Abstract
Background and aim:
Upper airway diseases are frequent and recognize different etiopathogenetic mechanisms, including infection, inflammation, and allergy. Therefore, topical treatments are preferable in comparison with systemic medications. Several delivery devices are available on the market, including nasal drops, syringes, sprays, nebulizers, and douches. However, it is clinically relevant to know the better way to use it.
Methods:
The present experience compared five different devices that were tested: i) a nasal dropper, ii) a standard nasal spray device, iii) a mucosal atomization device with a nozzle tip, iv) a nebulizer device, and v) a nasal douche. Saline solution with methylene blue was the marker to evaluate the intranasal distribution.
Results:
The findings showed an apparent difference in area distribution among these delivery devices.
Conclusion
The present experience showed that doctors should choose the most appropriate device for the current disease in clinical practice. (www.actabiomedica.it)
Keywords: upper airway diseases, intranasal therapy, delivery device, methylene blue, clinical practice
Introduction
Upper airway disorders are frequent medical conditions and recognize different etiopathogenetic mechanisms, including infection, inflammation, and allergy. In addition, these diseases may be of longer or shorter duration, i.e., have an acute, sub-acute, or chronic course. The most frequent conditions are rhinitis, rhinosinusitis, and rhinopharyngitis. In particular, it can be said that every individual has a pathology of this kind at least once a year. Therefore, managing upper airway disorders is a common clinical practice for any doctor, especially for otorhinolaryngologists, pediatricians, allergists, and general practitioners.
The advantage of airways is the accessibility to topical administration of treatments that allow high concentrations of the medications to be achieved, thus reducing side effects (1). However, many delivery devices are available on the market, including nasal drops, syringes, sprays, nebulizers, and douches (2). Theoretically, each device has advantages, disadvantages, and different applications. In addition, many factors affect the effectiveness of topical therapy, concerning device characteristics, patient anatomy and disease, medication composition, viscosity, thixotropy, and temperature (3). Moreover, the dimension of delivered particles is relevant. For example,> 10 µ diameter is indicated for the nose (4).
Consequently, choosing the ideal device represents a dilemma for many doctors. Literature provided some studies, but most were conducted using anatomic models (cadavers, replicas, casts) or radio nuclear imaging (5-7). Therefore, the present study was performed on one healthy volunteer (one of the authors) during a nasal endoscopy.
Materials and methods
Immediately before the endoscopic examination, a saline solution containing methylene blue was administered using one of the investigated delivery devices. Five different procedures were performed at least five days apart to complete the dye wash-out. Methylene blue 0.25% aqueous solution was used to visualize upper airways as it is commonly used (8).
Two frames were collected during endoscopy concerning the middle turbinate and ostio-meatal complex (OMC) and nasopharynx (NP) area. These clinically relevant anatomic sites serve as functional airway “control unit” and drain secretions from the anterior and posterior sinuses (9).
Five different devices were tested: i) a nasal dropper, ii) a standard nasal spray device containing mometasone furoate (Zhekort, Valeas, Milan, Italy), iii) a mucosal atomization device (MAD) with a nozzle tip, iv) a nebulizer device (RiNubes, ADL, Milan, Italy), and v) a nasal douche (Rinowash, AirLiquide MedicalSystem, Bovezzo, Italy).
As the volunteer was healthy and a co-author, the approval of an Ethics Committee was not used.
Results
Figure 1 shows the different area distributions observed after using the delivery devices.
Figure 1.
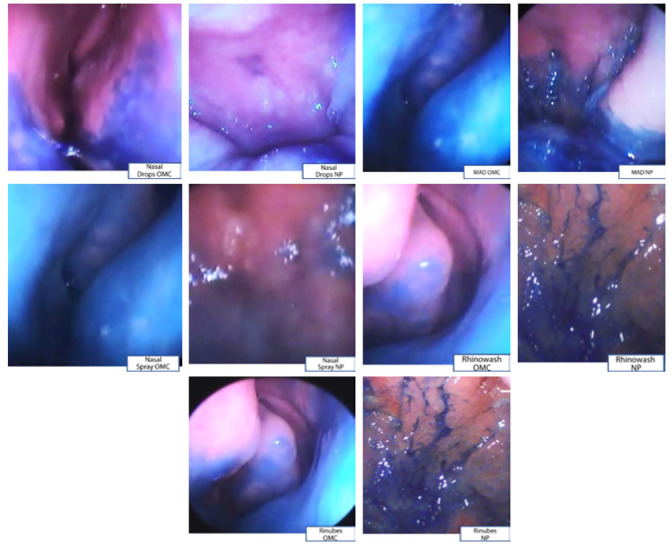
Frames of Ostio-meatal complex and (OCM) and nasopharynx (NP) for 5 nasal delivery devices: nasal drops, nasal spray, mucosal atomization device, nebulizer device, and nasal douche.
The nasal drops poorly dye both sites, and the nasal spray and mucosal atomization device dye both sites, but preferentially OMC.
The nebulizer device (RiNubes) and nasal douche (Rinowash) also stain both sites, but mostly NP.
The endoscopies were well tolerated, and no adverse reaction occurred.
Discussion
Topical medication for upper airways is generally recommended in clinical practice as it allows for an immediate treat the nose and neighboring structures using low medication dosages. Intranasal delivery can be used both to clean the airways (“nasal hygiene”) by removing thick secretions (using isotonic or hypertonic saline solutions) and to administer drugs (hyaluronic acid, corticosteroids, antihistamines, antibiotics...) to treat diseases (10). Many delivery devices are available, but there may be considerable differences between them in their ability to distribute the substance within the nasal cavities and the first airways. This aspect is crucial for choosing the most appropriate device. Although the topic is particularly practical and interests a wide range of physicians, the literature is minimal.
This is why we decided to conduct a pilot experience to compare five different delivery devices commonly used in daily clinical practice.
The findings demonstrated that the nasal devices are substantially different among them and provided various clinical indications. This information could be helpful in clinical practice since the choice of device depends on the pathology to be treated. Nasal spray and MAD allow an optimal dying of the anterior nasal cavity. This region is crucial for rhinitis (allergic, non-allergic, infectious) treatment and guarantees sinus ventilation and drainage: Rinubes and Rinowash electively color the nasopharynx. The nasopharynx collects secretions from the posterior sinus and includes the Waldeyer ring. Thus, medicating this region is relevant for rhinosinusitis, rhinopharyngitis, and adenoid disorders. However, it must be noted that these devices, but drops, can ensure a good deposition of medications to all areas.
Therefore, the delivery device choice mainly depends on the disease to be treated.
The current study had some limitations, including the limited number of participants (a co-author), the lack of patient enrollment, the assessment of two frames alone, and the lack of a measurement of dye progression time in the various areas. Therefore, there is the need of performing further studies with a robust methodology to confirm these preliminary results.
However, this study was performed in vivo, providing information reflecting what may occur in clinical practice.
In conclusion, nasal drops should be discouraged. In contrast, the nebulizer device and nasal douche are useful to medicate the nasopharynx: a crucial anatomic site as it also contains the “microbiological bank,” where bacterial biofilms grow. However, these devices still allow adequate distribution of the anterior nasal cavity. In addition, a nebulizer device is a specific device (an adapted syringe) that does not need to be connected to an aerosol machine. In contrast, nasal douche is an ampoule that requires to be connected.
Conclusions
A nasal spray and mucosal atomization device are preferable for rhinitis and rhinosinusitis as they allow perfect distribution in the anterior cavity. However, they also allow the NP to be reached. In addition, the mucosal atomization device, as well as the nebulizer device, is a modified syringe and, therefore, very easy to use anywhere.
Contribution of Authors:
The authors provided substantial contributions to the conception and design of the study, acquisition of the data, or analysis and interpretation of the data; 2) drafted the article or revised it critically for important intellectual content; and 3) gave the final approval of the version to be published.
Conflict of Interest:
The authors declare that they have no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Varricchio A, La Mantia I, Brunese FP, Ciprandi G. Inflammation, infection, and allergy of upper airways: new insights from national and real-world studies. It J Pediatr. 2020;46:18. doi: 10.1186/s13052-020-0782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger M, Ahmad N, Marple BF. The safety of intranasal steroids. Otolaryngol Head Neck Surgery. 2003;129:739–750. doi: 10.1016/j.otohns.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Moffa A, Costantino A, Rinaldi V, Sabatino L, Trecca EMC, Baptista P, et al. Nasal delivery devices: a comparative study on cadaver model. BioMed Res Int. 2019:4602651. doi: 10.1155/2019/4602651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA Guidance for Industry, Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products - Chemistry, Manufacturing, and Controls Documentation, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. 2002 [Google Scholar]
- Zhao K, Craig JR, Cohen NA. Sinus irrigations before and after surgery-visualization through computational fluid dynamics simulations. Laryngoscope. 2016;126:90–96. doi: 10.1002/lary.25666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube BL. Devices for aerosol delivery to treat sinusitis. JAerosol Med Pulm Drug Delivery. 2007;20:S5–S18. doi: 10.1089/jam.2007.0569. [DOI] [PubMed] [Google Scholar]
- Albu S. Novel drug-delivery systems for patients with chronic rhinosinusitis. Drug Design Develop Ther. 2012;6:125–132. doi: 10.2147/DDDT.S25199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil LM, Jefferson ND. Direct Visualization of Laryngeal Mucociliary Clearance in Adults. Ann Otol Rhinol Laryngol. 2019;128:1048–1053. doi: 10.1177/0003489419859376. [DOI] [PubMed] [Google Scholar]
- Varricchio A, Avvisati F, Varricchio AM, Tortoriello G, Ciprandi G. The nose and paranasal sinuses. Int J Immunopathol Pharmacol. 2010;23(1 Suppl):1–3. [PubMed] [Google Scholar]
- Ciprandi G, Gelardi M. Open and clean: the healthy nose. Acta Biomed. 2019;90(2-S):4–6. doi: 10.23750/abm.v90i2-S.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]


