Abstract
Purpose
Whether anemia type modifies the risk of pregnancy and newborn outcomes and the effectiveness of iron supplementation is unclear. We examined the association of iron deficiency anemia (IDA) and non-iron deficiency anemia (NIDA) on the risks of these outcomes and the extent to which anemia type modifies the impact of prenatal iron supplementation.
Methods
This was a secondary analysis of a placebo-controlled trial of iron supplementation among 1450 HIV-negative women in Tanzania. Eligibility criteria included gestational age < 27 weeks, hemoglobin > 85 g/L, and ferritin > 12 μg/L. Individuals were categorized as non-anemia, IDA or NIDA using hemoglobin, ferritin and CRP. Analyses were conducted using regression models and likelihood ratio tests.
Results
Compared to the non-anemia group, delivery hemoglobin was lower by 15 g/L (95% CI 10.9, 19.3) in the baseline IDA group, and 7.3 g/L (95% CI 3.1, 11.5) in the baseline NIDA group. The RRs of anemia severity, iron deficiency, placental malaria, stillbirths, perinatal mortality, birthweight, and preterm birth were not different among women in the baseline NIDA group (vs. non-anemia) compared to the baseline IDA group (vs. non-anemia). The difference in the mean delivery hemoglobin for iron supplementation and placebo arms was 8 g/L (95% CI 6, 11) in the non-anemia group, 7 g/L (95% CI 2, 13) in the NIDA group, and 16 g/L (95% CI 10, 22) in the IDA group.
Conclusion
Iron supplementation is effective even among pregnant women with NIDA.
Keywords: Iron, Anemia, Pregnancy, Iron deficiency, Inflammation
Introduction
Anemia contributes substantially to the disease burden worldwide, accounting for 59 million years lived with disability in 2019, with a much higher burden in low and middle-income countries (LMIC) [1]. Pregnant women and children are most commonly affected [2]. The prevalence of anemia among pregnant women in sub-Saharan Africa was approximately 44%, based on a recent analysis of nationally representative surveys [2]. Similar estimates have been reported in Tanzania [3]. Iron deficiency (ID) accounts for up to 37% of anemia cases among pregnant women in sub-Saharan Africa [4] and results from increased maternal and fetal iron demands for growth and metabolism that is unmatched by increased dietary iron intake and intestinal absorption [5]. The other causes of anemia are together referred to as non-iron deficiency anemia (NIDA) [6]. Anemia of inflammation (AI) is the most common cause of NIDA and contributes to up to 20% of anemia in regions where infections with HIV, malaria, and helminths are common [7, 8]. Non-AI causes of NIDA of public health significance include severe deficiencies of folate and vitamin B12 which cause megaloblastic anemia, vitamin A deficiency which reduces the incorporation of iron into red blood cells during erythropoiesis, and genetic causes such as sickle cell disease [9]. Indirect evidence suggests that approximately 27% of Tanzanian women have folate deficiency severe enough to cause megaloblastic anemia [10].
Iron deficiency anemia (IDA) is associated with increased risk of adverse outcomes for mothers and newborn, including increased risk of preterm birth and low birthweight [11]. However, the impact of NIDA on the risk of maternal and child health outcomes is unclear [12, 13]. For instance, AI is associated with increased production of pro-inflammatory cytokines and hepcidin [14, 15]. Elevated hepcidin increases iron sequestration in the reticuloendothelial system [14, 16], and reduces iron absorption in the gastrointestinal tract, which reduce bioavailability of adequate iron stores, and limits the effectiveness of iron supplementation [17]. These factors could potentially impair placental iron transfer and intrauterine growth among pregnant women, though population studies have been inconclusive [13]. Hyperhomocysteinemia among pregnant women with vitamin B12 deficiency is also associated with increased preterm birth and low birthweight risk, possibly due to impairments in maternal vascular function [18]. It is therefore imperative to understand the impact of NIDA on pregnancy outcomes as well as evaluate the extent to which NIDA may modify the effectiveness of iron supplement use, given that universal iron supplementation is the standard of antenatal care in many developing countries.
We investigated the impact of NIDA on maternal and newborn outcomes, compared to IDA, among pregnant HIV-negative women in Tanzania. The analyses aimed to assess the occurrence of anemia types among pregnant women in a malaria-endemic setting, to examine the association between anemia type and the risks of maternal and child outcomes, and determine the extent to which anemia type modifies the effect of iron supplementation on these outcomes.
Methods
We performed secondary data analyses from a randomized controlled trial (RCT) of pregnant women presenting to three antenatal clinics in Dar-es-Salaam, Tanzania. Dar-es-Salaam is Tanzania’s largest city with a population of about seven million people. Participants were eligible if they were 18–45 y old, HIV-negative, in their first or second pregnancy, presenting for antenatal care before 28 weeks gestation, iron-replete and not severely anemic (ferritin > 12 μg/L using a rapid test and hemoglobin > 85 g/L), and planning to stay in Dar-es-Salaam until delivery. HIV testing was based on two rapid assays, and discrepant results were confirmed using an enzyme-linked immunosorbent assay (ELISA). Participants were recruited between September 2010 and March 2013 and individually randomized to receive a daily oral dose of 60 mg elemental iron (200 mg of ferrous sulfate) or placebo (Tishcon Corp, New York, USA). The active and placebo tablets and packaging were indistinguishable from one another. Randomization was based on a computer-generated sequence in blocks of 20, and was created by a statistician who was not involved in the data collection. To preserve blinding, the regimen bottles were prelabeled before being issued to the study clinics. Participants were assigned the next numbered regimen bottle at randomization, and prelabeled regimen bottles with participants’ identification number at subsequent visits. The sample size of 1,500 was selected based on a desired statistical power of 80% at a 5% significance level to detect a ≥ 35% effect of iron supplementation on placental malaria at a background rate of 20%, assuming 10% loss to follow-up [19]. They were followed monthly until six weeks postpartum. In addition, participants received standard prenatal care, including 5 mg/dAY of folic acid and intermittent preventive treatment (IPT) for malaria [20]. Further details of screening, treatment assignment, and follow-up for the RCT [19] have been described elsewhere.
Study procedures
Interviewer-administered questionnaires were administered at enrollment to collect information on age, parity, date of last menstrual period, level of education completed, household assets, use of malaria prevention methods, cooking fuels used, and meat intake frequency. At enrollment, monthly pregnancy visits, delivery, and 6 weeks postpartum visits, detailed physical assessments were conducted by research physicians and nurses.
Maternal blood samples were collected at enrollment and at the time of delivery or within the first 48 h postpartum. On-call midwives documented birth outcomes. Enrollment was limited to women who planned to deliver in Dar es Salaam to prevent loss to follow-up. Participants were closely followed up, especially in the last weeks of pregnancy. Nonetheless, for women who later left the study area to be with extended family or delivered at a non-study facility, delivery blood samples could not be collected. As much as possible, birth outcome information for survival status, birthweight and gestational age (GA) at birth was later obtained by examining health records.
Enrollment and delivery blood samples were tested at the Muhimbili University Clinical Research laboratory for a complete blood count (CBC, AcT5 Diff AL, Beckman Coulter, FL, USA), serum ferritin concentration (Cobas Integra), and C-reactive protein concentration (CRP, Roche Diagnostics, Basil, Switzerland). A subset of participants with stored baseline and delivery samples was tested for soluble transferrin receptor concentration (Roche Diagnostics). Soluble transferrin receptor (sTfR)-ferritin index was estimated as a ratio of log10 of sTfR and ferritin. Participants with undetectable measurements of biomarkers were assigned the lowest detectable concentrations. Further details of the biomarkers’ testing, quality control, and precision have been previously reported [21]. Placental samples were collected at delivery, processed, and evaluated using both histopathologic analysis and polymerase chain reaction (PCR) as previously reported [19].
Case definitions and outcomes
Anemia was defined as hemoglobin < 110 g/L [22]. Inflammation was regarded as present if CRP > 5 mg/L [23]. Anemia type was defined based on the recent guidelines from the World Health Organization (WHO) [24]. Briefly, in the presence of anemia, IDA was defined as ferritin < 15 μg/L irrespective of inflammation and ferritin < 70 μg/L in the presence of inflammation. Individuals with anemia but not IDA were regarded as NIDA.
The sTfR-ferritin index was only available in a subset of participants and was used to re-classify anemia type in sensitivity analysis based on a modification of the approach proposed by Weiss and Goodnough [25]. In the presence of anemia, IDA was defined based on ferritin < 15 μg/L, or ferritin 15 – < 70 μg/L with either CRP > 5 mg/L or CRP ≤ 5 mg/L and sTfR-ferritin index ≥ 1.03[26]. AI was defined as anemia with either ferritin ≥ 70 μg/L or a combination of ferritin 15 – < 70 μg/L, CRP ≤ 5 mg/L, and sTfR-ferritin < 1.03 [26]. Individuals with missing sTfR testing (n = 114) could not be classified as either AI or IDA, and were categorized as Unknown. Using these sensitivity analyses, we directly assessed AI rather than using the broader definition of NIDA that includes AI and other causes of anemia, allowing more appropriate inference [25].
Maternal outcomes at delivery considered in the main analyses include hemoglobin concentration (continuous, in g/L), anemia (hemoglobin < 110 g/L), moderate to severe anemia (hemoglobin < 100 g/L), severe anemia (hemoglobin < 70 g/L), iron deficiency, anemia type (IDA and NIDA) and GA at delivery in weeks. In sensitivity analysis, AI was considered instead of NIDA. Placental malaria was defined as microscopic if diagnosed by histopathology, as submicroscopic if diagnosed by PCR, and as any, if diagnosed by at least one of the two methods. Child outcomes included stillbirths (baby born with no signs of life at or after 28 weeks gestation), birth weight (continuous, in grams), low birth weight (below 2500 g), small-for-gestational age (SGA, below 10th percentile for gestational age (GA), based on the INTERGROWTH standard [27]), severe SGA (below 3rd percentile for GA), preterm birth (< 37 weeks GA at delivery), very preterm birth (28–32 weeks GA at delivery), neonatal mortality (death of infant ≤ 28 days old), perinatal mortality (stillbirths and death of infant ≤ 7 days old).
Statistical analysis
The analyses of the association of anemia groups and adverse maternal and infant outcomes were restricted to participants in the placebo arm to enable the estimation of the association without the influence of assignment to supplementation. We evaluated the influence of baseline anemia groups (NIDA, IDA and non-anemia) on the risk of maternal and child outcomes from log-binomial regression models [28]. In a few instances, the models did not converge, and log-Poisson models, which provide consistent but not fully efficient estimates of the relative risk, and its confidence intervals were used [29]. Multinomial logistic regression models were used for the anemia group at delivery (non-anemia, IDA and NIDA) and RRs for the NIDA category presented, with IDA as the reference. All continuous outcomes—GA at delivery in weeks, hemoglobin concentrations at delivery, and birth weight in grams—were not normally distributed, and generalized linear regression models with robust standard errors were employed to obtain differences in the means between the groups and confidence intervals [30]. To assess whether the influence of baseline anemia vs. non-anemia is distinct from the influence of baseline anemia type (NIDA and IDA) vs. non-anemia, we compared a model with different RRs by baseline anemia type versus non-anemia with the model with RRs for anemia versus non-anemia using likelihood ratio tests. In sensitivity analyses, the analyses were repeated using AI, IDA, non-anemia and unknown anemia type groups, though the findings for the unknown anemia type are not reported.
The second set of analyses was conducted among all study participants to evaluate whether the effect of iron supplementation on maternal and child outcomes was modified by anemia type. Across baseline anemia groups, the influence of randomly assigned iron supplementation on maternal, placental, and newborn outcomes was examined in log-binomial, log-Poisson, and linear models, and the multinomial logistic regression for anemia group at delivery. Effect modification was evaluated by comparing a model with an interaction term for iron supplement use and anemia type, and comparing it to the model without the interaction term using the likelihood ratio test. Both models had main effects for iron supplement use and anemia group.
All models were adjusted for multiple covariates. These covariates were selected in the manner described by Hosmer and Lemeshow [31]. Briefly, baseline sociodemographic, nutritional, and hematologic variables that were significant at p < 0.25 in univariate models for delivery hemoglobin were considered for inclusion. Selected variables were included in the multivariate model and variables that were not significant at p < 0.05 in any of the models were excluded. Variables that caused > 20% change in the beta estimates were added back into the model along with variables that have been previously established in the literature to be important predictors (including number of household assets, meat intake, and twin gestation) [32–38]. To account for potential confounding that may vary by maternal age and GA at enrollment, interaction terms for categories of maternal age and GA at enrollment with each of the other covariates were considered for inclusion in the models based on likelihood ratio tests. Alternative functional forms (categorical and continuous) were also considered for maternal age, GA at enrollment, and BMI, and a final set of covariates were selected and applied to all analyses. Variables adjusted for include age (18–20, 21–25, and > 25 years), gravidity (1, 2), GA at enrollment (continuous, weeks), BMI at enrollment (continuous, kg/m2), number of household assets (0–1, 2–3, 4–5), meat consumption (< 90 g, ≥ 90 g per week), and twin gestation (yes, no).
Values presented in the text are medians (IQRs), means (± SD), mean (95% CI), and relative risks (95% CI). P-values were two-sided, and significance was set at < 0.05. None of the covariates was missing in > 5% of observations, 94% of participants had complete covariate data, and a complete case analysis was done. Statistical analyses were conducted in RStudio1.0.153 [39].
Sensitivity analyses
First, anemia type was re-classified using the sTfR-ferritin index as mentioned above, and the regression analyses were repeated to evaluate the influence of exposure definition as AI compared to NIDA in the primary analyses. Second, the analysis previously restricted to the placebo arm in the primary analysis was repeated in the complete dataset for comparison, further adjusting for the treatment arm the women were randomized to. Third, the primary analyses were also compared in singleton pregnancies only, i.e., excluding twin pregnancies, to exclude any possible influence of multiple gestations on the findings.
Ethics
Participants gave written informed consent at enrollment. Ethical approval for the primary trial and for secondary use of the data was obtained from the institutional review boards of the Harvard T.H. Chan School of Public Health (18,341-01) and Muhimbili University of Health and Allied Sciences (MU/DRP/AEC/Vol.XVI/144), and regulatory approval from the Tanzanian National Institute for Medical Research (NIMR), and the Tanzanian Food and Drug Administration (TFDA). The clinical trial was registered at clinicaltrials.gov (NCT01119612).
Results
Among 1,500 HIV-negative pregnant women randomized to iron supplements or placebo, 1,450 had hemoglobin, ferritin, and CRP measured at baseline and were included in the study (Fig. 1). The median age (IQR) of the women was 23 years (21, 26). They were enrolled at a median (IQR) GA of 18 weeks (15, 21) and received iron supplementation or placebo for 21 weeks (18, 25) until delivery (Table 1). The use of malaria prevention measures was predominant, especially bed-nets. The most commonly used cooking methods were charcoal (93%) and kerosene (62%).
Fig. 1.
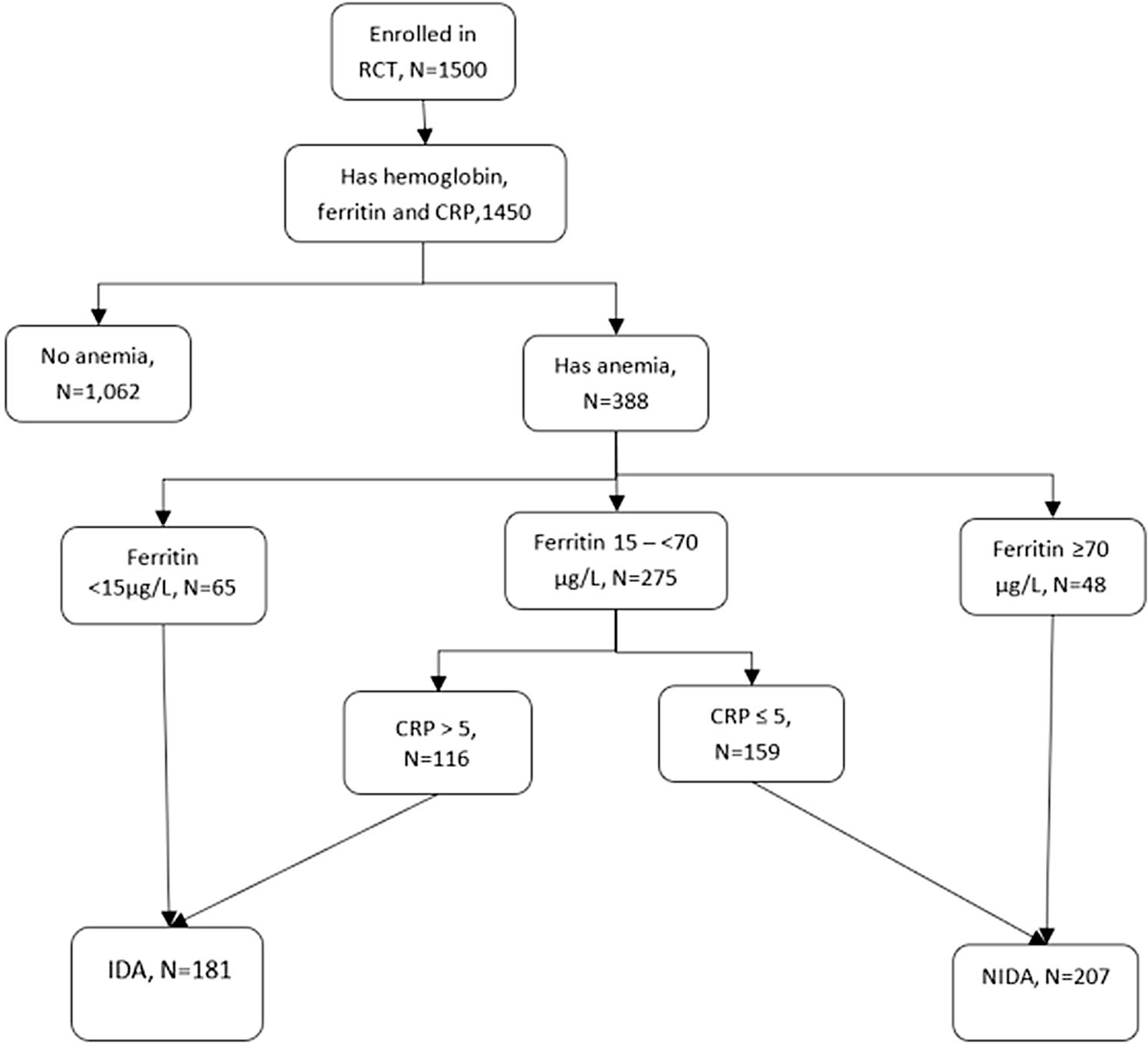
Flow chart of participant selection
Table 1.
Participant characteristics at enrollment in the trial population overall and stratified by randomized regimen (N = 1450)
| Characteristic | Total, N = 1450 n (%) | Iron, N = 726 n (%) | Placebo N = 724 n (%) |
|---|---|---|---|
|
| |||
| Age, years | |||
| Median (IQR) | 23 (21, 26) | 23 (21, 26) | 24 (21, 27) |
| 18 − < 20 y | 192 (13%) | 111 (15%) | 81 (11%) |
| 20 − < 25 y | 695 (48%) | 341 (47%) | 354 (49%) |
| 25 − ≤ 45 y | 563 (39%) | 274 (38%) | 289 (40%) |
| Gestational age, week | |||
| Median (IQR) | 18 (15, 21) | 18 (15, 21) | 18 (15, 21) |
| 4 − < 13 | 165 (12%) | 69 (10%) | 96 (13%) |
| 13 − < 20 | 745 (51%) | 378 (52%) | 367 (50%) |
| 20 − < 28 | 539 (37%) | 278 (38%) | 261 (36%) |
| District | |||
| Ilala | 920 (63%) | 459 (63%) | 461 (64%) |
| Kinondoni | 530 (37%) | 267 (18%) | 263 (18%) |
| Body mass index, kg/m2 | |||
| Median (IQR) | 23.7 (21.3, 26.8) | 23.6 (21.2, 26.8) | 23.8 (21.5, 26.8) |
| ≤ 18.5 | 67 (5%) | 31 (4%) | 36 (5%) |
| 18.5 − < 25 | 823 (57%) | 422 (59%) | 401 (56%) |
| 25 − < 30 | 393 (27%) | 181 (25%) | 212 (29%) |
| ≥ 30 | 159 (11%) | 87 (12%) | 72 (10%) |
| Meat intake, g/week | |||
| < 90 | 1039 (72%) | 520 (72%) | 519 (72%) |
| ≥ 90 | 411 (28%) | 206 (28%) | 205 (28%) |
| Season | |||
| Dry | 448 (31%) | 227 (32%) | 221 (31%) |
| Harvest | 452 (31%) | 224 (31%) | 228 (32%) |
| Long rains | 290 (20%) | 145 (20%) | 145 (20%) |
| Short rains | 260 (18%) | 130 (18%) | 130 (18%) |
| Use of malaria prevention methods | |||
| Any | 1353 (93%) | 674 (93%) | 679 (94%) |
| Bednet | 1289 (89%) | 645 (89%) | 644 (89%) |
| Insecticide-treated net | 862 (59%) | 436 (60%) | 426 (59%) |
| Fumigation | 390 (27%) | 188 (26%) | 202 (28%) |
| Mosquito coil | 132 (9%) | 67 (9%) | 65 (9%) |
| Cooking methods used regularly | |||
| Charcoal | 1354 (93%) | 680 (94%) | 674 (93%) |
| Kerosene or liquid paraffin | 893 (62%) | 448 (62%) | 445 (62%) |
| Gas | 103 (7%) | 49 (7%) | 54 (8%) |
| Electric | 44 (3%) | 24 (3%) | 20 (3%) |
| Firewood | 26 (2%) | 15 (2%) | 11 (2%) |
| Crop residue | 8 (1%) | 6 (1%) | 2 (0.3%) |
IQR interquartile range
Among all women in both arms of the study, the prevalence of anemia (hemoglobin < 110 g/L) at baseline was 27% (Table 2), and the median (IQR) hemoglobin was 116 g/L (109, 124). The prevalence of elevated CRP (> 5 mg/L) concentrations was 45% and the median (IQR) was 4.5 mg/L (2.1, 8.0). The median (IQR) of serum ferritin concentration was 30.1 μg/L (19.1, 49.1). Twelve percent of participants had serum ferritin < 15 μg/L. At baseline, IDA was present in 12% and NIDA in 14%.
Table 2.
Concentrations and levels of biomarkers at baseline
| Biomarkers | Total, N = 1450 n (%) | Iron, N = 726 n (%) | Placebo, N = 724 n (%) |
|---|---|---|---|
|
| |||
| Hemoglobin, n = 1450 | |||
| Median (IQR) | 116 (109, 124) | 116 (108, 125) | 117 (109, 124) |
| < 110 g/L | 388 (27%) | 202 (28%) | 186 (26%) |
| Ferritin, μg/L, n = 1450 | |||
| Median (IQR) | 30.1 (19.1, 49.1) | 30.6 (18.9, 47.8) | 30.0 (19.3, 51.0) |
| ≤ 15 | 173 (12%) | 86 (12%) | 87 (12%) |
| C-reactive protein, mg/L, n = 1450 | |||
| Median (IQR) | 4.5 (2.1, 8.0) | 4.7 (2.2, 8.3) | 4.3 (2.1, 7.7) |
| CRP > 5 | 659 (45%) | 348 (48%) | 311 (43%) |
| sTfR, mg/L, n = 3571 | |||
| Median (IQR) | 2.0 (1.1, 2.9) | 2.0 (1.2, 2.8) | 2.0 (1.1, 3.0) |
| > 4.4 | 25 (7%) | 11 (7%) | 14 (8%) |
| sTfR-Ferritin ratio, n = 357 | |||
| Median (IQR) | 1.3 (0.7, 2.1) | 1.4 (0.7, 2.0) | 1.3 (0.8, 2.1) |
| ≤ 1.03 | 226 (63%) | 109 (64%) | 117 (63%) |
| Anemia type, n = 14502 | |||
| Non-anemia | 1062 (73%) | 524 (72%) | 538 (74%) |
| IDA | 181 (13%) | 103 (14%) | 78 (11%) |
| NIDA | 207 (14%) | 99 (14%) | 108 (15%) |
| Anemia—alternative categories, n = 14503 | |||
| Non-anemia | 1062 (73%) | 524 (72%) | 538 (75%) |
| IDA | 207 (14%) | 115 (16%) | 92 (13%) |
| AI | 67 (5%) | 37 (5%) | 30 (4%) |
| Unknown | 114 (8%) | 50 (7%) | 64 (9%) |
AI anemia of inflammation, IDA iron deficiency anemia, IQR interquartile range, NIDA non-iron deficiency anemia
A subsample of participants was tested for sTfR
In the presence of anemia, IDA was defined as ferritin < 15 μg/L irrespective of inflammation and ferritin < 70 μg/L in the presence of inflammation. Individuals with anemia but not IDA were regarded as NIDA
In the presence of anemia, IDA was defined as either (1) ferritin < 15 μg/L, (2) ferritin 15 – < 70 μg/L and CRP > 5 mg/L, or (3) ferritin 15 – < 70 μg/L, CRP ≤ 5 mg/L and sTfR-ferritin ≥ 1.03. AI was defined as either (1) ferritin ≥ 70 μg/L, or (2) ferritin 15 – < 70 μg/L, CRP ≤ 5 mg/L and sTfR-ferritin < 1.0
In the placebo arm, the mean (95% CI) of hemoglobin was 109 g/L (107, 111) at delivery, and the prevalence of anemia, moderate anemia, and severe anemia among all the women were 49.9%, 26.7%, and 2.9%, respectively, at delivery (Table 3). The prevalence was 37.3% for IDA and 11.4% for NIDA at delivery. Among all women in the study, the mean (95% confidence interval (CI)) of hemoglobin was 113 g/L (112, 114) at delivery, and the prevalence of anemia, moderate anemia, and severe anemia among all the women in this study were 40.2%, 20.3%, and 2.7%, respectively, at delivery (Supplement 1).
Table 3.
Association of anemia group with adverse maternal hematologic and neonatal outcomes among HIV-negative pregnant women in the placebo arm of a randomized trial in Dar es Salaam, Tanzania, 2010–2013 (N = 724)
| Delivery outcome | Total, n = 724 | No anemia at baseline, n = 538 | Non-iron deficiency anemia (NIDA) at baseline, n = 108 | Iron deficiency anemia (IDA) at baseline, n = 78 | p-value for NIDA vs IDA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| n (%) or mean (95% CI) | n (%) or mean (95% CI) | Adjusted RR or MD (95% CI)2, 3, 4 | n (%) or mean (95% CI) | Adjusted RR or MD (95% CI)2, 3, 4 | p-value | n (%) or mean (95% CI) | Adjusted RR or MD (95% CI)2, 3, 4 | p-value | ||
|
| ||||||||||
| Maternal | ||||||||||
| Delivery hemoglobin, g/L, N = 483 | 109 (107, 111) | 112(110, 114) | Ref | 106 (102, 110) | −7.3 (−11.5, −3.1) | <0.001 | 96 (92, 101) | − 15.0 (−19.3, −10.9) | <0.001 | 0.005 |
| Anemia< 110 g/L | 49.9% | 42.4% | Ref | 61.3% | 1.42 (1.00, 1.99) | 0.05 | 84.6% | 1.97 (1.37, 2.79) | <0.001 | 0.13 |
| Moderate anemia < 100 g/L | 26.7% | 21.3% | Ref | 34.7% | 1.73 (1.06,2.74) | 0.02 | 51.9% | 2.50 (1.53, 4.00) | <0.001 | 0.18 |
| Severe anemia <70 g/L | 2.9% | 2.2% | Ref | 2.7% | 2.37 (0.45, 12.5) | 0.31 | 7.7% | 4.49 (1.05, 19.1) | 0.04 | 0.42 |
| Delivery ferritin, μg/L, N = 476 | 41.1 (37.4,44.7) | 42.0 (37.7, 46.3) | Ref | 39.0 (31.6,46.3) | 0.1 (−5.8, 6.0) | 0.64 | 38.2(25.0,51.5) | −3.6 (−9.4, 2.3) | 0.23 | 0.34 |
| ID at delivery | 73.2% | 74.0% | Ref | 65.8% | 0.90 (0.65, 1.22) | 0.50 | 78.8% | 1.07 (0.75, 1.50) | 0.72 | 0.44 |
| Anemia type, N = 472 | ||||||||||
| IDA, N = 176 | 37.3% | 33.2% | Ref | 38.4% | Ref | 64.0% | Ref | 0.004 | ||
| NIDA, N = 54 | 11.4% | 8.0% | Ref | 21.9% | 3.05 (1.34, 6.93) | 0.008 | 20.0% | 1.34 (0.53,3.37) | 0.54 | |
| Placental malaria, any, N = 493 | 6.5% | 6.4% | Ref | 7.6% | 1.29 (0.51, 3.28) | 0.72 | 5.5% | 0.80 (0.23, 2.80) | 0.59 | 0.49 |
| Submicroscopic | 4.7% | 4.5% | Ref | 7.6% | 1.94 (0.72, 5.23) | 0.23 | 1.8% | 0.27 (0.03, 2.23) | 0.19 | 0.04 |
| Microscopic | 2.0% | 1.9% | Ref | 1.3% | 0.74 (0.08, 6.63) | 0.82 | 3.6% | 3.23 (0.50, 21.0) | 0.22 | 0.24 |
| Newborn | ||||||||||
| Stillbirths, N = 702 | 4.7% | 3.8% | Ref | 3.9% | 1.34 (0.37, 3.78) | 0.61 | 10.3% | 3.10(1.16, 7.67) | 0.02 | 0.19 |
| Perinatal mortality, N = 702 | 6.3% | 5.4% | Ref | 5.8% | 1.23 (0.45, 2.91) | 0.66 | 12.8% | 2.61 (1.14, 5.61) | 0.02 | 0.17 |
| Neonatal mortality, N = 669 | 2.2% | 2.2% | Ref | 1.9% | 0.86 (0.18, 4.08) | 0.85 | 2.9% | 1.25 (0.26, 6.05) | 0.78 | 0.74 |
| Low birth weight, N = 636 | 11.8% | 12.4% | Ref | 10.6% | 0.73 (0.34, 1.39) | 0.36 | 8.7% | 0.64 (0.24, 1.43) | 0.32 | 0.79 |
| SGA, N = 635 | 16.4% | 18.8% | Ref | 8.5% | 0.40(0.18,0.79) | 0.02 | 10.1% | 0.54 (0.22, 1.11) | 0.13 | 0.62 |
| Birth weight, g, N = 636 | 3130 (3089, 3172) | 3133 (3084, 3183) | Ref | 3090 (2987, 3194) | 36 (−60, 132) | 0.46 | 3163 (3047, 3280) | 79 (−38, 195) | 0.18 | 0.57 |
| GA at delivery, weeks, N = 702 | 39.2 (39.0, 39.5) | 39.4 (39.1,39.5) | Ref | 38.5 (37.8, 39.1) | −0.7 (−1.4, −0.1) | 0.02 | 39.0(38.2, 39.8) | − 0.3 (−1.0, 0.4) | 0.43 | 0.20 |
| Preterm | 18.1% | 16.7% | Ref | 23.3% | 1.54 (0.94,2.45) | 0.07 | 20.5% | 1.40 (0.77, 2.42) | 0.24 | 0.78 |
| Very preterm | 2.7% | 1.5% | Ref | 4.9% | 3.08 (0.87, 10.1) | 0.07 | 7.7% | 4.42 (1.27, 14.2) | 0.01 | 0.62 |
Values in the column are the number of outcome events/number of observations (n/N) or mean (95% CI)
Values in the column are relative risks (RR) or mean difference (MD), with 95% confidence intervals
Log-Poisson regression models estimated to obtain the RR of critical pregnancy outcomes. RR > 1 implies the pregnancy outcome is more likely to occur among those who meet the definition of anemia of inflammation at baseline. RR < 1 implies the pregnancy outcome is less likely. Linear regression models estimated mean differences, reported with robust confidence intervals
Multivariable estimates were adjusted for age (18–20, 21–25 and > 25 years), and parity (0, 1), gestational age at enrollment (continuous, weeks), BMI (continuous, kg/m2), meat consumption (< 90 g, ≥ 90 g per week), number of household assets (0–5, 6–8, 9–10),and infant sex (male, female)
Anemia group at baseline was associated with the mean hemoglobin concentration at delivery (Table 3). Compared to those with no anemia at baseline, the mean hemoglobin concentration at delivery was lower by 7.3 g/L (95% CI 3.1–11.5) in those with NIDA and by 15.0 g/L (95% CI 10.9–19.3) in those with IDA, adjusted for covariates. The difference in the mean delivery hemoglobin was greater in the baseline IDA group than the baseline NIDA group (p-value = 0.005).
Anemia group at baseline was also associated with the presence and severity of maternal anemia at delivery among women in the placebo arm of the trial. Compared to those with no anemia at baseline, the adjusted risk of maternal anemia at delivery was 1.42-fold (95% CI 1.00–1.99) higher in those with NIDA at baseline and 1.97-fold higher in those with baseline IDA (95% CI 1.37–2.79). For moderate anemia at delivery, the risk was 1.73-fold (95% CI 1.06–2.74) higher in those with NIDA at baseline and 2.50-fold higher in those with IDA (95% CI 1.53–4.00), when compared to those with no anemia at baseline. IDA was associated with a 4.49-fold (95% CI 1.05, 19.1) higher risk of severe anemia at delivery while there was no association for NIDA (RR = 2.37; 95% CI 0.45–12.5). The adjusted RRs for anemia (any, moderate or severe) were not different in baseline IDA and NIDA groups.
Among women in the placebo arm, the mean (95% CI) of serum ferritin was 41.1 μg/L (37.4, 44.7) at delivery (Table 3). The prevalences of ID, IDA, and NIDA were 73.2%, 37.3%, and 11.4% at delivery, respectively. Maternal anemia group at baseline was also not associated with delivery ferritin concentration or with the presence of iron deficiency at delivery. Among women in the placebo arm, the risk ratio of maternal NIDA at delivery was different from the risk ratio of maternal IDA at delivery, both compared to the non-anemia group (p-value = 0.004). Compared to the baseline non-anemia group, pregnant women in the baseline NIDA group were 3.05-fold (95% CI 1.34, 6.93) more likely to have NIDA at delivery than they were to have IDA at delivery. Compared to the baseline non-anemia group, pregnant women in the baseline IDA group were 1.34-fold (95% CI 0.53, 3.37) more likely to have NIDA at delivery than they were to have IDA at delivery.
In the placebo arm, the incidence of submicroscopic placental malaria was 4.7% for submicroscopic placental malaria and 2.0% for microscopic placental malaria, and 6.5% for any placental malaria (Table 3). The RRs for submicroscopic and microscopic placental malaria were not different for baseline NIDA and IDA groups compared to baseline non-anemia.
The incidence of stillbirths, perinatal, and neonatal mortality was 4.7%, 6.3%, and 2.2% among women in the placebo arm. The incidence of low birth weight, preterm, and SGA was 11.8%, 18.1%, and 16.1%, respectively, and the mean (95% CI) birth weight was 3,130 g (3,089, 3,172) among women in the placebo arm. The mean (95% CI) GA at delivery was 39 wk (39, 40). Compared to the maternal non-anemia group, maternal IDA was associated with a 3.10-fold (95% CI 1.16, 7.67) higher risk of stillbirth and 2.61-fold (95% CI 1.15, 5.61) higher risk of perinatal mortality among pregnant women in the placebo arm. The duration of gestation was also shorter by 0.7 wk (95% CI 0.1, 1.4) among those with NIDA at baseline. Compared to those with no anemia, the risk of very preterm birth was higher by 3.08-fold (95% CI 0.87, 10.1) among individuals with NIDA and 4.42-fold (95% CI 1.27, 14.2) among individuals with IDA. The RR for newborn outcomes did not differ between baseline NIDA and IDA groups.
In subgroup analyses, the extent to which the effect of iron supplementation on the risk of maternal and newborn outcomes varies among the baseline anemia groups was explored (Table 4). The effect of iron supplementation on delivery hemoglobin differed among baseline anemia groups (p value < 0.001), being highest in the baseline IDA group. Delivery hemoglobin was higher in the iron-supplemented group (vs. placebo) by 16 g/L (95% CI 10, 22) in the baseline IDA group, but only 8 g/L (95% CI 6, 11) in the baseline non-anemia group and 7 g/L (95% CI 2, 13) in the baseline NIDA group. Iron supplementation reduced the risk of delivery anemia, iron deficiency, and IDA, across all categories, to varying extents, but the effect measures did not differ between women without anemia, women with IDA and women with NIDA at enrollment. The effect of iron supplementation on newborn outcomes did not differ across anemia groups either.
Table 4.
Effect modification of the efficacy of iron supplementation on the risk of adverse maternal and neonatal outcomes by anemia type among HIV-negative pregnant women in Dar es Salaam, Tanzania, 2010–2013 (N = 1450)
| Outcome | Non-anemia, N = 1062 | Non-iron deficiency anemia (NIDA) at baseline, N = 181 | Iron deficiency anemia at baseline, N = 207 | p value for interaction, any | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Iron, n (%) or mean (95% CI)1 | Placebo, n (%) or mean (95% CI)1 | Adjusted RR or MD (95% CI)2,3,4 | Iron, n (%) or mean (95% CI)1 | Placebo, n (%) or mean (95% CI)1 | Adjusted RR or MD (95% CI)2,3,4 | Iron, n (%) or mean (95% CI)1 | Placebo, n (%) or mean (95% CI)1 | Adjusted RR or MD (95% CI)2,3,4 | ||
|
| ||||||||||
| Maternal, at delivery | ||||||||||
| Hemoglobin, g/L, N = 950 | 120(117, 122) | 112(110, 114) | 8 (6, 11) | 112(107, 118) | 106 (102, 110) | 7 (2, 13) | 112(107, 117) | 96 (92, 101) | 16 (10, 22) | <0.0001 |
| Anemia< 110 g/L | 26.0% | 42.4% | 0.59 (0.45, 0.77) | 41.0% | 61.3% | 0.69 (0.41, 1.17) | 40.3% | 84.6% | 0.50 (0.30, 0.80) | 0.67 |
| Moderate anemia < 100 g/L | 10.8% | 21.3% | 0.48 (0.31, 0.72) | 23.0% | 34.7% | 0.57 (0.28, 1.15) | 19.4% | 51.9% | 0.41 (0.20, 0.78) | 0.52 |
| Severe anemia <70 g/L | 1.8% | 2.2% | 0.66 (0.21, 2.06) | 3.3% | 2.7% | 0.66 (0.07, 5.93) | 5.6% | 7.7% | 0.75 (0.17, 3.41) | 0.99 |
| Delivery ferritin, μg/L, N = 926 | 67.2 (61.4, 73.0) | 42.0 (37.7, 46.3) | 25.1 (18.0, 32.2) | 69.0 (55.1, 82.8) | 39.0 (31.6, 46.3) | 22.0 (2.8, 41.3) | 63.2 (50.6, 75.8) | 38.2 (25.0, 51.5) | 28.8 (13.2, 44.4) | 0.84 |
| ID at delivery | 51.3% | 74.0% | 0.70 (0.57, 0.85) | 45.2% | 65.8% | 0.68 (0.41, 1.12) | 55.9% | 78.8% | 0.77 (0.49, 1.23) | 0.97 |
| Anemia type, RR, N = 922 | ||||||||||
| Non-anemia | 76.7% | 58.7% | Ref | 60.0% | 39.7% | Ref | 63.2% | 16.0% | Ref | |
| IDA | 14.6% | 33.2% | 0.30 (0.20, 0.45) | 20.0% | 38.4% | 0.33 (0.13, 0.85) | 23.5% | 64.0% | 0.11 (0.04, 0.30) | 0.10 |
| NIDA | 8.7% | 8.0% | 0.85 (0.48, 1.53) | 20.0% | 21.9% | 0.64 (0.21, 1.96) | 13.2% | 20.0% | 0.14 (0.04, 0.55) | |
| Placental malaria, N = 971 | ||||||||||
| Any | 6.2% | 5.5% | 0.90 (0.49, 1.65) | 10.9% | 7.6% | 1.11 (0.36, 3.45) | 6.8% | 5.5% | 1.36 (0.30, 7.26) | 0.67 |
| Submicroscopic | 5.0% | 1.8% | 1.02 (0.50, 2.06) | 9.4% | 7.6% | 0.95 (0.29, 3.10) | 4.1% | 1.8% | Not est | 0.64 |
| Microscopic | 1.8% | 1.9% | 0.76 (0.23, 2.47) | 3.1% | 1.3% | 1.71 (0.11, 27.2) | 4.1% | 3.6% | 1.11 (0.16, 7.72) | 0.69 |
| Newborn | ||||||||||
| Stillbirths, N = 1386 | 3.7% | 3.8% | 0.86 (0.42, 1.74) | 5.1% | 3.9% | 1.23 (0.30, 4.99) | 3.0% | 10.4% | 0.33 (0.08, 1.40) | 0.30 |
| Perinatal mortality, N = 1385 | 6.4% | 5.4% | 1.08 (0.63, 1.88) | 8.2% | 5.8% | 1.69 (0.55, 5.55) | 4.0% | 13.0% | 0.34 (0.09, 1.12) | 0.12 |
| Neonatal mortality, N = 1326 | 3.0% | 2.2% | 1.37 (0.62, 3.00) | 3.2% | 2.0% | 1.68 (0.28, 10.1) | 1.0% | 2.9% | 0.37 (0.03, 4.11) | 0.56 |
| Low birth weight, N = 1261 | 12.0% | 12.4% | 0.98 (0.68, 1.42) | 11.6% | 10.6% | 1.85 (0.70, 5.49) | 16.5% | 8.8% | 1.11 (0.46, 2.67) | 0.51 |
| SGA, N = 1258 | 16.8% | 18.8% | 0.86 (0.63, 1.18) | 19.8% | 8.5% | 2.62 (1.08, 6.38) | 17% | 10.3% | 1.49 (0.58, 3.82) | 0.05 |
| Birth weight, g, N = 1303 | 3159 (3106, 3211) | 3134 (3085, 3183) | 20 (- 43, 84) | 3073 (2968, 3179) | 3090 (2987, 3194) | - 79 (- 216, 59) | 3124(3014, 3236) | 3163 (3047, 3280) | - 104 (- 258, 51) | 0.43 |
| GA at delivery, week, N = 1432 | 39.6 (39.3, 40.0) | 39.4 (39.1, 39.7) | 0.2 (- 0.2, 0.5) | 39.6 (39.0, 40.1) | 38.5 (37.8, 39.1) | 0.8 (- 0.1, 1.6) | 39.2(38.6, 39.7) | 39.0 (38.2, 39.8) | 0.1 (- 0.9, 1.0) | 0.01 |
| Preterm | 15.6% | 16.7% | 0.91 (0.66, 1.24) | 15.3% | 23.3% | 0.67 (0.33, 1.32) | 18.0% | 20.8% | 0.78 (0.38, 1.61) | 0.69 |
| Very preterm | 1.4% | 1.5% | 0.88 (0.30, 2.56) | 2.0% | 4.9% | 0.41 (0.07, 2.48) | 2.0% | 7.8% | 0.60 (0.06, 5.75) | 0.63 |
Values in the column are the number of outcome events/number of observations (n/N) or mean (95% CI)
Values in the column are relative risks (RR) or mean difference (MD), with 95% confidence intervals
Log-Poisson regression models were estimated to obtain the RR of critical pregnancy outcomes. RR > 1 implies the pregnancy outcome is more likely to occur among those who meet the definition of anemia of inflammation at baseline. RR < 1 implies the pregnancy outcome is less likely. Linear regression models estimated mean differences reported with robust confidence intervals
Multivariable estimates were adjusted for age (18–20, 21–25 and > 25 years), parity (0, 1), gestational age at enrollment (continuous, weeks), BMI (continuous, kg/m2), meat consumption (< 90 g, ≥ 90 g per week), number of household assets (0–5, 6–8, 9–10),and infant sex (male, female)
In further analyses (Supplement 1), we repeated the models in Tables 3 and 4 in the full cohort including pregnant women randomized to placebo and iron supplementation and there were no qualitative differences in the findings. There were also no meaningful differences in the findings when restricting the analysis to singleton pregnancies (Supplement 2).
Among 207 individuals with NIDA at baseline, 114 did not have sTfR-ferritin and could not be further re-classified as AI or IDA. Of the remaining 93 participants, 67 (72%) had AI. We re-considered maternal anemia groups with AI instead of NIDA, and the classification scheme is presented in Supplement 3. In models based on this classification (Supplement 4), AI was associated with an increased risk of severe anemia at delivery (RR = 4.49, 95% CI 1.08–18.7) in the placebo arm. The RRs for stillbirths were different in baseline AI and IDA groups (p-value = 0.003), with IDA being associated with a 2.22-fold (95% CI 1.37, 8.08) increased risk of stillbirths while AI was not associated with an increased risk of stillbirths. The remaining findings were similar as in Table 3, based on NIDA. The effect of iron supplementation on the risk of maternal and child outcomes did not differ among baseline anemia groups based on this classification (Supplement 5). Given that there were no meaningful differences between the findings in Tables 3 and 4 using data from the placebo arm alone and Supplement 1 using data from both arms, we repeated the analysis in Supplement 4 using both arms. We found that baseline AI was associated with increased risk of placental malaria (RR = 2.67; 95% CI 1.28, 5.56) and baseline IDA was associated with increased stillbirths (RR = 2.10; 95% CI 1.05, 4.24), compared to the baseline non-anemia group (Supplement 6). The other results were similar.
Discussion
We conducted a post hoc analysis to evaluate the association of anemia types with the risk of important maternal and perinatal outcomes among iron-replete pregnant women in Tanzania, using data from a recently completed randomized trial. We found relationships with the risk of maternal hematologic and infant outcomes at delivery. Both maternal IDA and NIDA at baseline were associated with maternal anemia at delivery, with NIDA at baseline being additionally associated with NIDA at delivery. In addition, IDA was associated with stillbirths, perinatal mortality and very preterm births. We also examined the interaction of maternal anemia type with iron supplementation and found that the efficacy of iron supplementation to prevent and treat maternal anemia does not depend on anemia type.
Characterizing NIDA as distinct from the more commonly studied IDA and understanding its possible impact on maternal and child health would be critical if its burden is substantial and if IDA and NIDA impact maternal and child health differently. ID is the most common cause of anemia among pregnant women globally, though its contribution to the anemia prevalence is substantially lower in populations with high burden of infections such as malaria, HIV and tuberculosis [40]. Anemia of inflammation accounts for a significant proportion of NIDA in malaria-endemic settings [6], and AI is the second most common cause of anemia globally [41]. Among participants whose anemia type could be characterized using ferritin, CRP and sTfR-ferritin ratio in our study, approximately 72% of those with NIDA had AI. AI is a composite of mild or moderate normochromic, normocytic anemia following defective iron metabolism and impaired erythrocyte production and survival caused by inflammation [41]. AI among pregnant Tanzanian women living in urban and peri-urban settlements could be due to chronic or repeated subclinical malarial inflammation, untreated helminth infections, or environmental exposures [42]. AI distorts the normal biological regulation of iron metabolism and erythrocyte production, predisposing anemia risk [16]. Non-AI causes of NIDA in this setting include deficiencies of folate and vitamin B12, disorders of hemoglobin structure such as sickle cell anemia, and numerous other less common etiologies. In this cohort of pregnant women in a malaria-endemic country selected to increase the likelihood that they were iron-replete, the prevalence of NIDA and IDA were 14% and 13%, respectively. In the absence of a standard definition for AI and due to limitations imposed by available data, we evaluated NIDA as a proxy for AI. Among those in whom we had biomarker measures with which to fully characterize AI, we found no substantial differences in the risk of maternal and infant outcomes, as well as the effect of iron supplementation to prevent adverse maternal and infant outcomes.
Notably, both NIDA and IDA at baseline were associated with an increased risk of maternal hematologic outcomes at delivery. Specifically, both NIDA and IDA were associated with an increased risk of delivery anemia. In addition, NIDA at baseline was associated with approximately three-fold increased risk of NIDA at delivery, while those with IDA at baseline had an elevated risk of anemia at delivery, irrespective of type. These current findings replicate and extend previous findings that baseline anemia, in the context of iron deficiency, may predict future anemia risk [21]. This is likely due to continued exposure to the same environmental circumstances such as poor nutrition, repeated malaria, or untreated helminth infection.
We hypothesized that both NIDA and IDA would increase the risk of newborn outcomes, especially stillbirth, intrauterine growth restriction (IUGR), and preterm birth. Both AI and IDA potentially reduce the placental transfer of iron, essential for fetal metabolism and growth [43]. In AI, elevated hepcidin binds to ferroportin on the basal surface of the placental syncytiotrophoblast, degrading it and dampening the transfer of placental iron stores to the fetus [44, 45]. Although the fetal iron transfer is prioritized over maternal iron requirements when maternal iron status is poor [46], women with IDA have depleted iron stores that are simply inadequate for adequate levels to be transferred to the fetus [12]. Second, inflammation in the context of AI could lead to adverse perinatal outcomes. Circulating levels of pro-inflammatory cytokines (such as interleukin-1β (IL-1β), tumor necrosis factor (TNF)) increase in the presence of malaria infection, and a pro-inflammatory cytokine pattern in the placenta is associated with an increased risk of fetal growth restriction and preterm birth [47, 48]. Other notable causes of NIDA such as the deficiencies of folate and vitamin B12 and sickle cell disease are also associated with fetal growth restriction, preterm birth, stillbirths and perinatal mortality [49, 50]. We found that NIDA and IDA were associated with approximately 3.1- and 4.4-fold increased risks of very preterm birth, respectively. We also found that IDA is associated with a 3.1- and 2.6-fold increased risk of stillbirth and perinatal mortality, respectively. While iron supplementation is the recommended strategy to prevent and treat IDA, AI and the other types of NIDA are treated by addressing the underlying etiology [51].
Iron supplementation is well known to improve hemoglobin concentration and prevent adverse maternal and newborn outcomes [21, 52]. These effects of iron supplementation are mediated by preventing maternal iron deficiency and increasing the pool of iron available for transfer to the fetus [53]. While iron supplementation is highly effective among pregnant women with IDA, the effect in pregnant women with NIDA appears dampened. For instance, iron supplementation reduced the risk of delivery anemia by 49% in the baseline IDA group but only by 31% in the baseline NIDA group. There is a plausible mechanistic basis to support the presence of differential effects. Humans obtain iron for cellular function from two sources—dietary iron intake and recycling of red cells. Systemic inflammation in the context of AI slows down gastrointestinal absorption of dietary iron by preventing iron export from intestinal enterocytes to the plasma and thereby limits the effectiveness of supplementation [51]. Among women with baseline IDA, iron supplementation was unexpectedly associated with fewer delivery NIDA cases, though the confidence interval was wide, and the estimate was considerably impacted by adjusting for the total number of household assets as a measure of socioeconomic status. We found that iron supplementation improved delivery hemoglobin and prevented delivery IDA across all baseline anemia groups without leading to worse outcomes in any subgroup—even pregnant women with NIDA at baseline appeared to benefit from iron supplementation. Universal iron supplementation of pregnant women may therefore be imperative to prevent delivery IDA and its untoward effects.
Our analysis has several strengths and limitations. We carefully evaluated baseline anemia groups based on NIDA, and compared our findings with AI among participants with additional biomarker data. We also explored the analyses in different populations—placebo arm alone, in iron and placebo arms, and in singleton pregnancies alone, and our findings were consistent. The primary trial screened out individuals who had serum ferritin < 12 μg/L and hemoglobin < 85 g/L; for ethical reasons these women were provided with iron per standard of care. The IDA group in this study is therefore not representative of the general population of pregnant women with IDA since those with the most severe IDA were more likely to have been excluded. Our results may, therefore, underestimate the association of IDA with adverse maternal and child outcomes. We modified a decision tree proposed by Weiss and Goodnough, using newly published WHO guidelines for the use of ferritin to diagnose IDA [24]. Our study may, therefore, be limited by misclassification of anemia categories, as evidenced by the slight changes in the results when the sTfR-ferritin ratio was used to further classify anemia categories partially. In this cohort, sTfR was not measured in a random sample. We considered potential confounding by demographic and clinical variables in detail and adjusted for important variables.
We explored whether the efficacy of iron supplementation varied across baseline anemia groups. Subgroup analyses such as these are often plagued by limited magnitude of effect, multiple testing, inconsistent effects and inadequate evidence from other settings to support them [54, 55]. It is also not always clear whether the findings of subgroup analyses are meaningful for clinical practice or policy [55]. We were unable to collect delivery samples in 10% of participants because they relocated for cultural reasons prior to the time of birth. The propensity to relocate was not likely related to the baseline anemia group, iron supplementation arm, or any of the newborn outcomes, and this missingness in the outcomes is unlikely to have biased our results. Studies among pregnant women and children suggest that high dose folate supplementation, such as in our study, could increase the risk of failure of IPT [56]. Most participants in our study used additional malaria prevention techniques such as insecticide treated bednets, and any failure of IPT is unlikely to have impacted our findings.
Our analysis was also limited by the lack of a standard definition for anemia of inflammation. We did not establish the sources of inflammation, though malaria, helminthiasis, and indoor air pollution are likely. Stratified analyses in a larger dataset examining these relationships in 1st versus 2nd trimesters across nutritional status and diet quality categories may clarify some of our findings. Importantly, future studies may also evaluate the degree to which comprehensive screen-and-treat programs for infections among pregnant women may modify the relationships we observed.
Conclusion
We examined the influence of NIDA during pregnancy in comparison to IDA and non-anemia on the risk of maternal and newborn outcomes among pregnant women without severe IDA and found that maternal anemia at baseline across both groups is associated with increased risk of anemia at delivery. We also found that iron supplementation led to substantial improvements in maternal hematologic status and newborn outcomes, and the magnitude of the effect varied across the groups for the delivery hemoglobin outcome. Iron supplementation prevented IDA across the baseline groups, without causing adverse consequences in the baseline NIDA group. Therefore, targeting iron supplementation to exclude pregnant women with NIDA may not be necessary.
Supplementary Material
Funding
This study was supported by a grant from the National Institute of Child Health and Human Development (NICHD U01 HD061232).CD was supported in part by K24DK104676 and 2P30 DK040561. The NIH did not have any role in the design of the study, data collection, data analysis, data interpretation, or writing of this report.
Abbreviations
- ACD
Anemia of chronic disease
- AI
Anemia of inflammation
- AIDS
Acquired Immune Deficiency Syndrome
- ART
Antiretroviral therapy
- BMI
Body Mass Index
- CBC
Complete blood count
- CI
Confidence Interval
- CKD
Chronic kidney disease
- CRP
C-reactive protein
- ELISA
Enzyme Linked Immunosorbent Assay
- HAART
Highly Active Antiretroviral Therapy
- HAND
HIV-associated neurocognitive disorder
- HIV
Human Immunodeficiency Virus
- HR
Hazard ratio
- ID
INTERGROWTH: International Fetal and Newborn Growth Consortium for the 21st Century
Iron deficiency
- IDA
Iron deficiency anemia
- IQR
Interquartile range
- IRA
Iron-restricted anemia
- LMIC
Low and Middle Income Countries
- MRC
Medical Research Council
- NIDA
Non-Iron Deficiency anemia
- NTBI
Non-transferrin bound iron
- OR
Odds ratio
- PCR
Polymerase chain reaction
- PLWHIV
People living with HIV
- RCT
Randomized controlled trial
- RR
Relative risk
- SD
Standard deviation
- SE
Standard error
- SGA
Small-for-gestational age
- sTfR
Soluble Transferrin receptor
- TB
Tuberculosis
- TIBC
Total Iron Binding Capacity
- ZPP
Zinc protoporphyrin
Footnotes
Declarations
Conflict of interest None of the authors have any conflict of interest.
Trial registration NCT01119612 (May 7, 2010).
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00394-022-03029-0.
References
- 1.Gardner W, Kassebaum N (2020) Global, regional, and national prevalence of anemia and its causes in 204 countries and territories, 1990–2019. Curr Develop Nutr 4(2):830–830 [Google Scholar]
- 2.Weze K, Abioye AI, Obiajunwa C, Omotayo M (2021) Spatio-temporal trends in anemia among pregnant women, adolescents and preschool children in sub-Saharan Africa. Public Health Nutrition 24(12):3648–3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Msuya SE, Hussein TH, Uriyo J, Sam NE, Stray-Pedersen B (2011) Anaemia among pregnant women in northern Tanzania: prevalence, risk factors and effect on perinatal outcomes. Tanzan J Health Res 13(1):33–39 [DOI] [PubMed] [Google Scholar]
- 4.Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, Moursi M, Donahue Angel M, Rohner F (2016) The Proportion of anemia associted with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients. 10.3390/nu8110693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw JG (2011) Friedman JF Iron deficiency anemia: focus on infectious diseases in lesser developed countries. Anemia 260380:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leenstra T, Acosta LP, Langdon GC, Manalo DL, Su L, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF (2006) Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte. Philippines Am J Clin Nutr 83(2):371–379 [DOI] [PubMed] [Google Scholar]
- 7.Leenstra T, Coutinho HM, Acosta LP, Langdon GC, Su L, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF (2006) Schistosoma japonicum reinfection after praziquantel treatment causes anemia associated with inflammation. Infect Immun 74(11):6398–6407. 10.1128/IAI.00757-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Broek NR, Letsky EA (2000) Etiology of anemia in pregnancy in south Malawi. Am J Clin Nutr 72(1 Suppl):247S–256S [DOI] [PubMed] [Google Scholar]
- 9.Bourassa MW, Osendarp SJM, Adu-Afarwuah S, Ahmed S, Ajello C, Bergeron G, Black R, Christian P, Cousens S, de Pee S, Dewey KG, Arifeen SE, Engle-Stone R, Fleet A, Gernand AD, Hoddinott J, Klemm R, Kraemer K, Kupka R, McLean E, Moore SE, Neufeld LM, Persson L-Å, Rasmussen KM, Shankar AH, Smith E, Sudfeld CR, Udomkesmalee E, Vosti SA (2019) Review of the evidence regarding the use of antenatal multiple micronutrient supplementation in low- and middle-income countries. Ann N Y Acad Sci 1444(1):6–21. 10.1111/nyas.14121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noor RA, Abioye AI, Ulenga N, Msham S, Kaishozi G, Gunaratna NS, Mwiru R, Smith E, Dhillon CN, Spiegelman D (2017) Large–scale wheat flour folic acid fortification program increases plasma folate levels among women of reproductive age in urban Tanzania. PLoS ONE 12(8):e0182099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen LH (2000) Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr 71(5):1280S–1284S. 10.1093/ajcn/71.5.1280s [DOI] [PubMed] [Google Scholar]
- 12.Abioye AI, McDonald EA, Park S, Ripp K, Bennett B, Wu HW, Pond-Tor S, Sagliba MJ, Amoylen AJ, Baltazar PI, Tallo V (2019) Maternal anemia type during pregnancy is associated with anemia risk among offspring during infancy. Pediatr Res 86(3):396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abioye AI, Park S, Ripp K, McDonald EA, Kurtis JD, Wu H, Pond-Tor S, Sharma S, Ernerudh J, Baltazar P, Acosta LP (2018) Anemia of inflammation during human pregnancy does not affect newborn iron endowment. J Nutrition 148(3):427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T (2004) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Investig 113(9):1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM, Mor G, Schulz L, Soares M, Spencer T, Strominger J, Way SS, Yoshinaga K (2015) Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol 16(4):328–334. 10.1038/ni.3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganz T, Nemeth E (2009) Iron sequestration and anemia of inflammation. Semin Hematol 46(4):387–393. 10.1053/j.seminhematol.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss G, Ganz T, Goodnough LT (2019) Anemia of inflammation. Blood 133(1):40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Luo D, Wang Q, Ma Y, Ping L, Wu T, Tang J, Peng D (2020) Serum homocysteine and folate concentrations in early pregnancy and subsequent events of adverse pregnancy outcome: the Sichuan Homocysteine study. BMC Pregnancy Childbirth 20(1):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etheredge AJ, Premji Z, Gunaratna NS, Abioye AI, Aboud S, Duggan C, Mongi R, Meloney L, Speigleman D, Roberts D, Hamer DH, Fawzi WW (2015) Iron supplementation among iron-replete and non-anemic pregnant women: a randomized placebo-controlled trial in Tanzania. JAMA Pediatr 169(10):947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mwakyusa DH, Mukama W, MOHSW-Tanzania (2008) The national road map strategic plan to accelerate reduction of maternal, newborn and child deaths in Tanzania 2008–2015. Ministry of Health and Social Welfare, Tanzania, Dar es Salaam. http://advancefamilyplanning.org/sites/default/files/resources/RMNCH%20Plan%202014%20to%202015.pdf. Accessed 28 Oct 2022 [Google Scholar]
- 21.Abioye AI, Aboud S, Premji Z, Etheredge AJ, Gunaratna NS, Sudfeld CR, Mongi R, Meloney L, Darling AM, Noor RA (2016) Iron supplementation affects hematologic biomarker concentrations and pregnancy outcomes among iron-deficient Tanzanian women–. J Nutr 146(6):1162–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO) (2011) Haemoglobin concentration for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. WHO, Geneva. https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf. Accessed 28 Oct 2022 [Google Scholar]
- 23.Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJ (2017) Adjusting ferritin concentrations for inflammation: biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Am J Clin Nutr 106(1):359S–371S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO) (2020) WHO Guideline: Use of ferritin concentrations to assess iron status in individuals and populations. https://www.who.int/docs/default-source/micronutrients/ferritin-guideline/ferritin-guidelines-brochure.pdf?sfvrsn=76a71b5a_4. Accessed June 3 2020 [PubMed]
- 25.Weiss G, Goodnough LT (2005) Anemia of chronic disease. N Engl J Med 352(10):1011–1023. 10.1056/NEJMra041809 [DOI] [PubMed] [Google Scholar]
- 26.Skikne BS, Punnonen K, Caldron PH, Bennett MT, Rehu M, Gasior GH, Chamberlin JS, Sullivan LA, Bray KR, Southwick PC (2011) Improved differential diagnosis of anemia of chronic disease and iron deficiency anemia: a prospective multicenter evaluation of soluble transferrin receptor and the sTfR/log ferritin index. Am J Hematol 86(11):923–927. 10.1002/ajh.22108 [DOI] [PubMed] [Google Scholar]
- 27.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, Lambert A, Papageorghiou AT, Carvalho M, Jaffer YA, Gravett MG, Purwar M, Frederick IO, Noble AJ, Pang R, Barros FC, Chumlea C, Bhutta ZA, Kennedy SH (2014) International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st Project. Lancet (Lond Engl) 384(9946):857–868. 10.1016/S0140-6736(14)60932-6 [DOI] [PubMed] [Google Scholar]
- 28.Spiegelman D, Hertzmark E (2005) Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 162(3):199–200 [DOI] [PubMed] [Google Scholar]
- 29.Zou G (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706 [DOI] [PubMed] [Google Scholar]
- 30.Hertzmark E, Spiegelman D (2010) The SAS ROBREG9 Macro.1–15 [Google Scholar]
- 31.Hosmer DW Jr, Lemeshow S, Sturdivant RX (2013) Applied logistic regression, vol 398. John Wiley and Sons [Google Scholar]
- 32.Black AK, Allen LH, Pelto GH, de Mata MP, Chávez A (1994) Iron, vitamin B-12 and folate status in Mexico: associated factors in men and women and during pregnancy and lactation. J Nutr 124(8):1179–1188 [DOI] [PubMed] [Google Scholar]
- 33.Cade JE, Moreton JA, O’Hara B, Greenwood DC, Moor J, Burley VJ, Kukalizch K, Bishop DT, Worwood M (2005) Diet and genetic factors associated with iron status in middle-aged women. Am J Clin Nutr 82(4):813–820 [DOI] [PubMed] [Google Scholar]
- 34.Spiegler J, Stichtenoth G, Weichert J, Konig IR, Schlaud M, VDW A, Olbertz D, Gurth H, Schiffmann JH, Bohnhorst B, Gortner L, Herting E, Gopel W (2013) Pregnancy risk factors for very premature delivery: what role do hypertension, obesity and diabetes play? Arch Gynecol Obstet 288(1):57–64. 10.1007/s00404-013-2739-6 [DOI] [PubMed] [Google Scholar]
- 35.McGregor JA, French JI, Richter R, Franco-Buff A, Johnson A, Hillier S, Judson FN, Todd JK (1990) Antenatal microbiologic and maternal risk factors associated with prematurity. Am J Obstet Gynecol 163(5 Pt 1):1465–1473 [DOI] [PubMed] [Google Scholar]
- 36.Cheong JL, Doyle LW (2012) Increasing rates of prematurity and epidemiology of late preterm birth. J Paediatr Child Health 48(9):784–788. 10.1111/j.1440-1754.2012.02536.x [DOI] [PubMed] [Google Scholar]
- 37.Melku M, Addis Z, Alem M (2014) Enawgaw B (2014) Prevalence and predictors of maternal anemia during pregnancy in Gondar, Northwest Ethiopia: an institutional based cross-sectional study. Anemia 108593:9. 10.1155/2014/108593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ou Z, Li Q, Liu W, Sun X (2011) Elevated hemoglobin A2 as a marker for BETA.-Thalassemia trait in pregnant women. Tohoku J Exp Med 223(3):223–226 [DOI] [PubMed] [Google Scholar]
- 39.RStudio Team (2015) RStudio: integrated development for R. RStudio, Inc, Boston, MA: URL rstudio com 42:14 [Google Scholar]
- 40.Wirth JP, Woodruff BA, Engle-Stone R, Namaste SM, Temple VJ, Petry N, Macdonald B, Suchdev PS, Rohner F, Aaron GJ (2017) Predictors of anemia in women of reproductive age: biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Am J Clin Nutr 106(Suppl 1):416s–427s. 10.3945/ajcn.116.143073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nemeth E, Ganz T (2014) Anemia of inflammation. Hematol Oncol Clin North Am 28(4):671–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith LE, Prendergast AJ, Turner PC, Humphrey JH, Stoltzfus RJ (2017) Aflatoxin exposure during pregnancy, maternal anemia, and adverse birth outcomes. Am J Trop Med Hyg 96(4):770–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynch S, Pfeiffer CM, Georgieff MK, Brittenham G, Fairweather-Tait S, Hurrell RF, McArdle HJ, Raiten DJ (2018) Biomarkers of nutrition for development (BOND)-iron review. J Nutr. 10.1093/jn/nxx036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sangkhae V, Fisher AL, Chua KJ, Ruchala P, Ganz T, Nemeth E (2020) Maternal hepcidin determines embryo iron homeostasis in mice. Blood 136(19):2206–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald EA, Gundogan F, Olveda RM, Bartnikas TB, Kurtis JD, Friedman JF (2022) Iron transport across the human placenta is regulated by hepcidin. Pediatr Res 92(2):396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gambling L, Czopek A, Andersen HS, Holtrop G, Srai SK, Krejpcio Z, McArdle HJ (2009) Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol 296(4):R1063–1070. 10.1152/ajpregu.90793.2008 [DOI] [PubMed] [Google Scholar]
- 47.Moormann AM, Sullivan AD, Rochford RA, Chensue SW, Bock PJ, Nyirenda T, Meshnick SR (1999) Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J Infect Dis 180(6):1987–1993 [DOI] [PubMed] [Google Scholar]
- 48.Abioye AI, McDonald EA, Park S, Joshi A, Kurtis JD, Wu H, Pond-Tor S, Sharma S, Ernerudh J, Baltazar P (2019) Maternal, placental and cord blood cytokines and the risk of adverse birth outcomes among pregnant women infected with Schistosoma japonicum in the Philippines. PLoS Negl Trop Dis 13(6):e0007371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boga C, Ozdogu H (2016) Pregnancy and sickle cell disease: a review of the current literature. Crit Rev Oncol Hematol 98:364–374 [DOI] [PubMed] [Google Scholar]
- 50.Green R, Allen LH, Bjørke-Monsen A-L, Brito A, Guéant J-L, Miller JW, Molloy AM, Nexo E, Stabler S, Toh B-H (2017) Vitamin B 12 deficiency. Nat Rev Dis Primers 3(1):1–20 [Google Scholar]
- 51.Ganz T (2019) Anemia of inflammation. N Engl J Med 381(12):1148–1157 [DOI] [PubMed] [Google Scholar]
- 52.Mwangi MN, Prentice AM, Verhoef H (2017) Safety and benefits of antenatal oral iron supplementation in low-income countries: a review. Br J Haematol 177(6):884–895. 10.1111/bjh.14584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McArdle HJ, Gambling L, Kennedy C (2014) Iron deficiency during pregnancy: the consequences for placental function and fetal outcome. Proc Nutr Soc 73(1):9–15. 10.1017/s0029665113003637 [DOI] [PubMed] [Google Scholar]
- 54.Oxman AD, Guyatt GH (1992) A consumer’s guide to subgroup analyses. Ann Intern Med 116(1):78–84 [DOI] [PubMed] [Google Scholar]
- 55.Sun X, Briel M, Walter SD, Guyatt GH (2010) Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 30:340. [DOI] [PubMed] [Google Scholar]
- 56.Verhoef H, Veenemans J, Mwangi MN, Prentice AM (2017) Safety and benefits of interventions to increase folate status in malaria-endemic areas. Br J Haematol 177(6):905–918. 10.1111/bjh.14618 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


