Abstract
Context:
Patients with spinal cord injury (SCI) are at high risk for venous thromboembolism (VTE). The risk factors for VTE in patients with SCI are complex.
Objective:
This meta-analysis was conducted to clarify the risk factors for VTE in patients with SCI.
Methods:
The Cochrane Library, PubMed, EBSCO, Web of Science, China National Knowledge Infrastructure (CNKI), China Biomedical Literature Database (CBM), Wanfang Med Data Database, and VIP Database were searched to identify studies reporting on risk factors for VTE in patients with SCI.
Results:
The meta-analysis included 25 studies. Findings showed that risk of VTE in patients with SCI was significantly associated with middle- and old-age (OR = 2.08, 95%CI, 1.47, 2.95), male sex (OR = 1.41, 95%CI, 1.26, 1.59), complete paralysis (OR = 3.69, 95%CI, 2.60, 5.24), personal/family history of venous thrombosis (OR = 1.95, 95%CI, 1.35, 2.81), history of smoking (OR = 2.67, 95%CI, 1.79, 3.98), lack of compression therapy (OR = 2.44, 95%CI, 1.59, 3.73), presence of lower limb/pelvic fracture (OR = 3.47, 95%CI, 1.79, 6.75), paraplegia (OR = 1.81, 95%CI, 1.49, 2.19), and diabetes (OR = 4.24, 95%CI, 2.75, 6.52).
Conclusion:
The meta-analysis identified 9 risk factors for VTE in patients with SCI. Healthcare providers should be aware of the risk factors for VTE when rehabilitating patients with SCI.
Keywords: Spinal cord injury, Venous thromboembolism, Risk factors, Meta-analysis
Introduction
Spinal cord injury (SCI) is associated with severe neurological and functional morbidity.1 Patients with SCI experience chronic medical complications, including venous thromboembolism (VTE), which constitutes deep venous thrombosis (DVT) and pulmonary embolism (PE).2–5 The incidence of DVT and PE in patients with SCI are estimated at 65% and 0%–18%, respectively, with most cases of VTE occurring within the 3 months following SCI.2,6
Patients with SCI are at high risk for VTE due to the presence of the three components of Virchow’s triad: endothelial dysfunction, altered blood flow (stasis), and hypercoagulability.7–9 Other factors that are associated with VTE include age, male sex, trauma, paraplegia, severity of injury, previous history of VTE, history of smoking, serum homocysteine (Hcy) level, serum D-dimer level, and factor V Leiden mutation.35,10–13
Understanding the risk factors for VTE in patients with SCI could lead to prevention; however, the evidence is controversial. One report showed that female sex is an independent risk factor for VTE in patients with SCI,14 and another indicated that serum D-dimer level is elevated in many conditions and has limited utility for confirming VTE.15 This meta-analysis was conducted to clarify the risk factors for VTE in patients with SCI. Findings should raise awareness of the risk factors for VTE in patients with SCI, and reduce the incidence of VTE in this patient population.
Methods
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16
Search strategy
Two authors (Bo Wei, Haiqiong Kang) independently searched the Cochrane Library, PubMed, EBSCO, Web of Science, and China National Knowledge Infrastructure (CNKI) databases, the China Biomedical Literature Database (CBM), the Wanfang Med Data Database and the VIP Database from inception to July 2020.
The search strategy for literature published in the English language included the following key words: ‘Spinal Cord Injuries’, ‘Paraplegia’, ‘Quadriplegia’, ‘Venous Thromboembolism’, ‘Venous Thrombosis’, ‘Pulmonary Embolism’ and ‘Risk Factors’ as the subject words, and ‘Spinal Cord Trauma’, ‘Spinal Cord Injury’, ‘Paraplegias’, ‘Tetraplegia’, ‘Venous Thromboses’, ‘Deep Vein Thrombosis’, ‘Pulmonary Embolisms’, ‘relative risk’, ‘risks’, ‘cohort studies’ as free words.
The search strategy for literature published in the Chinese language included the following key words: ‘spinal cord injury’, ‘paraplegia’, ‘limb paralysis’, ‘venous thromboembolism’, ‘risk factors’, ‘case–control study’, ‘cohort study’ as the subject words, and ‘Paraplegia’, ‘spastic paraplegia’, ‘quadriplegia’, ‘limb spasm’, ‘venous thrombosis’, ‘deep venous thrombosis’, ‘pulmonary embolism’, ‘influencing factors’, ‘etiology’, ‘related factors’ and ‘case–control’ as free words.
Inclusion and exclusion criteria
Inclusion criteria were: (1) case–control or cohort studies; (2) studies that included patients with confirmed diagnoses of SCI with or without (controls) VTE during the same period; and (3) studies that reported outcomes as odds ratios (ORs) and corresponding 95% confidence intervals (CIs).
Exclusion criteria were: (1) duplicate studies; (2) studies published in languages other than English or Chinese; (3) studies that lacked a control group; (4) studies with incomplete data; or (5) literature reviews or meta-analyses.
Data extraction
One author examined titles and abstracts and reviewed relevant full text articles to select eligible studies. The author extracted data from the eligible studies including details describing the study population and outcomes.
Assessment of methodological quality
Two authors (Bo Wei, Yuan Yuan) independently assessed the quality of eligible studies using the Newcastle-Ottawa Scale (NOS).17 The scale assessed quality of sample selection, comparability of cohorts, assessment of outcomes, adequacy of follow up, and drop-out rate. Studies with scores of 0–3, 4–6, and 7–9 were considered low, moderate, and high quality, respectively. Publication bias was explored using funnel plots.18
Statistical analysis
Statistical analyses were performed with Review Manager 5.3. A random-effects model was used to pool studies with significant heterogeneity, as determined by the Cochrane Q test (P ≤ 0.10) and the inconsistency index (I2 ≥ 50%), otherwise a fixed effect model was used. Sensitivity analysis was used to investigate sources of heterogeneity between studies.
Results
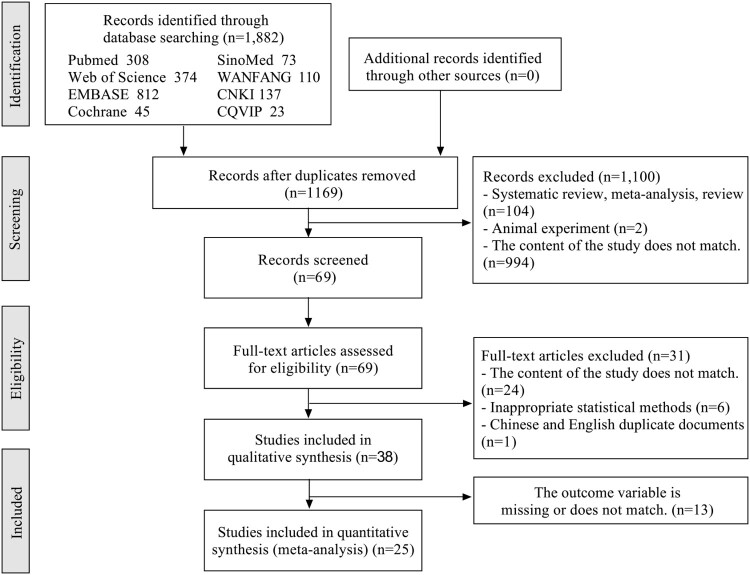
The searches identified 1,882 articles. Of these, 1,813 articles were excluded as they were duplicates, reviews, experiments in animals, or irrelevant. The full text of 69 articles was reviewed. Of these, 44 articles were excluded as they were duplicates, irrelevant, reported inappropriate statistical analyses, or were missing outcomes data. Finally, 25 articles were included in the meta-analysis (Fig. 1). The characteristics of the included studies are summarized in Table 1.
Figure 1.
Flow chart of study selection.
Table 1.
Characteristics of the included studies.
| Author | Year | Area | Study design | Admitted time | Onset time | Thrombus type | Case number | Control number | Risk factors* |
|---|---|---|---|---|---|---|---|---|---|
| Adrian A. Maung5 | 2011 | USA | Case control study | 2007–2008 | Not specified | VTE | 787 | 17515 | 1 2 30 31 32 33 34 35 36 |
| Selassie, A. W.23 | 2011 | USA | Case control study | 1998.1–2009.12 | Acute SCI | VTE | 138 | 3251 | 2 16 17 18 19 50 |
| Chao-Wei Wang12 | 2016 | China | Case control study | 2013–2014 | <24 h | DVT | 55 | 224 | 1 3 4 5 6 7 8 9 20 |
| Deborah Rubin-Asher29 | 2010 | Israel | Case control study | 1996.1–2003.5 | Acute SCI | VTE | 22 | 64 | 4 21 |
| Dong-Mei Wu19 | 2019 | China | Case control study | 2015.1–2018.9 | Acute SCI | DVT | 39 | 168 | 1 4 5 9 10 11 12 13 |
| JC de Campos Guerra11 | 2014 | Brazil | Case control study | 2011.1–2012.4 | Chronic SCI | DVT | 17 | 83 | 6 28 |
| Jong Geol Do74 | 2013 | Korea | Case control study | 2002.1–2011.7 | Acute SCI | DVT | 51 | 134 | 29 |
| Li Chengyan30 | 2018 | China | Case control study | 2015.12–2016.5 | Acute SCI | DVT | 14 | 133 | 4 27 60 |
| LIU Hong-wei21 | 2018 | China | Case control study | 2014.6–2017.6 | Acute SCI | DVT | 62 | 207 | 1 2 9 56 |
| Liu, W.33 | 2017 | China | Case control study | 2015.9–2016.8 | Not specified | DVT | 68 | 354 | 52 53 58 61 62 63 |
| Liu, Y. M.13 | 2018 | China | Case control study | 2013.5–2014.12 | <1w | DVT | 56 | 140 | 26 56 57 |
| Ma Yujuan14 | 2014 | China | Case control study | 2012.3–2014.7 | ≤1w | DVT | 9 | 40 | 2 58 |
| PAN Hongxia34 | 2018 | China | Case control study | 2013.12–2015.1 | Not specified | VTE | 82 | 83 | 12 26 52 |
| R Clements24 | 2017 | Australia | Case control study | 2010–2013 | Acute SCI | VTE | 47 | 175 | 2 23 24 25 26 |
| DVT | 30 | 192 | 23 25 26 27 | ||||||
| PE | 25 | 197 | 2 23 24 | ||||||
| Reza Ehsanian75 | 2019 | USA | Retrospective cohort study | 2009.12–2013.1 | Acute SCI | VTE | 48 | 234 | 54 55 |
| S Aito36 | 2007 | Italy | Case control study | 1999.7–2004.2 | Acute SCI | DVT | 43 | 46 | 6 22 |
| Seth Ahlquist76 | 2020 | USA | Case control study | 2013.1–2018.8 | Acute SCI | VTE | 12 | 67 | 53 |
| Tracey Jones25 | 2005 | USA | Retrospective cohort study | 1991.1–2001.12 | Acute SCI | VTE | 977 | 15263 | 2 26 27 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 |
| XIAO Long-yi22 | 2018 | China | Case control study | 2016.1–2017.12 | Acute SCI | DVT | 31 | 129 | 1 9 24 64 56 |
| Xin, K.26 | 2019 | China | Case control study | 2015.5–2017.5 | Acute SCI | DVT | 34 | 131 | 24 26 56 57 58 |
| Xuan Cheng31 | 2017 | China | Case control study | 2011.1–2015.12 | <24 h | DVT | 76 | 301 | 4 14 15 |
| Yang, K.27 | 2020 | China | Case control study | 2015.9–2016.8 | Not specified | DVT | 68 | 352 | 24 27 62 65 63 |
| Yu, X.20 | 2020 | China | Case control study | 2016.1–2017.12 | <48 h | DVT | 35 | 45 | 1 24 56 57 58 |
| Yu Yingying32 | 2020 | China | Case control study | 2016.1–2018.12 | Not specified | DVT | 48 | 252 | 26 56 57 |
| Zhu Xiaoguang28 | 2015 | China | Case control study | 2013.1–2013.12 | <48 h | DVT | 46 | 97 | 24 26 59 |
*Risk factors:1. Age; 2. Sex; 3. Hypercholesterolemia; 4. History of vein thrombosis; 5. CRP; 6. Homocysteine elevation (HCY elevation); 7. High-density lipoprotein (HDL); 8. Lipoprotein(a) (LP(a)); 9. D-dimer; 10. Macrophage migration inhibitory factor (MIF); 11. Clinical complications; 12. Rehabilitation therapy; 13. IL-6; 14. Chronic kidney disease; 15. Small intestinal bacterial overgrowth (SIBO); 16. Length of stay (LOS); 17. Level I hospital; 18. Level III hospital; 19. Discharge status (Deceased); 20. Fibrinogen; 21. Prothrombin mutation; 22. Inhibitor of plasminogen activator-1 (PAI-1); 23. Weight; 24. Motor paralysis complete or AIS A grade; 25. Time in days between injury and commencement of anticoagulant chemoprophylaxis (per day increase); 26. Associated lower limb or pelvic fracture; 27. Paraplegia; 28. Factor V Leiden; 29. absence of spasticity; 30. Injury Severity Score(ISS); 31. C5-7 vs C1-4; 32. T1-6 vs C1-4; 33. T7-12 vs C1-4; 34. Lumbar vs C1-4; 35. Traumatic brain injury (TBI); 36. Chest trauma; 37. African American; 38. 8–13 yrs; 39. 14–19 yrs; 40. 20–29 yrs; 41. >80 yrs; 42. Insurance status; 43. Elixhauser Comorbidity Index score; 44. Elixhauser Comorbidity Index score (2); 45. Elixhauser Comorbidity Index score (>3); 46. 250–350 beds; 47. <125 beds; 48. Tracheostomy; 49. Unspecified tetraplegia; 50. Incomplete tetraplegia; 51. Unspecified paraplegia; 52. Degree of injury; 53. Early use of low molecular weight heparin or heparin; 54. Low vitamin D without supplementation; 55. vitamin D supplementation; 56. Diabetes; 57. Smoking history; 58. Treatment without limb air pressure and ankle pump; 59. Combined with lumbar nerve injury; 60. Blood type; 61. Degree of education; 62. Abnormal urination; 63. DVT cognitive level; 64. Obesity; 65. No spouse.
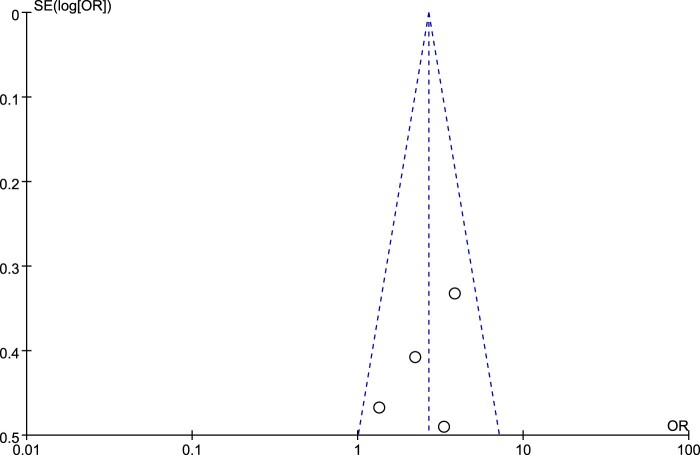
All articles were published between 2005 and 2020; 14 articles were published in the English language, and 11 articles were published in Chinese. Among the 25 studies, 23 were case–control studies and 2 were cohort studies. The studies involved a total of 2,865 cases of SCI with VTE and 39,488 controls. The methodological quality of the included studies was high (Table 2). There was no evidence of publication bias (for an example, please see Fig. 2).
Table 2.
Methodological quality of the included studies.
| References | Selection | Comparability | Outcome | NOS scores |
|---|---|---|---|---|
| Adrian A. Maung | ☆☆ | ☆☆ | ☆☆☆ | 7 |
| Selassie, A. W. | ☆☆ | ☆☆ | ☆☆☆ | 7 |
| Chao-Wei Wang | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Deborah Rubin-Asher | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Dong-Mei Wu | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| JC de Campos Guerra | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Jong Geol Do | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| R Clements | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Reza Ehsanian | ☆☆ | ☆☆ | ☆☆☆ | 7 |
| S Aito | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Seth Ahlquist | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Tracey Jones | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Xuan Cheng | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Yu, X. | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Li Chengyan | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| LIU Hong-wei | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Liu, W. | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Liu, Y. M. | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Ma Yujuan | ☆☆ | ☆☆ | ☆☆☆ | 7 |
| PAN Hongxia | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| XIAO Long-yi | ☆☆ | ☆☆ | ☆☆☆ | 7 |
| Xin, K. | ☆☆ | ☆☆ | ☆☆☆ | 7 |
| Yang, K. | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Yu Yingying | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Zhu Xiaoguang | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
Figure 2.
Funnel plot for history of smoking. The symmetrical funnel plot suggested there was no evidence of publication bias.
Risk factors for VTE
Age
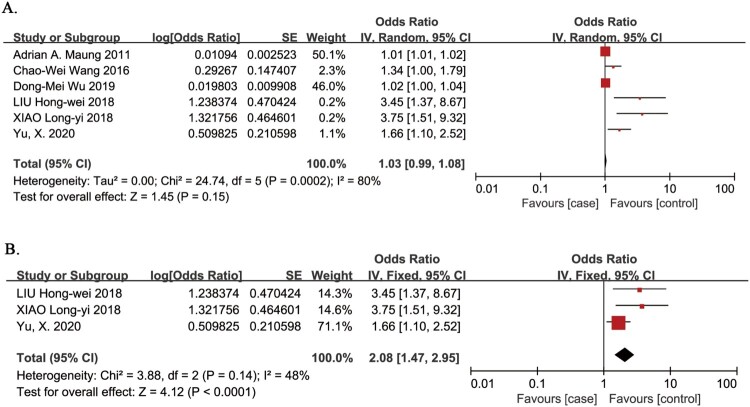
Age as a risk factor for VTE in patients with SCI was evaluated in 6 studies.5,12,19–22 The meta-analysis demonstrated no association between the risk of VTE in patients with SCI and age (OR 1.03, 95% CI 0.99, 1.08; P = 0.15; Fig. 3A). There was evidence of significant heterogeneity between studies (I2 = 80%, P = 0.0002). In a sensitivity analysis that omitted three studies that did not include age as a categorical variable,5,12,19 the risk of VTE in patients with SCI was significantly associated with age >45 years (OR 2.08, 95% CI 1.47, 2.95; P < 0.0001; Fig. 3B),20–22 and there was no evidence of heterogeneity between studies (I2 = 48%, P = 0.14).
Figure 3.
Age as a risk factor for VTE in patients with SCI. A. Primary analysis; there was no association between the risk of VTE in patients with SCI and age. There was evidence of significant heterogeneity between studies (I2 = 80%, P = 0.0002). B. Sensitivity analysis omitted three studies that did not include age as a categorical variable. The risk of VTE in patients with SCI was significantly associated with age >45 years. There was no evidence of heterogeneity between studies (I2 = 48%, P = 0.14).
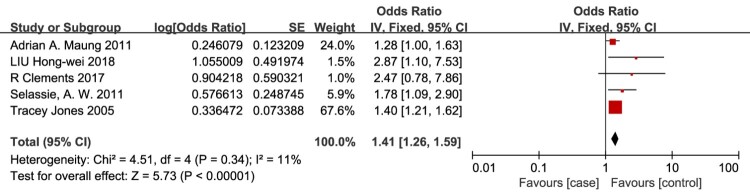
Male sex
Male sex as a risk factor for VTE in patients with SCI was evaluated in 5 studies.5,21,23–25 The meta-analysis demonstrated that the risk of VTE in patients with SCI was significantly associated with male sex (OR = 1.41, 95% CI, 1.26-1.59; P < 0.00001; Fig. 4), and there was no evidence of heterogeneity between studies (I2 = 11%, P = 0.34).
Figure 4.
Male sex as a risk factor for VTE in patients with SCI. The risk of VTE in patients with SCI was significantly associated with male sex. There was no evidence of heterogeneity between studies (I2 = 11%, P = 0.34).
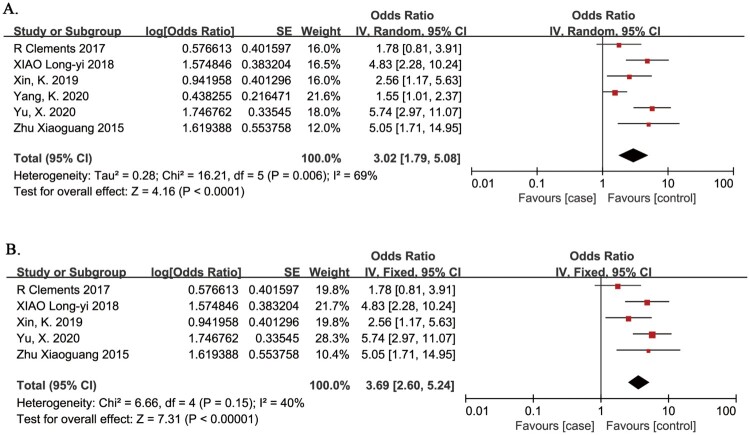
Complete paralysis
Complete paralysis as a risk factor for VTE in patients with SCI was evaluated in 6 studies.20,22,24,26–28 The meta-analysis demonstrated that the risk of VTE in patients with SCI was significantly associated with complete paralysis (OR 3.02, 95% CI 1.79, 5.08; P < 0.0001; Fig. 5A). There was evidence of significant heterogeneity between studies (I2 = 69%, P = 0.006). The source of heterogeneity was thought to arise from differing study populations. Four studies included subjects aged <45 years, and one study included subjects aged >45 years.32 In a sensitivity analysis, omission of this study confirmed that the risk of VTE in patients with SCI was significantly associated with complete paralysis (OR 3.69, 95% CI 2.60, 5.24; P < 0.00001; Fig. 5B), and there was no evidence of heterogeneity between studies (I2 = 40%, P = 0.15).32
Figure 5.
Complete paralysis as a risk factor for VTE in patients with SCI. A. Primary analysis; the risk of VTE in patients with SCI was significantly associated with complete paralysis. There was evidence of significant heterogeneity between studies (I2 = 69%, P = 0.006). B. Sensitivity analysis omitted a study in subjects aged >45 years. The risk of VTE in patients with SCI was significantly associated with complete paralysis. There was no evidence of heterogeneity between studies (I2 = 40%, P = 0.15).
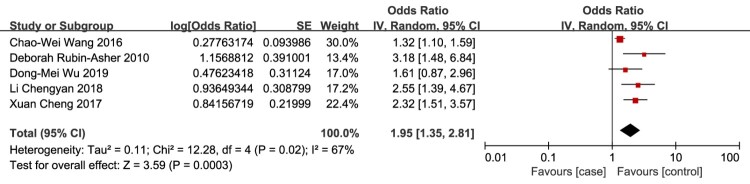
Personal / family history of venous thrombosis
Personal/family history of venous thrombosis as a risk factor for VTE in patients with SCI was evaluated in 5 studies.12,19,29–31 The meta-analysis demonstrated that the risk of VTE in patients with SCI was significantly associated with personal/family history of venous thrombosis (OR 1.95, 95% CI 1.35, 2.81; P < 0.0003; Fig. 6). There was evidence of significant heterogeneity between studies (I2 = 67%, P = 0.02). The source of the heterogeneity between studies was not obvious, but a fixed effect model confirmed the findings of the random effects model.
Figure 6.
Personal/family history of venous thrombosis as a risk factor for VTE in patients with SCI. The risk of VTE in patients with SCI was significantly associated with personal/family history of venous thrombosis. There was evidence of significant heterogeneity between studies (I2 = 67%, P = 0.02).
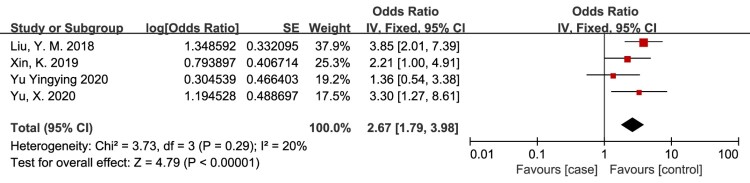
Smoking history
History of smoking as a risk factor for VTE in patients with SCI was evaluated in 4 studies.13,20,26,32 The meta-analysis demonstrated that the risk of VTE in patients with SCI was significantly associated with history of smoking (OR = 2.67, 95% CI, 1.79, 3.98; P < 0.00001; Fig. 7). There was no evidence of heterogeneity between studies (I2 = 20%, P = 0.29).
Figure 7.
History of smoking as a risk factor for VTE in patients with SCI. The risk of VTE in patients with SCI was significantly associated with history of smoking. There was no evidence of heterogeneity between studies (I2 = 20%, P = 0.29).
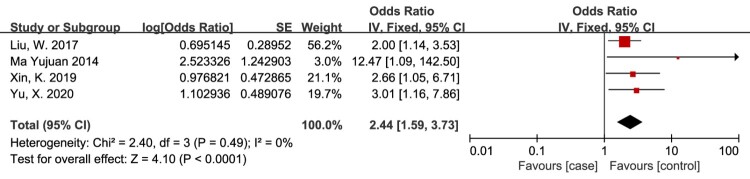
Lack of compression therapy
Treatment that did not include compression therapy as a risk factor for VTE in patients with SCI was evaluated in 4 studies.14,20,26,33 The meta-analysis demonstrated that the risk of VTE in patients with SCI was significantly associated with treatment that did not include compression therapy (OR = 2.44, 95% CI, 1.59, 3.73; P < 0.0001; Fig. 8). There was no evidence of heterogeneity between studies (I2 = 0%, P = 0.49).
Figure 8.
Lack of compression therapy as a risk factor for VTE in patients with SCI. The risk of VTE in patients with SCI was significantly associated with treatment that did not include compression therapy. There was no evidence of heterogeneity between studies.
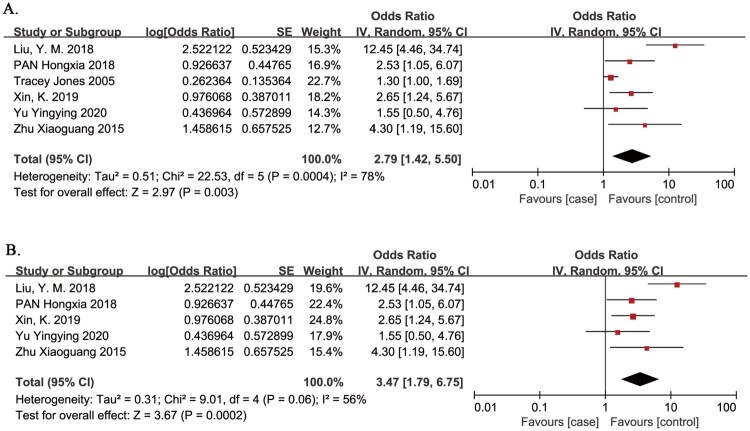
SCI combined with lower limb / pelvic fracture
The presence of a lower limb/pelvic fracture as a risk factor for VTE in patients with SCI was evaluated in 6 studies.13,25,26,28,32,34 The meta-analysis demonstrated that the risk of VTE in patients with SCI was significantly associated with the presence of a lower limb/pelvic fracture (OR = 2.79, 95% CI, 1.42, 5.50; P = 0.003; Fig. 9). There was evidence of significant heterogeneity between studies (I2 = 78%, P = 0.0004). One study proposed that trauma to a lower extremity was an independent risk factor for VTE, and did not include lower limb fracture.25 In a sensitivity analysis, omission of this study22 confirmed that the risk of VTE in patients with SCI was significantly associated with the presence of a lower limb/pelvic fracture (OR = 3.47, 95% CI, 1.79, 6.75; P = 0.0002). There was evidence of significant heterogeneity between studies (I2 = 56%, P = 0.06).
Figure 9.
Presence of a lower limb/pelvic fracture a risk factor for VTE in patients with SCI. A. Primary analysis; the risk of VTE in patients with SCI was significantly associated with the presence of a lower limb/pelvic fracture. There was evidence of significant heterogeneity between studies (I2 = 78%, P = 0.0004). B. Sensitivity analysis omitted one study that proposed trauma to a lower extremity was an independent risk factor for VTE, and did not include lower limb fracture. The risk of VTE in patients with SCI was significantly associated with the presence of a lower limb/pelvic fracture. There was evidence of significant heterogeneity between studies (I2 = 56%, P = 0.06).
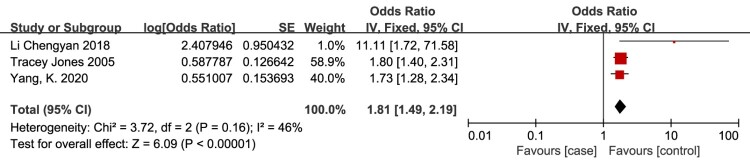
Paraplegia
Paraplegia is defined as injury below the cervical spinal cord (T1 and lower), and quadraplegia is defined as injury to the cervical spinal cord (C8 and higher).35 Paraplegia as a risk factor for VTE in patients with SCI was evaluated in 3 studies.25,27,30 The meta-analysis demonstrated that the risk of VTE in patients with SCI was significantly associated with paraplegia (OR = 1.81, 95% CI, 1.49, 2.19; P < 0.00001; Fig. 10). There was no evidence of heterogeneity between studies (I2 = 46%, P = 0.16).
Figure 10.
Paraplegia as a risk factor for VTE in patients with SCI. The risk of VTE in patients with SCI was significantly associated with paraplegia. There was no evidence of heterogeneity between studies (I2 = 46%, P = 0.16).
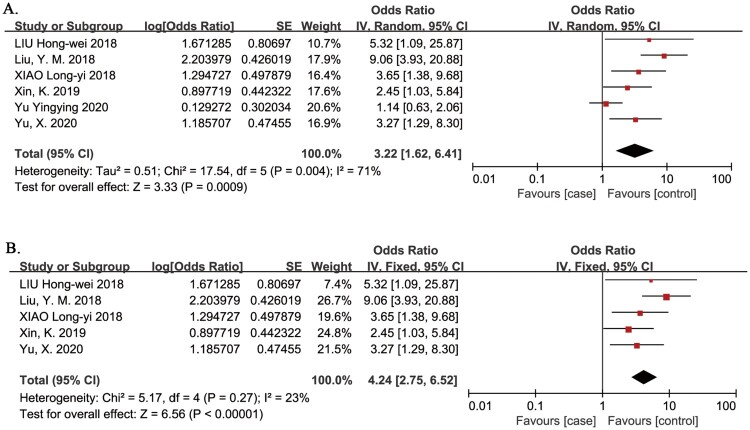
Diabetes
Diabetes as a risk factor for VTE in patients with SCI was evaluated in in 6 studies.13,20–22,26,32 The meta-analysis demonstrated that the risk of VTE in patients with SCI was significantly associated with diabetes (OR = 3.22, 95% CI, 1.62, 6.41; P = 0.0009; Fig. 11A). One study did not specify that patients had acute SCI.32 In a sensitivity analysis, omission of this study confirmed that the risk of VTE in patients with SCI was significantly associated with diabetes (OR = 4.24, 95% CI, 2.75, 6.52; P < 0.00001; Fig. 11B), and there was no evidence of heterogeneity between studies (I2 = 23%, P = 0.27).32
Figure 11.
Diabetes as a risk factor for VTE in patients with SCI. A. Primary analysis; the risk of VTE in patients with SCI was significantly associated with diabetes. There was evidence of significant heterogeneity between studies (I2 = 71%, P = 0.004). B. Sensitivity analysis omitted one study that did not specify that patients had acute SCI. The risk of VTE in patients with SCI was significantly associated with diabetes. There was no evidence of heterogeneity between studies (I2 = 23%, P = 0.27).
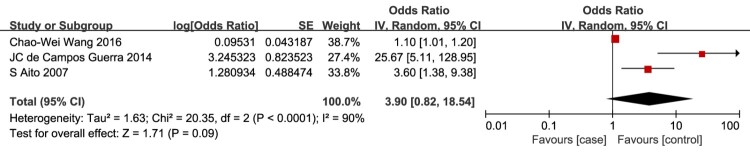
Elevated serum Hcy
Elevated serum Hcy level as a risk factor for VTE in patients with SCI was evaluated in 3 studies.11,12,36 The meta-analysis demonstrated no association between the risk of VTE in patients with SCI and elevated serum Hcy level (OR = 3.90, 95% CI, 0.82, 18.54; P = 0.09; Fig. 12).
Figure 12.
Elevated serum Hcy level as a risk factor for VTE in patients with SCI. There was no association between the risk of VTE in patients with SCI and elevated serum Hcy level.
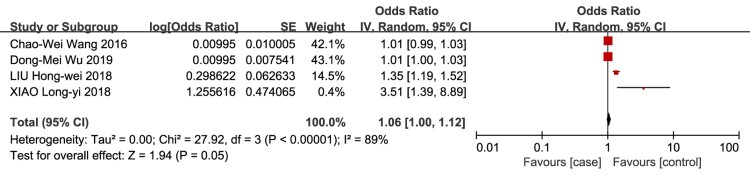
Elevated serum D-dimer
Elevated serum D-dimer level as a risk factor for VTE in patients with SCI was evaluated in 4 studies.12,19,21,22 The meta-analysis demonstrated no association between the risk of VTE in patients with SCI and elevated serum D-dimer level (OR = 1.06, 95% CI, 1.00, 1.12; P = 0.05; Fig. 13).
Figure 13.
Elevated serum D-dimer level as a risk factor for VTE in patients with SCI. There was no association between the risk of VTE in patients with SCI and elevated serum D-dimer level.
Overall, the meta-analysis identified 9 risk factors for VTE in patients with SCI, which are summarized in Table 3.
Table 3.
Risk factors for VTE in patients with SCI.
| Risk factors | Number of documents | OR | 95%CI | Heterogeneity test | Test for overall effect | |||
|---|---|---|---|---|---|---|---|---|
| Q | P | I2(%) | Z | P | ||||
| >45 years old | 3 | 2.08 | 1.47–2.95 | 3.88 | 0.14 | 48 | 4.12 | <0.0001 |
| Male sex | 5 | 1.41 | 1.26–1.59 | 4.51 | 0.34 | 11 | 5.73 | <0.00001 |
| Complete paralysis | 5 | 3.69 | 2.60–5.24 | 6.66 | 0.15 | 40 | 7.31 | <0.00001 |
| Personal / family history of VTE | 5 | 1.95 | 1.35–2.81 | 12.28 | 0.02 | 67 | 3.59 | 0.0003 |
| History of smoking | 4 | 2.67 | 1.79–3.98 | 3.73 | 0.29 | 20 | 4.79 | <0.00001 |
| Lack of compression therapy | 4 | 2.44 | 1.59–3.73 | 2.40 | 0.49 | 0 | 4.10 | <0.0001 |
| Presence of a lower limb /pelvic fracture | 5 | 3.47 | 1.79–6.75 | 9.01 | 0.06 | 56 | 3.67 | 0.0002 |
| Paraplegia | 3 | 1.81 | 1.49–2.19 | 3.72 | 0.16 | 46 | 6.09 | <0.00001 |
| Diabetes | 5 | 4.24 | 2.75–6.52 | 5.17 | 0.27 | 23 | 6.56 | <0.00001 |
Discussion
This meta-analysis identified age, male sex, complete paralysis, personal/family history of VTE, history of smoking, lack of compression therapy, presence of a lower limb/pelvic fracture, paraplegia, and diabetes as risk factors for VTE in patients with SCI.
Previous reports indicate that age is an independent risk factor for VTE in patients with SCI.3,6,37,38 Age less than 14 years was predictive of not developing VTE in patients with complete or incomplete SCI (OR = 0.2), 25 but the risk of developing VTE increased with age.4,5,10,39 In one study, the mean age of patients with SCI and VTE was 40 years, while the mean age of patients with SCI and no VTE was 32 years.6 In another study, age >45 years was a risk factor for VTE in patients with acute SCI (hazard ratio = 8.4).40 Accordingly, the results of our pooled analyses revealed that the risk of VTE in patients with SCI was significantly associated with age >45 years (OR = 2.08).
Some evidence suggests that male sex is an independent risk factor for VTE in patients with SCI.5,24,38,41–44 Consistent with this, findings from the present study confirmed that the risk of VTE in patients with SCI was significantly associated with male sex (OR = 1.41). The role of sex as an influencing factor in VTE is not clear. The increased incidence of VTE in men may be related to height, as individuals with longer limbs have a greater risk of developing DVT, 45,46 or hormonal factors.
Several studies show that severity of injury and loss of motor function are risk factors for VTE in patients with SCI, 4,5,10,25,47–49 while other reports suggest there is no difference in the incidence of DVT between patients with complete paralysis or incomplete paralysis.50 In one study, the risk of DVT was greater in patients with Frankel A SCI compared to patients with Frankel B, C or D SCI.38 Other studies showed that the risk of thrombosis was increased in patients with AIS Grade A injuries, where complete loss of motor or sensory function in the sacral segments S4–S5 means that the skeletal-muscle pump cannot aid in venous return.33,39 Our search identified no studies reporting on the incidence of DVT in lower AIS grades. The results of our pooled analyses revealed that the risk of VTE in patients with SCI was significantly associated with complete paralysis (OR = 3.69).
One study indicates that lack of compression therapy is a risk factor for VTE in patients with SCI.26 Skeletal muscles in the lower extremities become atrophied and fatigued and cannot maintain venous return following SCI, which can lead to VTE.33 Compression therapy can limit stasis in the paralyzed lower extremities and reduce the risk of VTE in patients with SCI.20,26,32,33 Consistent with this, findings from the present study confirmed that the risk of VTE in patients with SCI was significantly associated with lack of compression therapy (OR = 2.44).
Some evidence suggests that personal/family history of venous thrombosis is a risk factor for VTE, although the pathogenesis is unclear.12,31,40,51,52 Environmental and genetic factors may increase the risk of developing VTE in patients with acute SCI.12,53 One study proposed that 50% of patients with a personal or family history of VTE have a weak plasma anticoagulant response to activated protein C (APC), which is a serine protease with strong anticoagulant activity.54 Other studies showed that a point mutation at nucleotide 1691 of the Factor V gene leads to the formation of factor V Leiden (FVL), APC resistance, and thrombosis,55 and identified FVL and prothrombin 20210A (PT-20210A) variants as risk factors for VTE.56 The results of our pooled analyses confirmed that the risk of VTE in patients with SCI was significantly associated with personal/family history of venous thrombosis (OR = 1.95).
Several studies show that history of smoking is associated with VTE in the general population and in patients with various diseases.53,57–59 Nicotine and benzopyrene in tobacco may increase platelet activation, platelet aggregation and blood viscosity, induce vascular endothelial injury, and promote a hypercoagulable state. Oxidative stress and inflammation induced by smoking can directly act on the vascular endothelium and cause endothelial dysfunction. Together, these factors may increase the risk of VTE.13,20,26,32 Consistent with this, findings from the present study confirmed that the risk of VTE in patients with SCI was significantly associated with history of smoking (OR = 2.67).
Previous reports indicate that presence of a limb/pelvic fracture is an independent risk factor for VTE in patients with SCI.10,25,28,60,61 Lower limb/ pelvic fracture can destroy the integrity of blood vessels and damage vascular endothelial cells. Subsequent bed rest and surgical stress can reduce blood flow, trigger an inflammatory reaction, and increase blood viscosity, thus increasing the risk of DVT.13,26,32,34,62 Accordingly, the results of our pooled analyses revealed that the risk of VTE in patients with SCI was significantly associated with the presence of a lower limb/pelvic fracture (OR = 3.47).
Compared with quadraplegia, paraplegia may be a risk factor for VTE.5,6,10,25,40 This may be because mortality is higher in patients with quadraplegia compared to paraplegia; however, the influence of other factors, such as the degree of spasticity, presence of long bone and pelvic fractures and studies with small sample sizes, should also be considered.5,25,40 One report showed no difference in the incidence of VTE in patients with paraplegia or tetraplegia.63 Findings from the present study suggested that the risk of VTE in patients with SCI was significantly associated with paraplegia (OR = 1.81). Despite this, the findings should be interpreted with caution as the pooled analysis only included 3 studies.
There is growing evidence that diabetes is associated with an increased risk of VTE, although some meta-analyses show no significant association between diabetes and VTE in the general population.64–67 Hyperglycemia, hyperinsulinemia, and insulin resistance in patients with diabetes may damage endothelial cells and endothelial function, cause chronic inflammation, and promote hypercoagulability. Alternatively, the increased risk of VTE in individuals with diabetes may be due to confounding factors such as age, BMI, race, hypertension, dyslipidemia, or smoking rather than an inherent effect of diabetes.66,67 The results of our pooled analyses revealed that the risk of VTE in patients with SCI was significantly associated with diabetes (OR = 4.24).
Previous reports indicate that elevated serum Hcy levels are associated with an increased risk of VTE.12,68–70 However, findings from the present study suggested that the risk of VTE in patients with SCI was not associated with elevated serum Hcy. These disparate findings may be explained by our small sample size and the design of the included studies. One study analyzed elevated Hcy as a categorical rather than a continuous variable, and reported that serum Hcy levels of 15–30 μmol/L were associated with the risk of DVT.11
Our pooled analyses also revealed that the risk of VTE in patients with SCI was not associated with elevated serum D-dimer level. This is consistent with findings from other studies, which report that normal or low serum D-dimer levels are useful for excluding VTE.4,6,15,47,71,72
This meta-analysis had several limitations. First, several studies reported on the association of other factors with VTE, which were not included in our review due to a lack of data. Second, our pooled analyses for some factors included a small number of studies. Third, many older articles that do not report ORs were not included in the meta-analysis; therefore, potentially relevant risk factors for VTE may have been missed. In future, these articles may be included in a narrative review. Last, we did not differentiate between chronic and acute SCI, even though this may have clinical implications.73
In conclusion, this meta-analysis identified 9 risk factors for VTE in patients with SCI, including middle and old age, male sex, complete paralysis, personal/family history of venous thrombosis, history of smoking, lack of compression therapy, presence of a lower limb/pelvic fracture, paraplegia, and diabetes. Healthcare providers should be aware of the risk factors for VTE when rehabilitating patients with SCI.
Disclaimer statements
Contributors: None
Conflict of interest: The authors declare that they have no conflict of interest.
Funding Statement
None
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Richard-Denis A, Feldman DE, Thompson C, Mac-Thiong JM.. The impact of acute management on the occurrence of medical complications during the specialized spinal cord injury acute hospitalization following motor-complete cervical spinal cord injury. J Spinal Cord Med 2018;41(4):388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiGiorgio AM, Tsolinas R, Alazzeh M, Haefeli J, Talbott JF, Ferguson AR, et al. Safety and effectiveness of early chemical deep venous thrombosis prophylaxis after spinal cord injury: pilot prospective data. Neurosurg Focus 2017;43(5):E21. [DOI] [PubMed] [Google Scholar]
- 3.Piran S, Schulman S.. Incidence and risk factors for venous thromboembolism in patients with acute spinal cord injury: A retrospective study. Thromb Res 2016;147:97–101. [DOI] [PubMed] [Google Scholar]
- 4.Eichinger S, Eischer L, Sinkovec H, Wittgruber G, Traby L, Kammer M, et al. Risk of venous thromboembolism during rehabilitation of patients with spinal cord injury. PLoS One 2018;13(3):e0193735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maung AA, Schuster KM, Kaplan LJ, Maerz LL, Davis KA.. Risk of venous thromboembolism after spinal cord injury: not all levels are the same. J Trauma 2011;71(5):1241–5. [DOI] [PubMed] [Google Scholar]
- 6.Mackiewicz-Milewska M, Jung S, Kroszczynski AC, Mackiewicz-Nartowicz H, Serafin Z, Cisowska-Adamiak M, et al. Deep venous thrombosis in patients with chronic spinal cord injury. J Spinal Cord Med 2016;39(4):400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mammen E. Pathogenesis of venous thrombosis. Chest 1992;102:640S–644S. [DOI] [PubMed] [Google Scholar]
- 8.Horattas MC, Wright DJ, Fenton AH, Evans DM, Oddi MA, Kamienski RW, et al. Changing concepts of deep venous thrombosis of the upper extremity–report of a series and review of the literature. Surgery 1988;104(3):561–7. [PubMed] [Google Scholar]
- 9.Neuer RV. Fall von todlichen: emboli der lungenarterie. Arch Pathol Anat 1856;10:225–8. [Google Scholar]
- 10.Prevention of Venous Thromboembolism in Individuals with Spinal Cord Injury: Clinical Practice Guidelines for Health Care Providers, 3rd ed.: Consortium for Spinal Cord Medicine. Top Spinal Cord Inj Rehabil. 2016;22(3):209-40. [DOI] [PMC free article] [PubMed]
- 11.de Campos GJC, Mourão MA, França CN, Da RCD, Burattini MN.. Impact of coagulation in the development of thromboembolic events in patients with spinal cord injury. Spinal Cord 2014;52(4):327–32. [DOI] [PubMed] [Google Scholar]
- 12.Wang CW, Su LL, Tao SB, Ma PJ, Chang HG, Ji SB.. An increased serum level of lipoprotein(a) is a predictor for deep vein thrombosis in patients with spinal cord injuries. World Neurosurg 2016;87:607–12. [DOI] [PubMed] [Google Scholar]
- 13.Liu YM, Liu Y, Mao SF.. Risk factors of lower limb deep venous thrombosis in patients with spinal cord injury during rehabilitation. Chin J Gerontol 2018;38(5):1153–5. [Google Scholar]
- 14.Ma YJ, Cao LS, Cai XG, Zhuang WS, Yang Y, Zou LL.. Analysis of risk factors of lower extremity deep venous thrombosis in patients with early spinal cord injury. Chin J Phys Med Rehabil 2014;36(12):918–20. [Google Scholar]
- 15.Kearon C. Diagnosis of suspected venous thromboembolism. Hematology Am Soc Hematol Educ Program 2016;2016(1):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 18.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- 19.Wu DM, Zheng ZH, Wang S, Wen X, Han XR, Wang YJ, et al. Association between plasma macrophage migration inhibitor factor and deep vein thrombosis in patients with spinal cord injuries. Aging (Albany NY) 2019;11(8):2447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu X, Liu W, Zhang HW.. Analysis of related factors of deep venous thrombosis after spinal cord injury. China J Orthop Traumatol 2020;33(2):140–3. [DOI] [PubMed] [Google Scholar]
- 21.Liu HW, Liu L, Li J, Feng B, Yang DG, Feng YT.. Features and related factors for deep venous thrombosis in patients with traumatic paraplegia during early rehabilitation. Chin J Rehab Theory Pract 2018;24(2):191–5. [Google Scholar]
- 22.Xiao LY, Wen HL, Cao XL, Xuan TH, Deng R.. Clinical analysis of lower limb deep venous thrombosis after thoracolumbar spine fracture with spinal cord injury. J Gannan Med Univ 2018;38(11):1085–8. [Google Scholar]
- 23.Selassie AW, Varma A, Saunders LL.. Current trends in venous thromboembolism among persons hospitalized with acute traumatic spinal cord injury: does early access to rehabilitation matter? Arch Phys Med Rehabil 2011;92(10):1534–41. [DOI] [PubMed] [Google Scholar]
- 24.Clements R, Churilov L, Wahab AL, Ng LC.. Exploratory analysis of factors associated with venous thromboembolism in Victorian acute traumatic spinal cord-injured patients 2010–2013. Spinal Cord 2017;55(1):74–8. [DOI] [PubMed] [Google Scholar]
- 25.Jones T, Ugalde V, Franks P, Zhou H, White RH.. Venous thromboembolism after spinal cord injury: incidence, time course, and associated risk factors in 16,240 adults and children. Arch Phys Med Rehabil 2005;86(12):2240–7. [DOI] [PubMed] [Google Scholar]
- 26.Xin K, Wang TT.. Analysis of risk factors of postoperative lower limb deep venous thrombosis in patients with thoracolumbar spinal fracture complicated with spinal cord injury. J Cervicodynia Lumbodynia 2019;40(3):431–2. [Google Scholar]
- 27.Yang K, Zhao P, Xing FM, Zhang J, Wang FL, Zhang XL.. Influencing factors of lower limb venous thrombosis in middle-aged and elderly patients with spinal cord injury using blood circulation pump. Chin J Gerontol 2020;40(7):1552–4. [Google Scholar]
- 28.Zhu XG, Yang KC, Zhong WX, Wu W, Zhao G, Zhu JA.. Risk factors of deep vein thrombosis in patients with acute traumatic spinal cord injury. Chin J Emerg Med 2015;24(8):878–81. [Google Scholar]
- 29.Rubin-Asher D, Zeilig G, Ratner A, Asher I, Zivelin A, Seligsohn U, et al. Risk factors for failure of heparin thromboprophylaxis in patients with acute traumatic spinal cord injury. Thromb Res 2010;125(6):501–4. [DOI] [PubMed] [Google Scholar]
- 30.Li CY, Ning N, Qu JH, Liu N, He L, Li PF.. Analysis of the incidence and related factors of postoperative DVT in patients with acute spinal cord injury. Chin J Mod Nurs 2018;24(22):2673–6. [Google Scholar]
- 31.Cheng X, Zhang L, Xie NC, Xu HL, Lian YJ.. Association between small-intestinal bacterial overgrowth and deep vein thrombosis in patients with spinal cord injuries. J Thromb Haemost 2017;15(2):304–11. [DOI] [PubMed] [Google Scholar]
- 32.Yu YY, Xie L.. Analysis of related factors of lower limb deep venous thrombosis in patients with spinal cord injury treated with limb blood circulation pump. Chin J Prim Med Pharm 2020;27(7):841–5. [Google Scholar]
- 33.Liu W. The Situation and the Influence Factors of Blood Circulation Pump on Deep Venous Thrombosis in Patients with SCI. North China University of Technology. 2017. DOI:CNKI:CDMD:2.1017.741302.
- 34.Pan HX, Ding MF, Wei Q, He CQ.. Analysis of clinical factors of venous thrombosis in patients with spinal cord injury. Chin J Spine Spinal Cord 2018;28(1):57–61. [Google Scholar]
- 35.Karunakaran KD, He J, Zhao J, Cui JL, Zang YF, Zhang Z, et al. Differences in cortical gray matter atrophy of paraplegia and tetraplegia after complete spinal cord injury. J Neurotrauma 2019;36(12):2045–51. [DOI] [PubMed] [Google Scholar]
- 36.Aito S, Abbate R, Marcucci R, Cominelli E.. Endogenous risk factors for deep-vein thrombosis in patients with acute spinal cord injuries. Spinal Cord 2007;45(9):627–31. [DOI] [PubMed] [Google Scholar]
- 37.Caprini JA. Risk assessment as a guide to thrombosis prophylaxis. Curr Opin Pulm Med 2010;16(5):448–52. [DOI] [PubMed] [Google Scholar]
- 38.Waring WP, Karunas RS.. Acute spinal cord injuries and the incidence of clinically occurring thromboembolic disease. Paraplegia 1991;29(1):8–16. [DOI] [PubMed] [Google Scholar]
- 39.Hon B, Botticello A, Kirshblum S.. Duplex ultrasound surveillance for deep vein thrombosis after acute traumatic spinal cord injury at rehabilitation admission. J Spinal Cord Med 2020;43(3):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giorgi Pierfranceschi M, Donadini MP, Dentali F, Ageno W, Marazzi M, Bocchi R, et al. The short- and long-term risk of venous thromboembolism in patients with acute spinal cord injury: a prospective cohort study. Thromb Haemost 2013;109(1):34–8. [DOI] [PubMed] [Google Scholar]
- 41.Andreou ER, Koru-Sengul T, Linkins L, Bates SM, Ginsberg JS, Kearon C.. Differences in clinical presentation of deep vein thrombosis in men and women. J Thromb Haemost 2008;6(10):1713–19. [DOI] [PubMed] [Google Scholar]
- 42.Kyrle PA, Minar E, Bialonczyk C, Hirschl M, Weltermann A, Eichinger S.. The risk of recurrent venous thromboembolism in men and women. N Engl J Med 2004;350(25):2558–63. [DOI] [PubMed] [Google Scholar]
- 43.Montagnana M, Favaloro EJ, Franchini M, Guidi GC, Lippi G.. The role of ethnicity, age and gender in venous thromboembolism. J Thromb Thrombolysis 2010;29(4):489–96. [DOI] [PubMed] [Google Scholar]
- 44.White RH, Dager WE, Zhou H, Murin S.. Racial and gender differences in the incidence of recurrent venous thromboembolism. Thromb Haemost 2006;96(3):267–73. [DOI] [PubMed] [Google Scholar]
- 45.Faioni EM, Zighetti ML, Vozzo NP.. Sex, gender and venous thromboembolism: do we care enough? Blood Coagul Fibrinolysis 2018;29(8):663–7. [DOI] [PubMed] [Google Scholar]
- 46.Severinsen MT, Johnsen SP, Tjonneland A, Overvad K, Dethlefsen C, Kristensen SR.. Body height and sex-related differences in incidence of venous thromboembolism: a danish follow-up study. Eur J Intern Med 2010;21(4):268–72. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto S, Suda K, Iimoto S, Yasui K, Komatsu M, Ushiku C, et al. Prospective study of deep vein thrombosis in patients with spinal cord injury not receiving anticoagulant therapy. Spinal Cord 2015;53(4):306–9. [DOI] [PubMed] [Google Scholar]
- 48.Aito S, Gruppo Italiano Studio Epidemiologico Mielolesioni GG . Complications during the acute phase of traumatic spinal cord lesions. Spinal Cord 2003;41(11):629–35. [DOI] [PubMed] [Google Scholar]
- 49.Halim TA, Chhabra HS, Arora M, Kumar S.. Pharmacological prophylaxis for deep vein thrombosis in acute spinal cord injury: an Indian perspective. Spinal Cord 2014;52(7):547–50. [DOI] [PubMed] [Google Scholar]
- 50.Sugimoto Y, Ito Y, Tomioka M, Tanaka M, Hasegawa Y, Nakago K, et al. Deep venous thrombosis in patients with acute cervical spinal cord injury in a Japanese population: assessment with Doppler ultrasonography. J Orthop Sci 2009;14(4):374–6. [DOI] [PubMed] [Google Scholar]
- 51.Rubin-Asher D, Zeilig G, Ratner A, Asher I, Zivelin A, Seligsohn U, et al. Risk factors for failure of heparin thromboprophylaxis in patients with acute traumatic spinal cord injury. Thromb Res 2010;125(6):501–4. [DOI] [PubMed] [Google Scholar]
- 52.Zoller B, Li X, Sundquist J, Sundquist K.. Age- and gender-specific familial risks for venous thromboembolism: a nationwide epidemiological study based on hospitalizations in Sweden. Circulation 2011;124(9):1012–20. [DOI] [PubMed] [Google Scholar]
- 53.Suchon P, Resseguier N, Ibrahim M, Robin A, Venton G, Barthet MC, et al. Common risk factors add to inherited thrombophilia to predict venous thromboembolism risk in families. TH Open 2019;3(1):e28–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994;369(6475):64–7. [DOI] [PubMed] [Google Scholar]
- 55.Albagoush SA, Schmidt AE.. Factor V Leiden deficiency. Treasure Island (FL: ): StatPearls; 2020. [Google Scholar]
- 56.Varela ML, Adamczuk YP, Forastiero RR, Martinuzzo ME, Cerrato GS, Pombo G, et al. Major and potential prothrombotic genotypes in a cohort of patients with venous thromboembolism. Thromb Res 2001;104(5):317–24. [DOI] [PubMed] [Google Scholar]
- 57.Shah PH, Thompson RH, Boorjian SA, Lohse CM, Lyon TD, Shields RC, et al. Symptomatic venous thromboembolism is associated with inferior survival among patients undergoing nephrectomy with Inferior vena cava tumor thrombectomy for renal cell carcinoma. J Urol 2018;200(3):520–7. [DOI] [PubMed] [Google Scholar]
- 58.Kaddourah O, Numan L, Jeepalyam S, Abughanimeh O, Ghanimeh MA, Abuamr K.. Venous thromboembolism prophylaxis in inflammatory bowel disease flare-ups. Ann Gastroenterol 2019;32(6):578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng YJ, Liu ZH, Yao FJ, Zeng WT, Zheng DD, Dong YG, et al. Current and former smoking and risk for venous thromboembolism: a systematic review and meta-analysis. PLoS Med 2013;10(9):e1001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maxwell RA, Chavarria-Aguilar M, Cockerham WT, Lewis PL, Barker DE, Durham RM, et al. Routine prophylactic vena cava filtration is not indicated after acute spinal cord injury. J Trauma 2002;52(5):902–6. [DOI] [PubMed] [Google Scholar]
- 61.Chung WS, Lin CL, Chang SN, Chung HA, Sung FC, Kao CH.. Increased risk of deep vein thrombosis and pulmonary thromboembolism in patients with spinal cord injury: a nationwide cohort prospective study. Thromb Res 2014;133(4):579–84. [DOI] [PubMed] [Google Scholar]
- 62.Sen RK, Kumar A, Tripathy SK, Aggarwal S, Khandelwal N, Manoharan SR.. Risk of postoperative venous thromboembolism in Indian patients sustaining pelvi-acetabular injury. Int Orthop 2011;35(7):1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yelnik A, Dizien O, Bussel B, Schouman-Claeys E, Frija G, Pannier S, et al. Systematic lower limb phlebography in acute spinal cord injury in 147 patients. Paraplegia 1991;29(4):253–60. [DOI] [PubMed] [Google Scholar]
- 64.Peng YH, Lin YS, Chen CH, Tsai KY, Hung YC, Chen HJ, et al. Type 1 diabetes is associated with an increased risk of venous thromboembolism: A retrospective population-based cohort study. PLoS One 2020;15(1):e0226997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bai J, Ding X, Du X, Zhao X, Wang Z, Ma Z.. Diabetes is associated with increased risk of venous thromboembolism: a systematic review and meta-analysis. Thromb Res 2015;135(1):90–5. [DOI] [PubMed] [Google Scholar]
- 66.Gariani K, Mavrakanas T, Combescure C, Perrier A, Marti C.. Is diabetes mellitus a risk factor for venous thromboembolism? A systematic review and meta-analysis of case-control and cohort studies. Eur J Intern Med 2016;28:52–8. [DOI] [PubMed] [Google Scholar]
- 67.Bell EJ, Folsom AR, Lutsey PL, Selvin E, Zakai NA, Cushman M, et al. Diabetes mellitus and venous thromboembolism: A systematic review and meta-analysis. Diabetes Res Clin Pract 2016;111:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cattaneo M. Hyperhomocysteinemia and venous thromboembolism. Semin Thromb Hemost 2006;32(7):716–23. [DOI] [PubMed] [Google Scholar]
- 69.Hsu TS, Hsu LA, Chang CJ, Sun CF, Ko YL, Kuo CT, et al. Importance of hyperhomocysteinemia as a risk factor for venous thromboembolism in a Taiwanese population. A case-control study. Thromb Res 2001;102(5):387–95. [DOI] [PubMed] [Google Scholar]
- 70.Szlauer A, Mielimonka A, Glowacki R, Borowczyk K, Stachniuk J, Undas A.. Protein N-linked homocysteine is associated with recurrence of venous thromboembolism. Thromb Res 2015;136(5):911–6. [DOI] [PubMed] [Google Scholar]
- 71.Roussi J, Bentolila S, Boudaoud L, Casadevall N, Vallee C, Carlier R, et al. Contribution of D-Dimer determination in the exclusion of deep venous thrombosis in spinal cord injury patients. Spinal Cord 1999;37(8):548–52. [DOI] [PubMed] [Google Scholar]
- 72.Jianlong M, Diansheng Z, Jing R.. [Estimation of venous thromboembolism risk with thrombotic biomarkers in cancer patients]. Zhonghua Zhong Liu Za Zhi 2015;37(4):283–9. [PubMed] [Google Scholar]
- 73.de Campos Guerra JC, Mourao MA, Franca CN, da Rosa CD, Burattini MN.. Impact of coagulation in the development of thromboembolic events in patients with spinal cord injury. Spinal Cord 2014;52(4):327–32. [DOI] [PubMed] [Google Scholar]
- 74.Do JG, Kim DH, Sung DH.. Incidence of deep vein thrombosis after spinal cord injury in Korean patients at acute rehabilitation unit. J Korean Med Sci 2013;28(9):1382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ehsanian R, Timmerman MA, Wright JM, McKenna S, Dirlikov B, Crew J.. Venous thromboembolism is associated With lack of vitamin D supplementation in patients with spinal cord injury and low vitamin D levels. PM R 2019;11(2):125–34. [DOI] [PubMed] [Google Scholar]
- 76.Ahlquist S, Park HY, Kelley B, Holly L, Shamie AN, Park DY.. Venous thromboembolism chemoprophylaxis within 24 hours of surgery for spinal cord injury: is it safe and effective? Neurospine 2020;17(2):407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]