Abstract
Subarachnoid haemorrhage (SAH) is the third most common subtype of stroke. Incidence has decreased over past decades, possibly in part related to lifestyle changes such as smoking cessation and management of hypertension. Approximately a quarter of patients with SAH die before hospital admission; overall outcomes are improved in those admitted to hospital, but with elevated risk of long-term neuropsychiatric sequelae such as depression. The disease continues to have a major public health impact as the mean age of onset is in the mid-fifties, leading to many years of reduced quality of life. The clinical presentation varies, but severe, sudden onset of headache is the most common symptom, variably associated with meningismus, transient or prolonged unconsciousness, and focal neurological deficits including cranial nerve palsies and paresis. Diagnosis is made by CT scan of the head possibly followed by lumbar puncture. Aneurysms are commonly the underlying vascular cause of spontaneous SAH and are diagnosed by angiography. Emergent therapeutic interventions are focused on decreasing the risk of rebleeding (ie, preventing hypertension and correcting coagulopathies) and, most crucially, early aneurysm treatment using coil embolisation or clipping. Management of the disease is best delivered in specialised intensive care units and high-volume centres by a multidisciplinary team. Increasingly, early brain injury presenting as global cerebral oedema is recognised as a potential treatment target but, currently, disease management is largely focused on addressing secondary complications such as hydrocephalus, delayed cerebral ischaemia related to microvascular dysfunction and large vessel vasospasm, and medical complications such as stunned myocardium and hospital acquired infections.
Introduction
Spontaneous subarachnoid haemorrhage (SAH) is the third most common type of stroke and frequently related to aneurysmal rupture.1 In this Seminar, we will primarily focus on aneurysmal SAH, but spontaneous SAH that lacks imaging evidence of aneurysms will also be discussed whereas traumatic or other non-spontaneous causes will be omitted. There is a trend towards a decreasing worldwide incidence of SAH2,3 possibly related to public health interventions and lifestyle modifications, due to reduced rates of smoking and management of hypertension.3–6 Poor-grade SAH is associated with high morbidity and mortality and is managed aggressively with advanced neurosurgical, interventional neuroradiological, and neurocritical care strategies that have shown good functional outcomes.7–11 Novel strategies to prevent and treat early brain injury, delayed cerebral ischaemia, seizures, and other medical complications might hold promise to improve outcomes further. Common data elements to standardise research efforts for patients with SAH have been published.12
Epidemiology and risk factors
Worldwide, almost 500 000 patients have aneurysmal SAH annually.13 An incidence of 8 cases of spontaneous SAH per 100 000 person-years was reported in a meta-analysis including 75 studies from 32 countries (analysing 8176 patients, 67 746 051 person-years).3 Despite regional differences, SAH incidence has declined over past decades from a high of 10 per 100 000 person-years in 1980 to 6 in 2010, or by 1·7% annually between 1955 and 2014. The incidence changes differ by country and range between a decrease of 59% in Japan to 14% in North America, which might be related to differences in smoking prevalence.3 Early case fatality remains high, as up to one-quarter of patients with aneurysmal SAH do not reach the hospital or die in the emergency room.14
The underlying causes of decreasing SAH incidence are uncertain but are possibly related to improved management of hypertension and the cessation of smoking. On a population basis, the incidence of SAH—adjusted by age and sex—decreased by 7·1% per mm Hg reduction in systolic blood pressure and by 2·4% with every percent decrease in smoking prevalence.3 Aspirin use has been associated with a decreased risk of aneurysm rupture, but also with an increased risk of rebleeding once rupture has occurred.15 A notable exception to the overall decreasing rates of aneurysmal SAH is China. Stroke burden (including SAH) is increasing in China, particularly in rural areas, and is blamed on the quality of primary stroke prevention, socioeconomic factors, and the high prevalence of risk factors such as hypertension and smoking.6 Worldwide, women have a 1·3 times greater relative risk of developing SAH than men. Furthermore, the mean age of patients with SAH increased from 52 to 62 years between 1973 and 2002.7
The prevalence of unruptured intracranial aneurysms is estimated at approximately one in 20–30 adults16 (3·2%) in a population without diagnosed comorbidity. The mean age of patients with unruptured aneurysms is 50 years, and the prevalence is twice as high in women as in men.17 Widespread imaging for headaches and non-specific complaints has yielded large cohorts of patients with unruptured aneurysms. Familial clustering of intracranial aneurysms and aneurysmal SAH clearly suggest a genetic contribution and genome-wide association studies identified a number of loci with common variants.18 Part of the genetic correlation with intracranial aneurysms might be driven by genetic risk factors for potentially modifiable conditions and behaviours including smoking and hypertension.19 A genetic predisposition for insomnia raised the risk of both aneurysm formation and aneurysmal SAH, but these findings need replication. A positive family history of aneurysmal SAH, defined as two affected first-degree relatives, accounts for 11% of events whereas autosomal dominant polycystic kidney disease accounts for 0·3% of events.20
Hypertension, smoking, and female sex have been identified as the most consistent risk factors for unruptured intracranial aneurysms.4,5 Screening for intracranial aneurysms with MR or CT angiography might be beneficial in patients with two or more first-degree relatives who have had aneurysmal SAH.21 Among people without a history of aneurysm but with a family history, the rate of de novo aneurysm detection was 3% in individuals who had previously had two negative screens.21
Hypertension, older age, larger aneurysm size, posterior circulation aneurysm, and irregular aneurysm shape (ie, those with a daughter sac) have been implicated as risk factors for aneurysmal rupture.22–24 Based on pooled analysis of individual patient data from 8382 participants in six prospective cohort studies, a prediction tool for rupture of incidentally diagnosed aneurysms was developed called the PHASES score.22 The score generated by the prediction tool takes into account the population (P), hypertension (H), patient age (A), aneurysm size (S), earlier SAH (E), and site of aneurysm (S). Haemodynamic stress and vessel wall injury and the inhibition of its repair have all been linked to hypertension, smoking, cocaine ingestion, heavy alcohol use, oestrogen compounds, hyper-cholesterolaemia, and diabetes as risk factors for aneurysm formation and rupture.25–28
Clinical presentation, signs and symptoms, and diagnosis
The most common clinical presentation of spontaneous SAH is sudden onset of thunderclap headaches that are very painful, unexpected, and intense from the onset.29 These headaches are the only symptom in half of SAH cases. The presentation can be non-specific and headaches are one of the most common complaints of patients seen in an emergency room. Signs and symptoms in patients with aneurysmal SAH can occur due to mass effect or embolic strokes from the aneurysm itself, or from direct or indirect effects of the haemorrhage.
Loss of consciousness is seen in between 26% and 53% of patients with aneurysmal SAH,30–32 a range likely to be explained primarily by referral bias. This disorder of consciousness might be brief or more prolonged and is associated with poor clinical grade, more subarachnoid and intraventricular blood on admission CT, and higher frequency of global cerebral oedema. Other symptoms of aneurysmal SAH include neck pain or stiffness, emesis, focal cranial nerve abnormalities (ie, third nerve palsy with posterior communicating artery aneurysms), seizures, or focal supratentorial deficits such as hemiparesis. The neurological examination is categorised using World Federation of Neurological Surgeons33 or Hunt Hess34 scales (table).
Table:
Clinical and imaging scales
| Clinical grading scale | Imaging grading scale | |||
|---|---|---|---|---|
| WFNS scale | Hunt Hess scale | Modified Fisher scale | SEBES | |
| Prediction purpose | Outcome | Outcome | Delayed cerebral ischaemia | Quantify oedema |
| Range | 1–5 | 1–5 | 0–4 | 0–4 |
| Criteria | ·· | ·· | Quantify location and extent of SAH and IVH | At two levels in each hemisphere: (1) effacement of sulci or (2) disruption of grey-white matter |
| Grade | ||||
| 0 | ·· | ·· | No SAH | ·· |
| 1 | GCS 15 | Mild headache | Thin SAH, no IVH | ·· |
| 2 | GCS 13–14, no motor deficit | Moderate to severe headache, cranial nerve palsy, nuchal rigidity | Thin SAH, with IVH | ·· |
| 3 | GCS 13–14, with motor deficit | Lethargy or confusion, mild focal deficit | Thick SAH, no IVH | ·· |
| 4 | GCS 7–12 | Stupor, hemiparesis | Thick SAH, with IVH | ·· |
| 5 | GCS 3–6 | Coma, decerebrate posturing | ·· | ·· |
WFNS=World Federation of Neurological Surgeons. SEBES=subarachnoid hemorrhage early brain oedema score. GCS=Glasgow coma scale. SAH=subarachnoid haemorrhage. IVH=intraventricular haemorrhage.
Misdiagnosis of patients with good-grade SAH continues to be a problem, putting patients at higher risk of rebleeding, delayed cerebral ischaemia (DCI), and procedural complications.35 Particularly in resource-limited settings, clinically based decision instruments enable alert patients with headache that warrant additional diagnostic studies to be identified. The Ottawa SAH rule29 incorporates information on demographics and clinical presentation to trigger additional diagnostic testing: age 40 years or older, neck pain or stiffness, witnessed loss of consciousness, onset of headache during exertion, instantaneous onset of headache, or limited neck flexion on examination. This scale applies only to alert patients older than 15 years, with new severe non-traumatic headache reaching a maximum intensity within 1 h, and without new neurological deficits, a history of aneurysms, SAH, brain tumours, or recurrent headaches. This scale allows practitioners to rule out SAH without requiring a CT scan in some low-risk patients, or would prompt a CT scan to seek SAH or other bleeding in high-risk patients (sensitivity for SAH 100%, specificity 12·7%).36
Diagnostic investigations
Diagnosis of SAH is based on pre-test probability accounting for history and clinical examination. This traditionally relies on non-contrast head CT scanning (figure 1), followed by lumbar puncture if CT is negative for patients with clinical symptoms concerning for SAH. CT timing in relation to onset of clinical symptoms determines the sensitivity to identify subarachnoid blood. A CT within 6 h of a thunderclap headache can have 98·7% sensitivity.37 In a multicentre, implementation science study of 3672 patients combining the Ottawa SAH rule with information obtained from CT scans acquired within 6 h of headache onset, the diagnosis of SAH can be made with some reliability (sensitivity 95·5%, specificity 100%); importantly SAH might be missed in 5% of patients.36 Given the gravity of missing an aneurysmal SAH diagnosis and the high morbidity associated with rebleeding, these decision rules have limited impact for patients in resource-rich settings.38 Conditions that mimic SAH include pseudo SAH following primary cardiac arrest, which has a distinct CT appearance dissimilar to true SAH.39 MRI is currently not widely used to diagnose aneurysmal SAH, but some studies demonstrate a similar performance to CT.40
Figure 1: Neuroimaging of patients with aneurysmal subarachnoid haemorrhage.
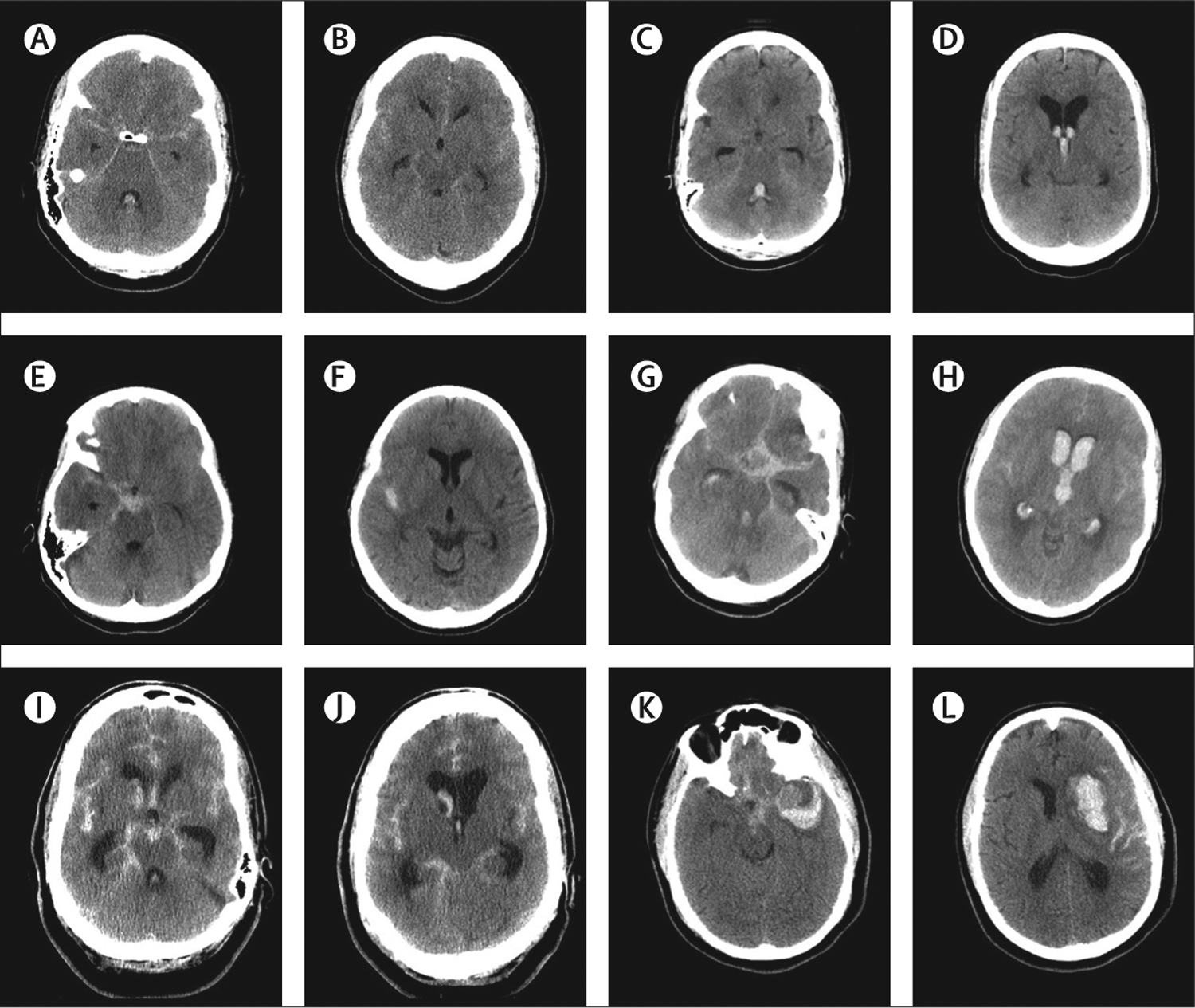
Head CT demonstrating modified Fisher scores 1 (A, B), 2 (C, D), 3 (E, F), 4 (G, H), hydrocephalus (I, J), and intracerebral haemorrhage (K, L).
Vascular imaging, such as CT angiography or digital subtraction angiography, should be obtained for all patients presenting without recent trauma or imaging evidence of SAH (typically head CT) or lumbar puncture. Angiography will not identify an aneurysm in approximately 15% of patients41 and half of these are perimesencephalic SAH.42 Repeat angiography reveals aneurysms in 2–8% of those with initially negative vascular imaging42 with a slightly higher incidence of aneurysms in those with a family history of aneurysmal SAH.43 30% of patients with SAH have multiple aneurysms44 and a ruptured aneurysm might be identified on the basis of blood location from the initial CT scan, and aneurysm size and morphological features.45,46
Perimesencephalic SAH
Perimesencephalic SAH is a type of non-aneurysmal spontaneous SAH with a distinct imaging appearance and a more benign clinical course. The diagnosis is made from a CT scan (obtained within 72 h of onset) and increasingly with a single high-quality CT angiogram.47 The CT scan demonstrates predominance of subarachnoid blood immediately anterior to the midbrain or pons, with smaller amounts of blood extending to the perimesencephalic and basal Sylvian cisterns, foramen magnum, interhemispheric fissure, and lateral ventricles. CT angiography excludes aneurysms as the bleeding cause. Additional diagnostic work-up (including digital subtraction angiography) is controversial as state-of-the-art CT angiography is highly sensitive for the detection of aneurysms.48 The incidence of perimesencephalic SAH is approximately 0·5 per 100 000 person-years and men are affected slightly more often than women (unlike aneurysmal SAH), but no clear risk factors have been identified. Complications such as rebleeding, hydrocephalus, DCI, and poor outcomes are rare.
Differential diagnosis
SAH diagnosis relies on obtaining an accurate history that then triggers the diagnostic work-up outlined above. The differential for patients with spontaneous SAH who have no aneurysm found on angiography is broad and includes but is not limited to perimesencephalic SAH, reversible cerebral vasoconstriction syndrome (RCVS),49 and cortical vein thrombosis. Trauma is the underlying cause in most patients who present with SAH, and trauma history might not always be obvious. The clinical course and management of patients with traumatic SAH is fundamentally different and not the focus of this Seminar. Importantly, aneurysmal SAH with associated loss of consciousness might cause secondary traumatic brain injury. The blood pattern in classic traumatic SAH is predominantly located in the cortical sulci50 whereas the location of aneurysmal SAH is usually in the basal cisterns. Although rare, intracranial artery dissection from trauma can also result in SAH.51 RCVS is a clinical diagnosis and acquiring an accurate history is crucial. The RCVS2-score takes into account thunderclap headache, carotid artery involvement, presence of vasoconstrictive triggers, sex, and presence of SAH and provides a structured approach to diagnosis of RCVS.49
Acute management
Aneurysm treatment
Despite differences in health-care systems, admission to high-volume centres—defined as institutions that care for at least 35 patients with SAH annually—is an important predictor of good outcomes.52–54 Aneurysm treatment within 72 h of symptom onset is recommended.55 Treatment within this timeframe compared with treatment within the first 24 h of symptom onset did not consistently improve outcomes in a meta-analysis of 4667 patients with poor-grade aneurysmal SAH,56 but earlier treatment remains an active topic of discussion.
Much of the initial management is focused on minimising the risk of rebleeding (figure 2). In natural history studies, this complication is seen in 8–23% of patients and carries a very high morbidity and mortality.57 Patients with a high clinical grade, hypertension, intracerebral or intraventricular haemorrhage, and larger aneurysms are at particular rebleeding risk, especially early after the bleed.58 Short-term antifibrinolytic therapy was considered as a strategy to reduce the rebleeding risk as most rebleeding occurs within 24 h of SAH onset.59 In the ultra-early tranexamic acid after subarachnoid haemorrhage study, 480 patients were randomised to receive 24 h of tranexamic acid (1 g bolus followed by 1 g given every 8 h) and 475 received standard care.60 Aneurysm repair was performed within 24 h with a median of 14 h from onset to treatment. There was no difference in the primary study endpoint, defined as a good clinical outcome (modified Rankin scale score 0–3) at 6 months, between treatment arms (odds ratio [OR] 0·86, 95% CI 0·66–1·12). Rebleeding after randomisation and before aneurysm treatment occurred at similar rates in both groups (10% with tranexamic acid and 14% in controls; OR 0·71, 95% CI 0·48–1·04). This trial demonstrated the lack of benefit from short-term tranexamic acid administration if aneurysm securement is early. Patients for whom early aneurysm treatment cannot be performed might still benefit from short-term antifibrinolytic therapy, but risks of this approach are uncertain given the available data. Hypertension is frequently encountered following SAH and might promote rebleeding. Most treatment protocols aim to keep systolic blood pressure below a threshold with cutoffs varying between 160 mm Hg and 180 mm Hg.55,61 The benefits of platelet transfusions outside of throm-bocytopenia remain uncertain.62
Figure 2: Management approach for patients with aneurysmal subarachnoid haemorrhage.
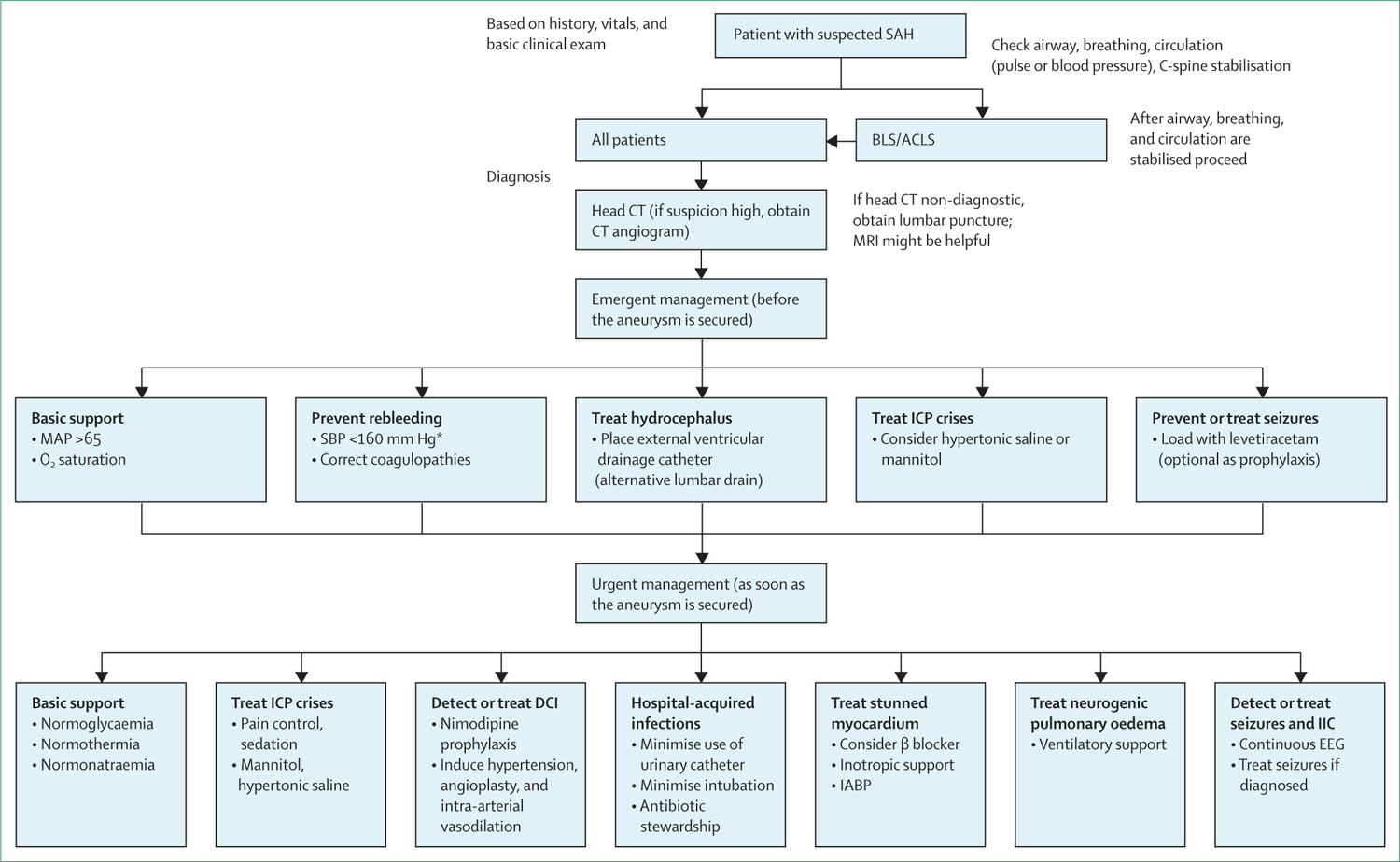
SAH=subarachnoid haemorrhage. BLS=basic life support. ACLS=advanced cardiac life support. ICP=intracranial pressure. MAP=mean arterial pressure. SBP=systolic blood pressure. DCI=delayed cerebral ischaemia. IIC=ictal-interictal continuum. IABP=intra-aortic balloon pump. EEG=electroencephalogram. *As per US guidelines (European guidelines recommend <180 mm Hg).
Aneurysm treatment is focused on preventing blood entry into the aneurysm (eg, clipping or coiling). The International Subarachnoid Aneurysm Trial (ISAT), the largest interventional randomised aneurysmal SAH clinical trial to date, randomly assigned 2143 patients with aneurysmal SAH to clipping or coil embolisation. Endovascular coiling was associated with a higher chance of independent survival than clipping 1 year after the haemorrhage and among patients suitable for both treatments,63 with a persistently lower chance of death or dependency measured by the modified Rankin scale score at 10 years.64 There was a persistent but small increase in rates of rebleeding among patients who had undergone coiling in a study with 10–18·8 year follow-up, but the risk of death and disability from rebleeding was small (one in 1397 patient-years in the endovascular group and one in 2041 patient-years in the neurosurgery group).64 Among ISAT patients who survived hospitalisation, there was no difference in functional recovery at 5 years65 or beyond.64 In post-hoc analysis, ISAT investigators suggested that pretreatment delays might have been to blame for higher in-hospital mortality for patients who underwent clipping.66 In a large multicentre examination of coiling versus clipping in clinical practice across 24 centres from Europe, USA, and Australia (n=9159), coiling was associated with higher 14-day case fatality and non-superior 90-day outcomes.67 Discussions are ongoing regarding the generalisability of these findings related to case ascertainment, group differences such as pretreatment delays, technical advances over the past two decades since the last enrolment into ISAT, and unbalanced operator experience. Apart from complications unique to clipping (eg, retraction injury) or coiling (eg, coil migration), all approaches might be complicated by intraprocedural rupture.
Advances in microsurgical and interventional neuroradiological techniques provide novel treatment options even for challenging aneurysms such as wide-necked or bifurcation aneurysms, blister aneurysms, or arteries arising from within the aneurysm. In selecting treatment approaches, physicians expertise as well as location of the aneurysm, aneurysm characteristics (ie, branch arteries incorporated into the aneurysm dome, size, neck width, fusiform shape, and calcification), and the parent artery anatomy (ie, bifurcation aneurysm or parent artery flow dynamics) must be considered. Intravascularly placed devices might need antiplatelet medications, which complicates management of patients with SAH who need ventricular drain or shunt placements. A comprehensive discussion of all possible neurosurgical and interventional neuroradiological treatment approaches is beyond the scope of this Seminar as innovative technological advances are constantly providing novel opportunities to manage challenging cases.
Several secondary complications can occur after aneurysmal SAH, including early brain injury, elevated intracranial pressure (ICP), and DCI. The underlying pathophysiology is complex, probably overlapping, and only partly understood. Frequently implicated mechanisms include inflammation, microvascular dysfunction, dysfunction of brain metabolism, haematological abnormalities, and cortical spreading depolarisation. Better understanding of these pathomechanisms might help apply targeted therapies in clinical practice.
Early brain injury
Early brain injury is an emerging concept of injury sustained within the first 72 h following aneurysmal rupture with global cerebral oedema as the most prominent imaging manifestation.68 Early brain injury is associated with cerebral metabolic distress,69 inflammation,70,71 platelet activation,71 and cortical spreading depolarisation.72 Animal models implicate early activation of the innate immune response followed by microglia and astrocytic activation73 with disruption of the blood–brain barrier.74 Early brain injury, as defined by imaging criteria, is common and the risk of poor outcomes is doubled in patients with early brain injury compared with patients without early brain injury.68 Brain oedema can be quantified using the subarachnoid haemorrhage early brain oedema score (SEBES),75 which was recently found to be associated with ICP and outcomes in a cohort of 745 patients.76 Novel treatments for early brain injury are being studied that might have a potentially larger impact than interventions aimed at vasospasm or DCI.
ICP, herniation, and hydrocephalus
Patients with poor-grade aneurysmal SAH can develop mass effect from intracerebral haemorrhage or brain oedema. Surgery to remove clots or hemicraniectomy can decrease mortality. Hypertonic saline is as effective as mannitol for treatment of increased ICP in aneurysmal SAH; however, the optimal dose and treatment strategy, or whether it improves outcomes, is unclear.77 Hydrocephalus (figure 1) is seen in 12–31% of patients with aneurysmal SAH depending on factors such as hospital ascertainment and presence of intraventricular blood,78,79 with casting of the fourth ventricle portending a poor prognosis.80 Emergent management includes placement of an external ventricular drainage catheter, which is complicated by tract haemorrhages in approximately 15% of patients,81 most being clinically insignificant. Little data exist on the safety of neurosurgical procedures for patients taking dual antiplatelets. A single-centre case series suggests that external ventricular drainage (EVD) placement with dual antiplatelets (which might be started after aneurysm treatment using stent-assisted coiling or flow-diverters) was associated with a higher risk of additional haemorrhage than without dual antiplatelets (27% vs 8%), but no increase in symptomatic bleeding was seen.82 In a preliminary, retrospective cohort study, the same investigators found no increase in bleeding rates in patients undergoing ventriculoperitoneal shunt placement with dual antiplatelets.83 Methodological limitations of this study do not allow conclusions about the safety of this approach. Ventriculitis might complicate the course in approximately a quarter of patients managed with a ventricular drain, but diagnosis is largely clinical due to the unreliability of standard biochemical and microbiological measures.84
DCI
DCI occurs in 20–30% of patients with aneurysmal SAH and continues to have a substantial impact on outcome.85,86 Traditionally, large vessel vasospasm induced by haemoglobin and other blood products had been thought to be the primary cause of DCI, but recent evidence suggests a more complex and multifactorial mechanistic understanding. Predictors of DCI and vasospasm include poor neurological examination (ie, high World Federation of Neurosurgical Surgeons score), high amounts of subarachnoid and ventricular blood, large aneurysm size, cigarette smoking, hyperglycaemia, hydrocephalus, pre-existing diabetes, and female sex.87,88 Patients with spontaneous SAH but no aneurysm detected on angiography rarely develop DCI, even if the blood pattern on CT scan suggests an underlying aneurysm.89 However, patients with non-aneurysmal SAH require cerebrospinal fluid (CSF) diversion at similar rates to those with aneurysmal SAH if the blood pattern on imaging is similar to the classic aneurysmal SAH pattern and at much higher rates when compared with patients with perimesencephalic SAH.89
Timely detection or, if possible, prediction of DCI is crucially important for management of DCI. A combination of clinical and radiological factors can both increase the pre-test probability and should factor into clinical monitoring for DCI, including loss of consciousness at ictus and high clinical grade with large amounts of subarachnoid and intraventricular blood (captured by scales including the Hijdra score and modified Fisher scale; figure 1).90–94 Diagnosis is primarily based on the clinical examination but, particularly in poor-grade patients, clinical changes can go undetected even in carefully monitored settings. Most protocols rely on serial neurological assessments for timely detection of DCI possibly supported by an assessment of vasospasm, such as transcranial doppler, CT angiography and CT perfusion studies, or digital subtraction angiography.95 Whereas transcranial doppler is less frequently used for the detection of vasospasm in the USA,96 a recent meta-analysis suggests some benefit over digital subtraction angiography97 (figure 3). Follow-up catheter angiography remains widely practised as it allows not only diagnosis, but also administration of vasodilators to prevent stroke.
Figure 3:
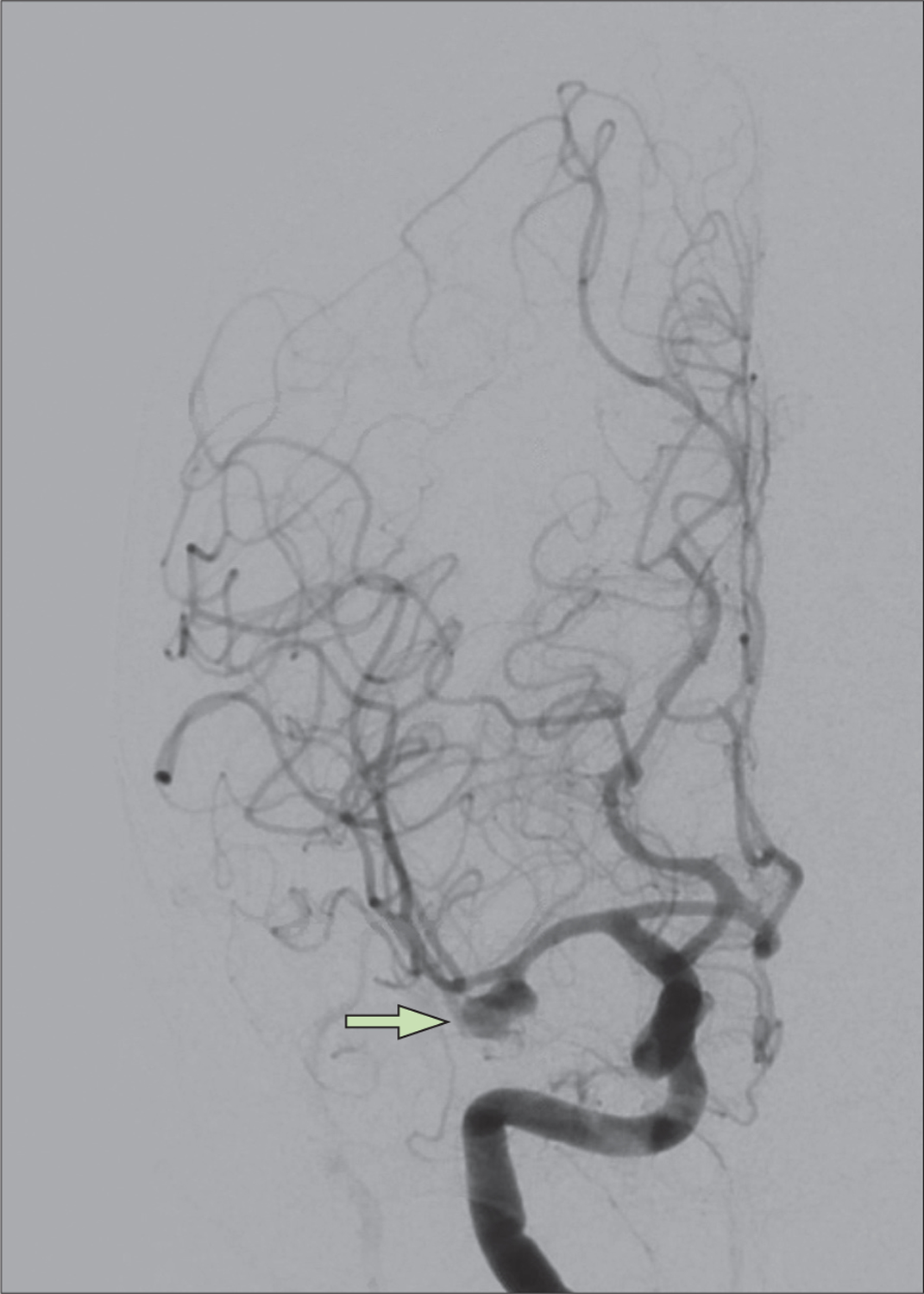
Digital subtraction angiography of patient demonstrating right, multilobulated middle cerebral artery bifurcation aneurysm
Several novel imaging and electrophysiological approaches have been introduced to detect DCI onset. Quantitative electroencephalogram (EEG) measures can be incorporated into prediction algorithms to generate pre-test probability scores for DCI for a patient on any given day.98 CT angiography alone might not be reliable for diagnosing vasospasm,99 but early CT perfusion might have promise for predicting DCI.100 Other approaches in comatose patients with SAH at high risk of DCI can incorporate invasive brain monitoring including brain tissue oxygen, cerebral microdialysis, electrocorticography, and intracortical EEG.101,102 Exploring physiological data recorded as part of general critical care, such as heart rate and blood pressure, might offer low-cost approaches to detect DCI in the future.103
DCI and outcomes did not improve despite lower vasospasm rates in the CONSCIOUS-2 and 3 trials,104,105 supporting the notion of a more complex underlying pathophysiology.106 Implicated mechanisms include vascular dysfunction with a larger focus on the microvasculature, systemic inflammation, cortical spreading depolarisation, and autoregulatory failure. The initial ICP elevation during aneurysm rupture with ictal global hypoperfusion has been implicated as a trigger for microvascular dysfunction. Additionally, microthrombosis in distal arteries might play a role.107
Prophylactic administration of oral nimodipine, but not other calcium-channel blockers, is supported by systematic meta-analyses based largely on one randomised clinical trial108 and is recommended in clinical guidelines. Despite having potentially advantageous bioavailability, sustained release microparticle formulations of nimodipine (EG-1962) did not decrease the rate of vasospasm or reduce poor outcomes.109 The long-term goal in the acute management of aneurysmal SAH includes the administration of targeted cerebro-protective agents110 but, other than nimodipine, none have thus far been identified.111
Some studies have indicated lower rates of DCI and better outcomes with low-dose heparin112 or antiplatelet therapy.113 Recent studies also suggest that the meningeal lymphatic system has a central role in the clearance of erythrocytes from the CSF in aneurysmal SAH.114 Little is known about the potential role of dysfunction of the paravascular glymphatic system in promoting neuroinflammation in DCI.115 Numerous studies have implicated inflammation in the pathophysiology of DCI but, on closer inspection, many investigators have explored vastly divergent mechanisms and insufficiently differentiated between physiological responses to the initial injury and pathophysiological events that further promote damage. Systemic pro-inflammatory cytokines and cellular mediators including myeloid and lymphoid lineage cells are elevated following aneurysmal SAH, correlating temporally with the development of DCI. Studies implicate microglial activation mediated by inflammation in brain injury, attributed to DCI and poor outcome.116,117 Spreading depolarisations are large depolarising waves that propagate slowly across the cortex and reflect metabolic collapse with underlying ATP-dependent ion pump breakdown.118 In SAH animal models and patients with aneurysmal SAH, spreading depolarisation has been implicated in the DCI mechanism,119 independent of angiographic vasospasm.120 Autoregulatory failure is seen as a downstream process in aneurysmal SAH and other acute brain injuries, probably related to several underlying mechanisms, further promoting brain injury.121 These insights into potential DCI mechanisms open up the prospect for novel approaches for DCI prevention and the hope to improve outcomes.
To treat patients with DCI, induced hypertension is practised widely even though high-level evidence to support this approach is lacking. A large retro spective, multicentre, observational study found induced hypertension to be an effective strategy for preventing DCI-related cerebral infarcts.122 A randomised controlled, open-label trial was stopped early due to lack of effect on cerebral perfusion and slow enrolment.123 Early clinical improvement in the group that underwent induced hypertension did not translate into better long-term functional outcomes. Hypervolaemia and haemodilution, part of the abandoned concept of triple-H therapy, might be harmful.124 Failure of medical treatment approaches to treating DCI might be predicted by neurogenic cardiac injury.125 Excessive induced hypertension can be complicated by posterior reversible encephalopathy syndrome (PRES),126 seizure, and ictal-interictal electrical activity.127 In an open-label, single-site pilot study (n=108), a targeted approach involving advanced haemodynamic monitoring with algorithmic goal-directed blood pressure interventions reduced DCI rates and improved functional outcomes at 3 months.128 The benefits of balloon angioplasty are controversial, but a recent case series suggests possible benefits from intra-arterial administration of vasodilators, if applied early and repeatedly in severe vasospasm.129 Early magnesium administration following SAH (within 6 h of the bleed) to supranormal levels was not beneficial in a meta-analysis of five randomised controlled trials.130
Seizures
Seizures might be seen at the time of haemorrhage, during acute hospitalisation, and as a long-term consequence of aneurysmal SAH. Underlying mechanisms for seizures following aneurysmal SAH are incompletely understood but might include neuroinflam mation131,132 and cerebral hyperaemia.127 Both phenomena might be seen immediately before seizure onset as well as during the seizure and feed into a vicious cycle promoting a pro-ictal state. Many in-hospital seizures are exclusively electrographic and some are recorded only on intracortical electrodes.133 Electrographic seizures following aneurysmal SAH have been associated with poor functional and cognitive outcomes.134 It remains unclear exactly how seizures affect outcome, but in acute brain injury, compensatory mechanisms for increased metabolic demand might be impaired.135 Long-term epilepsy is infrequent but associated with worse outcomes136 and is less likely in patients who underwent coil embolisation.137 Before aneurysm treatment, short-term seizure prophylaxis might be considered given the fear of rebleeding,61 but administration beyond this very early phase is highly controversial. Risk stratification using the seizure after aneurysmal subarachnoid haemorrhage risk (SAFARI) score might allow identification of high-risk patients who could benefit from prophylaxis, but this remains unproven. The SAFARI score takes into account patients aged 60 years and older, seizure before hospitalisation, ruptured anterior circulation aneurysm, and hydrocephalus requiring CSF diversion.138 A small, underpowered, prospective, single-centre, randomised, open-label trial (duration of prophylaxis after subarachnoid haemorrhage trial) suggests that prolonged seizure prophylaxis with levetiracetam might only benefit patients with aneurysmal SAH with imaging evidence of early brain injury.139
Medical complications
Medical complications are frequently seen in patients with severe aneurysmal SAH and have a major impact on outcomes.140,141 General critical care complications including bacteraemia, central line infections, urinary tract infections, aspiration pneumonia, and deep venous thrombosis are common and require meticulous critical care strategies for prevention, early detection, and targeted treatment.140 Hospital-acquired infections are an established contributor to poor outcomes and have been associated with nutritional deficiencies, specifically low glutamine levels.142 Normoglycaemia is a goal of critical care, but overly aggressive insulin strategies can lead to intracerebral hypoglycaemia in aneurysmal SAH.143 Anaemia has been associated with poor outcomes,144 but whereas guidelines61 support transfusions for patients who are clearly anaemic (haemoglobin <7 g/dL), higher haemoglibin targets are controversial.145 Adequate oxygenation is a cornerstone of resuscitation strategies, but hyperoxia should be prevented.146 Initiation of chemical deep vein thrombosis prophylaxis using unfractionated heparin within 24 h of aneurysm treatment is extrapolated from other patient populations and is supported by guidelines. It is associated with low bleeding risk,147 but controversy persists.148 Optimal fluid resuscitation strategies are unclear, and simply following the fluid balance is not as valuable as understanding volume status and cerebral blood flow. Higher fluid intake has been associated with hospital complications and poor outcome,149 but causality is unproven. Avoiding hypovolaemia is likely to be crucial, and fluid titration is encouraged to achieve this.
Cardiac dysfunction (biomarker, electrocardiographic, or wall-motion abnormality) is associated with greater risk of DCI and poor outcomes.150 Stress cardiomyopathy is common in patients with aneurysmal SAH, affecting the heart globally with severely decreased left ventricular ejection fraction or regional wall-motion abnormalities.151 The clinical challenge is to differentiate stress cardiomyopathy from acute coronary syndromes, as both disorders can share the characteristics of chest pain, electrocardiogram changes, and elevation in cardiac troponin.152 The admission electrocardiogram can be helpful in differentiating these.153
Respiratory dysfunction can develop, particularly in patients with high-grade aneurysmal SAH,154,155 and can result from neurogenic pulmonary oedema, fluid overload from DCI management, left heart dysfunction, and adult respiratory distress syndrome. Hyponatraemia following aneurysmal SAH due to the syndrome of inappropriate antidiuretic hormone or cerebral salt-wasting156,157 is common and complicated by longer stays in intensive care units and hospitals.158 In practice, it is difficult to differentiate between these and fluid restriction can be harmful for patients with aneurysmal SAH. Management focuses on maintaining euvolaemia. Hyponatraemia is of particular concern in patients with elevated ICP and brain swelling as it can promote both. For these patients, administration of sodium chloride solution (eg, 2% hypertonic saline) or fludro-cortisone can be pursued. Patients with aneurysmal SAH often have fevers, which has been linked to worse outcomes. Central fevers can occur with or without infections, leading to potentially unnecessary antibiotic administration in some patients.159 Fever prevention can reduce secondary brain injury160 and improve outcomes,161 as brain hypoxia worsens with fever after aneurysmal SAH. Pharmacological antipyretics might not be sufficient, so adjunctive strategies for normothermia might be needed.
Approximately 22% of patients with aneurysmal SAH who are acutely managed with EVDs will need permanent CSF diversion and require shunt placement, most commonly connecting the ventricles to the peritoneal space. Prevalence of shunt dependency varies widely (18–27%)162 and patients requiring a shunt have worse long-term functional outcomes.163 The optimum modes of CSF drainage (intermittent vs continuous), EVD weaning (rapid vs gradual), and EVD wean timing (following aneurysm securement vs time of vasospasm risk) are uncertain.164 Early mobilisation might be beneficial for all patients with aneurysmal SAH including those with EVDs.165
Timing of tracheostomy and percutaneous endoscopic gastrostomy is controversial. A retrospective case series of haemorrhagic stroke, including aneurysmal SAH, suggests that early tracheostomy and percutaneous endoscopic gastrostomy might be associated with decreased length of stay.166 However, no difference was seen in 6-month functional outcomes in 382 patients with severe ischaemic stroke, intracerebral haemorrhage, or aneurysmal SAH randomly assigned to early (≤5 days of intubation) versus delayed tracheostomy (≥10 days).167
Long-term management
After hospital discharge, patients with aneurysmal SAH should be followed up by a neurologist or neurosurgeon. Repeat vascular imaging is often obtained, especially in patients who had aneurysm coiling, given concerns about long-term durability.64 Given the technological advances in imaging technology, gadolinium-enhanced MR angiography is emerging as an alternative to digital subtraction angiography for follow-ups. Up to 8% of patients are readmitted within 30 days, and 14% of these have potentially preventable conditions (eg, dehydration, pneumonia, and urinary tract infection).168 Long-term antiseizure medications are only considered for patients with seizures during hospital admission or following hospital discharge. Patients with aneurysmal SAH frequently remain on long-term antipsychotic, pain, and antiseizure medications, which can be decreased with appropriate follow-up.169
Outcomes
Large meta-analyses have shown that case fatality rates of patients with aneurysmal SAH have decreased in the past decades by 0·8% per year, which might be linked to improved surgical and medical management.7 Similar to other acute brain injuries, most patients with poor-grade illness die following withdrawal of life-sustaining therapies.170 Up to a third of SAH survivors will experience decreased quality of life, attentional deficits, cognitive impairment, depression, and reduced activities of daily living.171,172 Poor cognitive outcomes have been linked to DCI, white matter abnormalities, and pituitary gland volume loss.173–175 Results from a large cohort of patients with aneurysmal SAH, who were followed up over 10 years within ISAT, suggested that quality of life in patients who underwent coiling was superior to those who underwent clipping.9 Standardised neuropsycho-logical assessments might predict long-term return to work in patients with aneurysmal SAH.176 The potential for good functional outcomes in patients with poor-grade aneurysmal SAH supports aggressive early resuscitation strategies.10
Prediction of long-term outcomes is challenging;177 however, families demand early guidance. Based on population statistics, prognostication scores such as the functional recovery expected after subarachnoid haemorrhage score,178 SAFIRE score,79 and subarachnoid haemorrhage international trialists score179 have been developed. Nihilism based on age should be avoided, as favourable outcomes are not infrequent in older adults.11 The limited relevance of these scores to prognosticating in individual patients cannot be overstated. To achieve more precise outcome predictions, a personalised medicine approach incorp orating biomarkers and genetics might be more promising.180
Differences in resource allocation are major drivers of disparities in aneurysmal SAH outcomes and need to be addressed in health-care reform efforts going forward.181 Improvements in neurological services including the application of a multidisciplinary approach, specialised intensive care units, and focused management protocols might be associated with better outcomes for patients with aneurysmal SAH in both high-income183 and low-and-middle income countries.184
Future directions
Common data elements aimed at standardisation of research efforts for patients with SAH will facilitate future studies.12
Aneurysm treatment
Worldwide, most aneurysms are treated with coil embolisation and ongoing trials are addressing the benefits of clipping versus coiling in patients eligible for clipping who were underrepresented or excluded from previous trials such as ISAT.185 Numerous ongoing trials are exploring the benefits of newly developed neurosurgical and neuroradiological technology to allow for treatment of the most challenging aneurysms.
Management of secondary complications
Patients with poor-grade aneurysmal SAH are at higher risk of occult DCI, and might warrant invasive multimodality neuromonitoring for brain hypoxia or ischaemia, or metabolic or electrographic derangements to trigger timely intervention.102 An optimal cerebral perfusion pressure (CPP) might exist for patients with high-grade aneurysmal SAH, below which there is increased risk of brain hypoxia and poor outcome.186 ICP and CPP monitoring alone might be insufficient to detect cerebral compromise, as brain hypoxia and metabolic crisis can exist with normal ICP.187 Invasive monitoring including cerebral microdialysis can identify ischaemic patterns that precede clinical deterioration (figure 4),188 and it might play an important role in detecting silent ischaemia in unconscious patients. Multimodality monitoring catheters should be inserted in the watershed anterior cerebral artery territory (anterior cerebral artery to middle cerebral artery) ipsilateral to the maximal blood load or to ruptured aneurysms (if symmetrical blood and on the non-dominant side).189 If placed in response to neurological deterioration, catheter placement is guided by the location of the tissue most at risk. Cortical spreading depolarisations on subdural electro-corticography reflect metabolic collapse. Prolonged clusters result in infarction,190 while the number of episodes is correlated with outcome.191 Technical guidance exists for what is currently a complex process of data acquisition, analysis, and interpretation,192 but it is not yet known whether treatment strategies are effective or improve outcome.
Figure 4: Patient with aneurysmal SAH and DCI diagnosed with multimodality monitoring and cognitive-motor dissociation.
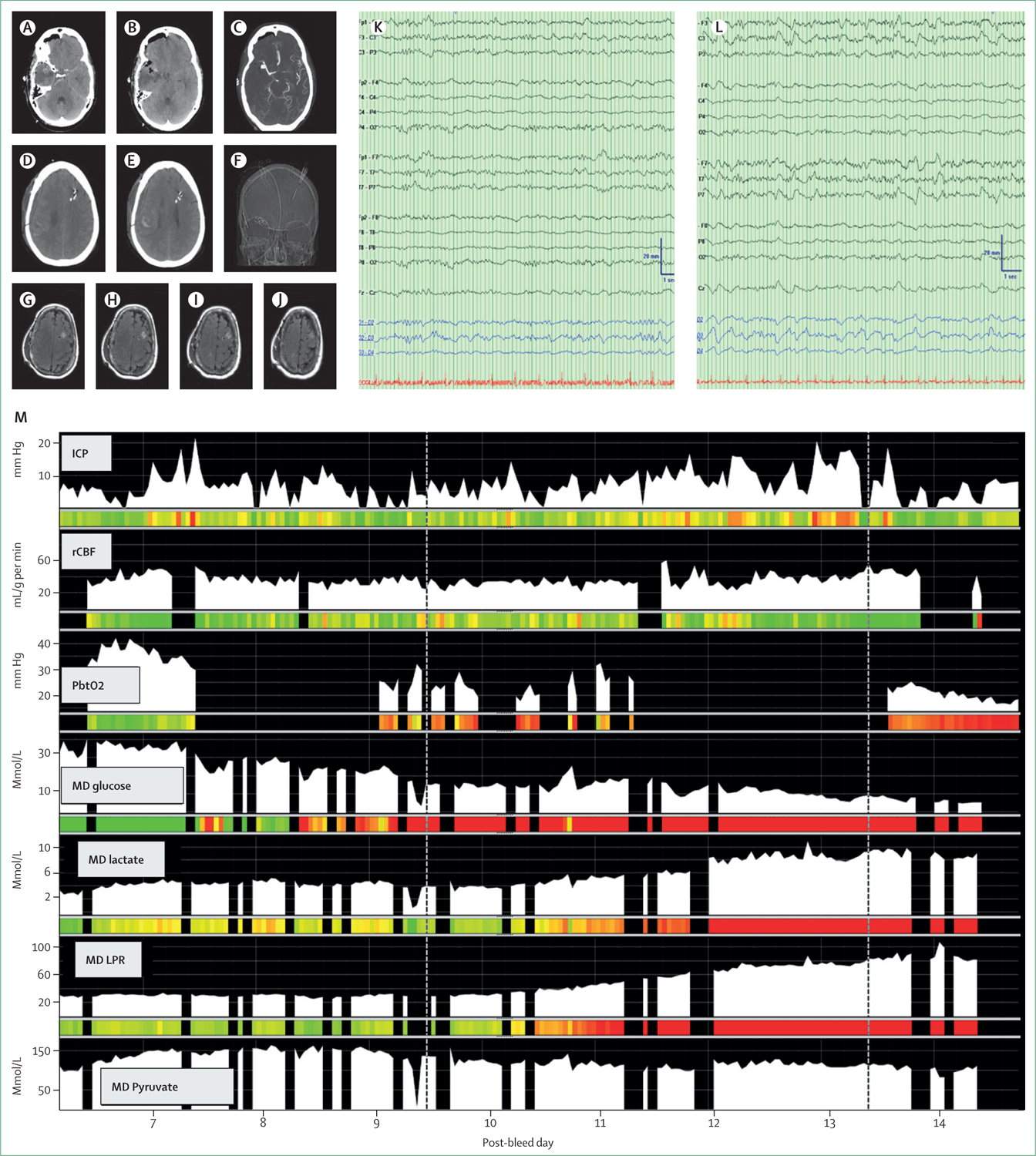
A 73-year-old woman who presented with severe headache and loss of consciousness. She was diagnosed with aneurysmal subarachnoid haemorrhage (SAH) (A, B; Hunt Hess 4, mFS 4) from an aneurysm at the right middle cerebral artery (CT angiography; C). Operative treatment failed at an outside hospital before she was transferred to a tertiary care centre. She arrived in deep coma with a coma recovery scale-revised score of 9 out of 23 that quickly deteriorated to 7. To support detection of delayed cerebral ischaemia (DCI), on post-bleed day 5 the decision was made to place a multimodality monitoring bundle into the left frontal lobe (placement on the right was impossible due to the previous craniotomy) including measurements for intracranial pressure (ICP), brain oxygenation (PbtO2), brain temperature, regional cerebral blood flow (rCBF), cerebral microdialysis (MD), and surface and intracortical electroencephalogram (EEG). Baseline EEG demonstrated a minor breach over the right hemisphere but otherwise symmetric EEG (K). On post-bleed day 10 she was diagnosed with DCI triggered by focal changes on surface EEG (L) and PbtO2, rCBF, cerebral glucose, pyruvate and rising lactate and lactate-pyruvate ratio (LPR) (M, first dotted line). On post-bleed day 13 she was diagnosed with cognitive motor dissociation using the motor command protocol while the coma recovery scale-revised remained unchanged at 7 (M, second dotted line). She was severely disabled at 3 months (Glasgow outcome scale-extended of 3) post-injury.
Prevention of ischaemic strokes related to DCI remains a focus of investigation. Following the clazosentan to overcome neurological ischaemia and infarction occurring after SAH (CONSCIOUS) trials, which demonstrated biological efficacy to prevent vasospasm but failed to show an effect on functional outcomes, clazosentan is currently being evaluated in an international placebo-controlled trial to determine the impact primarily on clinical deterioration due to DCI and secondarily on the risk of ischaemic strokes and 3-month functional outcomes. In Japan, clazosentan was approved for prevention of vasospasm, vasospasm-related cerebral infarction, and ischaemic symptoms after aneurysmal SAH in 2022, following the completion of two Japanese studies of endovascular coiling and surgical clipping, respectively.193 A small pilot trial of long-acting statins showed a signal for lower large vessel spasm without affecting DCI.194 Manipulation of the peripheral cellular immune response might offer novel targets, while non-specific, broad immunomodulatory therapies have largely been equivocal at best.111,195 Targeting spreading depolarisations might offer new therapeutic targets to reduce DCI including phosphodiesterase inhibitors,196 N-methyl-D-aspartate antagonists such as ketamine,197 and possibly valproic acid.198 Ongoing trials are investigating the benefit of intrathecal nicardipine,199 remote ischaemic preconditioning,200 stellate ganglion block,201 and neurapheresis using a lumbar drainage filtration system.202,203
Cerebral autoregulation, under normal conditions, aims to maintain constant nutrient and oxygen supply to the brain. This mechanism is impaired acutely after aneurysmal SAH and dynamic assessment of cerebral autoregulation using transcranial doppler or near-infrared spectroscopy might identify patients at risk of DCI.204 A pilot cohort of 31 patients showed that assessing personalised outer limits of cerebral autoregulation using invasive (ie, ICP) and non-invasive (ie, near infrared spectroscopy) measurements is feasible in aneurysmal SAH, and exceeding personalised limits of autoregulation was associated with poor functional outcomes.205 It is not yet known whether manipulating blood pressure to stay within optimal limits prevents DCI or improves outcomes. Advanced haemodynamic monitoring with algorithmic goal-directed blood pressure interventions reduced the rate of DCI and improved outcome at 3 months in a small randomised trial (n=108).128
Shunt prediction models206 and clinical trials investigating EVD management that focused on shortening EVD duration while decreasing shunt dependency have the potential to improve long-term outcomes.
Impaired consciousness is frequent after aneurysmal SAH particularly at the onset of bleeding30 and in patients with early clinical deterioration.207 Challenges for the precise assessment of consciousness are frequently encountered fluctuations in consciousness208 and the potential dangers associated with stopping sedation, such as brain tissue hypoxia and elevated ICP.209 However, precise assessments of consciousness are crucial as they heavily inform goals of care discussions and are fundamental for the detection of secondary complications, such as DCI. Measures beyond the behavioural examination have been explored. Computational analysis of the EEG at rest or in combination with clinical features correlates with the behavioural examination and might be able to support predictions of recovery of consciousness earlier than the clinical examination can.210 Functionally connected networks in the brain that underlie acute disorders of consciousness can be detected by use of resting-state MRI or coherence analysis of the EEG.211 EEG and MRI can help to identify brain activation of motor commands in clinically unresponsive patients, a phenomenon also known as cognitive motor dissociation or covert consciousness.212 39% of behaviourally unresponsive patients with SAH can be diagnosed with cognitive motor dissociation in the intensive care unit compared with approximately 15–20% of behaviourally unresponsive patients with other injuries such as traumatic brain injury, intracerebral haemorrhage, or cardiac arrest. Cognitive motor dissociation is independently associated with short-term recovery of consciousness and better 1-year functional recovery.213,214 Underlying mechanisms and how to support recovery are uncertain and currently being investigated. Potential treatment options include neurostimulants such as amantadine, but safety and efficacy in this patient population are unproven.
Ictal-interictal continuum
Invasive brain monitoring has shown that, in patients with aneurysmal SAH, seizures can be recorded from intracortical electrodes without a surface correlate and that seizures are associated with increased metabolic demand.215 In patients with epilepsy, increased regional cerebral blood flow compensates for elevated metabolic demand within seconds. This compensatory response can be delayed in patients with SAH and acute brain injury by up to 10 min, which is possibly related to impaired vasoreactivity. These highly localised seizures are associated with worse functional outcomes at 3 months when compared with seizures that are more widespread, and they probably represent a surrogate marker of neuronal disconnection. Following acute brain injury, the EEG might not only reveal seizures, but also EEG patterns that are clearly abnormal but do not meet classic seizure criteria. Examples of these patterns include rhythmic discharges such as lateralised or generalised periodic discharges, also known as the ictal-interictal continuum. Management of these patterns is controversial216 and invasive brain monitoring supports more aggressive treatment for high-frequency than low-frequency ictal-interictal discharges.217
Conclusion
Despite overall improvements in outcome, aneurysmal SAH continues to have a major public health impact with a mean age of onset in the mid-fifties, leading to many years of reduced quality of life. Active areas of investigation are in the categories of optimising early management approaches, goal-directed haemodynamic and perfusion-guided management, personalised medicine for prognostication, and cerebroprotection.
Search strategy and selection criteria.
We searched SCOPUS, Web of Science, Embase, MEDLINE, Cochrane Central Register of Controlled Trials, and PubMed (Jan 1, 2015–Feb 28, 2021) using the search terms “subarachnoid haemorrhage”, “subarachnoid hemorrhage”, “sub-arachnoid”, and “subarachnoid”. We selected publications in English regarding humans within the past 5 years that were commonly referenced as well as highly regarded older publications. We also searched reference lists of articles identified by this search strategy and selected the relevant articles. Review articles and book chapters are cited to provide readers with further details and references.
Acknowledgments
We thank E Sander Connolly, Chair of Neurosurgery at Columbia University, for providing a critical review and helpful comments for our manuscript. We would like to thank Gloria Willson, Librarian at the Health Sciences Library of Columbia University, for supporting our literature search.
Declaration of interests
JC is a minority shareholder at iCE Neurosystems. JC is supported by grant funding from the US National Institutes of Health (NIH; NS106014, NS112760) and the McDonnell Foundation. SP is supported by grant funding from the NIH (NS113055).
References
- 1.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009; 8: 355–69. [DOI] [PubMed] [Google Scholar]
- 2.De Rooij NK, Linn FHH, van der Plas JA, Algra A, Rinkel GJE. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 2007; 78: 1365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etminan N, Chang H-S, Hackenberg K, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population. JAMA Neurol 2019; 76: 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol 2016; 12: 699–713. [DOI] [PubMed] [Google Scholar]
- 5.Cras TY, Bos D, Arfan Ikram M, et al. Determinants of the presence and size of intracranial aneurysms in the general population: the Rotterdam study. Stroke 2020; 51: 2103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation 2017; 135: 759–71. [DOI] [PubMed] [Google Scholar]
- 7.Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 2009; 8: 635–42. [DOI] [PubMed] [Google Scholar]
- 8.Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366: 809–17. [DOI] [PubMed] [Google Scholar]
- 9.Hua X, Gray A, Wolstenholme J, et al. Survival, dependency, and health-related quality of life in patients with ruptured intracranial aneurysm: 10-year follow-up of the United Kingdom cohort of the international subarachnoid aneurysm trial. Neurosurgery 2021; 88: 252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ironside N, Buell TJ, Chen CJ, et al. High-grade aneurysmal subarachnoid hemorrhage: predictors of functional outcome. World Neurosurg 2019; 125: e723–28. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg J, Schoeni D, Mordasini P, et al. Survival and outcome after poor-grade aneurysmal subarachnoid hemorrhage in elderly patients. Stroke 2018; 49: 2883–89. [DOI] [PubMed] [Google Scholar]
- 12.Suarez JI, Sheikh MK, Macdonald RL, et al. Common data elements for unruptured intracranial aneurysms and subarachnoid hemorrhage clinical research: a national institute for neurological disorders and stroke and national library of medicine project. Neurocrit Care 2019; 30: 4–19. [DOI] [PubMed] [Google Scholar]
- 13.Hughes JD, Bond KM, Mekary RA, et al. Estimating the global incidence of aneurysmal subarachnoid hemorrhage: a systematic review for central nervous system vascular lesions and meta-analysis of ruptured aneurysms. World Neurosurg 2018; 115: 430–47. [DOI] [PubMed] [Google Scholar]
- 14.Korja M, Lehto H, Juvela S, Kaprio J. Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology 2016; 87: 1118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Can A, Rudy RF, Castro VM, et al. Association between aspirin dose and subarachnoid hemorrhage from saccular aneurysms: a case-control study. Neurology 2018; 91: 1175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korja M, Kaprio J. Controversies in epidemiology of intracranial aneurysms and SAH. Nat Rev Neurol 2016; 12: 50–55. [DOI] [PubMed] [Google Scholar]
- 17.Vlak MHM, Algra A, Brandenburg R, Rinkel GJE. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011; 10: 626–36. [DOI] [PubMed] [Google Scholar]
- 18.Bakker MK, van der Spek RAA, van Rheenen W, et al. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat Genet 2020; 52: 1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karhunen V, Bakker MK, Ruigrok YM, Gill D, Larsson SC. Modifiable risk factors for intracranial aneurysm and aneurysmal subarachnoid hemorrhage: a mendelian randomization study. J Am Heart Assoc 2021; 10: 22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruigrok YM, Buskens E, Rinkel GJE. Attributable risk of common and rare determinants of subarachnoid hemorrhage. Stroke 2001; 32: 1173–75. [DOI] [PubMed] [Google Scholar]
- 21.Bor ASE, Rinkel GJE, van Norden J, Wermer MJH. Long-term, serial screening for intracranial aneurysms in individuals with a family history of aneurysmal subarachnoid haemorrhage: a cohort study. Lancet Neurol 2014; 13: 385–92. [DOI] [PubMed] [Google Scholar]
- 22.Greving JP, Wermer MJH, Brown RD, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 2014; 13: 59–66. [DOI] [PubMed] [Google Scholar]
- 23.Wiebers DO. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003; 362: 103–10. [DOI] [PubMed] [Google Scholar]
- 24.UCAS Japan Investigators, Morita A, Kirino T, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 2012; 366: 2474–82. [DOI] [PubMed] [Google Scholar]
- 25.Lindbohm JV, Kaprio J, Jousilahti P, Salomaa V, Korja M. Sex, smoking, and risk for subarachnoid hemorrhage. Stroke 2016; 47: 1975–81. [DOI] [PubMed] [Google Scholar]
- 26.Larsson SC, Wallin A, Wolk A, Markus HS. Differing association of alcohol consumption with different stroke types: a systematic review and meta-analysis. BMC Med 2016; 14: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tominari S, Morita A, Ishibashi T, et al. Prediction model for 3-year rupture risk of unruptured cerebral aneurysms in Japanese patients. Ann Neurol 2015; 77: 1050–59. [DOI] [PubMed] [Google Scholar]
- 28.Andreasen TH, Bartek J, Andresen M, Springborg JB, Romner B. Modifiable risk factors for aneurysmal subarachnoid hemorrhage. Stroke 2013; 44: 3607–12. [DOI] [PubMed] [Google Scholar]
- 29.Perry JJ, Stiell IG, Sivilotti MLA, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA 2013; 310: 1248–55. [DOI] [PubMed] [Google Scholar]
- 30.Suwatcharangkoon S, Meyers E, Falo C, et al. Loss of consciousness at onset of subarachnoid hemorrhage as an important marker of early brain injury. JAMA Neurol 2016; 73: 28–35. [DOI] [PubMed] [Google Scholar]
- 31.Linn FHH, Kinkel GJE, Algra A, van Gijn J. Headache characteristics in subarachnoid haemorrhage and benign thunderclap headache. J Neurol Neurosurg Psychiatry 1998; 65: 791–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontanarosa PB. Recognition of subarachnoid hemorrhage. Ann Emerg Med 1989; 18: 1199–205. [DOI] [PubMed] [Google Scholar]
- 33.World Federation of Neurological Surgeons Committee. Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg 1988; 68: 985–86. [DOI] [PubMed] [Google Scholar]
- 34.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968; 28: 14–20. [DOI] [PubMed] [Google Scholar]
- 35.Kowalski RG, Claassen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA 2004; 291: 866–69. [DOI] [PubMed] [Google Scholar]
- 36.Perry JJ, Sivilotti MLA, Émond M, et al. Prospective implementation of the Ottawa subarachnoid hemorrhage rule and 6-hour computed tomography rule. Stroke 2020; 51: 424–30. [DOI] [PubMed] [Google Scholar]
- 37.Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage. Stroke 2016; 47: 750–55. [DOI] [PubMed] [Google Scholar]
- 38.Meurer WJ, Walsh B, Vilke GM, Coyne CJ. Clinical guidelines for the emergency department evaluation of subarachnoid hemorrhage. J Emerg Med 2016; 50: 696–701. [DOI] [PubMed] [Google Scholar]
- 39.Hasan TF, Duarte W, Akinduro OO, et al. Nonaneurysmal “pseudo-subarachnoid hemorrhage” computed tomography patterns: challenges in an acute decision-making heuristics. J Stroke Cerebrovasc Dis 2018; 27: 2319–26. [DOI] [PubMed] [Google Scholar]
- 40.Nelson SE, Sair HI, Stevens RD. Magnetic resonance imaging in aneurysmal subarachnoid hemorrhage: current evidence and future directions. Neurocrit Care 2018; 29: 241–52. [DOI] [PubMed] [Google Scholar]
- 41.Flaherty ML, Haverbusch M, Kissela B, et al. Perimesencephalic subarachnoid hemorrhage: incidence, risk factors, and outcome. J Stroke Cerebrovasc Dis 2005; 14: 267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohan M, Islim AI, Rasul FT, et al. Subarachnoid haemorrhage with negative initial neurovascular imaging: a systematic review and meta-analysis. Acta Neurochir 2019; 161: 2013–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai PMR, Ng I, Gormley WB, et al. Familial predisposition and differences in radiographic patterns in spontaneous nonaneurysmal subarachnoid hemorrhage. Neurosurgery 2021; 88: 413–19. [DOI] [PubMed] [Google Scholar]
- 44.McDowell MM, Zhao Y, Kellner CP, et al. Demographic and clinical predictors of multiple intracranial aneurysms in patients with subarachnoid hemorrhage. J Neurosurg 2018; 128: 961–68. [DOI] [PubMed] [Google Scholar]
- 45.Omodaka S, Endo H, Niizuma K, et al. Circumferential wall enhancement on magnetic resonance imaging is useful to identify rupture site in patients with multiple cerebral aneurysms. Neurosurgery 2018; 82: 638–44. [DOI] [PubMed] [Google Scholar]
- 46.Nehls DG, Flom RA, Carter LP, Spetzler RF. Multiple intracranial aneurysms: determining the site of rupture. J Neurosurg 1985; 63: 342–48. [DOI] [PubMed] [Google Scholar]
- 47.Mensing LA, Vergouwen MDI, Laban KG, et al. Perimesencephalic hemorrhage: a review of epidemiology, risk factors, presumed cause, clinical course, and outcome. Stroke 2018; 49: 1363–70. [DOI] [PubMed] [Google Scholar]
- 48.Agid R, Andersson T, Almqvist H, et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: when is digital subtraction angiography still needed? Am J Neuroradiol 2010; 31: 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocha EA, Topcuoglu MA, Silva GS, Singhal AB. RCVS2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology 2019; 92: 639–47. [DOI] [PubMed] [Google Scholar]
- 50.Charidimou A, Linn J, Vernooij MW, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 2015; 138: 2126–39. [DOI] [PubMed] [Google Scholar]
- 51.Debette S, Compter A, Labeyrie MA, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol 2015; 14: 640–54. [DOI] [PubMed] [Google Scholar]
- 52.Rush B, Romano K, Ashkanani M, McDermid RC, Celi LA. Impact of hospital case-volume on subarachnoid hemorrhage outcomes: a nationwide analysis adjusting for hemorrhage severity. J Crit Care 2017; 37: 240–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNeill L, English SW, Borg N, Matta BF, Menon DK. Effects of institutional caseload of subarachnoid hemorrhage on mortality: a secondary analysis of administrative data. Stroke 2013; 44: 647–52. [DOI] [PubMed] [Google Scholar]
- 54.Lindgren A, Burt S, Bragan Turner E, et al. Hospital case-volume is associated with case-fatality after aneurysmal subarachnoid hemorrhage. Int J Stroke 2019; 14: 282–89. [DOI] [PubMed] [Google Scholar]
- 55.Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European stroke organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 2013; 35: 93–112. [DOI] [PubMed] [Google Scholar]
- 56.Rawal S, Alcaide-Leon P, Macdonald RL, et al. Meta-analysis of timing of endovascular aneurysm treatment in subarachnoid haemorrhage: inconsistent results of early treatment within 1 day. J Neurol Neurosurg Psychiatry 2017; 88: 241–48. [DOI] [PubMed] [Google Scholar]
- 57.Larsen CC, Astrup J. Rebleeding after aneurysmal subarachnoid hemorrhage: a literature review. World Neurosurg 2013; 79: 307–12. [DOI] [PubMed] [Google Scholar]
- 58.Tang C, Zhang TS, Zhou LF. Risk factors for rebleeding of aneurysmal subarachnoid hemorrhage: a meta-analysis. PLoS One 2014; 9: e99536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hillman J, Fridriksson S, Nilsson O, Yu Z, Säveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg 2002; 97: 771–78. [DOI] [PubMed] [Google Scholar]
- 60.Post R, Germans MR, Tjerkstra MA, et al. Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet 2021; 397: 112–18. [DOI] [PubMed] [Google Scholar]
- 61.Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. Stroke 2012; 43: 1711–37. [DOI] [PubMed] [Google Scholar]
- 62.Post R, Tjerkstra MA, Middeldorp S, et al. Platelet transfusion in patients with aneurysmal subarachnoid hemorrhage is associated with poor clinical outcome. Sci Reports 2020; 10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molyneux A International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002; 360: 1267–74 [DOI] [PubMed] [Google Scholar]
- 64.Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RSC. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the international subarachnoid aneurysm trial (ISAT). Lancet 2015; 385: 691–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the international subarachnoid aneurysm trial (ISAT): long-term follow-up. Lancet Neurol 2009; 8: 427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Donkelaar CE, Bakker NA, Birks J, et al. Impact of treatment delay on outcome in the international subarachnoid aneurysm trial. Stroke 2020; 51: 1600–03. [DOI] [PubMed] [Google Scholar]
- 67.Lindgren A, Turner EB, Sillekens T, et al. Outcome after clipping and coiling for aneurysmal subarachnoid hemorrhage in clinical practice in Europe, USA, and Australia. Neurosurgery 2019; 84: 1019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke 2002; 33: 1225–32. [DOI] [PubMed] [Google Scholar]
- 69.Helbok R, Ko SB, Schmidt JM, et al. Global cerebral edema and brain metabolism after subarachnoid hemorrhage. Stroke 2011; 42: 1534–39. [DOI] [PubMed] [Google Scholar]
- 70.Savarraj J, Parsha K, Hergenroeder G, et al. Early brain injury associated with systemic inflammation after subarachnoid hemorrhage. Neurocrit Care 2017; 28: 203–11. [DOI] [PubMed] [Google Scholar]
- 71.Frontera JA, Provencio JJ, Sehba FA, et al. The role of platelet activation and inflammation in early brain injury following subarachnoid hemorrhage. Neurocrit Care 2016; 26: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eriksen N, Rostrup E, Fabricius M, et al. Early focal brain injury after subarachnoid hemorrhage correlates with spreading depolarizations. Neurology 2019; 92: 326–41. [DOI] [PubMed] [Google Scholar]
- 73.Gris T, Laplante P, Thebault P, et al. Innate immunity activation in the early brain injury period following subarachnoid hemorrhage. J Neuroinflammation 2019; 16: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keep RF, Andjelkovic AV, Xiang J, et al. Brain endothelial cell junctions after cerebral hemorrhage: changes, mechanisms and therapeutic targets. J Cereb Blood Flow Metab 2018; 38: 1255–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahn SH, Savarraj JP, Pervez M, et al. The subarachnoid hemorrhage early brain edema score predicts delayed cerebral ischemia and clinical outcomes. Neurosurgery 2018; 83: 137–45. [DOI] [PubMed] [Google Scholar]
- 76.Said M, Gümüs M, Herten A, et al. Subarachnoid hemorrhage early brain edema score (SEBES) as a radiographic marker of clinically relevant intracranial hypertension and unfavorable outcome after subarachnoid hemorrhage. Eur J Neurol 2021; 28: 4051–59. [DOI] [PubMed] [Google Scholar]
- 77.Pasarikovski CR, Alotaibi NM, Al-Mufti F, Macdonald RL. Hypertonic saline for increased intracranial pressure after aneurysmal subarachnoid hemorrhage: a systematic review. World Neurosurg 2017; 105: 1–6. [DOI] [PubMed] [Google Scholar]
- 78.Hasan D, Vermeulen M, Wijdicks EF, Hijdra A, van Gijn J. Management problems in acute hydrocephalus after subarachnoid hemorrhage. Stroke 1989; 20: 747–53. [DOI] [PubMed] [Google Scholar]
- 79.van Donkelaar CE, Bakker NA, Birks J, et al. Prediction of outcome after aneurysmal subarachnoid hemorrhage: development and validation of the SAFIRE grading scale. Stroke 2019; 50: 837–44. [DOI] [PubMed] [Google Scholar]
- 80.Catapano JS, Zabramski JM, Baranoski JF, et al. The prognostic significance of a cast fourth ventricle in ruptured aneurysm patients with intraventricular hemorrhage in the barrow ruptured aneurysm trial (BRAT). Neurosurgery 2019; 85: 275–82. [DOI] [PubMed] [Google Scholar]
- 81.Enriquez-Marulanda A, Ascanio LC, Salem MM, et al. Accuracy and safety of external ventricular drain placement by physician assistants and nurse practitioners in aneurysmal acute subarachnoid hemorrhage. Neurocrit Care 2018; 29: 435–42. [DOI] [PubMed] [Google Scholar]
- 82.Hudson JS, Prout BS, Nagahama Y, et al. External ventricular drain and hemorrhage in aneurysmal subarachnoid hemorrhage patients on dual antiplatelet therapy: a retrospective cohort study. Neurosurgery 2019; 84: 479–84. [DOI] [PubMed] [Google Scholar]
- 83.Hudson JS, Nagahama Y, Nakagawa D, et al. Hemorrhage associated with ventriculoperitoneal shunt placement in aneurysmal subarachnoid hemorrhage patients on a regimen of dual antiplatelet therapy: a retrospective analysis. J Neurosurg 2017; 129: 916–21. [DOI] [PubMed] [Google Scholar]
- 84.Dorresteijn KRIS, Jellema K, van de Beek D, Brouwer MC. Factors and measures predicting external CSF drain-associated ventriculitis: a review and meta-analysis. Neurology 2019; 93: 964–72. [DOI] [PubMed] [Google Scholar]
- 85.Galea JP, Dulhanty L, Patel HC. Predictors of outcome in aneurysmal subarachnoid hemorrhage patients: Observations from a multicenter data set. Stroke 2017; 48: 2958–63. [DOI] [PubMed] [Google Scholar]
- 86.Vergouwen MDI, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010; 41: 2391–95. [DOI] [PubMed] [Google Scholar]
- 87.Germans MR, Jaja BNR, de Oliviera Manoel AL, et al. Sex differences in delayed cerebral ischemia after subarachnoid hemorrhage. J Neurosurg 2018; 129: 458–64. [DOI] [PubMed] [Google Scholar]
- 88.de Rooij NK, Rinkel GJE, Dankbaar JW, Frijns CJM. Delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review of clinical, laboratory, and radiological predictors. Stroke 2013; 44: 43–54. [DOI] [PubMed] [Google Scholar]
- 89.Nesvick CL, Oushy S, Rinaldo L, Wijdicks EF, Lanzino G, Rabinstein AA. Clinical complications and outcomes of angiographically negative subarachnoid hemorrhage. Neurology 2019; 92: 2385–94. [DOI] [PubMed] [Google Scholar]
- 90.Claassen J, Bernardini GL, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke 2001; 32: 2012–20. [DOI] [PubMed] [Google Scholar]
- 91.Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery 2006; 59: 21–26. [DOI] [PubMed] [Google Scholar]
- 92.Hijdra A, Brouwers P, Vermeulen M, van Gijn J. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke 1990; 21: 1156–61. [DOI] [PubMed] [Google Scholar]
- 93.Kramer AH, Hehir M, Nathan B, et al. A comparison of 3 radiographic scales for the prediction of delayed ischemia and prognosis following subarachnoid hemorrhage. J Neurosurg 2008; 109: 199–207. [DOI] [PubMed] [Google Scholar]
- 94.van der Steen WE, Leemans EL, van den Berg R, et al. Radiological scales predicting delayed cerebral ischemia in subarachnoid hemorrhage: systematic review and meta-analysis. Neuroradiology 2019; 61: 247–56. [DOI] [PubMed] [Google Scholar]
- 95.Westermaier T, Pham M, Stetter C, et al. Value of transcranial doppler, perfusion-CT and neurological evaluation to forecast secondary ischemia after aneurysmal SAH. Neurocrit Care 2013; 20: 406–12. [DOI] [PubMed] [Google Scholar]
- 96.Kumar G, Albright KC, Donnelly JP, Shapshak AH, Harrigan MR. Trends in transcranial doppler monitoring in aneurysmal subarachnoid hemorrhage: a 10-year analysis of the nationwide inpatient sample. J Stroke Cerebrovasc Dis 2017; 26: 851–7. [DOI] [PubMed] [Google Scholar]
- 97.Kumar G, Dumitrascu OM, Chiang CC, O’Carroll CB, Alexandrov AV. Prediction of delayed cerebral ischemia with cerebral angiography: a meta-analysis. Neurocrit Care 2018; 30: 62–71. [DOI] [PubMed] [Google Scholar]
- 98.Rosenthal ES, Biswal S, Zafar SF, et al. Continuous electroencephalography predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective study of diagnostic accuracy. Ann Neurol 2018; 83: 958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Letourneau-Guillon L, Farzin B, Darsaut TE, et al. Reliability of CT angiography in cerebral vasospasm: a systematic review of the literature and an inter- and intraobserver study. AJNR Am J Neuroradiol 2020; 41: 612–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malinova V, Dolatowski K, Schramm P, Moerer O, Rohde V, Mielke D. Early whole-brain CT perfusion for detection of patients at risk for delayed cerebral ischemia after subarachnoid hemorrhage. J Neurosurg 2016; 125: 128–36. [DOI] [PubMed] [Google Scholar]
- 101.Foreman B, Albers D, Schmidt JM, et al. Intracortical electrophysiological correlates of blood flow after severe SAH: a multimodality monitoring study. J Cereb Blood Flow Metab 2018; 38: 506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Veldeman M, Albanna W, Weiss M, et al. Invasive multimodal neuromonitoring in aneurysmal subarachnoid hemorrhage: a systematic review. Stroke 2021; 52: 3624–32. [DOI] [PubMed] [Google Scholar]
- 103.Megjhani M, Terilli K, Weiss M, et al. Dynamic detection of delayed cerebral ischemia: a study in 3 centers. Stroke 2021; 52: 1370–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Macdonald RL, Higashida RT, Keller E, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol 2011; 10: 618–25. [DOI] [PubMed] [Google Scholar]
- 105.Mayer SA, Aldrich EF, Bruder N, et al. Thick and diffuse subarachnoid blood as a treatment effect modifier of clazosentan after subarachnoid hemorrhage. Stroke 2019; 50: 2738–44. [DOI] [PubMed] [Google Scholar]
- 106.Geraghty JR, Testai FD. Delayed cerebral ischemia after subarachnoid hemorrhage: beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep 2017; 19: 1–12. [DOI] [PubMed] [Google Scholar]
- 107.McBride DW, Blackburn SL, Peeyush Kumar T, Matsumura K, Zhang JH. The role of thromboinflammation in delayed cerebral ischemia after subarachnoid hemorrhage. Front Neurol 2017; 8: 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dorhout Mees SM, Rinkel GJE, Feigin VL, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007; 3: CD000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carlson AP, Hänggi D, Wong GK, et al. Single-dose intraventricular nimodipine microparticles versus oral nimodipine for aneurysmal subarachnoid hemorrhage. Stroke 2020; 51: 1142–49. [DOI] [PubMed] [Google Scholar]
- 110.Lyden PD. Cerebroprotection for acute ischemic stroke: looking ahead. Stroke 2021; 52: 3033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weiland J, Beez A, Westermaier T, Kunze E, Sirén AL, Lilla N. Neuroprotective strategies in aneurysmal subarachnoid hemorrhage (aSAH). Int J Mol Sci 2021; 22: 5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kole MJ, Wessell AP, Ugiliweneza B, et al. Low-dose intravenous heparin infusion after aneurysmal subarachnoid hemorrhage is associated with decreased risk of delayed neurological deficit and cerebral infarction. Neurosurgery 2020; 88: 523–30. [DOI] [PubMed] [Google Scholar]
- 113.Mees SD, van den Bergh WM, Algra A, Rinkel GJ. Antiplatelet therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007; 4: CD006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen J, Wang L, Xu H, et al. Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nat Commun 2020; 11: 3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luo C, Yao X, Li J, et al. Paravascular pathways contribute to vasculitis and neuroinflammation after subarachnoid hemorrhage independently of glymphatic control. Cell Death Dis 2016; 7: 2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hanafy KA. The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J Neuroinflammation 2013; 10: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takase H, Chou SHY, Hamanaka G, et al. Soluble vascular endothelial-cadherin in CSF after subarachnoid hemorrhage. Neurology 2020; 94: 1281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 2011; 17: 439–47. [DOI] [PubMed] [Google Scholar]
- 119.Dreier JP, Major S, Manning A, et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 2009; 132: 1866–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Woitzik J, Dreier JP, Hecht N, et al. Delayed cerebral ischemia and spreading depolarization in absence of angiographic vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2012; 32: 203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yundt KD, Grubb RL, Diringer MN, Powers WJ. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab 1998; 18: 419–24. [DOI] [PubMed] [Google Scholar]
- 122.Haegens NM, Gathier CS, Horn J, Coert BA, Verbaan D, van den Bergh WM. Induced hypertension in preventing cerebral infarction in delayed cerebral ischemia after subarachnoid hemorrhage. Stroke 2018; 49: 2630–36. [DOI] [PubMed] [Google Scholar]
- 123.Gathier CS, van den Bergh WM, van der Jagt M, et al. Induced hypertension for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke 2018; 49: 76–83. [DOI] [PubMed] [Google Scholar]
- 124.Vergouw LJM, Egal M, Bergmans B, et al. High early fluid input after aneurysmal subarachnoid hemorrhage: combined report of association with delayed cerebral ischemia and feasibility of cardiac output-guided fluid restriction. J Intensive Care Med 2020; 35: 161–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suwatcharangkoon S, De Marchis GM, Witsch J, et al. Medical treatment failure for symptomatic vasospasm after subarachnoid hemorrhage threatens long-term outcome. Stroke 2019; 50: 1696–702. [DOI] [PubMed] [Google Scholar]
- 126.Allen ML, Kulik T, Keyrouz SG, Dhar R. Posterior reversible encephalopathy syndrome as a complication of induced hypertension in subarachnoid hemorrhage: a case-control study. Neurosurgery 2019; 85: 223–30. [DOI] [PubMed] [Google Scholar]
- 127.Alkhachroum A, Megjhani M, Terilli K, et al. Hyperemia in subarachnoid hemorrhage patients is associated with an increased risk of seizures. J Cereb Blood Flow Metab 2020; 40: 1290–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anetsberger A, Gempt J, Blobner M, et al. Impact of goal-directed therapy on delayed ischemia after aneurysmal subarachnoid hemorrhage: randomized controlled trial. Stroke 2020; 51: 2287–96. [DOI] [PubMed] [Google Scholar]
- 129.Jabbarli R, Pierscianek D, Rölz R, et al. Endovascular treatment of cerebral vasospasm after subarachnoid hemorrhage. Neurology 2019; 93: 458–66. [DOI] [PubMed] [Google Scholar]
- 130.Dorhout Mees SM, Algra A, Wong GKC, et al. Early magnesium treatment after aneurysmal subarachnoid hemorrhage: individual patient data meta-analysis. Stroke 2015; 46: 3190–93. [DOI] [PubMed] [Google Scholar]
- 131.Claassen J, Albers D, Schmidt JM, et al. Nonconvulsive seizures in subarachnoid hemorrhage link inflammation and outcome. Ann Neurol 2014; 75: 771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lissak IA, Zafar SF, Westover MB, et al. Soluble ST2 Is associated with new epileptiform abnormalities following nontraumatic subarachnoid hemorrhage. Stroke 2020; 51: 1128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Waziri A, Claassen J, Morgan Stuart R, et al. Intracortical electroencephalography in acute brain injury. Ann Neurol 2009; 66: 366–77. [DOI] [PubMed] [Google Scholar]
- 134.De Marchis GM, Pugin D, Meyers E, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology 2016; 86: 253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol 2013; 74: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Claassen J, Peery S, Kreiter KT, et al. Predictors and clinical impact of epilepsy after subarachnoid hemorrhage. Neurology 2003; 60: 208–14. [DOI] [PubMed] [Google Scholar]
- 137.Molyneux AJ, Kerr RS, Yu L-M, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366: 809–17. [DOI] [PubMed] [Google Scholar]
- 138.Jaja BNR, Schweizer TA, Claassen J, Le Roux P, Mayer SA, Loch MacDonald R. The SAFARI score to assess the risk of convulsive seizure during admission for aneurysmal subarachnoid hemorrhage. Neurosurgery 2018; 82: 887–93. [DOI] [PubMed] [Google Scholar]
- 139.Human T, Diringer MN, Allen M, et al. A randomized trial of brief versus extended seizure prophylaxis after aneurysmal subarachnoid hemorrhage. Neurocrit Care 2018; 28: 169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dasenbrock HH, Rudy RF, Smith TR, et al. Hospital-acquired infections after aneurysmal subarachnoid hemorrhage: a nationwide analysis. World Neurosurg 2016; 88: 459–74. [DOI] [PubMed] [Google Scholar]
- 141.Hammer A, Ranaie G, Erbguth F, et al. Impact of complications and comorbidities on the intensive care length of stay after aneurysmal subarachnoid haemorrhage. Sci Rep 2020; 10: 6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Badjatia N, Cremers S, Claassen J, et al. Serum glutamine and hospital-acquired infections after aneurysmal subarachnoid hemorrhage. Neurology 2018; 91: 421–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oddo M, Schmidt JM, Carrera E, et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med 2008; 36: 3233–38. [DOI] [PubMed] [Google Scholar]
- 144.Ayling OGS, Ibrahim GM, Alotaibi NM, Gooderham PA, Loch Macdonald R. Anemia after aneurysmal subarachnoid hemorrhage is associated with poor outcome and death. Stroke 2018; 49: 1859–65. [DOI] [PubMed] [Google Scholar]
- 145.Kramer AH, Gurka MJ, Nathan B, Dumont AS, Kassell NF, Bleck TP. Complications associated with anemia and blood transfusion in patients with aneurysmal subarachnoid hemorrhage. Crit Care Med 2008; 36: 2070–75. [DOI] [PubMed] [Google Scholar]
- 146.Jeon SB, Choi HA, Badjatia N, et al. Hyperoxia may be related to delayed cerebral ischemia and poor outcome after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2014; 85: 1301–07. [DOI] [PubMed] [Google Scholar]
- 147.Manoel AL, Turkel-Parrella D, Germans M, et al. Safety of early pharmacological thromboprophylaxis after subarachnoid hemorrhage. Can J Neurol Sci 2014; 41: 554–61. [DOI] [PubMed] [Google Scholar]
- 148.Haines SJ. Commentary: what we might or might not know about venous thromboembolism prophylaxis in neurosurgery. Neurosurgery 2020; 86: 455–68. [DOI] [PubMed] [Google Scholar]
- 149.Rass V, Gaasch M, Kofler M, et al. Fluid intake but not fluid balance is associated with poor outcome in nontraumatic subarachnoid hemorrhage patients. Crit Care Med 2019; 47: 555–62. [DOI] [PubMed] [Google Scholar]
- 150.van der Bilt IAC, Hasan D, Vandertop WP, et al. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: a meta-analysis. Neurology 2009; 72: 635–42. [DOI] [PubMed] [Google Scholar]
- 151.Kerro A, Woods T, Chang JJ. Neurogenic stunned myocardium in subarachnoid hemorrhage. J Crit Care 2017; 38: 27–34. [DOI] [PubMed] [Google Scholar]
- 152.Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol 2018; 72: 1955–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Frangieh AH, Obeid S, Ghadri JR, et al. ECG criteria to differentiate between Takotsubo (stress) cardiomyopathy and myocardial infarction. J Am Heart Assoc 2016; 5: e003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mazeraud A, Robba C, Rebora P, et al. Acute distress respiratory syndrome after subarachnoid hemorrhage: incidence and impact on the outcome in a large multicenter, retrospective cohort. Neurocrit Care 2020; 34: 1000–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care 2012; 16: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hannon MJ, Behan LA, O’Brien MMC, et al. Hyponatremia following mild/moderate subarachnoid hemorrhage is due to SIAD and glucocorticoid deficiency and not cerebral salt wasting. J Clin Endocrinol Metab 2014; 99: 291–98. [DOI] [PubMed] [Google Scholar]
- 157.Kurokawa Y, Uede T, Ishiguro M, et al. Pathogenesis of hyponatremia following subarachnoid hemorrhage due to ruptured cerebral aneurysm. Surg Neurol 1996; 46: 500–08. [DOI] [PubMed] [Google Scholar]
- 158.Quinn L, Tian DH, Fitzgerald E, et al. The association between hyponatraemia and long-term functional outcome in patients with aneurysmal subarachnoid haemorrhage: a single centre prospective cohort study. J Clin Neurosci 2020; 78: 353–59. [DOI] [PubMed] [Google Scholar]
- 159.Oliveira–Filho J, Ezzeddine MA, Segal AZ, et al. Fever in subarachnoid hemorrhage. Neurology 2001; 56: 1299–304. [DOI] [PubMed] [Google Scholar]
- 160.Ianosi B, Rass V, Gaasch M, et al. An observational study on the use of intravenous non-opioid analgesics and antipyretics in poor-grade subarachnoid hemorrhage: effects on hemodynamics and systemic and brain temperature. Ther Hypothermia Temp Manag 2020; 10: 27–36. [DOI] [PubMed] [Google Scholar]
- 161.Badjatia N, Fernandez L, Schmidt JM, et al. Impact of induced normothermia on outcome after subarachnoid hemorrhage: a case-control study. Neurosurgery 2010; 66: 696–701. [DOI] [PubMed] [Google Scholar]
- 162.Di Russo P, Di Carlo DT, Lutenberg A, Morganti R, Evins AI, Perrini P. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg Sci 2020; 64: 181–89. [DOI] [PubMed] [Google Scholar]
- 163.Paisan GM, Ding D, Starke RM, Crowley RW, Liu KC. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: predictors and long-term functional outcomes. Neurosurgery 2018; 83: 393–402. [DOI] [PubMed] [Google Scholar]
- 164.Chung DY, Olson DM, John S, et al. Evidence-based management of external ventricular drains. Curr Neurol Neurosci Rep 2019; 19: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yataco RA, Arnold SM, Brown SM, et al. Early progressive mobilization of patients with external ventricular drains: safety and feasibility. Neurocrit Care 2018; 30: 414–20. [DOI] [PubMed] [Google Scholar]
- 166.McCann MR, Hatton KW, Vsevolozhskaya OA, Fraser JF. Earlier tracheostomy and percutaneous endoscopic gastrostomy in patients with hemorrhagic stroke: associated factors and effects on hospitalization. J Neurosurg 2019; 132: 87–93. [DOI] [PubMed] [Google Scholar]
- 167.Bösel J, Niesen WD, Salih F, et al. Effect of early vs standard approach to tracheostomy on functional outcome at 6 months among patients with severe stroke receiving mechanical ventilation: the SETPOINT2 randomized clinical trial. JAMA 2022; 327: 1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liang JW, Cifrese L, Ostojic LV, Shah SO, Dhamoon MS. Preventable readmissions and predictors of readmission after subarachnoid hemorrhage. Neurocrit Care 2018; 29: 336–43. [DOI] [PubMed] [Google Scholar]
- 169.Paavola JT, Väntti N, Junkkari A, et al. Antipsychotic use among 1144 patients after aneurysmal subarachnoid hemorrhage: a population-based case-control study. Stroke 2019; 50: 1711–18. [DOI] [PubMed] [Google Scholar]
- 170.Hoogmoed J, de Oliveira Manoel AL, Coert BA, et al. Why do patients with poor-grade subarachnoid hemorrhage die? World Neurosurg 2019; 131: 508–13. [DOI] [PubMed] [Google Scholar]
- 171.Taufique Z, May T, Meyers E, et al. Predictors of poor quality of life 1 year after subarachnoid hemorrhage. Neurosurgery 2016; 78: 256–64. [DOI] [PubMed] [Google Scholar]
- 172.Al-Khindi T, MacDonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke 2010; 41: 519–36. [DOI] [PubMed] [Google Scholar]
- 173.Reijmer YD, van den Heerik MS, Heinen R, et al. Microstructural white matter abnormalities and cognitive impairment after aneurysmal subarachnoid hemorrhage. Stroke 2018; 49: 2040–45. [DOI] [PubMed] [Google Scholar]
- 174.Huenges Wajer IMC, Hendriks ME, Witkamp TD, et al. The relationship between ischaemic brain lesions and cognitive outcome after aneurysmal subarachnoid haemorrhage. J Neurol 2019; 266: 2252–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rass V, Schoenherr E, Ianosi B-A, et al. Subarachnoid hemorrhage is followed by pituitary gland volume loss: a volumetric MRI observational study. Neurocrit Care 2019; 32: 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Buunk AM, Spikman JM, Metzemaekers JDM, van Dijk JMC, Groen RJM. Return to work after subarachnoid hemorrhage: the influence of cognitive deficits. PLoS One 2019; 14: e0220972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rinkel GJE, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol 2011; 10: 349–56. [DOI] [PubMed] [Google Scholar]
- 178.Witsch J, Frey HP, Patel S, et al. Prognostication of long-term outcomes after subarachnoid hemorrhage: the FRESH score. Ann Neurol 2016; 80: 46–58. [DOI] [PubMed] [Google Scholar]
- 179.Mascitelli JR, Cole T, Yoon S, et al. External validation of the subarachnoid hemorrhage international trialists (SAHIT) predictive model using the barrow ruptured aneurysm trial (BRAT) cohort. Neurosurgery 2020; 86: 101–06. [DOI] [PubMed] [Google Scholar]
- 180.Chou SHY, Macdonald RL, Keller E, et al. Biospecimens and molecular and cellular biomarkers in aneurysmal subarachnoid hemorrhage studies: common data elements and standard reporting recommendations. Neurocrit Care 2019; 30: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Strickland BA, Mert M, Ravina K, et al. Discrepancy in neurologic outcomes following aneurysmal subarachnoid hemorrhage as a function of socioeconomic class. World Neurosurg 2020; 138: 787–94. [DOI] [PubMed] [Google Scholar]
- 182.Egawa S, Hifumi T, Kawakita K, et al. Impact of neurointensivist-managed intensive care unit implementation on patient outcomes after aneurysmal subarachnoid hemorrhage. J Crit Care 2016; 32: 52–55. [DOI] [PubMed] [Google Scholar]
- 183.Samuels O, Webb A, Culler S, Martin K, Barrow D. Impact of a dedicated neurocritical care team in treating patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care 2011; 14: 334–40. [DOI] [PubMed] [Google Scholar]
- 184.Dhandapani S, Singh A, Singla N, et al. Has outcome of subarachnoid hemorrhage changed with improvements in neurosurgical services? Study of 2000 patients over 2 decades from India. Stroke 2018; 49: 2890–95. [DOI] [PubMed] [Google Scholar]
- 185.Darsaut TE, Roy D, Weill A, et al. A randomized trial of endovascular versus surgical management of ruptured intracranial aneurysms: interim results from ISAT2. Neurochirurgie 2019; 65: 370–76. [DOI] [PubMed] [Google Scholar]
- 186.Schmidt JM, Ko SB, Helbok R, et al. Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke 2011; 42: 1351–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen HI, Stiefel MF, Oddo M, et al. Detection of cerebral compromise with multimodality monitoring in patients with subarachnoid hemorrhage. Neurosurgery 2011; 69: 53–63. [DOI] [PubMed] [Google Scholar]
- 188.Skjøth-Rasmussen J, Schulz M, Kristensen SR, Bjerre P. Delayed neurological deficits detected by an ischemic pattern in the extracellular cerebral metabolites in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg 2004; 100: 8–15. [DOI] [PubMed] [Google Scholar]
- 189.Ulrich CT, Fung C, Vatter H, et al. Occurrence of vasospasm and infarction in relation to a focal monitoring sensor in patients after SAH: placing a bet when placing a probe? PLoS One 2013; 8: e62754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain 2006; 129: 3224–37. [DOI] [PubMed] [Google Scholar]
- 191.Sakowitz OW, Santos E, Nagel A, et al. Clusters of spreading depolarizations are associated with disturbed cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke 2013; 44: 220–23. [DOI] [PubMed] [Google Scholar]
- 192.Dreier JP, Fabricius M, Ayata C, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group: J Cereb Blood Flow Metab 2016; 37: 1595–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Endo H, Hagihara Y, Kimura N, et al. Effects of clazosentan on cerebral vasospasm-related morbidity and all-cause mortality after aneurysmal subarachnoid hemorrhage: two randomized phase 3 trials in Japanese patients. J Neurosurg 2022; 1: 1–11. [DOI] [PubMed] [Google Scholar]
- 194.Naraoka M, Matsuda N, Shimamura N, et al. Long-acting statin for aneurysmal subarachnoid hemorrhage: A randomized, double-blind, placebo-controlled trial. J Cereb Blood Flow Metab 2018; 38: 1190–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.de Oliveira Manoel AL, Macdonald RL. Neuroinflammation as a target for intervention in subarachnoid hemorrhage. Front Neurol 2018; 9: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sugimoto K, Nomura S, Shirao S, et al. Cilostazol decreases duration of spreading depolarization and spreading ischemia after aneurysmal subarachnoid hemorrhage. Ann Neurol 2018; 84: 873–85. [DOI] [PubMed] [Google Scholar]
- 197.Santos E, Olivares-Rivera A, Major S, et al. Lasting s-ketamine block of spreading depolarizations in subarachnoid hemorrhage: a retrospective cohort study. Crit Care 2019; 23: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hamming AM, van der Toorn A, Rudrapatna US, et al. Valproate reduces delayed brain injury in a rat model of subarachnoid hemorrhage. Stroke 2017; 48: 452–58. [DOI] [PubMed] [Google Scholar]
- 199.Hafeez S, Grandhi R. Systematic review of intrathecal nicardipine for the treatment of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Neurocrit Care 2019; 31: 399–405. [DOI] [PubMed] [Google Scholar]
- 200.Raval RN, Small O, Magsino K, et al. Remote ischemic preconditioning in subarachnoid hemorrhage: a prospective pilot trial. Neurocrit Care 2021; 34: 968–73. [DOI] [PubMed] [Google Scholar]
- 201.Zhang J, Nie Y, Pang Q, Zhang X, Wang Q, Tang J. Effects of stellate ganglion block on early brain injury in patients with subarachnoid hemorrhage: a randomised control trial. BMC Anesthesiol 2021; 21: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Blackburn SL, Grande AW, Swisher CB, Hauck EF, Jagadeesan B, Provencio JJ. Prospective trial of cerebrospinal fluid filtration after aneurysmal subarachnoid hemorrhage via lumbar catheter (PILLAR). Stroke 2019; 50: 2558–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Al-Tamimi YZ, Bhargava D, Feltbower RG, et al. Lumbar drainage of cerebrospinal fluid after aneurysmal subarachnoid hemorrhage: a prospective, randomized, controlled trial (LUMAS). Stroke 2012; 43: 677–82. [DOI] [PubMed] [Google Scholar]
- 204.Budohoski KP, Czosnyka M, Smielewski P, et al. Cerebral autoregulation after subarachnoid hemorrhage: comparison of three methods. J Cereb Blood Flow Metab 2013; 33: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Silverman A, Kodali S, Strander S, et al. Deviation from personalized blood pressure targets is associated with worse outcome after subarachnoid hemorrhage. Stroke 2019; 50: 2729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.García-Armengol R, de Pablo PP, Misis M, et al. Validation of shunt dependency prediction scores after aneurysmal spontaneous subarachnoid hemorrhage. Acta Neurochir 2021; 163: 743–51. [DOI] [PubMed] [Google Scholar]
- 207.Helbok R, Kurtz P, Vibbert M, et al. Early neurological deterioration after subarachnoid haemorrhage: risk factors and impact on outcome. J Neurol Neurosurg Psychiatry 2013; 84: 266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Reznik ME, Mahta A, Schmidt JM, et al. Duration of agitation, fluctuations of consciousness, and associations with outcome in patients with subarachnoid hemorrhage. Neurocrit Care 2018; 29: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Helbok R, Kurtz P, Schmidt MJ, et al. Effects of the neurological wake-up test on clinical examination, intracranial pressure, brain metabolism and brain tissue oxygenation in severely brain-injured patients. Crit Care 2012; 16: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Claassen J, Velazquez A, Meyers E, et al. Bedside quantitative electroencephalography improves assessment of consciousness in comatose subarachnoid hemorrhage patients. Ann Neurol 2016; 80: 541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mikell CB, Banks GP, Frey HP, et al. Frontal networks associated with command following after hemorrhagic stroke. Stroke 2015; 46: 49–57. [DOI] [PubMed] [Google Scholar]
- 212.Edlow BL, Chatelle C, Spencer CA, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017; 140: 2399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med 2019; 380: 2497–505. [DOI] [PubMed] [Google Scholar]
- 214.Egbebike J, Shen Q, Doyle K, et al. Cognitive-motor dissociation and time to functional recovery in patients with acute brain injury in the USA: a prospective observational cohort study. Lancet Neurol 2022; 21: 704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol 2013; 74: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zafar SF, Postma EN, Biswal S, et al. Effect of epileptiform abnormality burden on neurologic outcome and antiepileptic drug management after subarachnoid hemorrhage. Clin Neurophysiol 2018; 129: 2219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Witsch J, Frey HP, Schmidt JM, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol 2017; 74: 301–09. [DOI] [PMC free article] [PubMed] [Google Scholar]


