Abstract
PNX was described as an uncommon complication in COVID-19 patients but clinical risk predictors and the potential role in patient's outcome are still unclear. We assessed prevalence, risk predictors and mortality of PNX in hospitalized COVID- 19 with severe respiratory failure performing a retrospective observational analysis of 184 patients admitted to our COVID-19 Respiratory Unit in Vercelli from October 2020 to March 2021. We compared patients with and without PNX reporting prevalence, clinical and radiological features, comorbidities, and outcomes. Prevalence of PNX was 8.1% and mortality was >86% (13/15) significantly higher than in patients without PNX (56/169) (P < 0.001). PNX was more likely to occur in patients with a history of cognitive decline (HR: 31.18) who received non-invasive ventilation (NIV) (p < 0.0071) and with low P/F ratio (HR: 0.99, p = 0.004). Blood chemistry in the PNX subgroup compared to patients without PNX showed a significant increase in LDH (420 U/L vs 345 U/L, respectively p = 0.003), ferritin (1111 mg/dl vs 660 mg/dl, respectively p = 0.006) and decreased lymphocytes (HR: 4.440, p = 0.004). PNX may be associated with a worse prognosis in terms of mortality in COVID patients. Possible mechanisms may include the hyperinflammatory status associated with critical illness, the use of NIV, the severity of respiratory failure and cognitive impairment. We suggest, in selected patients showing low P/F ratio, cognitive impairment and metabolic cytokine storm, an early treatment of systemic inflammation in association with high-flow oxygen therapy as a safer alternative to NIV in order to avoid fatalities connected with PNX.
Keywords: Pneumothorax, COVID-19, Non-invasive ventilation, Risk predictors, Inflammatory state
1. Introduction
COVID-19, a new severe acute respiratory syndrome (SARS) due to SARS-CoV-2 coronavirus emerging in late 2019, is responsible for the global pandemic; with data, at time of writing (December 18, 2022), reporting over 649 million confirmed cases and over 6.6 million deaths globally, in the last 28 days, over 13.7 million cases and over 40 000 new fatalities [1]. At present, in Italy, and globally, the number of new weekly cases are decreasing but recently Italy faced up to a fourth wave of infection with significant pressures on hospital services. Being a virus with a specific tropism for the lower respiratory tract there is a real risk (in about 20% of affected patients) of developing the severe form of the disease with an acute respiratory distress syndrome (ARDS) resulting in high morbidity/mortality [[2], [3], [4], [5]]. Pneumothorax (PNX) has been observed as a potential complication of COVID-19 in patients at emergency department admission and also in ventilated patients [6]. During the second wave of the pandemic (from October 2020 to March 2021) in our COVID-19 semi-intensive care Respiratory Unit in Vercelli we treated COVID-19 patients with severe acute respiratory failure recording clinical, laboratory, radiological and medical prescriptions data. The aim of the index study was to investigate the prevalence and risk factors associated with the onset of PNX in a cohort of hospitalized COVID-19 patients with severe respiratory failure in order to find possible early risk predictors for its development and for a safer treatment of this complication.
2. Materials and methods
2.1. Study design and population
We performed a retrospective observational single centre evaluation of a cohort of 184 adult patients admitted to the COVID-19 semi-intensive care Respiratory Unit in Vercelli, Italy (Ospedale S. Andrea) from October 2020 to March 2021 for severe respiratory failure secondary to SARS-CoV-2 infection. This study was approved by the Institutional Review Board CE 67/20, in accordance with the principles of the Declaration of Helsinki. An informed consent was obtained. Inclusion criteria included a diagnosis of SARS-CoV-2 infection confirmed by real-time reverse transcription-polymerase chain reaction (RT-PCR) molecular testing from a nasopharyngeal swab. All patients presented a diagnosis of acute respiratory failure type I (paO2 <60 mmHg) and bilateral pneumonia as confirmed with chest radiography and/or computed tomography (CT) scan. They all received pharmacological treatment based on updated national guidelines [7]. We adopted non-invasive respiratory support devices following our local protocol. Patients were supported with conventional oxygen support by low-flow intranasal cannula or Venturi mask, high flow nasal cannula (HFNC) oxygen therapy, delivering humified oxygen through a nasal cannula with a flow delivery set at 60 l/min and temperature at 37 °C, then adjusted according to patient's tolerance and response, or non-invasive ventilation (NIV) with a pressure support ventilation modality with positive end-expiratory pressure (PEEP) of 10 cmH20 and pressure support (PS) of 8–12 cmH2O. NIV was intended as generic non-invasive ventilatory support. Presenting severe hypoxemic respiratory failure with compensated PH in all cases, patients were treated with CPAP, then upgraded if not responding. The criteria for choosing HFNC or NIV was taken according to the best clinical practice and considering clinical characteristics of the patients; the settings of each device were adjusted based on continuous cardiopulmonary monitoring and following patient's tolerance. In case of worsening of gas exchanges, neurological deterioration or hemodynamic instability, they were moved to the intensive care unit according to national guidelines [8]. The suspect of pneumothorax development was based on deterioration of clinical conditions, especially the worsening of gas exchanges (hypoxemia) despite oxygen support. Selected patients performed a specific radiographic study with chest radiography or CT scan to confirm the diagnosis. No patients were immunized with vaccine against COVID-19 as no vaccines were available at the time of the study.
2.2. Data collection and clinical variables
We collected clinical data of the enrolled patients including demographics, past medical history and comorbidities, smoking status, laboratory tests at patient arrival to emergency department, radiological documentation, clinical management, ventilatory support, length of hospitalization, the day of occurrence of PNX and the outcome of in-hospital stay (discharged or deceased). Demographics, past medical history and symptoms onset were extracted from the electronic patient registry. Cognitive decline diagnosis (including dementia and vascular encephalopathy) at admittance to our unit was recorded from anamnestic medical records. Patients with either mild or severe cognitive impairment were included in the study. Laboratory tests including lactate dehydrogenase (LDH) (U/L), ferritin (mcg/L), C-reactive protein (CRP) (mg/dL), blood platelet count, D-dimers (ng/mL), lymphocyte count and neutrophils/macrophages ratio, troponin, and PaO2/FiO2 (P/F) ratio at admission onto our unit were recorded using electronic schedules of emergency department. Lung patterns (consolidative, interstitial, pleural effusion, presence of excavations) on the computed tomography scan were evaluated and a qualitative assessment was performed. In case of a prevalent interstitial pattern a quantitative severity index was reported. All patients with PNX were treated with chest drainage and with appropriate intensive respiratory support. The principal outcome variable was the in-hospital prevalence of PNX.
2.3. Statistical methods
Variables were presented with frequencies and percentages for categorical variables and as median (1st-3rdquartile) for continuous variables. In time-to-event analyses the primary event of interest was the development of PNX in hospitalized COVID-19 patients. Patients were followed from the day of admission until the hospital discharge/death. Baseline time for analysis was considered the admittance day to our unit. In survival analyses, patients who were alive at discharge or died during hospitalization were considered censored observations. A univariate and multivariate Cox proportional-hazards model and hazard ratios with 95% confidence intervals were used to evaluate the association between clinical, radiological and laboratory variables and PNX development. To test non-proportionality, martingale residuals method was used. The variables included in the multivariate regression model respect proportionality assumption. We considered, as adjusting factors in the multivariable models, the statistically significant variables at the univariate analysis in our cohort (dementia, P/F, NIV support, LDH, ferritin, lymphocytes reduction) including age and sex. Using a stepwise selection procedure (entry probability - slentry 0.10, exit probability - slstay 0.05) dementia, P/F and low lymphocytes were selected as the most significant variables associated with PNX development. A p-value of <0.05 was considered significant. Statistical analyses were performed with SAS (SAS institute, Cary, NC, USA) software.
3. Results
3.1. Clinical characteristics of PNX subgroup patients
A total of 15/184 patients (8.1%) experienced during their hospitalization PNX with a median time lapse from admission of 9 (0–41) days. Their median age was 81 years (52–89), 6/15 patients were males (40%), 11/15 patients were current or former smokers (73.3%). Regarding the most common comorbidities 14 patients out of 15 had a history of cognitive impairment (93.3%), and 8/15 patients suffered from hypertension (53.5%). The most affected side of PNX was the right (12/15 patients), 2 patients developed PNX on the left and two patients had bilateral PNX. Moreover 8/15 patients developed associated pneumomediastinum. The totality of patients required chest drainage. Regarding respiratory support 13/15 patients during hospitalization received non-invasive ventilation with NIV before experiencing a PNX (86.6%). An analysis of blood chemistry values, revealed elevated CRP levels in all the patients (100%) with a median value of 7.1 mg/dl (0–42 mg/dl), 14 patients (93.3%) had elevated LDH levels with a median value of 420 U/L (120–1163 U/L), 13 patients (87%) had low leukocyte cell count (<4.5 × 103/μL), high neutrophils/leukocyte rate, high D-Dimer test with median value 455 ng/ml (75–1410 ng/ml) and high ferritin with median value of 1111 mcg/L (148–6256 mcg/L), and 12 patients had high troponin (80%). The median P/F ratio of pneumothorax group was 106 mmHg (38–400) (p = 0.004). We observed an in-hospital mortality significantly higher among PNX subgroup patients: the fatality rate was of 13/15 patients (86.7%). The admission characteristics and medical history of the whole cohort with PNX subgroup are shown in Table 1 , radiological and laboratory findings are shown in Table 2 .
Table 1.
Clinical characteristics of studied population.
| Variable | All n = 184 (100) | no PNX n = 169 (92) | PNX n = 15 [8] | HR (CI 95%) | P |
|---|---|---|---|---|---|
| Age, years (IQR) | 79 (69–86) | 79 (69–86) | 81 (74–84) | 1.007 (0.960–1.050) | >0.05 |
| Male sex, n (%) | 98 (53.3) | 92 (54.4) | 6 (40.0) | 0.619 (0.220–1.743) | >0.05 |
| Smoking abitude, n (%) | 96 (52.2) | 85 (50.2) | 11 (73.3) | 2.606 (0.829–8.194) | >0.05 |
| Obesity, n (%) | 79 (42.9) | 72 (42.6) | 7 (46.6) | 1.170 (0.420–3.260) | >0.05 |
| Comorbidities, n(%) | |||||
| Diabetes | 31 (16.8) | 29 (17.1) | 2 (13.3) | 0.672 (0.149–3.024) | >0.05 |
| Arterial hypertension | 97 (52.7) | 89 (52.6) | 8 (53.3) | 0.903 (0.324–2.513) | >0.05 |
| Heart disease | 44 (23.9) | 41 (24.2) | 3 (20.0) | 0.834 (0.233–2.990) | >0.05 |
| Kidney disease | 14 (7.6) | 12 (7.1) | 2 (13.3) | 1.476 (0.330–6.603) | >0.05 |
| Dementia | 54 (29.3) | 40 (23.6) | 14 (93.3) | 31.181 (4.089–237.793) | 0.0009 |
| Blood gas parameters and ventilatory support | |||||
| PaO2/FIO2(>200), n (%) | 124 (67.4) | 118 (69.8) | 6 (40.0) | 0.338 (0.119–0.959) | 0.041 |
| PaO2/FIO2 mmHg (IQR) | 262 (185–304) | 266 (192–304) | 106 (56–309) | 0.990 (0.986–0.997) | 0.004 |
| NIV, n (%) | 84 (45.4) | 71 (42.0) | 13 (86.6) | 7.970 (1.760–36.088) | 0.007 |
| Outcome | |||||
| Mortality at day 30, n (%) | 69 (37.5) | 56 (33.1) | 13 (86.6) | 5.097 (2.518 10.317) | <.0001 |
| Hospital stay days, n (IQR) | 13 [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]] | 14 [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]] | 9 [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]] | >0.05 | |
| Hospital stay days, min-max | (0–73) | (1–73) | (0–41) | >0.05 | |
| Day of PNX diagnosis, n | 9 | 9 | |||
Data are presented as number and percentage for dichotomous values or median and interquartile range (IQR) for continuous values.
Abbreviations: HR, Hazard Ratio; CI, confidence interval; PNX, Spontaneous pneumothorax; NIV, non-invasive ventilation; PaO2, arterial oxygen partial pressure; FiO2, fraction of inspired oxygen.
Table 2.
Radiological and laboratory data at admission of studied population.
| Variable | All n = 184 (100) | no PNX n = 169 (92) | PNX n = 15 [8] | HR (CI 95%) | p |
|---|---|---|---|---|---|
| Radiographic pattern | |||||
| Severe ground glass, n (%) | 78 (42.4) | 72 (42.6) | 6 (40.0) | 0.748 (0.265–2.113) | >0.05 |
| Pleural effusion, n (%) Right | 13 (7.1) | 11 (6.5) | 2 (13.3) | >0.05 | |
| Left | 14 (7.6) | 13 (7.6) | 1 (6.6) | >0.05 | |
| Bilateral | 39 (21.2) | 37 (21.8) | 2 (13.3) | >0.05 | |
| Escavation, n (%R) | 4 (2.2) | 4 (2.3) | 0 (0) | >0.05 | |
| Lung consolidations, n (%) | 49 (26.6) | 43 (25.4) | 6 (40) | 1.755 (0.624–4.940) | >0.05 |
| Inflammatory markers at admission | |||||
| LDH elevation, n (%) | 157 (85.3) | 144 (85.2) | 13 (86.6) | 1.074 (0.240–4.804) | >0.05 |
| LDH - U/L (IQR) | 346.5 (277.5–454.5) | 345 (277–444) | 420 (292–550) | 1.002 (1.000–1.004) | 0.003 |
| Ferritin elevation, n (%) | 169 (91.8) | 155 (91.7) | 14 (93.3) | 1.275 (0.166–9.816) | >0.05 |
| Ferritin - mcg/L (IQR) | 697 (256.5–850.5) | 660 (249–834) | 1111 (315–1.696) | 1.001 (1.000–1.001) | 0.006 |
| CRP elevation, n (%) | 178 (96.7) | 163 (96.4) | 15 (100) | >0.05 | |
| CRP - mg/dl (IQR) | 7.7 (3.6–13.3) | 7.9 (3.6–13.2) | 7.2 (4.0–15.3) | 0.990 (0.920–1.060) | >0.05 |
| PLT elevation, n (%) | 12 (6.5) | 11 (6.5) | 1 (6.6) | 0.872 (0.110–6.897) | >0.05 |
| PLT - x 10.000/mcl (IQR) | 205 (143–272) | 206 (143–272) | 177 (127–223) | 0.990 (0.990–1.004) | >0.05 |
| D-dimer elevation, n (%) | 152 (82.6) | 139 (82.2) | 13 (86.6) | 1.083 (0.241–4.869) | >0.05 |
| D-dimer - ng/ml (IQR) | 435 (271–870) | 434 (272–889) | 455 (270–602) | 0.990 (0.990–1.000) | >0.05 |
| Tn T, n (%) | 114 (62.0) | 102 (60.3) | 12 (80.0) | 2.066 (0.575–7.421) | >0.05 |
| Lymphocytes reduction, n (%) | 31 (16.8) | 24 (34.7) | 7 (46.6) | 4.440 (1.604–12.289) | 0.004 |
| N/Macrophages elevated ratio | 155 (84.2) | 142 (84.0) | 13 (86.6) | 1.006 (0.225–4.498) | >0.05 |
Data are presented as number and percentage for dichotomous values or median and interquartile range (IQR) for continuous values.
Abbreviations: HR, Hazard Ratio; CI, confidence interval; Tn, troponin; CRP, C-reactive protein; PTL, platelets; LDH, lactate dehydrogenase; N/M, neutrophils/macrophages ratio.
3.2. Clinical predictors of development of PNX
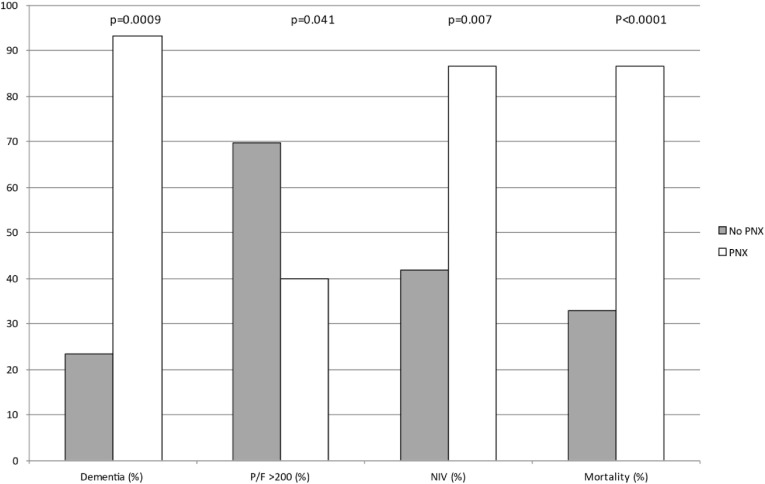
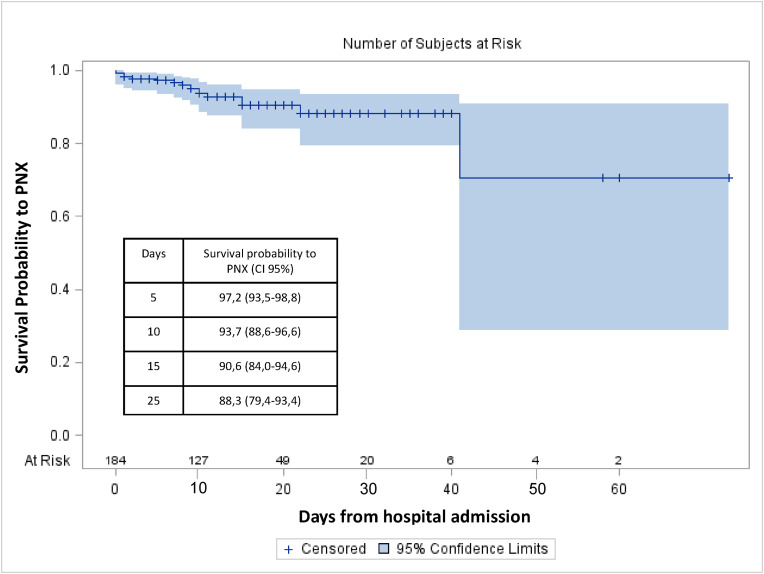
Considering the high fatality rate in the subgroup of PNX patients we tried to investigate more carefully this population to find any early clinical predictor that may be related to the development of PNX in our cohort of patients and possibly predict a poor prognosis. No significant differences were found comparing occurrence of PNX in females and males (10.5% vs 6.1%, respectively). We observed that older age, smoking habitude and obesity were associated with an increased risk of development of PNX, by univariate analysis (HR = 1.007; 95%CI, 0.960–1.050, HR = 2.606; 95%CI, 0.829–8.194 and HR = 1.170; 95%CI, 0.420–3.260, respectively). We evaluated the prevalence of underlying comorbidities among PNX patients finding that in our cohort chronic renal failure is associated with an increased risk of PNX development (HR = 1.476; 95%CI, 0.330–6.603) but in particular dementia was the only comorbidity associated with an increased risk to develop PNX reaching statistically significance (HR = 31.181; 95%CI, 4.089–237.793, p = 0.0009). With regards to the clinical presentation at admission, the P/F ratio, identifying a more severe respiratory failure at admission, predicted the occurrence of PNX along with the ventilatory support. In particular, patients with a low P/F ratio had an increased risk of developing a PNX, while patients with higher P/F (>200) showed a statistically significant reduced risk to develop PNX (HR = 0.338; 95%CI, 0.119–0.959; p = 0.041) even considering P/F as a continuous variable (HR = 0.990; 95%CI, 0.986–0.997; p = 0.004). Similarly, patients being treated (from admission to our unit) with non-invasive ventilation (NIV), when compared to those treated with ventilatory support including HFNC oxygen therapy or only conventional oxygen support by low-flow intranasal cannula or Venturi mask, showed a poorer prognosis with around 8 times higher risk of developing a PNX (HR = 7.970; 95% CI, 1.760–36.088; p = 0.007). A sub-analysis including patients with P/F ratio <200 showed for subjects with PNX an increased mortality risk when compared to patients without PNX (OR = 9.81; 95%CI, 1.945–49.471), this effect has been adjusted for disease severity in a multivariable logistic regression model. Statistically significant variables associated with PNX compared between the groups of patients with and without PNX are reported in Fig. 1 . Probability of PNX development during the time of observation in the cohort is reported in Fig. 2 .
Fig. 1.
Comparison between significant clinical characteristics of COVID 19 patients with PNX (white bars) and w/o PNX (grey bars). Abbreviations: PNX, pneumothorax; NIV, non-invasive ventilation; P/F, Arterial oxygen partial pressure/fraction of inspired oxygen.
Fig. 2.
Probability of PNX development during the time of observation in the cohort with pointwise confidence limits (CI 95%).
3.3. Radiological and laboratory predictors of PNX development
Among the laboratory variables at admission, we observed higher values of most inflammatory indices (LDH, ferritin, CRP, D-dimers, troponin, N/M ratio) in the PNX subgroup, some of them were associated with an increased risk of PNX development, but the most important predictor of PNX in our cohort were elevated ferritin (median value 1111 mcg/L; HR = 1.001; 95% CI, 1.000–1.001; p = 0.006) and LDH (median value 420 U/L; HR = 1.002; 95% CI, 1.000–1.004, p = 0.003) levels, and a low white cell count (HR = 4.440; 95% CI, 1.604–12.289, p = 0.004). There were no significant differences identified for radiological findings (severe ground-glass, excavation, pleural effusion) in the two groups even though we observed that a consolidative pattern is associated with an increased risk of occurrence of PNX (HR = 1.755; 95% CI, 0.624–4.940).
The multivariate regression model confirmed that dementia and low lymphocytes are risk factors for the development of PNX while a P/F > 200 is a protective factor. Table 3 .
Table 3.
Predictors of Pneumothorax development on multivariable logistic regression analysis according to prediction sets.
| Parameter | Parameter Estimate | Standard Error | Chi-Square | Pr > ChiSq | Hazard Ratio | 95% Hazard Ratio Confidence Limits | |
|---|---|---|---|---|---|---|---|
| Age | 0.00186 | 0.03587 | 0.0027 | 0.9586 | 1.002 | 0.934 | 1.075 |
| Sex | 0.39569 | 0.56826 | 0.4849 | 0.4862 | 1.485 | 0.488 | 4.524 |
| Dementia | 3.26344 | 1.04312 | 9.7877 | 0.0018 | 26.139 | 3.384 | 201.929 |
| Low lymphocytes | 1.57814 | 0.58117 | 7.3737 | 0.0066 | 4.846 | 1.551 | 15.138 |
| PaO2/FiO2 | −0.00716 | 0.00295 | 5.8989 | 0.0152 | 0.993 | 0.987 | 0.999 |
Hazard ratio (HR) with standard error (SE) and p values (p) have been reported.
Abbreviations: PaO2, arterial oxygen partial pressure; FiO2, fraction of inspired oxygen.
4. Discussion
In this study we analyzed the clinical characteristics and outcome of a cohort of patients hospitalized in an intermediate care unit of pneumology for severe COVID-19 infection and respiratory failure. We focused the analysis, in particular, on those who developed PNX as a potential complication of the infection. The occurrence of PNX as a complication in hospitalized COVID-19 patients, including non-ventilated patients, has been described during the pandemic period [[9], [10], [11], [12], [13]] and has been associated with a high incidence of mortality especially in critically ill patients developing ARDS [14]. However, currently, limited data is available on the incidence and outcome of this complication in COVID-19 patients and early predictors of PNX development in this specific population remain poorly described. The occurrence of PNX may also reflect a specific feature of COVID-19 severe infection resulting in the development of lung excavation and bullae [[15], [16], [17], [18], [19]]. In our cohort, 15/184 patients (8%) developed PNX and in this subgroup the fatality rate was much more higher confirming previous reports showing a worse prognosis in severe COVID-19 patients who develop PNX/PNM [[20], [21], [22], [23], [24]]. The fatality rate of the whole cohort of COVID patients was 37.5%. Data regarding the in-hospital mortality are in line with those reported in literature in Italy for that period in our geographic area [[25], [26], [27]]. Data of patients in Northern Italy, at the time, reported an in-hospital mortality close to 30% although most of reports were referring to patients in the intensive care unit (ICU) setting [28,29]. Recent reports showed an important variability in COVID-19 mortality rate in Italy for hospitalized patients depending on individual prognostic factors and also depending on the pandemic wave, being at a lower risk of death patients hospitalized after the first wave [30]. Older age seems to have, in general, an important impact on mortality in COVID-19 patients as reported in previous studies and meta-analyses [9,31,32] and, more specifically, some studies reported a correlation with older age and a negative prognosis in COVID-19 patients with PNX [15,33]. In our cohort the elevated rate of mortality may partly be explained by the older age of the patients (median age in PNX group 81 years) and partly by the severity of illness at admission. The development of PNX in our study was shown to be independent of gender and correlated with aging (HR 1.007). We noticed a similar length of hospitalization between surviving patients and deceased patients with a median time of hospitalization in the whole population of 14 days. Moreover, we investigated the clinical, radiological and biochemical characteristics shared in patients showing PNX in order to try to detect factors making them more prone to develop PNX. Evaluating the prevalence of underlying comorbidities, we observed that, interestingly, only cognitive impairment reached a statistically significant prevalence in patients with PNX (p = 0.0009). Recent data showed that dementia can predict severity of COVID-19 infection. In fact, patients with dementia are more exposed to COVID-19 infection and more likely to require hospitalization and to have severe sequelae or fatal outcomes compared with patients who do not [34]. Factors connecting dementia to the development of PNX are not completely clarified yet and, to our knowledge, has not yet been investigated before. We hypothesized that an altered mental status may be related to a misperception of health status and difficulties in communication conducting to a worst compliance to therapies, especially to mechanical ventilation, and a poor outcome, in particular in the presence of a serious complication like PNX. Analysing which clinical predictors might reveal patients at higher risk of PNX development we found that the most important clinical index was the P/F ratio, identifying that a more severe respiratory failure condition at admission was likely to predict PNX occurrence. In particular, patients with a low P/F ratio (<200) were more prone to develop PNX and had a poorer prognosis compared to survivors (median value 106 [56–309] vs 266 [192–304]; p = 0.004) [35,36]. Another important observation from our data suggests that ventilatory support was a predictor of PNX development. Patients receiving ventilation from hospital admission (related to the severity of the acute respiratory failure and/or to the need for respiratory support) were shown to develop PNX more frequently when compared with those receiving only conventional oxygen support or HFNC (p = 0.007). This may indicate a barotrauma mechanism although, recent evidence demonstrated that barotrauma may overlap with other specific lung damage that may increase the frailty of airway tissue amplifying the final result [20,24,[37], [38], [39], [40],42]. It is likely that, in our PNX subgroup, a specific phenotype of patients characterized by a particular severity of illness was selected. Clearly the overlapping of factors including the severity of Covid-19 related respiratory failure, the low P/F ratio, the development of ARDS, and the need for mechanical ventilation make it difficult to specifically attribute the cause of the onset of PNX or the high mortality rate observed in these patients. The role of mechanical ventilation has been hypothesized to contribute to barotrauma though this mechanism, independently, is unlikely to explain by itself the occurrence of PNX occurrence in these patients [41]. To support this observation, a recent systematic review and meta-analysis showed no evidence for distinct clinical phenotypes in patients with COVID-19-related ARDS compared to other causes of ARDS concluding that no change in conventional lung-protective ventilation strategies are warranted [43]. It can be hypothesized that inflammatory and ischemic damage in the smaller airways, together with prolonged mechanical ventilation that these patients frequently require, may lead to the increased risk of the development of PNX, moreover, lung co-infections may induce air leaks leading to spontaneous PNX [13]. Also, cough and/or airway obstruction by secretions may induce hyperinflation and endo-alveolar pressure with a Macklin effect [44]. Future studies with larger sample sizes are needed to address these questions and to better define the pathophysiology of lung injury induced by SARS-CoV-2, leading especially to PNX development and subsequently to find specific treatment strategies, and prevent and manage these dangerous complications. In line with the above-mentioned observations, among laboratory variables at admission, we observed in the PNX subgroup a higher value of many inflammatory indices, particularly lymphopenia, ferritin and LDH at admission seem to have a stronger association with PNX (p = 0.004, p = 0.006, p = 0.003, respectively). Compromised leukocyte's function may lead to uncontrolled lymphocyte traffic from peripheral blood to the lung, resulting in an increased immunological alveolar injury and, in turn, to an increased risk of pneumothorax [45]. Numerous studies have highlighted increased ferritin levels during COVID-19 infection and a meta-analysis on 18 studies showed that ferritin levels were significantly increased in severe patients and in non-survivors; moreover, patients with one or more comorbidities including thrombotic complications had significantly higher levels of ferritin compared to the others needing more intensive supportive care and mechanical ventilation [46]. High ferritin levels can trigger cell death by releasing reactive oxygen radicals and in COVID-19 may reflect cell damage and contribute to inflammation [47] and thrombotic events [48]. Various studies have identified the presence of high LDH levels as a prognostic biomarker with a high accuracy for predicting in-hospital mortality in severe and critically ill patients with COVID-19 [[49], [50], [51]] and a recent meta-analysis confirmed that LDH levels can be used as a COVID-19 severity marker and are a predictor of survival [52]. Finally, it has been recently argued that therapies specifically targeting neutrophilic inflammation in COVID-19 infection may have a positive effect, although no therapies have currently been investigated in large-scale clinical trials [53].
5. Limitations
Our study presents some limitations; the main limitation is the relatively small sample size of the PNX subgroup, but in line with previous reports in literature due to the difficulty to collect large number of cases of relatively infrequent complications. The retrospective nature of this investigation is another limitation. An observational case series cannot establish causality between COVID-19 and pneumothorax. Moreover, it is possible that some cases of pneumothorax are coincidental to COVID-19. However, given the relative frequency of this co-presentation it seems unlikely that this explains even a majority, of our patients. It must be noted that the particular severity of COVID-19 disease of the patients admitted to our centre does not allow us to generalize the prevalence and mortality data that we have observed to all general hospital departments. A limitation may be represented by the lack of collection of specific data relating to the methods of execution of mechanical ventilation in individual patients, however the nature of the digitized documents from which we drew data did not allow this type of evaluation. Moreover, the exact cause of death of patients was not collected, neither in patients experiencing PNX, and this aspect may not allow us to understand whether the deceased patients were directly due to the PNX or to other complications not directly associated.
6. Conclusions
PNX may be a not infrequent and serious complication of severe COVID-19 infection associated with a poor prognosis. Our findings are consistent with the possible mechanism of a hyperinflammatory state associated with the critical illness rather than the simple barotrauma effect resulting in the development of PNX in these patients. According to our experience, it seems reasonably to suggest for selected patients, particularly with cognitive impairment, that high-flow oxygen therapy, when applicable and indicated, or ventilation with protective lung settings may be a safer alternative to standard NIV in order to avoid the potentially fatal occurrence of pneumothorax. Moreover, early recognition of cytokine storm with specific inflammatory indexes and treatment of the inflammatory state in COVID patients may help to a better control of the infection and possibly to avoid the increased lung frailty in COVID/ARDS and its consequences. Further research is essential to identify early risk factors for this potentially fatal COVID-19 complication.
CRediT authorship contribution statement
B. Ragnoli: Conceptualization, Writing – original draft, preparation, All authors have read and agreed to the published version of the manuscript. T. Cena: statistical, Formal analysis, All authors have read and, agreed to the published version of the manuscript, Writing – review & editing. A. Radaeli: Conceptualization, All authors have read and agreed to the published version of the manuscript. P. Pochetti: Writing – original draft, preparation, All authors have read and agreed to the published version of the manuscript. . L. Conti: Writing – original draft, preparation, All authors have read and agreed to the published version of the manuscript. A. Calareso: Writing – original draft, preparation, All authors have read and agreed to the published version of the manuscript. . J. Morjaria: Writing – review & editing, All authors have read and agreed to the published version of the manuscript. Mario Malerba: Writing – review & editing, All authors have read and agreed to the published version of the manuscript.
References
- 1.European Centre for Disease Prevention and Control https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available online:
- 2.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy. JAMA. 2020;323:1545. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 5.Imperial College COVID-19 Response Team Report 17 - clinical characteristics and predictors of outcomes of hospitalised patients with COVID-19 in a London NHS Trust: a retrospective cohort study. www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-17-clinical/ Date last accessed: 14 May 2020. Date last updated: 29 April 2020.
- 6.Miró Ò., Llorens P., Jiménez S., Piñera P., Burillo-Putze G., Martín A., Martín-Sánchez F.J., García-Lamberetchs E.J., Jacob J., Alquézar-Arbé A. Spanish investigators on emergency situations team (SIESTA) network. Frequency, risk factors, clinical characteristics, and outcomes of spontaneous pneumothorax in patients with coronavirus disease 2019: a case-control, emergency medicine-based multicenter study. Chest. 2021;159:1241–1255. doi: 10.1016/j.chest.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.https://www.aifa.gov.it/aggiornamento-sui-farmaci-utilizzabili-per-il-trattamento- della-malattia-covid19 Available online:
- 8.https://www.flipsnack.com/SIAARTI/siaarti_raccomandazioni_per_la_gestione_del_paziente_criti/full-view. html Available online:
- 9.Zhou C., Gao C., Xie Y., et al. COVID-19 with spontaneous pneumomediastinum. Lancet Infect. Dis. 2020;20:510. doi: 10.1016/S1473-3099(20)30156-0. Published Online March 9, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loffi M, Regazzoni V, Sergi P et al. Spontaneous pneumomediastinum in COVID-19 pneumonia. Monaldi Arch. Chest Dis. 90 (4). 10.4081/monaldi.2020.1399. [DOI] [PubMed]
- 11.Wali A., Rizzo V., Bille A., Routledge T., et al. Pneumomediastinum following intubation in COVID-19 patients: a case series. Anaesthesia. 2020;75:1076–1081. doi: 10.1111/anae.15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg D.J., Naborsa C., Chandya D. Pneumothorax and pneumomediastinum in patients hospitalized with coronavirus disease 2019 (COVID-19) Heart Lung. 2021;50:386–387. doi: 10.1016/j.hrtlng.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elhakim T.S., Abdul H.S., Romero C.P., Rodriguez-Fuentes Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19 pneumonia: a rare case and literature review. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-239489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Duan J., Han X., et al. High incidence and mortality of pneumothorax in critically Ill patients with COVID-19. Heart Lung. 2021;50:37–43. doi: 10.1016/j.hrtlng.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinelli A.W., Ingle T., Newman J., et al. COVID-19 and pneumothorax: a multicentre retrospective case series. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.02697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong W., Agarwal P.P. Chest imaging appearance of COVID-19 infection. Radiol Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K., Zeng Y., Xie P., et al. COVID-19 with cystic features on computed tomography: a case report. Medicine. 2020;99 doi: 10.1097/MD.0000000000020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun R., Liu H., Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J. Radiol. 2020;21:541–544. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joynt G.M., Antonio G.E., Lam P., et al. Late-stage adult respiratory distress syndrome caused by severe acute respiratory syndrome: abnormal findings at thin-section CT. Radiology. 2004;230:339–346. doi: 10.1148/radiol.2303030894. [DOI] [PubMed] [Google Scholar]
- 20.Bonato M., Fraccaro A., Landini N. Pneumothorax and/or pneumomediastinum worsens the prognosis of COVID-19 patients with severe acute respiratory failure: a multicenter retrospective case-control study in the north-east of Italy. J. Clin. Med. 2021;10(21):4835. doi: 10.3390/jcm10214835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong W.H., Saha B.K., Hu K. The incidence, clinical characteristics, and outcomes of pneumothorax in hospitalized COVID-19 patients: a systematic review. Heart Lung. 2021;50:599–608. doi: 10.1016/j.hrtlng.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chopra A., Al-Tarbsheh A.H., Shah N.J., et al. Pneumothorax in critically ill patients with COVID-19 infection: incidence, clinical characteristics and outcomes in a case control multicenter study. Respir. Med. 2021;184 doi: 10.1016/j.rmed.2021.106464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marciniak S.J., James Farrell J., Rostron A., et al. COVID-19 pneumothorax in the UK: a prospective observational study using the ISARIC WHO clinical characterization protocol. Eur. Respir. J. 2021;58 doi: 10.1183/13993003.00929-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belletti A., Palumbo D., Alberto Zangrillo A., et al. Predictors of pneumothorax/pneumomediastinum in mechanically ventilated COVID-19 patients. J. Cardiothorac. Vasc. Anesth. 2021;35:3642–3651. doi: 10.1053/j.jvca.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellan M., Patti G., Hayden E., et al. Fatality rate and predictors of mortality in an Italian cohort of hospitalized COVID-19 patient. Scientifc Reports. 2020;10 doi: 10.1038/s41598-020-77698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertolotti M., Betti M., Ferrante D., et al. Mortality in Covid-19 patients hospitalized in a teaching hospital in Italy during the first 3 waves. EJPH. October 2022;32(3) doi: 10.1093/eurpub/ckac131.058. ckac131.058. [DOI] [Google Scholar]
- 27.De Rosa F.G., Palazzo A., Rosso T., et al. Risk factors for mortality in COVID-19 hospitalized patients in piedmont, Italy: results from the multicenter, regional, CORACLE registry. J. Clin. Med. 2021;10(9):1951. doi: 10.3390/jcm10091951. May. . Published online 2021 May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394Grasselli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grasselli G., Greco M., Zanella A., et al. M risk factors associated with mortality among patients with COVID-19 in intensive care units in lombardy, Italy. JAMA Intern. Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minnai F., De Bellis G., Dragani T.A., et al. COVID-19 mortality in Italy varies by patient age, sex and pandemic wave. Scientifc Reports. 2022;12:4604. doi: 10.1038/s41598-022-08573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonanad C., Sergio García-Blas S., Tarazona-Santabalbina F., et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. JAMDA. 2020;21:915e918. doi: 10.1016/j.jamda.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung C. Risk factors for predicting mortality in elderly patients with COVID-19: a review of clinical data in China. Mech. Ageing Dev. 2020;188 doi: 10.1016/j.mad.2020.111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Çoraplı G., Teki V. Evaluation of patients who developed pneumothorax due to COVID-19. Diagnostics. 2022;12:2140. doi: 10.3390/diagnostics12092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghafari M., Ansari H., Beladimoghadam N., et al. Neurological features and outcome in COVID-19: dementia can predict severe disease. J. Neurovirol. 2021;27:86–93. doi: 10.1007/s13365-020-00918-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson P.G., Qin L., Hon Puah. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020 Jul;213(2):54–56.e1. doi: 10.5694/mja2.50674. Epub 2020 Jun 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selickman J., Vrettou C.S., Mentzelopoulos S.D., Marini J.J. COVID-19-Related ARDS: key mechanistic features and treatments. J. Clin. Med. 2022;11:4896. doi: 10.3390/jcm11164896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guidi L., Palumbo D., De Cobelli F., et al. An increasing rate of pneumomediastinum in non-intubated COVID-19 patients: the role of steroids and a possible radiological predictor. Respiratory Investigation. 2022;60:865–e867. doi: 10.1016/j.resinv.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palumbo D., CampochiaroC BellettiA., et al. Pneumothorax/pneumomediastinum in non-intubated COVID-19 patients: differences between first and second Italian pandemic wave. Eur. J. Intern. Med. 2021;88:144–146. doi: 10.1016/j.ejim.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Battaglini D., Robba C., Ball L., Silva P.L., Cruz F.F., Pelosi P., et al. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: a narrative review. Br. J. Anaesth. 2021;127:353e64. doi: 10.1016/j.bja.2021.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shrestha D.B., Sedhai Y.R., Budhathoki P., et al. Pulmonary barotrauma in COVID-19: a systematic review and meta-analysis. Annals of Medicine and Surgery. 2022;73 doi: 10.1016/j.amsu.2021.103221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemmers D.H.L., Hilal M.A., Bnà C., et al. Pneumomediastinum and subcutaneous emphysema in COVID-19: barotrauma or lung frailty? ERJ Open Res. 2020;6:2020. doi: 10.1183/23120541.00385-2020. 00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tonelli R, Bruzzi G, Manicardi L et al. Risk factors for pulmonary air leak and clinical prognosis in patients with COVID-19 related acute respiratory failure: a retrospective matched control study. Front. Med. 9:848639.doi: 10.3389/fmed.2022.848639. [DOI] [PMC free article] [PubMed]
- 43.Reddy M.P., Subramaniam A., Chua C., et al. Respiratory system mechanics, gas exchange, and outcomes in mechanically ventilated patients with COVID-19-related acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir. Med. 2022;3(2022) doi: 10.1016/S2213-2600(22)00393-9. Published Online November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekhon M.S, Thiara S, Kanji H.D et al. Spontaneous pneumomediastinum in COVID-19: the Macklin effect? Am. J. Respir. Crit. Care Med. Vol 204, Iss 8, pp 989–990, Oct 15, 20s21. [DOI] [PMC free article] [PubMed]
- 45.Alon R., Sportiello M., Kozlovski S. Leukocyte trafficking to the lungs and beyond: lessons from influenza for COVID-19. Nat. Rev. Immunol. 2021;21:49–64. doi: 10.1038/s41577-020-00470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng L., Li H., Li L. Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J. Clin. Lab. Anal. 2020;34 doi: 10.1002/jcla.23618. 1 of 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonnweber Thomas, et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients' performance: a prospective observational cohort study. Respir. Res. 2020;21(1):276. doi: 10.1186/s12931-020-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malerba M., Barbieri M., Mondini L., Ruggero L., Trotta L., Beatrice Ragnoli. Interrelationship between thrombosis and COVID-19. Acta Scientific Medical Sciences. 2022;6(2):119–126. [Google Scholar]
- 49.Chang Li, Ye J., Chen Q., et al. Elevated Lactate Dehydrogenase (LDH) level as an independent risk factor for the severity and mortality of COVID-19. Aging (Albany NY) 2020 Aug 14;12(15):15670–15681. doi: 10.18632/aging.103770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henry B.M., Aggarwal G., Wong J., et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am. J. Emerg. Med. 2020 Sep;38(9):1722–1726. doi: 10.1016/j.ajem.2020.05.073. Epub 2020 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong X., Lu Sun L., Yan Li Y. Prognostic value of lactate dehydrogenase for in-hospital mortality in severe and critically ill patients with COVID-19. Int. J. Med. Sci. 2020;17(14):2225–2231. doi: 10.7150/ijms.47604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szarpak L., Ruetzler K., Safiejko K., et al. Lactate dehydrogenase level as a COVID-19 severity marker. AJEM (Am. J. Emerg. Med.) 2021;45:638–639. doi: 10.1016/j.ajem.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conrad C., Looney M.R. Is neutrophilic inflammation treatable in COVID-19? Lancet Respir. Med. 2022 Sep 2;S2213–2600(22) doi: 10.1016/S2213-2600(22)00293-4. 00293-00294. [DOI] [PMC free article] [PubMed] [Google Scholar]