Abstract
Levothyroxine monotherapy has been the standard of care for treatment of hypothyroidism for more than 40 years. However, patients treated with levothyroxine have relatively lower serum tri-iodothyronine (T3) concentrations than the general population, and symptoms of hypothyroidism persist for some patients despite normalisation of thyroidstimulating hormone (TSH) concentrations. The understanding that maintenance of normal T3 concentrations is the priority for the thyroid axis has redirected the clinical focus to serum T3 concentrations in patients with hypothyroidism. This Personal View explores whether it is currently feasible to identify patients who could be considered for liothyronine supplementation in combination with levothyroxine. Genetic profiling stands out as a potential future tool to identify patients who do not respond well to levothyroxine due to suboptimal peripheral thyroxine (T4) activation. Moreover, new slow-release liothyronine preparations are being developed to be trialled in these symptomatic patients, in an attempt to restore T3 concentrations and provide conclusive results for the use of T4 plus T3 combination therapy.
Introduction
Multiple mechanisms within the hypothalamus–pituitary– thyroid (HPT) axis help to maintain steady tri-iodothyronine (T3) concentrations within the normal range. Even after all thyroid hormone deiodinases have been inactivated, studies in mouse models indicate that the thyroid gland has the ability to step up T3 production, maintaining T3 concentrations.1,2 However, patients with primary hypothyroidism treated with levothyroxine monotherapy can have increased free thyroxine (T4) concentrations, lower free T3 concentrations, and an increased ratio of free T4 to free T3, despite normalisation of thyroid-stimulating hormone (TSH) concentrations.3,4 Moreover, there is mounting evidence of metabolic and clinical consequences that could be attributed to low serum T3 concentrations in cohorts of patients treated with levothyroxine despite normal TSH concentrations—eg, increased bodyweight, slower basal metabolic rate, elevated serum cholesterol concentrations, and statin utilisation.5–10
Patients treated with levothyroxine had a greater degree of dissatisfaction, poorer quality of life, and impaired cognition when compared with a control population, mostly due to hypothyroid symptoms11,12 and the persistence of metabolic alterations.5,8,13
The discrepancy between normal TSH and impaired peripheral thyroid hormone action in some levothyroxine-treated patients, suggests that the T4-to-T3 conversion by peripheral deiodinases is unable to restore appropriate intracellular T3 to all tissues. Thus, some patients treated with levothyroxine might benefit from T3 supplementation. Currently the role of T3 supplementation has not been established, mainly due to the fact that most previous trials comparing levothyroxine monotherapy with the combination of liothyronine plus levothyroxine have not focused on patients who remain symptomatic despite therapy, instead pooling all levothyroxine-treated patients together.14,15 However, a randomised, blinded cross-over study showed that the patients who were the most symptomatic while on therapy with levothyroxine were the ones who benefitted from therapy with liothyronine plus levothyroxine or desiccated thyroid extract (DTE), which also contains a combination of both hormones.16
This Personal View focuses on the potential importance of maintaining normal T3 concentrations in patients with hypothyroidism, and how to identify patients who could be considered for liothyronine supplementation in combination with levothyroxine.
How is T3 produced and cleared?
Physiology of T3 production in humans
In healthy people, the thyroid gland secretes about 10 nmol/kg of bodyweight of T4 and 0·7 nmol/kg of bodyweight of T3 daily.17 The thyroid gland is thus responsible for only 20% of the total daily T3 production; the remainder (about 25 μg daily) is derived from peripheral conversion of T4 to T3 through the action of type 1 deiodinase (DIO1) and, to a lesser extent, type 2 deiodinase (DIO2).18,19
Clearance of T3
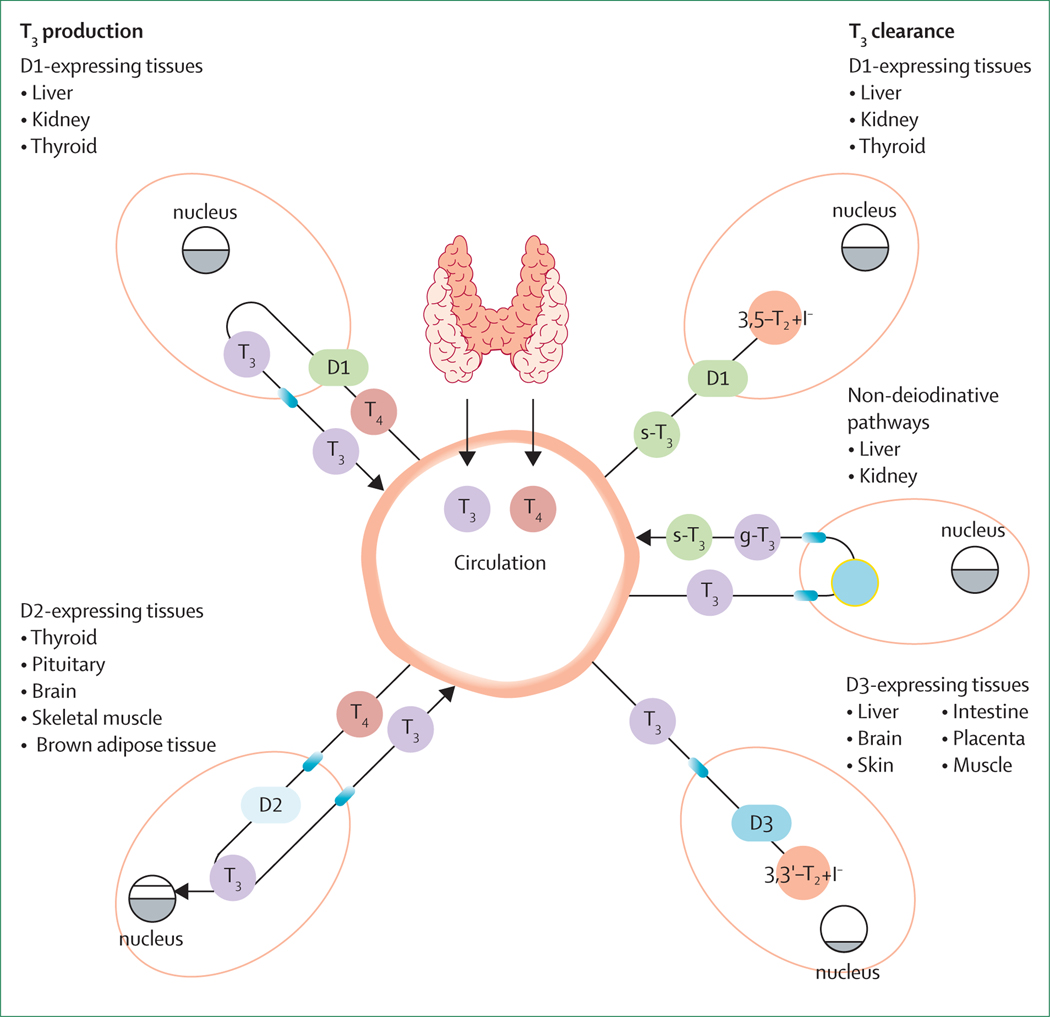
Two thirds of the T3 pool in our body is intracellular, and the main pathway by which T3 is cleared is through intracellular deiodination to 3,3’-diiodothyronine (T2) by type 3 deiodinase (DIO3). There are three T2 isomers, the inactive molecules 3,3’-T2 and 3’,5’-T2, and the active hormone 3,5-T2, which can derive from the outer ring deiodination of T3 by DIO1 and DIO2. Conversely, DIO3 inactivates T3 by inner ring deiodination to 3,3’-T2.20 T3 can also be metabolised through non-deiodinative pathways such as sulpho-conjugation, glucuronidation, or decarboxylation in the liver, but it is not clear how much these pathways contribute to the daily T3 economy.21 The figure shows a comprehensive overview of T3 production and clearance in humans.
Intraindividual serum T3 stability
Serum thyroid hormone concentrations present a low intraindividual variability over time in adult life. Steadystate thyroid hormone concentrations in an individual are determined by the specific setpoint of the HPT axis, which is affected by genetic and environmental factors.22 Serum T3 also has a circadian rhythmicity that follows the TSH circadian variation, with about 10% higher concentrations in the early morning hours.23 The HPT axis is also highly sensitive to changes in circulating thyroid hormone concentrations, with even minimal changes in serum T4 or T3 leading to major changes in TSH secretion.24 Although deiodinases play an important role in producing the bulk of T3, preclinical studies revealed that the inactivation of the DIO1 and DIO2 genes does not affect serum T3 concentrations.1,2 In these circumstances, the thyroid increases the relative output of both T4 and T3 due to an increase in TSH, ensuring that serum T3 remains within the normal range. Thus, although ordinarily the thyroidal T3 secretion and contribution to the circulating T3 pool are relatively small when compared with peripheral conversion via deiodination, this contribution is adjustable according to TSH concentrations. This mechanism is lost in patients with primary hypothyroidism due to the absence of thyroidal secretion of T3. Preserving serum T3 concentrations is important because circulating T3 is in equilibrium with tissue T3, which is responsible for most of the actions of thyroid hormone.25 Therefore, although there is twice as much T3 in the tissues as in the plasma, a decrease in serum T3 also reflects a reduction in thyroid hormone action in most tissues.
Rationale for T4-only supplementation
Treatment of hypothyroidism was developed around 1890 and was based on daily administration of desiccated thyroid extracts, later known as DTE. The doses were adjusted over time to avoid clinical signs and symptoms of thyrotoxicosis. This treatment was effective and was used successfully over the following 90 years. However, two issues eroded the confidence of physicians prescribing DTE. First, the standardisation of the amounts of T4 and T3 in the tablets was based on iodine content and not on hormonal content. Thus, great variability in the single hormone content was observed among tablets from different manufacturers, and even tablets produced by the same manufacturer. This issue has been resolved, and currently the preparations commercially available follow standardisation based on the United States Pharmacopeia—the organisation that sets the quality standard of drugs in the USA. The second issue was inconsistent stability of the medication—ie, shelf-life. Cases were reported in which patients were taking tablets containing no active thyroid hormone. Those two issues led physicians to pursue synthetic forms of thyroid hormone—levothyroxine and liothyronine—that were used, albeit infrequently, during the 1960s to treat patients with hypothyroidism. In 1970, at a time when physicians were empirically determining appropriate combination dosing for their patients, the seminal observation that humans are capable of converting T4 to T3 was made. This observation was interpreted as indicating that levothyroxine alone could restore thyroid hormone action, rapidly obviating the use of T3, and the standard of care became treatment with levothyroxine alone.26
Is levothyroxine capable of restoring normal serum T3 concentrations in every patient?
The first recorded analysis of serum T3 in levothyroxinetreated patients with normal serum TSH was in 1974.3 The authors identified approximately a 10% reduction in serum T3 and a similar corresponding elevation in serum T4. However not much attention was paid to this finding for the next 30 years. A number of studies revisited the topic and most supported the relatively lower serum T3 concentrations than the general population.14 Notably, a minority of the studies could not identify differences in serum T3 concentrations when comparing levothyroxinetreated patients with controls.27 In the 2010s, two large studies substantiated the 1974 findings,5,28 and this slight reduction in serum T3 and elevation in serum T4 in levothyroxine-treated patients is now recognised in the most recent European Thyroid Association and American Thyroid Association guidelines for the treatment of hypothyroidism.29,30
Is serum T3 measurement reliable?
Despite the biologically key role of T3, current guidelines do not recommend the use of the normal range of serum T3 as a therapeutic target in patients with hypothyroidism.30,31 Although a solid rationale for abandoning serum T3 is not available, it is partly due to a number of limitations in the reliability of T3 assays. T3 can be measured as total serum T3 or free T3—ie, unbound to the three binding proteins: thyroxine binding globulin, transthyretin, and albumin. The accuracy of any total T3 measurement can be affected by various factors that alter the circulating binding protein concentrations and ligand affinity. To overcome the potential biases due to binding protein variations, measurement of free T3 is preferable.32 However, unbound thyroid hormone concentrations can be difficult to accurately measure in clinical practice due to the numerous medical conditions or states that can impair measurement. Conditions in which free thyroid hormone measurement is impaired include: changes in binding proteins, pregnancy, renal failure, non-thyroidal illness, drugs, and the presence of heterophile and autoantibodies.33
In addition, the results of immunoassays might be altered by the presence of variations in binding protein concen trations or heterophile antibodies such as rheumatoid factor, and a number of studies reported a notable disagreement between the various commercially available assays on identical serum samples.34–36 Furthermore, immunoassays are unreliable at the extremes of normal T3 concentrations, particularly at the lower values,37 and the overestimation of free T3 at low concentrations might severely affect the clinical judgement of patients with hypothyroidism treated with levothyroxine who report persistent hypothyroid symptoms.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) has shown robust superiority in the reliability and precision of free T3 measurement compared with immunoassays, even in conditions of altered binding protein concentrations.34 It is important to note that quantification of free thyroid hormone by LC-MS/MS requires samples to be pretreated to remove protein-bound thyroid hormone, while leaving free thyroid hormone in the sample to be tested. Accordingly, equilibrium dialysis and ultrafiltration have become the standard techniques for sample preparation thanks to incremental improvements in speed, accuracy, and cost that have occurred over the past 15 years.38 Despite these improvements, direct analogue immunoassays without physical separation of free T3 from bounded T3 are still frequently used in clinical laboratories. In summary, the clinician must interpret any single T3 measurement within the clinical context in which the sample was obtained, and should consider how the measurement was made. The utility of LC-MS/MS to accurately measure free thyroid hormone concentrations has promise as its use becomes more commonplace in clinical laboratories, but for now it remains out of reach for most practicing clinicians.
When should liothyronine be considered for patients with hypothyroidism?
Thyroid hormone replacement therapy is meant to normalise thyroid hormone action in all tissues by restoring their physiological concentrations of thyroid hormone. Monotherapy with levothyroxine became the standard of care under the assumption that normalisation of serum TSH also signified normalisation of thyroid hormone in all other tissues. The first signs that this normalisation did not necessarily occur in all other tissues came from the early observation that some of the patients who were switched from DTE to levothyroxine redeveloped hypothyroid symptoms.39 This observation was later supported in studies that addressed cognition and quality of life in patients treated with levothyroxine.11,12 Evidence of metabolic dysfunction has also been obtained, and the levothyroxine-treated patients were found to weigh on average 4·5 kg more, and were more likely to be on statin therapy when compared with control individuals matched for age, sex, ethnic background, and TSH.5 Increased statin utilisation, when compared with matched euthyroid controls, was also observed in a large population study involving 1 758 955 of patients treated with levothyroxine.8 Accordingly, other studies showed that serum cholesterol concentrations were higher in levothyroxine-treated patients, despite the higher utilisation of statins.6,9,40
It is difficult to predict how much the lower serum T3 concentrations in levothyroxine-treated patients versus the general population contribute to the signs and symptoms mentioned previously. Although T3 is known to affect cognition, quality of life, and the metabolic variables previously discussed, it is also conceivable that most patients can compensate—at a cellular level—for the relatively lower serum T3, thus explaining why levothyroxine therapy is successful in most patients with hypo thyroidism. Nonetheless, the presence of comorbidities or conditions might play a role, exhausting the patient’s ability to compensate. For example, mutations and polymorphisms have been found in the genes encoding DIO1 and DIO2, compromising the ability of these enzymes to convert T4 to T3 (table).51 Predicting which patients would benefit from combination therapy with levothyroxine and liothyronine remains clinically challenging, particularly because residual hypothyroidlike symptoms are not specific to levothyroxine-treated patients with normal TSH, and might be seen in patients with diabetes, depression, sleep apnoea, vitamin D and B12 deficiency, chronic fatigue syndrome, and adrenal insufficiency—especially in patients with autoimmune thyroiditis.34 Only once non-thyroid disorders have been ruled out can the addition of liothyronine be considered. This process has been done empirically on a trial basis for patients who unequivocally did not get better with levothyroxine alone.30
Table:
Consequences of polymorphisms and mutations in deiodinases and thyroid hormone transporter genes on serum thyroid hormone concentrations
| Serum T3 | Serum T4 | Serum reverse T3 | TSH | Number of patients | |
|---|---|---|---|---|---|
| Polymorphism | |||||
| rs2235544 (DIOl)41 | Free T3 increased | Free T4 decreased | Decreased | Unchanged | 552 |
| rs12095080 (DIOl)42,43 | Total T3 increased | Unchanged | Unchanged | Unchanged | 995; 156 |
| rs11206244 (DIOl)42,43 | Total T3 decreased | Free T4 increased | Increased | Unchanged | 995; 156 |
| rs225014 (DIO2)44 | Free T3 decreased | Free T4 increased | NA | Unchanged | 140 |
| rs12885300 (DIO2)42,44 | Unchanged | Unchanged | Unchanged | Unchanged | 995; 140 |
| rs6647476 (MCT8*)45 | Free T3 and total T3 decreased | Unchanged | Unchanged | Unchanged | 2057 men |
| rs5937843 (MCT8*)45,46 | Unchanged | Free T4 decreased | Unchanged | Unchanged | 2057 men; 97 men |
| rs14399 (MCTlO†)45 | Unchanged | Unchanged | Unchanged | Unchanged | 3238 |
| rs17606253 (MCTlO†)47 | Unchanged | Unchanged | NA | Unchanged | 45 |
| rs10444412 (OATPlCl ‡)45,48 | Unchanged | Unchanged | Unchanged | Unchanged | 3238; 1192 |
| rs10770704 (OATPlCl‡)45,48 | Unchanged | Unchanged | Unchanged | Unchanged | 3238; 154 |
| rs36010656 (OATPlCl‡)48 | Unchanged | Unchanged | Increased | Unchanged | 154 |
| Mutation | |||||
| DIOl 49 | Unchanged | Unchanged | Increased | Increased | 8 |
| MCT8* (Allan-Herndon-Dudley syndrome)50 | Increased | Decreased | Decreased | Unchanged | 5 |
NA=not available. T3=tri-iodothyronine. T4=thyroxine. TSH=thyroid-stimulating hormone.
Also known as SLC16A2.
Also known as SLC16A10.
Also known as SLCO1C1.
Why do some clinicians have doubts of a role for T3 in therapy?
Results from clinical trials
There are more than 20 clinical trials and six metaanalyses that have compared therapy with levothyroxine versus therapy with liothyronine plus levothyroxine;14 however, no consensus as to the superiority of either therapy was achieved.14,15 The European Thyroid Association, British Thyroid Association, and American Thyroid Association issued a statement on the subject, explaining that most clinical trials did not focus on symptomatic patients treated with levothyroxine.14 Given that treatment with levothyroxine is successful in the majority of patients, most trials did not have sufficient statistical power to detect a potential beneficial effect provided by combination therapy to the subset of symptomatic patients. A 2021 prospective, blinded, randomised, cross-over clinical trial analysed 90 patients assigned to one of three therapy groups: levothyroxine, liothyronine plus levothyroxine, and DTE. Patients completed the thyroid symptom question naire (TSQ-36), quality of life general health questionnaire (GHQ-12), the Wechsler memory scale-version IV (VMS-IV), and the Beck Depression Inven tory (BDI). Subgroup analyses of the third of the most symptomatic patients on levothyroxine revealed strong preference for treatment containing either form of combination therapy, which improved performance on TSQ-36, GHQ-12, BDI, and visual memory index.16 Therefore this finding indicates the possibility that focusing on symptomatic patients might help to reach a consensus.
Current T3 replacement therapies
A major limitation of T3 replacement is the nonphysiological pharmacokinetics of currently available liothyronine preparations. The short-acting oral liothyronine leads to a sharp rise in serum T3 concentration, peaking 2–3 h after ingestion before rapidly decreasing to normal or low-normal concentrations for most of the day.52 In healthy individuals, concentrations of serum T3 remain relatively steady throughout the day, except for the minimal circadian rhythmicity.53 This sharp peak after ingestion of oral liothyronine might explain why some patients feel better following the dose, but report their condition worsening later in the day, leading to the recommendation that the liothyronine dose be split in two.54 A frequent concern about combination therapy is the potential long-term adverse effects, namely atrial fibrillation and osteoporosis, considering that therapy for hypothyroidism is lifelong. Although these sideeffects are valid concerns, no evidence currently exists suggesting that patients kept on combination therapy are more likely to develop such adverse reactions than patients on levothyroxine monotherapy. Despite the paucity of long-term studies of T3 therapy, a combined analysis of 20 clinical trials of combination therapy revealed that in a group of about 1000 patients receiving liothyronine plus levothyroxine for up to 1 year, the adverse reactions were similar to the group of patients receiving levothyroxine only.55 It is important to note that serum TSH concentrations in those patients under combination therapy were kept within the normal reference range. A large study of approximately 400 individuals taking combination therapy for up to 17 years in the Scottish region of Tayside also reported adverse reactions similar to those in patients treated with levothyroxine only.56 More studies about the safety of DTE are becoming available.16,57,58 In addition, a Swedish registry study of 575 461 individuals taking thyroid hormone replacement, of which 11 147 were using liothyronine, did not detect increased all-cause mortality or any cancer mortality compared with levothyroxine use, during a median follow-up time of 8·1 years.59
Future perspectives and conclusions
Slow-release liothyronine
The need for a liothyronine formulation with a pharmacokinetic profile that better mimics typical T3 physiology has led to the development of slow-release drug designs with various drug delivery methods. Proposed delivery methods include oral, subcutaneous, tissue-targeted, and regenerative therapies.60 More than 15 years ago, a proof-of-concept cross-over study of levothyroxine and a proprietary slow-release oral form of liothyronine showed a delayed, smaller serum T3 peak when compared with levothyroxine plus the standard liothyronine preparation.61 No data are available on patient symptoms or preferences during the trial. The use of T3 sulphate has also been tested in rats and humans with promising results in terms of safety and serum T3 stability.62,63 In this case, T3 is delivered to the circulation after deconjugation by liver and intestinal sulphatases. More recently, an oral preparation of a metal-coordinated form of liothyronine containing zinc, eg, poly-zinc-liothyronine, was shown to delay the serum T3 peak after dosing in rats, creating a longer period of relatively stable serum T3 concentrations.64 A phase 1 clinical trial to determine the safety and pharmacokinetic profile of poly-zincliothyronine in humans has been completed with satisfactory results— poly-zinc-liothyroinine showed an appropriate pharmacokinetic profile following a single daily admini stration, without any adverse events.65 If the safety and efficacy of slow-release preparations of liothyronine are shown, the next step will be to create clinical trials targeting levothyroxine-treated patients with persistent symptoms and metabolic abnormalities, to determine whether levothyroxine plus slow-release liothyronine preparations help to resolve the shortcomings of levothyroxine monotherapy in these patients.
Genetic profiling
Novel genome-wide association studies clearly show that serum thyroid hormone concentrations are strongly influenced by dozens of genes that encode for peripheral regulators of thyroid hormone action and metabolism.66,67 The plasma T3 concentration in an individual is the result of their specific proportion of thyroid T3 secretion and peripheral T3 production relative to clearance of T3. In patients with hypothyroidism, the loss of the thyroid gland and its adjustable contribution to the T3 pool leave the peripheral T4-to-T3 conversion as the only source of T3 production, which might compromise serum T3 concentrations. A major concern with the universal applicability of levothyroxine monotherapy came from the finding that some patients with hypothyroidism carry variants of thyroid hormone metabolism-related genes that might prevent normalisation of circulating and tissue T3 concentrations (table).68 In particular, this issue has been seen in people with the relatively common Thr92Ala substitution in DIO2 (D2-Ala), which has been associated with increased likelihood of persistent hypothyroid-like clinical manifestations in levothyroxine-treated patients despite normal TSH concentrations.5,6,69 Thyroidectomised levothyroxine-treated patients with the D2-Ala polymorphism have also been shown to have lower postsurgical serum T3 concentrations than their presurgical concentrations.44 The hypothesis of reduced enzymatic activity of D2-Ala has been further substantiated by results from invitro cellular assays44 and mice models,70 and by a study of 45 patients with hypothyroidism71 that suggested that patients with combined expression of Thr92Ala-DIO2 and MCT10 transporter gene rs17606253 variants might respond better to liothyronine plus levothyroxine than levothyroxine monotherapy.
The discovery that variants in deiodinases and thyroid hormone transporter genes are associated with subnormal T3 concentrations, persistent symptoms in levothyroxinetreated patients, and improvement in response to addition of liothyronine to therapy might indicate a viable way to identify the small but notable percentage of patient candidates who would benefit from liothyronine plus levothyroxine combined therapy. In this context, genotyping for transporters and deiodinase polymorphisms within prospective trials of liothyronine plus levothyroxine combination therapy is crucial. Given that the correlation between alterations in T3 concentrations and genetic polymorphisms derives from a small number of unreplicated studies, large studies will be required to provide more evidence. Hopefully, these studies will reveal a gene profile that can inform personalised thyroid hormone replacement therapy.
Figure: Schematic representation of T3 production and clearance in humans.
The thyroid gland secretes T4 and T3 with a molar ratio of about 14:1 and accounts for 20% of total daily T3 production.17 The rest of T3 body requirement derives from T4-to-T3 conversion by D1 and D2. D1 is mainly expressed in the liver, kidney, and thyroid gland and is located in the cell membrane. Almost the total amount of D1-derived T3 is released in the systemic circulation. D2 contributes to a lesser extent to serum T3 concentrations and operates in the intracellular space within the endoplasmic reticulum. D2-expressing tissues (namely, the thyroid and pituitary glands, brain, skeletal muscle, and brown adipose tissue) use D2 to increase their nuclear T3 concentrations on top of the serum-derived amount of T3.17,18 D3 is responsible for T3 inactivation through the conversion of 3,5,3’-T3 to 3,3’-T2. D3 is an intracellular enzyme expressed in the liver, brain, skin, intestine, placenta, and muscle. These tissues use D3 to lower the T3 nuclear concentration in response to specific cell needs. Liver and kidney can inactivate T3 in a non-deiodinative way by sulpho-conjugation (s-T3) or glucuronidation (g-T3) within the microsomes. S-T3 is released in the systemic circulation and becomes a strong substrate for D1, which converts it to the inactive 3,5-T2 sulphate and releases one iodide ion for the subsequent utilisation in the thyroid hormonogenesis.20 Grey area within nuclei represents nuclear T3 content. D1=type 1 deiodinases. D2=type 2 deiodinases. D3=type 3 deiodinases. T3=tri-iodothyronine. T4=thyroxine.
Search strategy and selection criteria.
This Personal View is based on a search of primary and review literature from PubMed with the terms “hypothyroidism”, “T3”, “liothyronine”, and “deiodinase”, among other keywords. PubMed searches were supplemented by Google Scholar and the our previous knowledge of the subject. We searched for clinical trials of the liothyronine plus levothyroxine combination and desiccated thyroid extract therapy in MEDLINE and the Cochrane Central Register of Controlled Trials, published in English from database inception to Oct 3, 2021, with the following search terms: “T3”, “T4”, or “LT3”, “LT4” or “DTE”, “desiccated thyroid extract” in combination with “combination therapy” or “combined therapy”. We also did a manual search of the reference lists of the selected articles to identify additional relevant papers not seen in the original search.
Footnotes
Declaration of interests
ACB is a consultant for AbbVie (makers of Synthroid), Allergan (makers of Armor Thyroid), and Synthonics, Sention Therapeutics, and Thyron (makers of non-clinically available products). All other authors declare no competing interests.
References
- 1.Christoffolete MA, Arrojo e Drigo R, Gazoni F, et al. Mice with impaired extrathyroidal thyroxine to 3,5,3′-triiodothyronine conversion maintain normal serum 3,5,3′-triiodothyronine concentrations. Endocrinology 2007; 148: 954–60. [DOI] [PubMed] [Google Scholar]
- 2.Galton VA, Schneider MJ, Clark AS, St Germain DL. Life without thyroxine to 3,5,3′-triiodothyronine conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology 2009; 150: 2957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stock JM, Surks MI, Oppenheimer JH. Replacement dosage of L-thyroxine in hypothyroidism. A re-evaluation. N Engl J Med 1974; 290: 529–33. [DOI] [PubMed] [Google Scholar]
- 4.Gereben B, McAninch EA, Ribeiro MO, Bianco AC. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol 2015; 11: 642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson SJ, McAninch EA, Bianco AC. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab 2016; 101: 4964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito M, Miyauchi A, Hisakado M, et al. Biochemical markers reflecting thyroid function in athyreotic patients on levothyroxine monotherapy. Thyroid 2017; 27: 484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larisch R, Midgley JEM, Dietrich JW, Hoermann R. Symptomatic relief is related to serum free triiodothyronine concentrations during follow-up in levothyroxine-treated patients with differentiated thyroid cancer. Exp Clin Endocrinol Diabetes 2018; 126: 546–52. [DOI] [PubMed] [Google Scholar]
- 8.Idrees T, Prieto WH, Casula S, et al. Use of statins among patients taking levothyroxine: an observational drug utilization study across sites. J Endocr Soc 2021; 5: bvab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAninch EA, Rajan KB, Miller CH, Bianco AC. Systemic thyroid hormone status during levothyroxine therapy in hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab 2018; published online Aug 15. 10.1210/jc.2018-01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridgway EC, Cooper DS, Walker H, et al. Therapy of primary hypothyroidism with L-triiodothyronine: discordant cardiac and pituitary responses. Clin Endocrinol (Oxf) 1980; 13: 479–88. [DOI] [PubMed] [Google Scholar]
- 11.Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol (Oxf) 2002; 57: 577–85. [DOI] [PubMed] [Google Scholar]
- 12.Wekking EM, Appelhof BC, Fliers E, et al. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur J Endocrinol 2005; 153: 747–53. [DOI] [PubMed] [Google Scholar]
- 13.Peterson SJ, Cappola AR, Castro MR, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid 2018; 28: 707–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid 2021; 31: 156–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Eur Thyroid J 2021; 10: 10–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+Liothyronine in hypothyroidism. J Clin Endocrinol Metab 2021; 106: e4400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 2002; 23: 38–89. [DOI] [PubMed] [Google Scholar]
- 18.Luongo C, Dentice M, Salvatore D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat Rev Endocrinol 2019; 15: 479–88. [DOI] [PubMed] [Google Scholar]
- 19.Geffner DL, Azukizawa M, Hershman JM. Propylthiouracil blocks extrathyroidal conversion of thyroxine to triiodothyronine and augments thyrotropin secretion in man. J Clin Invest 1975; 55: 224–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Köhrle J, Lehmphul I, Pietzner M, et al. 3,5-T2-a janus-faced thyroid hormone metabolite exerts both canonical t3-mimetic endocrine and intracrine hepatic action. Front Endocrinol (Lausanne) 2020; 10: 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancino G, Sibilio A, Luongo C, et al. The thyroid hormone inactivator enzyme, type 3 deiodinase, is essential for coordination of keratinocyte growth and differentiation. Thyroid 2020; 30: 1066–78. [DOI] [PubMed] [Google Scholar]
- 22.Medici M, Visser WE, Visser TJ, Peeters RP. Genetic determination of the hypothalamic-pituitary-thyroid axis: where do we stand? Endocr Rev 2015; 36: 214–44. [DOI] [PubMed] [Google Scholar]
- 23.Andersen S, Bruun NH, Pedersen KM, Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid 2003; 13: 1069–78. [DOI] [PubMed] [Google Scholar]
- 24.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 2002; 87: 1068–72. [DOI] [PubMed] [Google Scholar]
- 25.Gereben B, Zavacki AM, Ribich S, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 2008; 29: 898–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAninch EA, Bianco AC. The swinging pendulum in treatment for hypothyroidism: from (and toward?) combination therapy. Front Endocrinol (Lausanne) 2019; 10: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonklaas J, Davidson B, Bhagat S, Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA 2008; 299: 769–77. [DOI] [PubMed] [Google Scholar]
- 28.Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One 2011; 6: e22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J 2012; 1: 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid 2014; 24: 1670–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf) 2016; 84: 799–808. [DOI] [PubMed] [Google Scholar]
- 32.Thienpont LM, Van Uytfanghe K, Poppe K, Velkeniers B. Determination of free thyroid hormones. Best Pract Res Clin Endocrinol Metab 2013; 27: 689–700. [DOI] [PubMed] [Google Scholar]
- 33.van Deventer HE, Soldin SJ. The expanding role of tandem mass spectrometry in optimizing diagnosis and treatment of thyroid disease. Adv Clin Chem 2013; 61: 127–52. [DOI] [PubMed] [Google Scholar]
- 34.Jonklaas J, Sathasivam A, Wang H, Gu J, Burman KD, Soldin SJ. Total and free thyroxine and triiodothyronine: measurement discrepancies, particularly in inpatients. Clin Biochem 2014; 47: 1272–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonklaas J, Kahric-Janicic N, Soldin OP, Soldin SJ. Correlations of free thyroid hormones measured by tandem mass spectrometry and immunoassay with thyroid-stimulating hormone across 4 patient populations. Clin Chem 2009; 55: 1380–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernández JM, Soldevila B, Velasco I, et al. Reference intervals of thyroid function tests assessed by immunoassay and mass spectrometry in healthy pregnant women living in catalonia. J Clin Med 2021; 10: 2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gant Kanegusuku A, Araque KA, Nguyen H, Wei B, Hosseini S, Soldin SJ. The effect of specific binding proteins on immunoassay measurements of total and free thyroid hormones and cortisol. Ther Adv Endocrinol Metab 2021; 12: 2042018821989240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borsò M, Agretti P, Zucchi R, Saba A. Mass spectrometry in the diagnosis of thyroid disease and in the study of thyroid hormone metabolism. Mass Spectrom Rev 2020; published online NZov 25. 10.1002/mas.21673. [DOI] [PubMed] [Google Scholar]
- 39.Taylor S, Kapur M, Adie R. Combined thyroxine and triiodothyronine for thyroid replacement therapy. BMJ 1970; 2: 270–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YK, Lee H, Han S, et al. Association between thyroidstimulating hormone level after total thyroidectomy and hypercholesterolemia in female patients with differentiated thyroid cancer: a retrospective study. J Clin Med 2019; 8: E1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panicker V, Cluett C, Shields B, et al. A common variation in deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and triiodothyronine. J Clin Endocrinol Metab 2008; 93: 3075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jong FJ, Peeters RP, den Heijer T, et al. The association of polymorphisms in the type 1 and 2 deiodinase genes with circulating thyroid hormone parameters and atrophy of the medial temporal lobe. J Clin Endocrinol Metab 2007; 92: 636–40. [DOI] [PubMed] [Google Scholar]
- 43.Peeters RP, van Toor H, Klootwijk W, et al. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab 2003; 88: 2880–88. [DOI] [PubMed] [Google Scholar]
- 44.Castagna MG, Dentice M, Cantara S, et al. DIO2 Thr92Ala reduces deiodinase-2 activity and serum-T3 levels in thyroid-deficient patients. J Clin Endocrinol Metab 2017; 102: 1623–30. [DOI] [PubMed] [Google Scholar]
- 45.Roef GL, Rietzschel ER, De Meyer T, et al. Associations between single nucleotide polymorphisms in thyroid hormone transporter genes (MCT8, MCT10 and OATP1C1) and circulating thyroid hormones. Clin Chim Acta 2013; 425: 227–32. [DOI] [PubMed] [Google Scholar]
- 46.van der Deure WM, Peeters RP, Visser TJ. Genetic variation in thyroid hormone transporters. Best Pract Res Clin Endocrinol Metab 2007; 21: 339–50. [DOI] [PubMed] [Google Scholar]
- 47.Cantara S, Ricci C, Maino F, Marzocchi C, Pacini F, Castagna MG. Variants in MCT10 protein do not affect FT3 levels in athyreotic patients. Endocrine 2019; 66: 551–56. [DOI] [PubMed] [Google Scholar]
- 48.van der Deure WM, Hansen PS, Peeters RP, et al. Thyroid hormone transport and metabolism by organic anion transporter 1C1 and consequences of genetic variation. Endocrinology 2008; 149: 5307–14. [DOI] [PubMed] [Google Scholar]
- 49.França MM, German A, Fernandes GW, et al. Human type 1 iodothyronine deiodinase (DIO1) mutations cause abnormal thyroid hormone metabolism. Thyroid 2021; 31: 202–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friesema ECH, Grueters A, Biebermann H, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 2004; 364: 1435–37. [DOI] [PubMed] [Google Scholar]
- 51.Bianco AC, Kim BS. Pathophysiological relevance of deiodinase polymorphism. Curr Opin Endocrinol Diabetes Obes 2018; 25: 341–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saravanan P, Siddique H, Simmons DJ, Greenwood R, Dayan CM. Twenty-four hour hormone profiles of TSH, Free T3 and free T4 in hypothyroid patients on combined T3/T4 therapy. Exp Clin Endocrinol Diabetes 2007; 115: 261–67. [DOI] [PubMed] [Google Scholar]
- 53.Russell W, Harrison RF, Smith N, et al. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab 2008; 93: 2300–06. [DOI] [PubMed] [Google Scholar]
- 54.Wiersinga WM. Therapy of endocrine disease: T4 + T3 combination therapy: is there a true effect? Eur J Endocrinol 2017; 177: R287–96. [DOI] [PubMed] [Google Scholar]
- 55.Idrees T, Palmer S, Maciel RMB, Bianco AC. Liothyronine and desiccated thyroid extract in the treatment of hypothyroidism. Thyroid 2020; 30: 1399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leese GP, Soto-Pedre E, Donnelly LA. Liothyronine use in a 17 year observational population-based study—the tears study. Clin Endocrinol (Oxf) 2016; 85: 918–25. [DOI] [PubMed] [Google Scholar]
- 57.Hoang TD, Olsen CH, Mai VQ, Clyde PW, Shakir MK. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab 2013; 98: 1982–90. [DOI] [PubMed] [Google Scholar]
- 58.Tariq A, Wert Y, Cheriyath P, Joshi R. Effects of long-term combination LT4 and LT3 therapy for improving hypothyroidism and overall quality of life. South Med J 2018; 111: 363–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Planck T, Hedberg F, Calissendorff J, Nilsson A. Liothyronine use in hypothyroidism and its effects on cancer and mortality. Thyroid 2021; 31: 732–39. [DOI] [PubMed] [Google Scholar]
- 60.Idrees T, Price JD, Piccariello T, Bianco AC. Sustained release T3 therapy: animal models and translational applications. Front Endocrinol (Lausanne) 2019; 10: 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hennemann G, Docter R, Visser TJ, Postema PT, Krenning EP. Thyroxine plus low-dose, slow-release triiodothyronine replacement in hypothyroidism: proof of principle. Thyroid 2004; 14: 271–75. [DOI] [PubMed] [Google Scholar]
- 62.Santini F, Giannetti M, Ricco I, et al. Steady-state serum T3 concentrations for 48 hours following the oral administration of a single dose of 3,5,3′-triiodothyronine sulfate (T3S). Endocr Pract 2014; 20: 680–89. [DOI] [PubMed] [Google Scholar]
- 63.Santini F, Ceccarini G, Pelosini C, et al. Treatment of hypothyroid patients with L-thyroxine (L-T4) plus triiodothyronine sulfate (T3S). A phase II, open-label, single center, parallel groups study on therapeutic efficacy and tolerability. Front Endocrinol (Lausanne) 2019; 10: 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Da Conceição RR, Fernandes GW, Fonseca TL, Bocco BMLC, Bianco AC. Metal coordinated poly-zinc-liothyronine provides stable circulating triiodothyronine levels in hypothyroid rats. Thyroid 2018; 28: 1425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dumitrescu AM, Hanlon EC, Arosemena M, et al. Extended absorption of liothyronine from poly-zinc-liothyronine: results from a phase 1, double-blind, randomized, and controlled study in humans. Thyroid 2021; published online Dec 31. 10.1089/thy.2021.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porcu E, Medici M, Pistis G, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet 2013; 9: e1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teumer A, Chaker L, Groeneweg S, et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat Commun 2018; 9: 4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porcelli T, Salvatore D. Targeting the right population for T3 + T4 combined therapy: where are we now and where to next? Endocrine 2020; 69: 244–48. [DOI] [PubMed] [Google Scholar]
- 69.Panicker V, Saravanan P, Vaidya B, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab 2009; 94: 1623–29. [DOI] [PubMed] [Google Scholar]
- 70.Jo S, Fonseca TL, Bocco BMLC, et al. Type 2 deiodinase polymorphism causes ER stress and hypothyroidism in the brain. J Clin Invest 2019; 129: 230–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carlé A, Faber J, Steffensen R, Laurberg P, Nygaard B. Hypothyroid patients encoding combined MCT10 and DIO2 gene polymorphisms may prefer L-T3 + L-T4 combination treatment— data using a blind, randomized, clinical Study. Eur Thyroid J 2017; 6: 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]