Abstract
A flurry of recent research has centered on harnessing the power of nickel catalysis in organic synthesis. These efforts have been bolstered by contemporaneous development of well-defined nickel (pre)catalysts with diverse structure and reactivity. In this report, we present ten different bench-stable, 18-electron, formally zero-valent nickel–olefin complexes that are competent pre-catalysts in various reactions. Our investigation includes preparations of novel, bench stable Ni(COD)(L) complexes (COD = 1,5-cyclooctadiene), in which L = quinone, cyclopentadienone, thiophene-S-oxide, and fulvene. Characterization by NMR, IR, single-crystal X-ray diffraction, cyclic voltammetry, thermogravimetric analysis, and natural bond orbital analysis sheds light on the structure, bonding, and properties of these complexes. Applications in an assortment of nickel-catalyzed reactions underscore the complementary nature of the different pre-catalysts within this toolkit.
Keywords: catalyst toolkit, cross-coupling, ligand design, nickel, pre-catalyst
Graphical Abstract
A series of air-stable Ni(0) pre-catalysts of the general type Ni(COD)(L) are described, where L = thiophene oxide, quinone, cyclopentadienone, or fulvene. The properties of the complexes are analyzed through computational and experimental techniques. The precatalysts are competent in a variety of nickel-catalyzed reactions and enables rapid identification of precatalysts that overcome the limitations of both Ni(COD)2 and Ni(COD)(DQ).

Introduction
Homogeneous nickel catalysis research has witnessed a surge of interest in recent years. Various synthetic transformations capitalize on nickel’s unique combination of properties: namely, its ability to undergo facile oxidative addition, its capacity to maneuver through oxidation states via single-electron transfer processes, and the relatively sluggish nature of β-hydride elimination from alkylnickel intermediates.[1–3] Moreover, the lower economic and environmental costs of nickel compared to precious metals drive its widespread adoption.[4–6]
As the use of nickel in catalysis has increased, so has reliance upon the nickel pre- catalysts that are available to practitioners. Prominently used nickel pre-catalysts can be categorized based on the metal’s oxidation state: Ni(II) species, such as nickel halide salts and aryl and allyl oxidative addition complexes; and formally Ni(0) species, such as those pre-ligated with phosphine, N-heterocyclic carbene (NHC), nitrogen-based ligands, and olefin-bound variants used in in-situ ligation protocols.[7]
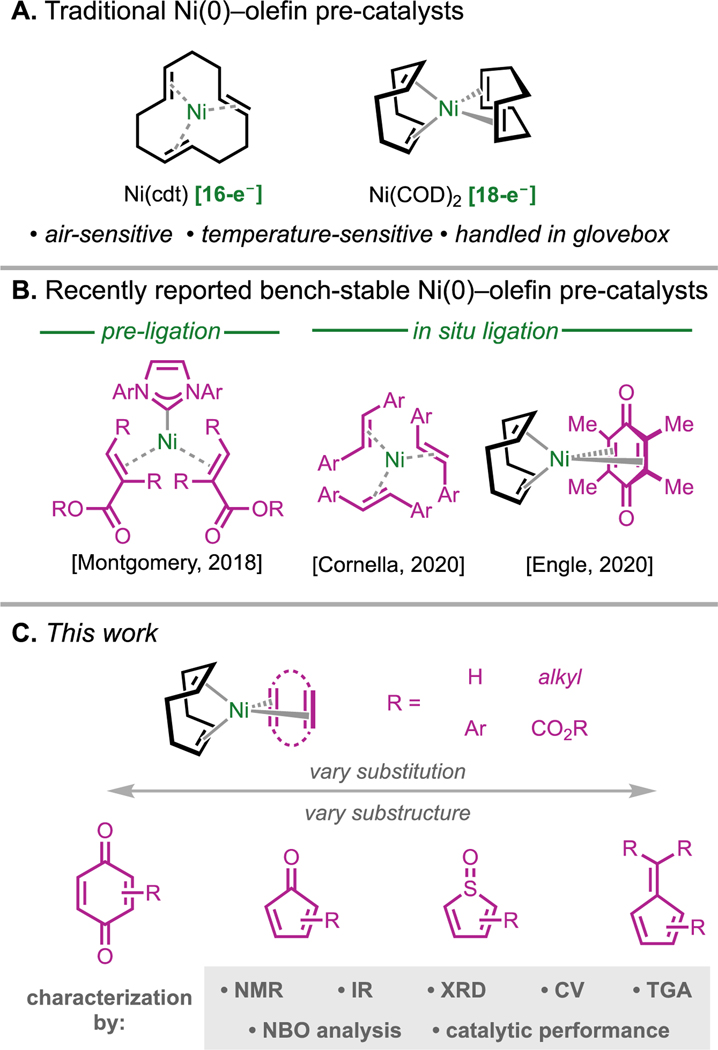
Comparing Ni(II) and Ni(0) pre-catalysts, Ni(0) complexes offer the advantage of directly entering the catalytic cycle through ligand exchange, without the need to undergo other elementary steps. While pre-ligated Ni(0) complexes, such as Montgomery’s Ni(NHC)(EDO) (EDO = electron-deficient olefin) series, have proven highly enabling,[8] Ni(COD)2 (COD = 1,5-cyclooctadiene) has dominated as the standard Ni(0) pre-catalyst due to the comparative lability of its COD ligands. This is especially true in the early stages of reaction optimization, owing to its commercial availability and ability to coordinate a broad assortment of ligands in situ. Nevertheless, its extreme sensitivity to oxygen, moisture, various solvents, and even moderate heat (Figure 1A)[9–11] necessitates handling in an inert atmosphere and long-term storage at low temperature.[12] This has hindered broad adoption of catalytic reactions that rely on Ni(COD)2, particularly in pharmaceutical process development.
Figure 1.
A. Traditionally used olefin-bound nickel(0) precatalysts, Ni(cdt) and Ni(COD)2, which are air- and temperature-sensitive. cdt = (1E,5E,9E)-cyclododecatriene, COD = 1,5-cyclooctadiene. B. Recent examples of bench-stable nickel(0)–olefin complexes. C. Overview of this study in which bench-stable nickel(0) complexes with various quinone, cyclopentadienone, thiophene sulfoxide, and fulvene ligands are developed.
Bench-stable complexes that are functionally equivalent to Ni(COD)2 for in situ ligation protocols are thus attractive targets for organometallic synthesis. To this end, our lab identified Ni(COD)(DQ) (DQ = duroquinone),[13] originally prepared by Schrauzer in 1962,[14] as an extraordinarily stable and versatile pre-catalyst for a number of important reactions, such as Suzuki–Miyaura cross-coupling, Buchwald–Hartwig amination, and Miyaura borylation.[13,15] However, we and others have found that Ni(COD)(DQ) is not always a suitable replacement for Ni(COD)2,[16] as some reactions required longer reaction times or did not proceed altogether.[17] We attribute this limitation to the low substitutional lability of DQ, which is especially problematic with weakly σ-donating ligands and points to the need for new pre-catalyst designs. In parallel to our work, Cornella and coworkers disclosed a series of 16-electron formally Ni(0)–tris(stilbene) [Ni(stb)3] complexes that function as bench-stable pre-catalysts for a variety of transformations.[18] Recently the Cornella lab demonstrated that modification of the substituents on the stilbene can lead to improved reactivity and stability.[19]
To address the shortcomings of Ni(COD)(DQ) and expand the reach of bench-stable 18-electron Ni(0) pre-catalysts, we hypothesized that modifying the Ni(COD)(L) architecture to new ligand scaffolds would unlock new reactivity and properties. Given the impact of ligand and catalyst screening kits in accelerating catalytic reaction development in academic and industrial settings, we reasoned that a toolkit of Ni(0) pre-catalysts would expedite the optimization and process development of synthetically useful reactions that rely on Ni(0) precursors without the need for an inert-atmosphere glovebox. To this end, we identified four tunable ligand families, quinones, cyclopentadienones, thiophene-S-oxides, and fulvenes, that form bench-stable Ni(COD)(L) complexes that function as effective pre-catalysts in various transformations (Figure 1C). Using an array of analytical techniques, the structural, electronic, and physical properties of these pre-catalysts are characterized, and their catalytic performance is compared across several reactions from the literature that employ a different ligands, solvents, and bases. The profound effect of the supporting ligand in modulating reactivity underscores the value of a library of robust Ni(0) pre-catalysts for reaction discovery and optimization.
Results and Discussion
1. Ligand Screening towards Identification of Bench-Stable Nickel(0) Complexes
To begin this study, we developed a protocol for assessing potential ligand candidates. This workflow evaluated ligands based on two criteria: (1) whether they formed a well-defined Ni(COD)(L) complex, and (2) whether the resulting complex was stable to work-up/purification procedures on the bench top. To these ends, ligands of interest were combined with Ni(COD)2 in 1:1 molar ratio in C6D6 under inert atmosphere,[20] and the resulting mixture was analyzed by 1H NMR (Figure 2A, see SI for details). The appearance of free COD along with new diastereotopic alkenyl 1H resonances from the remaining Ni-bound COD ligand indicated formation of a well-defined Ni(COD)(L) complex. In contrast, ineffective ligands led to unreacted starting materials, intractable mixtures, complete decomposition, or unassignable broad peaks.[21] Experiments showing evidence of Ni(COD)(L) formation were carried forward for preliminary stability assessment. Specifically, the crude reaction mixture was directly loaded onto a bed of silica gel under air, and elution with hexane/EtOAc was attempted.
Figure 2.
A. Graphical depiction of workflow for ligand screening (0.05 mmol) to form isolable, bench-stable, low-valent nickel complexes. B. Selected examples of unsuccessful ligands. Ligands that lead to no reaction, decomposition, and/or tentative formation of paramagnetic species are depicted in blue. Ligands that led to formation of desired nickel complex by 1H NMR where the resulting complexes were unstable to isolation attempts are depicted in green. C. Successfully synthesized bench-stable nickel complexes starting with 0.5 or 1.0 g Ni(COD)2 and corresponding isolated yields. Shaded boxes separate four different classes of ligand substructures (quinone, cylopentadienone, thiophene sulfoxide, fulvene). See SI for reaction conditions and additional unsuccessful ligands. Ar1 = 4-OMeC6H5, Ar2 = 4-CF3C6H5, E1 = CO2Me, E2 = CO2Et.
Utilizing this workflow, we screened 75 ligands spanning 13 structural classes (Figure 2B), and identified quinones, cyclopentadienones, thiophene-S-oxides, and fulvenes for further development, leading to the 10 stable nickel complexes shown in Figure 2C. Echoing earlier findings,[19] one successful series is based on quinone ligands. While we have previously shown that Ni(COD)(DQ) (1),[13] where the quinone bears tetramethyl substitution, is highly stable, unsubstituted benzoquinone (BQ) (L11) gives an intractable mixture,[22] pointing to the importance of the substituents on the quinone. With this in mind, we investigated different substitution patterns and groups with varying steric and electronic properties. 2,6-Disubstituted quinones (L12) did not form isolable complexes, and removal of a methyl group from DQ, as seen in 2,3,6-trimethyl-1,4-benzoquinone (L13) led to an unstable complex. In contrast, we found that dialkyl substitution at the 2,5-positions, as in bis(tert-butyl) (L2) and bis(cyclohexyl) (L3) resulted in isolable, bench-stable complexes 2 and 3, respectively, as red-orange solids (Figure 2C). Interestingly, aryl (L14–L16) and fluoro (L17) substituents led to decomposition, irrespective of the substitutions on the aryl ring, presumably due to their stronger oxidizing ability.[23] Broadly speaking, these empirical results reveal that C2-symmetric complexes containing alkyl-substituted quinone ligands are typically stable, reflecting an intricate interplay of redox behavior, steric hindrance, and symmetry of the quinone ligand. After gaining a better understanding of substituent effects on complexation of quinone-type ligands, we evaluated other ligand scaffolds. We tested various bidentate olefin ligands, including norbornadiene (L18) and [2.2.2]-bicylooctadienes (L19, L20) due to their precedented success as ligands in rhodium catalysis.[24,25] Though some of these gave the desired product, as detected by crude 1H NMR and/or single-crystal X-ray diffraction, none were readily isolable on preparative scale or bench-stable in the solid state. Ligands in which the two olefins were farther apart (L21) also proved to be unsuccessful, despite precedent demonstrating their coordination to low-valent palladium.[26]
We noted that this lack of success may be due to a mismatch of the bite angle of the olefin in relation to the relatively small size of the nickel center. We thus focused our attention on ligands containing an embedded conjugated 1,3-diene motif. We tested cyclopentadienones, reasoning that they might function analogously to quinones given their structural similarity. Notably, in 1986 Boleslawski and coworkers reported the synthesis and characterization of Ni(COD)(CPDH) (4);[27] however, its stability and catalytic reactivity have not been characterized to our knowledge. We were able to access Ni(COD)(CPDH) (4) as a dark-purple solid in quantitative yield, finding it to be highly stable to air and moisture. Other substituted tetraarylcyclopentadienone ligands with varied electronic properties, as exemplified by tetra(4-methoxyphenyl) and tetra(4-trifluoromethylphenyl) cyclopentadienone (L5 and L6, respectively), also coordinated. These ligands furnished the desired bench-stable nickel complexes 5 and 6. Contrary to our initial hypothesis that more electron-poor ligands would complex more effectively to electron-rich low-valent nickel, we instead observed that electron-withdrawing ester (L22, L23) and ketone (L24) substituents at the 2,5-positions of the cyclopentadienone scaffold resulted in no observable complex formation. We turned to alkyl substituted cyclopentadienones, but their propensity to dimerize upon formation precluded these efforts.
Among other cyclic 1,3-dienes, we found furans (L25, L26), phospholes (L27, L28), thiophenes (L29), and thiophene sulfones (L30) to be ineffective at complexation. We had initially expected thiophene sulfones to be successful due to their ability to coordinate to low-valent iron and cobalt.[28,29] However, tetraphenyl thiophene sulfone (L30) lead to decomposition of the ligand upon combination with Ni(COD)2. Instead, the corresponding tetraphenyl thiophene-S-oxide (L7) proved to be successful, resulting in bench-stable 18-electron complex 7. To the best of our knowledge, thiophene-S-oxide ligands have not previously been reported in complexation with nickel. Expanding on this result, we found that tetra(4-trifluoromethylphenyl) thiophene-S-oxide (L8) yielded the desired complex 8, and a 2,5-diester substituted thiophene-S-oxide (L9) yielded complex 9, in contrast to cyclopentadienone analogue L22. In another departure from trends observed with cyclopentadienone scaffolds, tetra(4-methoxyphenyl) thiophene-S-oxide (L31) did not produce an isolable nickel complex, which would have been analogous to complex 5. Tetraethyl thiophene-S-oxide (L32) was also unsuccessful. These results suggest that the Ni(COD)(cyclopentadienone) series tolerates moderately electron-poor to electron-rich ligands, while electron-neutral or -deficient ligands are required in the Ni(COD)( thiophene-S-oxide) series.
At this point, we questioned whether a double bond to an electron-withdrawing heteroatom, such as C=O and S=O, is necessary, or if a suitably substituted C=C bond could also function in an analogous fashion. Indeed, fulvene complex Ni(COD)(L33) had been previously synthesized and characterized by Behrens and coworkers in 1982, though its stability and catalytic activity were not described.[30] Upon testing diphenylfulvene (L33), we observed complexation by crude 1H NMR, but the resulting material did not pass our stability test, suggesting it would be necessary to tune the fulvene scaffold to identify a more stable variant. Electron-neutral, -donating, and -withdrawing substituents of the fulvene were unsuccessful (L34 & L35, See SI for details), except for 6,6-dicyano substitution, which resulted in some isolable complexes. Initially, we identified tetraphenyl-6,6-dicyano fulvene (L36) as a promising ligand but encountered reproducibility issues in the purification and long-term bench-stability of the resulting complex. To improve these properties, we found that replacement of the phenyl groups at the 1- and 4-positions with less bulky methyl groups (L10) resulted in an isolable, bench-stable nickel fulvene complex 10 (Figure 2C). Expansion to a 7-membered benzotropone motif (L37), resulted in an unstable complex.
2. Large-Scale Synthesis and Characterization
Figure 2C summarizes isolated and characterized bench-stable low-valent nickel complexes. We found that complexes 1–10 could be conveniently prepared on gram scale via ligand exchange from Ni(COD)2 (see Table S1 in the Supporting Information for additional data). Alternatively, we developed a two-step telescoped sequence from Ni(acac)2, which is more less user-friendly and less expensive than Ni(COD)2, and allows pre-catalyst synthesis without the use of a glovebox. As shown for two representative examples with commercially available ligands (L1 and L4), the desired complexes 1 and 4 were successfully prepared in good yield on gram scale by reduction of Ni(acac)2 with DIBAL–H (Figure 3) followed by solvent-swap and ligand introduction.
Figure 3.
Synthesis of complexes 1 and 4 from nickel(II) with DIBAL–H as reductant on gram-scale.
Complexes 1–10 were fully characterized by 1H NMR, 13C NMR, single-crystal X-ray diffraction, and IR.[31] By X-ray analysis, complexes across different ligand classes are largely isostructural in the solid-state. Common structural features of complexes 1–10 include a distorted tetrahedral geometry. The nickel center is coordinated to two central endocyclic olefins of the diene ligand and two olefinic moieties of COD, and the two sets of olefinic moieties in L and COD are oriented orthogonally to one another. σ-Donation from the filled carbonyl π-orbitals to the nickel center appears to be minimal in complexes 1–6. Furthermore, in all three thiophene-S-oxide-containing complexes 7–9, there is no evidence of σ-bonding from lone-pairs on sulfur or oxygen to the nickel center. In the solid-state structures, the sulfoxide S=O bond tilts away from the metal center, and the dihedral angle between the planar dienyl fragment and the S=O bond decreases 24–35° compared to the free ligand, becoming almost perpendicular. Due to fundamental structural differences across different ligand classes, comparisons of M-to-L bond lengths and C=C/C=O/S=O bond lengths among the entire collection of complexes are not especially meaningful. Instead, given that the Ni(COD) substructure is the only shared feature across the entire series, comparison of the average C=C bond lengths of bound COD provides a means of comparing the back donation ability of filled nickel d orbitals to the π*(C=C) orbital of COD as a function of the diene ligand (Table 1). Indeed, all of the complexes (1–10) exhibit shorter COD C=C bond lengths than Ni(COD)2 (<1.392(3) Å), consistent with a more electron-poor metal center and attenuated π-back-bonding. By the same token, larger average C=C-C bond angles of COD in these complexes reflect the decrease in sp3 hybridization character upon weaker back bonding. For aryl-containing ligand frameworks, electron-withdrawing groups result in shorter C=C bond lengths (entries 4–6, entry 7 vs entry 8). Values for IR stretching frequencies of carbonyl C=O bonds or S=O bonds are also listed for each complex. Unlike benzoquinone and cyclopentadienone-type ligands that show noticeable X=O bond length elongation upon coordination to nickel, changes in S=O bond length are minimal, which is consistent with NBO data (vide infra) that suggests minimal interaction between the S=O bond and the metal center.[32]
Table 1.
Selected X-ray structures and key characterization data for complexes 1–10.

|
Average values of multiple inequivalent bond lengths/angles with estimated standard deviations (ESDs) listed in parentheses; for details regarding the X-ray crystal structures, see SI and Ref. 30.
Density functional theory calculations for natural population analysis (NPA) were performed using NBO3
Calculated natural population analysis (NPA) charges of the complexes were next considered. While this method is limited in accuracy of capturing the true oxidation state of the metal center, it provides a useful readout of electronic trends across the series. The NPA charge at nickel for Ni(COD)2 was calculated to be 0.091, while we found this value to be slightly higher for catalysts 1–10 (Table 1). Within this collection, cyclopentadienone bound nickel centers in 4–6 were more positively charged with NPA charges of roughly 0.24, and thiophene-S-oxide bound nickel centers in 7–9 were the least positively charged with NPA charges of 0.15–0.18. Quinone bound nickel centers were found to be intermediary with charges of 0.21–0.23. Fulvene catalyst 10 had the highest NPA charge of 0.26.
We envisioned that the redox properties of the nickel center could be systematically tuned using ligands with disparate electronic properties. Thus, cyclic voltammetry (CV) studies of four representative nickel complexes (1, 4, 7, 10) and their corresponding ligands were taken in an electrolyte solution of 0.1 M TBAPF6 in DMF at 100 mV s−1. 1,4-Duroquinone (L1; Figure 4A) displays two reversible redox features at –1.23 V (all potentials calibrated vs Fc/Fc+) and –2.18 V, which are assigned to its sequential reduction to the radical anion and then the dianion, respectively.[33] Notably, the voltammograms of Ni(COD)(DQ) (1) exhibit different features depending on the direction of the scan. Scanning in the reductive direction from 0 V results in two major redox features at –1.87 V (forward scan) and +0.30 V (reverse scan; Figure 4A, red trace). We tentatively assign the irreversible wave at 0.30 V to the oxidation of the nickel center [formally from Ni(0) to Ni(I)], and the reversible feature at –1.90 V to a ligand (L1)-based single-electron reduction to a radical anion, while the nickel center maintains its formal oxidation state of Ni0. The cathodic shift (~700 mV) of ligated 1,4-duroquinone versus its unbound form is indicative of back-bonding of the low-valent nickel to the electron-deficient quinone. In contrast, when the CV scan was initiated oxidatively, we observed a new redox feature at –1.23 V (Figure 4A, blue trace). This quasi-reversible redox feature overlaps with the first reduction peak of free 1,4-duroquinone, suggesting that ligand dissociation takes place upon oxidation of Ni(COD)(DQ) at positive potentials. The irreversibility of this redox feature is likely because the reduced ligand can once again complex with Ni. These observations suggest that ligand dissociation of DQ takes place readily when nickel is at a relatively high oxidation state, creating available coordination sites for reaction substrates.
Figure 4.
A. Cyclic voltammograms of L1 (green) and complex 1 (reductive initiated scan: red, oxidative initiated scan: blue). Conditions: glassy carbon as working electrode, Ag/AgNO3 reference electrode, and platinum wire as counter electrode. 2.0 mM analyte in DMF (0.1 M TBAPF6). Scan rate: 100 mV/s. Potentials are referred vs the Fc+/Fc couple. B. Redox properties of nickel complex. Potential values for reversible features are reported as E1/2 and those for irreversible features are reported as half peak potential Ep/2. n.d. = not detected.
Redox properties of complex 4, 7 and 10 are summarized in Figure 4B, which display generally similar features as complex 1 (see SI for details). We compared redox activities of these complexes with their corresponding ligands and carried out CV scans in both reductive and oxidative directions for each complex to probe ligand dissociation. In each case, the reductively initiated scan of the Ni complex typically involves a ligand-based 1e-reduction and a metal-based 1e-oxidation. However, initiating the scan oxidatively results in more complex voltammograms featuring additional peaks, suggesting the formation of new species likely from oxidatively induced ligand dissociation.
Given the CV, X-ray diffraction, and computational data, complexes 1–10 are best described as Ni(0) centers engaging in moderate π-back-donation with two diene ligands. While one electronic structure possibility is a broken symmetry solution of Ni(I) engaging in anti-ferromagnetic coupling with a diene radical anion (BS(1,–1)), this is an unlikely scenario given the significant orbital overlap between nickel and the quinone/diene ligands. Broken symmetry is more characteristic of redox non-innocent ligands such as CO and other π-systems, in which the exact oxidation state of the metal is difficult to determine due to the significant mixing of metal and ligand orbitals. (In the case of complexes 1–10, the oxidation state of nickel likely lies on a continuum from one resonance extreme to the other, i.e., Ni(0) ↔ Ni(II).)[34,35] Further investigations into the precise electronic structure of these air-stable Ni(0) precatalyst is beyond the scope of this article.
Relevant physical properties of the complexes were next considered. In terms of air stability in the solid state, samples of complexes 1–10 capped under air at room temperature did not undergo any visible changes in appearance after 6 months. Similarly, there were no detectable changes in 1H NMR spectra of these complexes over this period (see SI). After >1 year, complexes 2 and 3 were observed to partially decompose when stored under air at room temperature. The thermal stability in both the solid and solution states was also assayed by thermogravimetric analysis (TGA) and variable temperature (VT) NMR, respectively (Figure 5 and SI). TGA and VT NMR data illustrated a range of stabilities with respect to increased temperature in the solid and solution states. While duroquinone complex 1 was found to be incredibly thermally stable, with >95% mass retained up to 200 °C, analogues 2 and 3 were less stable with >5% mass loss observed at 120 °C. The cyclopentadienone series (4–6) showed consistent stability by TGA, maintaining >95% initial weight past 200 °C. Thiophene-S-oxide containing complexes 7–9 demonstrated relative stability past 150 °C, followed by sharp decreases in weight at points between 150 °C and 200 °C. Fulvene complex 10 maintained more than 95% of its weight past 200 °C. Data with Ni(COD)2 was also collected for reference. Though Ni(COD)2 is reported to decompose under air at temperatures as low as 60 °C,[11] in these TGA experiments where air is excluded, we found >95% mass retention up to 140 °C. Headspace analysis of thermally decomposed Ni(COD)(DQ) (1) via GCMS indicated that COD is the primary component lost upon heating (see SI for details).
Figure 5.
A. Thermal gravimetric analysis (TGA) data for complexes 1–10 taken with a range of 20–300 °C at a rate of 10 °C per minute (N2 purge gas, platinum testing cell). Percent remaining weight (%) as a color gradient; B. Samples prepared under air with C6D6 as solvent under a temperature range of 25–60 °C covered with 5 °C increments. Bars indicate range of stability as indicated by lack of irreversible peak broadening.
Variable temperature NMR experiments were performed with a temperature range of 25–60 °C, with 5 °C increments. Complex 1 was found to be stable up to the maximum temperature examined. On the other hand, complexes 2 and 3 decomposed in solution past 30 °C as indicated by significant line broadening that did not resolve upon cooling, and observation of colorless precipitates. By VT NMR, cyclopentadienone complexes 4–6 were exceptionally stable in solution at increased temperatures. Interestingly, these complexes exhibited coalescence of the olefinic COD protons upon heating. For complexes 5 and 6, this coalescence occurred at ca. 55 °C, while for complex 4, coalescence occurred at a lower temperature of ca. 40 °C, concomitant with an intriguing re-separation of these peaks at ca. 55 °C. Complexes 7 and 8 were stable throughout our VT NMR experiments with only slight coalescence of the COD olefin peaks with increasing temperature. Complex 9 was stable in solution up to 55 °C.
3. Evaluation of Catalytic Performance
Prior to the development of the present toolkit, we found that Ni(COD)(DQ) (1) did not perform equivalently to Ni(COD)2 in some reaction systems,[16,17] likely due to differences in solubility, ligand exchange, or catalyst inhibition by liberated DQ. Complexes 1–10 were tested as pre-catalysts in real-world case studies (Figure 6). Our goal was to identify one or more effective pre-catalysts that would allow published transformations to be prepared outside of an inert atmosphere glovebox with minimal reoptimization. Thus, we initially tested the toolkit against two reactions from our labs that employ Ni(COD)2 as pre-catalyst: 1) arylamination of butenyl alcohols (Figure 6A) [34] and 2) decarboxylative cycloaddition[35] (Figure 6B). In nickel-catalyzed 1,2-arylamination of weakly-coordinating alkenyl alcohols, we did not observe any product with Ni(COD)(DQ) (1) but were able to obtain moderate to good yields with pre-catalysts 2, 3, 8, and 9. Unsurprisingly, the seemingly more inert cyclopentadienone complexes 4–6 were not active in this reaction. Thiophene-S-oxide pre-catalysts 8 and 9 performed well, while their more electron-rich counterpart 7 did not yield desired product. In the decarboxylative cycloaddition reaction (Figure 6B), we found that several complexes within the toolkit provided ≥95% yield after 48 h (1, 3, 8, 9). Reaction yield at 20 h provide a general idea of the comparative kinetics for these successful complexes. Ni(COD)(DQ) (1) performed well to give 65% yield after 20 h. However, we found that thiophene-S-oxide complex 8 gave 88% after 20 h. Cyclopentadienone complexes 4–5 and fulvene complex 10 performed poorly in this reaction.
Figure 6.
Evaluation of the catalytic performance of 1–10 in five model reactions. Yields obtained by 1H NMR with CH2Br2 as internal standard unless noted otherwise (n.d. = not detected). A. Arylamination of butenyl alcohols. B. Decarboxylative cycloaddition (yields obtained by LC based on authentic product standard). C. Ketone α-arylation. D. N-Allylation. E. Amination of aryl thioethers.
We also surveyed the recent literature for catalytic reactions reported with Ni(COD)2 that we found to give low to moderate yield with Ni(COD)(DQ) (1) in pilot experiments. In selecting representative reactions, we sought to test how the different precatalysts in the toolkit would perform with different bases, solvents, temperatures, and ligands, without any re-optimization of reported conditions. The α-crotylation of acetophenone as reported by Dong and Xing using Ni(COD)2 and IMes at 130 °C gave no product with Ni(COD)(DQ), but complex 8 resulted in 73% yield of product 13 (Figure 6C).[38] Cyclopentadienone 5 was the only other precatalyst to give even trace amounts of product. Because the metal carbene complex is pre-formed in this reaction, we partially attribute reaction success to solubility of the precatalyst in CPME. We were able to perform a glovebox-free setup of the reaction using 5 and isolate 13 in 87% yield. In another example, we found that Ni(COD)(DQ) performed poorly in the N-allylation of indole reported by Sauthier and coworkers but that complex 7 resulted in high yield of desired product 14.[39] Complexes 5 and 8 also outperformed Ni(COD)(DQ) in this reaction, and 7 could be used outside of the glovebox to prepare 14 in 62% isolated yield. Morandi and coworkers reported that the combination of Ni(COD)2 and DCYPE catalyzes the amination of thioethers in the presence of the strong base LiHMDS.[40] While Ni(COD)(DQ) performs moderately under these reaction conditions, precatalyst 7 gave desired product 15 in high yield and could be used outside of the glovebox to isolate 15 in 71% yield. These five examples demonstrate that the toolkit performs well among diverse reaction conditions and that it enables rapid identification of precatalysts that overcome the limitations of both Ni(COD)2 and Ni(COD)(DQ).
Conclusion
By taking advantage of the favorable complexation of diene ligands to form 18-electron complexes with low-valent nickel centers, we identified a new toolkit of 10 bench-stable Ni(0) pre-catalysts. This toolkit encompasses four different ligand substructures: quinones, cyclopentadienones, thiophene-S-oxides, and fulvenes. The complexes were characterized via NMR, IR, single crystal X-ray diffraction, XAS, CV, TGA, and natural bond orbital analysis. Reactivity was compared across five nickel-catalyzed reactions originally reported with Ni(COD)2, in each case leading to identification of a precatalyst that can be conveniently handled without an inert-atmosphere glovebox. The divergent performance of different pre-catalysts underscores the value of a screening kit of Ni(0) complexes to aid in reaction discovery and optimization. Ongoing efforts in our lab center on understanding ligand exchange processes with these complexes, mapping reaction performance trends in a comprehensive manner, and leveraging their unique properties in reaction design.
Supplementary Material
Acknowledgements
Financial support was provided by Bristol Myers Squibb, the National Science Foundation (CHE-2102550), and the National Institutes of Health (R01GM130928). We further thank the Schimmel Family Endowed Fellowship Fund for a Graduate Fellowship (C.Z.R.), the Kwanjeong Educational Foundation for a Graduate Fellowship (T.K.), and Bristol Myers Squibb for a Graduate Fellowship (Z.-Q. L.). We acknowledge Michelle Kubin for assistance with GCMS analysis and Dr. Michael A. Schmidt, Dr. Samantha N. MacMillan, and Prof. Kyle M. Lancaster for helpful discussion.
References
- [1].Ananikov VP, ACS Catal. 2015, 5, 1964–1971. [Google Scholar]
- [2].Tasker SZ, Standley EA, Jamison TF, Nature 2014, 509, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Diccianni JB, Diao T, Trends Chem. 2019, 1, 830–844. [Google Scholar]
- [4].Ludwig JR, Schindler CS, Chem 2017, 2, 313–316. [Google Scholar]
- [5].Holland PL, Chem 2017, 2, 443–444. [Google Scholar]
- [6].Bullock RM, Chem 2017, 2, 444–447. [Google Scholar]
- [7].a) Johnson SA, Dalton Trans. 2015, 44, 10905–10913; [DOI] [PubMed] [Google Scholar]; b) Hazari N, Melvin PR, Beromi MM, Nat. Rev. Chem. 2017, 1, 0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Nett AJ, Cañellas S, Higuchi Y, Robo MT, Kochkodan JM, Haynes II MT, Kampf JW, Montgomery J, ACS Catal. 2018, 8, 6606–6611; for pioneering reports on the use of a well-defined [Ni(NHC)2]2(COD) pre-catalyst, see: [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schaub T, Backes M, Radius U, J. Am. Chem. Soc. 2006, 128, 15964–15965; [DOI] [PubMed] [Google Scholar]; c) Zell T, Radius U, Z. Anor. Allg. Chem. 2011, 637, 1858–1862. [Google Scholar]
- [9].Wilke G, Angew. Chem. Int. Ed. 1988, 27, 185–206; Angew. Chem. 1988, 100, 189–211. [Google Scholar]
- [10].Schunn RA, Ittel SD, Cushing MA, Baker R, J Gilbert R, Madden DP, Inorg. Synth. 1990, 28, 94–98. [Google Scholar]
- [11].Wender PA, Smith TE, Duong HA, Louie J, Standley EA, Tasker SZ, in Encyclopedia of Reagents for Organic Synthesis; Wiley, 2015, 10.1002/047084289X.rb118.pub3. [DOI] [Google Scholar]
- [12].a) For benchtop-stable Ni(COD)2 using paraffin capsules, see:Dander JE, Weires NA, Garg NK, Org. Lett. 2016, 18, 3934–3936; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mehta MM, Boit TB, Dander JE, Garg NK, Org. Lett. 2020, 22, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Tran VT, Li Z-Q, Apolinar O, Derosa J, Joannou MV, Wisniewski SR, Eastgate MD, Engle KM, Angew. Chem. Int. Ed. 2020, 59, 7409–7413; Angew. Chem. 2020, 132, 7479–7483; [DOI] [PubMed] [Google Scholar]; b) Rubel CZ, Kim N, Engle KM in Encyclopedia of Reagents for Organic Synthesis; Wiley, 2022, 10.1002/047084289X.rn02446. [DOI] [Google Scholar]
- [14].Schrauzer GN, Thyret H, Naturforsch Z. 1962, 17b, 73–76. [Google Scholar]
- [15].a) For selected applications of Ni(COD)(DQ) in the recent literature, see: Jang Y, Lindsay VNG, Org. Lett. 2020, 22, 8872–8876; [DOI] [PubMed] [Google Scholar]; b) Reilly SW, Lam Y, Ren S, Strotman NA, J. Am. Chem. Soc. 2021, 143, 4817–4823; [DOI] [PubMed] [Google Scholar]; c) Cho IY, Kim WG, Jeon JH, Lee JW, Seo JK, Seo J, Hong SY, J. Org. Chem. 2021, 86, 9328–9343; [DOI] [PubMed] [Google Scholar]; d) Li Y, Shao Q, He H, Zhu C, Xue X-S, Xie J, Nat. Commun. 2022, 13, 10; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Roediger S, Leutenegger SU, Morandi B, Chem. Sci. 2022, 13, 7914–7919; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) You T, Li J, Org. Lett. 2022, 24, 6642–6646; [DOI] [PubMed] [Google Scholar]; g) Orchanian NM, Guizzo S, Steigerwald ML, Nuckolls C, Venkataraman L, Chem. Commun. 2022, DOI: 10.1039/D2CC03671A. [DOI] [Google Scholar]
- [16].a) Marchese AD, Wollenburg M, Mirabi B, Abel-Snape X, Whyte A, Glorius F, Lautens M, ACS Catal. 2020, 10, 4780–4785; [Google Scholar]; b) Li Z-Q, Fu Y, Deng R, Tran VT, Gao Y, Liu P, Engle KM, Angew. Chem. Int. Ed 2020, 59, 23306–23312; Angew. Chem. 2020, 132, 23506–23512; [DOI] [PubMed] [Google Scholar]; c) Marchese AD, Adrianov T, Köllen MF, Mirabi B, Lautens M, ACS Catal. 2021, 11, 925–931; [Google Scholar]; d) Zheng Y-L, Xie P-P, Daneshfar O, Houk KN, Hong X, Newman SG, Angew. Chem. Int. Ed. 2021, 60, 13476–13483; Angew. Chem. 2021, 133, 13588–13595; [DOI] [PubMed] [Google Scholar]; e) Kleinmans R, Apolinar O, Derosa J, Karunananda MK, Li Z-Q, Tran VT, Wisniewski SR, Engle KM, Org. Lett. 2021, 23, 5311–5316. [DOI] [PubMed] [Google Scholar]
- [17].Apolinar O, Tran VT, Kim N, Schmidt MA, Derosa J, Engle KM, ACS Catal. 2020, 10, 14234–14239. [Google Scholar]
- [18].Nattmann L, Saeb R, Nöthling N, Cornella J, Nat. Catal. 2020, 3, 6–13. [Google Scholar]
- [19].Nattmann L, Cornella J, Organometallics 2020, 39, 3295–3300. [Google Scholar]
- [20].In cases where low conversion to Ni(COD)(L) was observed in C6D6, additional experiments were conducted in more polar, aprotic solvents, such as toluene-d8, CD2Cl2, and acetone-d6. However, these additional experiments did not lead to identification of any additional promising ligands. [Google Scholar]
- [21].The physical appearance of unsuccessful complexation trials varied and included heterogeneous mixtures of different colors, such as black, brown, orange, yellow, or purple, often accompanied by formation of nickel black or nickel mirror.
- [22].Jain R, Kabir K, Gilroy JB, Mitchell KAR, Wong K, Hicks RG, Nature 2007, 445, 291–294. [DOI] [PubMed] [Google Scholar]
- [23].Huynh MT, Anson CW, Cavell AC, Stahl SS, Hammes-Schiffer S, J. Am. Chem. Soc. 2016, 138, 15903–15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Johnson JB, Rovis T, Angew. Chem. Int. Ed. 2008, 47, 840–871; Angew. Chem. 2008, 120, 852–884 [DOI] [PubMed] [Google Scholar]
- [25].Defieber C, Grützmacher H, Carreira EM, Angew. Chem. Int. Ed. 2008, 47, 4482–4502; Angew. Chem. 2008, 120, 4558–4579. [DOI] [PubMed] [Google Scholar]
- [26].Eastgate MD, Buono FG, Angew. Chem. Int. Ed. 2009, 48, 5958–5961; Angew. Chem. 2009, 121, 6072–6075. [DOI] [PubMed] [Google Scholar]
- [27].Eisch JJ, Galle JE, Aradi AA, Boleslawski MP, J. Organomet. Chem. 1986, 312, 399–416. [Google Scholar]
- [28].Albrecht R, Weiss E, J. Organomet. Chem. 1990, 399, 163–188. [Google Scholar]
- [29].Albrecht R, Weiss E, J. Organomet. Chem. 1991, 413, 355–377. [Google Scholar]
- [30].Deposition numbers 1972401 (1), 2099998 (2), 2033854 (3), 2015597 (4), 2033179 (5), 2015096 (6), 2015598 (7), 2033832 (8), 2025093 (9), 2049811 (10), L3 (2049810), L5 (2100570), L6 (2100569), L7 (2019514), L8 (2019515), L9 (2035558), SC1 (2020674), SC2 (2015097), SC3 (2129338), SC4 (2129337), SC5 (2129336), SC6 (2129339) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service. [Google Scholar]
- [31].Edelmann F, Lubke B, Behrens U, Chem. Ber. 1982, 115, 1325–1331. [Google Scholar]
- [32].Kim CK, Lee KA, Kim CK, Lee B-S, Lee HW, Chem. Phys. Lett. 2004, 391, 321–324. [Google Scholar]
- [33].Rieke RD, Saji T, Kujundzic N, J. Electroanal. Chem. 1979, 102, 397–405. [Google Scholar]
- [34].Chirik PJ, Inorg. Chem 2011, 50, 9737–9740. [DOI] [PubMed] [Google Scholar]
- [35].This is in sharp contrast to redox-active ligands where the oxidation state of the metal is readily determined and the unpaired electrons are in distinct, yet magnetically coupled molecular orbitals on both the metal and the ligand.
- [36].Kang T, Kim N, Cheng PT, Zhang H, Foo K, Engle KM, J. Am. Chem. Soc. 2021, 143, 13962–13970. [DOI] [PubMed] [Google Scholar]
- [37].Beutner GL, Hsiao Y, Razler T, Simmons EM, Wertjes W, Org. Lett. 2017, 19, 1052–1055. [DOI] [PubMed] [Google Scholar]
- [38].Chen T, Yang H, Yang Y, Dong G, Xing D, ACS Catal. 2020, 10, 4238–4243. [Google Scholar]
- [39].Mouhsine B, Karim A, Dumont C, Saint Pol A, Suisse I, M. Sauthier. Eur. J. Org. Chem. 2022, 2022, e202200042. [Google Scholar]
- [40].Bismuto A, Delcaillau T, Müller P, Morandi B. ACS Catal. 2020, 10, 4630–4639. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.