Abstract
Excessive exploitation, negligence, non-degradable nature, and physical and chemical properties of plastic waste have resulted in a massive pollution load into the environment. Consequently, plastic entres the food chain and can cause serious health issues in aquatic animals and humans. The present review summarizes currently reported techniques and approaches for the removal of plastic waste. Many techniques, such as adsorption, coagulation, photocatalysis, and microbial degradation, and approaches like reduction, reuse and recycling are potentially in trend and differ from each other in their efficiency and interaction mechanism. Moreover, substantial advantages and challenges associated with these techniques and approaches are highlighted to develop an understanding of the selection of possible ways for a sustainable future. Nevertheless, in addition to the reduction of plastic waste from the ecosystem, many alternative opportunities have also been explored to cash plastic waste. These fields include the synthesis of adsorbents for the removal of pollutants from aqueous and gaseous stream, their utility in clothing, waste to energy and fuel and in construction (road making). Substantial evidence can be observed in the reduction of plastic pollution from various ecosystems. In addition, it is important to develop an understanding of factors that need to be emphasized while considering alternative approaches and opportunities to cash plastic waste (like adsorbent, clothing, waste to energy and fuel). The thrust of this review is to provide readers with a comprehensive overview of the development status of techniques and approaches to overcome the global issue of plastic pollution and the outlook on the exploitation of this waste as resources.
Keywords: Plastic waste, Climate change, Adsorption, Coagulation, Photocatalysis, Reduction, Reuse, Recycling
Introduction
Nowadays, it is fair to say plastics can be regarded as a sweet poison for both humans and the environment. Undoubtedly, plastics are important in day-to-day life because of their longevity (mechanical properties, thermal properties, stability, and durability), resistance to decomposition, and low cost, which support their widespread applicability [1–3]. The pioneering work done by Belgian-American chemist Leo Baekeland in 1907 was remarkable, leading to the mass production of the first real synthetic plastic known as Bakelite [4, 5]. Countless new plastics and their groups with desirable properties have been realized and synthesized. Plastic materials can be used as an alternative in place of materials, glass, wood, and metals, for construction, decoration, packaging, coatings, drawing into filaments, and weaving. Later, after their commercialization in 1964, plastics occupied their place in every home, office, factory, and vehicle, and became an intrinsic part of human life [6, 7].
The plastic production rate continues to escalate, and global production dramatically reached 368 million metric tons in 2019 from 50 million metric tons in 1976. However, a short downturn can be observed in annual production in 2020 (367 million metric tons), which was predominantly the result of the COVID-19 pandemic. Among the number of plastics cumulatively produced, approximately 30% of plastics are still in use. Whereas approximately 55% of the total plastics were discarded, around 8% were incinerated, and only about 6% were recycled [8]. Before 1980, incineration and recycling methods were least prioritized or can say negligible.
After being used in specific applications for a period of time, plastics are considered as waste and ultimately discarded. With increasing population and development, the amount of discarded plastic increases at an alarming rate, and around 381 million tons of plastic polymers have been released [8]. Explosive population, industrial revolution, mismanagement approach, and physiochemical properties have led to an increase in discarded plastic waste [9, 10]. Consequently, they enter our nature and have a negative impact on the environment and human health with which they come in contact [11, 12]. As a result, their advantageous properties have now become a headache for researchers worldwide. Therefore, it is imperative to reduce the plastic waste load from the environmental ecosystem. The main thrust of the present article is to scratch the relevant information about the generation of plastic waste, disposal, along with the impact on nature, and mitigation. The initial desk research was conducted from various published articles and online sources such as Scopus Index databases and Science Citation Index Expanded (SCI-E), Environmental Performance Index (EPI), and United Nations Development Programme (UNEP) to gather relevant information. Article reflects a brief sketch of various techniques (such as adsorption, coagulation, photocatalysis, and microbial degradation) and approaches (such as reduction, reuse and recycling) that have withdrawn researchers’ attention to eliminate plastic pollution. Furthermore, their efficiency, interaction mechanism, advantages, and challenges are also explored. This article also highlights the alternative approaches and opportunities to cash plastic waste like adsorbent, clothing, waste to energy, and fuel along with prospects.
Synthesis and chemical nature
Depending on the starting materials and reaction involved in the synthesis different types of plastic polymers have been developed including: polyethylene terephthalate, high-density polyethylene, polyvinyl chloride, low-density polyethylene, polypropylene, polystyrene, and others [13–15].
Commercial plastic products take into account intricate mixtures of chemicals and probe additives with the aim of improving the basic mechanical, physical, or chemical properties of the plastic product as per the requirement. Table 1 summarizes the types of plastic, their application and properties. Additionally, additives shelter the polymer from the degrading effects of heat, light, heat, or bacteria and provide special characteristics such as improved surface appearance, reduced friction, and flame retardancy [16].
Table 1.
Summarize the plastic polymer groups, properties, their application, and recyclability
| Symbols | Types of plastics | Application | Properties | Recycled into | Comments | References |
|---|---|---|---|---|---|---|
 |
Polyethylene terephthalate (PETE) |
Soft drinks, water bottles, containers, salad dressing, biscuit trays, and salad domes | Clear, tough, solvent resistant, the barrier to gas and moisture softens at 80 °C | Pillow and sleeping bag filling, clothing, soft drink bottles, carpeting, building insulation | Can be used once (Single-use) | [17] |
 |
High-density polyethylene (HDPE) | Shopping bags, freezer bags, buckets, shampoo, milk bottles, ice cream containers, juice bottles, chemical and detergent bottles, rigid agricultural pipe, crates | Hard to semi-flexible, resistant to chemicals and moisture, waxy surface, opaque, softens at 75 °C, easily colored, processed, and formed | Recycling bins, compost bins, | These are reusable products | [18, 19] |
 |
Polyvinyl Chloride (PVC) Plasticized Polyvinyl chloride PVC-P |
Cosmetic container, plumbing pipes, fittings, electrical conduct, blister packs, wall cladding, roof sheeting, bottles, garden hose, shoe soles, cable sheathing, blood bags, and tubing |
Strong, tough, softens at 80 °C, can be clear, can be solvent welded Flexible, clear, elastic, can be solvent welded |
Compost bin |
These products may contain BPA. They cannot be used in a microwave oven |
[20, 21] |
 |
Low-density polyethylene (LDPE) | Refuse bags, irrigation tubing, mulch film, cling wrap, garbage bags, squeeze bottles | Soft flexible, waxy surface, translucent, softens at 70 °C, scratches easily | Bin liners, pallet sheets | These are reusable products | [22] |
 |
Polypropylene (PP) | Microwave dishes, lunch boxes, packaging tape, garden furniture, kettles, bottles and ice cream tubs, potato chip bags, straws | Hard and translucent, soften at 140 °C, translucent, withstand solvents, versatile | Pegs, bins, pipes, pallet sheets | These are reusable products | [23, 24] |
 |
Polystyrene (PS) Expanded polystyrene (PS-E) |
CD cases, plastic cutlery, imitation glassware, low-cost brittle toys, video cases/foamed polystyrene cups, protective packaging, building, and food insulation |
Clear, glassy rigid, opaque, semi-tough, soften at 95 °C, affected by fat, acids, and solvents, but resistant to alkalis, salt solutions, Low water absorption, when not pigmented is clear, are odor and taste-free Special types of PS are available for special applications |
Recycle bin | They cannot be used in a microwave oven | [25] |
 |
Other (polycarbonate, polylactide, acrylic, acrylonitrile butadiene, styrene, fiberglass, and nylon) |
Automotive and appliance components, computers, electronics, cooler bottles, packaging | Includes all resins and multi-materials (e.g., laminate) properties dependent on plastic or a combination of plastics | Recycle bins |
This product may contain BPA. They cannot be used in a microwave oven |
[26, 27] |
Structure of polymers
The plastic structure usually depends on the linkage of carbon atoms, either may be homopolymer or copolymer. The structures that include the linkage of carbon-to-carbon atoms are considered as homogeneous polymers, such as PP, polybutylene, PS, and polymethyl pentene [28]. However, if the linkage of the carbon atoms is interrupted by oxygen or nitrogen atoms, the structure known as a heterogeneous polymer, such as polyesters, nylons, and polycarbonates, is formed. In such structures, different elements can be attached to the carbon-to-carbon backbone, such as PVC containing attached chlorine atoms, and Teflon containing attached fluorine atoms [23, 29]. The plastics can also be altered by the insertion of some additives. In Table 2, some additives (used to alter the properties of commercial plastic polymers) are discussed along with their application.
Table 2.
Types of additives used to alter the properties of commercial plastic polymers are discussed along with their application
| Sample no | Additives | Application | References |
|---|---|---|---|
| 1 | Antioxidants | For plastic processing and outside application where weathering resistance is needed | [5] |
| 2 | Colorants | For colored plastic parts | [30] |
| 3 | Foaming agents | For expanded polystyrene cups and building board and polyurethane carpet underlayment | [31] |
| 4 | Plasticizers | used in wire insulation, flooring, gutters, and some films | [32] |
| 5 | Lubricants | Used for making fibers | [33] |
| 6 | Anti-stats | To reduce dust collection by static electricity attraction | [34] |
| 7 | Antimicrobials | Used for shower curtains and wall coverings | [35] |
| 8 | Flame retardants | To improve the safety of wire and cable coverings and cultured marble | [36] |
Consequences of using plastic
Generation of various pollutants and issues associated with plastic waste
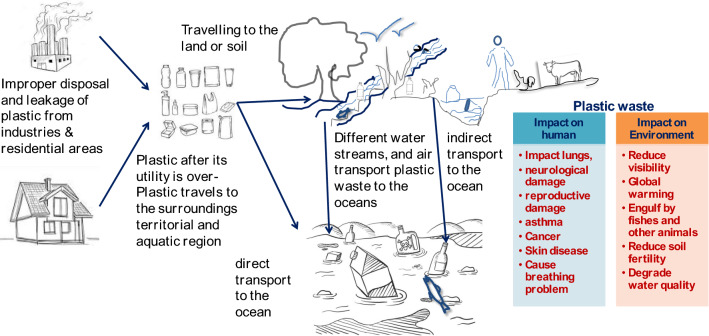
The disposed plastic waste materials react with their surroundings and release several degradation products, such as additives and polymers (of which they are made), throughout their lifetime [37]. Released chemicals (additives), plastic type, and their size determine the extent of the impact on the environment and human health [38, 39]. Plastic waste itself is solid waste that is classified as microplastic and microplastic. Waste with a diameter size > 20 mm is considered macroplastics, whereas, waste with an upper size limit of 5 mm in diameter is considered as microplastic. These microplastics are further characterized as primary microplastics (manufactured to have a size less than 5 mm) and secondary microplastics (resultant of degradation of macroplastics, found in textiles, medicines, and personal care products like facial and body scrubs) [40, 41]. Plastic after its utility is over is sent to landfills or burning in open or closed environments and is vulnerable to human health, air, water, and soil. When plastics are disposed over territorial areas, they turn out to be a prime source for solid waste problems, and as they ingress into water bodies (ultimately travel to an ocean), they become a serious threat to aquatic animals, as shown in Fig. 1 [42]. This illustration shows the pathway of traveling plastic waste from a residential or industrial area to their surroundings due to improper disposal. These plastic wastes (such as single-use plastics and others) float or move in surrounds either by air, water streams, and other activities (likes by animals), which finally end up in the ocean. As a result, this seriously impacts humans and other aquatic animals.
Fig. 1.
Illustration presents improper disposal and leakage of plastic after its utility is over and their impact
The fumes generated after burning plastic waste contain hazardous compounds like halogenated additives, PCBs, PVCs, fumes, dioxins, carbon monoxide (CO), arsenic, mercury, carbon dioxide (CO2), methane (CH4), and volatile organic compounds (VOCs) [37, 43, 44]. Some compounds like CO2 and CH4 are liable to stimulate the greenhouse effect, which increase the Earth’s temperature and contribute to climate change [45, 46].
Impact on environment and human
The human inhales byproduct of plastic waste burning in the atmosphere may include VOCs, dioxins, and CO, which may cause health-related issues such as cancer, endometriosis, birth defects, and child developmental disorders. These compounds can trigger neurological damage [47], reproductive issue [48], immune-related issue [49], asthma [50], and endocrine disruption [51, 52], even at low concentrations. Table 3 indicates various additives and other chemicals released from plastic components and their associated health issues.
Table 3.
Indicates various additives and other chemicals released from particular plastic burning and their associated health issues
| Sample no | Plastic/polymer | Additives/chemicals released after burning | Health effects | Environmental effects | References |
|---|---|---|---|---|---|
| 1 | PETE | Acetaldehyde | Erythema, coughing, pulmonary edema, and necrosis | Ozone precursors in the atmospheric environment | [53] |
| 2 | HDPE | Acetone | Irritation in eyes and respiratory tract | Can impact ozone production and OH in the upper troposphere | [54] |
| 3 | HDPE | Benzaldehyde | Irritates eyes, skin, and respiratory system limit brain functioning | Undergoes autoxidation to form benzoic acid upon exposure to air at room temperature | [55] |
| 4 | PVC | Polychlorinated dibenzo-dioxin | Carcinogenic irritates the skin, eyes, and respiratory system. changes in liver function, thyroid hormone levels, immune cell levels, and decreased performance in tests of learning and intelligence | Degradation of air quality | [56] |
| 5 | LDPE | CO2 | Shorting in breathing | Impact greenhouse gases, global warming | [57, 58] |
| 6 | PVC | Polychlorinated dibenzofuran | Irritates the eyes and the respiratory system, causes asthma | Degradation of air quality | [59] |
| 7 | PVC | Vinyl chloride | Carcinogenic, irritating to eyes, skin, and respiratory system. Effect on the central nervous system, liver, spleen, and blood-forming organs | Detoriate drinking water quality and air | [60] |
| 8 | PETE | Xylene | Irritates the eyes. Affect the central nervous system, reduce the level of consciousness, and impair learning ability | Can reduce air quality, especially indoor air | [61] |
| 9 | LDPE | Toluene | Irritates the eyes and the respiratory tract, sometimes may cause depression | Can reduce air quality, especially indoor air | [61] |
| 10 | LDPE | Ethylene | Throat, eye irritation, shortening in breathing | Emissions of greenhouse gases, acidification, and eco-toxicity (air and water) | [62] |
| 11 | LDPE | PP | Headache, dizziness, nausea, heartbeat irregularities, unconsciousness, and/or suffocation by asphyxiation | It has Pb and Cd that contaminate drinking water and soil | [63] |
| 12 | Low-density polyethylene | CH4 | Mood changes, slurred speech, vision problems, memory loss, nausea, vomiting, facial flushing, and headache | Effect amount of greenhouse gases and cause climate change | [62] |
| 13 | Polycarbonates | CO | Dizziness, headache, and decreased consciousness | It affects the amount of greenhouse gases | [64] |
| 14 | Polyvinyl chloride | Lead (Pb) | Anemia, weakness, and kidney and brain damage | Air becomes dusty | [65–67] |
Substances, such as Arsenic (As), Pb, Cadmium (Cd), and Mercury (Hg) are considered heavy metals, are sufficient to degrade water quality, and can reduce soil fertility at low concentrations [65, 68]. Moreover, we can frequently observe various plastic products (macroplastics or microplastics) in water bodies (rivers, streams, ponds, and oceans). These plastics are generated in the terrestrial region, end up in the oceans and degrade the health of the ecosystem [69–71]. Much evidence has been published that reflects aquatic species such as turtles, seabirds, fish, and marine mammals are suffering from entanglement, often associated with large animals when comparing to ingestion (visible in smaller animals) [72, 73]. In addition, micro-sized plastics also lead to inflammation, traverse cellular barriers, and even cross highly selective membranes such as the blood–brain barrier or the placenta. Within the cell, they sometimes trigger changes in gene expression and biochemical reactions. The signs of chemicals released during degradation may cause acute and chronic effects on living beings and the ecosystem as well. Some of the causes have been investigated as disruptions in the hormone system of vertebrates and invertebrates alike [74–77].
The presence of plastics or other larger bodies can be easily detected and separated from water bodies. Presently, microplastic (< 10–6) and nanoplastics (< 10–9) are considered as emerging contaminants [78], whose micro/nano size limits their detection in water bodies and no standardized method has been recognized for their effective identification and quantification. Various studies have documented the presence of microplastics in the guts of fishes and the stomachs of many terrestrial animals [76, 79–81].
Moreover, we have limited data documented on chemicals associated with plastics. However, our above paragraph concluded that every human and animal is now the host for some concentration of chemicals associated with plastics and can also be found in air, water, and soil. Some of them may be: Polychlorinated biphenyls (PCBs), organochlorine pesticides (DDTs, HCHs, and HCB), polycyclic aromatic hydrocarbons (PAHs), brominated flame retardants (PBDE and HBCD), phthalates, UV stabilizers, and antioxidants, which have been widely detected in microplastic samples from water [82–84].
Impact of plastic waste on environmental performance index (EPI) of nation
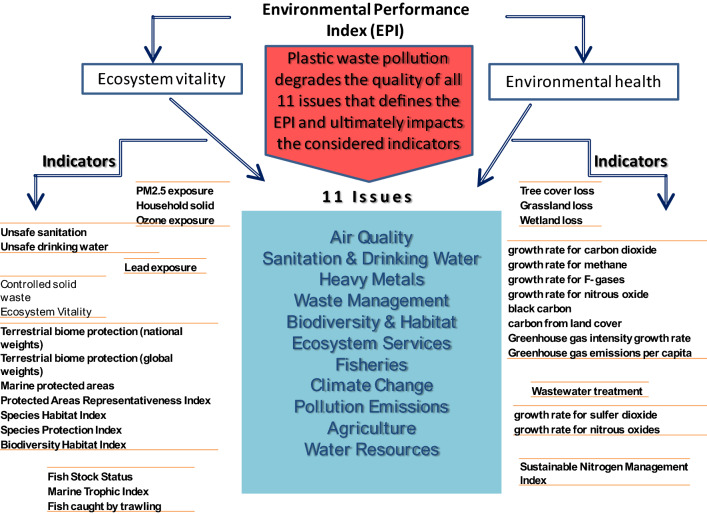
Plastic pollution directly or indirectly affects human health and the environment. On the other hand, plastic pollution also affects the environmental performance ranking of any nation. Environmental ranking indicates the quality of the environment and the efforts made to enhance the status of the environment of the nation. The considered indicators in EPI collectively depict the status of 10 years of the environmental pathway for any nation. Moreover, it indicates the best countries addressing the environmental challenges that every nation faces. Yale University and Columbia University, in collaboration with the World Economic Forum, developed a biennial index that was first published in 2002. It offers a scorecard, ranking 180 countries, using 32 performance indicators considering 11 issues that highlight the leaders and laggards in environmental performance. Furthermore, it also provides practical guidance for countries that aspire to move toward a sustainable future [85, 86]. Figure 2 indicates all 32 performance indicators considering 11 issues that highlight the environmental performance of any nation. This EPI reflects the status of the nation in dealing with environmental pollution to overcome. Notably, the indicators used to evaluate the EPI are the sectors that can be altered by plastic waste. Hence, with the perception of the nation, it is imperative to reduce the plastic load from the environment.
Fig. 2.
Environmental Performance Index (EPI)- 11 issues and 32 indicators to which plastic waste pollution has a significant impact
Different technologies and approaches for the removal of plastic
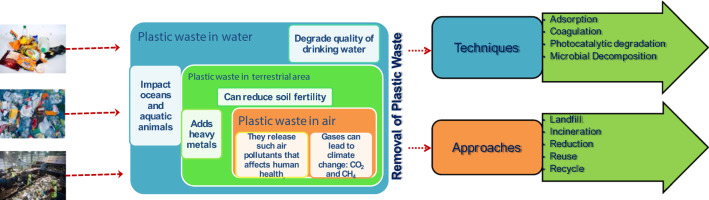
Since the last decade, efforts have been made to eliminate plastic pollution either by reducing it at their source or by removing it after their generation. Various technologies, such as adsorption [87, 88], photocatalytic degradation [89, 90], coagulation [91], and microbial decomposition [92, 93] have been introduced and investigated to reduce plastic loads [94]. Along with this, approaches such as landfill, incineration, and 3R (reduce, reuse, and recycle) have also been coped up [95]. In the context, Fig. 3 shows the many sources responsible for plastic waste in nature and their serious impact. Figure 3 also illustrates various techniques and approaches to reduce plastic waste.
Fig. 3.
Presence of plastic waste in the environment with serious impact on the environment and various techniques and approaches to get rid of the plastic load
Adsorption
Adsorption, among the oldest techniques, has been successfully explored for the removal of organic and inorganic pollutants from water and wastewater. This is the surface phenomenon where pollutants from the water or gaseous phase are transferred to the surface of solid materials, called adsorbents. In this phenomenon, the adsorbent plays a vital role in the adsorption of pollutants [96, 97]. Recently, the adsorption technique has gained a lot of attention for the removal of microplastics from water.
Yuan et al. used three-dimensional reduced graphene oxide for the removal of microplastics from water. Its adsorption performance was highly dependent on pH, ion concentration, adsorption time, initial concentration, and temperature, and its maximum adsorption capacity was calculated as 617.28 mg/g at 26 °C. The higher adsorption efficiency was the result of strong π–π force with the help of electrostatic attraction and physical retention [92].
Wang et al. studied a natural and biodegradable sponge material (through chemical cross-linking using plant protein) with high mechanical properties for the adsorption of microplastic. The prepared material shows a removal efficiency of up to 81.2% in the pH range of 6–9 with an initial microplastic concentration of 1 mg/L. They aim to develop good mechanical strength materials with a compressive strength of 176 kPa and Young’s modulus of 60.1 kPa. The mechanical properties of the sponge remained up to 90% even after 100 compression cycles at a compressive strain of 70%, which shows commendable fatigue-resistance close to that of commercial polyurethane sponges. The abundant active side chains on amino acid residues provided the protein sponge with a good capacity to adsorb microplastics. The adsorption kinetic study suggested that hydrophobic interactions and the intra-particle diffusion drove the adsorption process. The sponge possessed a highly interconnected porous structure (83%), thus showing fast adsorption ability to microplastics with 38% adsorbed onto the sponge within 10 s. The entrapped water can be released from the sponge simply by squeezing for cyclic use, and the fast adsorption ability was still maintained after 20 cycles [98].
Ye et al. demonstrated the facile and scalable fabrication of two types of bubble-propelled iron oxides-MnO2 core–shell micromotors (Fe3O4-MnO2 and Fe2O3-MnO2) for the removal of suspended microplastics. They used micro/nanoscale manipulation (MNM) in various environmental remediations, such as microplastic removal, via the synergy of catalytic degradation, surface adsorption, and adsorptive bubble separation mechanisms. The adsorptive bubbles was found to efficiently separate more than 10% of the suspended microplastics from the polluted water in 2 h [99].
Ye et al. demonstrated magnetic carbon nanotubes (M−CNTs) for the removal of microplastic from an aqueous phase. In this approach, M−CNTs were effectively adsorbed on microplastic materials such as PE, PET, and polyamide (PA). The formed MPs/M− CNTs composites were then separated by magnetic force from aqueous solutions. The results show that the prepared materials were more efficient for PE followed by PET and PA (maximum adsorption capacities were 1650, 1400, and 1100 M−CNTs mg/g, respectively). Furthermore, the recycling of adsorbed M−CNTs via thermal treatment (600 °C) makes them more efficient with the same magnetic properties. In the study, they used M-CNTs four times and were still able to remove ~ 80% of total MPs in the testing solution [100].
Kim et al. investigated the application of granular activated carbon (GAC) for the removal of plastic (microplastic). GAC was found to be effective for PE and PP, including 20–50 µm microplastics. These compounds are lighter than water, which is rarely removed in primary and biological treatments. About MP removal efficiency, similar results were obtained for the DWTP (drinking water treatment plant) equipped with the GAC filtration system (88% cumulative removal) [101]. Moreover, adsorbents prepared from plastic waste without the addition of any environmental-related problematic raw materials or products have gained much attention. Carbon nanostructure derived from plastic waste embedded with Co3Fe7/CoFe2O4 magnetic nanoparticles can be effectively used for the removal of pollutants such as methyl orange and methylene blue from the aqueous phase [102].
The adsorption technique was found to be effective for emerging pollutants in water and air stream. The removal of microplastics can be easily adsorbed and separated from wastewater through various micro, meso, and macroporous nanomaterials. These techniques are limited to plastic size >5 µm. The removal of microplastic from oceans is an untouchable field with this technique. Moreover, the regeneration of adsorbents is also considered a limitation, which allows researchers to look forward to a more effective technique.
Photocatalysis
Photocatalysis is an exciting approach for handling plastic waste, especially micro/nano plastics. This technique is a light-mediated redox process, in which appropriate light energy excites nanostructured semiconductors that lead to the creation of exciton pairs, which react with surrounding water/moisture to produce highly reactive species like superoxides and hydroxyl radicals that can effectively oxidize organic species, including polymers [103]. Various photocatalysts, such as zinc oxide (ZnO) and titanium oxide (TiO2), have been used to study the degradation of PVC, PET, PS, and PE.
Tofa et al. demonstrated the successful degradation of LDPE film using ZnO nanorod photocatalysts. The photo-mediated oxidation process was prevalent over the cracks and spots on the LDPE film after 175 h of exposure. As a result, under optimum light energy, photocatalyst excitement leads to the formation of hydroxyl radicals, which have a high oxidation capacity for degrading organic pollutants [104].
Shang et al. [105] investigated the photocatalytic degradation of PS plastic over copper phthalocyanine (CuPc) sensitized TiO2 photocatalyst (TiO2/CuPc) under fluorescent light irradiation. In their result, UV−VIS spectra show that TiO2/CuPc extends its photoresponse range to visible light, contrasting to only UV light absorption of pure TiO2. For PS photodegradation, they observed a higher PS weight loss rate, lower PS average molecular weight, fewer volatile organic compounds, and more CO2 in the PS−(TiO2/CuPc) system than in the PS−TiO2 system. Their study suggests the potential application of dye-sensitized TiO2 catalysts in the thorough photodegradation of PS plastic under fluorescent light. The photodegradation over TiO2/CuPc composite is more competent and efficient than that over pure TiO2. Zhao et al. [106] evaluated the same photocatalyst for the degradation of PE. Their results show that PE-TiO2/CuPc has a higher photocatalytic degradation rate than PE-TiO2. Furthermore, PS-TiO2/CuPc has slower degradation than PE-TiO2/CuPc due to the different molecular structures [105].
Fa et al. studied the photocatalytic degradation of polyethylene-oxidized polyethylene wax-TiO2 (PE-OPW-TiO2). In their study to improve the degradation efficiency, they used OPW to enhance the interaction between PE and modified TiO2 particles [107].
Wang et al. [108] prepared magnetic Au@mag@TiO2 micromotors using TiO2 particles coated with 10 nm nickel and 30 nm gold. The prepared photocatalytic micromotor can move efficiently in water and peroxide when irradiated with UV light, depending on a photocatalytic chemical reaction. In the study, they used polystyrene (PS) passive particles as a model system for microplastics, and in a mixture of both, the Au@Ni@TiO2 micromotors collectted polystyrene particles very efficiently through phoretic interactions in 1.67% hydrogen peroxide solution with 315 mW UV light. In the study, they used the second method, shoveling (pushing method), for the removal of microplastics from a 0.2% hydrogen peroxide solution [108].
Iqra Nabi investigated the photocatalytic degradation of PS and PE microplastic under UV light irradiation over TiO2 nanoparticle films. The study shows the complete mineralization (98.40%) of photocatalyst in 12 h made with Triton X-100. High photodegradation rates were observed after 36 h for PE, and CO2 was found to be the main end product [109].
Abdusalam Uheida et al. demonstrated that glass fiber substrates could be efficiently used to trap low-density microplastics such as PP from water. They used ZnO nanorods (ZnO NRs) immobilized onto glass fiber substrates for visible light to degrade the microplastic [110].
Degradation of types of plastic with UV–VIS light excited heterogeneous ZnO and TiO2 photocatalysts lead to the formation of such intermediate products that could be further exploited as raw materials for the chemical industry for the production of new petrochemical products or plastics or in organic synthesis. Our limited knowledge and few publications about the photocatalytic degradation of plastics, especially nano plastic is considered as an emerging issue of great concern.
Coagulation
Coagulation is the chemical method for the removal of solid particles from water by introducing small and highly charged molecules into the water to manipulate the electrostatic charges of the suspended particles in water [111]. Limited data are presented on the application of this technique for the removal of microplastics.
Ma et al. [112] investigated the micro-sized PE behavior during removal from water with the help of Fe-based salt. They observed that the traditional coagulation process can have higher removal for smaller PE particles. They evaluated the effect of anionic polyacrylamide on the solution, which played a significant role in increasing the removal efficiency, especially anionic polyacrylamide at high dosages (with efficiency up to 90.9%). This is because a dense floc was formed and had high adsorption ability due to the positively charged Fe-based flocs under neutral conditions. However, for ultrafiltration, microplastic were completely rejected, and slight membrane fouling was caused owing to their large particle size. After the coagulation, membrane flux was decreased; however, less severe membrane fouling was induced by flocs. This is the result of the heterogeneous nature of the cake layer caused by PE, even at high dosages of Fe-based salts. They concluded that the behavior exhibited during coagulation and ultrafiltration could potentially be applied in drinking water treatment [112]. Baiwen Ma [112] evaluated the removal efficiency of Al- and Fe-based salts for the removal of PE from water which is the main constituent of microplastics. They observed that Al-based salts dominated over Fe-based salts in PE removal efficiency. The smaller the PE particle size was, the higher the removal efficiency. However, a low removal efficiency was observed, even with a high Al-based salt dosage of 15 mmol/L (below 40%). Additionally, the removal efficiency was barely influenced by water conditions, such as ionic strength, and turbidity level [112].
Zhou et al. studied the removal of PS and PE microplastics using PAC and ferric chloride coagulation, where PAC showed higher removal efficiency than ferric chloride. Their work concluded that the removal efficiency of microplastics under alkaline conditions was higher than that under acidic conditions. Chlorine ion Cl− had little effect on the removal efficiency of microplastics, while SO42− and CO32− had inhibitory and promoting effects, respectively [113].
Horn [114] investigated the removal of the model microplastic spheres and microfibers in their study using alum as the coagulant. They used the coagulant, alum at concentrations between 5 mg/L and 10 mg/L Al to produce water with turbidity less than 1.0 NTU from solutions containing 5 mg/L microspheres with an initial turbidity of 16 NTU. The occurrence of surfactant at 20 mg/L in solution does not negatively impact the performance of coagulations of microspheres at low alum. However, they may have a small detrimental effect at high particle loading and high alum doses. They predicted that the stability of polyethylene microfibers in water was strongly influenced by surfactants. The smallest polyethylene fibers could be dispersed in solution by a surfactant and effectively removed via coagulation [115].
Darabi et al. [116] investigated the removal efficiency of micro-and nano plastics (180–125 μm) during drinking water treatment, particularly coagulation/flocculation combined with sedimentation (CFS) and granular filtration under ordinary working conditions at water treatment plants (WTPs). It also studied the interactions between biofilms and microplastics and the consequential impact on treatment efficiency. Generally, CFS was not sufficient to remove micro and nano plastics. The sedimentation rate of clean plastics was lower than 2.0% for all different sizes of plastic particles with the coagulant Al2(SO4)3. Even with the addition of coagulant aid (PolyDADMAC), the highest removal was only 13.6% for 45–53 μm particles. In contrast, granular filtration was much more effective at filtering out micro and nano plastics, from 86.9% to nearly complete removal (99.9% for particles larger than 100 μm). However, there existed a critical size (10–20 μm) where a significantly lower removal (86.9%) was observed. Biofilms were easily formed on microplastics. In addition, biofilm formation significantly increased the removal efficiency of CFS treatment from <2.0% to 16.5%. This work provides new knowledge to better understand the fate and transport of emerging micro-and nano plastic pollutants during drinking water treatment, which is of increasing concern due to the potential human exposure to micro-and nano plastics in drinking water [117].
Microorganism
Nowadays, researchers and scientists have shifted their effort to develop strains that could potentially help eliminate plastic waste. Various microorganisms, such as bacteria, and fungus, have been reported to potentially degrade plastic waste, especially microplastics [118, 119]. In microbial degradation, plastics decompose and end up as biomass, methane, carbon dioxide, water, and other inorganic compounds [120]. In a recent report, a fungus was examined that breaks down the plastic sponge and assimilates it like any other food. Such studies are in progress evaluating their efficiency on PET and polyurethane.
Paço et al. examined the naturally occurring fungus Zalerion Maritimum, that potentially biodegrade polyethylene plastics [121]. Other lab-engineered versions bacteria like E. coli are under investigation to transform terephthalic acid, a molecule derived from PET, into the culinary flavouring vanillin, via a series of chemical reactions. However, this study is still at a very early stage, and need to do more to find ways to make the process more efficient and economically viable. Bacterial strain Pseudomonas sp. TDA1 is under investigation and was originally found in a local rubbish dump to break down polyurethane using the enzyme. This bacterium multiplies its biomass, consumes around half the plastic, and releases the rest as carbon dioxide [122]. This method is the slowest among all removal methods and takes ample days for degradation, which depends on the characteristics of plastic waste, and its physical and chemical nature. In addition, environmental factors such as temperature, sunlight, ultraviolet rays, and atmospheric humidity, can influence their degradation rate [92, 93].
Tournier [123] prepared an improved PET hydrolase that depolymerizes 90& PET into monomers with a productivity of 16.7 g of terephthalate per liter per hour (200 g/kg of PET suspension, with an enzyme concentration of 3 mg/g of PET) in 10 h. The optimized PET hydrolases reported thus far, including an enzyme from the bacterium Ideonella sakaiensis strain 201-F6 (even assisted by a secondary enzyme) and this variant, attracted recent interest [123].
Enzyme or microbial conversion of PET to its constituent building blocks is interesting science and needs to be explored. In this context, French company Carbios produces engineered enzymes that could break down PET. Carbios teamed up with L'Oreal and Nestle to produce enzymatically recycled the world's first food-grade PET plastic. The study confirms that these enzymes do not cause contamination if the packaging is still dirty. Moreover, they are very specific and energy-efficient (they do not use much energy). However, this technology is limited to two polyesters, signifying around 75 million tonnes of annual production, compared to a global plastics production of around 350 million tonnes.
Kim et al. [101] investigated worms that can consume polystyrene to a strain of bacteria that lives in the larvae’s gut. They placed polystyrene as the only carbon source with 50 superworms in a chamber and observed that the worms had consumed around 70% of the plastic in the first 21 days. Furthermore, a strain of Pseudomonas aeruginosa bacteria was isolated from the gut of the worms and showed that is can grow directly on the surface of polystyrene and break it down. Finally, an enzyme called serine hydrolase was identified from the bacteria as a responsible candidate for most of the biodegradation [124]. Currently, studies are more concentrated on exploring enzymes and microorganisms that use plastics as their source of energy, as a result, they can degrade plastic waste completely.
Landfill and incineration
A few years before, the landfill has been the preferred approach for plastic waste management in which capital includes low investment, easy construction, easy disposal, convenience, and approachability [125, 126]. Communities generally dispose of their waste at a particular place near residence or segregate in different dustbins that are finally dumped at particular sites (city outskirt). Decades to decades of practice have now resulted in release of the plastic waste into the ocean, in which landfill practices have played a key role in their travel [52, 70]. A study conducted estimates that before 2015, more than 8.3 billion metric tonnes of plastics were produced worldwide, of which only 9% was recycled and the rest ended up in landfills or the environment [8, 127]. In 2018 and 2019, more than 7.2 million tons of plastic were sent to landfills, and various valuable elements were also thrown away. The amount of plastic in landfills increases with the increase in plastic every year (as we produce around 300 million tonnes of plastic waste every year and that is equivalent to the weight of the entire human population) [128–130]. Consequently, some of the associated toxic chemicals leach out from the landfill site to the soil and groundwater, causing severe health issues. Therefore, due to environmental and health concerns, and the loss of valuable substances, they are now the least preferred management option [131, 132]. Many countries have imposed restrictions on landfills and put extra effort to recover value substances that reduce the amount of plastic waste at landfill sites. This approach adopted by European state members reduced the plastic waste sent to landfills, which hoard approximately 5.7 million tons of resources, by 44% between 2006 and 2018 [133, 134].
Developing and underdeveloped countries still prefer landfills because of low investment, ease of construction, and approachability. On the other hand, low literacy, lack of awareness of the environment, and plastic and casual approaches could be reasons [135–138]. It is estimated that an astounding 6500 tonnes of plastic waste are going to landfills per day. Some developed countries, such as the USA and Canada, export their plastic wastes to developing countries for processing due to low-wage workers and cheap processing charges [139]. The exported plastic waste is turned into a useful product, and the rest is either sent to a landfill or disposed to the ocean. As a result, the volume of plastic waste becomes a nuisance for everyone.
To reduce the volume of waste, researchers have tried to capitalize on the incineration approach. Incineration is always practiced as an alternative to landfills in which waste is thermally treated under a controlled combustion process (rapid oxidation) to reduce the volume of waste and to recover energy [140, 141]. However, incineration could be considered an the efficient approach to reduce plastic, but a disadvantage associated with it is noxious fumes, which are more dangerous. The fumes release VOCs, polychlorinated biphenyls (PCBs), polyvinyl chloride, dioxins, furans, CO, and CO2 from the incinerator depending on the type of waste [142–146]. Among some of the gages are considered greenhouse gages that lead to climate change [46, 147]. In addition, the incineration approach requires high installation investment, operation, and maintenance that restrict its application for plastic waste removal [148, 149]. These conditions insist on the 3R approach, i.e., Reduce, Reuse and Recycle.
Reduce, reuse and recycle (3R approach)
Alongside the application of the above-discussed techniques, other approaches (such as Reduction, Reuse, and Recycle) to lower plastic waste have importance. In this context, an individual’s behavior and consciousness play a key role in controlling the plastic load in the environment. For any country, awareness and education are the two pillars on which the 3R approach stands and are supported by other factors, like population restriction, attitude, knowledge, and practices. Reduction is always considered the best way to manage any waste [150, 151]. The key factor is reducing the purchasing amount or restricting only to buying what we need. This approach is not only limited to saving resources and energy but also reduces the number of pollutant emissions and subsequently improves the environment. Unfortunately, due to the limited availability of alternatives to plastic, the topmost priority approach shifted to the least priority. In the reference, Table 4 summarizes the comparison of various techniques and approaches, which highlights their advantages and disadvantages. However, various governmental and non-government efforts have been made to avoid the utilization of plastic products. In some European countries like Germany, in 2016, the step forward is to curb plastic bags or to apply a fee of €0.05 to €0.50 (about $0.06 to $0.60) on purchase. The agreement was made by the Ministry, the German Retail Federation, and participating companies to restrict the use of plastic bags. In 2016, Switzerland government and the Canadian government charged consumers some taxes to ban plastic bags, (around $0.04 per single-use plastic bag and $0.02 per reusable bag). As a result, the demand for plastic bags drastically decreased by 80%. Some commendable efforts were made by Asian countries like Indonesia, in 2017 to completely phase out plastic bags by 2018 [152].
Table 4.
The comparison of the available techniques and approaches for the removal of plastic wastes
| Sample no | Techniques/Approaches | Advantages | Disadvantages |
|---|---|---|---|
| 1 | Adsorption | Low cost and low maintenance technique low, less energy consumption |
Effective for the micro plastic present in aqueous medium; Not effective for reducing high volume of plastic waste |
| 2 | Photocatalytic degradation |
Complete degradation of plastics like LDPE, PS; Effective for microplastics and nanoplastics |
High energy consumption; Not effective for reducing high volume of plastic waste |
| 3 | Coagulation | Effective for microplastics present in aqueous medium | Not effective for reducing high volume of plastic waste |
| 4 | Micro-organism | Breakdown the plastic sponge and assimilate it like any other food |
Produce GHGs like methane, carbon dioxide in decomposition; Decomposition may take ample days |
| 5 | Landfill |
Deals with high volume of plastic waste; Low cost; Reduced transportation distance; No energy consumption |
Leaching of chemicals to soil and ground water; Occupy large space |
| 6 | Incineration |
Reduce high volume of plastic waste; Energy recovery; Require minimum land area |
Require high capital cost; High energy consumption; Causes air pollution |
| 7 | 3 R- reduce/reuse/recycle |
Reduces the requirement of new plastic; Save money; Consumption of less energy in recycling rather than production of new plastics |
Unconsciousness can affect reuse of plastic waste |
| 8 | Conversion of plastic waste in carbon based materials |
Reduce volume of plastic waste; Availability of new materials at low cost |
Needs energy consumption; Limited to small group of population; Needs skilled and well trained personal for the conversion of plastic waste |
Reuse is another approach that may reduce the demand for the new plastic product. However, many people prefer the recycling or refuse approach over reuse but the reuse approach saves money, and conserves energy and resources [153–156]. It is observed that the reuse approach has been maximumly capitalized in developing countries like India, Pakistan, and Bangladesh, where one can reuse sandwiches, plastic grocery bags for small trash bags, and re-use plastic silverware [157–159]. Reuse is an efficient approach for the reduction of plastic waste load but recycling is considered more effective methodology to restrict the utilization of virgin materials in the manufacturing of plastic products. Further, recycling could save 40–100 MJ/kg (depending on the polymer) energy and reduce the depletion of fossil fuels [160]. However, many countries have debated the preferences among reuse or recycle, but it is observed that recycling generally takes less energy than making plastic from raw materials. Reusing is preferred to using plastic waste, which consumes less energy and fewer resources. In recycling, the formation of different products depends on the type of chemical used, and this approach opens new pathways for road construction, toilet making and information on pavement blocks. Plastic waste materials are used for making Plastone, which is used as a binder aggregating solid that can be used in the construction of the structure of toilet blocks and construction of pavement blocks. It is interesting to mention that for making a single Plastone block, 300 plastic carry bags and 4 to 6 PET bottles are used [161]. Their high transverse strength results in providing long life which can be used for flooring especially outdoors, in raising compound walls and lining of the canal [162, 163].
Conversion of plastics into carbon nanostructures for energy and environmental application
Beyond the removal of plastic waste and restrictions on its generation, the conversion of plastic waste into useful resources like carbon-based material is a growing field. In this context, plastic wastes can be thermally treated to obtain carbon materials using methods such as anoxic pyrolysis, catalytic carbonization, pressure carbonization, flash Joule heating, microwave treatment, molten salt methods [164, 165], and hydrothermal methods [166, 167]. These treatments are often applied to achieve the desired products, such as carbon-based solids, which may include carbon nanotubes (CNT) [168], graphene [96, 169], hierarchically porous carbon (HPC) [117, 170], and carbon nanosheets [171, 172]. Plastic-derived carbon nanostructures can be used in energy storage devices such as supercapacitors and batteries, which promote sustainable green energy systems.
The materials with distinctive features like high surface area, high conductivity, and porosity can be used as hydrogen evolution reaction (HER) catalysts and oxygen evolution reaction (OER) catalysts in the water-splitting process.
In recent years, there has been growing interest in making adsorbents from waste, especially using waste plastics. Many methodologies have been documented to prepare the activated carbons and their potential application as adsorbents for the removal of dyes, heavy metals, and pharmaceuticals from wastewater. Generally, PET and polyurethane foam (PUF) waste has been exploited for the synthesis of activated carbon and graphene. The synthesized adsorbents have been investigated for the adsorptive removal of various emerging pollutants, such as methylene blue, acid blue 25, p-nitrophenol (PNP), Fe, cephalexin (CEX), and CO2, from wastewater and air [173, 174]. This effort helps in managing plastic waste and releases pressure by removing various organic and inorganic pollutants from wastewater. However, in this approach, making adsorbents from plastic waste at a large scale has many constraints. Combustion is one of the major causes of air pollution, generating pollutants such as alkanes, PAHs (including triphenylbenzenes), acids (e.g., terephthalic and 4-hydroxybenzoic), and phthalates [175, 176]. In addition, the capital cost is another factor restricting plastic incineration [156].
However, in the search for alternative fuels and energy, the higher calorific value of plastics than wood-based oil, which is comparable to conventional diesel, has drawn attention over their exploitation [177]. Many researchers exploit this opportunity to better utilize plastic waste. In this pyrolysis, the methodology is used to derive a useful product in absence of oxygen. Shafferina Dayana Anuar Sharuddin [178] applied this method (at 500 °C in presence of nitrogen gas) to investigate the potential production of liquid fuel based on their different compositions of plastic mainly derived from non-recycled plastics (NRPs) [178]. Many studies have shown the application of thermochemical pyrolysis technology for the conversion of municipal mixed plastic waste (MMPW) into high-quality hydrocarbon fuel. To enhance their efficiency and fuel productivity, a low-cost catalyst, i.e., CAT-1 of 10% (in weight), was used. These catalysts, however, were more efficient for the conversion of municipal virgin plastic than MMPW.
Future aspects and suggestions
The industries involved in manufacturing any plastic product either at any stage of manufacturing (raw material collection, processing, and packaging) should reserve 25% surface area of the plastic product to advertise against the use of plastic products, employing at least 25% surface area of the product (Fig. 4).
Fig. 4.
Use 25% area to show the minimum usage of plastic products and their negative impact on human health and the environment
Even we can make alternations in clothing fashion. Here we suggest one more zip lock pocket in front of the jeans or pants to collect the wraps of chocolate, biscuits, chewing gums, etc. Daily collected plastic waste will be disposed of in the evening in proper waste collection dustbins. This approach avoids throwing waste plastic into our surroundings.
Implementation and consideration of plastic pollution as an indicator under the parameter of solid waste indicator in the environmental performance index will rank the countries based on their production and mitigation approaches. The best-ranked countries will be the path makers for lower-ranked countries. It is important to issue directions for the restricted utility of plastic at different shops which may include restaurants, confectionaries, hotels, vegetable shops, and especially unauthorized shops in developing countries. Customer and shop owner pay penalty schemes should be incorporated in the offence.
The implementation of a circular plastic economy could increase the reusability and recycling of plastic waste. It is observed that the linear plastic economy adopted sees 90% of products used once and then discarded. In a circular economy, plastic waste can be exploited as raw materials to develop and design various products such as pots, key rings, and decorative items. This concept helps to reduce the high volume of plastic and could be a source of income [96].
Unfortunately, an increase in pollution increases the product demand that ultimately directly or indirectly increases the utilization of plastic products and the high probability of plastic mismanagement. Hence, the population is the largest source of pollution; therefore, strategies should be developed and implemented to overcome such issues.
Traditional means of bleaching and screening are an effective approach for the removal of large plastic plastics. However, fabric nets of different sizes are applied at the source and exit in houses, industry, hospitals, and drainage to collect the entered plastic waste in the water stream.
In the chemical approach for plastic removal, different types of adsorbents such as pillared clays (PILCs), Porous Clay Hetersostructures (PCHs), MOFS, and activated carbon from different sources, could be potential candidates for microplastic removal.
Coupling different approaches, such as activated biological treatment followed by filtration and membrane filtration, may offer promising benefits.
Conclusions
The present review article provides an overview of the status of plastics and their waste, which are considered as one of the biggest threats to territorial life, aquatic life, and humans. Mishandling plastic waste releases toxic chemicals such as CO2, CH4, arsenic, and VOCs that may cause neurological damage, reproductive damage, immune damage, asthma, and endocrine disruption to humans even at low concentrations and contribute to climate change. Additionally, some by-products from plastic waste are considered hazardous to human health and can cause cancer, endometriosis, birth defects, and child developmental disorders. The plastic waste and its handling can affect the environmental performance ranking of any nation. Various technologies and approaches have been implemented to overcome plastic pollution. Adsorption technology, coagulation, photocatalysis, and microbial decomposition may remove plastics from the environment, although they are effective for microplastics. Understanding the interaction mechanism between plastic chemicals over adsorbents, coagulants, and photocatalyst. Nevertheless, landfill is always considered and prioritized for plastic waste management. Increasing volume of waste at landfills and adverse effects on soil, water, air, humans, territorial animals, and aquatic animals insist on incineration although it has a huge impact on the environment. Cause and effect pressurized to look after these approaches. Reduction, reuse, and recycling could be more effective in eliminating plastic waste, and coping with advanced technology offers enhanced efficiency. Reduction is considered the worth option but is an unavoidable approach, whereas the recycling approach is considered a better alternative in exploiting plastic waste in an alternate manner, such as adsorbents, fuels, energy, bricks, roads, and pavement, opening a new pathway to reduce their amount in nature as a waste. The implementation of a circular economy can play an effective role in reducing the volume of plastic waste which can be turned into many useful products. This concept can boost the economy of a nation and provide an alternative source of income. It is also important to couple technologies such as adsorption, coagulation, and photochemical degradation with the 3R approach to understand and develop better opportunities to save resources.
Declarations
Conflict of interest
The authors declare no potential conflict of interest regarding the publication of this work. In addition, ethical issues including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, and redundancy have been completely witnessed by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Prashant Pandey, Email: prashant24.rai@gmail.com.
Manisha Dhiman, Email: dmanisha518@gmail.com.
Ankur Kansal, Email: kansal2ankur@gmail.com.
Sarada Prasannan Subudhi, Email: spsubudhi@gmail.com.
References
- 1.Brydson JA. Plastics materials. Elsevier; 1999. [Google Scholar]
- 2.Chen GGQ. Plastics from bacteria: Natural functions and applications (Vol 14) Springer Science and Business Media; 2009. [Google Scholar]
- 3.Van den Tempel M. Mechanical properties of plastic-disperse systems at very small deformations. Journal of Colloid Science. 1961;16(3):284–296. doi: 10.1016/0095-8522(61)90005-8. [DOI] [Google Scholar]
- 4.Geyer R. A brief history of plastics. Mare Plasticum-The Plastic Sea. Germany: Springer; 2020. pp. 31–47. [Google Scholar]
- 5.Gilbert M. Plastics materials: Introduction and historical development Brydson's plastics materials. Cham: Elsevier; 2017. pp. 1–18. [Google Scholar]
- 6.Rodrigues M, Abrantes N, Gonçalves F, Nogueira H, Marques J, Gonçalves A. Impacts of plastic products used in daily life on the environment and human health: What is known? Environmental Toxicology and Pharmacology. 2019;72:103239. doi: 10.1016/j.etap.2019.103239. [DOI] [PubMed] [Google Scholar]
- 7.Thompson RC, Swan SH, Moore CJ, Vom Saal FS. Our plastic age. The Royal Society Publishing; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritchie, H., and Roser, M. 2018. Plastic pollution. Oxford, UK: Our world in data.
- 9.Meides N, Menzel T, Poetzschner BR, Löder MG, Mansfeld U, Strohriegl P, Senker JR. Reconstructing the environmental degradation of polystyrene by accelerated weathering. Environmental Science and Technology. 2021 doi: 10.1021/acs.est.0c07718. [DOI] [PubMed] [Google Scholar]
- 10.Singh P, Sharma V. Integrated plastic waste management: environmental and improved health approaches. Procedia Environmental Sciences. 2016;35:692–700. doi: 10.1016/j.proenv.2016.07.068. [DOI] [Google Scholar]
- 11.Lau WW, Shiran Y, Bailey RM, Cook E, Stuchtey MR, Koskella J, Murphy MB. Evaluating scenarios toward zero plastic pollution. Science. 2020;369(6510):1455–1461. doi: 10.1126/science.aba9475. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes CJ. Plastic pollution and potential solutions. Science Progress. 2018;101(3):207–260. doi: 10.3184/003685018X15294876706211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Encinar J, González J. Pyrolysis of synthetic polymers and plastic wastes. Kinetic study. Fuel Processing Technology. 2008;89(7):678–686. doi: 10.1016/j.fuproc.2007.12.011. [DOI] [Google Scholar]
- 14.Jung MR, Horgen FD, Orski SV, Rodriguez V, Beers KL, Balazs GH, Royer SJ. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Marine Pollution Bulletin. 2018;127:704–716. doi: 10.1016/j.marpolbul.2017.12.061. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz AE, Ligthart TN, Boukris E, Van Harmelen T. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: a review study. Marine Pollution Bulletin. 2019;143:92–100. doi: 10.1016/j.marpolbul.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Stipek J, Daoust H. Additives for plastics (Vol 5) New York, NY, USA: Springer; 2012. [Google Scholar]
- 17.Kawai F, Kawabata T, Oda M. Current state and perspectives related to the polyethylene terephthalate hydrolases available for biorecycling. ACS Sustainable Chemistry and Engineering. 2020;8(24):8894–8908. doi: 10.1021/acssuschemeng.0c01638. [DOI] [Google Scholar]
- 18.Adhikary KB, Pang S, Staiger MP. Dimensional stability and mechanical behaviour of wood–plastic composites based on recycled and virgin high-density polyethylene (HDPE) Composites, Part B: Engineering. 2008;39(5):807–815. doi: 10.1016/j.compositesb.2007.10.005. [DOI] [Google Scholar]
- 19.Kumar S, Panda AK, Singh RK. A review on tertiary recycling of high-density polyethylene to fuel. Resources, Conservation and Recycling. 2011;55(11):893–910. doi: 10.1016/j.resconrec.2011.05.005. [DOI] [Google Scholar]
- 20.Akovali G. Toxicity of building materials. Springer; 2012. Plastic materials: polyvinyl chloride (PVC) pp. 23–53. [Google Scholar]
- 21.Mercea PV, Losher C, Petrasch M, Toşa V. Migration of stabilizers and plasticizer from recycled polyvinylchloride. Journal of Vinyl and Additive Technology. 2018;24:E112–E124. doi: 10.1002/vnl.21609. [DOI] [Google Scholar]
- 22.Sogancioglu M, Yel E, Ahmetli G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. Journal of Cleaner Production. 2017;165:369–381. doi: 10.1016/j.jclepro.2017.07.157. [DOI] [Google Scholar]
- 23.Maddah HA. Polypropylene as a promising plastic: A review. American Journal of Polymer Science. 2016;6(1):1–11. [Google Scholar]
- 24.Ranjan VP, Goel S. Recyclability of polypropylene after exposure to four different environmental conditions. Resources, Conservation and Recycling. 2021;169:105494. doi: 10.1016/j.resconrec.2021.105494. [DOI] [Google Scholar]
- 25.Andrade BTNC, Bezerra AC, Calado CR. Adding value to polystyrene waste by chemically transforming it into sulfonated polystyrene. Matéria (Rio de Janeiro) 2019 doi: 10.1590/s1517-707620190003.0732. [DOI] [Google Scholar]
- 26.Gao J, Shen Y, Xu H. Investigations for the mechanical, macro-, and microstructural analyses of dissimilar submerged friction stir welding of acrylonitrile butadiene styrene and polycarbonate sheets. Proceedings of the Institution of Mechanical Engineers, Part B. 2016;230(7):1213–1220. doi: 10.1177/0954405415572663. [DOI] [Google Scholar]
- 27.Ţiplica G-S, Bucur L, Bucur G, Sălăvăstru CM. Kanerva’s occupational dermatology. Springer; 2020. Other plastics; pp. 821–839. [Google Scholar]
- 28.Lin L, Argon A. Structure and plastic deformation of polyethylene. Journal of Materials Science. 1994;29(2):294–323. doi: 10.1007/BF01162485. [DOI] [Google Scholar]
- 29.Aggarwal S. Structure and properties of block polymers and multiphase polymer systems: An overview of present status and future potential. Polymer. 1976;17(11):938–956. doi: 10.1016/0032-3861(76)90170-1. [DOI] [Google Scholar]
- 30.Harris RM. Coloring technology for plastics. Norwich, NY, USA: William Andrew; 1999. [Google Scholar]
- 31.Heck RL., III A review of commercially used chemical foaming agents for thermoplastic foams. Journal of Vinyl and Additive Technology. 1998;4(2):113–116. doi: 10.1002/vnl.10027. [DOI] [Google Scholar]
- 32.Wensing M, Uhde E, Salthammer T. Plastics additives in the indoor environment—Flame retardants and plasticizers. Science of the Total Environment. 2005;339(1–3):19–40. doi: 10.1016/j.scitotenv.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 33.GmbH C, Richter E. Lubricants for film manufacture. Plastics, Additives and Compounding. 2000;2(11):14–21. doi: 10.1016/S1464-391X(00)80092-6. [DOI] [Google Scholar]
- 34.Grob MC, Minder E. Permanent antistatic additives: new developments. Plastics, Additives and Compounding. 1999;1(3):20–26. doi: 10.1016/S1464-391X(99)80074-9. [DOI] [Google Scholar]
- 35.Jones A. Choosing antimicrobial additives for plastics. Plastics, Additives and Compounding. 2009;11(4):26–28. doi: 10.1016/S1464-391X(09)70109-6. [DOI] [Google Scholar]
- 36.Papazoglou ES. Flame retardants for plastics. Handbook of building materials for fire protection. New York: McGraw-Hill; 2004. pp. 41–488. [Google Scholar]
- 37.Verma R, Vinoda K, Papireddy M, Gowda A. Toxic pollutants from plastic waste-a review. Procedia Environmental Sciences. 2016;35:701–708. doi: 10.1016/j.proenv.2016.07.069. [DOI] [Google Scholar]
- 38.Alabi OA, Ologbonjaye KI, Awosolu O, Alalade OE. Public and environmental health effects of plastic wastes disposal: A review. Journal of Toxicology and Risk Assessment. 2019;5(021):1–13. [Google Scholar]
- 39.Rustagi N, Pradhan S, Singh R. Public health impact of plastics: An overview. Indian Journal of Occupational and Environmental Medicine. 2011;15(3):100. doi: 10.4103/0019-5278.93198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laskar N, Kumar U. Plastics and microplastics: A threat to environment. Environmental Technology and Innovation. 2019;14:100352. doi: 10.1016/j.eti.2019.100352. [DOI] [Google Scholar]
- 41.Lusher AL, Hernandez-Milian G, O'Brien J, Berrow S, O'Connor I, Officer R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True's beaked whale Mesoplodon mirus. Environmental Pollution. 2015;199:185–191. doi: 10.1016/j.envpol.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Avio CG, Gorbi S, Regoli F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Marine Environmental Research. 2017;128:2–11. doi: 10.1016/j.marenvres.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Cai C, Yu S, Liu Y, Tao S, Liu W. PBDE emission from E-wastes during the pyrolytic process: Emission factor, compositional profile, size distribution, and gas-particle partitioning. Environmental Pollution. 2018;235:419–428. doi: 10.1016/j.envpol.2017.12.068. [DOI] [PubMed] [Google Scholar]
- 44.Valavanidis A, Iliopoulos N, Gotsis G, Fiotakis K. Persistent free radicals, heavy metals and PAHs generated in particulate soot emissions and residue ash from controlled combustion of common types of plastic. Journal of Hazardous Materials. 2008;156(1–3):277–284. doi: 10.1016/j.jhazmat.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Kurdikar D, Fournet L, Slater SC, Paster M, Gruys KJ, Gerngross TU, Coulon R. Greenhouse gas profile of a plastic material derived from a genetically modified plant. Journal of Industrial Ecology. 2000;4(3):107–122. doi: 10.1162/108819800300106410. [DOI] [Google Scholar]
- 46.Shen M, Huang W, Chen M, Song B, Zeng G, Zhang Y. (Micro) plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. Journal of Cleaner Production. 2020;254:120138. doi: 10.1016/j.jclepro.2020.120138. [DOI] [Google Scholar]
- 47.Anwar Z, Anjum F, Ghayas S. Polycarbonate plastics and neurological disorders: From exposure to preventive interventions environmental contaminants and neurological disorders. Springer; 2021. pp. 147–183. [Google Scholar]
- 48.Amereh F, Babaei M, Eslami A, Fazelipour S, Rafiee M. The emerging risk of exposure to nano (micro) plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environmental Pollution. 2020;261:114158. doi: 10.1016/j.envpol.2020.114158. [DOI] [PubMed] [Google Scholar]
- 49.Navarro, G. P. P. 2020. Micro-plastics, nano-plastics and it’s components on the impact of human health. Doctoral dissertation, Lietuvos sveikatos mokslų universitetas.
- 50.Slovak A. Occupational asthma caused by a plastics blowing agent, azodicarbonamide. Thorax. 1981;36(12):906–909. doi: 10.1136/thx.36.12.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halden RU. Plastics and health risks. Annual Review of Public Health. 2010;31:179–194. doi: 10.1146/annurev.publhealth.012809.103714. [DOI] [PubMed] [Google Scholar]
- 52.Rajmohan KVS, Ramya C, Viswanathan MR, Varjani S. Plastic pollutants: effective waste management for pollution control and abatement. Current Opinion in Environmental Science and Health. 2019;12:72–84. doi: 10.1016/j.coesh.2019.08.006. [DOI] [Google Scholar]
- 53.Zhang H, Xu Y, Jia L, Xu M. Smog chamber study on the ozone formation potential of acetaldehyde. Advances in Atmospheric Sciences. 2021;38(7):1238–1251. doi: 10.1007/s00376-021-0407-5. [DOI] [Google Scholar]
- 54.Folkins I, Chatfield R. Impact of acetone on ozone production and OH in the upper troposphere at high NOX. Journal of Geophysical Research: Atmospheres. 2000;105(D9):11585–11599. doi: 10.1029/2000JD900067. [DOI] [Google Scholar]
- 55.Sankar M, Nowicka E, Carter E, Murphy DM, Knight DW, Bethell D, Hutchings GJ. The benzaldehyde oxidation paradox explained by the interception of peroxy radical by benzyl alcohol. Nature Communications. 2014;5(1):1–6. doi: 10.1038/ncomms4332. [DOI] [PubMed] [Google Scholar]
- 56.Zubair M, Adrees A. Air pollution-monitoring, quantification and removal of gases and particles. Intech Open; 2019. Dioxins and furans: emerging contaminants of air; pp. 111–125. [Google Scholar]
- 57.Amthor J. Respiration in a future, higher-CO2 world. Plant, Cell & Environment. 1991;14(1):13–20. doi: 10.1111/j.1365-3040.1991.tb01367.x. [DOI] [Google Scholar]
- 58.Yoshida N, Mizutani Y. Preparation of carbon dioxide for oxygen-18 determination of water by use of a plastic syringe. Analytical Chemistry. 1986;58(6):1273–1275. doi: 10.1021/ac00297a071. [DOI] [Google Scholar]
- 59.Nkwachukwu OI, Chima CH, Ikenna AO, Albert L. Focus on potential environmental issues on plastic world towards a sustainable plastic recycling in developing countries. International Journal of Industrial Chemistry. 2013;4(1):1–13. doi: 10.1186/2228-5547-4-34. [DOI] [Google Scholar]
- 60.Wagoner JK. Toxicity of vinyl chloride and poly (vinyl chloride): a critical review. Environmental Health Perspectives. 1983;52:61–66. doi: 10.1289/ehp.835261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lomonaco T, Manco E, Corti A, La Nasa J, Ghimenti S, Biagini D, Fuoco R. Release of harmful volatile organic compounds (VOCs) from photo-degraded plastic debris: A neglected source of environmental pollution. Journal of Hazardous Materials. 2020;394:122596. doi: 10.1016/j.jhazmat.2020.122596. [DOI] [PubMed] [Google Scholar]
- 62.Royer S-J, Ferrón S, Wilson ST, Karl DM. Production of methane and ethylene from plastic in the environment. PLoS ONE. 2018;13(8):e0200574. doi: 10.1371/journal.pone.0200574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross S, Evans D. The environmental effect of reusing and recycling a plastic-based packaging system. Journal of Cleaner Production. 2003;11(5):561–571. doi: 10.1016/S0959-6526(02)00089-6. [DOI] [Google Scholar]
- 64.Abelsohn A, Sanborn MD, Jessiman BJ, Weir E. Identifying and managing adverse environmental health effects: 6. Carbon Monoxide Poisoning. 2002;166(13):1685–1690. [PMC free article] [PubMed] [Google Scholar]
- 65.Boyle D, Catarino AI, Clark NJ, Henry TB. Polyvinyl chloride (PVC) plastic fragments release Pb additives that are bioavailable in zebrafish. Environmental Pollution. 2020;263:114422. doi: 10.1016/j.envpol.2020.114422. [DOI] [PubMed] [Google Scholar]
- 66.Casas JS, Sordo J. Lead: chemistry, analytical aspects, environmental impact and health effects. Elsevier; 2011. [Google Scholar]
- 67.Rosen JF. Adverse health effects of lead at low exposure levels: trends in the management of childhood lead poisoning. Toxicology. 1995;97(1–3):11–17. doi: 10.1016/0300-483X(94)02963-U. [DOI] [PubMed] [Google Scholar]
- 68.Saini, V., and Pandey, P. 2017. Natural husks as potential adsorbents for uptake of heavy metals. Millersville PA, USA: Materials Research Foundations.
- 69.Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Law KL. Plastic waste inputs from land into the ocean. Science. 2015;347(6223):768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 70.Kershaw P, Katsuhiko S, Lee S, Woodring D. Plastic debris in the ocean. United Nations Environment Programme; 2011. [Google Scholar]
- 71.Wabnitz C, Nichols WJ. Plastic pollution: An ocean emergency. Marine Turtle Newsletter. 2010;129:1. [Google Scholar]
- 72.Hoarau L, Ainley L, Jean C, Ciccione S. Ingestion and defecation of marine debris by loggerhead sea turtles, Caretta caretta, from by-catches in the South-West Indian Ocean. Marine Pollution Bulletin. 2014;84(1–2):90–96. doi: 10.1016/j.marpolbul.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 73.Lavers JL, Hutton I, Bond AL. Clinical pathology of plastic ingestion in marine birds and relationships with blood chemistry. Environmental Science and Technology. 2019;53(15):9224–9231. doi: 10.1021/acs.est.9b02098. [DOI] [PubMed] [Google Scholar]
- 74.Beaumont NJ, Aanesen M, Austen MC, Börger T, Clark JR, Cole M, Wyles KJ. Global ecological, social and economic impacts of marine plastic. Marine Pollution Bulletin. 2019;142:189–195. doi: 10.1016/j.marpolbul.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 75.Dris R, Imhof H, Sanchez W, Gasperi J, Galgani F, Tassin B, Laforsch C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-) plastic particles. Environmental chemistry. 2015;12(5):539–550. doi: 10.1071/EN14172. [DOI] [Google Scholar]
- 76.Gunaalan K, Fabbri E, Capolupo M. The hidden threat of plastic leachates: A critical review on their impacts on aquatic organisms. Water Research. 2020;184:116170. doi: 10.1016/j.watres.2020.116170. [DOI] [PubMed] [Google Scholar]
- 77.Peng L, Fu D, Qi H, Lan CQ, Yu H, Ge C. Micro-and nano-plastics in marine environment: Source, distribution and threats—A review. Science of the total environment. 2020;698:134254. doi: 10.1016/j.scitotenv.2019.134254. [DOI] [PubMed] [Google Scholar]
- 78.Chen, Z., Fang, J., Wei, W., Ngo, H.H., Guo, W., Ni, B.J. 2022. Emerging adsorbents for micro/nanoplastics removal from contaminated water: Advances and perspectives. Journal of Cleaner Production. 19: 133676.
- 79.Chae Y, An Y-J. Effects of micro-and nanoplastics on aquatic ecosystems: Current research trends and perspectives. Marine Pollution Bulletin. 2017;124(2):624–632. doi: 10.1016/j.marpolbul.2017.01.070. [DOI] [PubMed] [Google Scholar]
- 80.Franzellitti S, Canesi L, Auguste M, Wathsala RH, Fabbri E. Microplastic exposure and effects in aquatic organisms: A physiological perspective. Environmental Toxicology and Pharmacology. 2019;68:37–51. doi: 10.1016/j.etap.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Gao G, Zhao X, Jin P, Gao K, Beardall J. Current understanding and challenges for aquatic primary producers in a world with rising micro-and nano-plastic levels. Journal of Hazardous Materials. 2021;406:124685. doi: 10.1016/j.jhazmat.2020.124685. [DOI] [PubMed] [Google Scholar]
- 82.Vethaak AD, Leslie HA. Plastic debris is a human health issue. Cham: ACS Publications; 2016. [DOI] [PubMed] [Google Scholar]
- 83.Waring RH, Harris R, Mitchell S. Plastic contamination of the food chain: A threat to human health? Maturitas. 2018;115:64–68. doi: 10.1016/j.maturitas.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 84.Wright SL, Kelly FJ. Plastic and human health: a micro issue? Environmental Science and Technology. 2017;51(12):6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- 85.Index EP. Environmental performance index. New Haven, CT, USA: Yale University and Columbia University; 2018. [Google Scholar]
- 86.Ma Z, Ryberg MW, Wang P, Tang L, Chen W-Q. China’s import of waste PET bottles benefited global plastic circularity and environmental performance. ACS Sustainable Chemistry and Engineering. 2020;8(45):16861–16868. doi: 10.1021/acssuschemeng.0c05926. [DOI] [Google Scholar]
- 87.Tiwari E, Singh N, Khandelwal N, Monikh FA, Darbha GK. Application of Zn/Al layered double hydroxides for the removal of nano-scale plastic debris from aqueous systems. Journal of Hazardous Materials. 2020;397:122769. doi: 10.1016/j.jhazmat.2020.122769. [DOI] [PubMed] [Google Scholar]
- 88.Wei S, Kamali AR. Trifunctional mesoporous magnetic adsorbent-photocatalyst nanocomposite for efficient removal of potassium ethyl xanthate from mining wastewater. Journal of Water Process Engineering. 2022;1(49):103067. doi: 10.1016/j.jwpe.2022.103067. [DOI] [Google Scholar]
- 89.Nabi I, Ahmad F, Zhang L. Application of titanium dioxide for the photocatalytic degradation of macro-and micro-plastics: A review. Journal of Environmental Chemical Engineering. 2021;9:105964. doi: 10.1016/j.jece.2021.105964. [DOI] [Google Scholar]
- 90.Sarwan B, Acharya AD, Kaur S, Pare B. Visible light photocatalytic deterioration of polystyrene plastic using supported BiOCl nanoflower and nanodisk. European Polymer Journal. 2020;134:109793. doi: 10.1016/j.eurpolymj.2020.109793. [DOI] [Google Scholar]
- 91.Xu Q, Huang Q-S, Luo T-Y, Wu R-L, Wei W, Ni B-J. Coagulation removal and photocatalytic degradation of microplastics in urban waters. Chemical Engineering Journal. 2021;416:129123. doi: 10.1016/j.cej.2021.129123. [DOI] [Google Scholar]
- 92.Yuan F, Yue L, Zhao H, Wu H. Study on the adsorption of polystyrene microplastics by three-dimensional reduced graphene oxide. Water Science and Technology. 2020;81(10):2163–2175. doi: 10.2166/wst.2020.269. [DOI] [PubMed] [Google Scholar]
- 93.Yuan J, Ma J, Sun Y, Zhou T, Zhao Y, Yu F. Microbial degradation and other environmental aspects of microplastics/plastics. Science of the Total Environment. 2020;715:136968. doi: 10.1016/j.scitotenv.2020.136968. [DOI] [PubMed] [Google Scholar]
- 94.Kundu A, Shetti NP, Basu S, Reddy KR, Nadagouda MN, Aminabhavi TM. Identification and removal of micro-and nano-plastics: Efficient and cost-effective methods. Chemical Engineering Journal. 2021;421:129816. doi: 10.1016/j.cej.2021.129816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hopewell J, Dvorak R, Kosior E. Plastics recycling: Challenges and opportunities. Philosophical Transactions of the Royal Society B. 2009;364(1526):2115–2126. doi: 10.1098/rstb.2008.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pandey P, Saini VK. Green adsorbents for pollutant removal. Springer; 2018. Pillared interlayered clays for pollution remediation; pp. 353–376. [Google Scholar]
- 97.Pandey P, Saini VK. Pillared interlayered clays: sustainable materials for pollution abatement. Environmental Chemistry Letters. 2019;17(2):721–727. doi: 10.1007/s10311-018-00826-0. [DOI] [Google Scholar]
- 98.Wang Z, Sun C, Li F, Chen L. Fatigue resistance, re-usable and biodegradable sponge materials from plant protein with rapid water adsorption capacity for microplastics removal. Chemical Engineering Journal. 2021;415:129006. doi: 10.1016/j.cej.2021.129006. [DOI] [Google Scholar]
- 99.Ye H, Wang Y, Liu X, Xu D, Yuan H, Sun H, Ma X. Magnetically steerable iron oxides-manganese dioxide core–shell micromotors for organic and microplastic removals. Journal of Colloid and Interface Science. 2021;588:510–521. doi: 10.1016/j.jcis.2020.12.097. [DOI] [PubMed] [Google Scholar]
- 100.Tang Y, Zhang S, Su Y, Wu D, Zhao Y, Xie B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chemical Engineering Journal. 2021;406:126804. doi: 10.1016/j.cej.2020.126804. [DOI] [Google Scholar]
- 101.Kim KT, Park S. Enhancing microplastics removal from wastewater using electro-coagulation and granule-activated carbon with thermal regeneration. Processes. 2021;9(4):617. doi: 10.3390/pr9040617. [DOI] [Google Scholar]
- 102.Shi, H., Reza, M.O., Nikrityuk, P.A. 2021. The impact of swirling on the dynamics of a spouted bed. Powder Technology. 380: 143–51.
- 103.Ameta R, Solanki MS, Benjamin S, Ameta SC. Photocatalysis advanced oxidation processes for waste water treatment. Elsevier; 2018. pp. 135–175. [Google Scholar]
- 104.Tofa TS, Kunjali KL, Paul S, Dutta J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environmental Chemistry Letters. 2019;17(3):1341–1346. doi: 10.1007/s10311-019-00859-z. [DOI] [Google Scholar]
- 105.Shang J, Chai M, Zhu Y. Photocatalytic degradation of polystyrene plastic under fluorescent light. Environmental Science and Technology. 2003;37(19):4494–4499. doi: 10.1021/es0209464. [DOI] [PubMed] [Google Scholar]
- 106.Zhao, X., Li, Z., Chen, Y., Shi, L., Zhu, Y. 2008. Enhancement of photocatalytic degradation of polyethylene plastic with CuPc modified TiO2 photocatalyst under solar light irradiation. Applied Surface Science. 254 (6): 1825–1829.
- 107.Fa W, Yang C, Gong C, Peng T, Zan L. Enhanced photodegradation efficiency of polyethylene-TiO2 nanocomposite film with oxidized polyethylene wax. Journal of Applied Polymer Science. 2010;118(1):378–384. doi: 10.1002/app.32413. [DOI] [Google Scholar]
- 108.Wang L, Kaeppler A, Fischer D, Simmchen J. Photocatalytic TiO2 micromotors for removal of microplastics and suspended matter. ACS Applied Materials and Interfaces. 2019;11(36):32937–32944. doi: 10.1021/acsami.9b06128. [DOI] [PubMed] [Google Scholar]
- 109.Nabi I, Li K, Cheng H, Wang T, Liu Y, Ajmal S, Zhang L. Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. Iscience. 2020;23(7):101326. doi: 10.1016/j.isci.2020.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uheida A, Mejía HG, Abdel-Rehim M, Hamd W, Dutta J. Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. Journal of hazardous materials. 2021;406:124299. doi: 10.1016/j.jhazmat.2020.124299. [DOI] [PubMed] [Google Scholar]
- 111.O’Melia CR. The scientific basis of flocculation. Springer; 1978. Coagulation in wastewater treatment; pp. 219–268. [Google Scholar]
- 112.Ma B, Xue W, Ding Y, Hu C, Liu H, Qu J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. Journal of Environmental Sciences. 2019;78:267–275. doi: 10.1016/j.jes.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 113.Zhou G, Wang Q, Li J, Li Q, Xu H, Ye Q, Zhang J. Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: Performance and mechanism. Science of the Total Environment. 2021;752:141837. doi: 10.1016/j.scitotenv.2020.141837. [DOI] [PubMed] [Google Scholar]
- 114.Horn D, Miller M, Anderson S, Steele C. Microplastics are ubiquitous on California beaches and enter the coastal food web through consumption by Pacific mole crabs. Mar Pollut Bull. 2019;139:231–237. doi: 10.1016/j.marpolbul.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 115.Skaf DW, Punzi VL, Rolle JT, Kleinberg KA. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chemical Engineering Journal. 2020;386:123807. doi: 10.1016/j.cej.2019.123807. [DOI] [Google Scholar]
- 116.Darabi, M., Majeed, H., Diehl, A., Norton, J., Zhang, Y. 2021. A review of microplastics in aquatic sediments: occurrence, fate, transport, and ecological impact. Current Pollution Reports. 7 (1): 40–53.
- 117.Zhang Y, Diehl A, Lewandowski A, Gopalakrishnan K, Baker T. Removal efficiency of micro-and nanoplastics (180 nm–125 μm) during drinking water treatment. Science of the Total Environment. 2020;720:137383. doi: 10.1016/j.scitotenv.2020.137383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kyrikou I, Briassoulis D. Biodegradation of agricultural plastic films: a critical review. Journal of Polymers and the Environment. 2007;15(2):125–150. doi: 10.1007/s10924-007-0053-8. [DOI] [Google Scholar]
- 119.Shen M, Zeng G, Zhang Y, Wen X, Song B, Tang W. Can biotechnology strategies effectively manage environmental (micro) plastics? Science of the Total Environment. 2019;697:134200. doi: 10.1016/j.scitotenv.2019.134200. [DOI] [PubMed] [Google Scholar]
- 120.Kale SK, Deshmukh AG, Dudhare MS, Patil VB. Microbial degradation of plastic: A review. Journal of Biochemical Technology. 2015;6(2):952–961. [Google Scholar]
- 121.Paço A, Duarte K, da Costa JP, Santos PS, Pereira R, Pereira M, Rocha-Santos TA. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Science of the Total Environment. 2017;586:10–15. doi: 10.1016/j.scitotenv.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 122.Espinosa MJC, Blanco AC, Schmidgall T, Atanasoff-Kardjalieff AK, Kappelmeyer U, Tischler D, Eberlein C. Toward biorecycling: Isolation of a soil bacterium that grows on a polyurethane oligomer and monomer. Frontiers in Microbiology. 2020;11:404. doi: 10.3389/fmicb.2020.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tournier V, Topham C, Gilles A, David B, Folgoas C, Moya-Leclair E, Gavalda S. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020;580(7802):216–219. doi: 10.1038/s41586-020-2149-4. [DOI] [PubMed] [Google Scholar]
- 124.Kim HR, Lee HM, Yu HC, Jeon E, Lee S, Li J, Kim D-H. Biodegradation of polystyrene by Pseudomonas sp. isolated from the gut of superworms (larvae of Zophobas atratus ) Environmental Science and Technology. 2020;54(11):6987–6996. doi: 10.1021/acs.est.0c01495. [DOI] [PubMed] [Google Scholar]
- 125.Drzyzga O, Prieto A. Plastic waste management, a matter for the ‘community’. Microbial Biotechnology. 2019;12(1):66. doi: 10.1111/1751-7915.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamamoto T, Yasuhara A, Shiraishi H, Nakasugi O. Bisphenol A in hazardous waste landfill leachates. Chemosphere. 2001;42(4):415–418. doi: 10.1016/S0045-6535(00)00079-5. [DOI] [PubMed] [Google Scholar]
- 127.Dauvergne P. Why is the global governance of plastic failing the oceans? Global Environmental Change. 2018;51:22–31. doi: 10.1016/j.gloenvcha.2018.05.002. [DOI] [Google Scholar]
- 128.Haward M. Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nature Communications. 2018;9(1):1–3. doi: 10.1038/s41467-018-03104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Manchala M, Ramana BV. Beat the plastic pollution. International Journal of Environmental Planning and Development. 2020;6(2):1–6. [Google Scholar]
- 130.Sidhu BK, Desa BH. Plastics pollution: a new common concern of humankind? Environmental Policy and Law. 2018;48(5):252–255. doi: 10.3233/EPL-180084. [DOI] [Google Scholar]
- 131.Jalil MA, Mian MN, Rahman MK. Using plastic bags and its damaging impact on environment and agriculture: An alternative proposal. International Journal of Learning and Development. 2013;3(4):1–14. doi: 10.5296/ijld.v3i4.4137. [DOI] [Google Scholar]
- 132.Singh P, Verma R. Bioplastics: A green approach toward sustainable environment environmental microbiology and biotechnology. Springer; 2020. pp. 35–53. [Google Scholar]
- 133.Burlakovs J, Kriipsalu M, Porshnov D, Jani Y, Ozols V, Pehme K-M, Vincevica-Gaile Z. Gateway of landfilled plastic waste towards circular economy in Europe. Separations. 2019;6(2):25. doi: 10.3390/separations6020025. [DOI] [Google Scholar]
- 134.Žmak I, Hartmann C. Current state of the plastic waste recycling system in the European Union and in Germany. Tehnički Glasnik. 2017;11(3):138–142. [Google Scholar]
- 135.Guest H, Lotze HK, Wallace D. Youth and the sea: Ocean literacy in Nova Scotia, Canada. Marine Policy. 2015;58:98–107. doi: 10.1016/j.marpol.2015.04.007. [DOI] [Google Scholar]
- 136.Heidbreder LM, Bablok I, Drews S, Menzel C. Tackling the plastic problem: A review on perceptions, behaviors, and interventions. Science of the Total Environment. 2019;668:1077–1093. doi: 10.1016/j.scitotenv.2019.02.437. [DOI] [PubMed] [Google Scholar]
- 137.Singh KD, Mathur A. Plastic pollution in India: An evaluation of public awareness and consumption behaviour. OIDA International Journal of Sustainable Development. 2019;12(07):25–40. [Google Scholar]
- 138.Wath SB, Dutt P, Chakrabarti T. E-waste scenario in India, its management and implications. Environmental Monitoring and Assessment. 2011;172(1):249–262. doi: 10.1007/s10661-010-1331-9. [DOI] [PubMed] [Google Scholar]
- 139.Brooks AL, Wang S, Jambeck JR. The Chinese import ban and its impact on global plastic waste trade. Science Advances. 2018;4(6):eaat0131. doi: 10.1126/sciadv.aat0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Santoleri JJ, Reynolds J, Theodore L. Introduction to hazardous waste incineration. John Wiley & Sons; 2000. [Google Scholar]
- 141.Yang Z, Lü F, Zhang H, Wang W, Shao L, Ye J, He P. Is incineration the terminator of plastics and microplastics? Journal of Hazardous Materials. 2021;401:123429. doi: 10.1016/j.jhazmat.2020.123429. [DOI] [PubMed] [Google Scholar]
- 142.Ágnes N, Rajmund K. The environmental impact of plastic waste incineration. Academic and Applied Research in Military and Public Management Science. 2016;15(3):231–237. [Google Scholar]
- 143.Courtemanche B, Levendis YA. A laboratory study on the NO, NO2, SO2, CO and CO2 emissions from the combustion of pulverized coal, municipal waste plastics and tires. Fuel. 1998;77(3):183–196. doi: 10.1016/S0016-2361(97)00191-9. [DOI] [Google Scholar]
- 144.Huang D-Y, Zhou S-G, Hong W, Feng W-F, Tao L. Pollution characteristics of volatile organic compounds, polycyclic aromatic hydrocarbons and phthalate esters emitted from plastic wastes recycling granulation plants in Xingtan Town, South China. Atmospheric Environment. 2013;71:327–334. doi: 10.1016/j.atmosenv.2013.02.011. [DOI] [Google Scholar]
- 145.Li C-T, Zhuang H-K, Hsieh L-T, Lee W-J, Tsao M-C. PAH emission from the incineration of three plastic wastes. Environment International. 2001;27(1):61–67. doi: 10.1016/S0160-4120(01)00056-3. [DOI] [PubMed] [Google Scholar]
- 146.Linak WP, Wendt JO. Toxic metal emissions from incineration: mechanisms and control. Progress in Energy and Combustion Science. 1993;19(2):145–185. doi: 10.1016/0360-1285(93)90014-6. [DOI] [Google Scholar]
- 147.Bora RR, Wang R, You F. Waste polypropylene plastic recycling toward climate change mitigation and circular economy: energy, environmental, and technoeconomic perspectives. ACS Sustainable Chemistry and Engineering. 2020;8(43):16350–16363. doi: 10.1021/acssuschemeng.0c06311. [DOI] [Google Scholar]
- 148.Huiying Y. Analysis of the advantages and disadvantages of waste incineration and discussion on the standard of incineration. Solid State Technology. 2021;64(2):6415–6421. [Google Scholar]
- 149.Xiao-Yin, Y. 2003. Disadvantages of waste incineration technology and study on decoupling incineration technology. Environmental Protection Science.
- 150.Siddique R, Khatib J, Kaur I. Use of recycled plastic in concrete: A review. Waste Management. 2008;28(10):1835–1852. doi: 10.1016/j.wasman.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 151.White PR, Franke M, Hindle P. Integrated solid waste management: A lifecycle inventory. Springer Science & Business Media; 1995. [Google Scholar]
- 152.Puri HS, Mishra DS, Jindal V. Plastic waste management-issues solutions and case studies. Ministry of Housing and Urban Affairs Government of India; 2019. pp. 105–113. [Google Scholar]
- 153.Dan C, Xingyuan H, Peng W, Xiong L, Xianhua Z. Recycling effective ways of waste plastics. Engineering Plastics Application. 2012;40:92–94. [Google Scholar]
- 154.Thomas C, Sharp V. Understanding the normalisation of recycling behaviour and its implications for other pro-environmental behaviours: A review of social norms and recycling. Resources, Conservation and Recycling. 2013;79:11–20. doi: 10.1016/j.resconrec.2013.04.010. [DOI] [Google Scholar]
- 155.Tian S, Tang H, Wang Q, Yuan X, Ma Q, Wang M. Evaluation and optimization of blanket production from recycled polyethylene terephthalate based on the coordination of environment, economy, and society. Science of the Total Environment. 2021;772:145049. doi: 10.1016/j.scitotenv.2021.145049. [DOI] [PubMed] [Google Scholar]
- 156.Walker T, Gramlich D, Dumont-Bergeron A. The case for a plastic tax: a review of its benefits and disadvantages within a circular economy. Sustainability. 2020 doi: 10.1108/S2514-175920200000004010. [DOI] [Google Scholar]
- 157.Ahmed S, Mahmood Q, Elahi N, Nawab B. Current practices and futuristic options in plastic waste management in Pakistan. Central Asian Journal of Environmental Science and Technology Innovation. 2020;1(4):237–244. [Google Scholar]
- 158.Chowdhury AH, Mohammad N, Haque MRU, Hossain T. Developing 3Rs (reduce, reuse and recycle) strategy for waste management in the urban areas of Bangladesh: Socioeconomic and climate adoption mitigation option. IOSR Journal of Environmental Science, Toxicology and Food Technology. 2014;8(5):9–18. doi: 10.9790/2402-08510918. [DOI] [Google Scholar]
- 159.David A, Thangavel YD, Sankriti R. Recover, recycle and reuse: An efficient way to reduce the waste. International Journal of Mechanical and Production Engineering Research and Development (IJMPERD) 2019;9:2249–6890. [Google Scholar]
- 160.Prakash G, Pathak P. Intention to buy eco-friendly packaged products among young consumers of India: A study on developing nation. Journal of Cleaner Production. 2017;141:385–393. doi: 10.1016/j.jclepro.2016.09.116. [DOI] [Google Scholar]
- 161.Karpagavinayagam, P., Rajam, J.A., Suneetha, R.B., Vedhi, C. Prospects of carbon nanomaterial-based sensors for sustainable future. In Carbon nanomaterials-based sensors, pp. 417–428. Amsterdam, the Netherlands: Elsevier.
- 162.Bhattacharya, R., Chandrasekhar, K., Roy, P., et al. 2018. Challenges and opportunities: Plastic waste management in India.
- 163.Bura N. An overview of plastic waste management in India. Waste management and resource efficiency. Cham: Springer; 2019. pp. 935–943. [Google Scholar]
- 164.Kamali AR, Yang J, Sun Q. Molten salt conversion of polyethylene terephthalate waste into graphene nanostructures with high surface area and ultra-high electrical conductivity. Applied Surface Science. 2019;476:539–551. doi: 10.1016/j.apsusc.2019.01.119. [DOI] [Google Scholar]
- 165.Kamali AR, Yang J. Effect of molten salts on the structure, morphology and electrical conductivity of PET-derived carbon nanostructures. Polymer Degradation and Stability. 2020;177:109184. doi: 10.1016/j.polymdegradstab.2020.109184. [DOI] [Google Scholar]
- 166.Dai L, Karakas O, Cheng Y, Cobb K, Chen P, Ruan R. A review on carbon materials production from plastic wastes. Chemical Engineering Journal. 2022;12:139725. [Google Scholar]
- 167.Putra PH, Rozali S, Patah MF, Idris A. A review of microwave pyrolysis as a sustainable plastic waste management technique. Journal of Environmental Management. 2022;1(303):114240. doi: 10.1016/j.jenvman.2021.114240. [DOI] [PubMed] [Google Scholar]
- 168.Bazargan A, McKay G. A review–synthesis of carbon nanotubes from plastic wastes. Chemical Engineering Journal. 2012;195:377–391. doi: 10.1016/j.cej.2012.03.077. [DOI] [Google Scholar]
- 169.Vieira O, Ribeiro RS, de Tuesta JLD, Gomes HT, Silva AM. A systematic literature review on the conversion of plastic wastes into valuable 2D graphene-based materials. Chemical Engineering Journal. 2022;428:131399. doi: 10.1016/j.cej.2021.131399. [DOI] [Google Scholar]
- 170.Zhang H, Zhou XL, Shao LM, Lü F, He PJ. Hierarchical porous carbon spheres from low-density polyethylene for high-performance supercapacitors. ACS Sustainable Chemistry and Engineering. 2019;7:3801–3810. doi: 10.1021/acssuschemeng.8b04539. [DOI] [Google Scholar]
- 171.Gong J, Michalkiewicz B, Chen X, Mijowska E, Liu J, Jiang Z, Wen X, Tang T. Sustainable conversion of mixed plastics into porous carbon nanosheets with high performances in uptake of carbon dioxide and storage of hydrogen. ACS Sustainable Chemistry and Engineering. 2014;2:2837–2844. doi: 10.1021/sc500603h. [DOI] [Google Scholar]
- 172.Liu X, Ma C, Wen Y, Chen X, Zhao X, Tang T, Holze R, Mijowska E. Highly efficient conversion of waste plastic into thin carbon nanosheets for superior capacitive energy storage. Carbon. 2021;171:819–828. doi: 10.1016/j.carbon.2020.09.057. [DOI] [Google Scholar]
- 173.Kaur B, Gupta RK, Bhunia H. Chemically activated nanoporous carbon adsorbents from waste plastic for CO2 capture: Breakthrough adsorption study. Microporous and Mesoporous Materials. 2019;282:146–158. doi: 10.1016/j.micromeso.2019.03.025. [DOI] [Google Scholar]
- 174.Mendoza-Carrasco R, Cuerda-Correa EM, Alexandre-Franco MF, Fernández-González C, Gómez-Serrano V. Preparation of high-quality activated carbon from polyethyleneterephthalate (PET) bottle waste. Its use in the removal of pollutants in aqueous solution. Journal of Environmental Management. 2016;181:522–535. doi: 10.1016/j.jenvman.2016.06.070. [DOI] [PubMed] [Google Scholar]
- 175.Lazarevic D, Aoustin E, Buclet N, Brandt N. Plastic waste management in the context of a European recycling society: Comparing results and uncertainties in a life cycle perspective. Resources, Conservation and Recycling. 2010;55(2):246–259. doi: 10.1016/j.resconrec.2010.09.014. [DOI] [Google Scholar]
- 176.Simoneit BR, Medeiros PM, Didyk BM. Combustion products of plastics as indicators for refuse burning in the atmosphere. Environmental Science and Technology. 2005;39(18):6961–6970. doi: 10.1021/es050767x. [DOI] [PubMed] [Google Scholar]
- 177.Kumar A, Dash SK, Ahamed MS, Lingfa P. Study on conversion techniques of alternative fuels from waste plastics. Energy recovery processes from wastes. Springer; 2020. pp. 213–224. [Google Scholar]
- 178.Sharuddin SDA, Abnisa F, Daud WMAW, Aroua MK. Energy recovery from pyrolysis of plastic waste: Study on non-recycled plastics (NRP) data as the real measure of plastic waste. Energy Conversion and Management. 2017;148:925–934. doi: 10.1016/j.enconman.2017.06.046. [DOI] [Google Scholar]