Abstract
Background:
Epidemiological studies that investigate alterations in gut microbial composition associated with cognitive dysfunction are limited.
Objective:
To examine the association between the gut microbiota and subjective memory complaints (SMCs), a self-reported, validated indicator of cognitive dysfunction.
Methods:
In this cross-sectional study of 95 older women selected from the New York University Women’s Health Study (NYUWHS), we characterized the gut microbial composition using 16S rRNA gene sequencing. We estimated odds ratio (OR) from beta regression which approximates the ratio of mean relative abundances of individual bacterial taxon from phylum to genus levels by binary (2+ versus < 2) and continuous SMCs.
Results:
Women reporting 2 or more SMCs had higher relative abundances of genus Holdemania and family Desulfovibrionaceae compared with those reporting one or no complaint. Compared with women with < 2 SMCs, the relative abundances of Holdemania and family Desulfovibrionaceae were 2.09 times (OR: 2.09, 95% confidence interval [CI]: 1.38–3.17) and 2.10 times (OR: 2.10, 95% CI: 1.43–3.09) higher in women with 2+ SMCs, respectively (false discovery rate (FDR)-adjusted p = 0.038 and 0.010, respectively). A dose-response association was observed for genus Sutterella and family Desulfovibrionaceae. Every one-unit increase in SMCs was associated with 25% and 27% higher relative abundances of Sutterella (OR: 1.25; 95% CI: 1.11–1.40) and Desulfovibrionaceae (OR: 1.27; 95% CI: 1.13–1.42), respectively (FDR-adjusted p = 0.018 and 0.006, respectively).
Conclusion:
Our findings support an association between alterations in the gut bacterial composition and cognitive dysfunction.
Keywords: 16S RNA gene sequencing, cross-sectional, gut microbiota, NYUWHS, subjective memory complaints
INTRODUCTION
Alzheimer’s disease (AD), the most common form of dementia, affects an estimated 10% of adults over 65 in the United States [1]. AD and AD-related dementias (AD/ADRD) constitute a considerable public health challenge because of the current lack of effective treatment [2]. The pathologic process of AD begins decades before the emergence of clinical symptoms [3, 4]. A continuum between early, self-perceived changes in memory, here termed subjective memory complaints (SMCs), clinical manifestations of mild cognitive impairment (MCI) and predementia, has been documented [5]. SMCs have been associated with the presence of various pathological changes of AD, including amyloid-β) (Aβ) deposition [6–8], increases in white matter lesions [9, 10], and gray matter atrophy [11–13]. Also, SMCs strongly predict future dementia and AD [14–16]. Thus, the investigation of risk factors for SMCs may provide an impactful opportunity for elucidating the potential mechanism of AD/ADRD and facilitating early intervention to reduce or delay the onset of the early stages of dementia [17].
Emerging studies have provided evidence that gut microbiota may be associated with AD/ADRD [18] via bidirectional interactions within the microbiota-gut-brain axis [19, 20]. Gut microbes communicate to the central nervous system through neural, endocrine, immune, and microbial metabolite pathways [21, 22]. The brain can affect the community structure and function of the gut microbiota through the autonomic nervous system, by modulating regional gut motility, intestinal transit and secretion, and gut permeability, and potentially through the luminal secretion of hormones that directly modulate microbial gene expression [23]. Animal studies have shown alterations in the gut microbial composition in mouse models of AD, which could contribute to brain Aβ deposition and decrements in learning and memory [24–30]. Manipulation of gut microbiota in transgenic mouse models of AD can influence cerebral Aβ deposition [28]. However, epidemiologic studies investigating the role of gut microbiota in AD/ADRD are limited.
A few cross-sectional studies have demonstrated differences in the gut microbial composition between individuals with MCI, AD, or dementia and healthy controls [31–41] (Supplementary Table 1). Three studies reported an enrichment of Proteobacteria in individuals with AD or MCI compared with healthy controls [33–35], while some other studies reported inconsistent findings on bacteria in Bacteroidetes [31, 32, 34, 36, 37, 40, 41]. However, these studies are limited in size (n < 50) [31, 34, 40], did not use next generation sequencing to measure microbiome [36, 37], and/or included study subjects on medications or other interventions for AD/ADRD [32, 36, 37]. Only one hospital-based case-control study has assessed gut microbiota in relation to subjective cognitive decline [38]. We conducted a cross-sectional study to assess the association between gut microbiota and SMCs in 95 older women, recruited from a large prospective cohort study. We collected fecal samples and conducted 16S rRNA gene sequencing to quantify the gut microbial composition.
METHODS
Study population
The study population was derived from the New York University Women’s Health Study (NYUWHS), a prospective cohort study of 14,274 women aged 35–65 years enrolled in 1985–1991 at a mammography screening center in New York City [42]. Women were ineligible for enrollment if they had used hormonal medications or had been pregnant or lactating in the previous 6 months. At baseline, data on demographics, anthropometric measures (height and weight), medical history, reproductive and lifestyle variables were collected through self-administered questionnaires. Active contact with cohort participants is achieved through questionnaires mailed every 3–5 years and, for non-respondents, telephone calls.
For the present study, we selected 250 women who responded to the NYUWHS questionnaire mailed in December 2018 (2018 follow-up). After excluding known cases of cancer and women > 90 years of age, we sent an invitation letter and a consent form to 227 women along with a screening questionnaire to collect information on exclusion criteria, including current dialysis; history of major gastrointestinal tract surgery; and use of systemic antibiotics, treatment with oral, nasal, or injected corticosteroids, treatment with immunosuppressive drugs other than corticosteroids, cleansing of the large intestine, colonoscopy, and substantial weight change (> 20 lbs) in the previous 6 months. A total of 118 (52%) eligible women consented to participate and 101 (44%) women returned a stool sample. Participants received a gift card of $20 in the mail for their time and effort. The participation rate (44%), with the small amount of compensation, compares favorably with what has been reported in other cohorts (30–50%) that collected fecal samples [43–45].
At the time of fecal sample collection, participants completed a short questionnaire regarding current lifestyle (smoking, alcohol consumption, use of multivitamins and probiotics) and special diets (vegetarian, gluten-free, or other). All participants provided written informed consent to be involved in this study. All study procedures were approved by the institutional review board of NYU School of Medicine.
Assessment of SMCs
We assessed SMCs in the 2018 follow-up questionnaire based on 6 yes/no questions [7, 49], which have been validated against objective features of dementia, clinically established cognitive testing questionnaires for memory loss, and APOE ε4 genotype [7, 50–52]. The 6 questions were based on reports of 1) a recent change in memory; 2) having more trouble than usual remembering recent events; 3) remembering a short list of items; 4) following a group conversation or a plot in a TV program; 5) having difficulty understanding or following spoken instructions; and 6) having trouble finding one’s way around familiar streets. We gave 1 point for each “yes” and computed a SMCs score as the total number of complaints reported by each woman [7]; thus, SMCs scores ranged from 0 to 6.
Collection of stool samples
We developed a protocol adapting procedures used in the Multiethnic Cohort (MEC) study [45] that allow participants to collect a stool sample in the privacy of their own homes. Briefly, once a signed consent was returned, we sent the participants a stool collection kit and easy-to-follow instructions with photos for stool collection. The stool collection kit consisted of a stool collection container (white tub with lid; Fisher Scientific, MA), a toilet adaptor tray (Fisher Scientific, MA), a triple-slide Beckman Coulter Hemoccult II SENSA® card (Beckman Coulter, CA), a pair of exam gloves, and a Ziploc bag to save the card. Participants collected stool onto all the three slides of the card. This method produces reproducible and accurate 16S rRNA gene-derived microbiota data [46, 47], and exhibits stability at room temperature up to 8 weeks [48]. Samples were mailed directly to NYU following at-home collection and stored immediately at −80°C until analysis.
Covariates
Time-invariant variables (race, education, height) were from the baseline questionnaire. We used data on age (continuous, years), alcohol consumption and current use of multivitamin and probiotics collected at the time of fecal sample collection. Other self-reported covariates, including weight (continuous), hypertension (defined as use of antihypertensive medicine or self-reported diagnosis, yes/no), use of cholesterol lowering medication (yes/no), and use of antidepressants (yes/no) were from the 2018 follow-up questionnaire. Body mass index (BMI; kilogram per square meter) was calculated from height and weight. There were a limited number of women who reported current smoking (n = 1), diabetes (defined as use of insulin or diabetes pills or self-reported diagnosis, yes/no; n = 7), or special diets (vegetarian: n = 7, gluten-free: n = 5), and therefore these variables were not used.
Microbial assessment
From the 101 stool samples returned, one of the three sections from the cards containing the stool sample was cut and shipped on dry ice to Argonne National Laboratory for microbiome analysis. A second section from seven participants was included for quality control analyses. The samples underwent 16S rRNA gene sequencing at the Environmental Sample Preparation and Sequencing Facility of the Laboratory. Bacterial DNA was extracted using the Mo Bio PowerSoil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA) according to the manufacturer’s instructions. The V4 region of the 16S rRNA gene was PCR amplified with the 515F/806R primer pair [53, 54]. Six samples failed PCR amplification and were not further processed. PCR amplicons were purified using AMPure XP beads (Beckman Coulter, Brea, CA), pooled in equal amounts, and sequenced on the MiSeq platform (Illumina, San Diego, CA), as previously described [55].
Sequence read processing
Sequence reads were processed using QIIME 2 [56]. Briefly, sequence reads were demultiplexed and paired-end reads were joined, followed by standard quality filtering procedures [57]. We obtained an average of 28,148 reads per sample (range: 7,501–56,487; median: 27,453; interquartile range: 23,353–31,579). Next, the DADA2 pipeline [58] was applied, which uses sequence error profiles to obtain putative error-free sequences, referred to as amplicon sequence variants (ASVs). 0After building the ASV table and removing chimeras, taxonomy was assigned using the Ribosomal Database Project (RDP) Classifier (v2.13) [59] trained on the 99% OTUs in the Greengenes 13 8 database [60]. A phylogenetic tree was built using FastTree (v2.1.11) [61] from a multiple sequence alignment made with the PyNAST alignment tool [62]. The ASV tables was rarefied to 7,501 sequence reads per sample (the lowest sequencing depth among samples) prior to α- and β-diversity analysis. α-Diversity (within-subject diversity) was assessed using richness (the actual number of ASVs observed in a sample), Pielou’s evenness, and Shannon diversity index, which measures diversity by accounting for evenness and richness [63]. β-Diversity (between-subject diversity) was assessed using the Bray-Curtis dissimilarity, and unweighted and weighted UniFrac distances [64]. Principal coordinate analysis (PCoA) was used for visualization. The ASVs were agglomerated to phylum, class, order, family, and genus levels and relative abundance of bacterial taxa (total sum scaling) was calculated at each taxonomic rank. After removing taxa that a) were present in < 10% of samples, b) had mean relative abundance < 0.01%, and c) were unclassified at the family or genus level, a total of 9 phyla, 17 classes, 20 orders, 34 families, and 52 genera were included in final analyses.
Statistical analyses
We dichotomized the SMCs scores and compared women reporting two or more complaints with those reporting less than two complaints, as over 40% of the women reported at least one SMC. We also considered the SMCs as a continuous variable and compared participants with 2 and 3+ SMCs with those without SMCs. We calculated descriptive statistics for socio-demographic variables: a) mean and standard deviation for continuous variables and b) distribution (%) for categorical variables, by binary SMCs scores (2+ versus < 2). We used Fisher’s exact test and the Wilcoxon rank-sum test to detect group differences in categorical and continuous variables, respectively. We used linear regression models to test whether α-diversity differed by SMCs scores (2+ versus < 2 or 1, 2, 3+ versus 0), adjusting for age, BMI, and race. Permutational multivariate analysis of variance (PERMANOVA; ‘adonis’ function, ‘vegan’ package, R) was used to examine differences in the overall microbial community composition between SMCs strata (2+ versus < 2 or 1, 2, 3+ versus 0), adjusting for age, BMI, and race. We hypothesized that certain alterations in gut microbial composition may lead to decline in cognitive function. However, given the cross-sectional nature of the study, we could only assess whether the relative abundance of certain bacterial taxa was higher or lower in women with SMCs. We fit a beta regression model, which is appropriate to model a percentage response [65], to evaluate differences in the mean relative abundances (percentage response) of gut bacterial taxa associated with SMCs, adjusting for age, BMI, and race, using the “betareg” R package, as previously described [66]. Briefly, bacterial taxa with zero abundance were imputed as 10−6 because the logarithm of zero is undefined. For a given taxon with p mean relative abundance, the estimated odds ratio (OR) is a ratio of p/(1-p) in women with 2+ SMCs and p/(1-p) in women with < 2 SMCs. When p is small, as for most of the taxa under investigation (< 10% or < 5%), the OR approximates the ratio of the mean relative abundances by categories of SMCs. We thus interpreted the ORs as the ratios of mean relative abundances by the outcomes of interest. p values for these models were adjusted for multiple comparison at each taxonomic level separately by the false discovery rate (FDR) method [67]. Similar models were constructed using SMCs as a continuous independent variable to estimate OR associated with every one-unit increase in SMCs. For genera that were associated with SMCs after controlling for multiple comparisons, we further estimated ORs comparing women reporting any SMCs with women with no complaint. Sensitivity analyses were conducted with additional adjustment for education, and use of cholesterol lowering medication and antidepressants. We also conducted sensitivity analyses using a differential abundance analysis method based on read count data, namely, Analysis of Compositions of Microbiomes with Bias Correction (ANCOM-BC), which corrects for bias induced by differences in the unknown sampling fractions and identifies taxa that are differentially abundant according to the covariate of interest [68]. ANCOM-BC first estimates sampling fractions that are different across samples, and then models the log of read count data, in which zeros are replaced by pseudo-count 1, through a linear regression model including the estimated sampling fraction as an offset term.
RESULTS
Population characteristics
Among the invited participants, we compared women who provided a stool sample and were included in the present study with those who did not. Women who gave stool samples had a higher educational attainment (p < 0.01; Supplementary Table 2) and were also slightly younger (p = 0.07), compared with those who did not. The two groups did not differ significantly in other demographics, comorbidities, and SMCs scores. Participants in the present study were predominantly white (88.4%) and 78% were between the ages of 70–90 (mean: 76.0 ± 5.8 years old) at the time of fecal sample collection. Overall, 52.6% of the study participants reported no SMCs, 21.1% reported one, 14.7% reported two, and 11.7% reported three or more. Of the six SMCs included, a recent change in memory was the most common complaint, reported by 42.1% of the participants, followed by having more trouble than usual remembering recent events (20.2%) and a short list of items (18.5%), and having trouble finding one’s way on familiar streets (3.2%). Compared with women reporting < 2 SMCs, women reporting 2+ SMCs were more educated (Table 1, p = 0.09), less likely to use cholesterol lowering medication (p < 0.05), and more likely to use antidepressants (p = 0.07). No appreciable differences by SMCs were noted for the other demographic and lifestyle variables.
Table 1.
Distribution of lifestyle and demographic variables by subjective memory complaints scores (2+ versus < 2)
| Characteristics | Subjective memory complaints scores < 2 | Subjective memory complaints scores 2+ | p a |
|---|---|---|---|
| Total N | 70 | 25 | |
| Ageb (y), mean (SD) | 76.1 (5.9) | 75.7 (5.8) | 0.75 |
| Weightc (lbs), mean (SD) | 147.2 (37.3) | 150.9 (41.3) | 0.55 |
| BMIc (kg/m2), mean (SD) | 24.6 (4.8) | 24.5 (6.1) | 0.62 |
| Raced, n (%) | 0.28 | ||
| Caucasian | 60 (85.7%) | 24 (96.0%) | |
| Other | 10 (14.3%) | 1 (4.0%) | |
| Educationd, n (%) | 0.09 | ||
| Did not complete college | 20 (28.6%) | 2 (8.3%) | |
| Completed college | 13 (18.6%) | 4 (16.7%) | |
| Some schooling after college | 37 (52.8%) | 18 (75.0%) | |
| Any alcoholic drinke use per weekb, n (%) | 36 (54.5%) | 11 (44.0%) | 0.48 |
| Current multivitamin useb, n (%) | 36 (52.9%) | 9 (39.1%) | 0.34 |
| Current probioticf useb, n (%) | 11 (16.7%) | 4 (17.4%) | 1.00 |
| Current use of cholesterol lowering medicationc, n (%) | 35 (50.7%) | 4 (16.0%) | 0.004 |
| Hypertensionc, n (%) | 38 (54.3%) | 12 (48.0%) | 0.65 |
| Current antidepressant usec, n (%) | 6 (8.6%) | 6 (24.0%) | 0.07 |
Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
Data collected at the time of fecal sample collection.
Data from the 2018 follow-up questionnaire.
Data collected at baseline.
One drink equals to 12 oz of beer, 5 oz of wine, or 1½ ounces of liquor.
Pill, powder, or liquid.
α- and β-diversity in relation to SMCs
Women with 2+ SMCs did not differ significantly from those reporting < 2 SMCs in any of the α-diversity and β-diversity indices (Fig. 1 and Supplementary Figure 1). Pairwise comparisons between women with 1, 2, or 3+ SMCs and those without any complaint did not show any appreciable difference in α-diversity and β-diversity (data not shown).
Fig. 1.
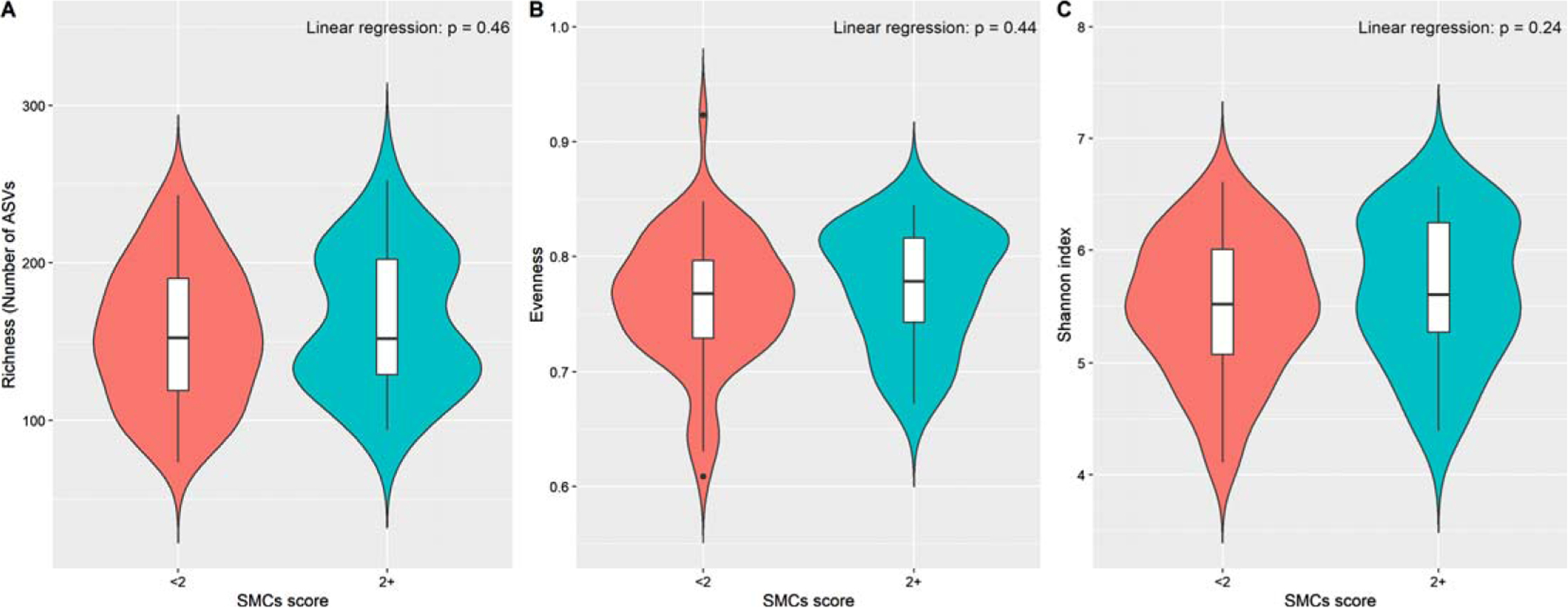
Alpha diversity indices of gut microbiota by subjective memory complaints scores (2+ versus < 2). Violin plots based on (A) richness (number of ASVs), (B) Evenness, and (C) Shannon diversity index. Linear regression was used to test the group difference, adjusting for age, BMI and race.
Taxa associated with SMCs
The relative abundances of 11 taxa were associated with SMCs at the nominal level when comparing women reporting 2+ SMCs with those reporting < 2 SMCs (Table 2), including the Betaproteobacteria-Burkholderiales-Alcaligenaceae-Sutterella and Deltaproteobacteria-Desulfovibrionales-Desulfovibrionaceae-Bilophila lineage within phylum Proteobacteria. After correction for multiple comparisons, genus Holdemania (family Erysipelotrichaceae) within phylum Firmicutes, class Deltaproteobacteria, order Desulfovibrionales, and family Desulfovibrionaceae were significantly enriched among women reporting 2+ SMCs compared with those reporting < 2 SMCs. The mean relative abundance of genus Holdemania was 2.09 times (OR: 2.09, 95% confidence interval [CI]: 1.38–3.17, FDR-adjusted p = 0.038) higher in women with 2+ SMCs, compared with women with < 2 SMCs. The mean relative abundance of the Deltaproteobacteria-Desulfovibrionales-Desulfovibrionaceae lineage was 2.10 times (OR: 2.10, 95% CI: 1.43–3.09, FDR-adjusted p = 0.005, 0.006, and 0.010, respectively) higher comparing women with 2+ SMCs with women who reported < 2 SMCs.
Table 2.
Gut microbial taxa associated with subjective memory complaintsa
| Taxa (class; order; family; genus) | Binary (2+ versus < 2) | Continuous | |||||
|---|---|---|---|---|---|---|---|
| Mean relative abundance, % | ORb (95% CI) | p | FDR adjusted pc | ORb (95% CI) | p | FDR adjusted pc | |
| Bacteroidetes | |||||||
| Bacteroidia; Bacteroidales; [Odoribacteraceae]; Odoribacter (genus) | 0.28 | 1.58 (1.03–2.43) | 0.038 | 0.399 | 1.18 (1.02–1.36) | 0.025 | 0.262 |
| Firmicutes | |||||||
| Clostridia; Clostridiales; Ruminococcaceae; Faecalibacterium (genus) | 7.07 | 1.50 (1.06–2.11) | 0.023 | 0.399 | 1.12 (1.00–1.25) | 0.053 | 0.393 |
| Clostridia; Clostridiales; Lachnospiraceae; Anaerostipes (genus) | 0.18 | 1.47 (0.95–2.25) | 0.085 | 0.628 | 1.15 (1.00–1.32) | 0.049 | 0.393 |
| Clostridia; Clostridiales; Veillonellaceae; Phascolarctobacterium (genus) | 0.26 | 0.69 (0.44–1.10) | 0.094 | 0.712 | 0.80 (0.68–0.95) | 0.014 | 0.180 |
| Erysipelotrichi; Erysipelotrichales; Erysipelotrichaceae; Holdemania (genus) | 0.04 | 2.09 (1.38–3.17) | 0.0007 | 0.038 | 1.24 (1.08–1.41) | 0.002 | 0.055 |
| Proteobacteria | 4.56 | 1.27 (0.89–1.83) | 0.193 | 0.998 | 1.19 (1.06–1.34) | 0.004 | 0.033 |
| Betaproteobacteria (class) | 1.43 | 1.61 (1.10–2.35) | 0.016 | 0.135 | 1.20 (1.07–1.35) | 0.002 | 0.021 |
| Betaproteobacteria; Burkholderiales (order) | 1.43 | 1.61 (1.10–2.35) | 0.016 | 0.158 | 1.20 (1.07–1.35) | 0.002 | 0.025 |
| Betaproteobacteria; Burkholderiales; Alcaligenaceae (family) | 1.38 | 1.76 (1.20–2.59) | 0.005 | 0.082 | 1.25 (1.11–1.40) | 0.0003 | 0.006 |
| Betaproteobacteria; Burkholderiales; Alcaligenaceae; Sutterella (genus) | 1.38 | 1.76 (1.20–2.59) | 0.005 | 0.131 | 1.25 (1.11–1.40) | 0.0003 | 0.018 |
| Deltaproteobacteria (class) | 0.35 | 2.10 (1.43–3.09) | 0.0003 | 0.005 | 1.27 (1.13–1.42) | 0.0002 | 0.003 |
| Deltaproteobacteria; Desulfovibrionales (order) | 0.35 | 2.10 (1.43–3.09) | 0.0003 | 0.006 | 1.27 (1.13–1.42) | 0.0002 | 0.003 |
| Deltaproteobacteria; Desulfovibrionales; Desulfovibrionaceae (family) | 0.35 | 2.10 (1.43–3.09) | 0.0003 | 0.010 | 1.27 (1.13–1.42) | 0.0002 | 0.006 |
| Deltaproteobacteria; Desulfovibrionales; Desulfovibrionaceae; Bilophila (genus) | 0.22 | 1.59 (1.03–2.43) | 0.038 | 0.399 | 1.22 (1.07–1.40) | 0.005 | 0.090 |
Taxa associated with either binary or continuous SMCs at the nominal level were shown in the table.
Adjusting for age, BMI, and race (White versus other).
FDR = 5%.
The associations between SMCs as a continuous score and the relative abundance of bacterial taxa were consistent with those with binary SMCs (Table 2). Specifically, after correction for multiple comparisons, the ORs for Proteobacteria and its downstream Betaproteobacteria-Burkholderiales-Alcaligenaceae-Sutterella and Deltaproteobacteria-Desulfovibrionales-Desulfovibrionaceae lineage associated with one-unit increase in SMCs were 1.19–1.27 (FDR-adjusted p = 0.003–0.033). Sensitivity analysis of additional adjustment for education, and use of cholesterol lowering medication and antidepressants in the model generated similar results. For instance, after correction for multiple comparisons, the mean relative abundances of genus Holdemania and family Desulfovibrionaceae were 2.57 times (OR: 2.57, 95% CI: 1.57–4.18, FDR-adjusted p = 0.016) and 2.05 times (OR: 2.05, 95% CI: 1.32–3.18, FDR-adjusted p = 0.062) higher in women reporting 2+ SMCs compared with women with < 2 SMCs.
Figure 2 depicts the enrichment of genus Holdemania and Sutterella in women reporting 1, 2, or 3+ SMCs compared with women without any complaint. For instance, the mean relative abundance of Holdemania was 2.36 times (OR: 2.36, 95% CI: 1.41–3.96) and 1.93 times (OR: 1.93, 95% CI: 1.08–3.45) higher in women reporting 2 and 3+ SMCs compared with women reporting no complaint, respectively. In addition, the mean relative abundance of Sutterella was 2.59 times (OR: 2.59, 95% CI: 1.56–4.30) higher in women reporting 3+ SMCs compared with women with no complaint.
Fig. 2.
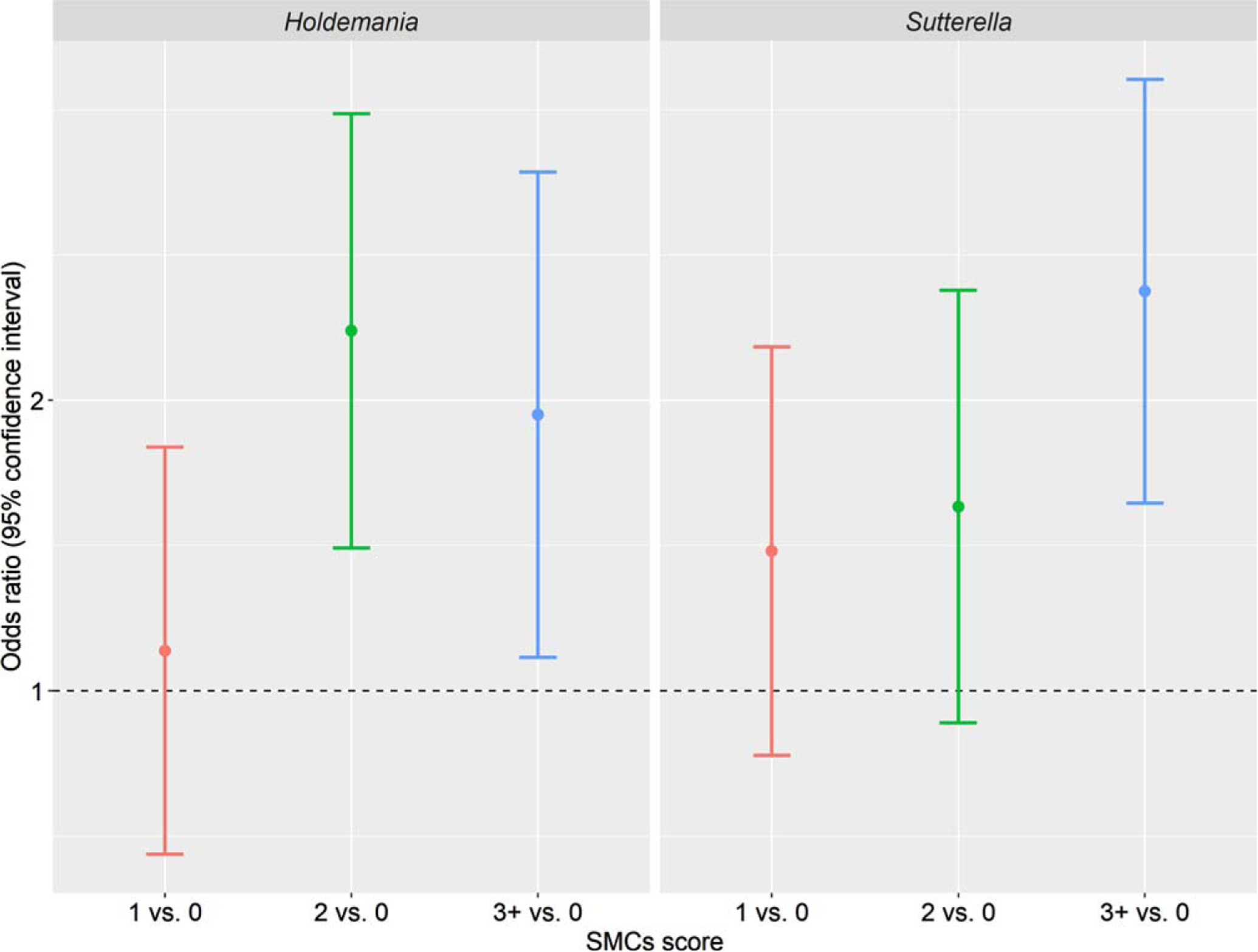
Odds ratios of mean relative abundance of Holdemania and Sutterella comparing women reporting 1, 2, or 3+ subjective memory complaints with women reporting no complaint. Beta regression was used to estimate the odds ratios, adjusting for age, BMI, and race.
Overall, the results from ANCOM-BC are consistent with those from beta regression at the nominal level, although none of the associations retained statistical significance after adjusting for multiple comparisons, possibly due to the conservative nature of the method. Specifically, ANCOM-BC also identified the genus Holdemania and the Deltaproteobacteria-Desulfovibrionales-Desulfovibrionaceae lineage as significantly enriched in women reporting 2+ SMCs compared with those reporting < 2 SMCs, with a fold-change ranging from 1.95–2.41 (p = 0.009–0.022). When SMCs were treated as a continuous variable, per-unit increase in the SMCs scores was associated with a fold-change of 1.23–1.35 in the read counts of the genus Holdemania, the Betaproteobacteria-Burkholderiales-Alcaligenaceae-Sutterella lineage, and the Deltaproteobacteria-Desulfovibrionales-Desulfovibrionaceae-Bilophila lineage (p = 0.004–0.039). Also consistent with beta regression, ANCOM-BC demonstrated that the read counts of Holdemania and Sutterella were higher among women with 2 or 3+ SMCs relative to those without any complaint (data not shown). For instance, the read counts of Sutterella were 3.13-fold (95% CI: 1.46–6.72; p = 0.003) higher in women reporting 3+ SMCs than those with no complaint, with a p for trend of 0.006.
DISCUSSION
In this study of older women from a large prospective cohort, we observed significant over-representation of the Deltaproteobacteria-Desulfovibrionales-Desulfovibrionaceae lineage within phylum Proteobacteria as well as genus Holdemania in women reporting 2 or more SMCs, compared to women reporting one or no complaint. Furthermore, increasing relative abundances of Proteobacteria and its downstream Betaproteobacteria-to-Sutterella and Deltaproteobacteria-Desulfovibrionales-Desulfovibrionaceae lineage were associated with increasing SMCs scores in a dose-dependent manner. The relative abundances of Holdemania and Sutterella were higher among women with 2 or 3+ SMCs relative to those without any complaint. These findings suggest that specific gut microbial taxa may have altered in women with SMCs.
One mechanism by which the gut bacteria may affect cognitive functions and promote neurodegeneration involves lipopolysaccharide (LPS), a pro-inflammatory endotoxin derived from the cell wall of gram-negative bacteria. Changes in gut microbiota composition can lead to increased intestinal barrier permeability, which allows for translocation of LPS to the circulation [69], impairing the blood-brain barrier and eliciting systemic inflammation [70] that promotes neuroinflammation, neuronal loss, and ultimately AD [71]. Moreover, LPS was found in amyloid plaques and around vessels in AD brain [72]. Our findings that increases in the phylum Proteobacteria and its downstream taxa were associated with SMCs may lend support to the neuroinflammation hypothesis, as Proteobacteria are LPS containing, gram-negative commensal bacteria. An increased abundance of Proteobacteria in the gut reflects an unstable structure of the gut microbial community and may serve as a biomarker for dysbiosis [73, 74]. Transgenic AD mice were characterized by an increase in Proteobacteria during aging [75]. In conventional mice re-colonized with microbiota from high-fat fed mice, proportions of Proteobacteria were negatively associated with learning and memory performance [24]. Enrichment of Proteobacteria has been reported in cognitively impaired, defined based on cognitive test performance, but neurologically healthy, community-dwelling older adults [34].
Within Proteobacteria, we found enrichment of the Deltaproteobacteria-to-Bilophila lineage in women reporting 2 or more SMCs. In mice, the abundance of family Desulfovibrionaceae was significantly higher in transgenic AD mice than in wild-type mice [76]. Bilophila was more abundant in AD individuals relative to age- and sex-matched control individuals [40]. We also found positive associations between the Betaproteobacteria-to-Sutterella lineage and SMCs. Interestingly, a cohort study of 1,551 largely female (90%) members of the TwinsUK British twin cohort (mean age 63, ranging 40–89) reported a significant association of class Betaproteobacteria and order Burkholderiales with poor cognitive function [39]. In addition, higher abundance of the family Alcaligeneceae was associated with poor performance across multiple cognitive tests in individuals with cirrhosis and hepatic encephalitis [77]. Alicaligenaceae are typically associated with opportunistic infections and known to degrade urea to ammonia, which may explain part of this association [78]. Previous studies have concluded that Sutterella species are possible pro-inflammatory agents [79]. Transgenic AD mice were characterized by an increase in Sutterella during aging [75]. Taken together, our results support the hypothesis that over-representation of the pro-inflammatory bacteria in the gut may occur in the early stage of preclinical AD.
In addition to the taxa in Proteobacteria, we also observed positive associations of genera Holdemania (phylum Firmicutes), Odoribacter (phylum Bacteroidetes), and Faecalibacterium (phylum Firmicutes) with SMCs, and an inverse association between genus Phascolarctobacterium and SMCs; however, only Holdemania retained statistical significance after adjustment for multiple comparisons. One study has reported that Holdemania was enriched in Parkinson’s disease patients [80]. In mice, the relative abundance of Holdemania was positively correlated with motor deficits [81], suggesting effects of Holdemania on neuropsychological disorders. The associations between other species and SMCs were supported by some but in contrary to other studies. For instance, genes of an Odoribacter species have been associated with the AD pathway [82], and increased relative abundance of this species was observed in transgenic AD mice [76] and in elderly AD subjects (mean > 80 years) [32]. Faecalibacterium and its metabolite butyrate, one of the gut microbiota-produced short-chain fatty acids (SCFAs), have well-documented anti-inflammatory properties [83]. A significant reduction in Faecalibacterium was reported in MCI [84] and AD subjects [32], and in subjects with subjective cognitive decline [38]. However, we observed that the relative abundance of Faecalibacterium was higher in women with SMCs. Phascolarctobacterium produces propionate, a less-studied SCFA that was reported to have equipotent anti-inflammatory effects as butyrate in vitro [85, 86]. It has been hypothesized that SCFAs may provide an alternative energy source to counteract neuronal damage [87] that contributes to neuronal dysfunction in AD [88]. SCFAs may also protect against blood-brain barrier permeability and modulate maturation and function of microglia in the brain [89]. However, a significant increase in Phascolarctobacterium was reported in MCI [35] and AD subjects [40] in other studies. Future larger studies are warranted to elucidate the role of these bacterial taxa across the stages of AD.
The present study has strengths including the use of 16S rRNA gene sequencing, the inclusion of participants from a prospective cohort study, and the use of validated questions for SMCs. Compared with previous studies, which often involved patients in the hospital, the participants in the present study did not have serious health conditions or existing AD, minimizing the impact of systemic behavioral or diet changes, or medication use due to AD/ADRD on the gut microbial composition. Also, the focus on a homogeneous population with respect to risk factors of cognitive impairment enhanced the internal validity of the study. The relatively small sample size and the lack of detailed information on diet and physical activity are limitations of the study. Stool samples were collected on average 7.6 months (93% within 12 months) after completion of the 2018 follow-up questionnaire. Cognitive impairment and SMCs are long-term conditions. Though stool samples were collected after the questionnaire, we consider the status and prevalence of SMCs remained similar during the period. Potential selection bias cannot be excluded as women who gave stool samples had a higher educational attainment than those who did not (Supplementary Table 2). To the extent that educational attainment is associated with both gut microbial composition and SMCs, it can bias the results. However, we did sensitivity analysis of additional adjustment for education in the model which generated similar results. Nevertheless, we cannot account for unmeasured factors that could potentially result in selection bias. In addition, the cross-sectional design does not allow us to assess whether the gut microbiota are associated with longitudinal changes in SMCs. Finally, lack of shotgun-sequenced metagenome data did not allow us to characterize metagenomic functions.
In summary, our findings provide supporting evidence of alterations in the gut microbial composition in association with self-perceived memory decline, characterized by an enrichment of pro-inflammatory taxa. The present study indicated feasibility of future fecal sample collection on a large scale in this aging population. Our observation that some of the SMCs-associated taxa were consistent with those found for AD/ADRD in the literature suggests a critical role of the gut microbiota in early stages of cognitive dysfunction. Future large or prospective studies with longitudinal data, objective measures of cognitive function, and shotgun metagenomic sequencing are needed to confirm the associations and evaluate the generalizability of the findings.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health grants [U01 CA182934].
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0011r1).
Footnotes
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220011.
REFERENCES
- [1].Alzheimer’s Association (2021) 2021 Alzheimer’s disease facts and figures. Alzheimers Dement 17, 327–406. [DOI] [PubMed] [Google Scholar]
- [2].Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M, Alzheimer’s Disease International (2005) Global prevalence of dementia: A Delphi consensus study. Lancet 366, 2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr., Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert MO, Holtzman DM, Kivipelto M, Lista S, Molinuevo JL, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR Jr., Proceedings of the Meeting of the International Working Group, the American Alzheimer’s Association on “The Preclinical State of AD, July, Washington DC USA (2016) Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement 12, 292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L (2014) Mild cognitive impairment: A concept in evolution. J Intern Med 275, 214–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Snitz BE, Weissfeld LA, Cohen AD, Lopez OL, Nebes RD, Aizenstein HJ, McDade E, Price JC, Mathis CA, Klunk WE (2015) Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am J Geriatr Psychiatry 23, 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA, Johnson KA, Rentz DM (2012) Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50, 2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ (2012) Subjective cognition and amyloid deposition imaging: A Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol 69, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Selnes P, Fjell AM, Gjerstad L, Bjornerud A, Wallin A, Due-Tonnessen P, Grambaite R, Stenset V, Fladby T (2012) White matter imaging changes in subjective and mild cognitive impairment. Alzheimers Dement 8, S112–121. [DOI] [PubMed] [Google Scholar]
- [10].Minett TS, Dean JL, Firbank M, English P, O’Brien JT (2005) Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. Am J Geriatr Psychiatry 13, 665–671. [DOI] [PubMed] [Google Scholar]
- [11].Peter J, Scheef L, Abdulkadir A, Boecker H, Heneka M, Wagner M, Koppara A, Kloppel S, Jessen F, Alzheimer’s Disease Neuroimaging Initiative (2014) Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimers Dement 10, 99–108. [DOI] [PubMed] [Google Scholar]
- [12].Schultz SA, Oh JM, Koscik RL, Dowling NM, Gallagher CL, Carlsson CM, Bendlin BB, LaRue A, Hermann BP, Rowley HA, Asthana S, Sager MA, Johnson SC, Okonkwo OC (2015) Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-aged adults at risk for AD. Alzheimers Dement (Amst) 1, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC (2006) Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 67, 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B (2014) Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr Scand 130, 439–451. [DOI] [PubMed] [Google Scholar]
- [15].Slot RER, Sikkes SAM, Berkhof J, Brodaty H, Buckley R, Cavedo E, Dardiotis E, Guillo-Benarous F, Hampel H, Kochan NA, Lista S, Luck T, Maruff P, Molinuevo JL, Kornhuber J, Reisberg B, Riedel-Heller SG, Risacher SL, Roehr S, Sachdev PS, Scarmeas N, Scheltens P, Shulman MB, Saykin AJ, Verfaillie SCJ, Visser PJ, Vos SJB, Wagner M, Wolfsgruber S, Jessen F; Alzheimer’s Disease Neuroimaging Initiative; DESCRIPA working group; INSIGHT-preAD study group; SCD-I working group, van der Flier WM (2019) Subjective cognitive decline and rates of incident Alzheimer’s disease and non-Alzheimer’s disease dementia. Alzheimers Dement 15, 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jonker C, Geerlings MI, Schmand B (2000) Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 15, 983–991. [DOI] [PubMed] [Google Scholar]
- [17].Sheng C, Yang K, Wang X, Li H, Li T, Lin L, Liu Y, Yang Q, Wang X, Wang X, Sun Y, Han Y (2020) Advances in non-pharmacological interventions for subjective cognitive decline: A systematic review and meta-analysis. J Alzheimers Dis 77, 903–920. [DOI] [PubMed] [Google Scholar]
- [18].Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG (2020) The gut microbiome in neurological disorders. Lancet Neurol 19, 179–194. [DOI] [PubMed] [Google Scholar]
- [19].Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O’Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG (2019) The microbiota-gut-brain axis. Physiol Rev 99, 1877–2013. [DOI] [PubMed] [Google Scholar]
- [20].Frohlich EE, Farzi A, Mayerhofer R, Reichmann F, Jacan A, Wagner B, Zinser E, Bordag N, Magnes C, Frohlich E, Kashofer K, Gorkiewicz G, Holzer P (2016) Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav Immun 56, 140–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K (2014) Gut microbes and the brain: Paradigm shift in neuroscience. J Neurosci 34, 15490–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cox LM, Weiner HL (2018) Microbiota signaling pathways that influence neurologic disease. Neurotherapeutics 15, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Martin CR, Osadchiy V, Kalani A, Mayer EA (2018) The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol 6, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard Et, Taylor CM, Welsh DA, Berthoud HR (2015) Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 77, 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].D’Amato A, Di Cesare Mannelli L, Lucarini E, Man AL, Le Gall G, Branca JJV, Ghelardini C, Amedei A, Bertelli E, Regoli M, Pacini A, Luciani G, Gallina P, Altera A, Narbad A, Gulisano M, Hoyles L, Vauzour D, Nicoletti C (2020) Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 8, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mao JH, Kim YM, Zhou YX, Hu D, Zhong C, Chang H, Brislawn CJ, Fansler S, Langley S, Wang Y, Peisl BYL, Celniker SE, Threadgill DW, Wilmes P, Orr G, Metz TO, Jansson JK, Snijders AM (2020) Genetic and metabolic links between the murine microbiome and memory. Microbiome 8, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, Musch MW, Liao F, Ward JF, Holtzman DM, Chang EB, Tanzi RE, Sisodia SS (2016) Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep 6, 30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Harach T, Marungruang N, Duthilleul N, Cheatham V, McCoy KD, Frisoni G, Neher JJ, Fak F, Jucker M, Lasser T, Bolmont T (2017) Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep 7, 41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang L, Wang Y, Xiayu X, Shi C, Chen W, Song N, Fu X, Zhou R, Xu YF, Huang L, Zhu H, Han Y, Qin C (2017) Altered gut microbiota in a mouse model of Alzheimer’s disease. J Alzheimers Dis 60, 1241–1257. [DOI] [PubMed] [Google Scholar]
- [30].Dodiya HB, Kuntz T, Shaik SM, Baufeld C, Leibowitz J, Zhang X, Gottel N, Zhang X, Butovsky O, Gilbert JA, Sisodia SS (2019) Sex-specific effects of microbiome perturbations on cerebral Abeta amyloidosis and microglia phenotypes. J Exp Med 216, 1542–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guo M, Peng J, Huang X, Xiao L, Huang F, Zuo Z (2021) Gut microbiome features of Chinese patients newly diagnosed with Alzheimer’s disease or mild cognitive impairment. J Alzheimers Dis 80, 299–310. [DOI] [PubMed] [Google Scholar]
- [32].Haran JP, Bhattarai SK, Foley SE, Dutta P, Ward DV, Bucci V, McCormick BA (2019) Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. mBio 10, e00632–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu P, Wu L, Peng G, Han Y, Tang R, Ge J, Zhang L, Jia L, Yue S, Zhou K, Li L, Luo B, Wang B (2019) Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun 80, 633–643. [DOI] [PubMed] [Google Scholar]
- [34].Manderino L, Carroll I, Azcarate-Peril MA, Rochette A, Heinberg L, Peat C, Steffen K, Mitchell J, Gunstad J (2017) Preliminary evidence for an association between the composition of the gut microbiome and cognitive function in neurologically healthy older adults. J Int Neuropsychol Soc 23, 700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nagpal R, Neth BJ, Wang S, Craft S, Yadav H (2019) Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 47, 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Saji N, Murotani K, Hisada T, Tsuduki T, Sugimoto T, Kimura A, Niida S, Toba K, Sakurai T (2019) The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: A cross-sectional study conducted in Japan. Sci Rep 9, 19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Saji N, Niida S, Murotani K, Hisada T, Tsuduki T, Sugimoto T, Kimura A, Toba K, Sakurai T (2019) Analysis of the relationship between the gut microbiome and dementia: A cross-sectional study conducted in Japan. Sci Rep 9, 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sheng C, Lin L, Lin H, Wang X, Han Y, Liu SL (2021) Altered gut microbiota in adults with subjective cognitive decline: The SILCODE Study. J Alzheimers Dis 82, 513–526. [DOI] [PubMed] [Google Scholar]
- [39].Verdi S, Jackson MA, Beaumont M, Bowyer RCE, Bell JT, Spector TD, Steves CJ (2018) An investigation into physical frailty as a link between the gut microbiome and cognitive health. Front Aging Neurosci 10, 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE (2017) Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7, 13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhuang ZQ, Shen LL, Li WW, Fu X, Zeng F, Gui L, Lu Y, Cai M, Zhu C, Tan YL, Zheng P, Li HY, Zhu J, Zhou HD, Bu XL, Wang YJ (2018) Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis 63, 1337–1346. [DOI] [PubMed] [Google Scholar]
- [42].Toniolo PG, Pasternack BS, Shore RE, Sonnenschein E, Koenig KL, Rosenberg C, Strax P, Strax S (1991) Endogenous hormones and breast cancer: A prospective cohort study. Breast Cancer Res Treat 18, S23–26. [DOI] [PubMed] [Google Scholar]
- [43].de Wit MA, Koopmans MP, Kortbeek LM, Wannet WJ, Vinje J, van Leusden F, Bartelds AI, van Duynhoven YT (2001) Sensor, a population-based cohort study on gastroenteritis in the Netherlands: Incidence and etiology. Am J Epidemiol 154, 666–674. [DOI] [PubMed] [Google Scholar]
- [44].Wheeler JG, Sethi D, Cowden JM, Wall PG, Rodrigues LC, Tompkins DS, Hudson MJ, Roderick PJ (1999) Study of infectious intestinal disease in England: Rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. BMJ 318, 1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fu BC, Randolph TW, Lim U, Monroe KR, Cheng I, Wilkens LR, Le Marchand L, Hullar MA, Lampe JW (2016) Characterization of the gut microbiome in epidemiologic studies: The multiethnic cohort experience. Ann Epidemiol 26, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sinha R, Chen J, Amir A, Vogtmann E, Shi J, Inman KS, Flores R, Sampson J, Knight R, Chia N (2016) Collecting fecal samples for microbiome analyses in epidemiology studies. Cancer Epidemiol Biomarkers Prev 25, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dominianni C, Wu J, Hayes RB, Ahn J (2014) Comparison of methods for fecal microbiome biospecimen collection. BMC Microbiol 14, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Song SJ, Amir A, Metcalf JL, Amato KR, Xu ZZ, Humphrey G, Knight R (2016) Preservation methods differ in fecal microbiome stability, affecting suitability for field studies. mSystems 1, e00021–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Go RC, Duke LW, Harrell LE, Cody H, Bassett SS, Folstein MF, Albert MS, Foster JL, Sharrow NA, Blacker D (1997) Development and validation of a Structured Telephone Interview for Dementia Assessment (STIDA): The NIMH Genetics Initiative. J Geriatr Psychiatry Neurol 10, 161–167. [DOI] [PubMed] [Google Scholar]
- [50].Donovan NJ, Amariglio RE, Zoller AS, Rudel RK, Gomez-Isla T, Blacker D, Hyman BT, Locascio JJ, Johnson KA, Sperling RA, Marshall GA, Rentz DM (2014) Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry 22, 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bhushan A, Fondell E, Ascherio A, Yuan C, Grodstein F, Willett W (2018) Adherence to Mediterranean diet and subjective cognitive function in men. Eur J Epidemiol 33, 223–234. [DOI] [PubMed] [Google Scholar]
- [52].Samieri C, Proust-Lima C, M MG, Okereke OI, Amariglio RE, Sperling RA, Rentz DM, Grodstein F (2014) Subjective cognitive concerns, episodic memory, and the APOE epsilon4 allele. Alzheimers Dement 10, 752–759 e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6, 1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108(Suppl 1), 4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Peters BA, Hayes RB, Goparaju C, Reid C, Pass HI, Ahn J (2019) The microbiome in lung cancer tissue and recurrence-free survival. Cancer Epidemiol Biomarkers Prev 28, 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodriguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS 2nd, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vazquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG (2013) Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10, 57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13, 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73, 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6, 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Price MN, Dehal PS, Arkin AP (2010) FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 5, e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R (2010) PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 26, 266–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jost L (2007) Partitioning diversity into independent alpha and beta components. Ecology 88, 2427–2439. [DOI] [PubMed] [Google Scholar]
- [64].Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R (2011) UniFrac: An effective distance metric for microbial community comparison. ISME J 5, 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ferrari S, Cribari-Neto F (2010) Beta regression for modelling rates and proportions. J Appl Stat 31, 799–815. [Google Scholar]
- [66].Nolan-Kenney R, Wu F, Hu J, Yang L, Kelly D, Li H, Jasmine F, Kibriya MG, Parvez F, Shaheen I, Sarwar G, Ahmed A, Eunus M, Islam T, Pei Z, Ahsan H, Chen Y (2020) The association between smoking and gut microbiome in Bangladesh. Nicotine Tob Res 22, 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B 57, 289–300. [Google Scholar]
- [68].Lin H, Peddada SD (2020) Analysis of compositions of microbiomes with bias correction. Nat Commun 11, 3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772. [DOI] [PubMed] [Google Scholar]
- [70].Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM (2005) Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci 25, 8843–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Megur A, Baltriukiene D, Bukelskiene V, Burokas A (2020) The microbiota-gut-brain axis and Alzheimer’s disease: Neuroinflammation is to blame? Nutrients 13, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhan X, Stamova B, Jin LW, DeCarli C, Phinney B, Sharp FR (2016) Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87, 2324–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Shin NR, Whon TW, Bae JW (2015) Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33, 496–503. [DOI] [PubMed] [Google Scholar]
- [74].Litvak Y, Byndloss MX, Tsolis RM, Baumler AJ (2017) Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr Opin Microbiol 39, 1–6. [DOI] [PubMed] [Google Scholar]
- [75].Bauerl C, Collado MC, Diaz Cuevas A, Vina J, Perez Martinez G (2018) Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett Appl Microbiol 66, 464–471. [DOI] [PubMed] [Google Scholar]
- [76].Shen L, Liu L, Ji HF (2017) Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J Alzheimers Dis 56, 385–390. [DOI] [PubMed] [Google Scholar]
- [77].Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM (2012) Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 302, G168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Obata T, Goto Y, Kunisawa J, Sato S, Sakamoto M, Setoyama H, Matsuki T, Nonaka K, Shibata N, Gohda M, Kagiyama Y, Nochi T, Yuki Y, Fukuyama Y, Mukai A, Shinzaki S, Fujihashi K, Sasakawa C, Iijima H, Goto M, Umesaki Y, Benno Y, Kiyono H (2010) Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci U S A 107, 7419–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hiippala K, Kainulainen V, Kalliomaki M, Arkkila P, Satokari R (2016) Mucosal prevalence and interactions with the epithelium indicate commensalism of Sutterella spp. Front Microbiol 7, 1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Qian Y, Yang X, Xu S, Wu C, Song Y, Qin N, Chen SD, Xiao Q (2018) Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun 70, 194–202. [DOI] [PubMed] [Google Scholar]
- [81].Jang JH, Yeom MJ, Ahn S, Oh JY, Ji S, Kim TH, Park HJ (2020) Acupuncture inhibits neuroinflammation and gut microbial dysbiosis in a mouse model of Parkinson’s disease. Brain Behav Immun 89, 641–655. [DOI] [PubMed] [Google Scholar]
- [82].(2021) KEGG. Alzheimer disease - Reference pathway - Odoribacter splanchnicus. Available at: https://www.kegg.jp/kegg-bin/show_pathway?category=Odoribacter%20splanchnicus&category_type=species&mapno=05010.
- [83].Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P (2008) Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105, 16731–16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ueda A, Shinkai S, Shiroma H, Taniguchi Y, Tsuchida S, Kariya T, Kawahara T, Kobayashi Y, Kohda N, Ushida K, Kitamura A, Yamada T (2021) Identification of Faecalibacterium prausnitzii strains for gut microbiome-based intervention in Alzheimer’s-type dementia. Cell Rep Med 2, 100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tedelind S, Westberg F, Kjerrulf M, Vidal A (2007) Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J Gastroenterol 13, 2826–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Filippone A, Lanza M, Campolo M, Casili G, Paterniti I, Cuzzocrea S, Esposito E (2020) The anti-inflammatory and antioxidant effects of sodium propionate. Int J Mol Sci 21, 3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, Rich KE, Switalski R, Mehta PD, Pratico D, Zinkowski R, Blennow K, de Leon MJ (2008) Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry 63, 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zilberter Y, Zilberter M (2017) The vicious circle of hypometabolism in neurodegenerative diseases: Ways and mechanisms of metabolic correction. J Neurosci Res 95, 2217–2235. [DOI] [PubMed] [Google Scholar]
- [89].Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermohlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


