, Schwentner C, Stenzl A, Bedke J. mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum. Vaccin. Immunother. 2014;10:3146–3152. doi: 10.4161/hv.29553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedland SJ, et al. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J. Clin. Oncol. 2007;25:1765–1771. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 24.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonarakis ES, et al. Changes in PSA kinetics predict metastasis- free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents: combined analysis of 4 phase II trials. Cancer. 2012;118:1533–1542. doi: 10.1002/cncr.26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soanes WA, Ablin RJ, Gonder MJ. Remission of metastatic lesions following cryosurgery in prostatic cancer: immunologic considerations. J. Urol. 1970;104:154–159. doi: 10.1016/S0022-5347(17)61690-2. [DOI] [PubMed] [Google Scholar]
- 27.Ablin RJ, Soanes WA, Gonder MJ. Elution of in vivo bound antiprostatic epithelial antibodies following multiple cryotherapy of carcinoma of prostate. Urology. 1973;2:276–279. doi: 10.1016/0090-4295(73)90463-9. [DOI] [PubMed] [Google Scholar]
- 28.Wolf PL, Vazquez J, Czajkowski NP, Rosenblatt M. A new method of active immunisation to autologous human tumour tissue. Lancet. 1967;2:905–909. doi: 10.1016/s0140-6736(67)90229-2. [DOI] [PubMed] [Google Scholar]
- 29.Rothauge CF, Kraushaar J, Gutschank S. Specific immunotherapy of prostate carcinoma [German] Urol. Int. 1983;38:84–90. doi: 10.1159/000280869. [DOI] [PubMed] [Google Scholar]
- 30.Kreutz FT, Schroeder G, Berger M. Long term clinical and biochemical outcomes following immunotherapy with an autologous immunomodulated vaccine in patients with prostate cancer. Cancer Res. 2013;73:Abst 473. doi: 10.1158/1538-7445.AM2013-473. [DOI] [Google Scholar]
- 31.Isaacs JT, Isaacs WB, Feitz WF, Scheres J. Establishment and characterization of seven Dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate. 1986;9:261–281. doi: 10.1002/pros.2990090306. [DOI] [PubMed] [Google Scholar]
- 32.Vieweg J, et al. Immunotherapy of prostate cancer in the Dunning rat model: use of cytokine gene modified tumor vaccines. Cancer Res. 1994;54:1760–1765. [PubMed] [Google Scholar]
- 33.Dranoff G, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Small EJ, et al. Granulocyte macrophage colony-stimulating factor–secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin. Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 35.Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol. Oncol. 2006;24:419–424. doi: 10.1016/j.urolonc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Higano CS, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 37.Simons JW, et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naive prostate cancer. Clin. Cancer Res. 2006;121:3394–3401. doi: 10.1158/1078-0432.CCR-06-0145. [DOI] [PubMed] [Google Scholar]
- 38.Ward JE, McNeel DG. GVAX: an allogeneic, whole-cell, GM-CSF-secreting cellular immunotherapy for the treatment of prostate cancer. Expert. Opin. Biol. Ther. 2007;7:1893–1902. doi: 10.1517/14712598.7.12.1893. [DOI] [PubMed] [Google Scholar]
- 39.Drake CG. Immunotherapy for prostate cancer: walk, don’t run. J. Clin. Oncol. 2009;27:4035–4037. doi: 10.1200/JCO.2009.22.2299. [DOI] [PubMed] [Google Scholar]
- 40.Hurwitz AA, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 41.van den Eertwegh AJ, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 42.Obradovic AZ, et al. T-cell infiltration and adaptive treg resistance in response to androgen deprivation with or without vaccination in localized prostate cancer. Clin. Cancer Res. 2020 doi: 10.1158/1078-0432.CCR-19-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fucikova J, et al. Phase I/II trial of dendritic cell-based active cellular immunotherapy with DCVAC/PCa in patients with rising PSA after primary prostatectomy or salvage radiotherapy for the treatment of prostate cancer. Cancer Immunol. Immunother. 2018;67:89–100. doi: 10.1007/s00262-017-2068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Podrazil M, et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2015;6:18192–18205. doi: 10.18632/oncotarget.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogelzang NJ, et al. Efficacy and safety of autologous dendritic cell-based immunotherapy, docetaxel, and prednisone vs placebo in patients with metastatic castration-resistant prostate cancer: the VIABLE phase 3 randomized clinical trial. JAMA Oncol. 2022;8:546–552. doi: 10.1001/jamaoncol.2021.7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maupas P, Goudeau A, Coursaget P, Drucker J, Bagros P. Hepatitis B vaccine: efficacy in high-risk settings, a two-year study. Intervirology. 1978;10:196–208. doi: 10.1159/000148983. [DOI] [PubMed] [Google Scholar]
- 47.Livingston P. Augmenting the immunogenicity of carbohydrate tumor antigens. Semin. Cancer Biol. 1995;6:357–366. doi: 10.1016/1044-579X(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, et al. Expression of potential target antigens for immunotherapy on primary and metastatic prostate cancers. Clin. Cancer Res. 1998;4:295–302. [PubMed] [Google Scholar]
- 49.Slovin SF, et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc. Natl Acad. Sci. USA. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slovin SF, et al. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol. Immunother. 2005;54:694–702. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slovin SF, et al. A polyvalent vaccine for high-risk prostate patients: “are more antigens better?”. Cancer Immunol. Immunother. 2007;56:1921–1930. doi: 10.1007/s00262-007-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slovin SF, et al. A bivalent conjugate vaccine in the treatment of biochemically relapsed prostate cancer: a study of glycosylated MUC-2-KLH and Globo H-KLH conjugate vaccines given with the new semi-synthetic saponin immunological adjuvant GPI-0100 OR QS-21. Vaccine. 2005;23:3114–3122. doi: 10.1016/j.vaccine.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 53.Tarr PE, et al. Evaluation of tolerability and antibody response after recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) and a single dose of recombinant hepatitis B vaccine. Vaccine. 1996;14:1199–1204. doi: 10.1016/S0264-410X(96)00031-X. [DOI] [PubMed] [Google Scholar]
- 54.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03579654 (2022).
- 55.Correale P, et al. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J. Natl Cancer Inst. 1997;89:293–300. doi: 10.1093/jnci/89.4.293. [DOI] [PubMed] [Google Scholar]
- 56.Xue BH, Zhang Y, Sosman JA, Peace DJ. Induction of human cytotoxic T lymphocytes specific for prostate-specific antigen. Prostate. 1997;30:73–78. doi: 10.1002/(SICI)1097-0045(19970201)30:2<73::AID-PROS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 57.Olson BM, et al. HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatase. Cancer Immunol. Immunother. 2010;59:943–953. doi: 10.1007/s00262-010-0820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsueda S, et al. Identification of peptide vaccine candidates for prostate cancer patients with HLA-A3 supertype alleles. Clin. Cancer Res. 2005;111:6933–6943. doi: 10.1158/1078-0432.CCR-05-0682. [DOI] [PubMed] [Google Scholar]
- 59.Machlenkin A, et al. Human CTL epitopes prostatic acid phosphatase-3 and six-transmembrane epithelial antigen of prostate-3 as candidates for prostate cancer immunotherapy. Cancer Res. 2005;65:6435–6442. doi: 10.1158/0008-5472.CAN-05-0133. [DOI] [PubMed] [Google Scholar]
- 60.Tjoa B, et al. Presentation of prostate tumor antigens by dendritic cells stimulates T-cell proliferation and cytotoxicity. Prostate. 1996;28:65–69. doi: 10.1002/(SICI)1097-0045(199601)28:1<65::AID-PROS9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 61.Kouiavskaia DV, et al. Vaccination with agonist peptide PSA: 154-163 (155L) derived from prostate specific antigen induced CD8 T-cell response to the native peptide PSA: 154-163 but failed to induce the reactivity against tumor targets expressing PSA: a phase 2 study in patients with recurrent prostate cancer. J. Immunother. 2009;32:655–666. doi: 10.1097/CJI.0b013e3181a80e0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McNeel DG, et al. Pilot study of an HLA-A2 peptide vaccine using flt3 ligand as a systemic vaccine adjuvant. J. Clin. Immunol. 2003;23:62–72. doi: 10.1023/A:1021904432489. [DOI] [PubMed] [Google Scholar]
- 63.Sasada T, Noguchi M, Yamada A, Itoh K. Personalized peptide vaccination: a novel immunotherapeutic approach for advanced cancer. Hum. Vaccin. Immunother. 2012;8:1309–1313. doi: 10.4161/hv.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noguchi M, et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol. Immunother. 2010;59:1001–1009. doi: 10.1007/s00262-010-0822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noguchi M, et al. A randomized phase II clinical trial of personalized peptide vaccination with metronomic low-dose cyclophosphamide in patients with metastatic castration-resistant prostate cancer. Cancer Immunol. Immunother. 2016;65:151–160. doi: 10.1007/s00262-015-1781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noguchi M, et al. A randomized phase III trial of personalized peptide vaccination for castrationresistant prostate cancer progressing after docetaxel. Oncol. Rep. 2021;45:159–168. doi: 10.3892/or.2020.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steinman R. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 68.Murphy GP, et al. Phase II prostate cancer vaccine trial: report of a study involving 37 patients with disease recurrence following primary treatment. Prostate. 1999;39:54–59. doi: 10.1002/(SICI)1097-0045(19990401)39:1<54::AID-PROS9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 69.Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate. 1996;29:371–380. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 70.Ardon H, et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol. Immunother. 2012;61:2033–2044. doi: 10.1007/s00262-012-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heiser A, et al. Induction of polyclonal prostate cancer-specific CTL using dendritic cells transfected with amplified tumor RNA. J. Immunol. 2001;166:2953–2960. doi: 10.4049/jimmunol.166.5.2953. [DOI] [PubMed] [Google Scholar]
- 72.Heiser A, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J. Clin. Invest. 2002;109:409–417. doi: 10.1172/JCI0214364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fong L, et al. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J. Immunol. 2001;167:7150–7156. doi: 10.4049/jimmunol.167.12.7150. [DOI] [PubMed] [Google Scholar]
- 74.Madan RA, et al. Putting the pieces together: completing the mechanism of action Jigsaw for Sipuleucel-T. J. Natl Cancer Inst. 2020;112:562–573. doi: 10.1093/jnci/djaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Small EJ, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J. Clin. Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 76.Burch PA, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 77.Small EJ, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 78.No Authors Listed. Sipuleucel-T: APC 8015, APC-8015, prostate cancer vaccine — Dendreon. Drugs R. D. 2006;7:197–201. doi: 10.2165/00126839-200607030-00006. [DOI] [PubMed] [Google Scholar]
- 79.Lee D. Autologous dendritic cells pulsed with prostatic acid phosphatase (APC8015) for patients with hormone-refractory prostate cancer with a Gleason score <or= 7. Clin. Prostate Cancer. 2003;2:81–83. doi: 10.1016/S1540-0352(11)70024-5. [DOI] [PubMed] [Google Scholar]
- 80.Higano CS, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 81.Williams BA, et al. Preoperative opioid informed consent and prescribing practices in children undergoing orthopaedic trauma surgery. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2022 doi: 10.5435/JAAOSGlobal-D-21-00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fong L, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J. Natl Cancer Inst. 2014 doi: 10.1093/jnci/dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lou DY, Fong L. Neoadjuvant therapy for localized prostate cancer: examining mechanism of action and efficacy within the tumor. Urol. Oncol. 2016;34:182–192. doi: 10.1016/j.urolonc.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beer TM, et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin. Cancer Res. 2011;17:4558–4567. doi: 10.1158/1078-0432.CCR-10-3223. [DOI] [PubMed] [Google Scholar]
- 85.Antonarakis ES, et al. Sequencing of sipuleucel-T and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: a phase II randomized trial. Clin. Cancer Res. 2017;23:2451–2459. doi: 10.1158/1078-0432.CCR-16-1780. [DOI] [PubMed] [Google Scholar]
- 86.Small EJ, et al. A randomized phase II trial of Sipuleucel-T with concurrent versus sequential abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2015;21:3862–3869. doi: 10.1158/1078-0432.CCR-15-0079. [DOI] [PubMed] [Google Scholar]
- 87.Twardowski P, et al. Randomized phase II trial of sipuleucel-T immunotherapy preceded by sensitizing radiation therapy and sipuleucel-T alone in patients with metastatic castrate resistant prostate cancer. Cancer Treat. Res. Commun. 2019;19:100116. doi: 10.1016/j.ctarc.2018.100116. [DOI] [PubMed] [Google Scholar]
- 88.Marshall CH, et al. Randomized phase II trial of Sipuleucel-T with or without radium-223 in men with bone-metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2021;27:1623–1630. doi: 10.1158/1078-0432.CCR-20-4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pachynski RK, et al. IL-7 expands lymphocyte populations and enhances immune responses to sipuleucel-T in patients with metastatic castration-resistant prostate cancer (mCRPC) J. Immunother. Cancer. 2021 doi: 10.1136/jitc-2021-002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dorff T, et al. Phase Ib study of patients with metastatic castrate-resistant prostate cancer treated with different sequencing regimens of atezolizumab and sipuleucel-T. J. Immunother. Cancer. 2021 doi: 10.1136/jitc-2021-002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scholz M, et al. Phase I clinical trial of sipuleucel-T combined with escalating doses of ipilimumab in progressive metastatic castrate-resistant prostate cancer. Immunotargets Ther. 2017;6:11–16. doi: 10.2147/ITT.S122497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jha GG, et al. A phase II randomized, double-blind study of sipuleucel-T followed by IDO pathway inhibitor, indoximod, or placebo in the treatment of patients with metastatic castration resistant prostate cancer (mCRPC) J. Clin. Oncol. 2017;35(Suppl. 15):3066. doi: 10.1200/JCO.2017.35.15_suppl.3066. [DOI] [Google Scholar]
- 93.Wargowski E, et al. Prime-boost vaccination targeting prostatic acid phosphatase (PAP) in patients with metastatic castration-resistant prostate cancer (mCRPC) using Sipuleucel-T and a DNA vaccine. J. Immunother. Cancer. 2018;6:21. doi: 10.1186/s40425-018-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saeed M, Kim K, Borkowski A, Pachynski RK. High dimensional analysis of sipuleucel T from metastatic castration resistant prostate cancer patients using mass cytometry. Cancer Res. 2022;82(Suppl. 12):4158. doi: 10.1158/1538-7445.AM2022-4158. [DOI] [Google Scholar]
- 95.Shanmugaraj B, et al. Bacterial and viral vectors as vaccine delivery vehicles for breast cancer therapy. Life Sci. 2020;250:117550. doi: 10.1016/j.lfs.2020.117550. [DOI] [PubMed] [Google Scholar]
- 96.McNeel DG. Prostate cancer immunotherapy. Curr. Opin. Urol. 2007;17:175–181. doi: 10.1097/MOU.0b013e3280eb10eb. [DOI] [PubMed] [Google Scholar]
- 97.El-Jesr M, Teir M, Maluquer de Motes C. Vaccinia virus activation and antagonism of cytosolic DNA sensing. Front. Immunol. 2020;11:568412. doi: 10.3389/fimmu.2020.568412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bernards R, et al. Effective tumor immunotherapy directed against an oncogene-encoded product using a vaccinia virus vector. Proc. Natl Acad. Sci. USA. 1987;84:6854–6858. doi: 10.1073/pnas.84.19.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mackett M, Smith GL, Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J. Virol. 1984;49:857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hodge JW, et al. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int. J. Cancer. 1995;63:231–237. doi: 10.1002/ijc.2910630215. [DOI] [PubMed] [Google Scholar]
- 101.Coupar BE, Andrew ME, Boyle DB. A general method for the construction of recombinant vaccinia viruses expressing multiple foreign genes. Gene. 1988;68:1–10. doi: 10.1016/0378-1119(88)90593-8. [DOI] [PubMed] [Google Scholar]
- 102.Bennink JR, Yewdell JW, Smith GL, Moller C, Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1984;311:578–579. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- 103.Hodge JW, McLaughlin JP, Kantor JA, Schlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15:759–768. doi: 10.1016/S0264-410X(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 104.Sanda MG, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/S0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 105.Eder JP, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin. Cancer Res. 2000;6:1632–1638. [PubMed] [Google Scholar]
- 106.Johnson LE, Frye TP, Chinnasamy N, Chinnasamy D, McNeel DG. Plasmid DNA vaccine encoding prostatic acid phosphatase is effective in eliciting autologous antigen-specific CD8+ T cells. Cancer Immunol. Immunother. 2007;56:885–895. doi: 10.1007/s00262-006-0241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hwang C, Sanda MG. Prospects and limitations of recombinant poxviruses for prostate cancer immunotherapy. Curr. Opin. Mol. Ther. 1999;1:471–479. [PubMed] [Google Scholar]
- 108.Kaufman HL, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2004;22:2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 109.Hodge JW, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 110.Dipaola R, et al. A phase I trial of Pox PSA vaccines (PROSTVAC(R)-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOMtrade mark) in patients with prostate cancer. J. Transl. Med. 2006;4:1. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parsons JK, et al. A randomized, double-blind, phase II trial of PSA-TRICOM (PROSTVAC) in patients with localized prostate cancer: the immunotherapy to prevent progression on active surveillance study. Eur. Urol. Focus. 2018;4:636–638. doi: 10.1016/j.euf.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.DiPaola RS, et al. A national multicenter phase 2 study of prostate-specific antigen (PSA) pox virus vaccine with sequential androgen ablation therapy in patients with PSA progression: ECOG 9802. Eur. Urol. 2015;68:365–371. doi: 10.1016/j.eururo.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McNeel DG, et al. Randomized phase II trial of docetaxel with or without PSA-TRICOM vaccine in patients with castrate-resistant metastatic prostate cancer: a trial of the ECOG-ACRIN cancer research group (E1809) Hum. Vaccin. Immunother. 2015;11:2469–2474. doi: 10.1080/21645515.2015.1062190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kantoff PW, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gulley JL, et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2019;37:1051–1061. doi: 10.1200/JCO.18.02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lasek W, Zapała Ł. Therapeutic metastatic prostate cancer vaccines: lessons learnt from urologic oncology. Cent. Eur. J. Urol. 2021;74:300–307. doi: 10.5173/ceju.2021.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Monge C, et al. Myocarditis in a patient treated with nivolumab and PROSTVAC: a case report. J. Immunother. Cancer. 2018;6:150. doi: 10.1186/s40425-018-0473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abdul Sater H, et al. Neoadjuvant PROSTVAC prior to radical prostatectomy enhances T-cell infiltration into the tumor immune microenvironment in men with prostate cancer. J. Immunother. Cancer. 2020 doi: 10.1136/jitc-2020-000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bolhassani A, Naderi N, Soleymani S. Prospects and progress of Listeria-based cancer vaccines. Expert Opin. Biol. Ther. 2017;17:1389–1400. doi: 10.1080/14712598.2017.1366446. [DOI] [PubMed] [Google Scholar]
- 120.Brockstedt DG, et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc. Natl Acad. Sci. USA. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Johnson LE, et al. Heterologous vaccination targeting prostatic acid phosphatase (PAP) using DNA and Listeria vaccines elicits superior anti-tumor immunity dependent on CD4+ T cells elicited by DNA priming. Oncoimmunology. 2018;7:e1456603. doi: 10.1080/2162402X.2018.1456603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Drake CG, et al. Safety and preliminary immunogenicity of JNJ-64041809, a live-attenuated, double-deleted Listeria monocytogenes-based immunotherapy, in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2021 doi: 10.1038/s41391-021-00402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stein MN, et al. ADXS31142 immunotherapy +/- pembrolizumab treatment for metastatic castration-resistant prostate cancer: open-label phase I/II KEYNOTE-046 study. Oncologist. 2022;27:453–461. doi: 10.1093/oncolo/oyac048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alam S, McNeel DG. DNA vaccines for the treatment of prostate cancer. Expert. Rev. Vaccines. 2010;9:731–745. doi: 10.1586/erv.10.64. [DOI] [PubMed] [Google Scholar]
- 125.Sheng D, et al. Oral S2-Ag85 DNA vaccine activated intestinal cell dsDNA and RNA sensors to promote the presentation of intestinal antigen. J. Immunol. Res. 2022 doi: 10.1155/2022/7200379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: TLR3 agonists in cancer therapy. Oncoimmunology. 2020;9:1771143. doi: 10.1080/2162402X.2020.1771143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fotin-Mleczek M, et al. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J. Immunother. 2011;34:1–15. doi: 10.1097/CJI.0b013e3181f7dbe8. [DOI] [PubMed] [Google Scholar]
- 128.Liu MA. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines. 2019 doi: 10.3390/vaccines7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pavlenko M, et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br. J. Cancer. 2004;91:688–694. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eriksson F, Totterman T, Maltais AK, Pisa P, Yachnin J. DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine. 2013;31:3843–3848. doi: 10.1016/j.vaccine.2013.06.063. [DOI] [PubMed] [Google Scholar]
- 131.McNeel DG, et al. Real-time immune monitoring to guide plasmid DNA vaccination schedule targeting prostatic acid phosphatase in patients with castration-resistant prostate cancer. Clin. Cancer Res. 2014;20:3692–3704. doi: 10.1158/1078-0432.CCR-14-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Becker JT, et al. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J. Immunother. 2010;33:639–647. doi: 10.1097/CJI.0b013e3181dda23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McNeel DG, et al. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J. Clin. Oncol. 2019;37:3507–3517. doi: 10.1200/JCO.19.01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 blockade restores antitumor efficacy following SSX2 epitope-modified DNA vaccine immunization. Cancer Immunol. Res. 2015;3:946–955. doi: 10.1158/2326-6066.CIR-14-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zahm CD, Colluru VT, McNeel DG. Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8+ T Cells. Cancer Immunol. Res. 2017;5:630–641. doi: 10.1158/2326-6066.CIR-16-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McNeel DG, et al. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2018;P9:25586–25596. doi: 10.18632/oncotarget.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McNeel DG, et al. Phase 2 trial of T-cell activation using MVI-816 and pembrolizumab in patients with metastatic, castration-resistant prostate cancer (mCRPC) J. Immunother. Cancer. 2022 doi: 10.1136/jitc-2021-004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03600350 (2022).
- 139.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04090528 (2022).
- 140.Chudley L, et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8+ T-cell responses and increases PSA doubling time. Cancer Immunol. Immunother. 2012;61:2161–2170. doi: 10.1007/s00262-012-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weber JS, et al. A phase 1 study of a vaccine targeting preferentially expressed antigen in melanoma and prostate-specific membrane antigen in patients with advanced solid tumors. J. Immunother. 2011;34:556–567. doi: 10.1097/CJI.0b013e3182280db1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shore ND, et al. CD8+ T cells impact rising PSA in biochemically relapsed cancer patients using immunotherapy targeting tumor-associated antigens. Mol. Ther. 2020 doi: 10.1016/j.ymthe.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03835533 (2022).
- 144.Olson BM, Johnson LE, McNeel DG. The androgen receptor: a biologically relevant vaccine target for the treatment of prostate cancer. Cancer Immunol. Immunother. 2013;62:585–596. doi: 10.1007/s00262-012-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Olson BM, et al. Safety and immunological efficacy of a DNA vaccine encoding the androgen receptor ligand-binding domain (AR-LBD) Prostate. 2017;77:812–821. doi: 10.1002/pros.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Olson BM, et al. Prostate cancer cells express more androgen receptor (AR) following androgen deprivation, improving recognition by AR-specific T cells. Cancer Immunol. Res. 2017;5:1074–1085. doi: 10.1158/2326-6066.CIR-16-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kyriakopoulos CE, et al. Multicenter phase I trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR, MVI-118) in patients with metastatic prostate cancer. Clin. Cancer Res. 2020;26:5162–5171. doi: 10.1158/1078-0432.CCR-20-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04989946 (2022).
- 149.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ott PA, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03532217 (2022).
- 152.Cho H, et al. Preclinical development of a vaccine-based immunotherapy regimen (VBIR) that induces potent and durable T cell responses to tumor-associated self-antigens. Cancer Immunol. Immunother. 2022 doi: 10.1007/s00262-022-03245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cho H, et al. Preclinical development of a vaccine-based immunotherapy regimen (VBIR) that induces potent and durable T cell responses to tumor-associated self-antigens. Cancer Immunol. Immunother. 2023;72:287–300. doi: 10.1007/s00262-022-03245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Autio KA, et al. First-in-human, phase I study of PF-06753512, a vaccine-based immunotherapy regimen (PrCa VBIR), in biochemical relapse (BCR) and metastatic castration-resistant prostate cancer (mCRPC) J. Clin. Oncol. 2021;39:2612–2612. doi: 10.1200/JCO.2021.39.15_suppl.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kubler H, et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J. Immunother. Cancer. 2015;3:26. doi: 10.1186/s40425-015-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stenzl A, et al. Results of the randomized, placebo-controlled phase I/IIB trial of CV9104, an mRNA based cancer immunotherapy, in patients with metastatic castration-resistant prostate cancer (mCRPC) Ann. Oncol. 2017;28:408–409. doi: 10.1093/annonc/mdx376.014. [DOI] [Google Scholar]
- 157.Gulley JL, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol. Res. 2014;2:133–141. doi: 10.1158/2326-6066.CIR-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sahin U, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 159.Cai Z, et al. Personalized neoantigen vaccine prevents postoperative recurrence in hepatocellular carcinoma patients with vascular invasion. Mol. Cancer. 2021;20:164. doi: 10.1186/s12943-021-01467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kloor M, et al. A frameshift peptide neoantigen-based vaccine for mismatch repair-deficient cancers: a phase I/IIa clinical trial. Clin. Cancer Res. 2020;26:4503–4510. doi: 10.1158/1078-0432.CCR-19-3517. [DOI] [PubMed] [Google Scholar]
- 161.Tuomela K, Ambrose AR, Davis DM. Escaping death: how cancer cells and infected cells resist cell-mediated cytotoxicity. Front. Immunol. 2022;13:867098. doi: 10.3389/fimmu.2022.867098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Koinis F, et al. Myeloid-derived suppressor cells in prostate cancer: present knowledge and future perspectives. Cells. 2021 doi: 10.3390/cells11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lopez-Bujanda ZA, et al. Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression. Nat. Cancer. 2021;2:803–818. doi: 10.1038/s43018-021-00227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zahm CD, Johnson LE, McNeel DG. Increased indoleamine 2,3-dioxygenase activity and expression in prostate cancer following targeted immunotherapy. Cancer Immunol. Immunother. 2019;68:1661–1669. doi: 10.1007/s00262-019-02394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nakamura T, et al. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;98:874–881. doi: 10.1111/j.1349-7006.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu T, et al. Targeting HIC1/TGF-β axis-shaped prostate cancer microenvironment restrains its progression. Cell Death Dis. 2022;13:624. doi: 10.1038/s41419-022-05086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Narayan V, et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 2022;28:724–734. doi: 10.1038/s41591-022-01726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hummel HD, et al. Pasotuxizumab, a BiTE((R)) immune therapy for castration-resistant prostate cancer: phase I, dose-escalation study findings. Immunotherapy. 2021;13:125–141. doi: 10.2217/imt-2020-0256. [DOI] [PubMed] [Google Scholar]
- 169.Karam A, et al. CAR-T cell therapy for solid tumors: are we still that far? A systematic review of literature. Cancer Invest. 2022;40:923–937. doi: 10.1080/07357907.2022.2125004. [DOI] [PubMed] [Google Scholar]
- 170.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04382898 (2022).
- 171.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02933255 (2022).
- 172.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03815942 (2022).
- 173.Zahm CD, Moseman JE, Delmastro LE, McNeel DG. PD-1 and LAG-3 blockade improves anti-tumor vaccine efficacy. Oncoimmunology. 2021;10:e1912892. doi: 10.1080/2162402X.2021.1912892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.